Abstract
Introduction
Nutraceuticals are a good means to lower cardiovascular risk. Having established a reasonable pharmacological background, a new nutraceutical combination should be tested in clinical trials.
Material and methods
This double-blind, placebo-controlled randomized clinical trial aims to evaluate the modulating effect, in a setting of controlled nutritional habits, of a combined food supplement with DIF1STAT (based on red yeast rice with a very low content of monacolins, linear aliphatic alcohols and niacin) and Olea europaea on plasma lipids and endothelial function, in a group of 40 healthy, moderately hypercholesterolemic patients in primary cardiovascular prevention.
Results
After 8 weeks of treatment, when compared to the placebo group, the active treated patients experienced significant improvements of different metabolic parameters and endothelial reactivity compared to placebo. The treated patients showed a statistically significant percentage change in total cholesterol (–12.25 delta% vs. –1.8%, p < 0.01), low-density lipoprotein (LDL) cholesterol (–28.7 delta% vs. –1.1%, p < 0.01), high-density lipoprotein (HDL) cholesterol (+4.99% vs. +0.9%, p < 0.05), non-HDL cholesterol (–16.02 delta% vs. –1.5%, p < 0.01), SUA (–12.96 delta%, p < 0.05) and endothelial reactivity (+6.73% vs. –1.4%, p < 0.01). In both groups, there was no case of intolerance and the safety parameters were unchanged.
Conclusions
The tested nutraceutical association is able to significantly improve different lipid parameters compared to placebo, and endothelial reactivity compared to baseline. Even if the study power appears to be adequate for the primary endpoints, the effect on endothelial function needs confirmation in a longer clinical trial.
Keywords: dietary supplements, cardiovascular benefit, monacolins, Olea europaea, Camellia sinensis
Introduction
Diet therapy is the first line of treatment of the patient with hypercholesterolemia, one of most prevalent cardiovascular risk factors. However, dietary intervention alone is usually able to reduce low-density lipoprotein (LDL) cholesterolemia in less than 10% of cases [1]. Therefore, the dietary supplementation with some effective lipid-lowering nutraceuticals could improve the dietary intervention efficacy [2]. In particular, red yeast rice is a commonly used and effective cholesterol-lowering nutraceutical, derived from the fermentation of red rice by the yeast Monascus purpureus that leads to the production of monacolins, natural 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors [3]. Even if very effective and tolerable, recently the European Food Safety Authority raised some concern over its higher dosages, so it was suggested to reduce the daily dose of monacolin K to less than 3 mg/day [4]. For this reason, it is commonly associated with other lipid-lowering nutraceuticals, for instance, Olea europaea, policosanol, a blend of sugar cane alcohols processed from Saccharum officinarum L, primarily composed of octacosanol (CH3-CH2(26)-CH2-OH), triacontanol (12%), and hexacosanol (7%). Policosanol increases fecal cholesterol and bile acid contents and deactivates 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase by AMP-activated protein kinase (AMPK) phosphorylation [5]. On the other hand, cholesterol’s ability to early impair vascular health is also mediated by its oxidation [6]. A number of dietary components exert an antioxidant effect on LDL and on endothelium that is strongly sensitive to exposure to the positive action of antioxidants [7, 8].
Olive oil and olive leaf extract are considered traditional remedies useful for the treatment of various conditions. More recently, experimental and clinical data seem to confirm the beneficial effects of olive derivates. In particular, the polyphenols present in olive leaves and pomace (cake) appear to have antioxidant, anti-inflammatory and anti-atherogenic effects [9]. The leaves of the olive plant are particularly rich in polyphenols, of which oleuropein and hydroxytyrosol are most characteristic [10].
Such polyphenols have been demonstrated to favorably modify a variety of cardiovascular risk factors:
benefits for plasma lipid levels and oxidative damage [11],
a role in the regulation of genes involved in the cholesterol efflux from cells to high-density lipoprotein (HDL) [12],
enhancement of HDL function [13].
Thus, it would be interesting to test the efficacy of combined lipid-lowering and antioxidant nutraceuticals on endothelial function in moderately hypercholesterolemic subjects.
In this context, the aim of this two-arm, double-blind randomized clinical trial is to comparatively test the effect of a combined lipid-lowering and antioxidant nutraceutical on lipid profile, markers of endothelial function, and estimated cardiovascular disease risk level in a group of 40 healthy adults with borderline LDL cholesterol levels.
