Abstract
Objective.
Somatic HER2 mutations occur in ~5% of cervical cancers and are considered oncogenic and associated with poor prognosis. Neratinib, an irreversible pan-HER tyrosine kinase inhibitor, is active in multiple HER2-mutant cancers. SUMMIT is a phase II basket trial investigating the efficacy and safety of neratinib in solid tumors.
Methods.
Patients with HER2-mutant, persistent, metastatic/recurrent cervical cancer with disease progression after platinum-based treatment for advanced/recurrent disease received oral neratinib 240 mg/day with mandatory loperamide prophylaxis during cycle 1. The primary endpoint was confirmed objective response rate (ORR). Secondary endpoints included: response duration (DOR); clinical benefit rate (CBR); progression-free survival (PFS); overall survival (OS); safety.
Results.
Sixteen eligible patients were enrolled; 10 (62.5%) had endocervical adenocarcinoma. The most common HER2 mutation was S310F (63% of patients). Three of 12 RECIST-measurable patients had confirmed partial responses (ORR 25%; 95%CI 5.5–57.2%); 3 had stable disease ≥16 weeks (CBR 50%; 95%CI 21.1–78.9%). DOR for responders were 5.6, 5.9, and 12.3 months. Median PFS was 7.0 months (95%CI 0.7–18.3 months); median OS was 16.8 months (95%CI 4.1–NE months). Diarrhea (75%), nausea (44%), and decreased appetite (38%) were the most common adverse events. One patient (6%) reported grade 3 diarrhea. There were no grade 4 events, and no diarrhea-related treatment discontinuations.
Conclusions.
Neratinib monotherapy showed evidence of activity in heavily pretreated patients with HER2-mutant cervical cancer, with no new safety signals. Given the few effective options for cervical cancer after platinum-based therapy failure, neratinib warrants further investigation in this molecularly defined patient population.
Trial registration number.
NCT01953926 (ClinicalTrials.gov), 2013–002872–42 (EudraCT).
Keywords: Cervical cancer, HER2 mutant, Neratinib, Clinical trial, Tyrosine kinase inhibitor
1. Introduction
Cervical cancer is a global health crisis [1] and the fourth most common malignant disease worldwide among women in terms of both incidence and mortality [2], with one woman dying of cervical cancer every 2 min [3]. In the United States, cervical cancer is the second most common cause of cancer death in women aged 20–39 years, leading to 10 premature deaths per week [4]. Although broad screening and the development of human papillomavirus vaccines have reduced the incidence of cervical cancer in some countries, 13% of patients are still diagnosed at an advanced stage [5]. Such patients are at high risk for locally recurrent and/or metastatic disease, which has a poor prognosis with median overall survival of 16.8 months; the 5-year overall survival (OS) rate for all disease stages is 68% [6].
Platinum-based chemotherapy plus bevacizumab is the standard first-line treatment for persistent, recurrent, and metastatic cervical cancer [7,8], but there is an increasing need for more effective therapies for patients who have progressed on or after platinum-based therapy [9]. In the US, the Food and Drug Administration (FDA) approved bevacizumab in 2014 as first-line treatment for persistent, recurrent, or metastatic cervical cancer, and approved pembrolizumab in 2018 for patients with recurrent or metastatic cervical cancer who had progressed on or after platinum-based chemotherapy and whose tumors have a PD-L1 combined positive score (CPS) ≥1. Currently there are no approved targeted treatments for cervical cancer.
HER2 is a member of the HER family of transmembrane receptor tyrosine kinases, which also includes HER1 (EGFR), HER3, and HER4. Increased HER2 expression and the resultant activation of its tyrosine kinase domain are associated with cellular transformation and HER2 is a validated therapeutic target in breast and esophagogastric cancers [10]. Somatic activating HER2 mutations are a recently identified class of oncogenic drivers that are present in a variety of solid tumor malignancies including bladder, colorectal, lung, breast, and cervical cancers [11–14]. Sequencing studies indicate that HER2 mutations are present in 3–6% of cervical cancers [15–18] and may be associated with a poor prognosis [16]. Given the clinical utility of HER2-targeted therapies in patients with breast, esophagogastric, endometrial, and lung cancers, the subset of patients with cervical cancers harboring HER2 mutations could potentially benefit from HER2-targeted therapies, such as irreversible pan-HER kinase inhibitors [15–17,19].
Neratinib is an oral, irreversible, pan-HER tyrosine kinase inhibitor (TKI) [20] that has demonstrated efficacy in the treatment of patients with early-stage or metastatic HER2-positive breast cancer [21–24]. Neratinib has also demonstrated potent inhibition of cell proliferation in HER2-mutant cervical cancer cell lines and potent inhibition of tumor growth in HER2-mutant cervical cancer xenograft models [17].
SUMMIT is a phase II basket study investigating the efficacy and safety of neratinib across a broad spectrum of cancer types in patients whose tumors harbor activating HER2 somatic mutations [25]. We report results from a cohort of heavily pretreated patients with HER2-mutant, metastatic cervical cancer receiving neratinib in SUMMIT.
2. Patients and methods
2.1. Study oversight
The SUMMIT study is being conducted in compliance with the principles of good clinical practice and in accordance with the International Conference on Harmonisation and the ethical principles of the Declaration of Helsinki. SUMMIT was approved by the independent ethics committee or institutional review board at each study site. All patients provided written informed consent prior to study entry.
