Abstract
Advances in the application of genomic technologies in clinical care have the potential to increase existing healthcare disparities. Studies have consistently shown that only a fraction of eligible patients with a family history of cancer receive recommended cancer genetic counseling and subsequent genetic testing. Care delivery models using pre-test and post-test counseling are not scalable, which contributes to barriers in accessing genetics services. These barriers are even more pronounced for patients in historically underserved populations. We have designed a multimodal intervention to improve subsequent cancer surveillance, by improving the identification of patients at risk for familial cancer syndromes, reducing barriers to genetic counseling/testing, and increasing patient understanding of complex genetic results. We are evaluating this intervention in two large, integrated healthcare systems that serve diverse patient populations (NCT03426878). The primary outcome is the number of diagnostic (hereditary cancer syndrome) findings. We are examining the clinical and personal utility of streamlined pathways to genetic testing using electronic medical record data, surveys, and qualitative interviews. We will assess downstream care utilization of individuals receiving usual clinical care vs. genetic testing through the study. We will evaluate the impacts of a literacy-focused genetic counseling approach versus usual care genetic counseling on care utilization and participant understanding, satisfaction, and family communication. By recruiting participants belonging to historically underserved populations, this study is uniquely positioned to evaluate the potential of a novel genetics care delivery program to reduce care disparities.
Keywords: genetics, genetic counseling, hereditary cancer, family history, underserved populations
Background
Individuals with hereditary cancer syndromes have up to an 80% lifetime risk of developing cancer.[1-5] About 1-2 in 200 individuals have a variant associated with one of the two most common hereditary cancer syndromes—Hereditary Breast and Ovarian Cancer syndrome (HBOC) and Lynch syndrome (LS).[1-5] Identifying these patients prior to cancer diagnosis facilitates preventive and risk-reducing measures that decrease morbidity and mortality. [6-12] Family history assessment and genetic testing for patients at risk for HBOC are now recommended in primary care by the United States Preventive Services Task Force (USPSTF).[13, 14] Despite this and the increasing availability of genetic testing, not all clinicians are aware of or implement the current recommendations.
Historically underserved populations experience barriers to cancer genetics services. Providers are less likely to evaluate their family history and refer them for genetic counseling, and they are less likely to receive genetic testing when appropriate.[15-18] Patients in community practice settings are less likely to receive genetics services than those receiving care in other practice settings, such as academic medical centers.[18-25] Communication about genetics and genomics between providers and patients is suboptimal.[26-29] Further, low health and genomic literacy present barriers for many English-speaking U.S. patients, and these barriers are exacerbated in Spanish-speaking patients. [30-33] Interventions are needed to improve access to genetics services for hereditary cancer syndromes, with a focus on improving access for historically underserved populations.
The Cancer Health Assessments Reaching Many (CHARM) study is part of the Clinical Sequencing Evidence-Generating Research (CSER) consortium, funded by the National Human Genome Research Institute (NHGRI) with co-funding from the National Institute on Minority Health and Health Disparities (NIMHD) and the National Cancer Institute (NCI).[34] CHARM is evaluating a series of interventions to address healthcare inequities in cancer genetics services among diverse populations. We endeavor to provide evidence and experience that health systems can use to increase access to genetics services for patients from all backgrounds. CHARM is recruiting a population of English- and Spanish-speaking patients, enriched for individuals from historically underserved backgrounds, in order to study the critical interactions among patients, family members, health practitioners, and laboratories that influence implementation of streamlined genomic services in these populations. The CHARM design addresses multiple barriers to equitable care in the current medical genetics services delivery model by implementing a multimodal intervention with 12 components (Figure 1).
Figure 1. Multimodal CHARM intervention altering the current genetics services delivery model.
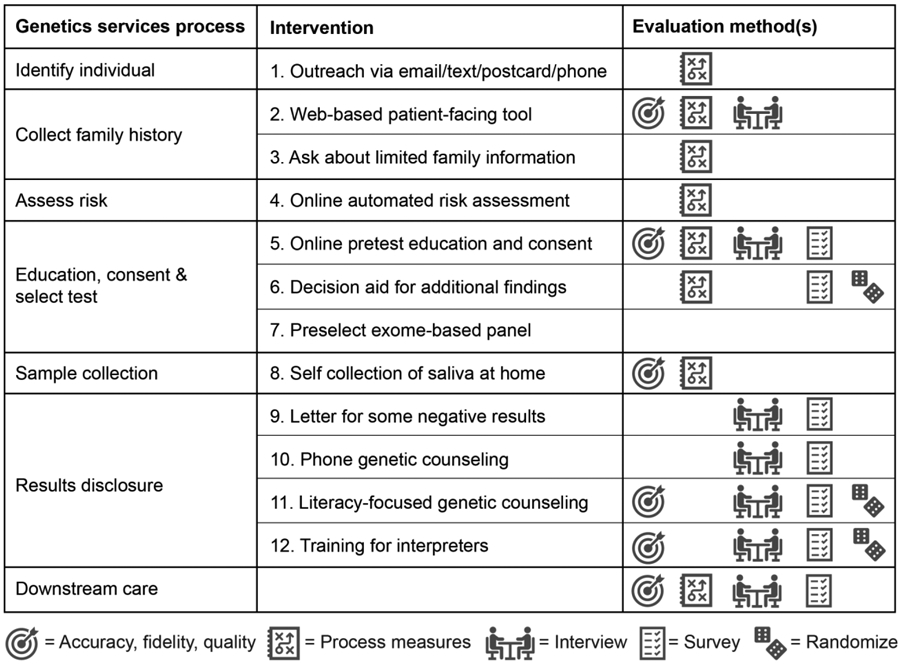
These components include a streamlined process for cancer risk assessment, genetics education, and consent for genetic testing through electronic tools. The electronic tools can be completed at the patient’s convenience either at home on a personal electronic device or in a clinical setting on a tablet. Further, we have trained CHARM study genetic counselors to implement a new mode of literacy-focused communication in post-test genetic counseling sessions, which we have termed the Accessible, Relational, Inclusive and Actionable (ARIA) model of genetic counseling. [35] Here we detail our protocol for testing the effectiveness and efficiency of this comprehensive risk assessment, genetic testing, and genetic counseling paradigm in a diverse patient population.
Overview of experimental design
Setting
Recruitment of participants is taking place at Kaiser Permanente Northwest (KPNW), an integrated healthcare system in the Portland, Oregon, metropolitan area, and Denver Health (DH), an integrated safety net health system with nine sites in Denver County, Colorado. KPNW serves approximately 25% of the population of Southwest Washington and Northwest Oregon. About 38% of members are racial/ethnic minorities. We are focusing recruitment in three KPNW clinics with the highest proportion of patients from racial/ethnic minorities and/or living in a census tract with low socioeconomic status as determined using geocoded census tract information and defined as a person living in a census tract where >20% of households are below the poverty level and/or >20% have less than a high school education; we are also focusing recruitment on patients with a documented need for Spanish interpretation (approximately 2%) as well as patients who have EMR indication of a primary language of Spanish. However, any KPNW patient is eligible to complete the risk assessment if they are a current health plan member aged 18-49 years.