Material and methods
Study design
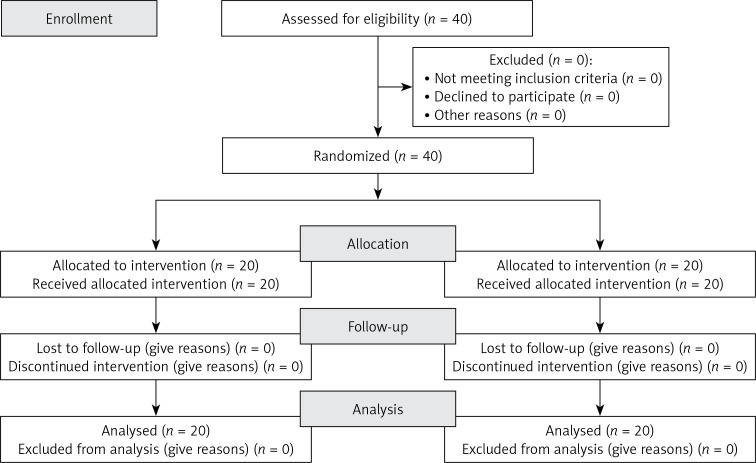
This single-center, double-blind, placebo-controlled randomized clinical trial included 40 healthy adult volunteers, aged 25–65 years, with borderline LDL cholesterol levels (LDL between 150 and 190 mg/dl as confirmed in at least two sequential checks) in primary cardiovascular prevention. Patients in secondary prevention for cardiovascular diseases, age < 25 or > 65 years, triglyceridemia > 400 mg/dl, taking lipid-lowering drugs or nutraceuticals, with chronic gastrointestinal disorders and under therapy for this disease, with clinically relevant modification of renal function or with any medical or surgical condition that makes the patient’s adherence to the study protocol complex or inconstant, were excluded from the study. The CONSORT flow diagram is reported in Figure 1. The study included a 1-month-long run-in period of diet standardization, followed by a 2-month-long period of active treatment or placebo. Patients received standard quantitative and qualitative nutritional advice to correct their negative behavioral habits, at the initial visit. In order to achieve an overall healthy diet for the length of the research, participants were highly advised to observe the general indications of a Mediterranean diet, limiting unhealthy consumption of dairy and red meat-derived items. Patients were highly advised to improve their physical fitness by exercising for at least 20 min 3 to 5 days a week. The study fully complied with the ethical guidelines of the Declaration of Helsinki and its protocol was approved by the Ethical Committee of the University of Bologna. All participants signed a written informed consent form to participate. The trial was carried out in line with the CONSORT statement (Figure 1).
Figure 1.
CONSORT flow diagram
Treatment
After 4 weeks of diet standardization, the participants were randomly assigned to receive two indistinguishable placebo or active substance capsules (kindly provided by Difass International, Cerasolo, Italy), one capsule per day. The tested nutraceutical contained DIF1STAT (red yeast rice, with monacolin K 2.5–2.9 mg/capsule, linear aliphatic alcohols titrated at 60% in octacosanols and niacin), Olea europaea total extract of pomace and leaves (titrated at 15% in oleuropein), Camellia sinensis extract and vitamins E, B6, B9, B12. Patients were instructed to take the capsules at the same time of day, after dinner. Randomization was performed with a 1 : 1 ratio and the blocks were stratified by sex and age. Each lot code (corresponding to medication or placebo) was placed on the dosage box according to an alphabetical code. Study personnel, investigators, and participants were all unaware of the group assignment. Codes were held in a closed envelope, which was not opened until the trial was concluded. Dose boxes were mixed and a blinded dose box was assigned to each patient. Treatment adherence was estimated by counting the amount of medication capsules at the defined date of visits. At the start of the trial, we weighed participants and provided them a container containing a 66-day supply of capsules, enough for the duration of the study. We advised patients to take their first dosage of a new treatment the day after they were provided it in the trial. For inventory purposes, all unused capsules were recalled. Care supplies were given away for free. At the beginning, in the middle and at the end of the study, patients’ medical histories, physical examinations, and laboratory tests were analyzed. Before and after the intervention time, endothelial reactivity was examined. Instrumental analyses were conducted by qualified professionals who adopted uniform procedures.
