Abstract
Aims:
The American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) updated the testing guideline in 2018 to address issues from uncommon HER2 fluorescence in situ hybridization (FISH) results according to the 2013 guideline. Next-generation sequencing (NGS) may be used to better classify patients. We aim to assess ERBB2 amplification status of invasive breast carcinoma with equivocal HER2 immunohistochemistry (IHC) results using NGS, focusing on Group 4 (HER2/CEP17 Ratio<2.0; Average HER2 Signals/cell ≥4.0 and <6.0).
Methods and Results:
We retrospectively reviewed HER2 FISH and NGS data of HER2 IHC-equivocal breast carcinomas at our center between January 2009 and September 2019, wherein all three assays were performed on the same tissue block, and compared HER2 FISH results, according to 2018 ASCO/CAP guideline, and ERBB2 amplification status by NGS. A total of 52 HER2 FISH and NGS results from 51 patients with HER2 IHC-equivocal breast carcinomas were reviewed. The cohort included 8 cases classified as 2018 ASCO/CAP ISH Group 1, 3 as Group 2, 3 as Group 3, 14 as Group 4 and 24 as Group 5. Thirteen of 14 (92.9%) Group 4 (HER2 negative) cases were classified as ERBB2 nonamplified using NGS; the discordant case was later classified as Group 1 with alternate sample FISH testing. NGS revealed no significant difference in somatic mutations or copy number alternations between Groups 4 and 5.
Conclusions:
Our NGS findings support the reclassification of HER2 FISH-equivocal cases as HER2 negative under the 2018 ASCO/CAP guideline.
Keywords: ASCO/CAP guideline, breast cancer, FISH, human epidermal growth factor receptor 2, next-generation sequencing
INTRODUCTION
Approximately 15% to 20% of primary invasive breast carcinomas show overexpression of human epidermal growth factor receptor 2 (HER2), a receptor tyrosine kinase, due to amplification of its encoding gene ERBB2.(1) HER2-amplification results in breast carcinoma with aggressive behavior and is associated with poor prognosis, compared to those in which HER2 is not amplified.(2) Development of HER2-targeted therapies, such as trastuzumab, lapatinib and pertuzumab, have been shown to be effective in achieving excellent outcomes in patients with HER2-amplified breast carcinoma.(3–5) Patients with HER2-negative breast carcinoma, on the other hand, have not shown clinical benefit with HER2-targeted therapeutics.(6, 7) Thus, accurate assessment of HER2 status is prudent for providing precise prognostic and predictive information for patients with breast carcinoma.
The US Food and Drug Administration (FDA) has approved immunohistochemistry (IHC) and in situ hybridization as methods for the determination of HER2 amplification status.(8) The American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) have issued guidelines for interpretation of HER2 test results by IHC and in situ hybridization.(9–11) However, given advances in molecular diagnostics, particularly with regard to next-generation sequencing (NGS) methods, the same assay may be utilized to provide both data on copy number alterations and somatic mutations.
Our group has previously published the validation for ERBB2 amplification assessment using a FDA-approved hybrid capture-based NGS assay [Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT)].(12) We also recently published our clinical and pathologic findings of cases reclassified under the updated 2018 ASCO/CAP HER2 testing guideline, focusing on the impact on patients in ISH Group 4.(13) The purpose of this retrospective study was to evaluate the ERBB2 amplification status of breast carcinoma cases using NGS at a single large tertiary-care cancer center. We examined the HER2 FISH results of invasive breast carcinoma cases with equivocal HER2 IHC, classified these cases using the 2018 ASCO/CAP guideline and evaluated the ERBB2 amplification status using MSK-IMPACT, with a focus on cases classified as ISH Group 4. In this study, we also assessed the clinical, pathologic and genomic features of our cohort.
MATERIALS AND METHODS
Study Population
This study was conducted under institutional review board approval. The pathology database of our institution was interrogated. All patients diagnosed between January 1, 2009 and September 30, 2019 with primary, recurrent or metastatic breast carcinoma with equivocal HER2 IHC results (2+ or 1+ to 2+), HER2 FISH testing, and NGS performed on the same paraffin-embedded, formalin-fixed tissue block were identified. All cases classified into 2018 ASCO/CAP ISH Groups 2, 3 and 4 were included in this study. For 2018 ASCO/CAP ISH Groups 1 and 5, breast carcinoma cases were selected from a cohort used in a prior validation study.(12) Clinical data, including patient age at primary diagnosis, tumor size, lymph node status, histologic subtype, status of estrogen receptor (ER) and progesterone receptor (PR), score of HER2 IHC, HER2 FISH results, administration of targeted anti-HER2 therapy, and clinical follow-up for patients, were recorded.
