Summary
Kodamaea ohmeri is a rare yeast pathogen that has recently emerged as an important cause of fungaemia in immunocompromised patients. In most cases, identification to the species level requires the adoption of new tools, including matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) or DNA sequencing. As K. ohmeri is a teleomorph of Candida guilliermondii var. membranaefaciens and its susceptibility to fluconazole is variable, a rapid and accurate identification of this yeast is important. Echinocandins may be the first choice for empiric therapy for this pathogen. We report the case of a 25-year-old male patient from North China who developed a catheter-related bloodstream infection caused by K. ohmeri. He was treated with caspofungin in hospital. He improved after removal of the central venous catheter and use of caspofungin as therapy. The K. ohmeri strain was identified using a MALDI-TOF mass spectrometer and a Vitek 2YST card. Definitive identification was obtained by a sequencing test of the internal transcribed spacer regions of the rDNA. Our patient findings, the first reported in mainland China, highlighted the diagnostic challenges associated with catheter-related bloodstream infection caused by fungi.
Keywords: Catheter-related bloodstream infection, Kodamaea ohmeri, Caspofungin, Case report
Introduction
Kodamaea ohmeri, previously known as Pichia ohmeri, is an ascomycetous yeast that belongs to the Saccharomycetaceae family [1]. K. ohmeri is an emerging opportunistic pathogen in clinical practice, and it should not be regarded as a blood culture contaminant [2]. In March 1998, the first case of fungaemia caused by K. ohmeri in an immunocompromised patient was reported in the USA [3]. Although the number of reported cases of this pathogen has recently increased, along with the high mortality rate, the appropriate therapy has not yet been established. In this report, we describe a case of catheter-related bloodstream infection (CRBSI) due to K. ohmeri that was successfully treated. The strain was identified using a matrix-assisted laser desorption/ionization time of flight (MALDI-TOF; Bruker, USA) mass spectrometer and VITEK (BioMerieux, France). Definitive identification was obtained using a sequencing test of the internal transcribed spacer (ITS) rDNA gene.
Presentation of case
A 25-year-old male patient was diagnosed with disturbance of consciousness, hypoxic-ischemic encephalopathy, pulmonary infection, and multiple organ dysfunction. On the 23rd July 2018, after drinking alcohol at noon, the patient worked in the sun for hours and in excess heat. The next morning, his family found him on the floor with signs of vomiting. He was admitted to the local hospital for a head CT, which showed no bleeding. His heart rate was 130 beats/min, body temperature was 39.5°C, and blood pressure was 120/60 mmHg. Laboratory results were as follows: white blood cells (WBC) 56.03 × 109/L, neutrophils 97.4%, haemoglobin 172 g/L, alanine aminotransferase (ALT) 6624 U/L, aspartate aminotransferase (AST) 6817 U/L, blood urea nitrogen (BUN) 14.8 mmol/L, creatine kinase (CK) 47080 U/L, lactate dehydrogenase (LDH) 8019 U/L, and procalcitonin (PCT) 25.68 ng/mL. A head multiple resonance image (MRI) showed a diffuse abnormal signal in the bilateral frontal cortex. Due to multi-organ failure, he was later transferred to the intensive care unit, where he was ventilated via a tracheostomy. On the 22nd August 2018, he was transferred to our hospital for further recovery. At the time of admission, the patient was unconscious, unable to communicate, and the muscle strength of his limbs was not coordinated. He was expectorating sputum and requiring intermittent assisted sputum aspiration. Sputum culture from the first hospital showed multidrug-resistant Acinetobacter baumannii. His body temperature gradually increased several days after admission, with his temperature recorded up to 39.5°C.
