Abstract
OBJECTIVE:
To identify and quantify risk factors for atonic postpartum hemorrhage.
DATA SOURCES:
PubMed, CINAHL, EMBASE, Web of Science, and and ClinicalTrials.gov databases were searched for English language studies with no restrictions on date or location. Studies included randomized trials, prospective or retrospective cohort studies, and case–control studies of pregnant patients who developed atonic postpartum hemorrhage and reported at least one risk factor.
METHODS OF STUDY SELECTION:
Title, abstract, and full-text screening were performed using the Raayan web application. Of 1,239 records screened, 27 studies were included in this review. Adjusted or unadjusted odds ratios (ORs), relative risks, or rate ratios were recorded or calculated. For each risk factor, a qualitative synthesis of low and moderate risk of bias studies classifies the risk factor as definite, likely, unclear, or not a risk factor. For risk factors with sufficiently homogeneous definitions and reference ranges, a quantitative meta-analysis of low and moderate risk of bias studies was implemented to estimate a combined OR.
TABULATION, INTEGRATION, AND RESULTS:
Forty-seven potential risk factors for atonic postpartum hemorrhage were identified in this review, of which 15 were judged definite or likely risk factors. The remaining 32 assessed risk factors showed no association with atonic postpartum hemorrhage or had conflicting or unclear evidence.
CONCLUSION:
A substantial proportion of postpartum hemorrhage occurs in the absence of recognized risk factors. Many risk factors for atonic hemorrhage included in current risk-assessment tools were confirmed, with the greatest risk conferred by prior postpartum hemorrhage of any etiology, placenta previa, placental abruption, uterine rupture, and multiple gestation. Novel risk factors not currently included in risk-assessment tools included hypertension, diabetes, and ethnicity. Obesity and magnesium were not associated with atonic postpartum hemorrhage in this review.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO, CRD42020157521.
Postpartum hemorrhage affects 3–10% of deliveries and accounts for nearly 20% of maternal deaths worldwide.1,2 Although there are many etiologies, uterine atony is the most common and accounts for nearly 70% of cases.3 Patients who experience postpartum hemorrhage can have increased morbidity and mortality, which can be attenuated by identifying patients at risk, early preparation, and increased vigilance.1,4 Risk stratification for postpartum hemorrhage is commonly performed using an assessment tool from one of several organizations, such as the California Maternal Quality Care Collaborative, Association of Women’s Health, Obstetric and Neonatal Nurses, or the American College of Obstetricians and Gynecologists.5–7 Although these risk-assessment tools have the support of major medical societies, recent evidence suggests that the tools have only moderate predictive value for severe hemorrhage in the highest risk groups and that a significant portion of hemorrhages (up to 43%) occur in those deemed low risk.8,9 This limitation may be partly due to the tools’ development via expert consensus opinion and a lack of systematically reviewed evidence to support or refute the included risk factors. A recently published meta-analysis that evaluated the association between maternal demographics and comorbidities, and postpartum hemorrhage evaluated only four potential risk factors and found body mass index, nulliparity, and hypertensive disorders to confer risk of postpartum hemorrhage.11 Additionally, existing tools assess the risk of all postpartum hemorrhage etiologies simultaneously, which may confound attempts at risk prediction because each etiology of postpartum hemorrhage is likely associated with different risk factors. Furthermore, current tools fail to account for the relative contribution of each risk factor or to provide mechanisms to quantify risk for a given patient when more than one risk factor is present. Because each etiology of postpartum hemorrhage likely has a unique set of risk factors, evaluating each etiology separately may improve the ability to delineate individual patient risk. The systematic identification and quantification of risk factors for atonic postpartum hemorrhage may allow the development of more reliable, weighted risk-stratification tools. Thus, this systematic review aimed to identify risk factors that increase the odds of a patient developing postpartum hemorrhage due only to uterine atony after vaginal or cesarean delivery.
SOURCES
This review follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement11 and was registered with the PROSPERO International Prospective Register for Systematic Reviews (registration number CRD42020157521).
The PubMed (National Library of Medicine), CI-NAHL (EBSCO), EMBASE (Ovid), Web of Science (Clarivate), and ClinicalTrials.gov (National Institutes of Health) databases were searched in November 2018 for English language studies with no restrictions on date or geographic location. PubMed MeSH headings included, but were not limited to, postpartum hemorrhage; uterine hemorrhage; uterine inertia; causality; epidemiology; incidence; methylergonovine; misoprostol; oxytocin; prevalence; probability; risk assessment; risk factors; and risk, in addition to corresponding keywords (full search strategies are shown in Appendix 1, available online at http://links.lww.com/AOG/C163). The PubMed search was translated for CINAHL, EMBASE, Web of Science, and clinicaltrials.gov.
STUDY SELECTION
Eligibility for inclusion was limited to randomized clinical trials, prospective or retrospective cohort studies, and case–control studies written in the English language with pregnant patients who developed postpartum uterine atony or atonic postpartum hemorrhage. Definitions of atonic postpartum hemorrhage varied by study and included some combination of clinical diagnoses, second-line uterotonic administration, estimated blood loss, need for transfusion, or International Classification of Diseases codes (Table 1). Studies were excluded if they reported postpartum hemorrhage data without specifying etiology or if they did not report the incidence of at least one risk factor for postpartum hemorrhage in a uterine atony subgroup. Case reports, case series, and unpublished meeting abstracts were excluded. Two blinded authors screened abstracts, and a third blinded author resolved conflicts. Full-text articles were obtained for all included abstracts. Full-text article review was conducted by the same two blinded authors, with conflicts resolved in the same manner. Title, abstract, and full-text screening were performed using the Raayan web application.12
Table 1.
Summary of Article Characteristics
| Author and Year | Study Country | Study Design | Study Years | No. of Patients | Rate of Atonic PPH n (%) | Mode of Delivery |
|---|---|---|---|---|---|---|
| Bateman et al, 20101 | United States | Retrospective cohort | 1995–2004 (2004 data for risk factor analysis) | 876,641 | Atonic PPH: 20,353 (2.3) | VD CD |
| Bateman et al, 201336 | United States | Retrospective cohort | 2000–2007 | 9,750 Calcium channel blocker: 1,226 | Atonic PPH: 201 (2.1) | VD CD |
| Bekabil et al, 201537 | Ethiopia | Prospective cohort | 2012–2013 | 432 Twin: 144 | Atony: 16 (3.7) | VD CD |
| Bryant* et al, 201238 | United States | Retrospective cohort | 2005–2008 | 2,488,974 | Atonic PPH: 53,266 (2.1) | VD CD |
| Callaghan et al, 201039 | United States | Retrospective cohort | 1994–2006 | 10,481,197 | Atonic PPH: 212, 00 (2) | VD CD |
| Chalouhi* etal, 201540 | United States | Retrospective cohort | 2009–2012 | 1,062 | Atony: 87 (8.2) | VD |
| Driessen et al, 201141 | France | Prospective cohort | 2004–2006 | 146,876 | Atonic PPH: 4,550 (3.1) | VD |
| Feerasta et al, 200042 | Pakistan | Retrospective case- control | 1987–1997 | 332 Case: 112, Control: 220 | NA (case-control) | VD |
| Foley et al, 201843 | United States | Retrospective cohort | 2015 | 402 | Atony: 195 (48.5) | CD |
| Definition of Atonic PPH | Risk Factors Assessed | Covariates Adjusted For | Exclusion Criteria |
|---|---|---|---|
| ICD code | Age, mode of delivery, HDP, diabetes, uterine leiomyoma, prior CD, polyhydramnios, chorioamnionitis, precipitous delivery, long labor, medical induction of labor, multiple gestation, stillbirth, antepartum hemorrhage, retained placenta | Logistic regression included all risk factors assessed | Missing data on maternal age |
| ICD code | Calcium channel blocker administration | Age, race, ethnicity, HDP, diabetes, obesity, renal disease, leiomyomas, prior CD, placenta previa, multiple gestation, mode of delivery, induction of labor | Preterm labor or preterm delivery, migraine, arrhythmia, angina, Raynaud, exposure to multiple antihypertensives, out of hospital delivery, no diagnosis of pre-existing or gestational hypertension |
| PPH: undefined Atony: undefined | Multiple gestation | None | None |
| ICD code | Race and ethnicity (African American, Hispanic, Asian or Pacific Islander) | Induction of labor, multiple gestation, polyhydramnios, diabetes, chorioamnionitis, stillbirth, grand multiparity, preeclampsia, obesity, leiomyomas, placental abruption, placenta previa, previous CD, chronic anemia, retained placenta, prolonged labor | Race or ethnicity data missing or coded as a category not included in the study |
| ICD code | Mode of delivery | Age | None |
| PPH: EBL Atony: uterotonic† | Ethnicity (Native American) | None | Multiple gestation, preterm delivery, coagulopathy |
| PPH: EBL or Hgb drop of more than 2 g per dL | Maternal age, BMI, prior PPH, fibroma, hydramnios, parity, multiple gestation, type of onset of labor, epidural analgesia, prolonged labor, oxytocin during labor, prolonged expulsive efforts, gestational age, instrumented VD, prophylactic uterotonics, birth weight | Logistic regression included all risk factors assessed | Incomplete data, CD, PPH of etiology other than atony |
| Atony: uterotonic† or other intervention,‡ case report form to elucidate etiology | |||
