Abstract
Designing implantable bioelectronic systems that continuously monitor physiological functions and simultaneously provide personalized therapeutic solutions for patients remains a persistent challenge across many applications ranging from neural systems to bioelectronic organs. Closed-loop systems typically consist of three functional blocks, namely, sensors, signal processors and actuators. An effective system, that can provide the necessary therapeutics, tailored to individual physiological factors requires a distributed network of sensors and actuators. While significant progress has been made, closed-loop systems still face many challenges before they can truly be considered as long-term solutions for many diseases. In this review, we consider three important criteria where materials play a critical role to enable implantable closed-loop systems: Specificity, Biocompatibility and Connectivity. We look at the progress made in each of these fields with respect to a specific application and outline the challenges in creating bioelectronic technologies for the future.
Introduction:
Bioelectronic medicine promises to dramatically improve the standard of care by providing therapies that can automatically adjust throughout the day to provide the optimal therapeutic benefit to each patient. Ideally, these systems would provide “closed-loop” control of physiological state variables to maintain values within a therapeutic window. For example, to regulate glucose levels, a feedback control system would use biosensors to accurately estimate the current glucose levels and compare these values to a desired setpoint. Based on the difference between the desired glucose levels and the measured values, a controller would then determine how to stimulate (or actuate) biological processes that directly or indirectly affect insulin production. The new glucose levels would then be measured by the biosensors, and the process would repeat, creating a closed loop control system that can maintain physiological processes within a desired range (Fig. 1). Accurate implementation of this real-time feedback control strategy, however, is complicated by the complexity of human physiology. Coupling between the nervous, circulatory, and gastro-intestinal systems (each one with complex non-linear dynamics) creates a system of systems, the state of which is difficult to estimate, much less control for therapeutic benefit.
Figure 1:
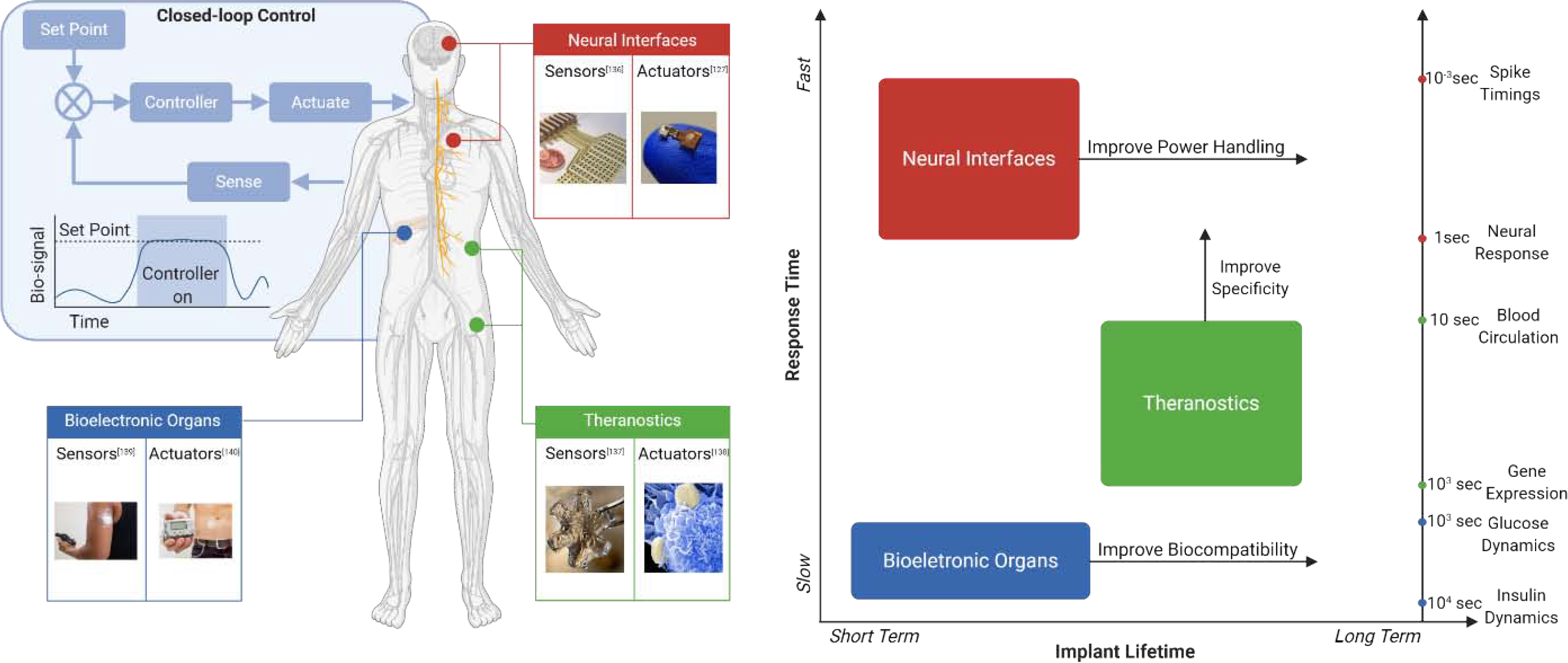
Distributed sensors and actuators for monitoring and regulating physiological processes
To create effective control systems that function within the complexity of human physiology it will likely be necessary to measure and actuate many types of physiological signals at multiple locations throughout the body. This requirement highlights the need to develop a distributed network of bioelectronic sensors and actuators. The signals measured by these sensors may include biochemical (eg. glucose, pH), thermal, mechanical (eg. strain, pressure) and electrical signals (ECG, EEG), with each providing critical information about physiological states that can be altered by the presence of disease. Similarly, electrical, mechanical, and biochemical actuators should be able to regulate physiological processes like neural activity, organ function, or biomolecule production. There are, however, significant challenges in our ability to create networks of bioelectronic sensors and actuators that can interface with target tissues and organs for continuous monitoring and control over long periods of time.
Here we focus on three key performance criteria where materials play a critical role in enabling a network of bioelectronic sensors and actuators: Specificity, Biocompatibility and Connectivity. These three criteria can present very different challenges depending on the application, creating a large and complex design space for developing bioelectronic implants. To highlight how these performance criteria can drive materials and device development, we focus on three specific bioelectronic applications described below: Artificial bioelectronic organs, Theranostics, and Neural Interfaces.
1. Artificial bioelectronic organs (e.g. Artificial Pancreas):
One example of a bioelectronic organ is the ‘artificial pancreas’ -- a system considered the holy grail of diabetes therapy. This system would provide closed-loop control of glucose by sensing glucose in blood and injecting an appropriate amount of insulin to regulate that level using a feedback control system [1].
While many prototypes have been developed and are being tested in clinical settings [2–8], the bottleneck for developing a commercially viable closed-loop system has been creating a long-lasting implantable sensor and implantable pump. Intravenous sensing and intravenous delivery, which represents an ideal system with minimum time lag is currently used only in critically ill patients [1,9]. Realization of a fully implantable closed-loop system for glucose control, requires development of new Biocompatible sensors and methods of insulin delivery for chronic implants.
2. Theranostics:
Theranostics are systems that combine therapeutics and diagnostics. In this system, biomolecules or drugs are measured by implanted sensors and therapeutic drugs are released by the actuators. Conventional therapies for metabolic diseases, cancer, and other disorders often rely on small molecule drugs. These therapies have significant limitations because the effects of drugs vary for each individual based on their genetic makeup, underlying conditions, or other factors [10]. To improve the effectiveness of existing drug therapies, there has been a paradigm shift to a more personalized treatment model. These models would ideally consider drug pharmacokinetics to optimize the timing, dosage and location of drug delivery for each individual’s physiological state [11]. Closed-loop systems to sense circulating biomolecules and release appropriate drug doses in real-time would be an important development, towards this goal. To control dosage, location and timing of drug delivery, many materials and nanoparticles have been developed for on-demand drug delivery. These materials are designed to release drugs in response to changes in environments--such as changes in pH and temperature, specific biomolecules or redox potential [12]. Many of the strategies applied have the advantage of being implantable and adaptable for diseases ranging from inflammatory, autoimmune to metabolic diseases [13]. Their widespread use however has been inhibited by their relatively low specificity when applied in closed-loop systems. Depending on their mode of sensing, theranostic sensors suffer from low specificity due to microenvironmental irregularities, biofouling of sensor surfaces, and low signal to noise ratios.
3. Neural Interfaces:
Neural interfaces consist of sensing, signal processing and actuating elements to provide therapies for neural disorders. Currently most neuromodulation devices use open-loop schemes. These schemes work well for some disorders such as Parkinson’s where stimulation pulse parameters and delivery location, which are the most important parameters, can be preprogrammed and remain safe and effective for many years with little adjustment [14,15, 16]. However, in the cases of disorders such as epilepsy, there is a need for detection of seizures and timely delivery of appropriate stimulation pulses. Many attempts have been made towards the creation of a closed-loop system for such applications [17, 18, 19]. While the development of these systems share the challenges of the other two categories including, development of chronically implantable sensors and actuators that are accurate and stable, one of the most difficult challenges faced for closed-loop neural interfaces is in the signal processing block. To design truly implantable closed-loop solutions, there is a need for the development of data handling systems. For example, in the case of seizure detection, several algorithms have been developed over the last 15 years [20–24]. Both analytical and machine learning classifiers have been investigated with some success. A major group of algorithms are based on time-domain feature and pattern recognition. Spatiotemporal correlation is utilized to perform seizure prediction [20]. In frequency-based methods spectral information is extracted from recorded EEG signals. Such methods involve preprocessing and normalization of data after which the best features are extracted and fed to a support vector machine for classification [25]. To design a reliable therapeutic device, the algorithm must be integrated into a chronically implantable device that can perform signal recording and processing over a long period of time. Two separate approaches have been explored for processing. In one approach (local computation), feedback control algorithms can be implemented in implanted hardware like an application specific integrated circuit (ASIC), which typically has a limited power and computational budget [26–30]. In the second approach (remote computation), data is transmitted to an external device like a mobile phone with significantly greater computational capacity and power budget [31]. Clinical requirements of on-time reaction to an upcoming neurological event such as an epilepsy seizure place a special importance on sensitivity, specificity, and low-latency. Therefore, computation power, algorithm complexity, power consumption, and data transmission become important design parameters. The trade-off between these parameters and the desired performance often dictates how much of the feedback control algorithm is implemented locally in the implanted hardware vs. remotely in an external device. Increasingly, new sensors and actuators have focused on miniaturization to improve chronic longevity, which has led to development of wireless, battery-free technologies. As a result, there is a growing need to improve the wireless Connectivity of these tiny sensing and actuating motes to provide power and communication with remote hardware systems that implement feedback control algorithms.