HER2 IHC and FISH
As per our institutional standard practice, HER2 IHC (4B5, Ventana, Tucson, Arizona, USA) was performed on all primary invasive, recurrent and metastatic breast carcinoma cases and scored as negative (0, 1+), equivocal (2+ or 1+ to 2+) or positive (3+), according to the ASCO/CAP guideline.(10, 11) In all cases where HER2 FISH was conducted, deparaffinized tissue sections were examined using a FDA-approved HER2 dual-probe FISH assay (HER2 IQFISH pharmDx, DAKO, Glostrup, Denmark; or PathVysion HER2 DNA Probe Kit, Vysis, Abbott Molecular, Des Plaines, Illinois, USA). HER2 FISH results were reported as negative, equivocal, or positive according to the 2013 or 2018 ASCO/CAP guideline, depending on date of testing.(10, 11) For cases performed prior to the 2018 ASCO/CAP guideline, HER2 FISH results were reclassified into 5 ISH categories, as defined by the updated guideline: HER2/CEP17 ratio 2.0 or higher and average HER2 copy number 4.0 or higher (Group 1), HER2/CEP17 ratio 2.0 or higher and average HER2 copy number lower than 4.0 (Group 2), HER2/CEP17 ratio lower than 2.0 and average HER2 copy number 6.0 or higher (Group 3), HER2/CEP17 ratio lower than 2.0 and average HER2 copy number 4.0 or higher and lower than 6.0 (Group 4), and HER2/CEP17 ratio lower than 2.0 and average HER2 copy number lower than 4.0 (Group 5). HER2 IHC and FISH testing was performed on alternate tumor block(s) from either the same specimen, different specimen, or both, for select cases either due to new tissue sampling or clinical request; in cases classified into ISH Groups 2, 3, and 4 and wherein additional HER2 FISH was performed those results were documented and compared separately with the FISH results of the block on which all 3 assays were performed.
Next-Generation Sequencing Analysis and Bioinformatics Pipeline to Detect Copy Number Alterations
Tumor and matched germline DNA of cases was subjected to targeted next-generation sequencing analysis using the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) assay, focusing on up to 468 key cancer-associated oncogenes and tumor-suppressor genes.(14) Methods of the copy number pipeline using the MSK-IMPACT assay have been previously described.(12, 14) Copy number gains were interpreted along with overall copy number profile and tumor purity. For ERBB2, fold change (FC) of greater than or equal to 2.0 and FC of greater than or equal to 1.5 and less than 2.0 with P-value less than 0.05 were considered as amplified or copy number gain, respectively, as determined in the validation study by Ross et al.(12)
Statistical Analysis
Differences between groups in categorical variables were calculated with the χ2 test and Fisher exact test, where applicable, and in noncategorical variables by using the Student t test. Statistical significance was established at P < .05. Multiple comparisons adjustment was performed using the Benjamini-Hochberg procedure with a corrected false discovery rate cut-off of .05.
RESULTS
A total of 52 FISH and NGS results from invasive breast carcinomas with equivocal HER2 IHC results from 51 patients, all women, were retrieved and reviewed. The clinical and pathologic findings are summarized in Table 1. Using the 2018 ASCO/CAP HER2 testing guideline, our study cohort was composed of 8 (15.4%) Group 1 cases from 8 patients, 3 (5.8%) Group 2 cases from 3 patients, 3 (5.8%) Group 3 cases from 3 patients, 14 (26.9%) Group 4 cases from 14 patients and 24 (46.2%) Group 5 cases from 23 patients. There were no statistically significant differences in patient age at primary diagnosis, pathologic tumor stage, primary tumor histology, histologic grade, hormone receptor status or administration of HER2-targeted therapy among the five 2018 ASCO/CAP ISH groups. At a median follow-up of 20.9 months, 12 of 14 Group 4 patients were alive at the time of last clinical follow-up, and 2 of 14 patients had died of disease.
Table 1.
Clinical and pathologic features of 52 breast carcinoma cases from 51 patients by 2018 American Society of Clinical Oncology/College of American Pathologists In-Situ Hybridization group.
| Feature, No. | 2018 ASCO/CAP HER2 ISH Group | |||||
|---|---|---|---|---|---|---|
| Group 1 (n = 8) | Group 2 (n = 3) | Group 3 (n = 3) | Group 4 (n = 14) | Group 5 (n = 24) | P | |
| Age at primary diagnosis, median (range), years | 56 (52–67) | 38 (35–65) | 51 (42–69) | 47 (28–67) | 50 (33–80) | .24 |
| HER2/CEP17 ratio, median (range) | 2.25 (2.10–3.90) | 2.27 (2.10–4.00) | 1.40 (0.75–1.60) | 1.59 (1.00–1.83) | 1.20 (1.00–1.70) | <.001a |
| HER2 copy number, median (range) | 5.25 (4.30–8.30) | 3.70 (2.13–3.88) | 6.30 (6.00–6.60) | 4.43 (4.00–5.92) | 2.80 (1.00–3.92) | <.001a |
| ERBB2 fold change, median (range) | 1.76 (1.52–3.18) | 2.73 (1.41–3.35) | 1.23 (1.27–3.14) | 1.23 (−1.06–1.98)b | 1.01 (−1.34–1.56) | <.001a |
| Pathologic tumor stage | .76 | |||||
| T1 | 4/8 | 1/3 | 1/3 | 5/14 | 7/23 | |
| T2 | 2/8 | 1/3 | 1/3 | 8/14 | 12/23 | |
| T3 | 1/8 | 1/3 | 1/3 | 1/14 | 2/23 | |
| Not available | 1/8 | 0 | 0 | 0 | 2/23 | |
| Primary tumor histology | .44 | |||||
| Invasive carcinoma NST | 4/8 | 3/3 | 1/3 | 11/14 | 13/23 | |
| Invasive lobular carcinoma, classic or pleomorphic | 1/8 | 0 | 0 | 0 | 3/23 | |
| Otherc | 2/8 | 0 | 2/3 | 3/14 | 3/23 | |
| Data not available | 1/8 | 0 | 0 | 0 | 4/23 | |
| Histologic grade | .05 | |||||
| 1 | 0 | 0 | 0 | 1/14 | 0 | |
| 2 | 2/8 | 0 | 1/3 | 1/14 | 11/23 | |
| 3 | 4/8 | 3/3 | 2/3 | 12/14 | 8/23 | |
| Not available | 2/8 | 0 | 0 | 0 | 4/23 | |
| Hormone receptor status | .40 | |||||
| Positive | 7/8 | 3/3 | 3/3 | 11/14 | 23/24 | |
| Negative | 1/8 | 0 | 0 | 3/14 | 1/24 | |
| HER2-targeted therapy | <.001a | |||||
| Received | 7/8 | 3/3 | 2/3 | 4/14 | 2/23 | |
| Did not receive | 0/8 | 0 | 1/3 | 10/14 | 21/23 | |
| Data not available | 1/8 | 0 | 0 | 0 | 0 | |
Abbreviations: ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; HER2, human epidermal growth factor receptor 2; ISH, in-situ hybridization; NST, no special type
Statistically significant P value < .05 by Student t test or Fisher’s exact test, where applicable.