Assessments
Patients’ personal history, dietary and smoking habits, physical activity, and pharmacological treatment were evaluated with a validated semi-quantitative questionnaire. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Height and weight were measured by standard procedures to the nearest 0.1 cm and 0.1 kg respectively, with subjects standing erect with eyes directed straight ahead, wearing light clothes, and with bare feet. Waist circumference (WC) was measured at the end of a normal expiration, in a horizontal plane at the midpoint between the inferior margin of the last rib and the superior iliac crest. The biochemical analyses were carried out on venous blood, withdrawn in the morning from the basilic vein. Subjects had fasted for at least 12 h at the time of sampling. All the hematochemical measurements were centrally performed in our department’s laboratory. Plasma used was obtained by addition of Na2EDTA (1 mg/ml) and centrifuged at 3000 rpm for 15 min at 48°C. All the laboratory analyses were performed by trained personnel immediately after centrifugation, in accordance with standardized methods largely described elsewhere [14]. The hematochemistry variables investigated were: total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), serum uric acid (SUA), creatinine (Cr), alanine transaminase (ALT), aspartate transaminase (AST), γ-glutamyltransferase (γ-GT), and creatinine phosphokinase (CPK). Glomerular filtration rate (GFR) was estimated with the CKD-epi equation [15]. Systolic (SBP) and diastolic blood pressure (DBP) values were detected in each subject supine and at rest, with a cuff applied at the right upper arm, using the Vicorder apparatus (Skidmore Medical Ltd, Bristol, UK), which is a validated oscillometric device guaranteeing excellent intra- and interoperator reliability [16]. Endothelial function was evaluated through flow-mediated dilatation (FMD) of the right brachial artery, using the Endocheck (BC Biomedical Laboratories Ltd, Vancouver, BC, Canada), which is embedded in the Vicorder system. The test was carried out before the morning treatment intake, with patients in supine position and having abstained from cigarette smoking and caffeinated beverages for at least 12 h. The brachial pulse volume (PV) waveforms were recorded for 10 s at baseline and then during reactive hyperemia, which was provoked through PV displacement, obtained by inflating up to 200 mm Hg for 5 min a cuff positioned distally around the forearm. When the cuff was released, the PV waveforms were recorded again for 3 min. The percentage PV displacement was calculated as the percentage change from baseline to peak dilatation [17]. All of the hemodynamic measurements were performed by a trained physician who was blinded to the treatment groups.
Statistical analysis
Data were analyzed using intention to treat by means of SPSS Statistics version 21.0 (IBM Corporation, Armonk, NY, USA) for Windows.
A one-sample t test was used to compare the values obtained before and after the treatment administration; a two-sample t test was used for between-group comparisons. All data were expressed as mean ± standard deviation (SD). Every test was two-tailed. P-values less than 0.05 were regarded as statistically significant. Tukey’s correction was carried out for multiple comparisons. Sample size was calculated for both primary outcomes (LDL-C decrease). Considering a type I error of 0.05 and a power of 0.80 and expecting a minimum LDL-C reduction of 10.0 mg/dl with an SD of 0.1, and considering a 10% dropout rate, we calculated the need to enroll 40 patients (20 per arm). This calculation was also valid to detect significant changes in endothelial reactivity. The normal distribution of the tested parameters was evaluated by the Kolmogorov-Smirnov test. The baseline characteristics of the population were described by the independent t test and the v2 test, followed by Fisher’s exact test for categorical variables. Every continuous parameter was compared by repeated-measures analysis of variance (ANOVA). The intervention effects were adjusted for all of the considered potential confounders by the analysis of covariance (ANCOVA). ANOVA was performed to assess the significance within and between groups. The statistical significance of the independent effects of treatments on the other variables was determined by the use of the ANCOVA.