Specificity:
Specificity is the ability of a system to discriminate between a target signal and other confounding signals. Measurement of the target with high specificity combined with a temporal resolution that can accurately track the system dynamics provides the “sensing” block of closed-loop control systems (Fig. 1, top left). In addition to high specificity, and temporal resolution these sensors should be able to maintain their specificity over a physiologically relevant concentration range. This requirement faces different challenges depending on types of signals being measured.
As an example, bioelectronic application where specificity is a major design criterion, we consider theranostics. Because theranostic systems can be applied to many different physiological conditions the associated biosensors span a variety of signals that must be detected with high specificity. In this section, we examine sensors used in four different theranostic systems to highlight the advances and challenges related to highly specific biosensors. Figure 2 shows the various theranostic modalities and the barriers to their specificity.
Figure 2:
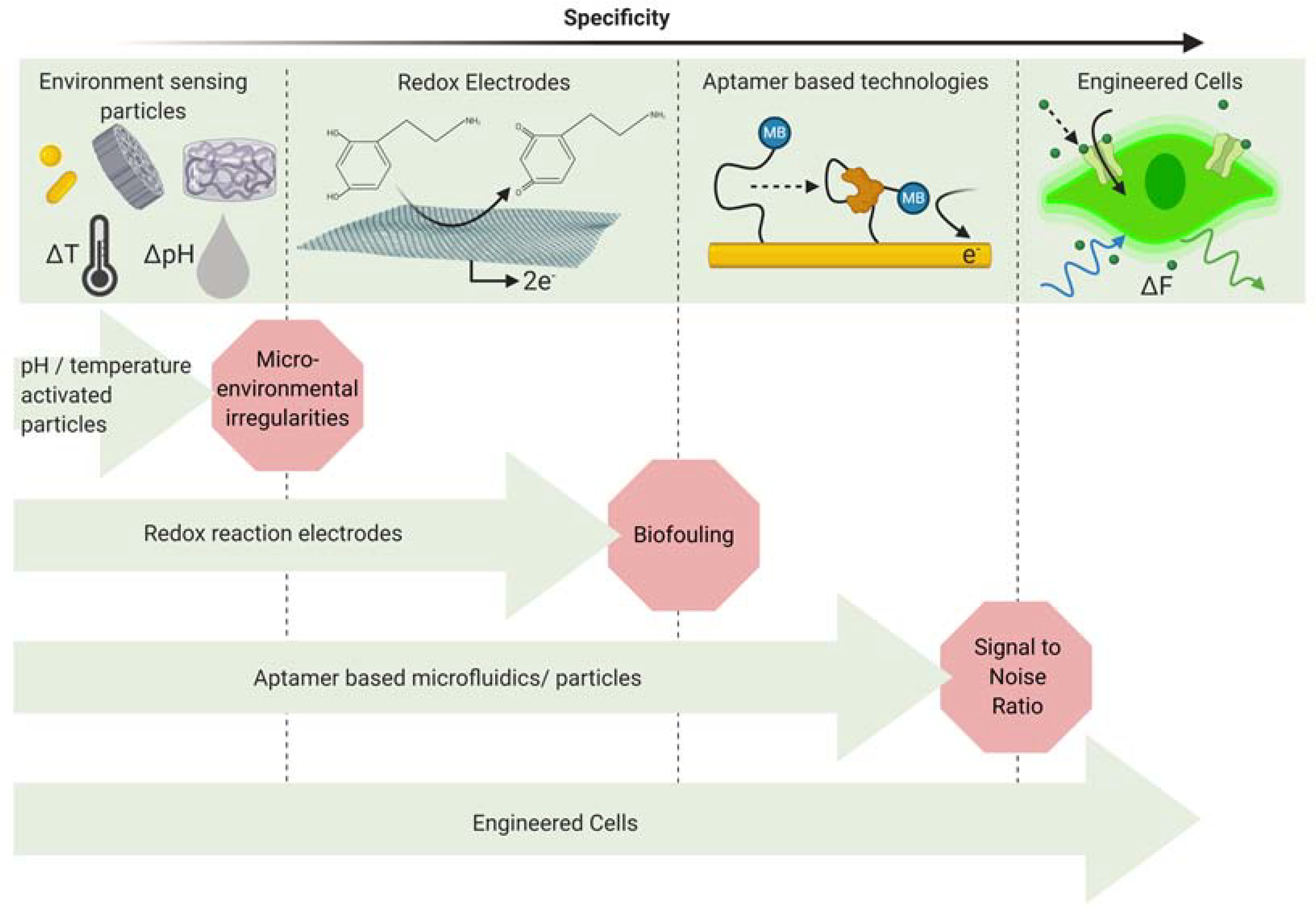
Microenvironmental irregularities present the first challenge for systems that depend on sensing of environmental factors such as temperature and pH. For implantable electrodes, biofouling can lead to reduced specificity which affects the performance of the system. Aptamer based microfluidic systems are highly specific but face the challenge of low signal to noise ratio when implanted in the body. Engineered cells represent a new direction for the development of highly specific theranostic systems.
Broadly, biosensors are composed of two main parts, a sensing element that recognizes a biological event and a transducer element that transfers the signal from the sensor to the next block in the system [32, 33]. The materials selected for construction of sensors implemented in a specific closed-loop system depends on a number of factors such as the type of signal, the tissue to be monitored, the positional requirements of the sensor and the type of transduction between the sensor and actuator.
The signal to be sensed can be a direct or indirect measurement of the disease state. Drug delivery systems that release therapeutics in response to an environmental stimulus are generally equipped with polymeric materials that are sensitive to pH [34], redox potential [35], temperature,[34] or specific biomolecules[36]. In all cases, the stimulus causes physical or chemical changes in the carrier, which lead to release of the encapsulated agents. Polymer science has been used in conjunction with pharmaceutical science to create novel systems that can specifically sense certain signals and release encapsulated drugs in response. This has created an entire class of ‘smart polymers’ that can be designed to be a complete system which senses the signal of interest and delivers the related therapeutic load in a controlled manner. Both natural and synthetic biodegradable polymers have been used in such applications. Polyester based polymers including polylactic acid (PLA), poly glycolic acid (PGA) and their co-polymers have become the gold standard demonstrated by over 500 patents [37]. The mechanical, thermal and biological properties of PLA are easily altered by altering its stereochemistry, making these polymers suitable for sensing a variety of signals. These properties are especially useful in the treatment of cancer. Tumor physiology differs markedly from normal tissue physiology. Tumors are characterized by a more acidic extracellular environment with a pH of 6.5 as compared to the normal physiological pH of 7.4 [38]. Anionic or cationic polymers are incorporated into grafted or block copolymers composed of a component that ionizes at a pH less than physiological pH. These copolymer networks swell or deswell to release drugs when they sense an acidic pH. Another characteristic of the tumor microenvironment is abnormal vasculature. Particles have been developed that take advantage of this property and accumulate in the tumor to deliver drugs in the specific location [39]. However, these approaches which depend on the measurement of environmental factors face the issue of low specificity due to irregularities in the surrounding microenvironment.
Another approach for realizing bioelectronic theranostic systems has been the use of modified electrodes. The sensing electrode is activated by biological signals which can range from small molecules to proteins and bacterial cells. The sensing electrode generates a reductive potential and current, which stimulates dissolution of an Fe3+-cross-linked alginate matrix on the second connected electrode resulting in the release of loaded biochemical species with different functionalities [40]. The method suffers in realtime measurement in whole blood due to fouling from blood cells and high-molecular weight clotting factors. These systems show promise of being applicable in a wide variety of applications but require modifications to achieve robust performance when implanted in the body.
Aptamer-based sensors utilizing structure switching nucleic acids have recently received much attention. These types of sensors provide high specificity [41]. In such systems, a conformation-changing aptamer probe is covalently attached at one terminus to an integrated electrode and the other end if modified with a redox reporter. When it binds with its target molecule, the probe undergoes a conformational rearrangement that modulates the redox current and generates an electrochemical signal. The conformational change is reversible, which makes these types of sensors useful for continuous, label-free, sensitive with rapid kinetics. Only binding of the target triggers a conformational change and non-specific binding of other interfering molecules does not generate a signal, making this method highly specific. The electrochemical signal can be used to drive a Micro-Electro-Mechanical-System (MEMS) based drug delivery component [42]. Techniques created for the electronics industry have enabled fabrication of valves, pumps, mixers, micro-reservoirs, which have been used to make miniaturized devices. These implantable devices can sense, mix, and pump small volumes of fluids [43], some of which are commercially available [44]. These advances have been applied in theranostic systems to create miniaturized devices that are implantable for continuous sensing and delivery of drugs [42].
Despite excellent specificity, there are several challenges when aptamer-based sensors are applied in closed-loop systems, the most difficult to overcome being that of low Signal to Noise Ratio (SNR). Aptamer based sensors perform well when used in undiluted blood serum [45] but require large sampling volumes in undiluted blood making it difficult to use in completely implantable closed loop systems.