| PPH: undefined Atony: undefined† | Parity, stillbirth or neonatal death, prior PPH, prior CD, HDP, antenatal anemia, multiple gestation, history of preterm labor, use of tocolytics, diabetes, polyhydramnios, amnionitis, uterine leiomyomas, induced labor, augmented labor, prolonged labor, mode of delivery, precipitous delivery, epidural, macrosomia | Logistic regression included all risk factors assessed | None |
| PPH: EBL, Hgb drop, transfusion Atony: uterotonic | Predelivery oxytocin exposure | Nulliparity, hypertension, diabetes, multiple gestation, polyhydramnios, premature rupture of membranes, preterm rupture of membranes, prolonged rupture of membranes, placenta previa, placental abruption, chorioamnionitis, macrosomia | General anesthesia, postpartum oxytocin dose not charted |
| Author and Year | Study Country | Study Design | Study Years | No. of Patients | Rate of Atonic PPH n (%) | Mode of Delivery |
|---|---|---|---|---|---|---|
| Grotegut* et al, 201144 | United States | Retrospective case- control | 2000–2004 | 108 Case: 54, control: 54 | NA (case-control) | VD CD |
| Guillaume et al, 201545 | France | Retrospective cohort | 2011–2013 | 7,810 | Atonic PPH: 202 (2.6) | VD |
| Harvey* et al, 201746 | United States | Retrospective cohort | 1995–2013 | 243,693 | Atonic PPH: 8,962 (3.7) | VD CD |
| Joseph et al, 201547 | Canada | Retrospective case- control | 1998–2009 | 138,704 Case: 6,378, control: 31,795 | NA (case-control) | VD CD |
| Kahr et al, 201848 | Switzerland | Prospective cohort | 2015–2016 | 1,487 | Atony: 71 (4.8) | VD CD |
| Kovacheva et al, 201349 | United States | Retrospective cohort | 2009 | 345 | Atony: 35 (10) | CD |
| Lao et al, 201450 | Hong Kong | Retrospective cohort | 1998–2008 | 64,886 | Atonic PPH: 1,194 (1.8) | VD CD |
| Looft et al, 201751 | Sweden | Retrospective cohort | 2008–2014 | 57,267 | Atonic PPH: 2,659 (4.6) | VD |
| Definition of Atonic PPH | Risk Factors Assessed | Covariates Adjusted For | Exclusion Criteria |
|---|---|---|---|
| PPH: ICD+chart review for transfusion | Predelivery oxytocin exposure, race, BMI, admission hematocrit, induction status, preeclampsia, magnesium therapy, chorioamnionitis | Race, BMI, admission hematocrit, induction status, magnesium treatment, chorioamnionitis | None |
| Atony: uterotonic† or other intervention‡ | |||
| PPH: EBL | Cord blood collection | Age, grand multiparity, diabetes, induction of labor, long labor, oxytocin augmentation, prophylactic postpartum oxytocin, instrumented delivery, birth weight, vaginal tearing, placental retention | Multiple gestation, gestational age less than 37 wk |
| Atony: medical team diagnosis | |||
| ICD code | Ethnicity (Native Hawaiians and Other Pacific Islanders, Asian, White) | Age, delivery type, prior CD, smoking, obesity, substance abuse, multiple gestation, multiparity, HDP, diabetes, labor induction, polyhydramnios, chorioamnionitis, placental abruption, placenta previa, prolonged labor | Non-Hawaii residents, records lacking race-ethnicity data, race-ethnicity other than Native Hawaiians and Other Pacific Islanders, Asian, or White |
| ICD code | Maternal medication use (antidepressants, aspirin, nonsteroidal antiinflammatory drugs, beta-agonists, and antihistamines), multiple pregnancies, placenta previa or abruption, polyhydramnios, prolonged labor, preeclampsia or eclampsia, epidural, labor induction, perineal trauma, uterine rupture, mode of delivery, chorioamnionitis, alcohol use disorder, liver disease, thrombocytopenia, asthma | Age, welfare status, rural vs urban residence, prior CD, alcoholism, liver disease, thrombocytopenia, multiple gestation, preeclampsia, polyhydramnios, placenta previa, placental abruption, epidural analgesia, labor induction, prolonged first stage, prolonged second stage, mode of delivery, uterine rupture, cervical laceration, severe perineal tear, chorioamnionitis | None |
| PPH: EBL or Hgb drop Atony: undefined | Blood group O | None | Gestational age less than 22 wk, primary coagulopathy or blood-clotting disorder, no informed consent, maternal age younger than 18 y |
| PPH: Difference between preoperative and postoperative hematocrit Atony: uterotonic† | Serum uric acid level | Uric acid level, use of magnesium, duration of oxytocin, polyhydramnios, oligohydramnios, chorioamnionitis, abnormal placentation, gestational age, birth weight | Multiple gestation, no serum uric acid measured within 24 hours of CD, incomplete medical records, general anesthesia, pre-existing coagulation abnormalities, hyperuricemia before pregnancy |
| PPH: EBL Atony: undefined | Maternal age | None | Maternal age younger than 20 y, gestational age less than 24 wk, multiple gestation, spontaneous miscarriage, therapeutic abortion, incomplete data |
| ICD code | Length of the second stage Length of pushing | Age, height, BMI, smoking, induction of labor, oxytocin use during first stage of labor, gestational age, birth weight | Noncephalic presentation, CD, missing data |
| Author and Year | Study Country | Study Design | Study Years | No. of Patients | Rate of Atonic PPH n (%) | Mode of Delivery |
|---|---|---|---|---|---|---|
| Lutomski et al, 201252 | Ireland | Retrospective cohort | 1999–2009 | 649,019 | Atonic PPH: 12,800 (2.0) | VD CD |
| Marshall* et al, 201753 | United States | Retrospective cohort | 2012–2013 | 1,352,691 | Atonic PPH: 31,549 (2.3) | VD CD |
| Mehrabadi et al, 201354 | Canada | Retrospective cohort | 2001–2010 | 371,193 | Atonic PPH: Total number not reported | VD CD |
| Oberg et al, 201455 | Sweden | Retrospective cohort | 1997–2009 | 538,332 women, 914,939 pregnancies | Atonic PPH: 17,818 (1.9) | VD (atonic PPH data analyzed VD only) |
| Regalia et al, 200156 | Italy | Retrospective cohort | 1995–1999 | 10,756 | Atony: 427 (4.0) | VD |
| Siddiqui* et al, 201757 | United States | Retrospective cohort | 2002–2013 | 21,898,501 | Atonic PPH: 499,189 (2.3) | VD CD |
| Tran et al, 201758 | Canada | Retrospective cohort | 2013–2015 | 490 | Atonic PPH: 80 (16) | CD |
| Vendittelli et al, 201659 | France | Prospective cohort | 2011 | 129,110 | Atonic PPH: 2,490 (1.9) | VD CD |
| Waheed et al, 201360 | Pakistan | Retrospective cohort | 2006–2009 | 8,713 | Atonic PPH: 93 (1.1) | VD CD |
| Definition of Atonic PPH | Risk Factors Assessed | Covariates Adjusted For | Exclusion Criteria |
|---|---|---|---|
| ICD code | Maternal age, hypertension, diabetes, mode of delivery, multiple gestation, large fetus, episiotomy, genital tract trauma, prolonged first stage, prolonged second stage, placental disorders, amniotic cavity infection, polyhydramnios | Age, marital status, socioeconomic status, hypertension, diabetes, mode of delivery, induction of labor, multiple gestation, macrosomia, episiotomy, genital tract trauma, prolonged labor, placenta previa, vasa previa, placental abruption, chorioamnionitis, polyhydramnios | None |
| ICD code | Race, parity | None | Incomplete data |
| ICD code | Maternal age, parity, birthweight, BMI, gestational age, smoking status, multiple gestation, mode of delivery, prior CD, epidural analgesia, induction, oxytocin augmentation, uterine rupture, third or fourth degree perineal tear, high vaginal laceration, cervical laceration, placenta previa, placental abruption, breech, transverse lie, polyhydramnios, prolonged first stage, prolonged second stage, preeclampsia, chorioamnionitis, forceps, vacuum, forceps and vacuum | Logistic regression included all risk factors assessed | Incomplete data |
| ICD code | History of PPH according to type | Year of birth, age, civil status, country of origin, chronic hypertension, diabetes, coagulopathy, leiomyomas | Multiple gestation |
| PPH: undefined | Maternal age, BMI, gestational age, parity, fetal weight, prior atony, prior CD or uterine scar, uterine leiomyomas, preeclampsia, fever in labor, hydramnios, labor induction, labor velocity of progress, use of Kristeller maneuvers, nonvertex cephalic presentation | Logistic regression included all risk factors assessed | Multiple gestation, placental risk factors for PPH, gestational age less than 35 wk, CD, incomplete data |
| Atony: undefined | |||
| ICD code | Ethnicity | None | Ethnicity other than Caucasian or Asian American and Pacific Islander |
| PPH: EBL and drop in hct | Length of the oxytocin recovery period, BMI | Preeclampsia, chorioamnionitis, morbid obesity, macrosomia, multiple gestation, polyhydramnios, oxytocin induction, dose and duration of oxytocin augmentation | Any mode of delivery other than intrapartum CD for labor arrest, women not receiving 2h or more of oxytocin augmentation during labor, gestational age less than 37 or more than 41 wk, PPH cause other than atony, incomplete data, general anesthesia |
| Atony: uterotonic† or other intervention‡ | |||
| PPH: EBL | Mode of delivery | None | None |
| Atony: undefined | |||
| PPH: EBL or change in vital signs | Mode of delivery | None | None |
| Atony: medical team diagnosis, uterine massage, uterotonic† |
| Author and Year | Study Country | Study Design | Study Years | No. of Patients | Rate of Atonic PPH n (%) | Mode of Delivery |
|---|---|---|---|---|---|---|
| Wetta* et al, 201361 | United States | Secondary analysis of randomized controlled trial | 2008–2010 | 1,798 | Atony: 118 (6.6) | VD |
| Definition of Atonic PPH | Risk Factors Assessed | Covariates Adjusted For | Exclusion Criteria |
|---|---|---|---|
| PPH: EBL Atony: medical team diagnosis | Age, oxytocin dose, BMI, ethnicity, race, augmentation, induction, birthweight, parity, preeclampsia, magnesium use, twin gestation, chorioamnionitis, hydramnios, amnioinfusion, epidural anesthesia, breastfeeding, artificial rupture of membranes | Logistic regression included all risk factors assessed | Gestational age less than 24 wk, CD, fetal demise, pulmonary edema, coagulopathy, cardiomyopathy |
PPH, postpartum hemorrhage; VD, vaginal delivery; CD, cesarean delivery; ICD, International Classification of Diseases; HDP, hypertensive disorders of pregnancy; EBL, estimated blood loss; Hgb, hemoglobin; BMI, body mass index; hct, hematocrit; NA, not applicable.