The DH health system contains a regional trauma center and provides preventive, primary, and acute health care to almost one-third of Denver County residents. DH has nine federally qualified neighborhood health centers with approximately 160,000 unique users, of whom approximately 81% are publicly insured or uninsured, 69% are racial/ethnic minorities and about 21% of the 18-49 year old demographic speak Spanish primarily or exclusively. DH is a health system serving a low-resource area, meaning that individuals at higher risk are less likely to be able to receive genetic testing, even when they have been identified as eligible, both due to access (DH does not offer genetic testing) and due to health coverage constraints. In order to close the recognized genetics care gap related to testing access at DH and to strive for health justice, recruitment at DH is focused on individuals identified as at potentially higher risk based on cancer screening uptake outside of clinical cancer-screening guidelines for the general population and those referred by their providers based on assessment of risk.
Study aims
The CHARM study has 4 overarching aims:
(1) Implement a hereditary cancer risk assessment program in 880 healthy 18-49-year-old adults at risk of a hereditary cancer syndrome in primary care settings, with stakeholder input, and offer exome sequencing to clarify risk.
(2) Evaluate and tailor for diverse populations the critical interactions in the program, including the consent process, choices for selecting additional findings, genetic counseling results disclosure approach, and participant and primary care provider response to results disclosure.
(3) Evaluate the clinical and personal utility of using exome sequencing to diagnose individuals with hereditary cancer syndromes and provide additional findings, including healthcare utilization and adherence to recommended care.
(4) Address pragmatic and ethical challenges to the integration of genomic medicine into clinical and health systems decision-making.
Inclusion and exclusion criteria
The CHARM study has inclusion and exclusion criteria at 3 different points: 1) to complete the risk assessment, 2) joining the genetic testing portion of the study, and 3) for family members to receive targeted testing for variant(s) identified by the CHARM study (Table 1).
Table 1:
Inclusion and exclusion criteria for completing the risk assessment and obtaining genetic testing through the study and for family member genetic testing
| Study participants | Family member genetic testing |
|
|---|---|---|
| Inclusion |
Criteria to take risk
assessment: 1. KPNW or DH patient 2. Age 18-49 3. English or Spanish speaker Additional criterion for genetic testing through study: 1. Screen at risk for a hereditary cancer syndrome via the risk assessment algorithms OR have limited family structure or knowledge of family history[36, 37] 2. No disclosure on the risk assessment of prior testing for germline variants predisposing to LS or HBOC and no healthcare record of prior testing |
1. First or second degree relative of a study
participant with a pathogenic or likely pathogenic variant in a gene for
a hereditary cancer syndrome or a medically actionable additional
finding. 2. Over the age of 18 3. English or Spanish speaker 4. Lives in the United States |
| Exclusion |
Criteria to take risk
assessment: 1. Not an English or Spanish speaker 2. Unable to provide informed consent 3. Patients that don’t want their results placed in their medical record Additional criterion for genetic testing through study: 1. Does not screen at risk for a hereditary cancer syndrome via the risk assessment algorithms AND does not have limited family structure or knowledge of family history 2. Disclosure on the risk assessment of prior testing for germline variants predisposing to LS or HBOC or healthcare record of prior testing |
1. Under the age of 18 2. Previous positive result for variant identified in CHARM participant 3. Not an English or Spanish speaker 4. Lives outside of the United States |
Interventions
CHARM is a multimodal intervention, designed to increase uptake and retention at each step of the clinical genomic service pathway, from patient identification and risk assessment through result disclosure (Figure 1). The study processes are presented in Figure 2. To create accessible Spanish-language versions of our materials, materials were developed in English with input from Spanish speakers to ensure effective translation prior to further cultural adaptation and translation into Spanish. All English materials were subject to reduced literacy adaptation by the study team and then iterative review by a seven-member English-speaking patient advisory committee to ensure cultural competency and accessibility. Spanish materials were translated by Dr. Nangel Lindberg, a CHARM co-investigator who is a certified translator with over 30 years of experience in culturally-adapted translations of research materials. This experience includes English speakers of limited literacy and Spanish-speaking participants from diverse socioeconomic and cultural backgrounds. Dr. Lindberg’s translations and cultural adaptations were subject to review by a ten-member Spanish-speaking patient advisory committee who provided input on further cultural adaptations. To ensure accessibility of study materials for patients of all literacy levels, whenever possible we crafted materials at a maximum 6th grade reading level in both English and Spanish. Further, bilingual research staff are available in clinic and by phone to read all documents to the participant when needed. The KPNW IRB approved this study, and all collaborating IRBs ceded to KPNW except Dana Farber Cancer Institute and Columbia University, who approved the study separately.
Figure 2. Study schema depicting patient-study interactions in the CHARM study.
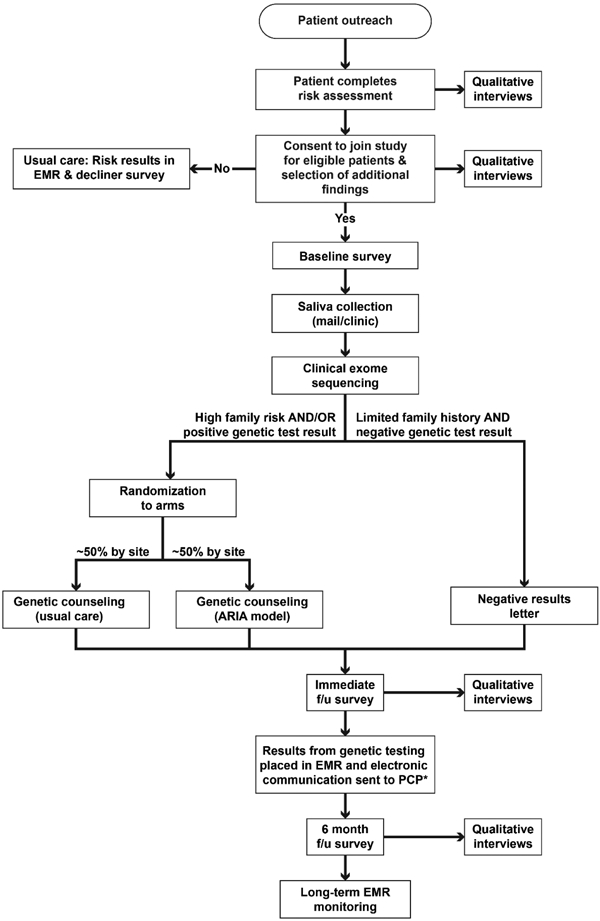
Abbreviations: f/u =follow-up.
Recruitment
We aim to enroll and complete clinical exome sequencing for 880 individuals at risk for hereditary cancer syndromes. In order to reach this target, we expect to recruit ~22,000 participants to complete the risk assessment portion of the study. This target assumes 10% of individuals who complete the risk assessment are eligible (namely, that high-risk individuals do not self-select to participate at higher rates) and that less than 50% of eligible participants elect to receive genetic testing through the study. Recruitment methods vary by site. As the primary recruitment method, KPNW uses one email followed by one text message for patients age 18-49 with upcoming visits at recruitment clinic sites. Secondary recruitment methods include one email followed by one text message for patients likely to be at higher risk for a hereditary cancer syndrome (see description of ‘high-risk’ below) and an in-person recruitment booth at select clinics. DH does not allow email or text message outreach for research purposes, so the primary recruitment approaches are a combination of postcards followed by a phone call for primary care patients who met criteria for being at potentially higher risk, and provider referrals for known high-risk patients (Figure 1; intervention 1). As a low-resource setting, DH does not provide genetic counseling or genetic testing outside of pregnancy and many patients cannot afford genetic testing via referral to a local university; therefore, we encouraged health care providers with suspected HBOC/LS patients to refer them directly to the study. As part of our efforts to reach Spanish-speaking individuals, we queried the EMR at both KPNW and DH to identify and perform outreach in Spanish to patients who regularly use Spanish interpretation services.