In addition to these modalities, recent advances in synthetic biology have highlighted the potential use of biohybrid systems for theranostic solutions. Reliable, efficient, and predictable engineering of cells presents an opportunity to develop even more specific biosensors by leveraging the natural receptor proteins that have evolved to specifically bind biochemical targets with the complex in vivo environment. For example, cells have been engineered to express G protein coupled receptors that upon binding the target biomolecule change their fluorescence or drive the production of reporter fluorophores. Examples of these genetically engineered biochemical sensors include sensors to report specific neuromodulators such as acetylcholine [46], dopamine and norepinephrine [47] in vivo. Engineered cells that sense biochemicals have also been used as part of cell-based cancer therapies and have the potential to be used as part of a closed-loop bioelectronic device. For example, CAR-T cells have been used to treat acute lymphoid leukemia [48] and neuroblastoma [49]. Apart from cancer theranostics, engineered cells are being applied to address metabolic disorders. Cells to constantly monitor blood lipid levels were implanted in obese mice using semipermeable immunoprotective hydrogels. These cells express clinically approved peptide hormone pramlintide to suppress appetite in relation to sensed blood fatty acid levels. The human dopamine receptor 1 (GPCR) has been used to sense external stimuli and secreting therapeutic molecules. This scheme has been applied in tumor lysis syndrome [50] and diabetic ketoacidosis [51]. Challenges with this approach include the need to either modify the host tissue using viral vectors or introducing genetically modified cells. Direct modification of the host presents a more challenging regulatory pathway, however, there are more than 2000 clinical trials worldwide, that are using viral gene therapy. An additional challenge is that any sensing modality based on gene transcription will have a long latency between binding the biomolecule and reporting that concentration via a reporter protein. The time between binding and gene expression is typically on the order of minutes. As a result, only physiological processes that vary more slowly than this sensing latency could be controlled using transcription-based biosensors. Finally, the reporter (typically a fluorescence or electronic) signal must be converted into an electronic signal to complete the closed-loop system. Thus, the sensing interface includes both a synthetic biological part and a bioelectronic part creating a biohybrid system [52]. Despite the additional engineering required to create these biohybrids, the specificity afforded by synthetic biology makes them an attractive approach for closed-loop bioelectronics that must function within the complex physiological environment in vivo.
Biohybrids based on synthetic biology also offer an opportunity to create interfaces that deliver select biochemical signals and can act as actuators in a feedback control system. These engineered cells can also produce multiple different biochemical therapies, overcoming the limitations of one disease-one drug approach. For example, engineering cells to express light-sensitive ion channels or proteins that respond to different wavelengths one can drive production of select biomolecules by using different colors of light [53–57].
Biocompatibility:
For the purposes of discussing closed-loop bioelectronics we define biocompatibility as the ability of sensors and actuators to safely stimulate/record the target physiological process for the period of time necessary for the system to perform the desired task. There are two main considerations when assessing the biocompatibility of a device: 1) biofunctionality- the effect on the performance of the implant due to its continuous interaction with biological tissue and 2) biosafety-the risk to the health of the patient due to the implanted device. The intended function of the biointerface will place different requirements for stability and longevity. From a regulatory standpoint biosafety is one of the most important criteria when designing implantable devices.
As a case study for the importance of biocompatibility we consider a bioelectronic organ: the artificial pancreas, which regulates insulin levels based on a measurement of blood glucose, is one of the most studied topics in this field. While there have been rapid improvements in all the components involved in this system, biocompatibility remains the most important challenge to the realization of a chronically implanted artificial pancreas. Figure 3 shows the advances over time in the design of artificial pancreas and the advances made in fabrication of materials to improve biocompatibility. The developments of these two fields in parallel have led to the possibility of a chronically implantable artificial pancreas.
Figure 3:
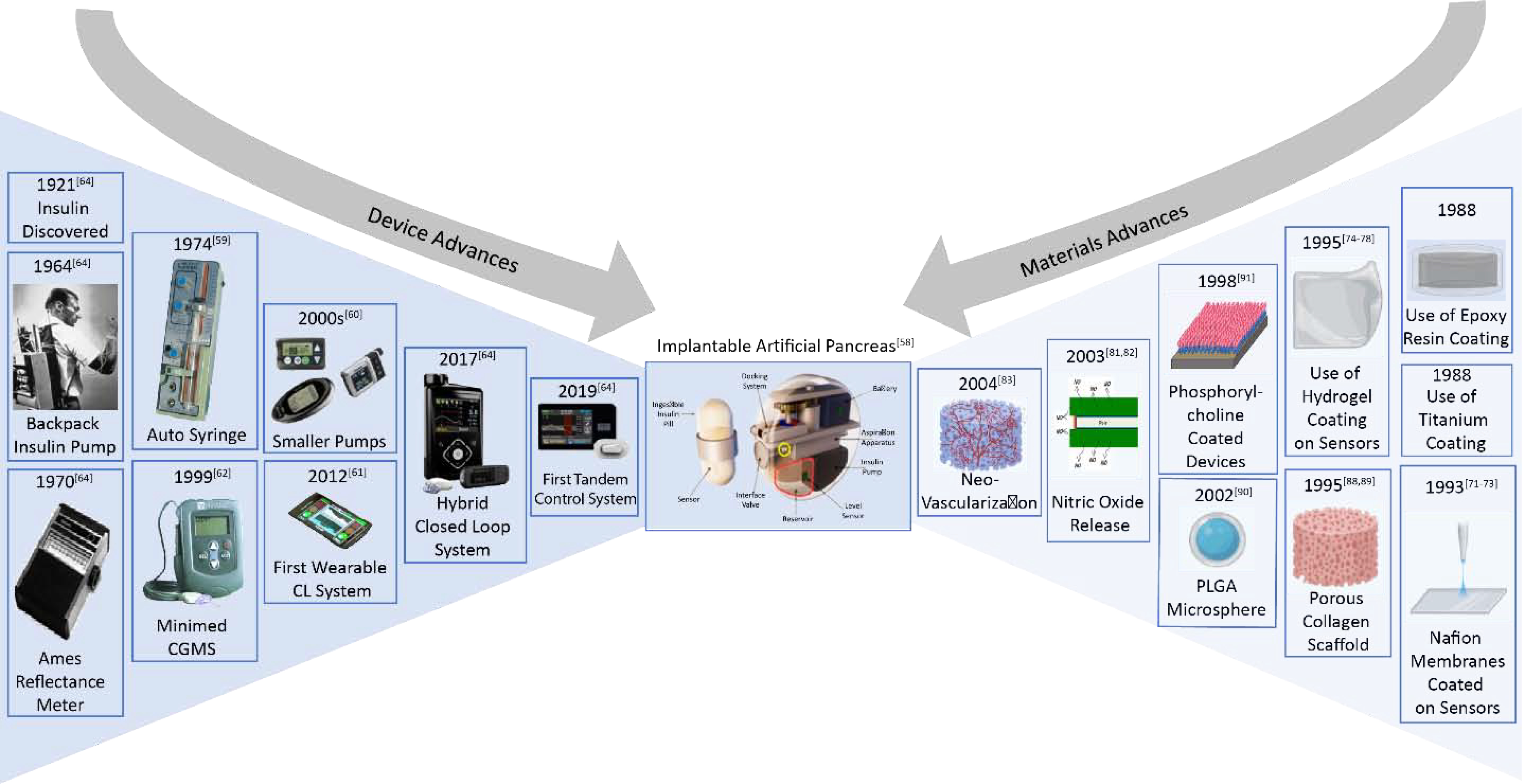
To create a Chronically Implantable Artificial Pancreas it is necessary to combine the developments made over decades in improving the device performances specifically for glucose regulation application with advances in materials developed for improving biocompatibility of implanted devices.
The first attempt of a closed loop system was made in 1964 by Kadish. Continuous real-time glucose monitoring was used with an on-off system for intravenous infusion of insulin and glucagon in a diabetic patient. Since then many efforts have been made to develop a true closed-loop system [58–61]. Regulatory approval of Continuous Subcutaneous Insulin Infusion (CSII) has led to the miniaturization and improved reliability of insulin pumps [62]. Many novel algorithms and control paradigms have been postulated for this specific application [63]. Advances have been made in all areas to move towards a truly closed loop system. Many prototypes have been developed and are being tested in clinical settings. Research prototypes of the artificial pancreas adopting the subcutaneous route include a control algorithm, a modular system supporting wireless connection to a range of glucose sensors and insulin pumps, each having potential for further advancement. Despite success of several prototypes, chronically implanted artificial pancreas has yet to be realized as a commercial product. A major barrier to this is the ability to implant all the components of this system. Current CGM sensors measure interstitial glucose levels using subcutaneous sensor elements. The designs and materials used for the construction of CGM sensors have ongoing problems due to compression of tissue around the sensors leading to false alarms. Implantable glucose sensors are still under development [64]. Efforts are being made to use intravenous sensors and intraperitoneal insulin infusion. There is limited experience with implantable pumps has hindered their widespread application [65]. There have been many complications with implantable pumps including ‘pump-pocket’ infections and device failures [66].
To understand the challenges in the development of biocompatible devices, we look at the body’s response to foreign objects. Biofouling which occurs shortly after implantation, consists of non-specific cell/protein adsorption locally around the implant. Device implantation can lead to both acute and chronic inflammatory responses. Attachment of proteins such as albumin and fibrinogen, associated with acute inflammatory response, to the implanted device can further lead to chronic inflammatory response. In this, macrophages play a key role in Foreign Body Giant Cell (FBGC) formation and production of degradative enzymes and inflammatory mediators. Inflammatory responses lead to the formation of a fibrous capsule surrounding the implant. This fibrous wall in combination with the FBGC, creates a barrier surrounding the implant, which can hinder its performance.
In the case of glucose monitoring systems, despite outstanding advances in in vitro functionality of sensors, in vivo applicability has been difficult due to gradual loss of sensor functionality after implantation. It has been overwhelmingly observed that biofouling contributes to a decrease in sensitivity when the sensor is initially implanted [67, 68]. Another possibility is that there may be changes in the diffusion characteristics of the tissue due to inflammatory response which affects the concentration of glucose in the immediate vicinity of the sensor [69].