Article evaluates race or ethnicity as risk factor for atonic postpartum hemorrhage. Additional information regarding classifications of race or ethnicity used, who classified patients, whether options were defined by participants or investigators, reasons race or ethnicity were assessed, missing data, and “other” categories is presented in Appendix 4, http://links.lww.com/AOG/C163.
Uterotonic administration was in addition to standard oxytocin therapy.
“Other interventions” included the use of a Bakri balloon, uterine massage, uterine artery embolization, or surgical interventions.
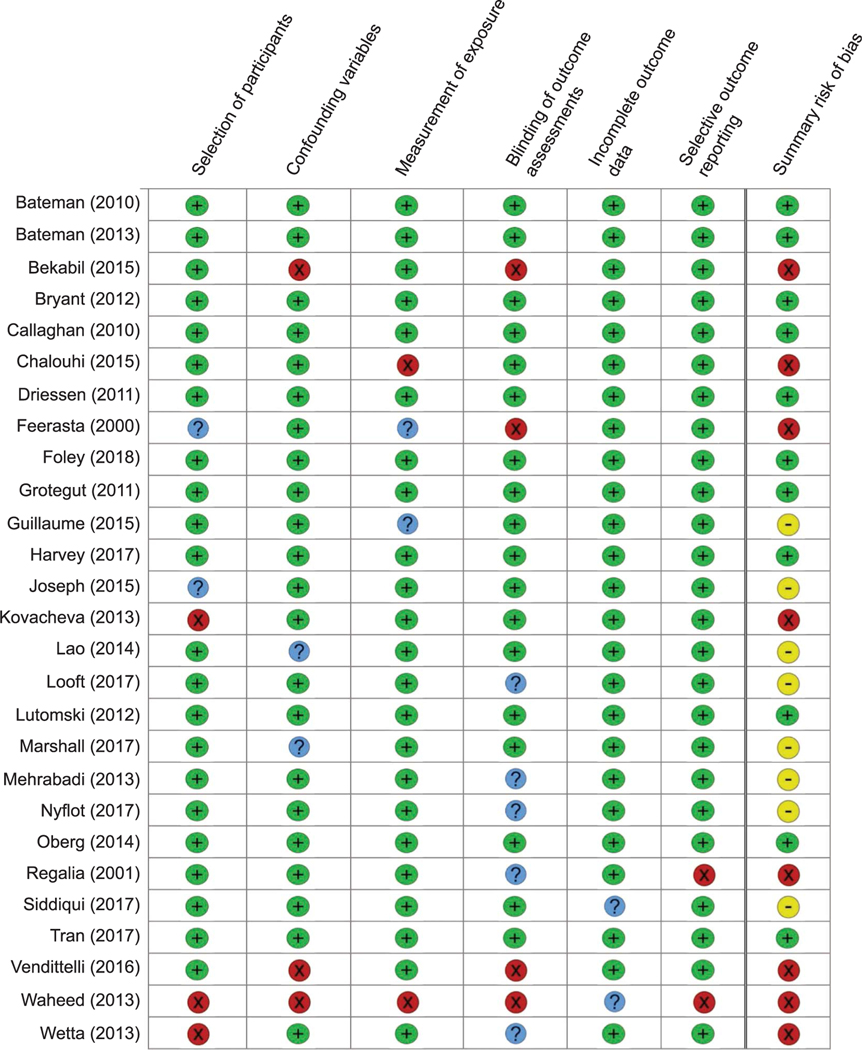
The risk of bias for each included study was assessed using the Risk of Bias Assessment Tool for Nonrandomized Studies.13 This tool was chosen because of the nonrandomized nature of all included studies as well as its scope in assessing multiple sources of bias, including those related to selection, performance, detection, attrition, and reporting. Six domains of risk of bias were assessed, including: 1) selection of participants, 2) confounding variables, 3) measurement of exposure, 4) blinding of outcome assessments, 5) incomplete outcome data, and 6) selective outcome reporting. Bias scoring proceeded after training each scorer on the assessment tool to ensure good inter-rater reliability correlation. Two authors (D.H.C., H.B.E.) independently assessed the risk of bias for each study, with disagreements resolved by a third blinded author (J.R.B.). Studies were found to have a low risk of bias if they attained a low-risk classification in all six evaluated domains. Studies were considered a moderate risk of bias if at least one domain was rated unclear risk (but no domains were rated high risk). Finally, studies were found to have a high risk of bias if at least one domain was rated high risk.
All data were collected independently by co-investigators into a study spreadsheet and verified by a separate author. In addition to baseline study characteristics including country of origin, study design, the number of patients, and inclusion and exclusion criteria, data were collected on all risk factors for atonic postpartum hemorrhage reported in the study. For each risk factor, adjusted or unadjusted odds ratios (aOR, uOR), relative risks (aRR, uRR), or rate ratios (arr, urr) were recorded when available. If not explicitly reported, uOR and 95% CI were calculated with a 2×2 table using the number of patients with and without a given risk factor who developed atonic postpartum hemorrhage.14 The primary outcome assessed in all studies was the OR, RR, or rr of risk factors associated with atonic postpartum hemorrhage. For each identified risk factor, low and moderate risk of bias studies were synthesized qualitatively to label each risk factor as definite, likely, unclear, or not a risk factor based on the number of total studies evaluating the factor and percentage of studies showing a positive association (Box 1). For risk factors with sufficiently homogeneous definitions and reference ranges, a quantitative meta-analysis of low and moderate risk of bias studies was implemented to estimate a combined OR. The inverse variance random effects method was used for meta-analysis.15 This method requires only effect estimates (ie, OR or risk ratios) and their SEs. The SEs were estimated by backtransforming the 95% confidence limits using the standard normal distribution. The DerSimonian-Laird estimator was used to estimate the between-study variance in effects.16 Owing to the rarity of uterine atony (and, thus, the near-equivalence of the OR and RR), studies that reported a risk ratio were included. Adjusted and unadjusted risk ratios and ORs were included. The I2 statistic and a P-value against the null hypothesis (ie, I250) are reported for each subgroup. The I2 statistic represents the percentage of variability in the effect estimates that is attributable to study heterogeneity (compared with sampling heterogeneity). Meta-analysis was implemented in R 3.6.3 (https://www.r-project.org), using the “metafor” 2.4 add-on package.
Box 1. Defining the Strength of a Risk Factor.
Definite
All low and moderate risk of bias studies positive (at least three studies)
Majority (more than 50%) low and moderate risk of bias studies positive (at least five studies)
Likely
All low and moderate risk of bias studies positive (two studies)
Majority (more than 50%) low and moderate risk of bias studies positive (2–4 studies)
Unclear
All low and moderate risk of bias studies positive (one study)
Low and moderate risk of bias studies show mixed or conflicting results
A majority (more than 50%) of studies negative but at least one low or moderate risk of bias study positive
Not a risk factor
No low or moderate risk of bias studies positive
RESULTS
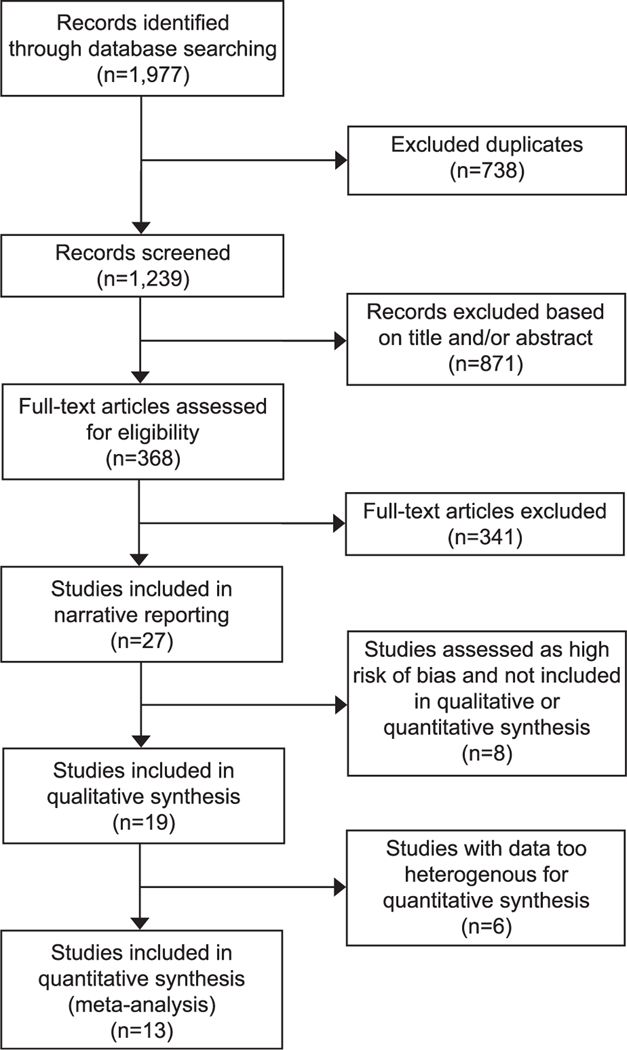
The database search revealed 1,977 total records, and 1,239 records were screened after removing duplicates. After excluding 871 records based on title and abstract review, 368 full-text articles were assessed for eligibility (Fig. 1). Twenty-seven studies reported data for at least one risk factor for atonic postpartum hemorrhage and were included in the narrative summary. Of those, 19 studies contributed data to the qualitative synthesis and 13 to the meta-analysis. Table 1 summarizes the study design, patient population, study definitions of postpartum hemorrhage and uterine atony, and the risk factors assessed in the included studies. The scoring for risk of bias for each study is reported in Figure 2.