At both KP and DH, patients identified for outreach on the basis of being potentially higher risk are defined as those receiving cancer screening outside of general population risk screening guidelines (e.g., screening colonoscopy prior to age 50), those with an HBOC- or LS-related cancer diagnosis prior to age 50, those with a documentation of a family history of HBOC- or LS-related cancers, and any patient who received a referral to genetics services due to cancer but did not ultimately receive genetic testing.
Online Interactive risk assessment
We invite patients to complete an electronic patient-facing family history risk assessment for LS and HBOC (Figure 1; intervention 2). To increase accessibility, the electronic tool was optimized for display on multiple electronic device types, including mobile devices such as tablets and phones. Tablets are available at clinic recruitment sites for participants without access to these devices, and a phone number is provided on mailed recruitment materials for participants to call the study; bilingual study staff are available to complete the application over the phone for the participant. Following a single page consent for the risk assessment, participants answer questions about their personal and family history of LS and HBOC-related cancers. Additionally, we ask participants who do not screen at clinically significant risk based on family history about limited family structure or limited family history knowledge (Figure 1; intervention 3). The electronic tool, which we adapted for patient-facing use among English- and Spanish-speaking participants with limited literacy, leverages two clinically validated risk assessment algorithms (PREMM5™ and B-RST™ 3.0) to assess participant risk for LS and HBOC, respectively.[38, 39] We created a third, unique algorithm designed to assess limited family knowledge and limited family structure, as acknowledged in NCCN guidelines.[36, 37]
Participants are considered eligible for genetic testing through the CHARM study with any of the following: (1) B-RST score of high or moderate risk, (2) PREMM5 score of ≥2.5% risk of LS genetic variant, or (3) a limited family history knowledge or family structure on the basis of the novel CHARM algorithm. Risk is automatically calculated from participant input, and all participants, regardless of eligibility, are immediately presented a summary of their risk assessment results and next clinical steps (Figure 1; intervention 4). We also inform eligible participants that they can request genetics services through their health care provider even if they do not enroll in the genetic testing portion of the study.
Online consent to genetic testing and study enrollment
In place of pre-test genetics education and counseling with a genetic counselor, patients who are eligible for genetic testing through CHARM are presented with an online education and consent application. The application informs participants about genetic testing and the study, describes their options for receiving genetic testing, and provides informed consent information (Figure 1; intervention 5).Although the genetics education and consent information is provided electronically, participants can download a PDF copy or request a paper copy from the study. We included custom illustrations[40] depicting key concepts about genetic testing and the CHARM study and incorporated pre-recorded audio voiceover options (in English and Spanish) for all written text in the online genetics education and consent module. In the education and consent module following determination of eligibility, participants are informed about and agree to (1) interest in genetic testing (generally), (2) obtaining genetic testing and cancer genetic test results through CHARM as well as being contacted for surveys and interviews; and (3) release of personal health information. Following the education and consent module for cancer genetic testing, we offer participants the option to receive additional findings (findings outside of the diagnostic genes for hereditary cancer risk) including medically actionable results for all participants and carrier results for some participants (Supplement 1). Participants can choose all, some, or none of the additional finding results.
A subset of English-speaking participants are randomized, block-stratified by site, to receive a decision aid that helps them decide if they want medically actionable additional findings, through completion of a seven-item values clarification exercise referred to as the Optional Results Choice Aid (ORCA). Based on responses to these items, participants receive summative guidance about the directionality of their responses, with a suggestion about what they might decide. (Figure 1; intervention 6).[41] Individuals who are randomized to not receive the decision aid complete the values clarification exercise questions on the baseline survey after their selection of additional findings.
Genetic testing: clinical exome sequencing
CHARM uses an exome-based panel (Figure 1; intervention 7), which includes the clinically relevant portion of the genome (about 5,000 of the 20,000 genes in the human genome). Following consent, participants receive a saliva self-collection kit by mail or in person (Figure 1; intervention 8) and can return the kit via prepaid mail or by returning it to study staff. Sequencing is completed at a CLIA-certified laboratory at the University of Washington, using a predefined gene list for variant reporting (Supplement 1). We report pathogenic, likely pathogenic, and variants of uncertain significance in 39 genes association with a hereditary predisposition to cancer (diagnostic; Supplement 1). For participants who consent to receipt of additional findings, we report pathogenic and loss of function, likely pathogenic variants in 77 medically actionable genes, and, for a subset of participants, pathogenic variants in 14 carrier genes (collectively, additional findings). Appropriate first- and second-degree relatives of individuals with pathogenic and likely pathogenic variants in cancer genes or medically actionable secondary findings are eligible for cascade testing through the study. Participants with secondary carrier findings are provided information for their family members and partners for appropriate clinical follow-up.
Genetic variant interpretation
Sequencing results are interpreted by the CLIA-certified laboratory at the University of Washington that performs the sequencing. Variants are classified using the American College of Medical Genetics and Genomics recommendations for variant interpretation. [42]Variants classified as VUS in a cancer gene associated with the participant reported personal and/or family history are reported to study participants and documented in the medical record to facilitate future updates by the health system when variants are reclassified. Variant reclassifications during the course of the study result in participant notification and entry of an updated laboratory report into the electronic health record.
Results disclosure interventions
Some participants with a limited family history receive negative results by letter, provided in English or Spanish based on patient’s preference for risk assessment and consent (Figure 1; intervention 9). For all individuals with positive results and for those individuals with negative results but at clinically significant risk on B-RST™ 3.0 or PREMM5™, result disclosure is conducted by telephone by board-certified genetic counselors (Figure 1; intervention 10).
We randomly assign participants (block-stratified by site) to receive results by phone via one of two approaches: usual care genetic counseling or the ARIA model (Figure 1; intervention 11). Genetic counselors (n=2) in the ARIA arm received training (seven 1-hour sessions) and ongoing support through case reviews to use evidence-based techniques for effective communication with individuals of limited health literacy and effective approaches to working with Spanish-language interpreters.[35] Genetic counselors (n=2) in the usual care arm use traditional genetic counseling communication methods and were not privy to nor received any specialized training for the study. As part of their training, all genetic counselors receive education to support the competent provision of culturally responsive and respectful care, and the genetic counselors disclosing results through the study have ongoing training available at their institutions and through their professional organizations. Participants are informed via consent that we are evaluating different ways to communicate results, but they are blinded to the genetic counseling arm to which they are assigned.
English-speaking genetic counselors conduct counseling visits for Spanish-speaking participants in partnership with professional interpreters. We also developed and are evaluating a training course in exome sequencing for healthcare interpreters (Figure 1; intervention 12). We randomly assigned 24 interpreters into two groups: the intervention group receives the training prior to providing interpretation services for the study; the control group receives the training after completing the interpretations.