Different approaches have been applied to overcome issues surrounding the FBR. Numerous coatings have been developed to reduce tissue reactions around sensors. In one study, a three-dimensional porous collagen scaffold was prepared by cross-linking collagen with nordihydroguaiaretic acid. Devices coated with this material were shown to be stable for four weeks upon incubation with collagenase solution [70]. Nafion membranes, an ionomer made from a sulfonated tetrafluoroethylene copolymer, have been used as coating material for sensors. Results have indicated unimpaired sensor function and longer lifetimes for coated devices [71–73]. Other hydrogel coatings include poly (hydroxyethyl methacrylate) [74, 75], PEG [76], polyurethane [77] and PVA [78]. Coatings made from these materials can be polar, uncharged, water-swellable, flexible aqueous layers that can be used to mask the surface of the device while improving functionality and lifetime. Water soluble analytes like glucose can diffuse through the polymer gel and the porosity of the gel can be controlled by controlling the cross-link density, giving these gels an advantage in implantable sensor devices.
Another approach is the use of anti-inflammatory drugs in the vicinity of the implant. Dexamethasone and a superoxide dimutase mimic (SODm) have been used previously [78–80]. However, these have not been widely applied since the use of a non-native drug can cause other systemic effects. To overcome this, glucose sensors that release nitric oxide to modulate inflammatory response have been developed. These have shown some promise in reducing inflammation for the length of time that the nitric oxide was released [81,82].
A suggested strategy for improving function has been neovascularization of the implant site to overcome fibrosis-associated vessel regression which is assumed to be a major factor in the loss of biosensor function in vivo. The most basic method to achieve vascularization is to infuse Vascular Endothelial Growth Factor (VEGF) with the device. This has been shown to marginally improve the sensor function [83]. To improve on these initial results, there has been an explosion of research in methods of delivering angiogenic growth factors [84–87]. Based on research in the fields of oncology and angiogenesis, it was found that certain types of porous materials when fabricated with a specific pore size, caused a prolific growth of new vessels into the region of the implanted device. Polyvinyl alcohol sponge and expanded polytetrafluoroethylene have been used to create scaffolds with controlled pore sizes [88,89]. In another experiment biodegradable poly lactide-glycolic acid (PLGA) was impregnated with VEGF to promote vascularization. This approach was found to be much more effective than using porous scaffolds or VEGF infusions alone [90].
Recently, there has been great interest in the use of biomimicry for fabrication of biomaterials for packaging of implantable devices. Phosphorylcholine (PC) has been used as a phospholipid membrane placed over devices. The idea behind this concept is to mimic the mammalian phospholipid cell membrane to avoid an FBR. PC-coated devices have shown reduced neutrophil binding [91], less platelet activation and reduced thrombin formation in vitro [92]. PC coated polymers in rabbits found a lower degree of fibrosis at 13 weeks [93] and a PC coated glucose sensor has passed cytotoxicity and biocompatibility tests [94]. Dopamine like materials have been used, which are capable of adherently coating many diverse substrates in a conformal manner. Chitosan has also shown promising results with many favorable characteristics like homeostasis and antibacterial properties [95–97].
Foreign body response encompasses many complex processes. The biocompatibility of materials used in implantable devices depends on the ability of the materials to overcome this response while maintaining implant function and minimizing risk for the patient. Biocompatibility still remains a major challenge for most implantable devices and is a major bottleneck in the implementation of the well-studied artificial pancreas system. No material has yet been found that is capable of completely solving the challenges faced due to biocompatibility. There have been many developments which show promising results.
Connectivity:
For closed-loop systems, data must be transferred between the sensors, actuators, and controller. The controller typically performs most of the signal processing and computation and does not necessarily need to be implanted in the body. In either case, chronic implants typically need remote mechanisms to tune and reprogram the controller. In addition, the controller, sensors, and actuators, must be powered by batteries, or by wireless energy harvesting. Together, these requirements emphasize the importance of data and power transfer for closed-loop bioelectronics.
Data and power transfer are particularly important for neural interfaces since they often deal with high-bandwidth data and power-hungry applications. In most cases the amount of low-latency processing that must be done for a closed-loop system cannot fit entirely in miniaturized (mm-scale) implantable devices. Instead, free-floating mm-sized sensors and actuators must receive data and power wirelessly. In this section we look at various schemes that have been explored to improve connectivity in the field of neural interfaces.
For chronically implantable closed loop systems, algorithms and miniaturized hardware need to be developed that can fit into a scheme of on-chip implementation with a limited power and computational budget. Powering these devices efficiently becomes especially important when trying to increase the lifetimes of implants and reduce their size by eliminating onboard batteries. These batteries not only increase the device footprint, but also require follow-up surgeries once the battery discharges or in case of lead migrations and failure [98]. For these schemes, sensors and actuators need to be developed with the capability of wireless data and power transfer.
Many different schemes have been developed for data and power transfer in the field of neural interfaces. Inductive coupling [99–101], ultrasonics [102–104], mid-field [105,106] and far-field [107,108] electromagnetic coupling have been proposed for wireless power transfer.
i). Near-Field Inductive Coupling:
This is the oldest and most studied strategy applied in implantable devices. It works on the principle of electromagnetic coupling. A transmitter placed close to the body produces a time-varying magnetic field which produces an Electro-Magnetic Field (EMF) in an implanted receiver coil. Inductive coupling has been used with proven results in FDA approved implanted devices [108,109]. Over the years many improvements have been made in the designs of coils and implants to improve the efficiency of inductive coupling. Some challenges still remain however, some of which are inherent to the technology. Efficiency is one of the most important challenges. Near field coupling usually results in sub-optimal efficiency since the link has to be designed for a particular load; however, many implants represent a dynamic load that changes depending on their activity state. Misalignment of implants which are very common due to the nature of the application, present another challenge for inductively coupled devices. Inductive coupling is greatly affected by misalignments and artifacts. It is also desirable for neural implants to be mechanically flexible. Real-time flexion of coils causes detuning. Additional circuitry is required for matching with the tuning capacitor circuitry. From a materials standpoint, copper wires provide excellent performance, however since copper ions released from metal can have toxic effects [110], care must be taken to hermetically package these coils, or one can find alternative materials. Other materials are often used in place of copper, which leads to lower performance due to higher electronic resistance. Many techniques have been applied to overcome these drawbacks. To improve efficiency, energy storage capacitors are used on the load side to present an optimal load despite varying demands and source decoupling is achieved by using high quality factor coils. Coil alignment using permanent magnets is used to reduce misalignment effects [111,108]. To overcome detuning in flexible implants, miniature coils with smaller inductance per area have been suggested [112, 113]. To continue using copper coils, silicone, glass and ceramic are used for packaging implants. These proposed solutions while mitigating the drawbacks of the technology, come with other trade-offs. For example, Use of permanent magnets leads to incompatibility of implants with MRI, miniaturization of coils leads to performance degradation, replacing copper by other materials leads to higher resistivity while continuing to use copper requires hermetic sealing of devices which can increase the footprint of the device. To create mm-sized bioelectronic implants that have advantages compared to NIC several alternative wireless data and power transfer technologies have been explored.
ii). Ultrasonics:
Ultrasound waves of frequencies greater than 20kHz are used for transfer of energy to implanted devices. An ultrasonic oscillator is used as a transmitter. It generates acoustic waves, usually within the frequency range of 200 kHz to 1.2 MHz. It is coupled through tissues to a receiver, which is a piezoelectric energy harvester implanted inside the body. Data can be uplinked from the implanted device through sensing the ultrasonic backscatter. The efficiency of ultrasound power transfer depends on various factors such as transducer losses, receiver losses, tissue absorption, losses due to the acoustic impedance matching layer and rectifier losses. Several design factors have to be considered for realizing an efficient ultrasonic system ranging from the choice of optimum operating frequency, transducer material, location of the implanted device, acoustic impedance matching at transmitter and receiver, to the power conditioning stages which all affect the power transmission efficiency. System level designs for powering active implanted devices, such as cochlear, pacemakers, and neurostimulators have been proposed in [114, 115, 116, 117, 118] as an alternative to its EM powering counterparts. Some of the challenges in the use of ultrasonics include varying acoustic impedance in different body tissues and at the interface between air and tissue, safety issues due to long term tissue vibrations and the separation between the transmitter and receiver leading to large swings in efficiency. Use of ultrasound energy transfer technology has become a popular alternative recently due to improvements in the performance of piezoelectric materials. Use of Lead Zirconate Titanate (PZT) as an ultrasonic transducer results in high electromechanical energy conversion. Many studies have shown improvements in piezoelectric properties of Polyvinylidene fluoride (PVDF) with the addition of other materials such as zinc oxide [119].
iii). Mid-field:
This has been suggested to overcome the shortcomings of near-field inductive coupling for miniature implants in which the receiver size is smaller than the wavelength. In the case of near-field inductive coupling, when the two coils are placed in a multi tissue layer environment and separated by a distance of a few centimeters, the wireless power transfer efficiency is very low at frequencies less than a few MHz. Maximized efficiency of distant miniature implants is achieved by combining near field inductive and far field radiative modes of transmitter at low GHz mid-field frequency range. The optimal mid-field frequency is then chosen based on implant depth and types of tissues to converge the transmitted waves at the position of the implanted receiver coils. Mid-field resonant coupling is an emerging scheme proposed for powering deep-seated microimplants [120]. Although the overall efficiency is improved compared to NIC, the delivered power levels are still in the range of a few milliwatts. One potential challenge with mid-field approaches is that the tissue absorbs electromagnetic energy at the 1–3GHz frequencies typically used, which has been reported to cause tissue heating of a few degrees C [121]. Safety limits therefore place the upper bound on the amount of energy that can be transferred with this approach, and long-term chronic use remains to be demonstrated.
iv). Far-field electromagnetic coupling:
In this scheme, the receiver antenna is placed at a large distance from the transmitter and receives power through radiating electromagnetic waves. This strategy has been investigated and applied for long-range power transmission in free space but has not been widely applied in implanted devices primarily because the body absorbs electromagnetic radiation in the common RF bands. As a result, only superficial antennas within the first few mm of the surface receive enough power to operate most bioelectronic devices for neural applications [122, 123]. For some low-power applications like cardiac pacing RF power has been proposed, but the antenna size required to efficiently capture GHz frequency energy prevents sub-mm miniaturization [124].
v). Magnetoelectric (ME):
While magnetoelectrics have been commonly studied in the context of sensing electromagnetic signals, recent studies have begun to look at magnetoelectrics as a form of wireless power transfer. [125] Magnetoelectric materials are composites of magnetostrictive and piezoelectric materials. In this modality, energy is converted from an alternating magnetic field into strain by the magnetostrictive material. The strain is then transferred to a coupled piezoelectric layer which induces an electric field. Because magnetic fields in the less than 500kHz frequency range do not suffer from significant absorption by the body or impedance mismatches between air/bone/ tissue, ME can serve as an efficient power transfer modality for implantable devices. Furthermore, in comparison to near field inductive coupling which also uses magnetic fields, ME devices are less affected by spatial and angular misalignment and have a higher power density [126] Like NIC data and power is transmitted using an electromagnetic coil that generates the alternating magnetic field, operating at single digit mT field strengths. The carrier magnetic field frequencies span 100 kHz - 500 kHz with in vivo demonstrations at 300–400 kHz [127]. Similar to ultrasound, material improvements can improve the efficiency and power density of this wireless power technique. Long term chronic studies and data uplink has not been demonstrated.