Results
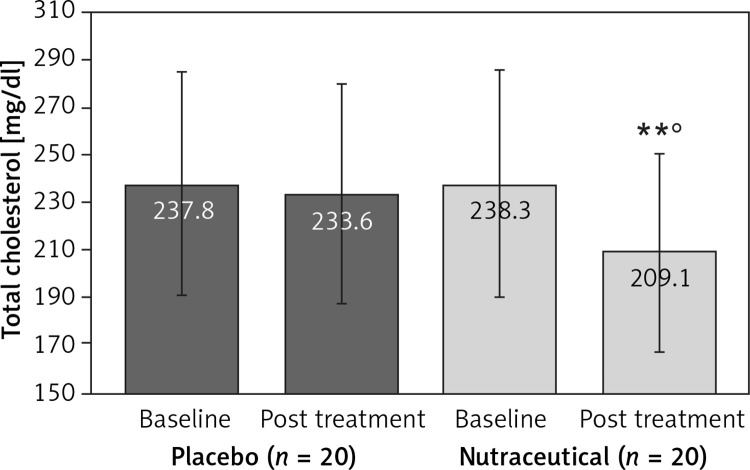
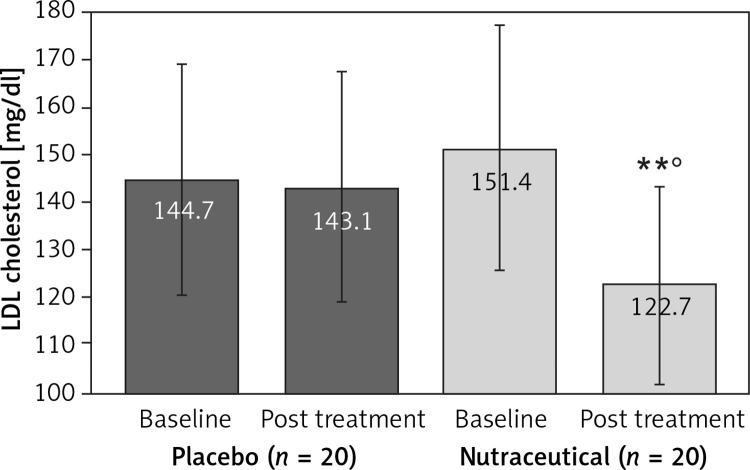
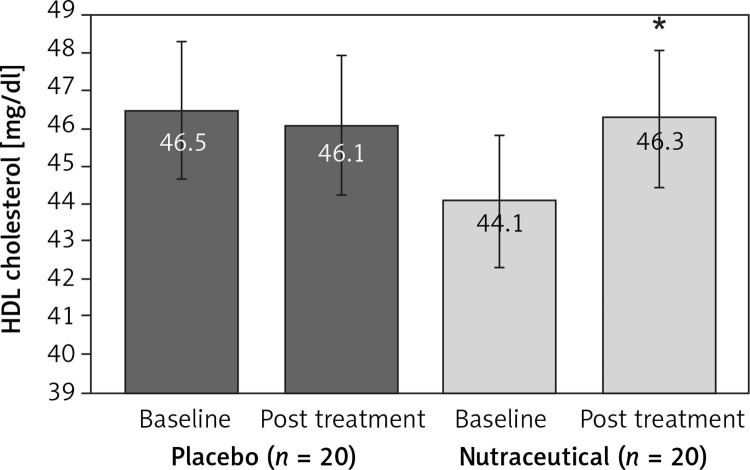
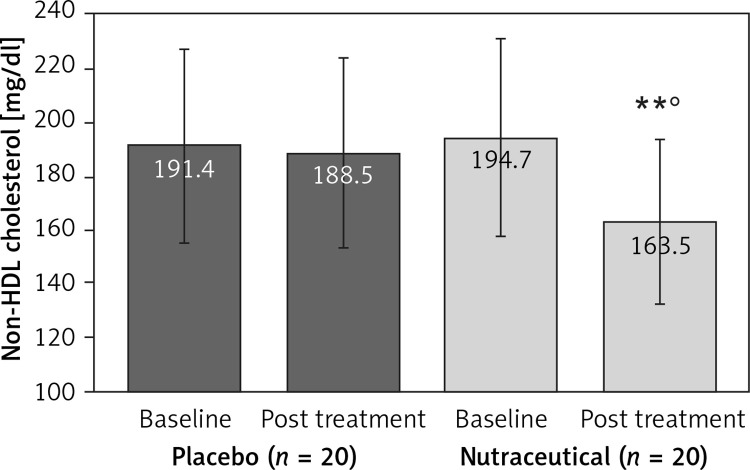
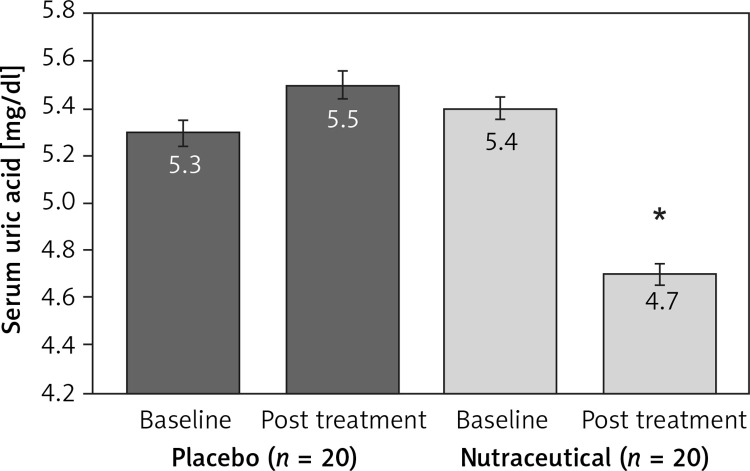
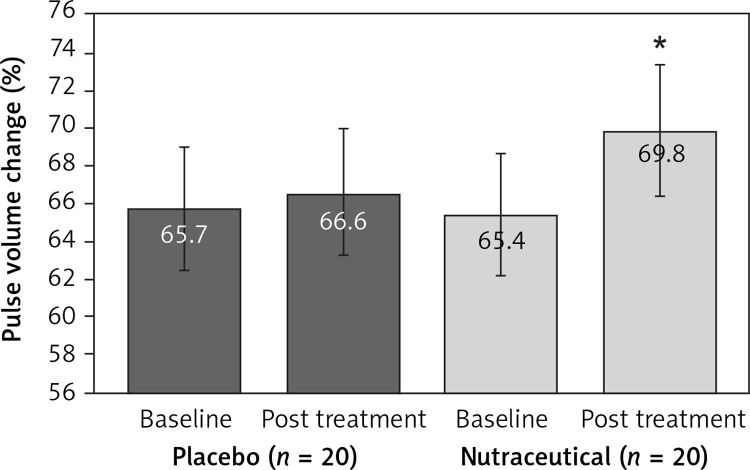
All subjects enrolled (19 men and 21 women) underwent the study according to the study plan. (Figure 1). The tested nutraceutical was given to 20 (50%) patients and placebo to 20 (50%) patients at randomization. Compliance was 100%. Considering the assumed products, the final sex-related distribution did not show any significant difference (χ2 < 0.001, p > 0.05). The two groups were well balanced for all of the parameters that were considered at the beginning of the study (p > 0.05 for all) (Table I). There were no subjective or laboratory negative events in either patients or the placebo group. The enrolled participants (T0) reported to have maintained similar dietary patterns from the first visit to the end of the trial, with no significant changes in energy intake, total cholesterol, or total saturated fatty acid consumption (Table I). At the end of the study, after 8 weeks of treatment (T1), in both groups, placebo and active treatment, there were no statistically significant changes in anthropometric parameters such as waist circumference, BMI, and body weight. Similarly, there were no statistically significant differences in the active group and placebo group in blood pressure, triglyceridemia, creatinine, alanine transaminase, aspartate transaminase, γ-glutamyltransferase, and creatinine phosphokinase. The treated patients showed a statistically significant percentage change in total cholesterol (–12.25 delta% vs. –1.8%, p < 0.01) (Figure 2), LDL cholesterol (–28.7 delta% vs. –1.1%, p < 0.01) (Figure 3), HDL cholesterol (+4.99% vs. +0.9%, p < 0.05) (Figure 4), non-HDL cholesterol (–16.02 delta% vs. –1.5%, p < 0.01) (Figure 5), SUA (–12.96 delta%, p < 0.05) (Figure 6) and endothelial reactivity (+6.73% vs. –1.4%, p < 0.01) (Figure 7, Table I).
Table I.
Baseline (T0) and post-treatment (T1) levels of the main parameters evaluated at the end of the intervention period (after 8 weeks of placebo or active treatment)
| Parameter | Placebo (N = 20) | Nutraceutical (N = 20) | ||