Following disclosure, all testing results and genetic counseling notes are documented in the medical record and deidentified data is deposited into ClinVar and AnVIL. Study genetic counselors facilitate follow-up care for at-risk participants by coordinating clinical hand-offs to clinicians within the participants’ health care system.
Evaluation of Study Outcomes: Process and Outcome Evaluations
We will evaluate the implementation of each intervention in our genetics healthcare delivery model using a variety of process and outcome measures gathered through qualitative and quantitative approaches. In cancer-related genetic testing, miscomprehension – especially of VUS – in tested patients and their referring providers has been reported, and it has been shown in some studies that counselees’ perception of risk, rather than their actual genetic risk status, is the driving factor in medical actions.[43-45] Thus, our assessments of patient-reported outcomes (e.g., understanding, satisfaction) as well as healthcare outcomes (namely, care utilization) will include the entire study sample..
Electronic tracking
All participant engagements with the study, including interactions with the online risk assessment and consent, are recorded electronically either automatically or manually by study staff in an integrated, secure study tracking system.
Surveys
Participants receiving genetic testing complete three surveys: a baseline survey and two follow-up surveys after results disclosure. Over half of the survey measures – such as subjective understanding of results, patient assessment of communication, and satisfaction of communication mode (each as a measure of genetic counseling mode success) – we collect from participants are from harmonized measures used across all six CSER consortium projects [46] (Table 2). We also ask participants about reasons for study participation, healthcare barriers, genetics communication, and genetics self-efficacy, family environment, and expanded demographics (e.g., formal education history, sexual orientation, gender identity, income, and insurance status). Additionally, we assess CHARM-specific outcomes such as decisional conflict and regret, satisfaction with information delivery about test results (i.e., participant satisfaction with genetic counseling mode), recall of test result as a measure of genetic counseling success, patient understanding of the utility of the test result, and quality of Spanish language interpretation. We invited potential participants who are eligible for genetic testing but decline to enroll and consent to complete a ‘decliner’ survey (Table 2). We administer surveys and capture all survey item responses via Research Electronic Data Capture (REDCap) tools hosted at KPNW.[47, 48] We import participant baseline completion events into the secure study tracking system.
Table 2.
Patient-reported outcome measures
| Domain | Measure | Survey Timepoint |
|---|---|---|
| Reasons for participating | Novela | BL |
| Concerns about participation | Novela | BL |
| Barriers to genetic testing | Novela | BL |
| Quality of life[49] | Short Form Survey 12 (SF12)b | BL, FU2 |
| Health literacy[50] | Brief Health Literacy screening Tool (BRIEF)b | BL |
| Subjective numeracy[51] | Shortened Subjective Numeracy Scale (SNS-3)b | BL |
| Health-Related locus of control[52] | Multidimensional Health Locus of Control (MHLC)a | BL |
| Access to care[53] | Medical Expenditure Panel Survey Household Component (MEPS-HC)b | BL |
| Communication self-efficacy[54] | Adapted from Ask, Understand, Remember Assessment (AURA)a | BL |
| Genetic self-efficacy[55] | Adapted from Kaphingst et al.a | BL, FU1, FU2 |
| Cancer/Genetics knowledge[56] | Adapted from Rose et al.a | BL, FU1, FU2 |
| Distrust[57] | Health Care System Distrust Scaleb | D, BL |
| Family environment[58] | McMaster family assessment devicea | BL |
| Information engagement[59] | Health information orientation scalea | BL |
| Understanding of consent | Novela | BL, FU1, FU2 |
| Religiosity | Multidimensional measure of religiousness/spiritualitya | D, BL |
| Satisfaction with communication mode | Novelb | FU1 |
| Quality of interpretation | Adapted from IPC Interpersonal processes of carea | FU1 |
| Subjective understanding of results | Novelb | FU1, FU2 |
| Feelings about results[60] | Feelings About genomiC Testing Results (FACToR)b | FU1, FU2 |
| Satisfaction with results | Novelb | FU1 |
| Patient-reported personal utility[61] | Personal Utility (PrU)b | FU1, FU2 |
| Family communication | Novelb | FU2 |
| Understanding of utility of results | Novela | FU1, FU2 |
| Patient assessment of communication[62] | Adapted from Patient Assessment of cancer Communication Experiences (PACE)b | FU1c |
| Information seeking Version 1 | Novelb | FU1d |
| Information seeking Version 2 | Novelb | FU2d |
| Patient-initiated actions | Novelb | FU2 |
| Follow through on medical actions | Novelb | FU2 |
| Recall of test result | Novela | FU1, FU2 |
| Satisfaction with information delivery | Novela | FU1c |
| Decisional regret[63] | Decision regret scalea | FU1 |
| Decisional conflict[64] | Decisional conflict scalea | BL |
| Decision aid knowledge questions | Novela | BL |
| Values self-assessment | Novela | BLe |
| Reasons for declining | Novelb | D |
Abbreviations: BL = baseline, FU1 = immediate follow-up survey, FU2 = 6 month follow-up survey
CHARM specific measures. Novel measures were created by study team members with relevant expertise and face validated
CSER consortium harmonized measure[46]
Administered only to participants with a telephone result disclosure
Portions of these questions only administered to participants with a positive result
Administered on BL survey only to participants who were not randomized to the decision aid arm. Those who were randomized to the decision aid arm responded to the questions during the consent process.
Fidelity of genetic counseling to the ARIA model or usual care
All counseling sessions are audio recorded and assessed to ensure fidelity to the two counseling approaches. We randomly assess a selection of audio recordings from each quarter, evenly distributed across the four genetic counselors. We developed a coding scheme to assess fidelity to each counseling approach and each recording is dual-coded.
Use of the electronic medical record (EMR)
At the time of identification for recruitment, potential participant demographic data available in the EMR is imported into the study tracking system for participants at KP; DH participants have demographic data added to tracking from the EMR at time of consent. When participants do not answer the demographics questions on the baseline or decliner surveys in REDCap, study analyses utilize data obtained from the study tracking system. Care utilization of participants in the genetic testing portion of the study and individuals eligible for, but who decline, genetic testing through the study will be drawn from procedure codes in the EMR and evaluated against recommendations provided by study genetic counselors.