Fig. 1.
Flow diagram of studies identified for systematic review.
Ende. Atonic Hemorrhage Risk Systematic Review. Obstet Gynecol 2021.
Fig. 2.
Risk of bias assessment. Risk of bias assessments were performed using the Risk of Bias Assessment Tool for Nonrandomized Studies.13 Green (+) indicates low risk of bias, blue (?) indicates unclear risk of bias, and red (X) indicates high risk of bias. For the summary of risk of bias, studies were considered overall low risk of bias (green +) if they were classified as low risk in all six domains. Studies were considered a moderate risk of bias (yellow —) if at least one domain was rated unclear risk (but no domains were rated high risk) and high risk (red X) if at least one domain was rated high risk.
Ende. Atonic Hemorrhage Risk Systematic Review. Obstet Gynecol 2021.
A total of 47 unique risk factors were identified in the search: 15 relating to maternal history or demographics, 11 to maternal comorbidities, six pregnancy-related factors, eight labor-related factors, and seven delivery-related factors. For qualitative comparison, the authors characterized each risk factor as definite, likely, unclear, or not a risk factor based on the number of low and moderate risk of bias studies that showed statistically significant evidence that the risk factor was associated with atony (Box 1).
Six variables were found to be definite risk factors for atonic postpartum hemorrhage, based on either all low and moderate risk of bias studies showing a positive association (if at least three studies) or the majority of low and moderate risk of bias studies showing a positive association (if at least five studies). Definite risk factors included being of Asian race, a history of prior postpartum hemorrhage of any etiology in a previous pregnancy, preexisting or gestational diabetes mellitus, placental disorders (including retained placenta, placenta previa, vasa previa, and placental abruption, but excluding abnormal placentation), prolonged labor (as defined in each study and presented in Appendix 2, available online at http://links.lww.com/AOG/C163), and genital tract trauma sustained during delivery. An additional nine variables were deemed likely associated with atonic postpartum hemorrhage, and these included being of Hispanic ethnicity, nulliparity, hypertensive diseases of pregnancy, multiple gestation, chorioamnionitis, uterine rupture, predelivery oxytocin exposure, induction of labor, and instrumented vaginal delivery. Some variables that are traditionally considered risk factors for postpartum hemorrhage were not found to be associated specifically with atonic postpartum hemorrhage in this review but may confer risk for hemorrhage due to other etiologies. These included maternal obesity, leiomyomas, polyhydramnios, prolonged second stage of labor, magnesium exposure, and cesarean delivery. Meta-analysis was implemented for 24 risk factors with at least two low or moderate risk of bias studies demonstrating homogeneous risk factor definitions and reference ranges (Figs. 3–7). Table 2 displays a summary of qualitative and quantitative (where applicable) results for each risk factor. Appendix 2 (http://links.lww.com/AOG/C163), includes a complete list of articles that report on each risk factor and the statistical associations reported, therein. A narrative summary of all risk factors, with the explanations of qualitative and quantitative synthesis results for each factor can be found in Appendix 3, available online at http://links.lww.com/AOG/C163. Further details on studies reporting on race or ethnicity as risk factors for atonic postpartum hemorrhage, including who classified each patient’s race, classifications used, and data on missing data and “other” classifications, can be found in Appendix 4, available online at http://links.lww.com/AOG/C163.
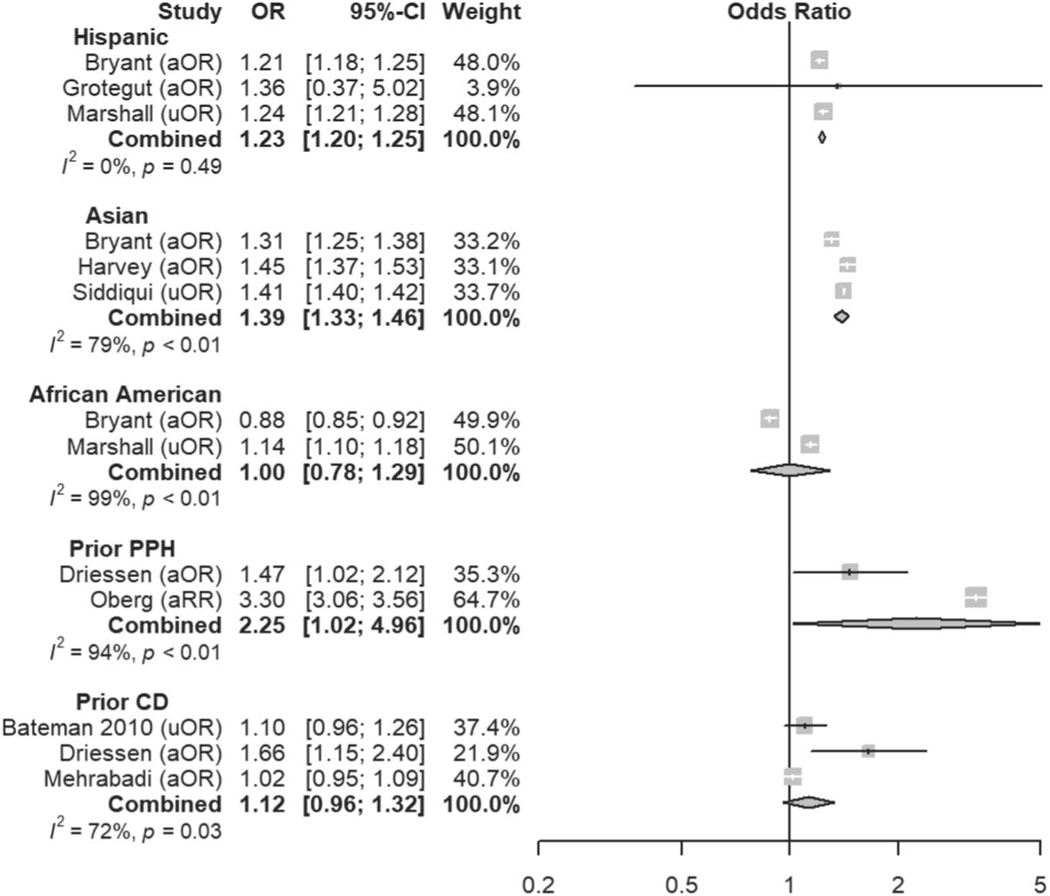
Fig. 3.
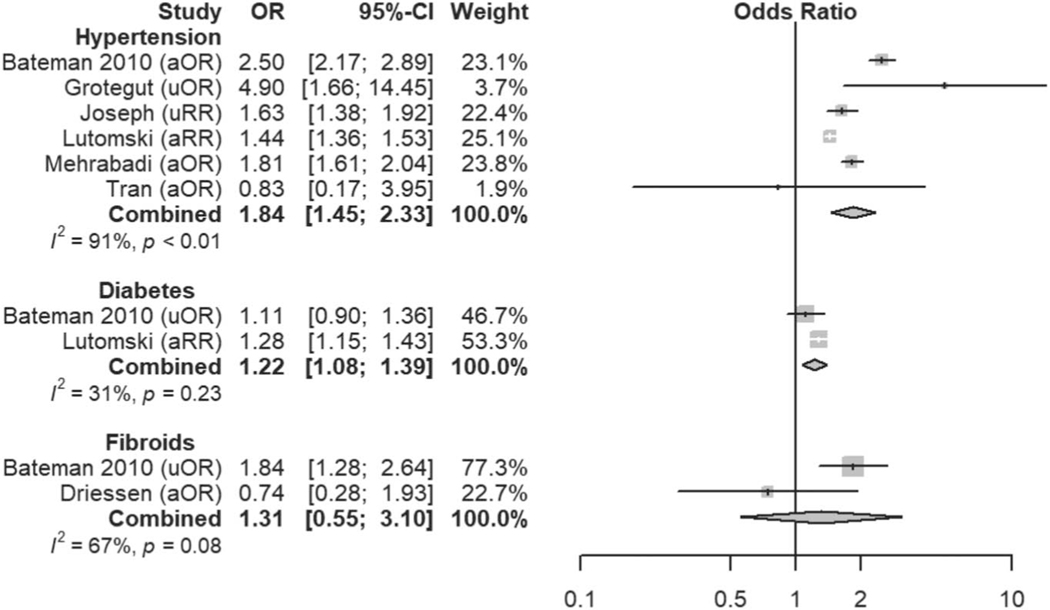
Meta-analysis of history and demographic risk factors. Forest plots of odds ratios (ORs) that were included in the quantitative meta-analysis and the associated overall ORs. For each OR, the size of the gray square region is proportional to the corresponding study weight. Diamond-shaped intervals represent the overall ORs. I2 represents the fraction of variability among the individual ORs that cannot be explained by sampling variability. aOR, adjusted odds ratio; uOR, unadjusted odds ratio; aRR, adjusted relative risk; PPH, postpartum hemorrhage; CD, cesarean delivery.