2.2. Patient eligibility
Eligible patients were women aged ≥18 years with histologically confirmed metastatic cervical cancer for whom no curative treatment existed and who had a likely pathogenic mutation in HER2. Patients were also required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, with adequate hematopoietic, hepatic, kidney, and cardiac function (defined as a left ventricular ejection fraction ≥50%). Key exclusion criteria included prior therapy with HER2-directed TKIs and prior radiotherapy ≤14 days before treatment initiation. Patients with treated and/or asymptomatic brain metastases were eligible. HER2 mutations were identified by testing at each participating site; tissue- or plasma-based sequencing assays performed by a Clinical Laboratory Improvement Amendments-certified or regionally equivalent laboratory were accepted.
2.3. Study design and treatment
SUMMIT is an open-label, single-arm, multicohort, multitumor, phase II, basket trial being conducted at 57 centers internationally (NCT01953926; EudraCT 2013–002872–42). Eligible patients received oral neratinib 240 mg once daily with food (recommended to be taken in the morning) on a continuous basis, with mandatory loperamide prophylaxis during cycle 1 (12 mg/day on days 1–14; 8 mg/day on days 15–28) and then as needed thereafter but not exceeding 16 mg/day. Patients were treated until disease progression, unacceptable toxicity, or withdrawal of consent.
2.4. Assessments
The primary endpoint was the confirmed objective response rate (ORR). Secondary endpoints included duration of response (DOR), clinical benefit rate (CBR), progression-free survival (PFS), OS, and safety. Tumor response was assessed locally every 8 weeks by computed tomography (CT), magnetic resonance imaging, and/or fluorodeoxyglucose-positron-emission tomography (FDG-PET). Adverse events, classified according to Common Terminology Criteria for Adverse Events (version 4.0), were monitored from the first dose until day 28 after discontinuation of study treatment.
2.5. Statistical analyses
The data cutoff for this report was February 2020. Baseline characteristics, efficacy, and safety were summarized in the safety analysis set, which included all patients who received at least one dose of neratinib. Efficacy analyses of tumor response data were also performed for patients in the Response Evaluation Criteria in Solid Tumors (RECIST) efficacy evaluable set, which included patients with RECIST-measurable disease at baseline who had at least one post-baseline tumor assessment per RECIST or who discontinued treatment prior to the first scheduled post-baseline tumor assessment. One patient in the safety analysis set was only evaluable by FDG-PET, therefore their tumor responses were evaluated by PET Response Criteria (PERCIST). All other patients in the safety analysis set and all patients in the RECIST efficacy evaluable set were evaluated by RECIST (version 1.1). PFS and OS were estimated using the Kaplan–Meier method. The Clopper–Pearson method was used to calculate 95% confidence intervals (CIs) for ORR and CBR. All statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient demographics and characteristics
Sixteen patients with histologically confirmed metastatic cervical cancer were enrolled and comprised the safety analysis set; 12 of these patients were evaluable for efficacy by RECIST (Fig. S1). All patients had documented evidence of a somatic HER2 mutation at the time of enrollment, as determined by a local, tumor tissue-based, next-generation sequencing assay. The median age of patients was 55 years (range 29–64 years), the majority (81%) were white, had endocervical adenocarcinoma (62.5%), and an ECOG performance status of 1 (63%) (Table 1). Further details on patient/disease characteristics and previous treatments are provided in Table S1.
Table 1.
Demographics and patient characteristics – safety analysis set (N = 16).
| HER2-mutant cervical cohort (N = 16) | |
|---|---|
| Median age (range), years | 55.0 (29–64) |
| Race, n (%) | |
| White | 13 (81.3) |
| Asian | 1 (6.3) |
| Black | 1 (6.3) |
| Other | 1 (6.3) |
| ECOG performance status, n (%) | |
| 0 | 6 (37.5) |
| 1 | 10 (62.5) |
| FIGO stage at diagnosisa, n (%) | |
| I | 7 (43.8) |
| II | 3 (18.8) |
| IIIB | 2 (12.5) |
| IV | 4 (25.0) |
| Histology, n (%) | |
| Endocervical adenocarcinoma | 10 (62.5) |
| Squamous cell carcinoma | 3 (18.8) |
| Adenocarcinoma | 2 (12.5) |
| Gastric type adenocarcinoma | 1 (6.3) |
| Median time from development of metastatic disease to enrollment (range), years | 1.2 (0.1–8.4) |
| Previous therapeutic interventionsb, n (%) | |
| Cisplatin | 5 (31.3)a |
| Carboplatin | 10 (62.5) |
| Paclitaxel | 15 (93.8) |
| Bevacizumab | 11 (68.8) |
| Topotecan | 2 (12.5) |
| Pembrolizumab | 2 (12.5) |
| Prior chemoradiation, n (%) | 6 (37.5) |
| Prior surgery, n (%) | 12 (75.0) |
ECOG: Eastern Cooperative Oncology Group; FIGO: International Federation of Gynecology and Obstetrics.
Five patients reported receiving cisplatin without concurrent radiation; of these, two had also previously received chemoradiation with cisplatin.
A complete list of previous systemic therapies is available in Supplementary Table S1.
All patients had previously been treated with platinum-based chemotherapy; 11 patients (69%) had previously received bevacizumab, and 2 (13%) had received pembrolizumab. The median number of prior systemic chemotherapy regimens was 2 (range 1–3) and 6 patients (38%) had received prior chemoradiation. Nine patients had persistent disease and seven had reoccurred. The most common HER2 variant was the hotspot S310F/Y mutation, which was identified in 10 of the patients (63%) (Fig. S2). The high prevalence of S310 mutation in this population of patients with cervical cancer with oncogenic HER2 mutations is consistent with prior reports [15,16,26].
