Summary
Background
Mobile telephones (henceforth ‘phones’) have become an essential part of everyday life in both healthcare and community settings. However, the widespread use of mobile phones in healthcare facilities is of concern because they can act as vehicles for transmitting pathogenic bacteria. This study aimed to investigate the bacterial contamination of mobile phones of healthcare workers (HCWs) at the University Teaching Hospital, Lusaka, Zambia.
Methods
This cross-sectional study, from May to July 2019, involved 117 HCWs. A self-administered questionnaire was used to gather sociodemographic and phone usage data. The mobile phones of HCWs were swabbed for culture and antimicrobial susceptibility testing.
Results
The overall prevalence of mobile phone contamination was 79%. The predominant isolates were coagulase-negative staphylococci (50%), Staphylococcus aureus (24.5%) and Bacillus spp. (14.3%). Other isolates were Escherichia coli, Acinetobacter spp., Pseudomonas spp., Klebsiella sp. and Proteus sp. Most isolates were susceptible to tetracycline, gentamicin and cotrimoxazole, while all Gram-positive organisms were resistant to penicillin. Meticillin resistance was detected in 25% and 48% of S. aureus and coagulase-negative staphylococci isolates, respectively. No significant association was found between mobile phone contamination and age group, gender, profession, mobile phone disinfection or work area.
Conclusion
Mobile phones of HCWs carry potentially pathogenic bacteria and can be a source of healthcare-associated infections in healthcare settings. Hence, regulations regarding the use of mobile phones need to be developed, especially in critical areas, to reduce the dissemination of pathogenic bacteria from hands to phones and, potentially, to patients.
Keywords: Mobile phones, Healthcare workers, Healthcare-associated infections, Bacterial contamination, Meticillin resistance
Introduction
Mobile telephones (henceforth ‘phones’) have become one of the most indispensable accessories of professional and social life [1,2]. A mobile or cellular phone is a long-range, portable electronic device for personal communication [1]. In under two decades, mobile phones have gone from being uncommon, costly pieces of equipment used mainly by the business elite, to common, low-cost personal items. In many countries, mobile phones outnumber landline telephones, as many adults and children now own their own personal mobile phones [3]. Mobile phones are popular with healthcare workers (HCWs) and patients alike [4,5], with approximately 98% of HCWs owning a mobile phone and 84.5% bringing them to work every day [6]. Their popularity is due to ease of access, low cost, user-friendliness and potential to be carried anywhere. With all the benefits of mobile phones, it is easy to overlook the health hazard they might pose to their users [5].
The constant use of mobile phones by HCWs and the lack of disinfection make them possible routes for transmission of bacterial pathogens, including multi-drug-resistant organisms [7,8]. The mobile phones of HCWs can serve as reservoirs of healthcare-associated pathogens and other organisms [9], particularly bacteria associated with skin colonization, due to the moisture and ideal temperature of the human body, particularly the palms of the hands [10]. These factors, and the heat generated by mobile phones, contribute to harbouring bacteria on the device. In addition, these bacterial organisms can survive on inanimate surfaces for weeks [11,12]. Hence, mobile phones may cause microbial cross-contamination between HCWs and patients, and may be a source of healthcare-acquired infections (HAIs) [[13], [14], [15]]. HAIs are a common threat to patient safety throughout the world, especially in low- and middle-income countries, as they lead to substantial morbidity, mortality and increased healthcare costs [16,17].
Studies have reported the isolation of various bacterial species from the surfaces of mobile phones, with coagulase-negative staphylococci (CoNS), a normal skin commensal, being the most common [10,18,19]. Potentially pathogenic organisms such as meticillin-susceptible Staphylococcus aureus, meticillin-resistant S. aureus (MRSA), Escherichia coli, Corynebacterium spp., Enterococcus faecalis, Clostridium perfringens, Klebsiella spp., Enterobacter spp., Pseudomonas spp., Aeromonas spp., Acinetobacter spp. and Stenotrophomonas maltophilia have also been reported [9,20], and may be potential threats to infection control practices, increasing the rate of HAIs [21].
While studies elsewhere have documented the role of mobile phones in the transmission of HAIs, there is no documented evidence that similar studies have been undertaken in Zambia. In addition, as it has been established that contamination of mobile phones varies geographically and also within different institutions or communities, there is a need to determine contamination rates in Zambian settings [22]. Furthermore, mobile phones are used without restriction in hospitals, and the majority of HCWs do not disinfect their mobile phones regularly [[23], [24], [25]]. Therefore, this study aimed to investigate the bacterial contamination of mobile phones of HCWs at the University Teaching Hospital, Lusaka, Zambia.
