Abstract
The effects of childhood exposure to perfluoroalkyl substances (PFASs) on lung function remain mostly unknown. Previous research indicates that children living or going to school near the World Trade Center (WTC) disaster were exposed to high levels of PFASs, among other toxic chemicals. To explore the effects of PFAS exposure on lung function, we measured serum PFASs in a cohort of children from the WTC Health Registry and a matched control group. Perfluorooctanesulfonate had the highest median concentrations in both groups (WTCHR = 3.72 ng/mL, Comparison = 2.75 ng/mL), while the lowest median concentrations were seen for perfluoroundecanoic acid (WTCHR = 0.12 ng/mL, Comparison = 0.01 ng/mL). Lung function outcomes were measured by spirometry, plethysmography, and oscillometry. Asthma diagnosis and serum eosinophil count were also recorded. We examined the relationships of each PFAS with lung function parameters and eosinophil count using linear regressions. Odds ratios for asthma were obtained for each PFAS using logistic regression. The effect of total PFASs on these outcomes was also assessed. All regression models were adjusted for sex, race/ethnicity, age, body mass index (BMI) and tobacco smoke exposure. We found that serum PFASs were not statistically associated with the measured lung function parameters, asthma diagnosis, or eosinophil count in this cohort (p < 0.05). These findings highlight the need for more longitudinal studies to explore the long-term effects of childhood PFAS exposure on lung function past adolescence and early adulthood.
Keywords: Perfluoroalkyl substances (PFASs), Children, Asthma, Lung function, World Trade Center disaster
1. Introduction
The World Trade Center disaster on September 11, 2001 exposed the nearby population to a large amount of dust-borne chemicals. Chemicals in the dust cloud, including perfluoroalkyl substances (PFASs), had the potential to be inhaled directly or ingested through food after dust settled onto surfaces in people’s homes. PFASs are of concern, as they are known to accumulate in human tissues (Sanchez Garcia et al., 2018; Perez et al., 2013) and exposure is quite common in drinking water (Ericson et al., 2009). Previously, we showed that children selected from the World Trade Center Health Registry (WTCHR) had significantly higher median levels of serum PFASs compared to a sociodemographically matched non-WTCHR group (p < 0.0001 for PFHxS, PFOS, PFOA, PFNA, and PFDA) (Trasande et al., 2017).
The effects of PFASs on lung function, specifically asthma, are of interest since a typical route of exposure to these chemicals is through inhalation (Yao et al., 2018). Asthma is characterized by airway hyperresponsiveness and remodeling caused by airway wall inflammation. Inflammation can occur via several pathways including TH-cell dysregulation favoring a shift from TH1-cells to TH2-cells and increased production of pro-inflammatory cytokines (Silkoff et al., 2015; Fireman, 2003). A few toxicological studies in mice support the association between PFASs and induced inflammation, reporting increased pro-inflammatory cytokines (Zheng et al., 2011) after oral exposure to 5 mg/kg/d perfluorooctanesulfonate (PFOS) and increased TH2-type cytokines (Singh et al., 2012) after dermal exposure to 10 mg/kg perfluorooctanoic acid (PFOA). Similar studies also show decreasing immunoglobulin M (IgM) and increasing immunoglobulin E (IgE) levels characteristic of asthma and allergy after 1–50 mg/kg PFOA exposure (Singh et al., 2012; Dewitt et al., 2008; Fairley et al., 2007). However, other toxicological studies indicate cytokine inhibition following PFAS exposure and no effect on airway hyperresponsiveness (Corsini et al., 2012; Ryu et al., 2014).
Epidemiologic studies of PFASs and lung function also lack clarity. Several studies show higher mean serum PFAS levels in children with asthma compared to non-asthmatic children (Zhu et al., 2016; Qin et al., 2017; Humblet et al., 2014). Higher serum PFAS concentrations have also been associated with increased TH2 cytokine levels in males (Zhu et al., 2016) and increased serum IgE concentrations in both males and females (Dong et al., 2013), suggesting that PFASs may induce inflammation. Still, other population-based studies have reported either no association (Stein et al., 2016) or inverse associations (Humblet et al., 2014) between asthma and PFAS concentrations.