Inferential statistical analysis
We will evaluate the risk assessment tool (interventions 2-4) in the following ways: (1) evaluate interrater agreement between genetic counselor family history data and the risk assessment tool (convergent validity); (2) determine predictors of time spent on the risk assessment tool using multivariable linear regression, and (3) index the overall agreement between accurate risk tool stratifications and risk stratifications produced from family history available in the EMR. We will determine whether the decision aid (intervention 6) improves informed values congruence using multivariable logistic regression. To compare the reportable findings yield between those with different family history knowledge and risk scores, we will use multivariable logistic regression (intervention 7). Comparing the yield between those with sufficient family history (standard thresholds=1) and those with incomplete family history (=0), a significant odds ratio that is greater than one would support the hypothesis that those with sufficient family history information have a higher yield of reportable findings. Using the projected sample size of 880 and assuming that 25% of participants qualify through incomplete family history data, and that 20% of participants who meet standard criteria have a reportable finding, the power is >80% to detect an odds ratio as small as 2. We will test for the superiority of the ARIA genetic counseling approach using ANCOVA (intervention 11). Using multivariable logistic and negative binomial regression, will compare uptake of recommended downstream risk-management and health care visits between (1) ARIA and usual care genetic counseling approaches (intervention 11) and (2) CHARM study interventions and usual care (wholistic evaluation). Regression analyses will be conducted in all participants and in those receiving reportable test results, exclusively. For all multivariable regression and ANCOVA analyses of participants in the genetic testing portion of the study, arm will be the independent variable and the randomization stratification factor of site (0=DH, 1=KP) will be the covariate. At a sample size of 880, we have 80% power to detect a Cohen’s d of .15 comparing patient reported outcomes from the two genetic counseling arms. Evaluations of non-randomized components of the multimodal CHARM intervention will be adjusted on the basis of measured covariates, including demographics and patient-reported data.
Quantitative evaluation of CHARM study Intervention
An overview of the CHARM study evaluation leveraging quantitative data is presented in Table 3.
Table 3.
Quantitative CHARM study evaluations leveraging data from the CHARM tracking system, EMR, and/or participant reported outcomes on CHARM surveys
| Intervention-specific evaluations | |||
|---|---|---|---|
| Intervention(s) | Process Evaluation(s) | Outcome Evaluation(s) | Clinical Trials Outcome |
| 1: Outreach via email/text/postcard/phone | - Number receiving each outreach method,
number of times contacted, number who engage with the online web
tool - Non-responders and responder characteristics |
- Response rate | N/A |
| 2-4: Web-based patient facing tool; ask about limited family information; online automated risk assessment | - Proportion incompleters - Risk assessment outcomes |
- Time spent on the tool - Accuracy of reported family history |
N/A |
| 5: Online pretest education and consent | - Proportion of eligible individuals
consenting to genetic testing - Time spent on the consent module |
- Time spent on consent | N/A |
| 6: Decision aid for medically actionable additional findings | - Decision aid selections and choices for
additional findings - Time spent on decision aid |
- Informed, values-congruent decision making | N/A |
| 7: Preselect exome-based panel | N/A | - Number and type of findings (P/LP/VUS) (descriptive) |
Primary study outcome - Number and type of findings in genes related to hereditary cancer syndromes Secondary study outcomes - Number and type of findings in genes related to medically actionable hereditary conditions - Number and type of findings in genes related to common carrier conditions |
| 8: Self collection of saliva at home | - Proportion of samples mailed | - Proportion of inadequate and successful first or second samples | N/A |
| 9: Letter for some negative results | - Participant characteristics - Proportion of participants receiving a letter |
- Understanding, utility, and satisfaction |
Secondary study
outcomes: - Participant understanding of recommended care - Participant understanding of genetic test results - Participant satisfaction with genetic counseling - Family communication - Personal utility |
| 10: Phone genetic counseling | - Participant characteristics - Proportion of participants receiving genetic counseling by phone |
- Understanding, utility, and satisfaction | |
| 11: Literacy-focused genetic counseling (ARIA model) | - Characteristics of individuals in the two
arms - Fidelity to the two counseling modes |
- Understanding, satisfaction, assessment of communication | |
| 12: Training for interpreters | - Interpreter characteristics and characteristics of participants utilizing interpreter services | - Quality and accuracy of
interpretation - Interpreter knowledge and self-efficacy - Participant understanding and satisfaction |
|
| Wholistic evaluations of study interventions | |||
| Utilization | - CHARM participant characteristics | - Uptake and adherence to recommended downstream cancer prevention measures |
Secondary study
outcome: Healthcare utilization |
| Provider experiences with care of CHARM participants | - Provider characteristics | - Satisfaction and understanding | N/A |
| LGBTQ+ participant family communication | - Characteristics of LGBTQ+ participants in comparison to CHARM study population | - Family communication | N/A |
Interviews
We are also using semi-structured qualitative interviews to assess participant, provider, and interpreter experiences with CHARM interventions at various points in the study (Table 4), including after risk assessment, after declining to join the genetic testing portion of the study, after consent to genetic testing through the study, immediately after result disclosure, and 6 months after result disclosure. These interviews assess participant, provider, and interpreter experiences with multiple aspects of the CHARM study intervention (Table 4). A unique codebook will be developed using inductive and deductive techniques for each set of qualitative interviews to identify common themes.[65, 66]
Table 4.
Qualitative interview approach
| Intervention(s) Evaluated | Interview Focus | Key domains | Time point |
|---|---|---|---|
| 1: Outreach via
email/text/postcard/phone 5: Online pretest education and consent Wholistic evaluations of study interventions |
Participant feelings of respect during research procedures | -Respect in the clinical
setting -Respect during recruitment and consent -Decision-making process -Trust in medical research |
After consent and before result disclosure OR within 4 weeks of disclosure of negative result |
| 2-4: Web-based patient facing tool; ask about limited family information; online automated risk assessment | Participant experience with risk assessment | -Reasons for incompletion or taking a long
time to complete -Understanding discrepancies between risk assessment responses and family history disclosed to genetic counselor -Acceptability -Application design |
After risk assessment |
| 5: Online pretest education and consent | Participant experience with consent for study enrollment | -Decision making -Understanding of consent and data sharing -Information quality -Emotional response -Application design and flow of consent |
After consent and before result disclosure |
| 10: Phone genetic counseling 11: Literacy-focused genetic counseling (ARIA model) 12: Training for interpreters Wholistic evaluations of study interventions |
Participant opinions of personal utility of genetic testing | -Impact on clinical care, affective state,
cognitive state, and life planning -Social impact |
3+ months after result disclosure |
| 10: Phone genetic counseling 11: Literacy-focused genetic counseling (ARIA model) 12: Training for interpreters |
Participant experience with result disclosure | (2 week interviews): -Familiarity with genetic testing -Understanding of test results and care recommendations -Perceptions of genetic counseling communication -Perceptions of Spanish interpretation -Uncertainty (6 month interviews): -Family communication -Communication with providers -Experience with downstream care |
Within 4 weeks of result disclosure and 6 months after result disclosure |
| 12: Training for interpreters | Interpreter experience | -Experience as an
interpreter -Experience interpreting for study -Perceptions of genetic counselors’ communication -Perceptions of interpreter training |
After 2 completed interpretations |
| Wholistic evaluations of study interventions | LGBTQ+ participant experience | -Impact of LGBTQ+ identity on family
relationships and family history sharing -Impact of LGBTQ+ identity on sharing of study results |
After result disclosure |
| Wholistic evaluations of study interventions | Provider experience | -Provider understanding of
result -Communication with patient -Plan to manage patient care -Prior experience and uncertainty of some genetic results -View of risk assessment tool |
After medical encounter with participant OR 6 months post result disclosure |
Discussion
In 2013, the USPSTF recommended family history screening for breast and ovarian cancer risk in primary care and recently reaffirmed that recommendation.[14, 67] However, these recommended screenings are under-implemented in current clinical practice, representing the first care gap for patients in the hereditary cancer genetics services delivery pipeline.[18-25] This care gap is wider for patients from medically underserved populations, who have a lower likelihood of both referral for genetic counseling and receipt of genetic testing when indicated.[15-18] The current genetics services delivery model may be thought of as a ‘leaky pipeline,’ especially for individuals who face systemic barriers to care access. Interventions are needed at multiple points to remove barriers created by the current structure and improve the equity of genetics services delivery. However, any intervention designed to increase appropriate referrals and genetic testing will contribute to an already strained genetics services delivery system.[68-74] The current genetics services delivery model is resource and labor intensive, typically requiring both pre- and post-test genetic counseling visits (often in-person), and there is a limited availability of genetic counselors to meet this demand. [68, 70, 71, 73, 74] As such, interventions need to both address the “leaky pipeline” and reimagine how genetics care is delivered in order to alleviate strain on the health system and make widespread genetic testing scalable even in low-resource areas. To address the issue of patients lost to follow-up at each step in the genetics services pipeline and to alleviate health system impact of increasing genetics referrals, the CHARM study is a evaluating a multimodal intervention acting at all stages of the current genetics services delivery model.