|---|---|---|---|---|
| Baseline (T0) | Post-treatment (T1) | Baseline (T0) | Post-treatment (T1) | |
| Age [years] | 53.4 ±4.1 | 54.1 ±4.4 | 52.6 ±5.0 | 54.5 ±5.2 |
| Weight [kg] | 64.3 ±5.4 | 63.8 ±5.4 | 63.4 ±4.2 | 62.7 ±4.3 |
| Waist circumference [cm] | 90.7 ±4.5 | 89.6 ±4.3 | 88.3 ±7.1 | 87.9 ±6.5 |
| Body mass index [kg/m2] | 23.7 ±1.7 | 23.6 ±1.5 | 22.8 ±2.2 | 22.6 ±2.3 |
| Systolic blood pressure [mm Hg] | 136.3 ±4.8 | 134.9 ±4.3 | 134.5 ±5.6 | 135.7 ±4.3 |
| Diastolic blood pressure [mm Hg] | 88.6 ±3.2 | 86.9 ±2.4 | 87.3 ±5.2 | 86.9 ±5.5 |
| Total cholesterol [mg/dl] | 237.8 ±21.7 | 233.6 ±18.5 | 238.3 ±23.0 | 209.1 ±16.8**° |
| HDL cholesterol [mg/dl] | 46.5 ±4.5 | 46.1 ±3.7 | 44.1 ±3.7 | 46.3 ±3.8* |
| LDL cholesterol [mg/dl] | 144.7 ±17.9 | 143.1 ±16.6 | 151.4 ±16.5 | 122.7 ±14.4**° |
| Non HDL cholesterol [mg/dl] | 191.4 ±20.7 | 188.5 ±18.9 | 194.7 ±21.4 | 163.5 ±19.7**° |
| Triglycerides [mg/dl] | 235.4 ±23.7 | 228.5 ±17.9 | 216.8 ±16.1 | 205.8 ±19.9 |
| AST [mg/dl] | 24.5 ±4.2 | 25.3 ±4.4 | 23.7 ±4.8 | 23.1 ±3.3 |
| ALT [mg/dl] | 21.9 ±3.4 | 22.1 ±3.9 | 22.0 ±4.3 | 23.3 ±3.8 |
| γ-GT [mg/dl] | 34.3 ±4.4 | 35.1 ±3.7 | 32.9 ±4.5 | 30.3 ±3.6 |
| Serum uric acid [mg/dl] | 5.3 ±0.9 | 5.5 ±1.0 | 5.4 ±0.8 | 4.7 ±0.9* |
| Serum creatinine [mg/dl] | 0.7 ±0.3 | 0.8 ±0.4 | 0.7 ±0.4 | 0.7 ±0.5 |
| CPK [U/ml] | 111.3 ±31.3 | 121.9 ±35.1 | 104.7 ±29.2 | 106.1 ±28.9 |
| Pulse volume change (%) | 65.7 ±5.3 | 66.6 ±5.5 | 65.4 ±6.2 | 69.8 ±5.1* |
p < 0.05 vs. baseline;
p < 0.01 vs. baseline;
p < 0.05 vs. placebo.
ALT – alanine transaminase, AST – aspartate transaminase, ansaminase, γ-GT – γ-glutamyltransferase, CPK – creatinine phosphokinase.
Figure 2.
Change in total cholesterol value: **p < 0.01 vs. baseline; °p < 0.05 vs. placebo
Figure 3.
Change in LDL cholesterol value: **p < 0.01 vs. baseline; °p < 0.05 vs. placebo
Figure 4.
Change in HDL cholesterol value: **p < 0.05 vs. baseline
Figure 5.
Change in non-HDL cholesterol value: **p < 0.01 vs. baseline; °p < 0.05 vs. placebo
Figure 6.
Change in serum uric acid value: **p < 0.05 vs. baseline
Figure 7.
Change in pulse volume change value: **p < 0.05 vs. baseline
Discussion