In our cohort, we observed that World Trade Center dust cloud exposure was associated with higher asthma incidence after September 11, 2001 among exposed children compared to the non-exposed group (Trye et al., 2018). To understand how PFASs in particular may be contributing to the difference in asthma incidence, we analyzed exposure to individual serum PFASs and total PFAS levels for asthma diagnosis among both WTC-exposed and non-exposed individuals. We then explored associations between PFAS levels and spirometry, plethysmography and oscillometry, all of which indicate lung function status. We also considered how serum PFAS levels were associated with absolute eosinophil count, since elevated eosinophils have been considered indicative of asthma (Silkoff et al., 2015). We hypothesized that exposure to individual and combined PFASs would adversely affect (1) asthma diagnosis after September 11, 2001, (2) major lung function outcomes, and (3) absolute eosinophil levels in exposed children.
2. Methods
2.1. Study population
Participants of the WTCHR who were born between September 11, 1993 and September 10, 2001 were included in the WTCHR arm of the study. To be considered for the WTCHR, subjects needed to live or attend school south of Canal Street or be present south of Chambers Street on September 11, 2001. We included WTCHR participants that had responded to the most recent WTCHR questionnaire cycle (2011–2012). The comparison group consisted of individuals who were not eligible for the WTCHR, who did not report living or attending school south of Canal Street or being present south of Chambers Street on September 11, 2001, and who were born in the same time frame noted above. Recruitment strategies for both groups have been described previously (Trasande et al., 2017; Koshy et al., 2017). Potential participants were excluded if they indicated having a serious heart or lung disease, heart or lung surgery, an active upper respiratory infection, or pregnancy. Subjects who were unable to attend scheduled visit dates and follow study protocols were also excluded. The comparison group was chosen based on a table of the desired frequencies of controls by age (0–2, 3–5 or 6–8 years-old on 9/11/2001), sex, race (Caucasian, African American, Asian, Other), ethnicity (Hispanic, non-Hispanic), and family annual income (< $25,000, ≥$25,000). All subjects were between the ages of 13 and 22 years at the time of pulmonary function measurements and blood draw, which occurred between February 20, 2014 and March 21, 2016.
2.2. Pulmonary function measurements
Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were collected through spirometry (Jaeger Masterscreen IOS; Carefusion, Yorba Linda, CA) and assessed by the National Health and Nutrition Examination Survey (NHANES) III reference equations (Beydon, 2006). Plethysmography was used to measure functional residual capacity (FRC). Inspiratory capacity and expiratory reserve volume, both measured by spirometry, were used with FRC to calculate total lung capacity (TLC) and residual volume (RV). The forced oscillation technique (FOT) was used to measure resistance at frequencies of 5 Hz (R5) and 20 Hz (R20) (Jaeger Masterscreen IOS; Carefusion, Yorba Linda, CA). Frequency dependence of resistance (FDR) was calculated as the difference between R5 and R20 (R5-R20) to assess distal airway function (Oppenheimer et al., 2009; van den Elshout et al., 1994). Each test was conducted a minimum of 3 and a maximum of 5 times and all test results were evaluated by two investigators to ensure reproducibility.
Information on asthma diagnosis was obtained from questions derived from NHANES. Participants that reported asthma diagnosis were asked to report on the time of diagnosis (before or after 9/11/2001) (Akinbami et al., 2003). Non-fasting blood samples collected via venipuncture provided total eosinophils from the whole blood count differential (Trasande et al., 2017).
2.3. Exposure variables
Blood samples were collected via venipuncture from the selected WTCHR and comparison subjects. PFASs were measured in serum using a solid phase extraction (SPE) procedure and high-performance liquid chromatograph interfaced with an electrospray tandem mass spectrometer. These methods have been described previously (Trasande et al., 2017; Kannan et al., 2004; Taniyasu et al., 2005). Eleven PFASs were measured: perfluorohexanesulfonic acid (PFHxS); n-methyl perfluorooctanesulfonamido acetic acid (N-meFOSAA); perfluorooctane sulfonamide (PFOSA); perfluorooctanesulfonate (PFOS); perfluorodecanesulfonate (PFDS); perfluoroheptanoic acid (PFHpA); perfluorooctanoic acid (PFOA); perfluorononanoic acid (PFNA); perfluorodecanoic acid (PFDA); perfluoroundecanoic (PFUnDA); and perfluorododecanoic acid (PFDoDA). The limit of detection (LOD) for all PFASs was 0.02 ng/mL apart from N-meFOSAA (LOD = 0.07 ng/mL), PFOS (LOD = 0.03 ng/mL), and PFOA (LOD = 0.05 ng/mL).