Methods
Study design, site and population
A cross-sectional study involving 117 HCWs (25 clinicians, 71 nurses and 21 laboratory personnel) at the University Teaching Hospital, Lusaka was conducted from May to July 2019. The University Teaching Hospital is the largest tertiary care and teaching hospital in Lusaka, which is the capital city of Zambia. The hospital offers specialized care to millions of residents and the country at large. This study included both male and female participants from the intensive care units (ICUs) (main ICU and neonatal ICU), paediatric operating theatre and general wards (admission, medical and surgical).
Data and sample collection
A self-administered questionnaire was used to collect data on sociodemographic variables, (e.g. age, sex and profession), in addition to mobile-phone-related questions (e.g. frequency of mobile phone disinfection and use of mobile phone at work). Following completion of self-administered questionnaires, the HCWs' mobile phones were swabbed using sterile swabs. Before taking a swab, both hands of the swab collector were cleaned using an alcohol-based hand sanitizer, and sterile powder-free disposable gloves were worn (per sample) throughout the work to prevent cross-contamination. The swabs were moistened with sterile normal saline before swabbing over the exposed surfaces of the mobile phone. The keypad, touch screen, earpad and back of the phone were swabbed as these are the areas that have frequent contact with the user. Samples were inoculated in tryptic soy broth (TSB) media, given unique identification numbers and transported to the laboratory. Thereafter, the mobile phone was disinfected using alcohol-based wipes and handed back to the user.
Isolation and identification of bacteria from mobile phones
The TSB media was incubated aerobically at 35–37oC for 24 h, and then inoculated on MacConkey, chocolate and blood agar. The plates were incubated at 35–37oC for 18–24 h, after which the plates were examined for growth and colony morphology. Pure isolated colonies were differentiated using Gram stain into Gram-negative and Gram-positive bacteria. The Gram-positive cocci were subjected to a catalase test, and those that were catalase-positive were further subjected to a coagulase test. This was undertaken to differentiate S. aureus from CoNS. Gram-negative isolates were identified using Simmons' citrate test, triple sugar iron agar, indole tests, lysine iron agar and oxidase test. The latter was employed to differentiate oxidase-positive Gram-negative bacteria (such as Pseudomonas spp. and Vibrio spp.) from oxidase-negative Enterobacterales.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of the isolates was determined using the Kirby–Bauer disc diffusion method on Mueller–Hinton agar, according to the 2019 guidelines of the Clinical and Laboratory Standards Institute (CLSI) [26]. Pure colonies (one to two colonies) of the organisms to be tested were added to a sterile tube containing 2 mL of normal saline, and mixed gently until a homogeneous suspension was formed. The turbidity of bacterial suspension was standardized using 0.5 McFarland standards. A sterile cotton swab was dipped into the suspension and inoculated over the entire surface of Mueller–Hinton agar (Oxoid, Basingstoke, UK). The Mueller–Hinton plates were left at room temperature to air dry for 3–5 min, after which antimicrobial discs were placed on the surface of the agar using sterilized forceps. The plates were then inverted carefully and incubated at 35–37°C for 18–24 h. Quality control was performed using reference strains E. coli ATCC 25922 and S. aureus ATCC 25923 according to the CLSI guidelines [26]. After 18–24 h, a metric ruler was used to measure the diameter of the zone of inhibition for each antibiotic disc tested. The measurements obtained were compared with the standard tables in the CLSI guidelines [26].
Data analysis
Data were entered into Excel 2016 (Microsoft Corp., Redmond, WA, USA) and analysed using Python 3.7 for Mac. Baseline demographic characteristics, mobile phone contamination and organisms isolated were analysed using descriptive statistics, and presented as frequencies and percentages in tables and graphs. The Chi-squared test of independence was performed to determine the relationships between mobile phone contamination and other variables, including profession, age group, gender, mobile phone disinfection and work area. Also, the Mann–Whitney U-test was conducted to determine the common types of bacteria isolated in the study.
Ethical considerations
Ethical approval to conduct the study was obtained from the University of Zambia Health Sciences Research Ethics Committee (Approval No. 20190217097). Verbal and written consent were obtained from all study participants.