The approaches to genetic assessment used in CHARM allow for a healthcare system to potentially reach a much larger portion of the patient population to assess risk, because they do not rely on contact with a healthcare provider (Figure 1, intervention 1). Similarly, a provider is not required to collect the family history data, because participants interact with a patient-facing risk assessment tool (Figure 1, intervention 2). The CHARM study also provides a way to expand eligibility for genetic testing on the basis of an assessment of limited family history knowledge or structure based on current guidance,[36, 37] which is more likely to impact patients from some historically underserved populations (Figure 1, intervention 3).[75-79] Through automated assessment and immediate communication of risk status, the study intervention seeks to provide guideline-adherent risk communication to participants, rather than relying on a provider to recognize risk from patient-provided family history during a medical appointment. Together, these interventions are designed to support systematic and efficient risk assessment and patient identification for referral (Figure 1, interventions 2-4). These barriers disproportionately impact medically underserved individuals and produce strain on the care system taxing already limited medical appointment time.[15-25]
With limited availability of genetic counselors and increased genetics services demand,[66, 69, 70, 72, 73] pre-test counseling and appropriate genetic test selection pose a bottleneck to genetic testing receipt. To address this bottleneck, our intervention delivers pre-test genetic testing education and consent in a web-based electronic patient-facing application (Figure 1, intervention 5) and also provides a decision aid designed to assist participants in selection of the receipt of medically actionable additional findings to be congruent with their values (Figure 1, intervention 6).
All participants in the study are receiving a large exome-based panel for cancer-related genes as well as medically actionable additional findings, which removes the need for patient-specific panel selection (Figure 1, intervention 7). However, a larger panel does introduce the possibility of additional reportable findings and may increase the identification of variants of uncertain significance or with unclear implications for the familial phenotype.
To reduce patient travel and time burden, a barrier that disproportionately impacts medically underserved populations,[80, 81] participants are able to self-complete a saliva collection kit at their convenience in their home and return it via prepaid postal envelope rather than needing to visit a clinical laboratory for a blood draw (Figure 1, intervention 8). Similarly, all participants receive genetic test results remotely by letter (Figure 1, intervention 9) or phone (Figure 1, intervention 10), which we anticipate will both reduce burden on the care system and remove the barrier posed by requiring travel to specialty centers. Further, the study is evaluating a novel genetic counseling approach (ARIA model) to address barriers to communication in limited-literacy populations (Figure 1, intervention 11). Finally, language barriers account for significant disparities in healthcare receipt, especially in genomics,[80] and the study is evaluating interpreter training methodology designed to ensure adequate communication of results by interpreters (Figure 1, intervention 12).
The multimodal intervention of the CHARM study provides a pathway to genetic testing that is scalable, removes the barrier of multiple appointments and repeated care system contacts, and reduces travel burden by making it possible to complete every step of the intervention outside of an in-person healthcare setting. All participant-facing materials were culturally adapted and translated into Spanish at a 6th-grade reading level. Individually, each aspect of the CHARM intervention is designed to remove or reduce a systemic barrier to genetics services access and/or to alleviate stress on the healthcare system to make widespread genetic testing more scalable. Our study design will evaluate the success of each component of this multimodal intervention in terms of both patient and care system impact, starting from screening and ending with result disclosure. While eliminating disparities in genetics services delivery does not end at diagnosis of a cancer syndrome, the CHARM study represents an important step in evaluating evidence-based scalable strategies that address inequities in the current medical genetics services delivery model. The evidence generated will include data on adherence to appropriate care based on genetic test results. These results may also provide hypothesis-generating data on the equity in receipt of downstream care that can guide the design of future studies to close downstream healthcare gaps. As part of the CSER consortium, our study also will contribute important evidence about sequencing in underserved populations, including best practices for variant interpretation, results reporting, and ethical, legal, and social implications in these groups.
Supplementary Material
Acknowledgements
The authors acknowledge Neon Brooks, PhD for editing assistance and Angela Paolucci and Ana Reyes for administrative assistance.
Funding
The Clinical Sequencing Evidence-Generating Research (CSER) consortium is funded by the National Human Genome Research Institute (NHGRI) with co-funding from the National Institute on Minority Health and Health Disparities (NIMHD) and the National Cancer Institute (NCI), supported by U01HG007292; MPIs: Wilfond, Goddard and U24HG007307 (Coordinating Center).