Ende. Atonic Hemorrhage Risk Systematic Review. Obstet Gynecol 2021.
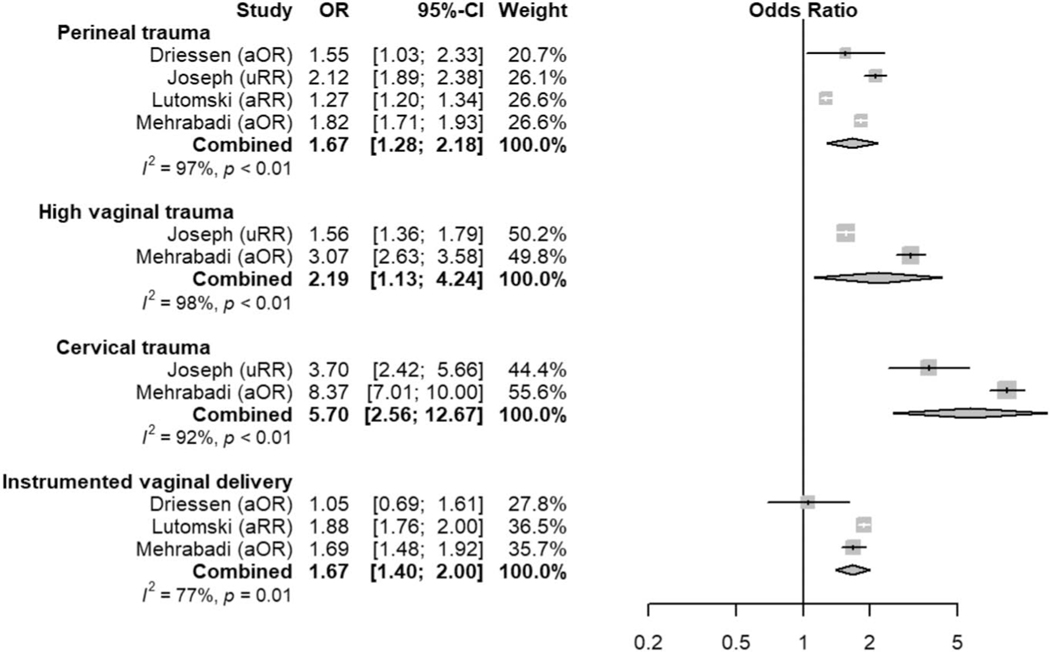
Fig. 7.
Meta-analysis of delivery-related risk factors. Forest plots of odds ratios (ORs) that were included in the quantitative meta-analysis and the associated overall ORs. For each OR, the size of the gray square region is proportional to the corresponding study weight. Diamond-shaped intervals represent the overall ORs. I2 represents the fraction of variability among the individual ORs that cannot be explained by sampling variability. aOR, adjusted odds ratio; aRR, adjusted relative risk; uRR, unadjusted relative risk.
Ende. Atonic Hemorrhage Risk Systematic Review. Obstet Gynecol 2021.
Table 2.
Summary of Qualitative and Quantitative Results for Assessed Risk Factors
| Risk Factors | Qualitative Synthesis Result | Meta-Analysis Result [OR (95% CI)] |
|---|---|---|
| History and demographic | ||
| Young age | Unclear | Not applicable |
| Old age | Unclear | Not applicable |
| Hispanic | Likely | 1.23 (1.20–1.25) |
| Asian | Definite | 1.39 (1.33–1.46) |
| Native American | Not | Not applicable |
| Black or African American | Unclear | 1.00 (0.78–1.29) |
| Nulliparity | Likely | Not applicable |
| Prior postpartum hemorrhage | Definite | 2.25 (1.02–4.96) |
| Prior cesarean delivery | Unclear | 1.12 (0.96–1.32) |
| Blood group O | Not | Not applicable |
| Calcium channel blocker exposure | Not | Not applicable |
| Antidepressant exposure | Unclear | Not applicable |
| Aspirin exposure | Not | Not applicable |
| Nonsteroidal antiinflammatory drug exposure | Not | Not applicable |
| Doxylamine exposure | Not | Not applicable |
| Maternal comorbidity | ||
| Hypertension | Likely | 1.84 (1.45–2.33) |
| Diabetes | Definite | 1.22 (1.08–1.39) |
| Anemia | Unclear | Not applicable |
| Obesity | Not | Not applicable |
| Leiomyomas | Unclear | 1.31 (0.55–3.10) |
| Liver disease | Not | Not applicable |
| Thrombocytopenia | Not | Not applicable |
| Asthma | Not | Not applicable |
| Elevated uric acid | Unclear | Not applicable |
| Alcohol use disorder | Not | Not applicable |
| Smoking | Not | Not applicable |
| Pregnancy-related | ||
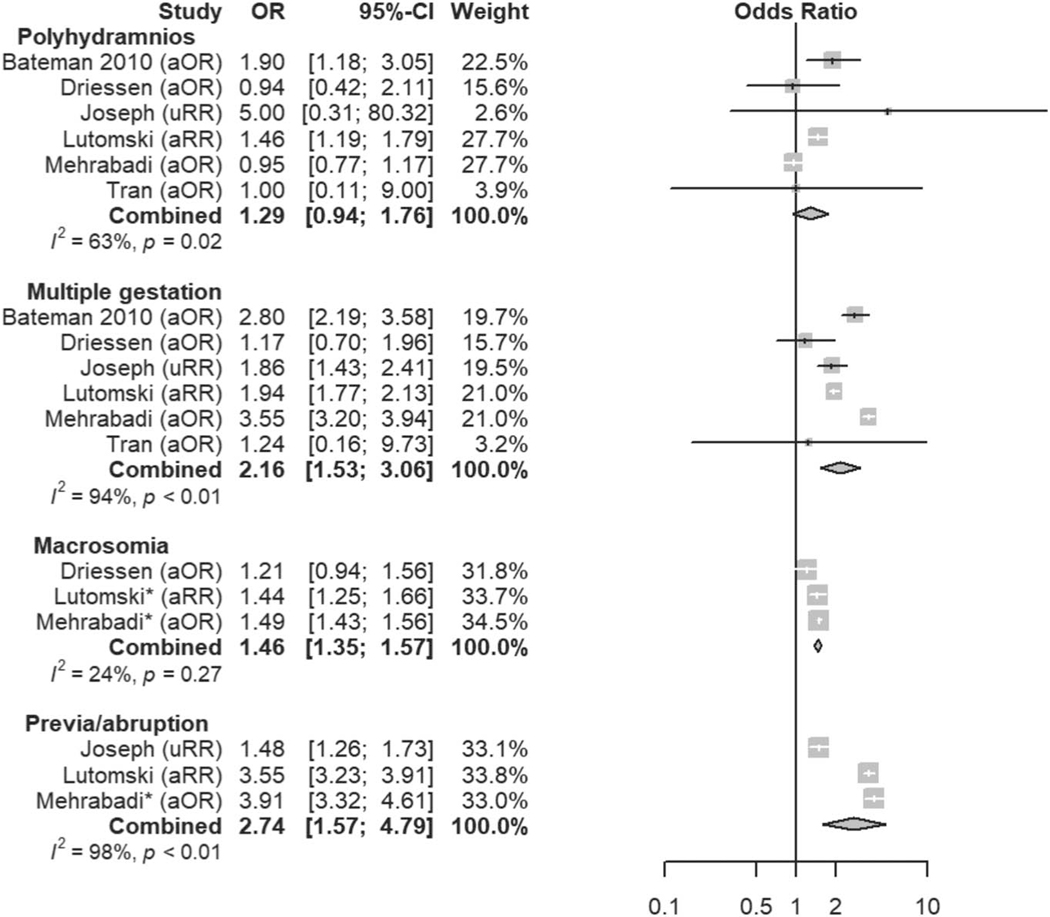
| Polyhydramnios | Unclear | 1.29 (0.94–1.76) |
| Multiple gestation | Likely | 2.16 (1.53–3.06) |
| Malpresentation | Unclear | Not applicable |
| Macrosomia | Unclear | 1.46 (1.35–1.57) (more than 4,000 g) |
| Placental disorders (previa or abruption) | Definite | 2.74 (1.57–4.79) |
| Antepartum hemorrhage | Unclear | Not applicable |
| Stillbirth | Unclear | Not applicable |
| Labor-related | ||
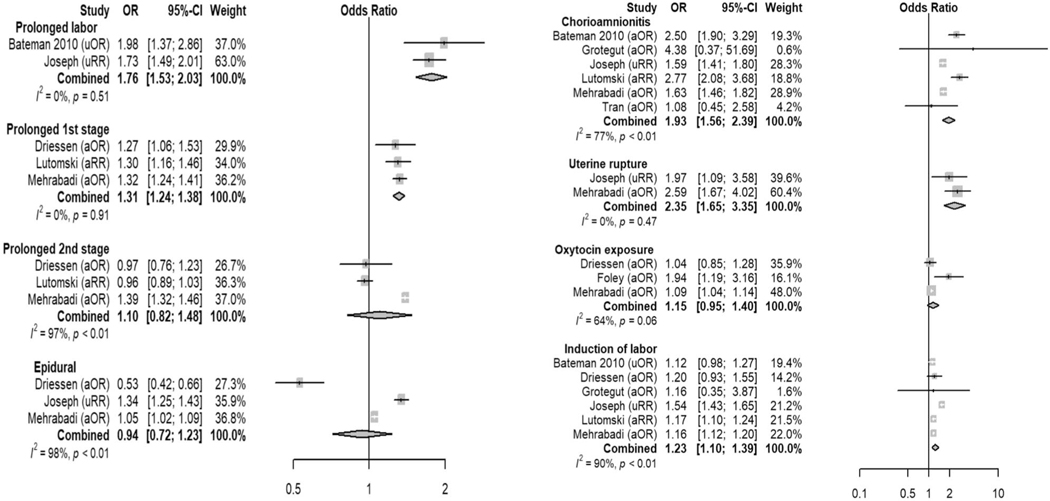
| Chorioamnionitis | Likely | 1.93 (1.56–2.39) |
| Uterine rupture | Likely | 2.35 (1.65–3.35) |
| Predelivery oxytocin exposure | Likely | 1.15 (0.95–1.40) |
| Induction of labor | Likely | 1.23 (1.10–1.39) |
| Prolonged labor | Definite | 1.76 (1.53–2.03) (all labor) |
| 1.31 (1.24–1.38) (1st stage) | ||
| 1.10 (0.82–1.48) (2nd stage) | ||
| Epidural | Unclear | 0.94 (0.72–1.23) |
| Magnesium exposure | Not | Not applicable |
| Tocolytic exposure | Not | Not applicable |
| Delivery-related | ||
| Gestational age | Not | Not applicable |
| Genital tract trauma | Definite | 1.67 (1.28–2.18) (perineal laceration) |
| 2.19 (1.13–4.24) (high vaginal laceration) | ||
| 5.70 (2.56–12.67) (cervical laceration) | ||
| Instrumented vaginal delivery | Likely | 1.67 (1.40–2.00) |
| Cesarean delivery | Unclear | Not applicable |
| Cord blood collection | Not | Not applicable |
| Breastfeeding | Not | Not applicable |
OR, odds ratio.