Figure 4 shows the various technologies being developed for power and data transfer.
Figure 4:
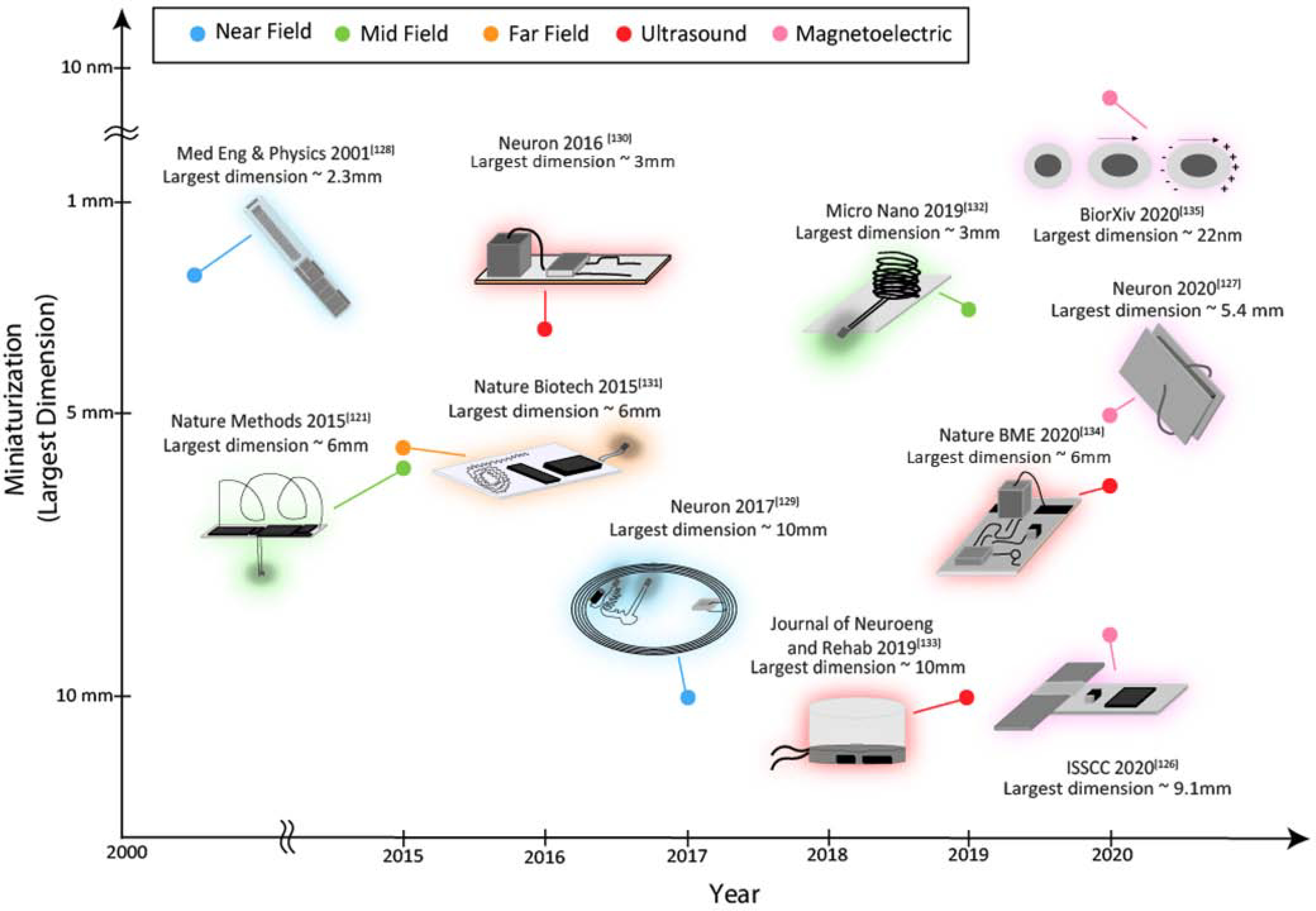
Developments in miniaturization of implantable neural stimulation systems using various wireless power transfer modalities
Conclusion:
Devices that are capable of real-time closed loop therapies can revolutionize how patients are treated and drastically improve health care outcomes. As we described above, specificity, biocompatibility and connectivity are three major design criteria that affect the performance of closed-loop bioelectronic systems, and the importance of each factor often depends on the application.
Specificity of sensors plays a critical role in development of theranostic systems. Many new materials have been developed to be responsive to highly specific stimuli. These materials still suffer from drawbacks such as low signal to noise ratio. Recently, great strides have been made in using advancements in synthetic biology by creating engineered cells for theranostic applications which shows the potential to greatly improve the performance of theranostic systems.
Biocompatibility is a key criterion for all implantable devices. In mature systems such as artificial pancreas, in which there have been many improvements over the years to create a completely implantable device, biocompatibility remains a bottleneck for chronic implantation. Over the years, concurrent advancements in biocompatible materials and miniaturization technologies have led to the possibility of being able to create a chronically implantable artificial pancreas.
Connectivity becomes critical as devices are miniaturized to create networks of distributed sensors and actuators. This is especially true for neural interfaces, which typically require low-latency, high power, and significant computational budgets. Many different schemes have been explored for wireless power and data transfer with each having its own advantages. The development and optimization of new materials affects all of the different criteria. New thin film encapsulation materials have the potential to replace the relatively large hermetically sealed ceramic/titanium boxes. More robust long-term tests, however, will be needed to verify the reliability of these thin film coatings, especially in covering devices that can potentially have complex geometries. Novel piezoelectric materials can expand device power budgets and allow for greater power transfer efficiencies at larger depths within the body.
In this paper we focus on three applications to demonstrate the role played by specificity, biocompatibility, and connectivity considerations in the design of closed-loop implantable systems. These closed-loop systems, however, show tremendous promise in a broad range of applications including wound healing, gastric dysfunction, cardiopulmonary and metabolic disorders.
While factors like specificity, biocompatibility, and connectivity are important for all bioelectronic systems, there is no singular best technology solution. Instead the choice of sensor, encapsulation, and data/power delivery depends heavily on the application. This highlights the importance of systems engineering along with materials and device development when developing closed-loop bioelectronic technologies for the future. For example, implantation depth and location will affect which wireless power technique can and should be used. Concentration ranges of interest can determine which biosensors are optimal for the application. Nevertheless, individual technological advances in specificity, biocompatibility, and connectivity will create a more powerful suite of component technologies that can be assembled into the type of advanced closed-loop bioelectronic medicines we imagine for the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).Hovorka R, 2006. Continuous glucose monitoring and closed-loop systems. Diabetic Medicine, 23, 1–12, DOI: 10.1111/j.1464-5491.2005.01672.x [DOI] [PubMed] [Google Scholar]
- 2).Schaller H, et al. , 2001. MPC algorithm controls blood glucose in patients with Type 1 diabetes mellitus under fasting conditions using IV-SC route. Diabetes Technology Meeting. San Francisco; A48. [Google Scholar]
- 3).Canonico V, et al. , 2002. Evaluation of a feedback model based on simulated interstitial glucose for continuous insulin infusion. Diabetologia, 45: 995. [Google Scholar]
- 4).Vering T, 2004. Minimally invasive control loop system for SC-SC control on patients with type 1 diabetes.” Diabetes Technology & Therapeutics, 6: 278. [Google Scholar]
- 5).Castle JR et al. , 2010. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care, 33, 1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Atlas E, Nimri R, Miller S, Grunberg EA & Phillip M, 2010. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care, 33, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Hovorka R et al. , 2010. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 375, 743–751. [DOI] [PubMed] [Google Scholar]
- 8).Renard E, Place J, Cantwell M, Chevassus H & Palerm CC, 2010. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas. Diabetes Care, 33, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Goldberg PA, Siegel MD, Russell RR, Sherwin RS, Halickman JI, Cooper DA et al. , 2004. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technology & Therapeutics, 6: 339–347. [DOI] [PubMed] [Google Scholar]
- 10).Kaye AD, Garcia AJ, Hall OM, et al. , 2019. Update on the pharmacogenomics of pain management. Pharmgenomics and Personalized Medicine, 12:125–143. July 3. doi: 10.2147/PGPM.S179152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Shah RR, Shah BR, 2012. Personalized medicine: is it a pharmacogenetic mirage? British Journal of Clinical Pharmacology. 74, 698–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Knipe JM, Peppas NA, 2014. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regenerative Biomaterials, Volume 1, Issue 1, November, Pages 57–65, 10.1093/rb/rbu006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Graya M, et al. , 2018. Implantable biosensors and their contribution to the future of precision medicine. The Veterinary Journal. 239, 21–29. 10.1016/j.tvjl.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 14).Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. , 2011. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Archives of Neurology, 68:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Volkmann J, Moro E, Pahwa R, 2006. Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Movement Disorders, 21(S14): S284–9. [DOI] [PubMed] [Google Scholar]
- 16).Schuepbach WMM, et al. , 2013. Neurostimulation for Parkinson’s disease with early motor complications. New England Journal of Medicine, 368:610–22. [DOI] [PubMed] [Google Scholar]
- 17).Morrell M, 2006. Brain stimulation for epilepsy: Can scheduled or responsive neurostimulation stop seizures? Current Opinion Neurology, vol.19, pp. 164–168, 2006. [DOI] [PubMed] [Google Scholar]
- 18).Good LB, Sabesan S, et al. , 2009. Control of synchronization of brain dynamics leads to control of epileptic seizures in rodents. International Journal of Neural Systems, vol. 19, pp. 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Mormann F, Andrzejak RG, Elger CE, and Lehnertz K, 2007. Seizure prediction: The long and winding road. Brain, Vol. 130, no. 2, pp. 314–333, September. [DOI] [PubMed] [Google Scholar]
- 20).Williamson JR, Bliss DW, Browne D, and Narayanan J, 2012. Seizure prediction using EEG spatiotemporal correlation structure. Epilepsy & Behavior, vol. 25, pp. 230–238. [DOI] [PubMed] [Google Scholar]
- 21).Li S, Zhou W, Yuan Q, and Liu Y, 2013. Seizure prediction using spike rate of intracranial EEG. IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 21, no. 6, pp. 880–886, November. [DOI] [PubMed] [Google Scholar]
- 22).Eftekhar A, Juffali W, El-Imad J, Constandinou T, and Toumazou C, 2014. Ngram-derived pattern recognition for the detection and prediction of epileptic seizures. PLoS One, vol. 9, Art. no. e96235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Zheng Y, Wang G, Li K, Bao G, and Wang J, 2014. Epileptic seizure prediction using phase synchronization based on bivariate empirical mode decomposition. Clinical Neurophysiology, vol. 125, pp. 1104–1111. [DOI] [PubMed] [Google Scholar]
- 24).Bandarabadi M, Teixeira C, Rasekhi J, and Dourado A, 2015. Epileptic seizure prediction using relative spectral power features. Clinical Neurophysiology, vol. 126, no. 2, pp. 237–248. [DOI] [PubMed] [Google Scholar]
- 25).Park Y, Luo L, Parhi K, and Netoff T, 2011. Seizure prediction with spectral power of EEG using cost-sensitive support vector machines. Epilepsia, vol. 52, pp. 1761–1770. [DOI] [PubMed] [Google Scholar]