Footnotes
Conflict of Interest
All authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garber JE, Offit K. Hereditary cancer predisposition syndromes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(2):276–92. [DOI] [PubMed] [Google Scholar]
- 2.Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer syndromes. Oncogene. 2004;23(38):6445–70. [DOI] [PubMed] [Google Scholar]
- 3.Petrucelli N, Daly MB, Feldman GL. BRCA1 and BRCA2 Hereditary Breast and Ovarian Cancer. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al. , editors. GeneReviews(R). Seattle, WA: University of Washington, Seattle; 1993. [Google Scholar]
- 4.Randall LM, Pothuri B. The genetic prediction of risk for gynecologic cancers. Gynecologic oncology. 2016;141(1):10–6. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein JH, Enns R, Heidelbaugh J, Barkun A. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Lynch Syndrome. Gastroenterology. 2015;149(3):777–82; quiz e16-7. [DOI] [PubMed] [Google Scholar]
- 6.Nelson HD, Fu R, Goddard K, Mitchell JP, Okinaka-Hu L, Pappas M, et al. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Systematic Review to Update the US Preventive Services Task Force Recommendation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 7.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genetics in medicine : official journal of the American College of Medical Genetics. 2009;11(1):42–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly MB, Pilarski R, Axilbund JE, Berry M, Buys SS, Crawford B, et al. Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2015. Journal of the National Comprehensive Cancer Network : JNCCN. 2016;14(2):153–62. [DOI] [PubMed] [Google Scholar]
- 9.Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. The American journal of gastroenterology. 2014;109(8):1159–79. [DOI] [PubMed] [Google Scholar]
- 10.National Collaborating Centre for Cancer. National Institute for Health and Clinical Excellence: Guidance. Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. Cardiff, UK.: National Collaborating Centre for Cancer (UK);National Collaborating Centre for Cancer; 2013. [PubMed] [Google Scholar]
- 11.Schofield L, Grieu F, Goldblatt J, Amanuel B, lacopetta B. A state-wide population-based program for detection of lynch syndrome based upon immunohistochemical and molecular testing of colorectal tumours. Familial cancer. 2012;11(1):1–6. [DOI] [PubMed] [Google Scholar]
- 12.Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62(6):812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyer VA. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–81. [DOI] [PubMed] [Google Scholar]
- 14.Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-Related Cancer: US Preventive Services Task Force recommendation statement. Jama. 2019;322(7):652–65. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy AM, Bristol M, Domchek SM, Groeneveld PW, Kim Y, Motanya UN, et al. Health Care Segregation, Physician Recommendation, and Racial Disparities in BRCA1/2 Testing Among Women With Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(22):2610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shields AE, Burke W, Levy DE. Differential use of available genetic tests among primary care physicians in the United States: results of a national survey. Genetics in medicine : official journal of the American College of Medical Genetics. 2008;10(6):404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cragun D, Weidner A, Lewis C, Bonner D, Kim J, Vadaparampil ST, et al. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123(13):2497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer LA, Anderson ME, Lacour RA, Suri A, Daniels MS, Urbauer DL, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: missed opportunities. Obstet Gynecol. 2010;115(5):945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delikurt T, Williamson GR, Anastasiadou V, Skirton H. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet. 2015;23(6):739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn BS, Wood ME, Ashikaga T, Stockdale A, Dana GS, Naud S. Primary care physicians' use of family history for cancer risk assessment. BMC Fam Pract. 2010;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murff HJ, Byrne D, Syngal S. Cancer risk assessment: quality and impact of the family history interview. Am J Prev Med. 2004;27(3):239–45. [DOI] [PubMed] [Google Scholar]
- 22.Nippert I, Harris HJ, Julian-Reynier C, Kristoffersson U, Ten Kate LP, Anionwu E, et al. Confidence of primary care physicians in their ability to carry out basic medical genetic tasks-a European survey in five countries-Part 1. J Community Genet. 2011;2(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plat AW, Kroon AA, Van Schayck CP, De Leeuw PW, Stoffers HE. Obtaining the family history for common, multifactorial diseases by family physicians. A descriptive systematic review. Eur J Gen Pract. 2009;15(4):231–42. [DOI] [PubMed] [Google Scholar]
- 24.Sin M, McGuinness JE, Trivedi MS, Vanegas A, Silverman TB, Crew KD, et al. Automatic Genetic Risk Assessment Calculation Using Breast Cancer Family History Data from the EHR compared to Self-Report. AMIA Annu Symp Proc. 2018;2018:970–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Wood ME, Kadlubek P, Pham TH, Wollins DS, Lu KH, Weitzel JN, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(8):824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng JKY, Guerra C, Pasick RJ, Schillinger D, Luce J, Joseph G. Cancer genetic counseling communication with low-income Chinese immigrants. J Community Genet. 2018;9(3):263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph G, Pasick RJ, Schillinger D, Luce J, Guerra C, Cheng JKY. Information Mismatch: Cancer Risk Counseling with Diverse Underserved Patients. Journal of genetic counseling. 2017;26(5):1090–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamara D, Weil J, Youngblom J, Guerra C, Joseph G. Cancer Counseling of Low-Income Limited English Proficient Latina Women Using Medical Interpreters: Implications for Shared Decision-Making. Journal of genetic counseling. 2018;27(1):155–68. [DOI] [PubMed] [Google Scholar]
- 29.Lara-Otero K, Weil J, Guerra C, Cheng JKY, Youngblom J, Joseph G. Genetic Counselor and Healthcare Interpreter Perspectives on the Role of Interpreters in Cancer Genetic Counseling. Health Commun. 2019;34(13):1608–18. [DOI] [PubMed] [Google Scholar]
- 30.Hurle B, Citrin T, Jenkins JF, Kaphingst KA, Lamb N, Roseman JE, et al. What does it mean to be genomically literate?: National Human Genome Research Institute Meeting Report. Genetics in medicine : official journal of the American College of Medical Genetics. 2013;15(8):658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutner M, Greenberg E, Jin Y, Paulsen C. The health literacy of America’s adults: results from the 2003 National Assessment of Adult Literacy National Center for Education Statistics. 2006;NCES; 2006–483. [Google Scholar]
- 32.Rodríguez SA, Roter DL, Castillo-Salgado C, Hooker GW, Erby LH. Translation and validation of a Spanish-language genetic health literacy screening tool. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2015;34(2):120–9. [DOI] [PubMed] [Google Scholar]
- 33.Pacyna JE, Shaibi GQ, Lee A, Byrne JO, Cuellar I, Sutton EJ, et al. Increasing access to individualized medicine: a matched-cohort study examining Latino participant experiences of genomic screening. Genetics in medicine : official journal of the American College of Medical Genetics. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amendola LM, Berg JS, Horowitz CR, Angelo F, Bensen JT, Biesecker BB, et al. The Clinical Sequencing Evidence-Generating Research Consortium: Integrating Genomic Sequencing in Diverse and Medically Underserved Populations. American journal of human genetics. 2018;103(3):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riddle L, Amendola LM, Gilmore M, Guerra C, Biesecker BB, Kauffman TL. Development and early implementation of a literacy-focused genetic counseling model for disclosure of clinical exome sequencing results Patient Education and Counseling. Patient education and counseling. 2021. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Comprehenisve Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Colorectal2019; March.2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf [Google Scholar]
- 37.National Comprehenisve Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic2020; January.2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [Google Scholar]
- 38.Bellcross C, Hermstad A, Tallo C, Stanislaw C. Validation of Version 3.0 of the Breast Cancer Genetics Referral Screening Tool (B-RST™). Genetics in medicine : official journal of the American College of Medical Genetics. 2019;21(1):181–4. [DOI] [PubMed] [Google Scholar]
- 39.Kastrinos F, Uno H, Ukaegbu C, Alvero C, McFarland A, Yurgelun MB, et al. Development and Validation of the PREMM(5) Model for Comprehensive Risk Assessment of Lynch Syndrome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(19):2165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraft SA, Porter KM, Duenas DM, Guerra C, Joseph G, Lee SS, et al. Participant Reactions to a Literacy-Focused, Web-Based Informed Consent Approach for a Genomic Implementation Study. AJOB Empir Bioeth. 2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freed AS, Gruß I, McMullen CK, Leo MC, Kauffman TL, Porter KM, et al. A decision aid for additional findings in genomic sequencing: Development and pilot testing. Patient education and counseling. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine : official journal of the American College of Medical Genetics. 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuntini R, Ferrari S, Bonora E, Buscherini F, Bertonazzi B, Grippa M, et al. Dealing With BRCA1/2 Unclassified Variants in a Cancer Genetics Clinic: Does Cosegregation Analysis Help? Front Genet. 2018;9:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vos J, Oosterwijk JC, Gomez-Garcia E, Menko FH, Collee MJ, van Asperen CJ, et al. Exploring the short-term impact of DNA-testing in breast cancer patients: the counselees' perception matters, but the actual BRCA1/2 result does not. Patient education and counseling. 2012;86(2):239–51. [DOI] [PubMed] [Google Scholar]
- 45.Richter S, Haroun I, Graham TC, Eisen A, Kiss A, Warner E. Variants of unknown significance in BRCA testing: impact on risk perception, worry, prevention and counseling. Ann Oncol. 2013;24 Suppl 8:viii69–viii74. [DOI] [PubMed] [Google Scholar]
- 46.Goddard KAB, Angelo F, Ackerman SL, Berg J, Biesecker BB, Danila MI, et al. Lessons learned about harmonizing survey measures for the CSER consortium. Journal of Clinical and Translational Science. 2020(April):1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 50.Haun J, Noland Dodd VJ, Graham-Pole J, Rienzo B, Donaldson P. Testing a health literacy screening tool: Implications for utilization of a BRIEF health literacy indicator. Federal Practitioner. 2009;26(12):24–31. [Google Scholar]
- 51.McNaughton CD, Cavanaugh KL, Kripalani S, Rothman RL, Wallston KA. Validation of a short, 3-item version of the subjective numeracy scale. Med Decis Making. 2015;35(8):932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallston KA. Multidimensional health locus of control (MHLC) scales 2019. Available from: https://nursing.vanderbilt.edu/projects/wallstonk/index.php. [DOI] [PubMed]
- 53.Agency for Healthcare Research and Quality (AHRQ). Medicare Expenditure Panel Survey (MEPS)-Household Component (HC), access to care section 2019; (P18R5/P19R3/P20R1). Available from: https://meps.ahrq.gov/survey_comp/hc_ques_sections.jsp.