Results
Demographic characteristics
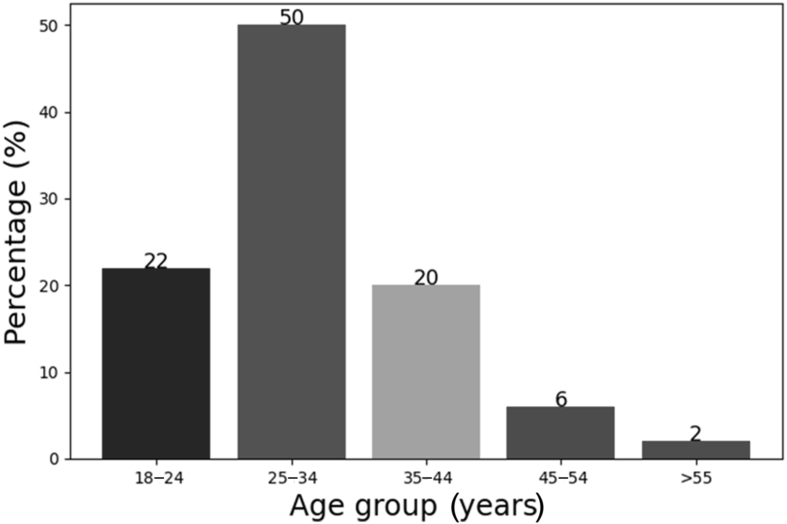
One hundred and seventeen participants were recruited into this study; of these, 75 (64%) were female. The majority were nurses [N=71 (61%)], followed by laboratory personnel [N=25 (21%)] and clinicians [N=21 (18%)]. The participants were divided into different age groups, and their distribution is shown in Figure 1. Data on the work area of the participants are shown in Table I.
Figure 1.
Age distribution of study participants.
Table I.
Frequencies and percentages of healthcare workers in different work areas (N=117)
| Work area | Frequency | Percentage (%) |
|---|---|---|
| Paediatrics theatre | 15 | 13 |
| NICU | 10 | 9 |
| Admission | 4 | 3 |
| MICU | 5 | 4 |
| Maternity ward | 11 | 9 |
| Medical wards | 26 | 22 |
| Surgical wards | 21 | 18 |
| Biochemistry laboratory | 4 | 3 |
| Histopathology laboratory | 4 | 3 |
| Parasitology laboratory | 6 | 5 |
| Bacteriology laboratory | 6 | 5 |
| Haematology laboratory | 5 | 4 |
NICU, neonatal intensive care unit; MICU, main intensive care unit.
Mobile phone usage and disinfection
The majority of participants (38%) reported use of their mobile phone <10 times; 36% of participants used their phone 10–20 times, 21% used their phone >20 times, and only 4% reported that they did not use their phone at work. Regarding disinfection, 92 (78%) of the participants disinfected their mobile phone occasionally, 15 (17%) never disinfected their phone, and only eight (7%) always disinfected their phone.
Mobile phone contamination and prevalence of bacteria on various phone surfaces
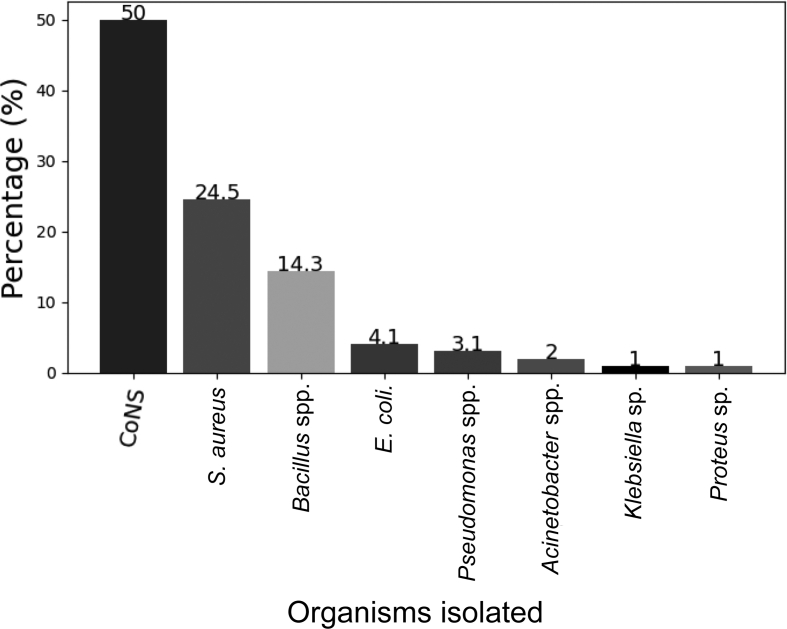
Bacterial contamination was found on 92 (79%) mobile phones belonging to the study participants. Of the bacteria isolated, 49 (50%) were CoNS, 24 (24.5%) were S. aureus, 14 (14.3%) were Bacillus spp., four (4.1%) were Escherichia coli, three (3.1%) were Pseudomonas spp., two (2%) were Acinetobacter spp., one (1%) was a Klebsiella sp. and one (1%) was a Proteus sp., as shown in Figure 2. Gram-positive bacteria were isolated more often than Gram-negative bacteria (P=0.036).
Figure 2.
Distribution of bacteria isolated from mobile phones of healthcare workers. CoNS, coagulase-negative staphylococci; S. aureus, Staphylococcus aureus; E. coli, Escherichia coli.