DISCUSSION
This systematic review highlights several previously unrecognized risk factors for atonic postpartum hemorrhage that are not included in current risk-assessment tools, and it questions whether other risk factors used in current tools are, in fact, supported by published evidence. This review also provides further evidence supporting the validity of many risk factors already included in risk-stratification tools and provides quantitative estimates of their contribution to atonic postpartum hemorrhage risk. Although some risk factors presented may cause postpartum hemorrhage independently, for example genital tract trauma or abnormal placentation, these factors also appear to contribute to uterine atony, and, thus, are captured in this review.
Perhaps most importantly, current risk-assessment tools fail to include some definite or likely risk factors that appear to be well-established in the literature. Hispanic ethnicity (OR 1.23, 95% CI 1.20–1.25) and Asian race (OR 1.39, 95% 1.33–1.46) were associated with atonic postpartum hemorrhage in this review and are missing from current risk-assessment tools. The ORs for ethnicity and race are comparable with those for commonly cited risk factors, including induction of labor (OR 1.23, 95% 1.10–1.39) and macrosomia (OR 1.46, 95% 1.35–1.57). Given the mounting evidence of disparities in the care of minority women, we may be failing to anticipate the increased risk of postpartum hemorrhage in these populations.17 These associations do not imply causation, and it is unclear whether the underlying mechanism of these relationships are biological, social, or a byproduct of disparities or systematic racism.18 Specific attention must be paid when including race or ethnicity in risk-prediction systems, because recent evidence suggests that these algorithms may perpetuate health care disparities.19,20
Hypertensive disease (OR 1.84, 95% 1.45–2.33) and diabetes mellitus (OR 1.22, 95% 1.08–1.39), both of which are known to be associated with vascular and perfusion abnormalities, also emerged as previously underappreciated risk factors for atonic postpartum hemorrhage.21–23 We hypothesize that the vascular pathophysiology of these diseases may contribute directly to the development of atony and hemorrhage; however, genetic susceptibility to both atony and hypertension or diabetes in specific patient populations may also explain this association and certainly warrants further investigation.24,25 Interestingly, high vaginal lacerations and cervical trauma are not included in current risk-assessment tools. However, high vaginal and cervical lacerations demonstrated higher OR than either instrumented delivery or perineal trauma, both of which are included in the Association of Women’s Health, Obstetric and Neonatal Nurses’ risk-stratification tool.6 Although nulliparity and prolonged first stage of labor were found to be associated with atonic postpartum hemorrhage and are not included in the current risk-assessment tools, they may be associated with other risk factors that are included, such as induction of labor, oxytocin use, and chorioamnionitis. Of these factors, chorioamnionitis demonstrated the highest OR of 1.93 (95% CI 1.56–2.39) and may contribute significantly to an increased risk associated with nulliparity or prolonged first stage of labor. Finally, uterine rupture was found to be associated with atonic postpartum hemorrhage. Although not specifically described in the studies that reported this association, hemorrhage attributable to uterine atony presumably occurred after repair of the uterine defect.
Additionally, some risk factors currently included in risk-assessment tools are not supported by this literature review of atonic postpartum hemorrhage, although an association with other etiologies of postpartum hemorrhage would not be captured by this study. Prolonged second stage of labor (which is included in nearly all risk-assessment tools) did not emerge as a definite risk factor for atonic postpartum hemorrhage in our systematic review and meta-analysis, with combined OR 1.10 (95% CI 0.82–1.48). Polyhydramnios did not emerge as a risk factor for atonic postpartum hemorrhage in quantitative analysis, with combined OR 1.29 (95% CI 0.94–1.76), despite the historical identification of polyhydramnios as a cause of atonic postpartum hemorrhage due to uterine overdistention. Macrosomia and multiple gestation, which are implicated by a similar mechanism, did demonstrate increased risk with combined OR of 1.46 (95% CI 1.35–1.57) and 2.16 (95% CI 1.53–3.06), respectively. Uterine overdistention alone, however, may not fully explain the variability in the risk of atony that is observed between these three conditions. For example, multiple gestation pregnancies are frequently due to in vitro fertilization, which has been associated with increased risk of postpartum hemorrhage possibly due to abnormalities of placental implantation and endometrial function.26 Additionally, there may be some underlying immunologic, vascular, or genetic pathophysiology that overlaps with infertility and atonic postpartum hemorrhage.27–30
Obesity and magnesium exposure are additional factors included in most risk-assessment tools; however, neither showed associations with atonic postpartum hemorrhage in this review. It is possible that the underlying conditions (ie, diabetes mellitus, hypertension) are the real risk factors, as discussed above, leaving the clinical impression that obesity and magnesium increase postpartum hemorrhage risk and leading some studies to find a positive effect. Given the retrospective nature of most included studies, however, magnesium exposure and dosing may have been inadequately captured, hindering the ability to demonstrate an association with atonic postpartum hemorrhage.
Cesarean delivery and uterine leiomyomas are included in the risk-assessment tools but were not found to be associated with atonic postpartum hemorrhage by either qualitative or quantitative analysis. Because intrapartum cesarean delivery is associated with factors (eg, prolonged labor and oxytocin exposure) shown to increase the risk of atonic postpartum hemorrhage, this may contribute to a positive association in studies or a clinical impression that cesarean delivery is significantly associated with atonic postpartum hemorrhage. The occurrence of leiomyomas varies significantly in size, location, vascularity, and previous surgical interventions, which may influence the ability to determine the significance of leiomyomas as a risk factor in published studies.
Finally, many risk factors already included in widely adopted risk-assessment tools are further confirmed in this systematic review as associated with atonic postpartum hemorrhage—prior postpartum hemorrhage, multiple gestation, placental disorders (previa or abruption), chorioamnionitis, predelivery oxytocin exposure, induction of labor, prolonged labor, perineal trauma, and instrumented vaginal delivery. Although some of these factors (eg, abnormal placentation, birth canal trauma) are etiologies for postpartum hemorrhage themselves, the evidence presented here suggests that they are additionally associated with the development of uterine atony. This evidence further supports the inclusion of these factors when stratifying risk, and our data may help health care professionals improve tools by assigning a quantitative weight to risk factors with a stronger association with atonic postpartum hemorrhage. Additionally, current tools likely can be further improved by more nuanced definitions of some factors shown to have a robust association with atonic postpartum hemorrhage. For example, further delineation of genital tract trauma may be warranted; although episiotomy or perineal tear (OR 1.67, 95% 1.28–2.18) is included in some current risk-assessment tools, high vaginal laceration and cervical laceration are not (OR 2.19, 95% 1.13–4.24, and 5.70, 95% 2.56–12.67, respectively).