For the Group 4 case showing 1.98 fold change, an alternate tumor sample was tested with HER2 fluorescence in-situ hybridization and shown to be HER2 amplified, classified as 2018 ASCO/CAP ISH Group 1.
Other category encompasses cases of invasive ductal carcinoma with special histologic features, including apocrine, micropapillary and mucinous features, and cases of invasive mammary carcinoma, wherein carcinoma shared features of both ductal and lobular carcinoma.
Assessment of ERBB2 Amplification Status by Next-Generation Sequencing Compared to HER2 FISH Results
Comparison of the overall assessment of ERBB2 amplification status by NGS and FISH assays is summarized in Table 2. By NGS, all 8 (100%) 2018 ASCO/CAP ISH Group 1 cases were classified as ERBB2-amplified/gain, 2 of 3 (66.7%) Group 2 cases as ERBB2-amplified/gain, 1 of 3 (33.3%) Group 3 cases as ERBB2-amplified/gain, 1 of 14 (7.1%) Group 4 cases as ERBB2-amplified/gain, and no (0%) cases of Group 5 as ERBB2-amplified/gain. For Group 1 cases, 5 demonstrated copy number gain of ERBB2 by NGS, and 3 displayed amplification. For Group 2 cases, one case showed copy number gain of ERBB2 by NGS, while the other displayed amplification. The Group 3 case demonstrated amplification of ERBB2 by NGS. The Group 4 case showed copy number gain of ERBB2 by NGS. Figure 1 depicts an example of the HER2 IHC, HER2 FISH and copy number plot for a representative Group 4 case with no ERBB2 amplification detected by NGS.
Table 2.
Comparison of ERBB2 amplification status by next-generation sequencing and HER2 fluorescence in situ hybridization results from 52 breast carcinoma cases from 51 patients.
| NGS result by MSK-IMPACT assay | 2018 ASCO/CAP HER2 ISH Group | ||||
|---|---|---|---|---|---|
| Group 1 (n = 8) | Group 2 (n = 3) | Group 3 (n = 3) | Group 4 (n = 14) | Group 5 (n = 24) | |
| Nonamplified | 0 | 1 | 2 | 13 | 24 |
| Amplified/Gain | 8 | 2 | 1 | 1 | 0 |
Abbreviations: ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; HER2, human epidermal growth factor receptor 2; ISH, in situ hybridization; MSK-IMPACT, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets; NGS, next-generation sequencing
Figure 1.