Factors associated with mobile phone contamination
The relationships between mobile phone contamination and other variables (profession, age group, gender and disinfection) were not significant, as shown in Table II.
Table II.
Relationship between mobile phone contamination and study variables
| Study variables (N=117) | χ2 | P-value |
|---|---|---|
| Profession | 0.2 | 0.92 |
| Age group | 1.2 | 0.88 |
| Gender | 0.05 | 0.82 |
| Mobile phone disinfection | 1.4 | 0.49 |
| Work area | 17.9 | 0.081 |
Antimicrobial susceptibility patterns of bacterial isolates
The antimicrobial susceptibility profiles of the isolates are shown in Table III. Most of the isolates were susceptible to first-line antimicrobial agents, except penicillin which showed 100% resistance for all Gram-positive isolates. S. aureus was susceptible to ciprofloxacin (88%), clindamycin (88%), gentamicin (84%), tetracycline (84%), cotrimoxazole (50%) and erythromycin (50%). The susceptibility patterns of CoNS are shown in Table III. Resistance to cefoxitin was detected in 25% (6/24) of S. aureus and 48% (21/49) of CoNS (Table III). Two-thirds of Pseudomonas spp. were resistant to ciprofloxacin and gentamicin (Table III).
Table III.
Antimicrobial susceptibility profiles of bacterial isolates from the mobile phones of healthcare workers at the University Teaching Hospital, Lusaka, Zambia
| Organism | Antimicrobial susceptibility (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S/R | CIP | CN | COT | CTX | E | FOX | GEN | P | TE | |
| CoNS | S | 88 | 88 | 50 | - | 50 | 52 | 84 | 0 | 84 |
| R | 12 | 12 | 50 | - | 50 | 48 | 16 | 100 | 16 | |
| S. aureus | S | 100 | 75 | 92 | - | 37.5 | 75 | 95.8 | 0 | 83.3 |
| R | 0 | 25 | 8 | - | 62.5 | 25 | 4.2 | 100 | 16.7 | |
| E. coli | S | 75 | - | 50 | 100 | - | - | 100 | - | 75 |
| R | 25 | - | 50 | 0 | - | - | 0 | - | 25 | |
| Klebsiella sp. | S | 100 | - | 100 | 100 | - | - | 100 | - | 100 |
| R | 0 | - | 0 | 0 | - | - | 0 | - | 0 | |
| Proteus sp. | S | 100 | - | 100 | 100 | - | - | 100 | - | 100 |
| R | 0 | - | 0 | 0 | - | - | 0 | - | 0 | |
| Acinetobacter spp. | S | 100 | - | 50 | 100 | - | - | 100 | - | 100 |
| R | 0 | - | 50 | 0 | - | - | 0 | - | 0 | |
| Pseudomonas spp. | S | 33.3 | - | - | - | - | - | 33.3 | - | - |
| R | 66.7 | - | - | - | - | - | 66.7 | - | - | |
CTX, cefotaxime; CIP, ciprofloxacin; CN, clindamycin; COT, cotrimoxazole; E, erythromycin; FOX, cefoxitin; GEN, gentamicin; P, penicillin; TE, tetracycline; CoNS, coagulase-negative staphylococci; -, not tested against the drug; S%, percentage susceptible to the antimicrobial agent; R%, percentage resistant to the antimicrobial agent.
Discussion
This study aimed to investigate the bacterial contamination of mobile phones of HCWs at the University Teaching Hospital, Lusaka. The study found that the mobile phones of HCWs generally harbour several bacterial organisms, including MRSA, and therefore represent a potential threat in the transmission of HAIs. The potential for transmission of healthcare-associated pathogens via electronic devices (e.g. personal digital assistants, handheld computers and bedside applications) has been reported previously [13,[27], [28], [29]].
This study showed that the rate of bacterial contamination of mobile phones of HCWs was 79%. Comparable results have been reported from other studies conducted in India (south and south-western regions) [30,31], Ethiopia [2,20], Egypt [20], Turkey [13] and Nepal [32], in which the number of contaminated devices ranged between 70% and 100%. However, other studies conducted in Ethiopia and the western region of India reported lower contamination levels of 30% and 62%, respectively [5,19]. The differences in contamination levels may be attributed to differences in geographical locations, methods used, sample sizes, mobile phone handling and hygiene practices of the sampled populations.