- 26).Azin M, Guggenmos D, Barbay S, Nudo R, and Mohseni P, 2011. A miniaturized system for spike-triggered intracortical micro stimulation in an ambulatory rat. IEEE Transactions on Biomedical Engineering, vol. 58, no. 9, pp. 2589–2597, September. [DOI] [PubMed] [Google Scholar]
- 27).Avestruz A et al. , 2008. A 5 μW/channel spectral analysis IC for chronic bidirectional brain machine interfaces. IEEE Journal of Solid-State Circuits, vol. 43, no. 12, pp. 3006–3024, December. [Google Scholar]
- 28).Chae M, Yang Z, Yuce MR, Hoang L, and Liu W,2009. A 128-channel 6 mW wireless neural recording IC with spike feature extraction and UWB transmitter. IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 17, no. 4, pp. 312–321, August. [DOI] [PubMed] [Google Scholar]
- 29).Chen T et al. , 2010. 1.4 μW/channel 16-channel EEG/ECoG processor for smart brain sensor SoC. Proceedings of IEEE Symposium on VLSI Circuits, June, pp. 21–22. [Google Scholar]
- 30).Shoaran M, Pollo C, Schindler K, and Schmid A, 2015. A fully-integrated IC with 0.85 μW/Channel consumption for epileptic EEG detection. IEEE Transactions on Circuits and Systems II: Express Briefs, vol. 62, no. 2, pp. 114–118, February. [Google Scholar]
- 31).Kassiri H, Tonekaboni S, Salam MT, et al. , 2017. Closed-Loop Neurostimulators: A Survey and A Seizure-Predicting Design Example for Intractable Epilepsy Treatment. IEEE Transactions on Biomedical Circuits and Systems.11(5):1026–1040. doi: 10.1109/TBCAS.2017.2694638 [DOI] [PubMed] [Google Scholar]
- 32).Turner APF, 2013. Biosensors: sense and sensibility. Chemical Society Reviews 42, 3184–3196. [DOI] [PubMed] [Google Scholar]
- 33).Mehrotra P, 2016. Biosensors and their applications – a review. Journal of Oral Biology and Craniofacial Research 6, 153–159, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Liechty WB, Caldorera-Moore M, Phillips MA, Schoener C, Peppas NA, 2011. Advanced molecular design of biopolymers for transmucosal and intracellular delivery of chemotherapeutic agents and biological therapeutics. Journal of Controlled Release, Volume 155, Issue 2, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Zalipsky S, Qazen M, Walker JA, Mullah N, Quinn YP, Huang SK, 1999. New detachable poly (ethylene glycol) conjugates: Cysteine-cleavable lipopolymers regenerating natural phospholipid, diacyl phosphatidylethanolamine. Bioconjugate Chemistry, 10, 703–707. [DOI] [PubMed] [Google Scholar]
- 36).Caldorera-Moore M, Peppas NA, 2009. Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Advanced Drug Delivery Reviews, 61, 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM, 1999. Polymeric systems for controlled drug release. Chemical Reviews, 99:3181–3198. [DOI] [PubMed] [Google Scholar]
- 38).Caldorera-Moore ME, Liechty WB, and Peppas NA, 2011. Responsive Theranostic Systems: Integration of Diagnostic Imaging Agents and Responsive Controlled Release Drug Delivery Carriers. Accounts of Chemical Research, 44 (10), 1061–1070 DOI: 10.1021/ar2001777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Yang S, Gao H, 2017. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacological Research, Volume 126, December, Pages 97–108. [DOI] [PubMed] [Google Scholar]
- 40).Katz E, Pingarrón JM, Mailloux S, Guz N, Gamella M, Melman G, and Melman A, 2015. Substance Release Triggered by Biomolecular Signals in Bioelectronic Systems. Journal of Physical Chemistry Letters, 6, 8, 1340–1347. [DOI] [PubMed] [Google Scholar]
- 41).Schoukroun-Barnes LR, Macazo FC, Gutierrez B, Lottermoser J, Liu J, and White RJ, 2016. Reagentless, Structure-Switching, Electrochemical Aptamer-Based Sensors. Annual Review of Analytical Chemistry, 9:1, 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Xu H, Wang C, Wang C, Zoval J, Madou M, 2006. Polymer actuator valves toward controlled drug delivery application. Biosensors and Bioelectronics, Vol. 21, Iss. 11, 15 May, Pages 2094–2099. [DOI] [PubMed] [Google Scholar]
- 43).Nisar A, Afzulpurkar N, Mahaisavariya B, Tuantranont A, 2008. MEMS-based micropumps in drug delivery and biomedical applications. Sensors and Actuators B: Chemical, 130, 917–942. [Google Scholar]
- 44).Ngoepe M, Choonara Y, Tyagi C, Tomar L, Du Toit LC, Kumar P, Ndesendo V, Pillay V, 2013. Integration of biosensors and drug delivery technologies for early detection and chronic management of illness. Sensors 13, 7680–7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Mage P, Ferguson B, Maliniak D, et al. , 2017. Closed-loop control of circulating drug levels in live animals. Nature Biomedical Engineering, 1, 0070. 10.1038/s41551-017-0070. [DOI] [Google Scholar]
- 46).Nguyen QT, Schroeder LF, Mank M, Muller A, Taylor P, Griesbeck O, Kleinfeld D, 2010. An in vivo biosensor for neurotransmitter release and in situ receptor activity. Nature Neuroscience, 13:127–132. [PubMed: 20010818] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Muller A, Joseph V, Slesinger PA, Kleinfeld D, 2014. Cell-based reporters reveal in vivo dynamics of dopamine and norepinephrine release in murine cortex. Nature Methods, 11:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH, 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New England Journal of Medicine, 368, 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, Brenner MK, 2008. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Medicine, 14, 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Kemmer C, Gitzinger M, Daoud-El Baba M, Djonov V, Stelling J, Fussenegger M, 2010. Self-sufficient control of urate homeostasis in mice by a synthetic circuit. Nature Biotechnology, 28, 355–360. [DOI] [PubMed] [Google Scholar]
- 51).Auslander D, Auslander S, Charpin-El Hamri G, Sedlmayer F, Muller M, Frey O, Hierlemann A, Stelling J, Fussenegger M, 2014. A synthetic multifunctional mammalian pH sensor and CO2 transgene-control device. Molecular Cell, 55 (2014) 397–408. [DOI] [PubMed] [Google Scholar]
- 52).Kojima R, Bojar D, Rizzi G et al. , 2018. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nature Communications, 9, 1305. 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Ye H, Daoud-El Baba M, Peng RW, Fussenegger M, 2011. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 332, 1565–1568. [DOI] [PubMed] [Google Scholar]
- 54).Stanley SA, Sauer J, Kane RS, Dordick JS, Friedman JM, 2015. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nature Medicine, 21, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, Friedman JM, 2012. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science, 336, 604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Folcher M, Oesterle S, Zwicky K, Thekkottil T, Heymoz J, Hohmann M, Christen M, Daoud El-Baba M, Buchmann P, Fussenegger M, 2014. Mind-controlled transgene expression by a wireless-powered optogenetic designer cell implant. Nature Communications, 5, 5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Kojima R, Aubel D, Fussenegger M, 2016. Toward a world of theranostic medication: Programming biological sentinel systems for therapeutic intervention. Advanced Drug Delivery Reviews, October;105(Pt A):66–76. DOI: 10.1016/j.addr.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 58).Signore MA, Pascali CD, Rescio G, Taurino A, Dario P, Lacovacci V, Siciliano P, Martucci C, Melissano E, Quaranta F, Francioso L, 2018. Fabrication of AlN-Based Flexible Piezoelectric Pressure Sensor to Integrate into an Artificial Pancreas. Proceedings, 2018, 2, 1037. [Google Scholar]
- 59).Clemens AH, Chang PH & Myers RW, 1977. The development of Biostator, a Glucose Controlled Insulin Infusion System (GCIIS). Hormone & Metabolic Research, 7, 23–33. [PubMed] [Google Scholar]
- 60).Renard E, Costalat G, Chevassus H & Bringer J, 2006. Closed loop insulin delivery using implanted insulin pumps and sensors in type 1 diabetic patients. Diabetes Research and Clinical Practice, 74 (Suppl. 2), S173–S177. [Google Scholar]
- 61).Kovatchev BP et al. (2013) Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care, 36, 1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Hovorka R, 2011. Closed-loop insulin delivery: from bench to clinical practice. Nature Reviews Endocrinology, 7(7):385–395. Published 2011 Feb 22. doi: 10.1038/nrendo.2011.32 [DOI] [PubMed] [Google Scholar]
- 63).Baysal N, Cameron F, Buckingham BA, Wilson DM, Chase HP, Maahs DM, Bequette BW, 2014. In Home Closed-Loop Study Group (IHCL): A novel method to detect pressure-induced sensor attenuations (PISA) in an artificial pancreas. Journal of Diabetes Science and Technology, 8:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Kovatchev B, 2019. A Century of Diabetes Technology: Signals, Models, and Artificial Pancreas Control. Trends in Endocrinology & Metabolism, July, Vol. 30, No. 7. [DOI] [PubMed] [Google Scholar]