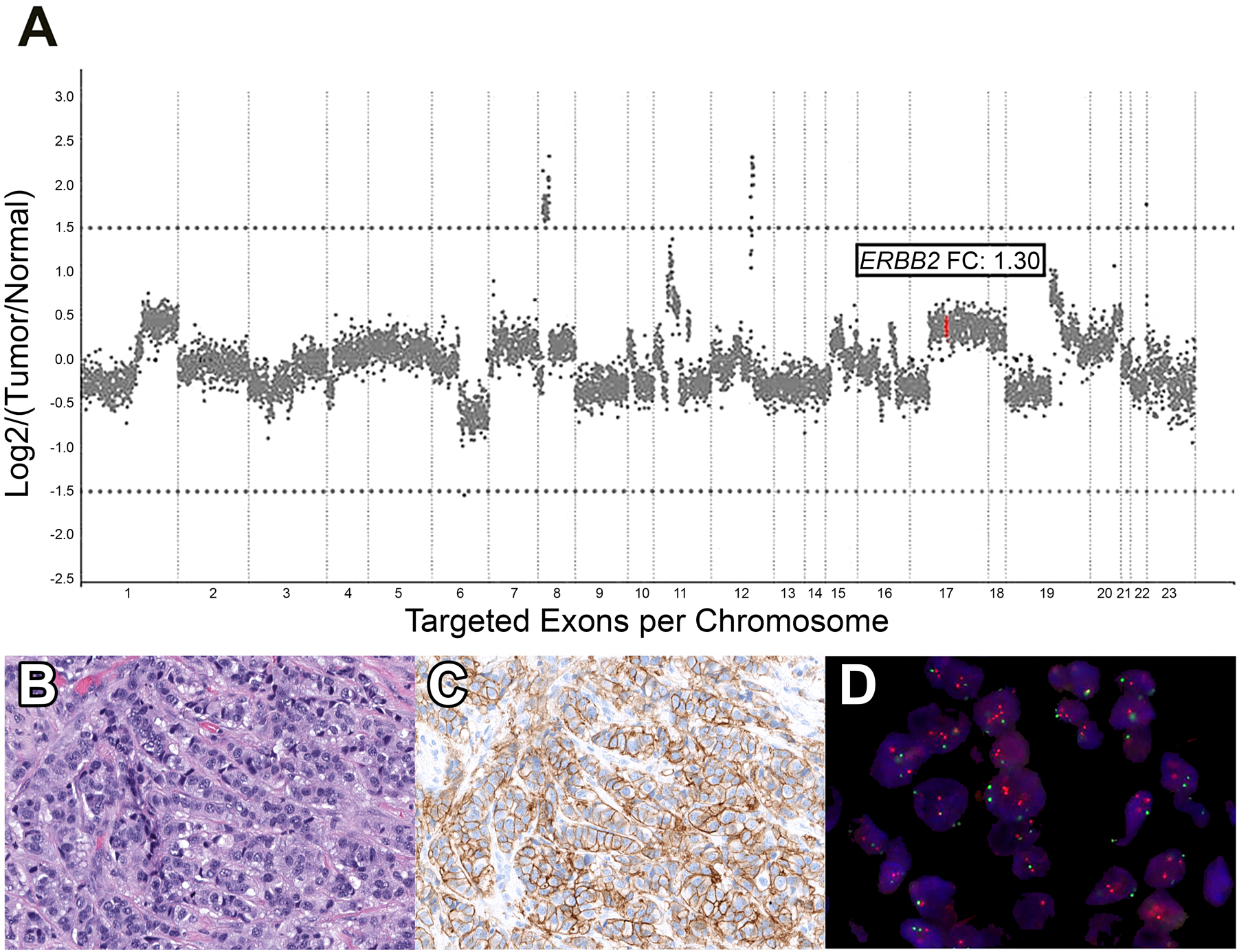
Representative 2018 ASCO/CAP ISH Group 4 case. A. Copy number plot determined by Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) next-generation sequencing assay, with each dot representing a probe set, and y-axis values showing the normalized log2 transformed fold change (FC) of tumor versus normal. In this Group 4 case, ERBB2 (red dots) FC is 1.30, indicating no amplification. B. Hematoxylin and eosin-stained section displaying invasive carcinoma of no special type. C. HER2 immunohistochemical-stained slide showing equivocal results (score, 2+) with complete membranous staining of weak to moderate intensity D. HER2 fluorescence in situ hybridization (red signal, HER2; green signal, CEP17), demonstrating a HER2/CEP17 ratio of 1.6 and average HER2 copy number of 4.2, classified as 2018 ASCO/CAP ISH Group 4.
For 8 of 14 Group 4 cases, HER2 FISH results were also available on alternate tumor samples from different tumor blocks from the same specimen (n = 2), different specimens (n = 5) or both (n = 1). In 5 cases for which ERBB2 was nonamplified by MSK-IMPACT, alternate tumor sample testing on a different block from the same specimen (n = 3) or different specimen (n = 2) revealed HER2 FISH results also classified as Group 4. In one case for which ERBB2 was nonamplified by MSK-IMPACT, testing on two alternate tumor samples from different specimens showed HER2 FISH results classified as Group 4 and Group 5. In one case for which ERBB2 was nonamplified by MSK-IMPACT, testing on two alternate tumor samples from different specimens demonstrated HER2 FISH results classified as Group 5. In the one case for which ERBB2 copy number gain was called using MSK-IMPACT, testing on an alternate specimen revealed HER2 FISH results classified as Group 1 (HER2/CEP17 ratio of 2.4 and average HER2 copy number of 5.3). In this discordant case, the sample with HER2 FISH results classified as Group 4 was from a metastatic hepatic lesion, whereas the sample that showed Group 1 results was of the mastectomy specimen of the primary tumor (further discussed below).
Results of FISH testing on alternate samples are summarized in Table 3. For all 3 Group 2 cases, HER2 FISH results were available on alternate tumor samples from different specimens. In each of the 2 Group 2 cases for which ERBB2 amplification was called using MSK-IMPACT, alternate tumor sample testing on two different specimens demonstrated HER2 FISH results classified as Group 1. In the Group 2 case for which ERBB2 was not amplified by MSK-IMPACT, HER2 FISH testing on an alternate specimen revealed results classified as Group 5 (HER2/CEP17 ratio of 1.2 and average HER2 copy number of 3.5).
Table 3.