The most common bacterial mobile phone contaminants isolated in this study were CoNS (50%). This finding was in agreement with studies conducted in different parts of the world, which reported CoNS to be the predominant mobile phone contaminant (although with different isolation rates) [13,31,[33], [34], [35]]. In the present study, CoNS isolates were lower (50%) compared with reports from Italy (97%) [36], Iran (82.4%) [37], Pakistan (62%), [38] Saudi Arabia (60.5%) [8] and Ethiopia (58.8%) [2], and higher compared with reports from India (30.5%) [31], Ethiopia (37.1%) and Nigeria (42.9%) [33]. CoNS are normal skin flora and are relatively harmless in otherwise healthy individuals. However, they have been implicated in several HAIs, such as bacteraemia in immunocompromised patients, neonates, and surgical wound infections in patients with implanted valve prosthetic devices and catheters [39,40]. Additionally, CoNS have been shown to resist drying (they can remain viable for months on inanimate surfaces), and can multiply rapidly in warm environments [6,41].
The isolation rate of S. aureus (24%) from mobile phones of HCWs was in line with other studies conducted in India and Ethiopia, which reported rates of 29.5% and 14.4%, respectively [2,21]. Conversely, a study conducted in Ethiopia demonstrated a higher isolation rate of S. aureus, with the authors reporting that it was the predominant organism isolated [19]. In Kuwait, Heyba et al. [42] found a much lower percentage of S. aureus (1.9%) on mobile phones. S. aureus is frequently carried by healthy individuals on the skin and mucous membranes [2,43]. A previous study on hand and nasal carriage of S. aureus at the University Teaching Hospital, Lusaka found an overall carriage rate of 17.1% [43]. Carriers serve as a source of infection to themselves and others; for example, by direct contact or by contamination of fomites [15,44]. S. aureus is one of the most common causes of HAIs, often of wounds (surgical) or bacteraemia associated with catheters [45].
The isolation of Bacillus spp. (14%) in this study confirms its ubiquitous nature as well as the ability of its spores to resist environmental changes, and withstand dry heat and certain chemical disinfectants for moderate periods. Among the least isolated bacterial organisms in this study were E. coli (4.1%), Pseudomonas spp. (3.1%), Acinetobacter spp. (2%), Klebsiella sp. (1%) and Proteus sp. (1%). These findings are in line with a study conducted in Nigeria [33]. Additionally, these bacterial organisms have been reported to be the most common cause of HAIs in various healthcare settings [46,47]. Of great concern is the isolation of Pseudomonas and Acinetobacter spp. from the mobile phones of HCWs, as these organisms are known to be multi-drug-resistant healthcare-associated pathogens. Furthermore, the isolation of Acinetobacter spp. on mobile phones in this study is not surprising as they have been isolated from numerous sources in hospital environments in outbreak and non-outbreak settings [41]. The presence of E. coli suggests faecal contamination (a direct indicator that other Enterobacterales could be carried on mobile phones) [48,49], as shown by the presence of Klebsiella sp. in this study.
In this study, bacterial contamination of mobile phones was not influenced by profession, age group, gender, mobile phone disinfection or work area. This finding supports other studies which found no significant correlation between mobile phone contamination and other variables such as gender, age, use of mobile phone in work area [6,50], disinfection practices and restriction of mobile phone use at work [8]. Although the present study found no significant difference between bacterial colonization of mobile phones and mobile phone disinfection, several studies have reported a positive association between these two factors [2,42]. The difference could be attributed to the lack of standardized guidelines regarding mobile phone disinfection in healthcare settings, leading to improper disinfection of mobile phones, as well as the reliability of participants' responses.
Antimicrobial resistance is the most serious health threat for patients [45]. Most of the isolates in this study were resistant to at least one antimicrobial agent; notably, all Gram-positive isolates were resistant to penicillin. S. aureus and CoNS were 50% and 62.5% resistant to erythromycin, respectively. In addition, MRSA and meticillin-resistant CoNS (MRCoNS) were isolated in this study. This may indicate that mobile phones of HCWs are a habitat for drug-resistant pathogens which may be difficult to treat. These findings were similar to other studies conducted in different regions, such as South Asia and the Middle East [21,42,45,51]. Pseudomonas spp. showed high levels of resistance to the antibiotics tested. This resistance pattern could be because Pseudomonas spp. have intrinsic and acquired resistance as well as specific mutations (e.g. biofilm-mediated resistance and formation of multi-drug-tolerant persister cells that enhance survival in the presence of antibiotics) [52]. A similar study conducted in Ethiopia showed that Pseudomonas spp. was commonly multi-drug-resistant [2].
Nonetheless, some antimicrobial agents showed favourable activity against the isolates detected. For instance, most CoNS and S. aureus were susceptible to clindamycin, ciprofloxacin, gentamicin and tetracycline. Variation in antibiotic resistance patterns in different geographic areas, or during different time periods in the same place, may depend on the antibiotic policy of the hospital at that particular time. It may also be due to different bacterial strains, hospital environments, ease of availability of certain drugs (without prescription), dosages and indiscriminate/prolonged use of common antibiotics.