3.2. Efficacy
One patient had a complete response (PERCIST) and 3 had partial responses (RECIST), for a confirmed ORR of 25.0% (95% CI 7.3–52.4%; Table 2) in the safety analysis set. Among the 12 patients with RECIST-measurable disease in the efficacy analysis set, 3 patients with endocervical adenocarcinoma had a confirmed objective partial response (ORR 25%; 95% CI 5.5–57.2%) and 3 additional patients had stable disease lasting ≥16 weeks (CBR 50%; 95% CI 22.1–78.9%; Table 2). Further details on responses to treatment are shown in Table S1.
Table 2.
Efficacy summary – safety analysis set (N = 16) and RECIST efficacy evaluable patients (N = 12).
| Efficacy endpoint | Safety analysis seta (N = 16) | RECIST efficacy evaluable patients (N = 12) |
|---|---|---|
| Objective response (confirmed), n (%) | 4 (25.0) | 3 (25.0) |
| CRb | 1 (6.3) | 0 |
| PR | 3 (18.8) | 3 (25.0) |
| Objective response rate, % (95% CI) | 25.0 (7.3–52.4) | 25.0 (5.5–57.2) |
| Duration of response, months | 3.7c, 5.6. 5.9, 12.3 | 5.6,5.9,12.3 |
| Clinical benefit rate, % (95% CI) | 43.8 (19.8–70.1) | 50.0 (21.1–78.9) |
| Median PFS, months (95% CI) | 7.0 (1.0–18.3)d | |
| Median OS, months (95% CI) | 16.8(4.1-NE)d | |
CI: confidence interval; CR: complete response; NE: not estimable; OS: overall survival; PFS: progression-free survival; PR: partial response; RECIST: Response Evaluation Criteria in Solid Tumors.
Not all patients had RECIST-measurable disease or post-baseline tumor assessments.
Confirmed by PERCIST.
Response ongoing.
PFS and OS calculated in all patients who received at least one dose of neratinib (N = 16).
The 3 patients who achieved a partial response all had reductions in tumor size >50% (−58.3%, −81.4%, and −86.7%; Fig. 1). The durations of response for the 3 responders were 5.6, 5.9, and 12.3 months. The specific HER2 mutation, duration of treatment, and best response for each of the 16 patients are shown in Fig. 2. Treatment duration ranged from 1 to >168 weeks. At the time of analysis, neratinib treatment was ongoing in 5 patients and 8 patients had died.
Fig. 1.
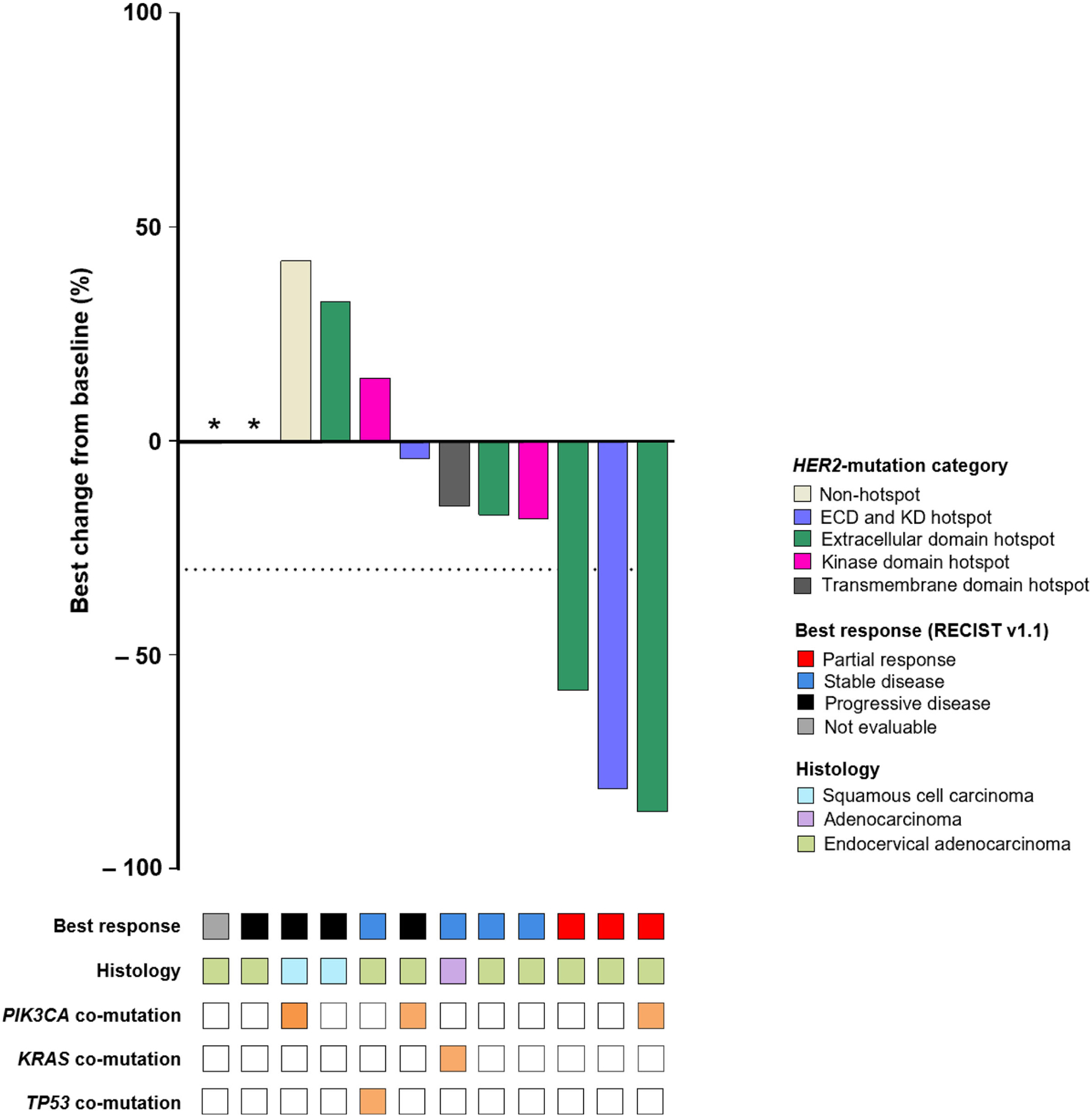
Best change in tumor size and characteristics in RECIST efficacy evaluable patients (N = 12). Response based on investigator tumor assessments by RECIST (version 1.1). Only the most common co-mutations, as reported by local testing at time of enrollment, are shown. *Patient developed new lesion (progressive disease) and had no post-baseline target lesion measurement. ECD: extracellular domain; KD: kinase domain; RECIST: Response Evaluation Criteria in Solid Tumors.