Summary of HER2 fluorescence in situ hybridization results and ERBB2 assessment by next-generation sequencing from matched tumor block and HER2 fluorescence in situ hybridization results of alternate tumor block from 13 patients.
| Study Patient | HER2 FISH result, matched block | ERBB2 Status by NGS, matched block | HER2 FISH result, alternate sample(s) |
|---|---|---|---|
| 1 | Group 2 | Amplified | Group 1 |
| 2 | Group 2 | Amplified | Group 1 |
| 3 | Group 2 | Nonamplified | Group 5 |
| 4 | Group 3 | Nonamplified | Group 5 |
| 5 | Group 3 | Amplified | Group 5 |
| 6 | Group 4 | Nonamplified | Group 4 |
| 7 | Group 4 | Nonamplified | Group 4 |
| 8 | Group 4 | Nonamplified | Group 4 |
| 9 | Group 4 | Nonamplified | Group 4 |
| 10 | Group 4 | Nonamplified | Group 4 |
| 11 | Group 4 | Nonamplified | Group 4, Group 5 |
| 12 | Group 4 | Nonamplified | Group 5, Group 5 |
| 13 | Group 4 | Copy number gain | Group 1 |
Abbreviations: FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; NGS, next-generation sequencing
For 2 of 3 Group 3 cases, HER2 FISH results were available on alternate tumor samples from different specimens. In one case for which ERBB2 was not amplified using MSK-IMPACT, alternate tumor sample testing showed HER2 FISH results classified as Group 5 (HER2/CEP17 ratio of 1.0 and average HER2 copy number of 3.0). In one case for which ERBB2 amplification was detected by MSK-IMPACT, HER2 FISH testing on an alternate specimen (a metastasis) demonstrated results classified as Group 5 (HER2/CEP17 ratio of 1.0 and average HER2 copy number of 2.8). The patient did receive HER2-targeted therapy, on the basis of the initial HER2 FISH results for the primary tumor (HER2/CEP17 ratio of 1.6 and average HER2 copy number of 6.6), but ultimately succumbed to her metastatic disease after multiple lines of chemotherapy.
Mutational Analysis by Next-Generation Sequencing Reveals Significant Differences in Copy Number Alterations Between 2018 ASCO/CAP ISH Group 1 and 4 Cases
Somatic mutations and copy number alterations in 2018 ASCO/CAP Groups 1, 4 and 5 cases by MSK-IMPACT are shown in Figure 2. There was a significant association with copy number alterations in ERBB2 and 2018 ASCO/CAP ISH Group 1, compared to Group 4, on univariate analysis (P < .001) and when adjusted for multiple comparisons (P = .01). There was also a significant association with copy number alterations in RARA and Group 1, compared to Group 4, on univariate analysis (P = .04), but not significant when adjusted for multiple comparisons (P = .68). In all 5 2018 ASCO/CAP ISH Groups 1 and 4 cases with RARA amplification, there was concurrent amplification of ERBB2 seen. There were no significant differences in somatic mutations or copy number alternations between 2018 ASCO/CAP ISH Groups 4 and 5 (Figure 2).
Figure 2.
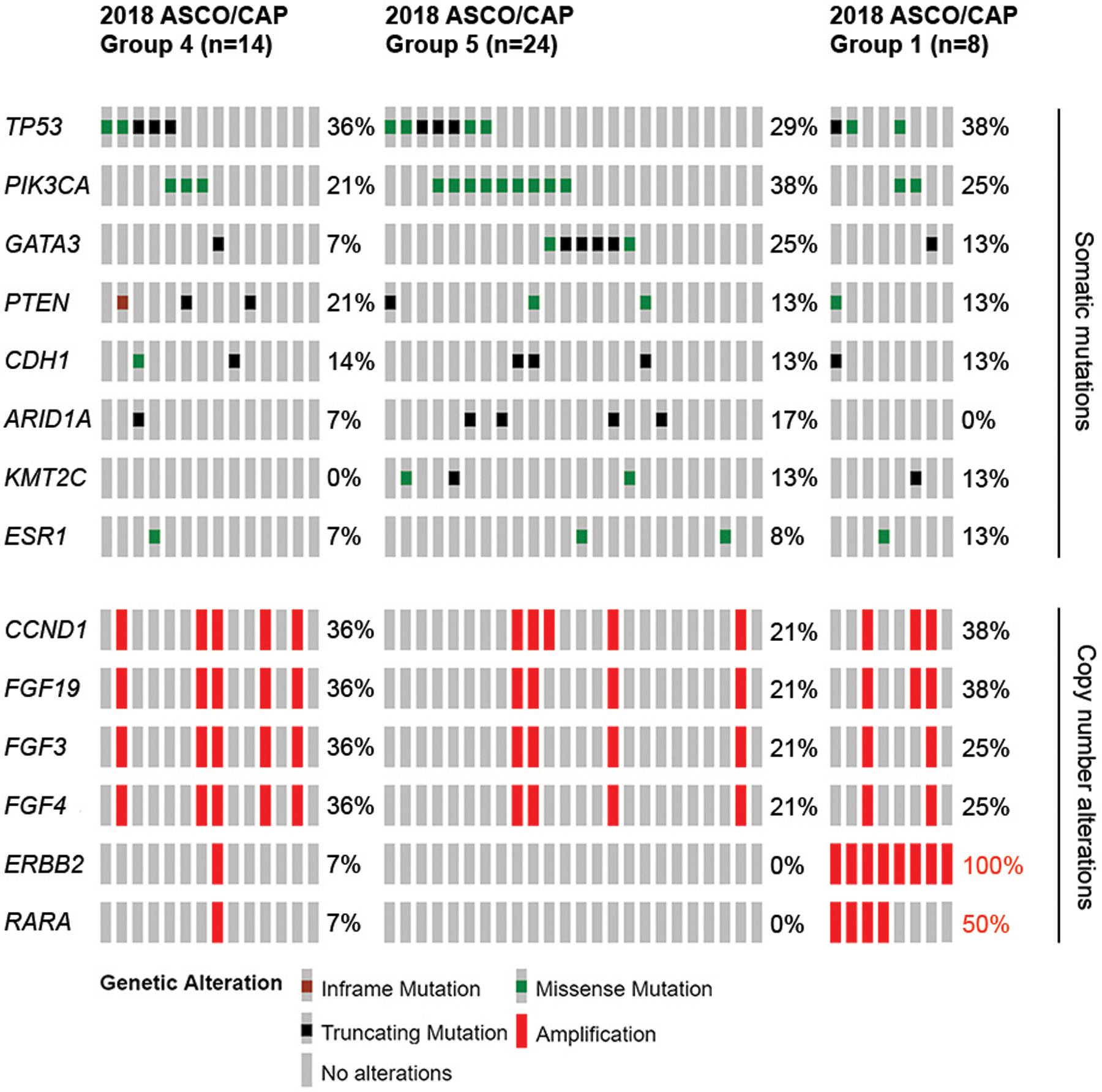
Somatic mutations and copy number alterations identified by Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) next-generation sequencing assay in 14 ASCO/CAP ISH Group 4, 24 Group 5 and 8 Group 1 breast carcinoma cases. Cases are represented in columns, and genes are displayed in rows. Alteration types are color-coded according the legend. Copy number alterations in ERBB2 and RARA were significant associated with Group 1 cases on univariate analysis with Fisher exact test (P < .001) and when adjusted for multiple comparisons (P = .01). Values written in red denote significant P < .05.