In conclusion, this study found that the mobile phones of HCWs were contaminated with potentially pathogenic bacteria that have been implicated in HAIs. CoNS and S. aureus (including MRCoNS and MRSA), Bacillus spp., E. coli, Acinetobacter spp., Pseudomonas spp., Klebsiella sp. and Proteus sp. were isolated during the study. Also, all Gram-positive isolates were resistant to penicillin. Clindamycin, tetracycline, cefotaxime, ciprofloxacin and gentamicin were the most effective antibiotics. The high levels of bacterial contamination of mobile phones of HCWs observed in this study highlights the need for regulations on the use of mobile phones in healthcare settings. Furthermore, studies regarding effective and efficient methods for mobile phone decontamination/disinfection should be conducted.
Funding sources
None.
Credit author statement
Conceived and designed the experiments: ANM, AK and MTS. Performed the experiments: ANM. Analysed the data: ANM, AK and PN. Supervision of Research: AK and MTS. Contributed reagents/materials/analysis tools and critical reading and editing of manuscript: KY, RN, PN and JN. Writing - original draft: ANM. Writing – review and editing: ANM, AK, MTS, PN, KY, RN, JN. All authors read and approved the final manuscript.
Conflict of interest statement
None declared.
Acknowledgements
The authors wish to thank the HCWs for their participation in this study. The authors also thank members of staff at the Ridgeway Campus and the University Teaching Hospital Microbiology Laboratories, and the University Teaching Hospital management for the permission to conduct the study at their healthcare facility.
References
- 1.Jaya Madhuri R., Saraswathi M., Mahitha G., Bhargavi M., Deepika S., Vijaya Lakshmi G. Bacterial contamination of mobile phones and computers in microbiological laboratories. Eur J Biotechnol Biosci. 2015;3:51–55. [Google Scholar]
- 2.Bodena D., Teklemariam Z., Balakrishnan S., Tesfa T. Bacterial contamination of mobile phones of health professionals in eastern Ethiopia: antimicrobial susceptibility and associated factors. Trop Med Health. 2019;47:15. doi: 10.1186/s41182-019-0144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Abdalall A.H. Isolation and identification of microbes associated with mobile phones in Dammam in eastern Saudi Arabia. J Family Community Med. 2010;17:11–14. doi: 10.4103/1319-1683.68783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramesh J., Carter A., Campbell M., Gibbons N., Powlett C., Moseley H., Sr. Use of mobile phones by medical staff at Queen Elizabeth Hospital, Barbados: evidence for both benefit and harm. J Hosp Infect. 2008;70:160–165. doi: 10.1016/j.jhin.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Rana R., Joshi S., Lakhani S., Kaur M., Patel P. Cell phones – homes for microbes. Int J Biol Med Res. 2013;4:3403–3406. [Google Scholar]
- 6.Brady R., Wasson A., Stirling I., McAllister C., Damani N. Is your phone bugged? The incidence of bacteria known to cause nosocomial infection on healthcare workers' mobile phones. J Hosp Infect. 2006;62:123–125. doi: 10.1016/j.jhin.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Famurewa O., David O. Cell phone: a medium of transmission of bacterial pathogens. World Rural Observ. 2009;1:69–72. [Google Scholar]
- 8.Banawas S., Abdel-Hadi A., Alaidarous M., Alshehri B., Bin Dukhyil A.A., Alsaweed M. Multidrug-resistant bacteria associated with cell phones of healthcare professionals in selected hospitals in Saudi Arabia. Can J Infect Dis Med Microbiol. 2018;2018:6598918. doi: 10.1155/2018/6598918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selim H.S., Abaza A.F. Microbial contamination of mobile phones in a health care setting in Alexandria, Egypt. GMS Hyg Infect Control. 2015;10:Doc03. doi: 10.3205/dgkh000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagoe D.N., Gyande V.K., Ansah E.O. Bacterial contamination of mobile phones: when your mobile phone could transmit more than just a call. Webmed Cent Microbiol. 2011;2:1–12. [Google Scholar]