Olive oil components have been demonstrated to contribute to the cardiovascular protective effect of the Mediterranean diet [18]. In particular, the Prevención con Dieta Mediterránea (PREDIMED) trial [18] has demonstrated the role of extra-virgin olive oil in preventing cardiovascular events and mortality in high cardiovascular risk patients [19], primarily through positive effects on blood pressure [20] and lipid profile [21], in particular reducing the oxidized low-density lipoprotein (LDL) level [22], and improving inflammation markers and endothelial function [23, 24]. The bioactive components of olive oil considered to be the main factor responsible for all these positive effects on human health are the phenolic compounds [25]. The most investigated are tyrosol, hydroxytyrosol and oleuropein [26]. They seem to exert their protective effects even when taken as dietary supplements [27].
Camellia sinensis use is also associated with a more favorable cardiovascular disease risk profile [28]. The positive impact of green tea on cardio-metabolic health is related to its high content of polyphenols, in particular catechins, such as epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epigallocatechin-3-gallate (EGCG) [29]. EGCG is the most prevalent catechin present in green tea, representing approximately 30–50% of the green tea catechin content, is an ester that forms from the reaction of epigallocatechin and gallic acid and acts as an antioxidant and reduces inflammation [30, 31].
Our findings confirm the expected ability of DIF1STAT combining lipid-lowering components, Olea europaea total extract and Camellia sinensis derived polyphenols, to improve both lipid profile and endothelial function. In particular, we observed an impressive LDL-C cholesterol reduction: a similar long-term reduction in LDL-C level achieved with statins has been estimated to be related to a 20% cardiovascular risk reduction [32]. The result of endothelial reactivity improvement is also potentially of interest since in a recent meta-analysis, a 1% improvement in the flow-mediated dilation test was associated with a 12% reduction in cardiovascular risk [33]. Finally, we observed a small lowering action of the tested nutraceutical on SUA that represents an emerging risk factor for cardiovascular diseases [34].