Fig. 2.
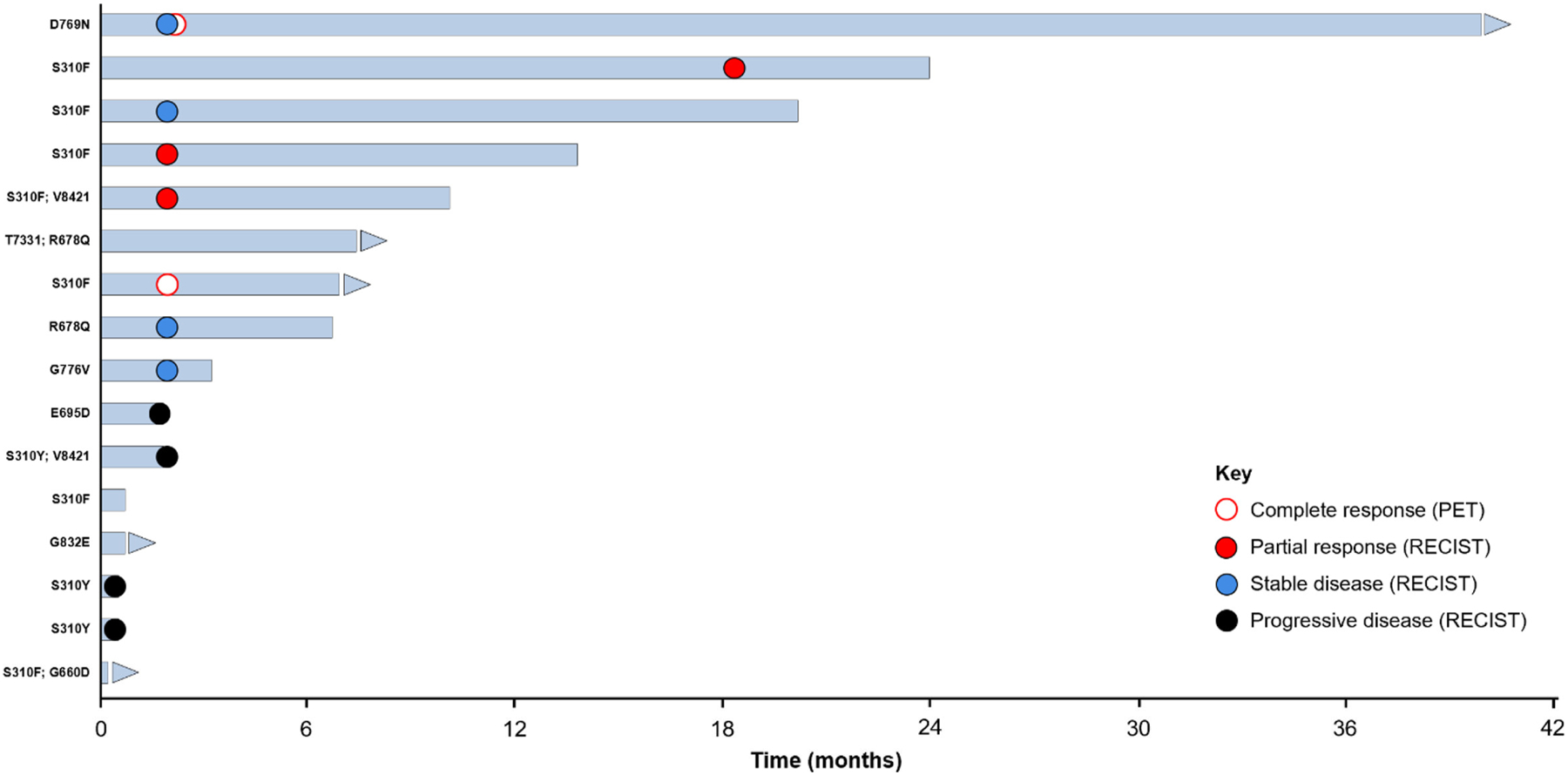
Duration of treatment and best response in all patients per RECIST or PERCIST (N = 16). Response based on investigator assessment. CT: computed tomography; PET: positron-emission tomography; RECIST: Response Evaluation Criteria in Solid Tumors.
All 3 patients who had a partial response had tumors with HER2 mutations at position S310 (S310F/Y) and one had a second HER2 V842I co-mutation. Co-mutations in other genes were also identified in these 3 patients, including an oncogenic PIK3CA E545K mutation.
Median PFS was 7.0 months (95% CI 1.0–18.3 months) and median OS was 16.8 months (95% CI 4.1 months–not estimable; Table 2 and Fig. 3). Six- and 12-month estimates for PFS were 52.8% (95% CI 23.4–75.5%) and 35.2% (95% CI 11.2–60.7%), respectively. Corresponding estimates for OS were 77.9% (95% CI 45.9–92.3%) and 60.6% (95% CI 29.2–81.6%), respectively.