- 11.Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber D.J., Rutala W.A., Miller M.B., Huslage K., Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 13.Ulger F., Esen S., Dilek A., Yanik K., Gunaydin M., Leblebicioglu H. Are we aware how contaminated our mobile phones with nosocomial pathogens? Ann Clin Microbiol Antimicrob. 2009;8:7. doi: 10.1186/1476-0711-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brady R.R., Fraser S.F., Dunlop M.G., Paterson-Brown S., Gibb A.P. Bacterial contamination of mobile communication devices in the operative environment. J Hosp Infect. 2007;66:397–398. doi: 10.1016/j.jhin.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Brady R.R., Hunt A.C., Visvanathan A., Rodrigues M.A., Graham C., Rae C. Mobile phone technology and hospitalized patients: a cross-sectional surveillance study of bacterial colonization, and patient opinions and behaviours. Clin Microbiol Infect. 2011;17:830–835. doi: 10.1111/j.1469-0691.2011.03493.x. [DOI] [PubMed] [Google Scholar]
- 16.Burke J.P. Infection control – a problem for patient safety. N Engl J Med. 2003;348:651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 17.Allegranzi B., Nejad S.B., Combescure C., Graafmans W., Attar H., Donaldson L. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 18.Kumar B.V., Hobani Y.H., Abdulhaq A., Jerah A.A., Hakami O.M., Eltigani M. Prevalence of antibacterial resistant bacterial contaminants from mobile phones of hospital inpatients. Libyan J Med. 2014;9:25451. doi: 10.3402/ljm.v9.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaka T., Misgana G., Feye B., Kassa R. Bacterial isolates from cell phones and hands of health care workers: a cross sectional study in pediatric wards at Black Lion Hospital, Addis Ababa, Ethiopia. J Bacteriol Parasitol. 2016;7:2. [Google Scholar]
- 20.Shahaby A., Awad N., El-Tarras A., Bahobial A. Mobile phone as potential reservoirs of bacterial pathogens. Afr J Biotechnol. 2012;11:15896–15904. [Google Scholar]
- 21.Misgana G.M., Abdissa K., Abebe G. Bacterial contamination of mobile phones of health care workers at Jimma University Specialized Hospital, Jimma, South West Ethiopia. Int J Infect Control. 2014;11:1–8. [Google Scholar]
- 22.Oluduro A.O., Ubani E., Ofoezie I. Bacterial assessment of electronic hardware user interfaces in Ile-Ife, Nigeria. Rev Cienc Farm Basica Ap. 2011;32:323–334. [Google Scholar]
- 23.Bhardwaj N., Khatri M., Bhardwaj S.K., Sonne C., Deep A., Kim K.H. A review on mobile phones as bacterial reservoirs in healthcare environments and potential device decontamination approaches. Environ Res. 2020;186:109569. doi: 10.1016/j.envres.2020.109569. [DOI] [PubMed] [Google Scholar]
- 24.Julian T., Singh A., Rousseau J., Weese J.S. Methicillin-resistant staphylococcal contamination of cellular phones of personnel in a veterinary teaching hospital. BMC Res Notes. 2012;5:193. doi: 10.1186/1756-0500-5-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loyola S., Gutierrez L.R., Horna G., Petersen K., Agapito J., Osada J. Extended-spectrum β-lactamase-producing Enterobacteriaceae in cell phones of health care workers from Peruvian pediatric and neonatal intensive care units. Am J Infect Control. 2016;44:910–916. doi: 10.1016/j.ajic.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute . 29th ed. CLSI Supplement M100; Wayne, PA: 2019. Performance standards for antimicrobial susceptibility testing. CLSI. [Google Scholar]
- 27.Manning M.L., Davis J., Sparnon E., Ballard R.M. iPads, droids, and bugs: infection prevention for mobile handheld devices at the point of care. Am J Infect Control. 2013;41:1073–1076. doi: 10.1016/j.ajic.2013.03.304. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy K., Laban K.L., Barrett K.E., Talbot D.C. Detection of viruses and body fluids which may contain viruses in the domestic environment. Epidemiol Infect. 1998;121:673–680. doi: 10.1017/s0950268898001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaacs D., Daley A., Dalton D., Hardiman R., Nallusamy R. Swabbing computers in search of nosocomial bacteria. Pediatr Infect Dis J. 1998;17:533. doi: 10.1097/00006454-199806000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Chawla K., Mukhopadhayay C., Gurung B., Bhate P., Bairy I. Bacterial ‘cell’phones: do cell phones carry potential pathogens? Online J Health Allied Sci. 2009;8 [Google Scholar]
- 31.Bhumbla U., Ahmad S., Mathur D., Bandey L., Mathur G. Study on microbial contamination of mobile phones and their role in nosocomial infections in a tertiary hospital of south India. Asian J Pharm Clin Res. 2016;9:201–202. [Google Scholar]
- 32.Karkee P., Madhup S., Humagain P., Thaku N., Timilsina B. Mobile phone: a possible vector of bacterial transmission in hospital setting. Kathmandu Univ Med J. 2017;59:217–221. [PubMed] [Google Scholar]