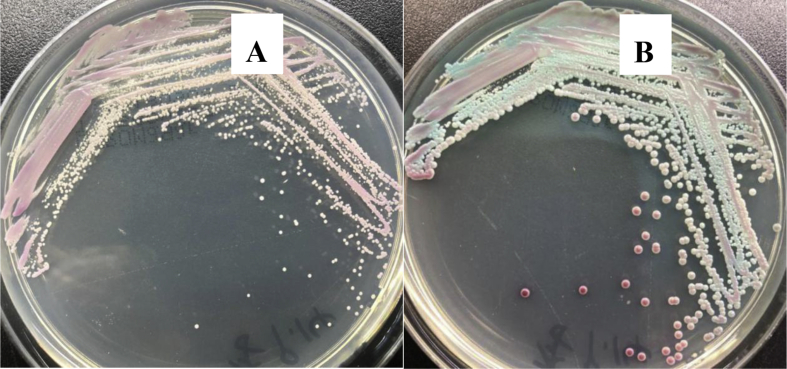
The central venous catheter (CVC) and urinary catheter were removed on 6th September 2018 and the CVC was sent to the clinical laboratory to be cultured on blood agar (Zhengzhou, Autobio) and Sabouraud dextrose agar (Tianjin, Jinzhang). There were white, rugged colonies visible on blood agar after 24-h culture (Figure 1). As shown in Figure 2, the colony was directly smeared on wet mount and Gram stain. Microscopically, there were numerous spores seen on direct wet mount (40×) and Gram stain (100×) (Figure 2). Then the colony was trans-cultured to CHROMagar plates (CHROMagar, France) in an incubator at 35°C and 5% CO2. From 24 h to 3 days of culture, the colour of the colonies changed from pink to blue (Figure 3). After 48 h, the colonies on blood agar were further identified as K. ohmeri using a MicrolexLT/SH mass spectrometer (Bruker, USA) with a score of 1.548, and the same result was obtained with the use of biochemical methods (Vitek 2 YST, bioMèrieux, France). Subsequently, the colonies were also identified by sequencing the ITS regions from the ribosomal DNA with fungus universal primers. All primer synthesis and sequencing was done commercially by BeiJing Biomed, China The sequencing primers were ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS2 (5′-TCCTCCGCTTATTGATATGC-3′). The Basic Local Alignment Search Tool (BLAST) alignment was compared to the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast). The result shared 99% similarity with K. ohmeri XS4 (GenBank accession: KY178312.1).
Figure 1.
Swabbed inoculum of central venous catheter on blood agar plate shows appearance of colonies after (A) 24 h, and (B) 3 days of incubation.
Figure 2.
Direct wet mount (40×) (A) and Gram stain under microscope (100×) (B).
Figure 3.
Swabbed inoculum of central venous catheter on CHROMagar-plate shows the appearance of colonies after (A) 24 h, and (B) 3 days of incubation.
The susceptibility to antifungal agents was tested using the ATB Fungus 3 system (bioMèrieux, France) according to the manufacturer's instructions; minimum inhibitory concentration (MIC) of the anti-fungal agents were as follows: amphotericin B <0.5 μg/mL, fluconazole 64 μg/mL, voriconazole 2 μg/mL, 5-flucytosine ≤4 μg/mL, and itraconazole 0.5 μg/mL. Results showed that the organism was susceptible to 5-flucytosine and amphotericin B but resistant to fluconazole. The susceptibility of the isolate to caspofungin was checked with E-test (Zhengzhou, Autobio). It showed susceptibility at MIC 0.125 μg/mL.
Ceftazidime (1 g q8h i.v.) and tigecycline (50 mg q12 i.v.) were used according to sputum culture and antibiotic susceptibility. A nonpruritic red rash appeared on his body and limbs after 3 days, which may have been caused by infection or allergy. The antibiotic treatment was adjusted to moxifloxacin (0.4 g OD i.v.), vancomycin (1 g BD i.v.), tinidazole (0.4 g OD i.v.), and caspofungin (50 mg OD i.v.). Meanwhile, CVC culture revealed K. ohmeri >100 colony-forming units (cfu)/5 cm on 10th September 2018. After consultation, only caspofungin and antihistamines were continued. Blood cultures obtained throughout his hospital stay were negative. The patient recovered after 30 days' therapy with caspofungin.
Discussion
K. ohmeri is a rare pathogen. Previously known as Pichia ohmeri and Yamadazyma ohmeri, K. ohmeri is an ascosporogenous yeast and a teleomorph of Candida guilliermondii var. membranaefaciens that belongs to class Ascomycetes and the Saccharomycetaceae family. The genus Kodamaea currently comprises five species: K. anthrophila, K. kakaduensis, K. laetipori, K. nitidulidarum, and K. ohmeri [1].When the first case of the fungus was isolated from a pleural effusion, it was initially treated as a contaminant [2]. K. ohmeri is reported to cause higher mortality (50%) in paediatric populations compared with patients with C. tropicalis fungaemia (24%) or non-fungaemia (18.2%) [4]. MALDI-TOF MS is a new proteomic approach that allows for rapid and accurate identification of rare fungi, as well as bacteria [5]. High mortality due to K. ohmeri infection may be related to the virulence factors of the organism, although no study has yet been conducted to confirm this. Thirty-eight (25.7%) of 148 phenotypically identified C. tropicalis isolates were confirmed as K. ohmeri by sequencing [6]. The high rate of fungaemia due to non-albicans Candida species has been observed, with almost 79–80% in both female and male populations in one centre in New Delhi, India [7]. Predisposing factors for K. ohmeri infection include diabetes mellitus, long-term broad-spectrum antibiotic therapy, use of intravenous catheters, malignancy, organ transplants, and intravenous drug use [8]. In an epidemiological study in an Indian tertiary care centre (2008–2009), Chakrabarti et al. showed that, in comparison to C. tropicalis fungaemia, piperacillin-tazobactam use was the only significant risk factor for acquiring K. ohmeri fungaemia in hospital. Whereas, when comparing K. ohmeri fungaemia and non-fungaemia groups, prolonged hospital stay, piperacillin-tazobactam use, endotracheal intubation, and mechanical ventilation were significant risk factors for the development of K. ohmeri fungaemia [6].