- 65).Gin H, Renard E, Melki V, Boivin S, Schaepelynck-Belicar P, Guerci B et al. , 2003. Combined improvements in implantable pump technology and insulin stability allow safe and effective long-term intraperitoneal insulin delivery in type 1 diabetic patients: the EVADIAC experience. Diabetes & Metabolism, 29: 602–607. [DOI] [PubMed] [Google Scholar]
- 66).Kerner W, Kiwit M, Linke B, 1993. The function of a hydrogen peroxide-detecting electroenzymatic glucose electrode is markedly impaired in human sub-cutaneous tissue. Biosensors and Bioelectronics, 8, 473. [DOI] [PubMed] [Google Scholar]
- 67).Elbicki JM, Weber SG, 1988. Ultrafiltration of human serum to determine the size of species that poison voltametric electrodes. Biosensors, 4, 251–257. [DOI] [PubMed] [Google Scholar]
- 68).Gerritsen M, Jansen JA, Dros A, Vriezema DM, Sommerdijk NAJM, Nolte RJM, Lutterman JA, Hovell SWFFV, Gaag AVD, 2000. Influence of inflammatory cells and serum on the performance of implantable glucose sensors. Journal of Biomedical Materials Research, 54, 69–75. [DOI] [PubMed] [Google Scholar]
- 69).Ju YM, Yu B, Koob TJ, Moussy Y, Moussy F, 2008. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. Journal of Biomedical Materials Research Part A, 87(1):136–46. [DOI] [PubMed] [Google Scholar]
- 70).Moussy F, Harrison DJ, O’Brien DW, Rajotte RV, 1993. Performance of subcutaneously implanted needle-type glucose sensors employing a novel trilayer coating. Analytical Chemistry, 65(15):2072–7. [DOI] [PubMed] [Google Scholar]
- 71).Valdes TI, Moussy F, 1999. A ferric chloride pre-treatment to prevent calcification of Nafion membrane used for implantable biosensors. Biosensors and Bioelectronics, 14(6):579–85. [DOI] [PubMed] [Google Scholar]
- 72).Park JK, Tran PH, Chao JK, Ghodadra R, Rangarajan R, Thakor NV, 1998. In vivo nitric oxide sensor using non-conducting polymer-modified carbon fiber. Biosensors and Bioelectronics, 13(11):1187–95. [DOI] [PubMed] [Google Scholar]
- 73).Yu B, Wang C, Ju YM, West L, Harmon J, Moussy Y, Moussy F, 2008. Use of hydrogel coating to improve the performance of implanted glucose sensors. Biosensors and Bioelectronics, 23(8):1278–84. [DOI] [PubMed] [Google Scholar]
- 74).Quinn CP, Pathak CP, Heller A, Hubbell JA, 1995. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly (ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials, 16(5):389–96. [DOI] [PubMed] [Google Scholar]
- 75).Espadas-Torre C, Meyerhoff ME, 1995. Thrombogenic properties of untreated and poly (ethylene oxide)-modified polymeric matrices useful for preparing intraarterial ion-selective electrodes. Analytical Chemistry, 67(18):3108–14. [DOI] [PubMed] [Google Scholar]
- 76).Rigby G, Ahmed S, Horseman G, Vadgama P, 1999. In vivo glucose monitoring with open microflow--influences of fluid composition and preliminary evaluation in man. Analytica Chimica Acta, 385: 23–32. [Google Scholar]
- 77).Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess DJ, 2005. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. The AAPS Journal, 7(1): E231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Udipi K, Ornberg RL, Thurmond KB II, Settle SL, Forster D, Riley D, 2000. Modification of inflammatory response to implanted biomedical materials in vivo by surface bound superoxide dismutase mimics. Journal of Biomedical Materials Research, 51, 549–560. [DOI] [PubMed] [Google Scholar]
- 79).Hickey T, Kreutzer D, Burgess DJ, Moussy F, 2002. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. Journal of Biomedical Materials Research, 61(2):180–7. [DOI] [PubMed] [Google Scholar]
- 80).Frost MC, Batchelor MM, Lee Y, Zhang H, Kang Y, Oh B, Wilson GS, Gifford R, Rudich SM, Meyerhoff ME, 2003. Preparation and characterization of implantable sensors with nitric oxide release coatings. Microchemical Journal, 74, 277–288. [Google Scholar]
- 81).Shin JH, Marxer SM, Schoenfisch MH, 2004. Nitric oxide-releasing sol–gel article/polyurethane glucose biosensors. Analytical Chemistry, 76, 4543–4549. [DOI] [PubMed] [Google Scholar]
- 82).Ward WK, Wood MD, Casey HM, Quinn MJ, Federiuk IF, 2004. The effect of local subcutaneous delivery of vascular endothelial growth factor on the function of a chronically implanted amperometric glucose sensor. Diabetes Technology & Therapeutics, 6(2):137–45. [DOI] [PubMed] [Google Scholar]
- 83).Klueh U, Dorsky DI, Kreutzer DL, 2003. Use of vascular endothelial cell growth factor gene transfer to enhance implantable sensor function in vivo. Journal of Biomedical Materials Research A, 67(4):1072–86. [DOI] [PubMed] [Google Scholar]
- 84).Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM, 2007. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. Journal of Biomedical Materials Research A, 81(4):858–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM, 2007. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response Journal of Biomedical Materials Research A, 81(4):858–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Patil SD, Papadmitrakopoulos F, Burgess DJ, 2007. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. Journal of Controlled Release, 117(1):68–79. [DOI] [PubMed] [Google Scholar]
- 87).Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC, 1995. Neovascularization of synthetic membranes directed by membrane microarchitecture. Journal of Biomedical Materials Research, 29(12):1517–24. [DOI] [PubMed] [Google Scholar]
- 88).Sharkawy AA, Klitzman B, Truskey GA, Reichert WM, 1998. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. Journal of Biomedical Materials Research, 40(4):598–605. [DOI] [PubMed] [Google Scholar]
- 89).Ward WK, Slobodzian EP, Tiekotter KL, Wood MD, 2002. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials, 23(21):4185–92. [DOI] [PubMed] [Google Scholar]
- 90).Yung LY, Cooper SL, 1998. Neutrophil adhesion on phosphorylcholine-containing polyurethanes. Biomaterials, 19(1–3):31–40. [DOI] [PubMed] [Google Scholar]
- 91).Van der Heiden AP, Willems GM, Lindhout T, Pijpers AP, Koole LH, 1998. Adsorption of proteins onto poly (ether urethane) with a phosphorylcholine moiety and influence of preadsorbed phospholipid. Journal of Biomedical Materials Research, 40(2):195–203. [DOI] [PubMed] [Google Scholar]
- 92).Goreish HH, Lewis AL, Rose S, Lloyd AW, 2004. The effect of phosphorylcholine-coated materials on the inflammatory response and fibrous capsule formation: in vitro and in vivo observations Journal of Biomedical Materials Research Part A, 68(1):1–9. [DOI] [PubMed] [Google Scholar]
- 93).Mang A, Pill J, Gretz N, Kränzlin B, Buck H, Schoemaker M, Petrich W, 2005. Biocompatibility of an electrochemical sensor for continuous glucose monitoring in subcutaneous tissue. Diabetes Technology & Therapeutics, 7(1):163–73. [DOI] [PubMed] [Google Scholar]
- 94).Belalia R, Grelier S, Benaissa M, Coma V, 2008. New bioactive biomaterials based on quaternized chitosan. Journal of Agricultural and Food Chemistry, 56(5):1582–8. [DOI] [PubMed] [Google Scholar]
- 95).Xie H, Khajanchee YS, Teach JS, Shaffer BS, 2008. Use of a chitosan-based hemostatic dressing in laparoscopic partial nephrectomy. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 85(1):267–71. [DOI] [PubMed] [Google Scholar]
- 96).Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, Kwon IC, 2003. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: implications for cartilage tissue engineering. Journal of Controlled Release, 91(3):365–74. [DOI] [PubMed] [Google Scholar]
- 97).Hauser RG, 2005. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. Journal of the American College of Cardiology, vol. 45, no. 12, pp. 2022–2025, 2005. [DOI] [PubMed] [Google Scholar]
- 98).RamRakhyani AK, Mirabbasi S, and Chiao M, 2011. Design and optimization of resonance-based efficient wireless power delivery systems for biomedical implants. IEEE Transactions on Biomedical Circuits and Systems, vol. 5, no. 1, pp. 48–63, February. [DOI] [PubMed] [Google Scholar]
- 99).Jegadeesan R, and Guo Y-X, 2012. Topology selection and efficiency improvement of inductive power links. IEEE Transactions on Antennas and Propagation, vol. 60, no. 10, pp. 4846–4854, October. [Google Scholar]
- 100).Jow U-M, and Ghovanloo M, 2009. Modeling and optimization of printed spiral coils in air, saline, and muscle tissue environments. IEEE Transactions on Biomedical Circuits and Systems, vol. 3, no. 5, pp. 339–347, October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Ozeri S, and Shmilovitz D, 2010. Ultrasonic transcutaneous energy transfer for powering implanted devices. Ultrasonics, vol. 50, no. 6, pp. 556–566. [DOI] [PubMed] [Google Scholar]