The data for oxytocin exposure, which also are already included in current risk-assessment tools, presented the most significant challenge in the interpretation of results for this systematic review. Although qualitative synthesis suggested an association between predelivery oxytocin exposure and atonic postpartum hemorrhage, the meta-analysis failed to confirm the association (combined OR 1.15, 95% 0.95–1.40). Importantly, two low risk of bias studies were not included in the meta-analysis because of heterogeneity in defining the exposure and reference ranges (eg, evaluating oxytocin per unit dose). With conflicting results, it is essential also to consider the mechanism by which oxytocin likely contributes to atonic postpartum hemorrhage, namely that tachyphylaxis to oxytocin occurs during labor, thereby decreasing efficacy of the primary drug used for the active management the third stage of labor.31–33 Further investigation is needed to determine the cut-offs for the amount or duration of oxytocin that leads to an increased risk of atony.
Although the risk-assessment tools may be refined by adding, keeping or removing risk factors, the individual weights and whether each individually or in combination defines an individual patient’s postpartum hemorrhage risk as moderate or high remains undetermined. When preparing and allocating resources for potential postpartum hemorrhage, the use of risk scores that weigh the variable contribution of risk factors may be preferable to the use of tools that merely collate a list of diagnoses with many associated or overlapping risk factors. To this end, recent headway has been made in using statistical modeling and machine learning to predict postpartum hemorrhage.34 Notably, however, a substantial proportion of postpartum hemorrhage occurs in the absence of recognized risk factors.
The strengths of this systematic review include the systematic approach to identifying all publications that included risk factors for atonic postpartum hemorrhage and the division of risk factors into maternal, pregnancy-related, labor-related, and delivery-related conditions to provide a logical progression of possible etiologies of atonic postpartum hemorrhage. Finally, we evaluated each variable, both qualitatively and quantitatively, to summarize a large number of studies in a comprehensive review. The results of this systematic review and meta-analysis, however, must be considered in the context of several limitations. First, we had to accept the authors’ definitions of atonic postpartum hemorrhage, which varied from study to study. The most common definitions in large database studies were rather consistent, using International Classification of Diseases codes for postpartum hemorrhage due to atony. The variability occurred primarily in the smaller studies, which used definitions of postpartum hemorrhage that included estimated blood loss, postpartum hemoglobin or hematocrit drop, and clinical diagnosis, with additional definitions for the diagnosis of atony including uterotonic administration, medical team diagnosis, or other surgical interventions. These definitions represent a spectrum of pathology, and each study’s definition may have affected the magnitude of associations of any given risk factor. Second, because all authors were fluent only in English, we restricted our search to English-language studies, which contributed to publication bias. Third, for thoroughness we did include in the narrative summary studies with a high risk of bias; however, we included only studies with a low or moderate risk of bias when evaluating each risk factor, both qualitatively and quantitatively. Fourth, the types of effect estimates reported and the factors used to adjust for potential confounding were heterogeneous across studies. Additionally, nearly all reported ORs and RRs reflect weak associations (range 1–2), which are likely below the discriminatory ability of the included cohort and case–control studies. These observations may result from unmeasured bias or confounding.35 Lastly, to summarize risk factors qualitatively, we had to provide a unique definition to categorize a risk factor as definite, likely, or unclear. Although these definitions were created de novo for this review, a post hoc evaluation revealed that for those variables designated definite risk factors, all (six of six) showed statistically significant combined OR. Of the variables designated likely, eight of nine were amenable to meta-analysis, and seven showed statistically significant increased odds of atonic postpartum hemorrhage. Finally, 15 variables were classified as unclear risk factors, with only six of those demonstrating data homogeneous enough for meta-analysis, and of those, only one (macrosomia) demonstrated a statistically significant OR.
In summary, this systematic review and meta-analysis of risk factors for postpartum hemorrhage due to uterine atony provides critical insights to help guide future obstetric care. By more narrowly defining our focus to only atonic postpartum hemorrhage, we aimed to provide more definitive evidence supporting or refuting presumed risk factors, given that each etiology of postpartum hemorrhage likely has a unique set of contributing factors. These findings should lead researchers and clinicians to refine current risk-assessment tools further. Future tools should include weighted values for risk factors or modeling risk levels by the etiology of postpartum hemorrhage. Finally, the genetic basis for postpartum hemorrhage warrants further investigation and eventual inclusion into risk-assessment tools.
Supplementary Material
Fig. 4.
Meta-analysis of maternal comorbidity risk factors. Forest plots of odds ratios (ORs) that were included in the quantitative meta-analysis and the associated overall ORs. For each OR, the size of the gray square region is proportional to the corresponding study weight. Diamond-shaped intervals represent the overall ORs. I2 represents the fraction of variability among the individual ORs that cannot be explained by sampling variability. aOR, adjusted odds ratio; uOR, unadjusted odds ratio; aRR, adjusted relative risk; uRR, unadjusted relative risk.
Ende. Atonic Hemorrhage Risk Systematic Review. Obstet Gynecol 2021.
Fig. 5.
Meta-analysis of pregnancy-related risk factors. Forest plots of odds ratios (ORs) that were included in the quantitative meta-analysis and the associated overall ORs. For each OR, the size of the gray square region is proportional to the corresponding study weight. Diamond-shaped intervals represent the overall ORs. I2 represents the fraction of variability among the individual ORs that cannot be explained by sampling variability. aOR, adjusted odds ratio; aRR, adjusted relative risk; uRR, unadjusted relative risk.
Ende. Atonic Hemorrhage Risk Systematic Review. Obstet Gynecol 2021.
Fig. 6.
Meta-analysis of labor-related risk factors. Forest plots of odds ratios (ORs) that were included in the quantitative meta-analysis and the associated overall ORs. For each OR, the size of the gray square region is proportional to the corresponding study weight. Diamond-shaped intervals represent the overall ORs. I2 represents the fraction of variability among the individual ORs that cannot be explained by sampling variability. aOR, adjusted odds ratio; uOR, unadjusted odds ratio; aRR, adjusted relative risk; uRR, unadjusted relative risk.
Ende. Atonic Hemorrhage Risk Systematic Review. Obstet Gynecol 2021.
Acknowledgments
Sarah S. Osmundson is supported by grant number K23DA047476 from the National Institute of Drug Abuse.
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg 2010;110:1368–73. doi: 10.1213/ANE.0b013e3181d74898 [DOI] [PubMed] [Google Scholar]
- 2.Sosa CG, Althabe F, Belizán JM, Buekens P. Risk factors for postpartum hemorrhage in vaginal deliveries in a LatinAmerican population. Obstet Gynecol 2009;113:1313–9. doi: 10.1097/AOG.0b013e3181a66b05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oyelese Y, Scorza WE, Mastrolia R, Smulian JC. Postpartum hemorrhage. Obstet Gynecol Clin North Am 2007;34:421–41, x. doi: 10.1016/j.ogc.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 4.Girard T, Mörtl M, Schlembach D. New approaches to obstetric hemorrhage: the postpartum hemorrhage consensus algorithm. Curr Opin Anaesthesiol 2014;27:267–74. doi: 10.1097/ACO.0000000000000081 [DOI] [PubMed] [Google Scholar]
- 5.Bingham D, Melsop K, Main E. CMQCC Obstetric hemorrhage hospital level implementation guide. Stanford University; 2010 [Google Scholar]
- 6.The AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. Accessed June 8, 2020. https://mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH_Risk_Assessment_Table-7-17-15.pdf
- 7.American College of Obstetricians and Gynecologists. Maternal safety bundle for obstetric hemorrhage. Accessed May 26, 2020. https://www.acog.org/community/districts-and-sections/district-ii/programs-and-resources/safe-motherhood-initiative/obstetric-hemorrhage
- 8.Kawakita T, Mokhtari N, Huang JC, Landy HJ. Evaluation of risk-assessment tools for severe postpartum hemorrhage in women undergoing cesarean delivery. Obstet Gynecol 2019; 134:1308–16. doi: 10.1097/AOG.0000000000003574 [DOI] [PubMed] [Google Scholar]
- 9.Dilla AJ, Waters JH, Yazer MH. Clinical validation of risk stratification criteria for peripartum hemorrhage. Obstet Gynecol 2013;122:120–6. doi: 10.1097/AOG.0b013e3182941c78 [DOI] [PubMed] [Google Scholar]