HER2-Targeted Therapy in 2018 ASCO/CAP Group 4 Patients
Therapy with anti-HER2 targeted treatment was administered to 4 of 14 (28.6%) patients with breast carcinoma showing 2018 ASCO/CAP HER2 ISH Group 4 results. One patient received such targeted treatment in the neoadjuvant and adjuvant settings, and 3 as the adjuvant therapy. The patient with Group 4 results treated with neoadjuvant HER2-targeted therapy was the discrepant case mentioned above. She was a 35-year-old woman, who presented with a palpable, 5 cm breast mass and de novo metastatic disease to the bone, liver and lung. Biopsy of a hepatic nodule, performed at another institution, showed metastatic carcinoma of mammary origin. At that same institution, HER2 IHC showed 2+ staining, and HER2 FISH revealed amplification (HER2/CEP17 ratio of 2.7 and average HER2 copy number of 5.5). Based on these findings, the patient received docetaxel, trastuzumab and pertuzumab in the neoadjuvant setting. Of note, HER2 FISH performed at our institution on the same tissue sample of the hepatic nodule showed 2+ HER2 IHC staining and, at the time of the procedure, HER2 FISH equivocal results, now classified as 2018 ASCO/CAP ISH Group 4 (HER2/CEP17 ratio of 1.6 and average HER2 copy number of 5.2). The patient subsequently underwent mastectomy, which showed a 6.8 cm, poorly differentiated invasive carcinoma of no special type with minimal response to neoadjuvant chemotherapy. HER2 IHC was equivocal (2+), and HER2 FISH demonstrated amplification, classified as Group 1 (HER2/CEP17 ratio of 2.4 and average HER2 copy number of 5.3).
Three patients with Group 4 results received adjuvant HER2-targeted therapy. In 2 cases, HER2 FISH tests performed on different specimens were interpreted at the referring institution as amplified, and the patients received therapy at that institution on the basis of those results. In one case, the patient responded well; the other patient had disease progression with malignant pleural effusion. In the remaining one case, adjuvant therapy was given due to intertumoral heterogeneity, where the patient had multifocal disease, and additional HER2 FISH testing on a separate lesion revealed approximately 10% of tumor cells with amplification. The patient responded well to targeted HER2 therapy. Of the patients with 2018 ASCO/CAP ISH Group 4 results who did not receive HER2-targeted therapy, 9 of 10 (90.0%) remained disease-free after their initial treatment, with a median follow-up time of 17 months (range, 2 to 31 months).
DISCUSSION
In this retrospective single-institution study of invasive breast carcinomas with equivocal HER2 IHC results, we have demonstrated the correlation between the 2018 ASCO/CAP ISH guideline and ERBB2 copy number analysis using next-generation sequencing in cases where all three assays were performed on the same tissue block. Using NGS, we revealed a significant difference in copy number alterations in ERBB2 and RARA between 2018 ASCO/CAP Groups 1 and 4. Furthermore, copy number analysis by NGS demonstrated ERBB2 amplification in a case where IHC was equivocal and FISH results were classified as Group 4.