In this case, the susceptibility testing revealed fluconazole resistance, intermediate resistance to voriconazole and itraconazole, and 5-flucytosine and amphotericin B susceptibility. Chiu et al. [9] reported a case in which the K. ohmeri isolate was sensitive to fluconazole in vitro, but the patient did not clinically respond to therapy. Instead, the patient responded when fluconazole was changed to caspofungin therapy. Because fluconazole resistance was demonstrated in the clinical isolates, fluconazole would be a poor initial choice of antifungal therapy. Further, there is an increased risk of nephrotoxicity with higher doses of amphotericin B [10]. Little information is available for the susceptibility testing of K. ohmeri isolates with echinocandins, and there is even less clinical data in the literature on their efficacy in the treatment of K. ohmeri infections. In this case, due to renal dysfunction, caspofungin was administered instead of amphotericin B.
In conclusion, K. ohmeri infection in the setting of an underlying immunocompromised state contributes to an increased risk of mortality. Both antifungal therapy and removal of CVCs or implanted medical devices are essential when treating K. ohmeri fungaemia. Both our patient findings and literature review highlighted that CRBSIs caused by K. ohmeri provide challenges for laboratories.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding sources
None.
References
- 1.MycoBank . 2004. Fungal databases nomenclature and species banks online taxonomic novelties submission; p. 9. Utrecht: Netherlands. [Google Scholar]
- 2.Kurtzman C.P., Fell J.W. Elsevier Science; Amsterdam: 1998. The yeasts: a taxonomic study; pp. 273–352. [Google Scholar]
- 3.Bergman M.M., Gagnon D., Doern G.V. Pichia ohmeri fungemia. Diagn Microbiol Infect Dis. 1998;30(3):229–231. doi: 10.1016/s0732-8893(97)00233-2. [DOI] [PubMed] [Google Scholar]
- 4.De Barros J.D., Do Nascimento S.M., De Araújo F.J., Braz Rde F., Andrade V.S., Theelen B. Kodamaea (Pichia) ohmeri fungemia in a paediatric patient admitted in a public hospital. Med Mycol. 2009;47:775–779. doi: 10.3109/13693780902980467. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh A.K., Paul S., Sood P., Rudramurthy S.M., Rajbanshi A., Jillwin T.J. Matrix-assisted laser desorption ionization time-of-flight mass pectrometry for the rapid identification of yeasts causing bloodstream infections. Clin Microbiol Infect. 2015 Apr;21(4):372–378. doi: 10.1016/j.cmi.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti A., Rudramurthy S.M., Kale P., Hariprasath P., Dhaliwal M., Singhi S. Epidemiological study of a large cluster of fungaemia cases due to Kodamaea ohmeri in an Indian tertiary care centre. Clin Microbiol Infect. 2014 Feb;20(2):O83–O89. doi: 10.1111/1469-0691.12337. [DOI] [PubMed] [Google Scholar]
- 7.Xess I., Jain N., Hasan F., Mandal P., Banerjee U. Epidemiology of candidemia in a tertiary care centre of north India: 5-year study. Infection. 2007;35:256–259. doi: 10.1007/s15010-007-6144-6. [DOI] [PubMed] [Google Scholar]
- 8.Menon T., Herrera M., Periasamy S., Palanivelu V., Sikhamani R., Wickes B. Oral candidiasis caused by Kodamaea ohmeri in a HIV patient in Chennai, India. Mycoses. 2010 Sep;53(5):458–459. doi: 10.1111/j.1439-0507.2009.01731.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiu C.H., Wang Y.C., Shang S.T., Chang F.Y. Kodamaea ohmeri fungemia successfully treated with caspofungin. Int J Antimicrob Agents. 2010 Jan;35(1):98–99. doi: 10.1016/j.ijantimicag.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Harbarth S., Pestotnik S.L., Lloyd J.F., Burke J.P., Samore M.H. The epidemiology of nephrotoxicity associated with conventional amphotericin B therapy. Am J Med. 2001;111(7):528–534. doi: 10.1016/s0002-9343(01)00928-7. [DOI] [PubMed] [Google Scholar]