Fig. 3.
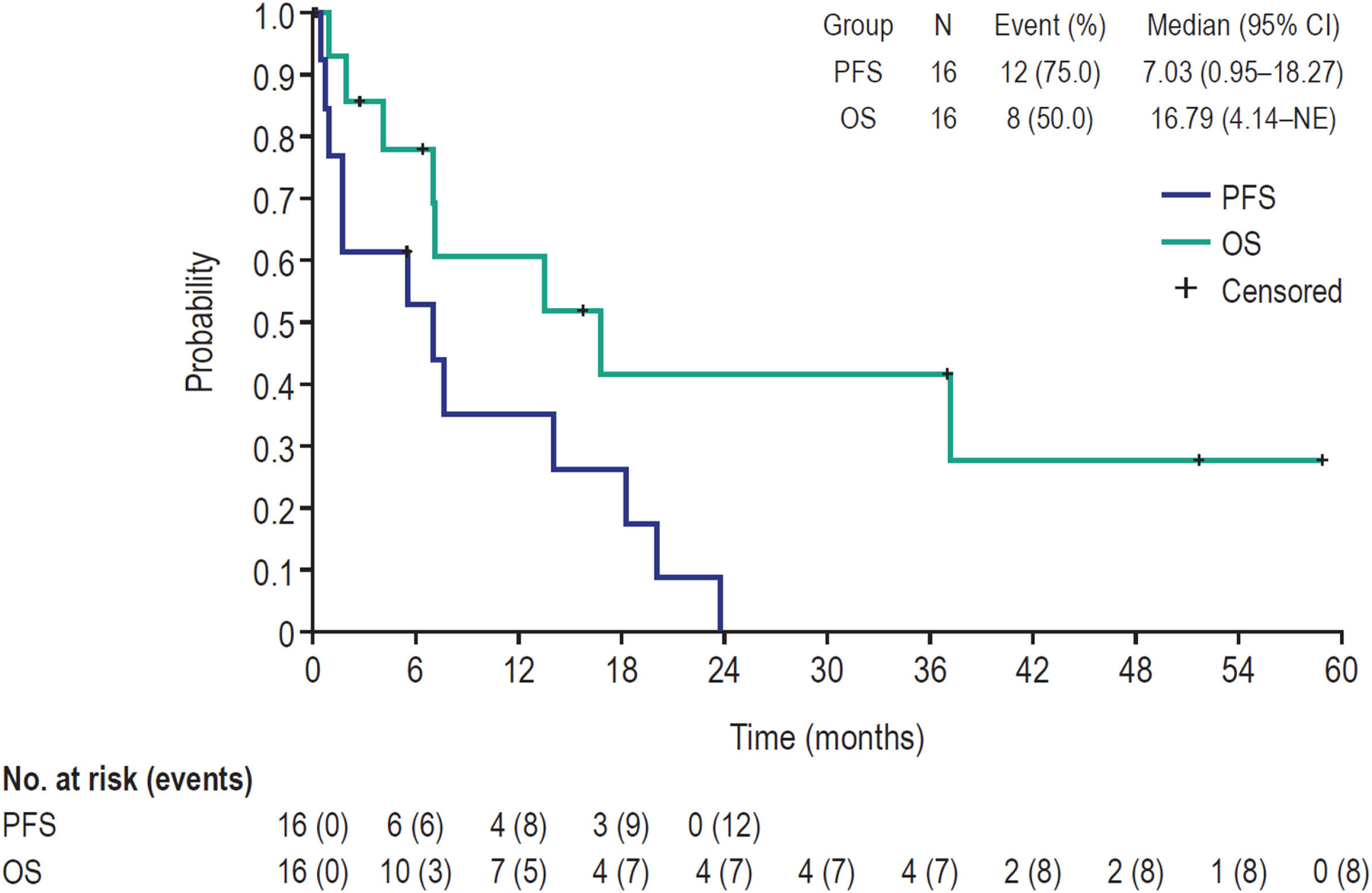
Kaplan–Meier estimates of PFS and OS in safety analysis set (N = 16). NE: not estimable; OS: overall survival; PFS: progression-free survival.
3.3. Safety
Diarrhea (75%), nausea (44%), and decreased appetite (38%) were the most common adverse events (Table 3). Twelve of the 16 patients reported having diarrhea (grades 1–3) and were treated with medication, primarily loperamide. One patient (6%) reported grade 3 diarrhea lasting 1 day, but there were no grade 4 diarrhea events and no treatment discontinuations due to diarrhea (Table S2).
Table 3.
Most common treatment-related adverse events in safety analysis set (N = 16).