- 10.Durmaz A, Komurcu N. Relationship between maternal characteristics and postpartum hemorrhage: a meta-analysis study. J Nurs Res 2018;26:362–72. doi: 10.1097/jnr.0000000000000245 [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 12.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013;66:408–14. doi: 10.1016/j.jclinepi.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 14.Morris JA, Gardner MJ. Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J (Clin Res Ed) 1988;296:1313–6. doi: 10.1136/bmj.296.6632.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97–111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17.Leonard SA, Main EK, Scott KA, Profit J, Carmichael SL Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol 2019;33:30–6. doi: 10.1016/j.annepidem.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan JB, Bennett T. Use of race and ethnicity in biomedical publication. JAMA 2003;289:2709–16. doi: 10.1001/jama.289.20.2709 [DOI] [PubMed] [Google Scholar]
- 19.Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019;366:447–53. doi: 10.1126/science.aax2342 [DOI] [PubMed] [Google Scholar]
- 20.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight - reconsidering the use of race correction in clinical algorithms. N Engl J Med 2020;383:874–82. doi: 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
- 21.Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am J Physiol Ren Physiol 2020;318:F1315–26. doi: 10.1152/ajprenal.00071.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014;2014:406960. doi: 10.1155/2014/406960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C Macrovascular complications in patients with diabetes and Prediabetes. Biomed Res Int 2017;2017:7839101. doi: 10.1155/2017/7839101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luft FC. Molecular genetics of human hypertension. Curr Opin Cardiol 2020;35:249–57. doi: 10.1097/HCO.0000000000000722 [DOI] [PubMed] [Google Scholar]
- 25.Gaulton KJ. Mechanisms of type 2 diabetes risk loci. Curr Diab Rep 2017;17:72. doi: 10.1007/s11892-017-0908-x [DOI] [PubMed] [Google Scholar]
- 26.Nyfløt LT, Sandven I, Stray-Pedersen B, Pettersen S, Al-Zirqi I, Rosenberg M, et al. Risk factors for severe postpartum hemorrhage: a case-control study. BMC Pregnancy Childbirth 2017; 17:17. doi: 10.1186/s12884-016-1217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escobar MF, Hincapie MA, Barona JS. Immunological role of the maternal uterine microbiota in postpartum hemorrhage. Front Immunol 2020;11:504. doi: 10.3389/fimmu.2020.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehsani M, Mohammadnia-Afrouzi M, Mirzakhani M. Female unexplained infertility: a disease with imbalanced adaptive immunity. J Hum Reprod Sci 2019;12:274–82. doi: 10.4103/jhrs.JHRS_30_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am 2012;39:535–49. doi: 10.1016/j.ogc.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanbo T, Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet Gynecol Scand 2017;96:659–67. doi: 10.1111/aogs.13082 [DOI] [PubMed] [Google Scholar]
- 31.Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Curr Opin Anaesthesiol 2011;24:255–61. doi: 10.1097/ACO.0b013e328345331c [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. World Health Organization; 2012. [PubMed] [Google Scholar]
- 33.Gülmezoglu AM, Lumbiganon P, Landoulsi S, Widmer M, Abdel-Aleem H, Festin M, et al. Active management of the third stage of labour with and without controlled cord traction: a randomised, controlled, non-inferiority trial. Lancet 2012; 379:1721–7. doi: 10.1016/S0140-6736(12)60206-2 [DOI] [PubMed] [Google Scholar]
- 34.Venkatesh KK, Strauss RA, Grotegut CA, Heine RP, Chescheir NC, Stringer JSA, et al. Machine learning and statistical models to predict postpartum hemorrhage. Obstet Gynecol 2020;135: 935–44. doi: 10.1097/AOG.0000000000003759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol 2012;120:920–7. doi: 10.1097/AOG.0b013e31826af61a [DOI] [PubMed] [Google Scholar]
- 36.Bateman BT, Hernandez-Diaz S, Huybrechts KF, Palmsten K, Mogun H, Ecker JL, et al. Outpatient calcium-channel blockers and the risk of postpartum haemorrhage: a cohort study. BJOG 2013;120:1668–7. doi: 10.1111/1471-0528.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekabil TT, Tsaedu FA, Debelew GT. Maternal complications of twin deliveries in Jimma University Specialized Hospital, Southwest Ethiopia: a facility-based cohort study. Gaziantep Med J 2015;21:84–9. [Google Scholar]
- 38.Bryant A, Mhyre JM, Leffert LR. The association of maternal race and ethnicity and the risk of postpartum hemorrhage. Anesth Analg 2012;115:1127–36. doi: 10.1213/ANE.0b013e3182691e62 [DOI] [PubMed] [Google Scholar]
- 39.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol 2010;202:353–6. doi: 10.1016/j.ajog.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 40.Chalouhi SE, Tarutis J, Barros G. Risk of postpartum hemorrhage among Native American women. Int J Gynaecol Obstet 2015;131:269–72. doi: 10.1016/j.ijgo.2015.05.037 [DOI] [PubMed] [Google Scholar]
- 41.Driessen M, Bouvier-Colle MH, Dupont C, Khoshnood B, Rudigoz RC, Deneux-Tharaux C Postpartum hemorrhage resulting from uterine atony after vaginal delivery: factors associated with severity. Obstet Gynecol 2011;117:21–31. doi: 10.1097/AOG.0b013e318202c845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feerasta SH, Motiei A, Motiwala S, Zuberi NF. Uterine atony at a tertiary care hospital in Pakistan: a risk factor analysis. JPMA J Pakistan Med Assoc 2000;50:132–6. [PubMed] [Google Scholar]
- 43.Foley A, Gunter A, Nunes KJ, Shahul S, Scavone BM Patients undergoing cesarean delivery after exposure to oxytocin during labor require higher postpartum oxytocin doses. Anesth Analg 2018;126:920–4. doi: 10.1213/ANE.0000000000002401 [DOI] [PubMed] [Google Scholar]
- 44.Grotegut CA, Paglia MJ, Johnson LN. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am J Obstet Gynecol 2011;204:56.e1–6. doi: 10.1016/j.ajog.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillaume A, Sananès N, Poirier V, Gaudineau A, Fritz G, Boudier E, et al. Benefits of cord blood collection in the prevention of post-partum hemorrhage: a cohort study. J Matern Fetal Neonatal Med 2015;28:2111–4. doi: 10.3109/14767058.2014.979401 [DOI] [PubMed] [Google Scholar]
- 46.Harvey SA, Lim E, Gandhi KR. Racial-ethnic disparities in postpartum hemorrhage in Native Hawaiians, Pacific Islanders, and Asians. J Asia Pac Med Public Health 2017;76:128–32. [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph KS, Sheehy O, Mehrabadi A. Can drug effects explain the recent temporal increase in atonic postpartum haemorrhage? Paediatric perinatal Epidemiol 2015;29:220–31. doi: 10.1111/ppe.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahr MK, Franke D, Brun R. Blood group O: a novel risk factor for increased postpartum blood loss? Haemophilia 2018;24:e207–12. doi: 10.1111/hae.13537 [DOI] [PubMed] [Google Scholar]
- 49.Kovacheva VP, Soens MA, Tsen LC. Serum uric acid as a novel marker for uterine atony and post-spinal vasopressor use during cesarean delivery. Int J Obstet Anesth 2013;22: 200–8. doi: 10.1016/j.ijoa.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 50.Lao TT, Sahota DS, Cheng YK. Advanced maternal age and postpartum hemorrhage - risk factor or red herring?. J Matern Fetal Neonatal Med 2014;27:243–6. doi: 10.3109/14767058.2013.807240 [DOI] [PubMed] [Google Scholar]
- 51.Looft E, Simic M, Ahlberg M, Snowden JM, Cheng YW, Stephansson O Duration of second stage of labour at term and Pushing time: risk factors for postpartum haemorrhage. Paediatr Perinat Epidemiol 2017;31:126–33. doi: 10.1111/ppe.12344 [DOI] [PubMed] [Google Scholar]
- 52.Lutomski JE, Byrne BM, Devane D, Greene RA. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG 2012;119:306–14. doi: 10.1111/j.1471-0528.2011.03198.x [DOI] [PubMed] [Google Scholar]
- 53.Marshall AL, Durani U, Bartley A, Hagen CE, Ashrani A, Rose C, et al. The impact of postpartum hemorrhage on hospital length of stay and inpatient mortality: a National Inpatient Sample-based analysis. Am J Obstet Gynecol 2017;217:344. e1–6. doi: 10.1016/j.ajog.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 54.Mehrabadi A, Hutcheon JA, Lee L, Kramer MS, Liston RM, Joseph KS Epidemiological investigation of a temporal increase in atonic postpartum haemorrhage: a population-based retrospective cohort study. BJOG 2013;120:853–62. doi: 10.1111/1471-0528.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberg AS, Hernandez-Diaz S, Palmsten K, Almqvist C, Bateman BT Patterns of recurrence of postpartum hemorrhage in a large population-based cohort. Am J Obstet Gynecol 2014;210: 229–8. doi: 10.1016/j.ajog.2013.10.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Regalia AL, Acmet E, Limonta Ghezzi GV. Logistic regression analysis highlighted previous atony, pre-eclampsia, oxytocin induction and augmentation of labour, macrosomia, slow labour, Kristeller manoevres to be risk factors for uterine atony. Ital J Gynaecol Obstet 2001;3:120–23. [Google Scholar]
- 57.Siddiqui M, Minhaj M, Mueller A, Tung A, Scavone B, Rana S, et al. Increased Perinatal morbidity and mortality among Asian American and Pacific Islander women in the United States. Anesth Analg 2017;124:879–86. doi: 10.1213/ANE.0000000000001778 [DOI] [PubMed] [Google Scholar]
- 58.Tran G, Kanczuk M, Balki M. The association between the time from oxytocin cessation during labour to cesarean delivery and postpartum blood loss: a retrospective cohort study. Can J Anaesth 2017;64:820–7. doi: 10.1007/s12630-017-0874-4 [DOI] [PubMed] [Google Scholar]
- 59.Vendittelli F, Barasinski C, Pereira B, Lémery D. Incidence of immediate postpartum hemorrhages in French maternity units: a prospective observational study (HERA study). BMC Pregnancy Childbirth 2016;16:242. doi: 10.1186/s12884-016-1008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waheed G, Toheed R, Mansha M, Bin Ayub T. Comparison of causes of postpartum haemorrhage following vaginal deliveries and caesarean sections in a tertiary care hospital of Pakistan. Pakistan J Med Health Sci 2013;7:5885–9. [Google Scholar]
- 61.Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. Am J Obstet Gynecol 2013;209:51–6. doi: 10.1016/j.ajog.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.