- 33.Nwankwo E.O., Ekwunife N., Mofolorunsho K.C. Nosocomial pathogens associated with the mobile phones of healthcare workers in a hospital in Anyigba, Kogi state, Nigeria. J Epidemiol Glob Health. 2014;4:135–140. doi: 10.1016/j.jegh.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadhem H., Abed Ali A., Hassan O. Isolation and identification of bacteria isolated from different parts of cell phones. World J Exp Biosci. 2016;4:29–31. [Google Scholar]
- 35.Chaman R., Nargeseyan S., Jannesar R., Ravangard S., Nikbakht G. Survey of prevalence and types of bacterial contamination of mobile phones of personnel employed in major wards of educational hospitals in Yasuj. J Fundam Appl Sci. 2018;10 [Google Scholar]
- 36.Galazzi A., Panigada M., Broggi E., Grancini A., Adamini I., Binda F. Microbiological colonization of healthcare workers' mobile phones in a tertiary-level Italian intensive care unit. Intensive Crit Care Nurs. 2019;52:17–21. doi: 10.1016/j.iccn.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Sedighi I., Alikhani M.Y., Ramezani S., Nazari M., Nejad A.S.M. Bacterial contamination of mobile phones of health care providers in a teaching hospital in Hamadan Province, Iran. Arch Clin Infect Dis. 2015;10 [Google Scholar]
- 38.Qureshi N.Q., Mufarrih S.H., Irfan S., Rashid R.H., Zubairi A.J., Sadruddin A. Mobile phones in the orthopedic operating room: microbial colonization and antimicrobial resistance. World J Orthop. 2020;11:252–264. doi: 10.5312/wjo.v11.i5.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker K., Heilmann C., Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naaz S., Madhavi K., Mai K., Sureka R.K. Microbial contamination of mobile phones a potential threat to the patients: a cross sectional study. Int J Curr Microbiol Appl Sci. 2019;8:1267–1274. [Google Scholar]
- 41.Borer A., Gilad J., Smolyakov R., Eskira S., Peled N., Porat N. Cell phones and acinetobacter transmission. Emerg Infect Dis. 2005;11:1160–1161. doi: 10.3201/eid1107.050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heyba M., Ismaiel M., Alotaibi A., Mahmoud M., Baqer H., Safar A. Microbiological contamination of mobile phones of clinicians in intensive care units and neonatal care units in public hospitals in Kuwait. BMC Infect Dis. 2015;15:434. doi: 10.1186/s12879-015-1172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakolwa G., Samutela M.T., Kwenda G., Mulundu G., Mwansa J., Hang'ombe B.M. Carriage rate and antimicrobial resistance profiles of Staphylococcus aureus among healthcare workers at a large tertiary referral hospital in Lusaka, Zambia. Sci Afr. 2019;5 [Google Scholar]
- 44.Debnath T., Bhowmik S., Islam T., Hassan Chowdhury M.M. Presence of multidrug-resistant bacteria on mobile phones of healthcare workers accelerates the spread of nosocomial infection and regarded as a threat to public health in Bangladesh. J Microsc Ultrastruct. 2018;16:165–169. doi: 10.4103/JMAU.JMAU_30_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morubagal R.R., Shivappa S.G., Mahale R.P., Neelambike S.M. Study of bacterial flora associated with mobile phones of healthcare workers and non-healthcare workers. Iran J Microbiol. 2017;9:143–151. [PMC free article] [PubMed] [Google Scholar]
- 46.Demissie M., Lulseged S. The prevalence of nosocomial infections and associated risk factors in pediatric patients in Tikur Anbessa Hospital. Ethiop J Ped Child Health. 2009;5:1–14. [Google Scholar]
- 47.Rosenthal V.D., Al-Abdely H.M., El-Kholy A.A., AlKhawaja S.A.A., Leblebicioglu H., Mehta Y. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010–2015: device-associated module. Am J Infect Control. 2016;44:1495–1504. doi: 10.1016/j.ajic.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Karabay O., Koçoglu E., Tahtaci M. The role of mobile phones in the spread of bacteria associated with nosocomial infections. J Infect Dev Ctries. 2007;1:72–73. [Google Scholar]
- 49.Tolera M., Abate D., Dheresa M., Marami D. Bacterial nosocomial infections and antimicrobial susceptibility pattern among patients admitted at Hiwot Fana Specialized University Hospital, eastern Ethiopia. Adv Med. 2018;2018:2127814. doi: 10.1155/2018/2127814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldblatt J.G., Krief I., Klonsky T., Haller D., Milloul V., Sixsmith D.M. Use of cellular telephones and transmission of pathogens by medical staff in New York and Israel. Infect Control Hosp Epidemiol. 2007;28:500–503. doi: 10.1086/513446. [DOI] [PubMed] [Google Scholar]
- 51.Pal S., Juyal D., Adekhandi S., Sharma M., Prakash R., Sharma N. Mobile phones: reservoirs for the transmission of nosocomial pathogens. Adv Biomed Res. 2015;4:144. doi: 10.4103/2277-9175.161553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]