| Adverse event, n (%) | Any grade | Grade 3/4 |
|---|---|---|
| Diarrhea | 12 (75.0) | 1 (6.3) |
| Nausea | 7 (43.8) | 0 |
| Decreased appetite | 6 (37.5) | 0 |
| Abdominal pain | 5 (31.3) | 1 (6.3) |
| Constipation | 5 (31.3) | 0 |
| Dyspnea | 4 (25.0) | 0 |
| Dry skin | 3 (18.8) | 0 |
| Epistaxis | 3 (18.8) | 0 |
| Headache | 3 (18.8) | 0 |
| Malaise | 3 (18.8) | 0 |
| Edema peripheral | 3 (18.8) | 0 |
| Pain | 3 (18.8) | 0 |
| Vomiting | 3 (18.8) | 0 |
| Anxiety | 2 (12.5) | 0 |
| Asthenia | 2 (12.5) | 1 (6.3) |
| Back pain | 2 (12.5) | 1 (6.3) |
| Cystitis | 2 (12.5) | 1 (6.3) |
| Dermatitis acneiform | 2 (12.5) | 0 |
| Dry mouth | 2 (12.5) | 0 |
| Dyspepsia | 2 (12.5) | 0 |
| Fatigue | 2 (12.5) | 0 |
| Insomnia | 2 (12.5) | 0 |
| Muscle spasms | 2 (12.5) | 0 |
| Muscular weakness | 2 (12.5) | 0 |
| Pain in extremity | 2 (12.5) | 0 |
| Rash maculo-papular | 2 (12.5) | 0 |
4. Discussion
Although early detection and preventive vaccination have reduced the risk of cervical cancer in some countries, locally recurrent and/or metastatic cervical cancer has a dismal prognosis [6]. First-line therapy is platinum chemotherapy with or without bevacizumab; unfortunately, cytotoxic agents tested in the second-line setting have been associated with ORRs of <10% and PFS of only 3 months [27]. Recently, the anti-PD1 antibody pembrolizumab was approved by the FDA for use after failure of prior platinum-based therapy based on a response rate of 12% [28,29]. Retrospective studies have identified oncogenic HER2 mutations (mainly codon S310 missense substitutions) and gene amplifications in 3–6% [15–17] and 1–12% [30] of cervical cancers, respectively. Other studies have reported that HER2 alterations are enriched in adenocarcinomas [31,32]. Notably, the incidence of HER2 mutations may be greater in patients with more advanced cervical cancer as these mutations may be associated with a worse prognosis or an adaptive mechanism of tumor survival, as seen in breast cancer [33,34].
Preclinical studies indicate that HER2 mutations can induce cellular transformation, and cancer cells expressing oncogenic HER2 mutations have been shown to be sensitive to selective HER kinase inhibitors [11–14]. The most prevalent HER2 mutations in cervical cancer are codon S310 mutations, which are located in the extracellular domain and induce kinase activation through increased receptor dimerization [14–17]. S310 mutations are highly sensitive to neratinib inhibition, which results in potent tumor inhibition in xenograft models [17]. In this study, HER2-mutant cervical cancers were predominantly of an adenocarcinoma histotype, which is consistent with other sequencing studies [15,17]. The increased prevalence of HER2 mutations in adenocarcinoma compared with squamous carcinoma, as seen in cervical cancer, is similar to the patterns observed with other oncogenic driver mutations including EGFR, ALK, RET, and ROS in non-small cell lung cancer [35].
With the exception of pembrolizumab, which is approved in PD-L1 CPS-positive patients who have progressed on or after prior platinum-based chemotherapy, there is still an unmet need for effective and well-tolerated therapies for use in the second-line cervical cancer setting and beyond. Targeting HER2-mutant cervical cancer with neratinib may represent the first precision medicine strategy for patients with advanced/metastatic cervical cancer. Neratinib was generally well tolerated in the SUMMIT study and no new safety signals were identified. The efficacy of neratinib monotherapy was encouraging and, even though cross-trial comparisons are problematic, the ORR of 25%, CBR of 50%, and median PFS of 7.0 months observed in SUMMIT compare favorably with those reported for platinum-based chemotherapy plus bevacizumab [27,36] and other investigational agents tested in this setting including newer anti-VEGF and anti-PD-L1 therapies, such as apatinib [18,37], nivolumab [38], and pembrolizumab [28,29].
Tumor molecular profiling with the goal of guiding treatment selection is now standard of care in multiple solid tumor types but is not currently performed routinely in patients with metastatic cervical cancer. The promising clinical activity of neratinib in the current study suggests that tumor molecular profiling could provide a much-needed indication of treatment options for patients with platinum-refractory advanced cervical cancer. HER2 mutations can also be detected in tumor DNA circulating in plasma [39,40], and observational protocols such as HER-Seq (NCT03786107), which use convenient blood-based screening, are currently underway to identify patients who are suitable to participate in neratinib clinical trials.
Limitations of this analysis were the small sample size due to the scarcity of patients with metastatic cervical cancer, the lack of routine genomic screening in patients with advanced cervical cancer, and the non-randomized, open-label design of the SUMMIT basket trial. Nevertheless, it is important to acknowledge that 16 patients with cervical cancer and HER2 mutations were enrolled, with a subset demonstrating clinical benefit from neratinib treatment. Enrollment is continuing in this study to better define the response rate following neratinib therapy in various rare cancer types, including cervical cancer.
In conclusion, HER2 mutations are an important class of oncogenic drivers in cervical cancer, especially in adenocarcinoma. Neratinib was well tolerated and showed promising clinical efficacy in heavily pretreated patients with metastatic cervical cancer. Given the paucity of available treatment options for this population, these results warrant further investigation and confirmation in larger clinical trials.
Supplementary Material
HIGHLIGHTS.
Somatic HER2 mutations are a newly identified class of oncogenic drivers in several solid cancers.
HER2 mutations have an estimated incidence of 5% in cervical cancer and may be associated with a poor prognosis.
A subset of HER2 mutations are sensitive to inhibition by neratinib, an irreversible pan-HER tyrosine kinase inhibitor.
Neratinib monotherapy showed promising efficacy in heavily pretreated patients with HER2-mutant, cervical cancer.
Acknowledgments
Medical writing assistance was provided by Lee Miller of Miller Medical Communications Ltd. This work was funded by Puma Biotechnology Inc.
Funding
This work was supported by Puma Biotechnology Inc. 10880 Wilshire Blvd, Suite 2150, Los Angeles, CA 90024, USA.