Our study found 13 of 14 (92.9%) 2018 ASCO/CAP ISH Group 4 cases which had concordant nonamplified results by HER2 FISH and NGS assessment of ERBB2 copy number alterations. In the case with discordant results, HER2 FISH testing on a core-needle biopsy of metastatic lesion showed no amplification, yet NGS copy number analysis demonstrated ERBB2 copy number gain; subsequent HER2 FISH testing of the primary tumor indeed revealed amplification, classified as 2018 ASCO/CAP ISH Group 1. Intratumoral heterogeneity alone cannot explain the discordant results, as the same tissue block was used for both FISH and NGS testing. Discordant HER2 FISH results have been shown to stem from low-level amplifications.(15) In this case, NGS revealed a copy number gain of ERBB2, which may have contributed to the discrepant results. Studies comparing HER2 FISH and ERBB2 copy number by NGS remain limited. Yang and colleagues(16) investigated the concordance between ERBB2 copy number alterations, HER2 FISH and HER2 IHC; however, the study was not limited to cases with equivocal HER2 results. The group found one of 17 (6%) cases with 2018 ASCO/CAP Group 4 results and ERBB2 amplification by NGS; though, in that case, HER2 IHC showed 3+ staining.(16)
Our study identified significant association between ERBB2 amplification and RARA amplification. Retinoic acid receptor alpha gene, or RARA, is located on chromosome 17q21.2, adjacent to the ERBB2 gene.(17) In our study, we found amplification of RARA in 56% (5/9 samples) of cases with ERBB2 amplification by next-generation sequencing. The intimate relationship between RARA and HER2 genes has been previously noted.(18–20) Troxell and colleagues(18) found in RARA amplification by FISH in 71% (5/7 samples) of cases with 3+ HER2 IHC staining. Likewise, Varga et al.(19) also found RARA amplification by FISH in 71% (10/14 samples) of HER2-amplified cases. RARA has been used as an alternative chromosome 17 probe in equivocal cases of HER2 FISH (although no longer recommended by the updated ASCO/CAP guidelines); however, given its proximity to ERBB2 and resultant frequent co-amplification, as shown in this study, it often provides little discriminatory value in such cases.(21) RARA is more notable for its role in the characteristic chromosomal translocation in acute promyelocytic leukemia of t(15;17)(q24;q21), which leads to the fusion of RARA gene to the promyelocytic leukemia gene, or PML.(22–24)
With regard to HER2-targeted therapy, controversy existed regarding management of patients with HER2 FISH–equivocal cases under the 2013 ASCO/CAP guideline. The practice at our center has been not to treat patients in this subgroup with HER2-targeted therapy, with the exception of the aforementioned circumstances (see Results, HER2-Targeted Therapy in 2018 ASCO/CAP Group 4 Patients). As shown in our study, 90% of patients who did not receive HER2-targeted therapy remained disease-free; although, the clinical follow-up time for these patients is short, and these results are limited in their uncontrolled and retrospective nature. In the small number of patients with ASCO/CAP ISH Group 4 results who did receive anti-HER2 therapy, 2 patients showed response to anti-HER2 therapy, while 1 patient had disease progression.
This study has some limitations. Firstly, this study is limited by number of cases. For the purposes of this study, we wanted to directly compare IHC, FISH, and NGS results without interference by intratumoral heterogeneity and pre-analytical variables by selecting cases on which all three assays were performed on the same tumor block. Secondly, our study is limited by its retrospective nature. Furthermore, evaluation of patient outcomes would be best ascertained in the prospective setting. Despite these limitations, our cohort reveals the advantages of next-generation sequencing for copy number assessment of ERBB2 status in patients with HER2 IHC-equivocal breast carcinoma, accurately identifying those in whom targeted therapy would be beneficial.
The findings as described in this limited study and in the validation study by Ross et al.(12) support NGS as a viable alternative to FISH testing. NGS offers several advantages over FISH, including objective assessment of ERBB2 status and valuable comprehensive genomic data that may guide clinical management and options for targeted therapy, even on limited samples. For example, in this present study’s patient cohort, PIK3CA mutations were uncovered in tumors of some patients, for whom FDA-approved, PI3K inhibitor alpelisib may be offered as therapy.(25) Additionally, ESR1 mutations were also identified in samples of select study patients, in whom these mutations result in acquired resistance to endocrine therapy and an alternate treatment may be warranted.(26) Furthermore, FISH, unlike NGS, is limited by specific requirements for fixation method and time, exhibits differences in sensitivity and specificity amongst various antibodies, shows interobserver and intraobserver variations in assay interpretation, and susceptible to interpretation issues involving CEP17 copy number, stemming from preparation issues (i.e., tissue thickness and nuclear truncation), normal cellular processes (i.e., cell division), and aneuploidy of chromosome 17.(12) NGS is immune to the latter, as copy number data is interpreted on a genomic level rather than using a single gene locus for reference.(12) On the other hand, a disadvantage of NGS from a clinical standpoint is its turnaround time, compared to that of FISH.
Herein we have revealed the molecular findings associated with the 2018 ASCO/CAP HER2 FISH Groups 1, 4 and 5. In rare instances, NGS may reveal bona fide ERBB2 amplification where neither HER2 IHC nor HER2 FISH are able to detect such amplification. Our data demonstrate that patients with Group 4 breast carcinoma show no ERBB2 amplification by next-generation sequencing analysis, supporting its reclassification from HER2 FISH-equivocal to HER2 FISH-negative.