- 102).Suzuki S. n., Kimura S, Katane T, Saotome H, Saito O, and Kobayashi K, 2002. Power and interactive information transmission to implanted medical device using ultrasonic. Japanese Journal of Applied Physics, vol. 41, no. 5S, pp. 3865–3866. [Google Scholar]
- 103).Maleki T, Cao N, Song SH, Kao C, Ko S-C, and Ziaie B, 2011. An ultrasonically powered implantable micro-oxygen generator (IMOG). IEEE Transactions on Biomedical Engineering, vol. 58, no. 11, pp. 3104–3111, November. [DOI] [PubMed] [Google Scholar]
- 104).Poon AS, O’Driscoll S, and Meng TH, 2010. Optimal frequency for wireless power transmission into dispersive tissue. IEEE Transactions on Antennas and Propagation, vol. 58, no. 5, pp. 1739–1750, May. [Google Scholar]
- 105).Ho JS, et al. , 2014. Wireless power transfer to deep tissue microimplants. Proceedings of the National Academy of Sciences, vol. 111, no. 22, pp. 7974–7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106).Liu C, Guo Y-X, Sun H, and Xiao S, 2014. Design and safety considerations of an implantable rectenna for far-field wireless power transfer. IEEE Transactions on Antennas and Propagation, vol. 62, no. 11, pp. 5798–5806, November. [Google Scholar]
- 107).Falkenstein E, Roberg M, and Popovic Z, 2012. Low-power wireless power delivery. IEEE Transactions on Microwave Theory and Techniques, vol. 60, no. 7, pp. 2277–2286, July. [Google Scholar]
- 108).Hochmair I, et al. , 2006. Med-el cochlear implants: State of the art and a glimpse into the future. Trends in Amplification, vol. 10, no. 4, pp. 201–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109).Patrick JF, Busby PA, and Gibson PJ, 2006. The development of the nucleus freedom cochlear implant system. Trends in Amplification, vol. 10, no. 4, pp. 175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Cortizo MC, Lorenzo de Mele MF, 2004. Cytotoxicity of copper ions released from metal. Biological Trace Element Research, 102, 129–141. 10.1385/BTER:102:1-3:129 [DOI] [PubMed] [Google Scholar]
- 111).Zeng F-G, Rebscher S, Harrison W, Sun X, and Feng H, 2008. Cochlear implants: System design, integration, and evaluation. IEEE Reviews in Biomedical Engineering, vol. 1, pp. 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112).Qusba A, RamRakhyani A, So J-H, Hayes G, Dickey M, and Lazzi G, 2014. On the design of microfluidic implant coil for flexible telemetry system. IEEE Sensors Journal, vol. 14, no. 4, pp. 1074–1080, April. [Google Scholar]
- 113).Kim R-H, et al. , 2012. Materials and designs for wirelessly powered implantable light-emitting systems. Small, vol. 8, no. 18, pp. 2812–2818. [DOI] [PubMed] [Google Scholar]
- 114).Ozeri S and Shmilovitz D, 2010. Ultrasonic transcutaneous energy transfer for powering implanted devices. Ultrasonics, vol. 50, no. 6, pp. 556–566. [DOI] [PubMed] [Google Scholar]
- 115).Maleki T, Cao N, Song SH, Kao C, Ko S-C, and Ziaie B, 2011. An ultrasonically powered implantable micro-oxygen generator (IMOG). IEEE Transactions on Biomedical Engineering, vol. 58, no. 11, pp. 3104–111, November. [DOI] [PubMed] [Google Scholar]
- 116).Ozeri S, Shmilovitz D, Singer S, and Wang C-C, 2010. Ultrasonic transcutaneous energy transfer using a continuous wave 650 khz gaussian shaded transmitter. Ultrasonics, vol. 50, no. 7, pp. 666–674. [DOI] [PubMed] [Google Scholar]
- 117).Shmilovitz D, Ozeri S, Wang C-C, and Spivak B, 2014. Noninvasive control of the power transferred to an implanted device by an ultrasonic transcutaneous energy transfer link. IEEE Transactions on Biomedical Engineering, vol. 61, no. 4, pp. 995–1004, April. [DOI] [PubMed] [Google Scholar]
- 118).Leadbetter J, Brown J, and Adamson R, 2013. The design of ultrasonic lead magnesium niobate-lead titanate composite transducers for power and signal delivery to implanted hearing aids. Journal of Acoustical Society of America, vol. 133, no. 5, pp. 3268–3268. [Google Scholar]
- 119).Dodds JS, Meyers FN, and Loh KJ, 2012. Piezoelectric Characterization of PVDF-TrFE Thin Films Enhanced with ZnO Nanoparticles. IEEE Sensors Journal, vol. 12, no. 6, pp. 1889–1890, June. doi: 10.1109/JSEN.2011.2182043. [DOI] [Google Scholar]
- 120).Ho JS, et al. , 2014. Wireless power transfer to deep tissue microimplants. Proceedings of the National Academy of Sciences, vol. 111, no. 22, pp. 7974–7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121).Montgomery K, Yeh A, Ho J et al. , 2015. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nature Methods 12, 969–974. 10.1038/nmeth.3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Lee J et al. , 2019. An Implantable Wireless Network of Distributed Microscale Sensors for Neural Applications. 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA, USA, pp. 871–874, doi: 10.1109/NER.2019.8717023 [DOI] [Google Scholar]
- 123).Khalifa A et al. , 2018. The Microbead: A Highly Miniaturized Wirelessly Powered Implantable Neural Stimulating System. in IEEE Transactions on Biomedical Circuits and Systems, vol. 12, no. 3, pp. 521–531, June. doi: 10.1109/TBCAS.2018.2802443. [DOI] [PubMed] [Google Scholar]
- 124).Lyu H, John M, Burkland D et al. , 2020. Synchronized Biventricular Heart Pacing in a Closed-chest Porcine Model based on Wirelessly Powered Leadless Pacemakers. Scientific Reports, 10, 2067. 10.1038/s41598-020-59017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Zhao P, Zhao Z, Hunter D, Suchoski R, Gao C, Mathews S, Wuttig M, and Takeuchi I, 2009. Fabrication and characterization of all-thin-film magnetoelectric sensors. Applied Physics Letters, 94, 243507. 10.1063/1.3157281 [DOI] [Google Scholar]
- 126).Yu Z, et al. , 2020. 34.3 An 8.2mm3 Implantable Neurostimulator with Magnetoelectric Power and Data Transfer. 2020 IEEE International Solid- State Circuits Conference - (ISSCC), San Francisco, CA, USA, pp. 510–512. doi: 10.1109/ISSCC19947.2020.9062931. [DOI] [Google Scholar]
- 127).Singer A, Dutta S, Lewis E, Chen Z, Chen JC, Verma N, Avants B, Feldman AK, O’Malley J, Beierlein M, Kemere C, Robinson JT, 2020. Magnetoelectric Materials for Miniature, Wireless Neural Stimulation at Therapeutic Frequencies. Neuron, Volume 107, Issue 4, 19 August, Pages 631–643.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128).Loeb GE, Peck RA, Moore WH, Hood K, 2001. BION system for distributed neural prosthetic interfaces. Medical Engineering & Physics, 23, 9–18. [DOI] [PubMed] [Google Scholar]
- 129).Shin G et al. , 2017. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron, vol. 93, no. 3, pp. 509–521. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130).Seo D, et al. , 2016. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron, vol. 91, no. 3, pp. 529–539, August. [DOI] [PubMed] [Google Scholar]
- 131).Park SI, et al. , 2015. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nature Biotechnology, vol. 33, no. 12, pp. 1280–1286, December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132).Khan W, Jia Y, Madi F et al. , 2019. Inductively coupled, mm-sized, single channel optical neurostimulator with intensity enhancer. Microsystems & Nanoengineering 5, 23. 10.1038/s41378-019-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Alam M, Li S, Ahmed RU et al. , 2019. Development of a battery-free ultrasonically powered functional electrical stimulator for movement restoration after paralyzing spinal cord injury. Journal of NeuroEngineering and Rehabilitation, 16, 36. 10.1186/s12984-019-0501-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Piech DK, Johnson BC, Shen K et al. , 2020. A wireless millimeter-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nature Biomedical Engineering, 4, 207–222. 10.1038/s41551-020-0518-9. [DOI] [PubMed] [Google Scholar]
- 135).Kozielski KL, et al. , 2020. Injectable Nanoelectrodes Enable Wireless Deep Brain Stimulation of Native Tissue in Freely Moving Mice, bioRxiv 2020.03.14.978676. doi: 10.1101/2020.03.14.978676 [DOI] [Google Scholar]
- 136).Rubehn B, et al. 2009. A MEMS-based flexible multichannel ECoG-electrode array. Journal of Neural Engineering, Volume 6, Number 3. [DOI] [PubMed] [Google Scholar]
- 137).Iverson NM, et al. , 2013. In vivo biosensing via tissue-localizable near-infrared-fluorescent single-walled carbon nanotubes. Nature Nanotechnology, DOI: 10.1038/nnano.2013.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138).Melendez B, et al. , Developing Novel Vaccine Delivery Systems for Cancer Therapy, Nanotechnology Image Library.
- 139).Brown G, 2020. What Is a CGM (Continuous Glucose Monitor) and How Do I Choose One? [Photograph]. https://www.healthline.com/diabetesmine/what-is-continuous-glucose-monitor-and-choosing-one
- 140).Zaiets R, Diabetic man with an insulin pump connected in his abdomen and holding the insulin pump at his hands. [Photograph]. Shutterstock. photo ID: 1212108649 [Google Scholar]