Footnotes
Declaration of Competing Interest
A. Oaknin: has received advisory board honoraria from Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Inmunogen, Genmab, and Deciphera and travel/accommodation support from Roche, AstraZeneca, and PharmaMar.
C.F. Friedman: has received institutional research funding from Bristol-Myers Squibb, Merck, and Genentech, advisory board honoraria from AstraZeneca, and serves on steering committees for the Genentech MyPathway and the Merck LYNK-002 studies (compensation waived).
L.D. Roman: has received advisory board honoraria from Tempus Labs and is a consultant for Quantgene.
A. D’Souza: has no competing interests.
I. Brana: has received institutional research funding from Puma Biotechnology Inc.
F. Clement-Bidard: has received advisory board honoraria from Pfizer, Novartis, Eli Lilly, Amgen, and AstraZeneca.
J. Goldman: has received institutional research funding from Puma Biotechnology Inc.
E. A. Alvarez: has received advisory board honoraria from Eisai Co. Inc. and ArQule Inc. and has been a medical consultant for Tracon Pharmaceuticals, Inc.
V. Boni: has received advisory board honoraria from Loxo Oncology and Ideaya.
A.C. ElNaggar: has received institutional research funding from Caris Life Sciences and advisory board honoraria from AstraZeneca, Clovis Oncology, Leap Therapeutics, Tesaro/GSK, and AbbVie Pharmaceuticals.
R. Passalacqua: has received advisory board/speaker honoraria from Amgen, Astellas, Bayer, BMS, Ipsen, Janssen, Novartis, Sanofi-Aventis, Roche, MSD, and Pierre-Fabre.
K.T.M. Do: has received advisory board honoraria from QED Therapeutics.
A.D. Santin: has received advisory board honoraria from Merck and Tesaro and has received institutional research funding from Puma Biotechnology Inc., Immunomedics, Tesaro, Boehringer Ingelheim, and Genentech.
K. Keyvanjah: is an employee and shareholder of Puma Biotechnology Inc.
F. Xu: is an employee and shareholder of Puma Biotechnology Inc.
L.D. Eli: is an employee and shareholder of Puma Biotechnology Inc.
A.S. Lalani: is an employee and shareholder of Puma Biotechnology Inc.
R.P. Bryce: is an employee and shareholder of Puma Biotechnology Inc.
D.M. Hyman: has acted in a consulting/advisory role for Atara Biotherapeutics, Chugai Pharma, CytomX Therapeutics, Boehringer Ingelheim, AstraZeneca, Pfizer, Bayer, and Genentech, and has received institutional research funding from Loxo Oncology, Puma Biotechnology Inc., and AstraZeneca. He is currently employed by Loxo Oncology/Eli Lilly.
F. Meric-Bernstam: has received institutional research funding from Novartis, AstraZeneca, Calithera, Aileron, Bayer, Jounce, CytoMx, eFFEC-TOR, Zymeworks, Puma Biotechnology Inc., Curis, Millennium, Daiichi Sankyo, AbbVie, Guardant Health, Takeda, and GlaxoSmithKline, grants/travel-related fees from Taiho, Genentech, Debiopharm Group, and Pfizer, consultancy fees from Pieris, Dialectica, Sumitomo Dainippon, Samsung Bioepis, Aduro, OrigiMed, Xencor, Jackson Laboratory, Zymeworks, and Parexel International, advisory board fees from Inflection Biosciences, GRAIL, Darwin Health, Clearlight Diagnostics, Spectrum, Mersana, and Seattle Genetics.
D.H. Solit: has acted in a consulting/advisory role for Loxo Oncology, Pfizer, Illumina, Vivideon Therapeutics, QED Therapeutics, and Lilly Oncology.
B.J. Monk: has received consultancy fees from Puma Biotechnology Inc.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2020.07.025.
References
- [1].Small W Jr., Bacon MA, Bajaj A, et al. , Cervical cancer: a global health crisis, Cancer 123 (2017) 2404–2412. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J. Clin 68 (2018) 394–424. [DOI] [PubMed] [Google Scholar]
- [3].FIGO World Congress, International Federation of Gynecology and Obstetrics Global Declaration on cervical Cancer elimination, Rev. Bras. Ginecol. Obstet 41 (2019) 102–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA Cancer J. Clin 70 (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [5].Li H, Wu X, Cheng X, Advances in diagnosis and treatment of metastatic cervical cancer, J. Gynecol. Oncol 27 (2016), e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Orbegoso C, Murali K, Banerjee S, The current status of immunotherapy for cervical cancer, Rep. Pract. Oncol. Radiother 23 (2018) 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tewari KS, Sill MW, Long HJ 3rd, et al. , Improved survival with bevacizumab in advanced cervical cancer, N. Engl. J. Med 370 (2014) 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tewari KS, Sill MW, Penson RT, et al. , Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240), Lancet 390 (2017) 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tewari KS, Monk BJ, Evidence-based treatment paradigms for management of invasive cervical carcinoma, J. Clin. Oncol 37 (2019) 2472–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moasser MM, The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis, Oncogene 26 (2007) 6469–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bose R, Kavuri SM, Searleman AC, et al. , Activating HER2 mutations in HER2 gene amplification negative breast cancer, Cancer Discov. 3 (2013) 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cocco E, Lopez S, Santin AD, Scaltriti M, Prevalence and role of HER2 mutations in cancer, Pharmacol. Ther 199 (2019) 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Connell CM, Doherty GJ, Activating HER2 mutations as emerging targets in multiple solid cancers, ESMO Open 2 (2017), e000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Greulich H, Kaplan B, Mertins P, et al. , Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ojesina AI, Lichtenstein L, Freeman SS, et al. , Landscape of genomic alterations in cervical carcinomas, Nature 506 (2014) 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xiang L, Jiang W, Ye S, et al. , ERBB2 mutation: a promising target in non-squamous cervical cancer, Gynecol. Oncol 148 (2018) 311–316. [DOI] [PubMed] [Google Scholar]