Our study has some limitations. The first one is the relatively small sample size of the enrolled cohort. However, the study power appears to be adequate for the primary endpoints, as previously stated. Also, the short duration of the trial is a limitation, but it is in line with other studies including metabolically active nutraceutical compounds. Another limitation of the present study is that we adopted a controlled diet instead of offering broad dietary guidelines and this likely reduced the beneficial benefits of food in general in particular with reference to the improvement of anthropometric parameters such as body weight and waist circumference; on the other hand, it more closely resembles general practice. Furthermore, we did not look for improvements in signaling molecules including bradykinin, adenosine, vascular endothelial growth factor, serotonin, or NO synthase, which are all linked to endothelial activity, and we restricted our investigation to the direct measurement of endothelial reactivity. However, the main value and novelty of our study is to have tested the medium-term effect of low monacolin dosage combined with antioxidants derived from Olea europaea and Camellia sinensis on lipid profile and endothelial function at the same time. Of course, larger and long-term studies are required to confirm this observation.
In conclusion, the tested nutraceutical association is able to improve lipid profile, endothelial function and SUA in a cohort of mildly hypercholesterolemic subjects. Even if the study power appears to be adequate for the primary endpoints, particularly the effect on endothelial function needs confirmation in a longer clinical trial.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Poli A, Barbagallo CM, Cicero AFG, et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol Res. 2018;134:51–60. doi: 10.1016/j.phrs.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Cicero AFG, Colletti A, Bajraktari G, et al. Lipid lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel. Arch Med Sci. 2017;13:965–1005. doi: 10.5114/aoms.2017.69326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cicero AFG, Fogacci F, Zambon A. Red yeast rice for hypercholesterolemia: JACC Focus Seminar. J Am Coll Cardiol. 2021;77:620–8. doi: 10.1016/j.jacc.2020.11.056. [DOI] [PubMed] [Google Scholar]
- 4.EFSA ANS Panel (EFSA Panel Food Additives and Nutrient Sources added to Food) Younes M, Aggett P, Aguilar F, et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018;16:5368. doi: 10.2903/j.efsa.2018.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nam DE, Yun JM, Kim D, Kim OK. Policosanol attenuates cholesterol synthesis via AMPK activation in hypercholesterolemic rats. J Med Food. 2019;22:1110–7. doi: 10.1089/jmf.2019.4491. [DOI] [PubMed] [Google Scholar]
- 6.Poznyak AV, Nikiforov NG, Markin AM, et al. Overview of OxLDL and its impact on cardiovascular health: focus on atherosclerosis. Front Pharmacol. 2021;11:613780. doi: 10.3389/fphar.2020.613780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang X, Zhao Z, Zhang W, et al. Natural antioxidants improve the vulnerability of cardiomyocytes and vascular endothelial cells under stress conditions: a focus on mitochondrial quality control. Oxid Med Cell Longev. 2021;2021:6620677. doi: 10.1155/2021/6620677. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Cicero AFG, Caliceti C, Fogacci F, et al. Effect of apple polyphenols on vascular oxidative stress and endothelium function: a translational study. Mol Nutr Food Res. 2017:61. doi: 10.1002/mnfr.201700373. [DOI] [PubMed] [Google Scholar]
- 9.Barbaro B, Toietta G, Maggio R, et al. Effects of the olive-derived polyphenol oleuropein on human health. Int J Mol Sci. 2014;15:18508–24. doi: 10.3390/ijms151018508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lockyer S, Rowland I, Spencer JPE, Yaqoob P, Stonehouse W. Impact of phenolicrich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: a randomised controlled trial. Eur J Nutr. 2017;56:1421–32. doi: 10.1007/s00394-016-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covas MI, Nyyssönen K, Poulsen HE, et al. The effect of polyphenols in olive oil on heart disease risk factors. Ann Intern Med. 2006;145:333–41. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 12.Farràs M, Valls RM, Fernández-Castillejo S, et al. Olive oil polyphenols enhance the expression of cholesterol efflux related genes in vivo in humans. A randomized controlled trial. J Nutr Biochem. 2013;24:1334–9. doi: 10.1016/j.jnutbio.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Hernáez A, Fernández-Castillejo S, Farràs M, et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: a randomized controlled trial. Arterioscler Thromb Vasc Biol. 2014;34:2115–9. doi: 10.1161/ATVBAHA.114.303374. [DOI] [PubMed] [Google Scholar]