ACKNOWLEDGEMENTS
Source of Funding Support:
This research was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and supported by the Marie-Josée and Henry R Kravis Center for Molecular Oncology.
Declaration of Conflicting Interests:
The authors listed below have the following potential conflicts of interest to disclose:
P. Razavi has received honoraria for consulting/advisory board for Novartis, AstraZeneca, Foundation Medicine and institutional research support from GRAIL, Inc.
M. Ladanyi has received honoraria for ad hoc advisory board participation from Merck, Astra-Zeneca, Bristol Myers Squibb, Takeda, Lilly Oncology and Bayer, and research support from LOXO Oncology, Merus, and Helsinn Therapeutics.
All remaining authors have declared no conflicts of interest.
Footnotes
The abstract for this paper was presented at the 109th annual meeting of the United States and Canadian Academy of Pathology on March 3, 2020 in Los Angeles, California, United States.
REFERENCES
- 1.Witton CJ, Reeves JR, Going JJ, et al. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200(3):290–7. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14(4):320–68. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. [DOI] [PubMed] [Google Scholar]
- 4.Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19(1):115–26. [DOI] [PubMed] [Google Scholar]
- 5.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Press MF, Finn RS, Cameron D, et al. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res. 2008;14(23):7861–70. [DOI] [PubMed] [Google Scholar]
- 7.Gianni L, Llado A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(7):1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga Z, Noske A, Ramach C, et al. Assessment of HER2 status in breast cancer: overall positivity rate and accuracy by fluorescence in situ hybridization and immunohistochemistry in a single institution over 12 years: a quality control study. BMC Cancer. 2013;13:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45. [DOI] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–22. [DOI] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 12.Ross DS, Zehir A, Cheng DT, et al. Next-Generation Assessment of Human Epidermal Growth Factor Receptor 2 (ERBB2) Amplification Status: Clinical Validation in the Context of a Hybrid Capture-Based, Comprehensive Solid Tumor Genomic Profiling Assay. J Mol Diagn. 2017;19(2):244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoda RS, Brogi E, Xu J, et al. Impact of the 2018 American Society of Clinical Oncology/College of American Pathologists HER2 Guideline Updates on HER2 Assessment in Breast Cancer With Equivocal HER2 Immunohistochemistry Results With Focus on Cases With HER2/CEP17 Ratio <2.0 and Average HER2 Copy Number >/=4.0 and <6.0. Arch Pathol Lab Med. 2020;144(5):597–601. [DOI] [PubMed] [Google Scholar]
- 14.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna WM, Kwok K. Chromogenic in-situ hybridization: a viable alternative to fluorescence in-situ hybridization in the HER2 testing algorithm. Mod Pathol. 2006;19(4):481–7. [DOI] [PubMed] [Google Scholar]
- 16.Yang SR, Bouhlal Y, De La Vega FM, et al. Integrated genomic characterization of ERBB2/HER2 alterations in invasive breast carcinoma: a focus on unusual FISH groups. Mod Pathol. 2020; 10.1038/s41379-020-0504-5. [DOI] [PubMed] [Google Scholar]
- 17.Mattei MG, Petkovich M, Mattei JF, et al. Mapping of the human retinoic acid receptor to the q21 band of chromosome 17. Hum Genet. 1988;80(2):186–8. [DOI] [PubMed] [Google Scholar]
- 18.Troxell ML, Bangs CD, Lawce HJ, et al. Evaluation of Her-2/neu status in carcinomas with amplified chromosome 17 centromere locus. Am J Clin Pathol. 2006;126(5):709–16. [DOI] [PubMed] [Google Scholar]
- 19.Varga Z, Tubbs RR, Wang Z, et al. Co-amplification of the HER2 gene and chromosome 17 centromere: a potential diagnostic pitfall in HER2 testing in breast cancer. Breast Cancer Res Treat. 2012;132(3):925–35. [DOI] [PubMed] [Google Scholar]
- 20.Lamy PJ, Fina F, Bascoul-Mollevi C, et al. Quantification and clinical relevance of gene amplification at chromosome 17q12-q21 in human epidermal growth factor receptor 2-amplified breast cancers. Breast Cancer Res. 2011;13(1):R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agersborg S, Mixon C, Nguyen T, et al. Immunohistochemistry and alternative FISH testing in breast cancer with HER2 equivocal amplification. Breast Cancer Res Treat. 2018;170(2):321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golomb HM, Rowley J, Vardiman J, et al. Partial deletion of long arm of chromosome 17: a specific abnormality in acute promyelocytic leukemia? Arch Intern Med. 1976;136(7):825–8. [PubMed] [Google Scholar]
- 23.Chomienne C, Ballerini P, Balitrand N, et al. The retinoic acid receptor alpha gene is rearranged in retinoic acid-sensitive promyelocytic leukemias. Leukemia. 1990;4(12):802–7. [PubMed] [Google Scholar]
- 24.Longo L, Pandolfi PP, Biondi A, et al. Rearrangements and aberrant expression of the retinoic acid receptor alpha gene in acute promyelocytic leukemias. J Exp Med. 1990;172(6):1571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019;380(20):1929–40. [DOI] [PubMed] [Google Scholar]
- 26.Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45(12):1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