- 54.Clayman ML, Pandit AU, Bergeron AR, Cameron KA, Ross E, Wolf MS. Ask, understand, remember: a brief measure of patient communication self-efficacy within clinical encounters. J Health Commun. 2010;15 Suppl 2:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaphingst KA, McBride CM, Wade C, Alford SH, Brody LC, Baxevanis AD. Consumers' use of web-based information and their decisions about multiplex genetic susceptibility testing. J Med Internet Res. 2010;12(3):e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rose A, Peters N, Shea JA, Armstrong K. The association between knowledge and attitudes about genetic testing for cancer risk in the United States. J Health Commun. 2005;10(4):309–21. [DOI] [PubMed] [Google Scholar]
- 57.Shea JA, Micco E, Dean LT, McMurphy S, Schwartz JS, Armstrong K. Development of a revised Health Care System Distrust scale. J Gen Intern Med. 2008;23(6):727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boterhoven de Haan KL, Hafekost J, Lawrence D, Sawyer MG, Zubrick SR. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015;54(1):116–23. [DOI] [PubMed] [Google Scholar]
- 59.DuBenske LL, Burke Beckjord E, Hawkins RP, Gustafson DH. Psychometric evaluation of the Health Information Orientation Scale: a brief measure for assessing health information engagement and apprehension. J Health Psychol. 2009;14(6):721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li M, Bennette CS, Amendola LM, Ragan Hart M, Heagerty P, Comstock B, et al. The feelings about genomiC Testing Results (FACToR) questionnaire: development and preliminary validation. Journal of genetic counseling. 2018. [DOI] [PubMed] [Google Scholar]
- 61.Kohler JN, Turbitt E, Lewis KL, Wilfond BS, Jamal L, Peay HL, et al. Defining personal utility in genomics: A Delphi study. Clinical genetics. 2017;92(3):290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazor KM, Street RL Jr., Sue VM, Williams AE, Rabin BA, Arora NK. Assessing patients' experiences with communication across the cancer care continuum. Patient education and counseling. 2016;99(8):1343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Connor AM. User Manual – Decision Regret Scale 1996 (2003). Available from: http://decisionaid/ohri.ca/docs/develop/User_Manuals/UM_Regret_Scale.pdf.
- 64.O’Connor AM. User Manual – Decisional Conflict Scale (10 item question format) 1993 (2010). Available from: http://decisionaid/ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf
- 65.Charmaz K Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: SAGE Publications; 2006. [Google Scholar]
- 66.Saldaña J The Coding Manual for Qualitative Researchers. 3rd ed. London: SAGE; 2015. [Google Scholar]
- 67.Nelson HD, Pappas M, Cantor A, Haney E, Holmes R. Risk assessment, genetic counseling, and genetic testing for BRCA-Related Cancer in women: updated evidence report and systematic review for the US Preventive Services Task Force. Jama. 2019;322(7):666–85. [DOI] [PubMed] [Google Scholar]
- 68.Buchanan AH, Rahm AK, Williams JL. Alternate service delivery models in cancer genetic counseling: a mini-review. Front Oncol. 2016;6:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hooker G, Buchanan A, Rhoads K, Vogel Postula KJ, Quillin J, Summerour P. Presented abstracts from the Thirty Third Annual Education Conference of the National Society of Genetic Counselors (New Orleans, LA, Sept 2014): Large scale changes in cancer genetic testing with variable integration of expanded gene panels. J Genet Counsel. 2014;23(6):1070–1. [Google Scholar]
- 70.McCuaig JM, Armel SR, Care M, Volenik A, Kim RH, Metcalfe KA. Next-generation service delivery: a scoping review of patient outcomes associated with alternative models of genetic counseling and genetic testing for hereditary cancer. Cancers (Basel). 2018;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. American journal of medical genetics Part C, Seminars in medical genetics. 2018;178(1):24–37. [DOI] [PubMed] [Google Scholar]
- 72.The National Society of Genetic Counselors (NSGC). Professional status survey 2018: Service delivery and access to care. 2018. [Google Scholar]
- 73.Trepanier A, Allain D. Models of service delivery for cancer genetic risk assessment and counseling. Journal of genetic counseling. 2013;23. [DOI] [PubMed] [Google Scholar]
- 74.Wham D, Vu T, Chan-Smutko G, Kobelka C, Urbauer D, Heald B. Assessment of clinical practices among cancer genetic counselors. Familial cancer. 2010;9(3):459–68. [DOI] [PubMed] [Google Scholar]
- 75.Ashida S, Goodman MS, Stafford J, Lachance C, Kaphingst KA. Perceived familiarity with and importance of family health history among a medically underserved population. J Community Genet. 2012;3(4):285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaphingst KA, Blanchard M, Milam L, Pokharel M, Elrick A, Goodman MS. Relationships Between Health Literacy and Genomics-Related Knowledge, Self-Efficacy, Perceived Importance, and Communication in a Medically Underserved Population. J Health Commun. 2016;21 Suppl 1(Suppl 1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaphingst KA, Lachance CR, Gepp A, D'Anna LH, Rios-Ellis B. Educating underserved Latino communities about family health history using lay health advisors. Public Health Genomics. 2011;14(4-5):211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roberts MC, Krakow M, Wheldon CW, Silver MI. Differences in family health history knowledge among bisexual and lesbian women. LGBT Health. 2019;6(3):134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slattery ML, Murtaugh MA, Lanier AP, Ma K-N, Ferucci ED, Etzel RA, et al. Family health history and health behaviors in Alaska native and American Indian people. J Health Care Poor Underserved. 2009;20(3):678–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Canedo JR, Wilkins CH, Senft N, Romero A, Bonnet K, Schlundt D. Barriers and facilitators to dissemination and adoption of precision medicine among Hispanics/Latinos. BMC Public Health. 2020;20(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38(5):976–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