- 14.Cicero AFG, Fogacci F, Veronesi M, et al. A randomized placebo-controlled clinical trial to evaluate the medium-term effects of oat fibers on human health: the Beta-Glucan Effects on Lipid Profile, Glycemia and inTestinal Health (BELT) Study. Nutrients. 2020;12:686. doi: 10.3390/nu12030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicero AFG, Fogacci F, Bove M, Giovannini M, Borghi C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytother Res. 2019;33:2094–101. doi: 10.1002/ptr.6402. [DOI] [PubMed] [Google Scholar]
- 16.McGreevy C, Barry M, Bennett K, et al. Repeatability of the measurement of aortic pulse wave velocity (aPWV) in the clinical assessment of arterial stiffness in community-dwelling older patients using the Vicorder(®) device. Scand J Clin Lab Invest. 2013;73:269–73. doi: 10.3109/00365513.2013.770162. [DOI] [PubMed] [Google Scholar]
- 17.Cicero AF, Colletti A, Rosticci M, Grandi E, Borghi C. Efficacy and tolerability of a combined lipid-lowering nutraceutical on cholesterolemia, hs-CRP level and endothelial function in moderately hypercholesterolemic subjects. J Biol Regul Homeost Agents. 2016;30:593–8. [PubMed] [Google Scholar]
- 18.Guasch-Ferré M, Hu FB, Martínez-González MA, et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014;12:78. doi: 10.1186/1741-7015-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 20.Toledo E, Hu FB, Estruch R, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med. 2013;11:207. doi: 10.1186/1741-7015-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghobadi S, Hassanzadeh-Rostami Z, Mohammadian F, et al. Comparison of blood lipid-lowering effects of olive oil and other plant oils: a systematic review and meta-analysis of 27 randomized placebo-controlled clinical trials. Crit Rev Food Sci Nutr. 2019;59:2110–24. doi: 10.1080/10408398.2018.1438349. [DOI] [PubMed] [Google Scholar]
- 22.Castañer O, Fitó M, López-Sabater MC, et al. The effect of olive oil polyphenols on antibodies against oxidized LDL. A randomized clinical trial. Clin Nutr. 2011;30:490–3. doi: 10.1016/j.clnu.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Schwingshackl L, Christoph M, Hoffmann G. Effects of olive oil on markers of inflammation and endothelial function – a systematic review and meta-analysis. Nutrients. 2015;7:7651–75. doi: 10.3390/nu7095356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis CR, Hodgson JM, Woodman R, Bryan J, Wilson C, Murphy KJ. Mediterranean diet lowers blood pressure and improves endothelial function: results from the MedLey randomized intervention trial. Am J Clin Nutr. 2017;105:1305–13. doi: 10.3945/ajcn.116.146803. [DOI] [PubMed] [Google Scholar]
- 25.Bulotta S, Celano M, Lepore SM, et al. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J Transl Med. 2014;12:219. doi: 10.1186/s12967-014-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boronat A, Mateus J, Soldevila-Domenech N, et al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic Biol Med. 2019;143:471–81. doi: 10.1016/j.freeradbiomed.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Carnevale R, Silvestri R, Loffredo L, et al. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br J Clin Pharmacol. 2018;84:1566–74. doi: 10.1111/bcp.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung M, Zhao N, Wang D, et al. Dose-response relation between tea consumption and risk of cardiovascular disease and all-cause mortality: a systematic review and meta-analysis of population-based studies. Adv Nutr. 2020;11:790–814. doi: 10.1093/advances/nmaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochman J, Jakubczyk K, Antoniewicz J, Mruk H, Janda K. Health benefits and chemical composition of matcha green tea: a review. Molecules. 2020;26:85. doi: 10.3390/molecules26010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamagata K. Protective effect of epigallocatechin gallate on endothelial disorders in atherosclerosis. J Cardiovasc Pharmacol. 2020;75:292–8. doi: 10.1097/FJC.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 31.Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green tea catechins. Int J Mol Sci. 2020;21:1744. doi: 10.3390/ijms21051744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cholesterol Treatment Trialists’ (CTT) Collaboration. Fulcher J, O’Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzawa Y, Kwon TG, Lennon RJ, et al. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4:e002270. doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borghi C, Cicero AFG. Serum uric acid and cardiometabolic disease: another brick in the wall? Hypertension. 2017;69:1011–3. doi: 10.1161/HYPERTENSIONAHA.117.09081. [DOI] [PubMed] [Google Scholar]