- [17].Zammataro L, Lopez S, Bellone S, et al. , Whole exome sequencing of cervical carcinomas identifies activating ERBB2 and PIK3CA mutations as targets for combination therapy, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 22730–22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang L Chen L, Yu H, Phase II study of apatinib, a novel tyrosine kinase inhibitor targeting tumor angiogenesis, as second-line treatment for recurrent or advanced cervical cancer patients, Investig. New Drugs (2019. October 21) 10.1007/s10637-019-00858-5 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- [19].Cancer Genome Atlas Research Network, Albert Einstein College of Medicine, Analytical Biological Services, et al. , Integrated genomic and molecular characterization of cervical cancer, Nature. 543 (2017) 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rabindran SK, Discafani CM, Rosfjord EC, et al. , Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase, Cancer Res. 64 (2004) 3958–3965. [DOI] [PubMed] [Google Scholar]
- [21].Chan A, Delaloge S, Holmes FA, et al. , Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial, Lancet Oncol. 17 (2016) 367–377. [DOI] [PubMed] [Google Scholar]
- [22].Martin M, Holmes FA, Ejlertsen B, et al. , Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial, Lancet Oncol. 18 (2017)1688–1700. [DOI] [PubMed] [Google Scholar]
- [23].Awada A, Colomer L, Inoue K, et al. , Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial, JAMA. Oncol 2 (2016) 1557–1564. [DOI] [PubMed] [Google Scholar]
- [24].Saura C, Oliveira M, Feng Y-H, et al. , Neratinib + capecitabine vs lapatinib + capecitabine in HER2+ metastatic breast cancer previously treated with two or more HER2-directed regimens: phase III NALA trial, J. Clin. Oncol (2020) July 17; doi: 10.1200/JCO.20.00147. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hyman DM, Piha-Paul SA, Won H, et al. , HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554 (2018) 189–194 (Erratum in: Nature. 566 (2019) E11-E12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].cBioPortal for Cancer Genomics, Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma, TCGA Firehose Legacy; cBioPortal for Cancer Genomics, MSK Clinical Sequencing Cohort, Cervical Cancer, https://www.cbioportal.org/study/summary?id=cesc_tcga.
- [27].McLachlan J, Boussios S, Okines A, et al. , The impact of systemic therapy beyond first-line treatment for advanced cervical cancer, Clin. Oncol 29 (2017) 153–160. [DOI] [PubMed] [Google Scholar]
- [28].Chung HG, Ros W, Delord JP, et al. , Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study, J. Clin. Oncol 37 (2019) 1470–1478. [DOI] [PubMed] [Google Scholar]
- [29].Frenel JS, Le Tourneau C, O’Neil B, et al. , Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial, J. Clin. Oncol 35 (2017) 4035–4041. [DOI] [PubMed] [Google Scholar]
- [30].Yan M, Parker BA, Schwab R, Kurzrock R, HER2 aberrations in cancer: implications for therapy, Cancer Treat. Rev 40 (2014) 770–780. [DOI] [PubMed] [Google Scholar]
- [31].Cerami E, Gao J, Dogrusoz U, et al. , The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data, Cancer Discov. 2 (2012) 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zehir A, Benayed R, Shah RH, et al. , Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients, Nat. Med 23 (2017) 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nayar U, Cohen O, Kapstad C, et al. , Acquired HER2 mutations in ER+ metastatic breast cancer confer resistance to estrogen receptor-directed therapies, Nat. Genet 51 (2019) 207–216. [DOI] [PubMed] [Google Scholar]
- [34].Razavi P, Chang MT, Xu G, et al. , The genomic landscape of endocrine-resistant advanced breast cancers, Cancer Cell 34 (2018) 427–438 (e6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Minuti G, D’Incecco A, Cappuzzo F, Targeted therapy for NSCLC with driver mutations, Expert. Opin. Biol. Ther 13 (2013) 1401–1412. [DOI] [PubMed] [Google Scholar]
- [36].Moon JY, Song IC, Ko YB, Lee HJ, The combination of cisplatin and topotecan as a second-line treatment for patients with advanced/recurrent uterine cervix cancer, Medicine 97 (2018), e0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guo Q, Sun Y, Kong E, et al. , Apatinib combined with chemotherapy or concurrent chemo-brachytherapy in patients with recurrent or advanced cervical cancer: A phase 2, randomized controlled, prospective study, Medicine (Baltimore) 99 (2020) (e19372). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Naumann RW, Hollebecque A, Meyer T, et al. , Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial, J. Clin. Oncol 37 (2019) 2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ma CX, Bose R, Gao F, et al. , Neratinib efficacy and circulating tumor DNA detection of HER2 mutations in HER2 nonamplified metastatic breast cancer, Clin. Cancer Res 23 (2017) 5687–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wardley AM, Kilburn L, Kernaghan S, et al. , Results from plasmaMATCH trial treatment Cohort B: a phase II trial of neratinib plus fulvestrant in ER positive breast cancer or neratinib alone in ER negative breast cancer in patient with a ERBB2 (HER2) mutation identified via ctDNA screening (CRUK/15/010). Proceedings of the 2019 San Antonio Breast Cancer Symposium; 2019 Dec 10–14; San Antonio, TX. Philadelphia (PA): AACR, Cancer Res. 80 (4 Suppl) (2020) (Abstract No. P1-19-07). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