2.4. Covariates
Tobacco exposure was based on salivary cotinine measures and was categorized as low (< 0.15 ng/mL salivary cotinine), medium (< 2.32 ng/mL and ≥0.15 ng/mL cotinine), and high (≥2.32 ng/mL cotinine). Salivary cotinine measurements were obtained using a highly reliable (r = 0.99 compared with serum) and sensitive (LOD = 0.15 ng/mL) test from Salimetrics, Inc. (State College, PA). Subjects missing salivary cotinine measures were assigned to the low exposure group if they reported neither smoking nor secondhand smoke exposure. Those who reported not smoking but exposure to secondhand smoke were assigned to the medium exposure category.
Body mass index (BMI) was calculated as (weight in kg)/(height in m) (Perez et al., 2013; Ogden et al., 2002). Measurements for weight (in pounds) and height (in inches) were taken using calibrated stadiometers (Shorr Productions, Olney, MD) and scales (Seca model 881; Seca Corp., Hanover, MD). Participants over the age of 19 were categorized into the following groups: normal (BMI ≤ 25), overweight (25 < BMI ≤ 30), or obese (BMI ≥ 30) (CDC, 2017). Participants ages 19 and younger were categorized into BMI groups based on BMI-for-age z-score: normal (BMI z-score < 1), overweight (BMI z-score > 1 and ≤ 2), and obese (BMI z-score > 2) (WHO, 2019). Sex, age, and race identification data were collected in general questionnaires. Dust exposures were collected via questionnaire. Participants experienced WTC cloud dust exposure if they were caught in the WTC dust or debris cloud the morning after the buildings collapsed on 9/11. Participants experienced home dust exposure if they lived in an apartment or home in which WTC dust or debris was visible on surfaces for a year after the incident. Psychological trauma was determined by answering yes to one of the following seven items: sight of either tower collapse, sight of injured people, sight of dead bodies, sight of people falling out of buildings, physical injury to self, need to depart home/work for safety, and worry about safety of a loved one (Comer et al., 2010).
2.5. Statistical analysis
All analyses were performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). Population characteristics were compared between the WTCHR and comparison groups after exclusion of those without a blood draw or any lung function data. Chi-square tests were used to compare sociodemographic characteristics between the two populations. The Fisher Exact Test was used to compare dust cloud, home dust, and trauma from the World Trade Center disaster. Age was analyzed using the Wilcoxon Rank-Sum Test.
PFASs that were below the LOD in 50% or more of the study population (PFOSA, PFDS, PFHpA, PFDoDA, N-MeFOSAA) were not considered for analysis in the present study, as these would require separate models not explored here (Helsel, 2012). PFHxS, PFOS, PFOA, and PFNA were present in 100% of the samples. For PFDA and PFUnDA, concentrations below the LOD were entered as LOD/√2 (Helsel, 2012). PFAS levels were then log-transformed to correct for positive skewedness. Absolute eosinophil count was calculated by multiplying the percentage of eosinophils by the white blood cell count and was then log-transformed to correct for positive skewedness.
Components of the spirometry, plethysmography, oscillometry tests and absolute eosinophil count were analyzed by univariable and multivariable linear regression. Odds ratios were obtained for asthma diagnoses after September 11, 2001 using univariable and multivariable logistic regression. Race and sex were included as covariates, as these characteristics may affect asthma and pulmonary outcomes and were significantly different between the WTCHR group, who on average experienced greater PFAS exposure, and the comparison group. Although age did not differ significantly between the two groups, we included it in the multivariable model due to its influence on pulmonary function and its possible effect on exposure to and retention of PFASs in the body.
Sex and race/ethnicity were included in all multivariable linear and logistic regression models due to significant differences between the exposed and comparison cohorts. Although age and tobacco exposure did not differ significantly between the two groups, both were included in the model due to their recorded effects on lung function and asthma (Oberg et al., 2011; Jones et al., 2011). BMI was included in all regression models as well, as it is known to affect spirometry, plethysmography, and oscillometry test outcomes (Oppenheimer et al., 2014; van de Kant et al., 2016) and because PFASs bind to human tissue (Sanchez Garcia et al., 2018). These covariates were assessed for collinearity. The linear and logistic regressions used all complete cases for the specified exposure and outcome measure. Comparison and exposure group designation were not included in the regression models. All hypothesis tests were 2-sided and assessed at a 5% significance level.
3. Results
Fig. 1 shows the number of participants that were included in the analysis from the parent study population. Participants from the complete cohort (n = 412) were excluded if they were missing either PFAS data (n = 96) or lung function data (n = 29). The participants included in this paper’s analysis (n = 287) were less likely to report exposure to WTC dust in the home than participants excluded from the analysis (n = 125) (Table S1).
Fig. 1.
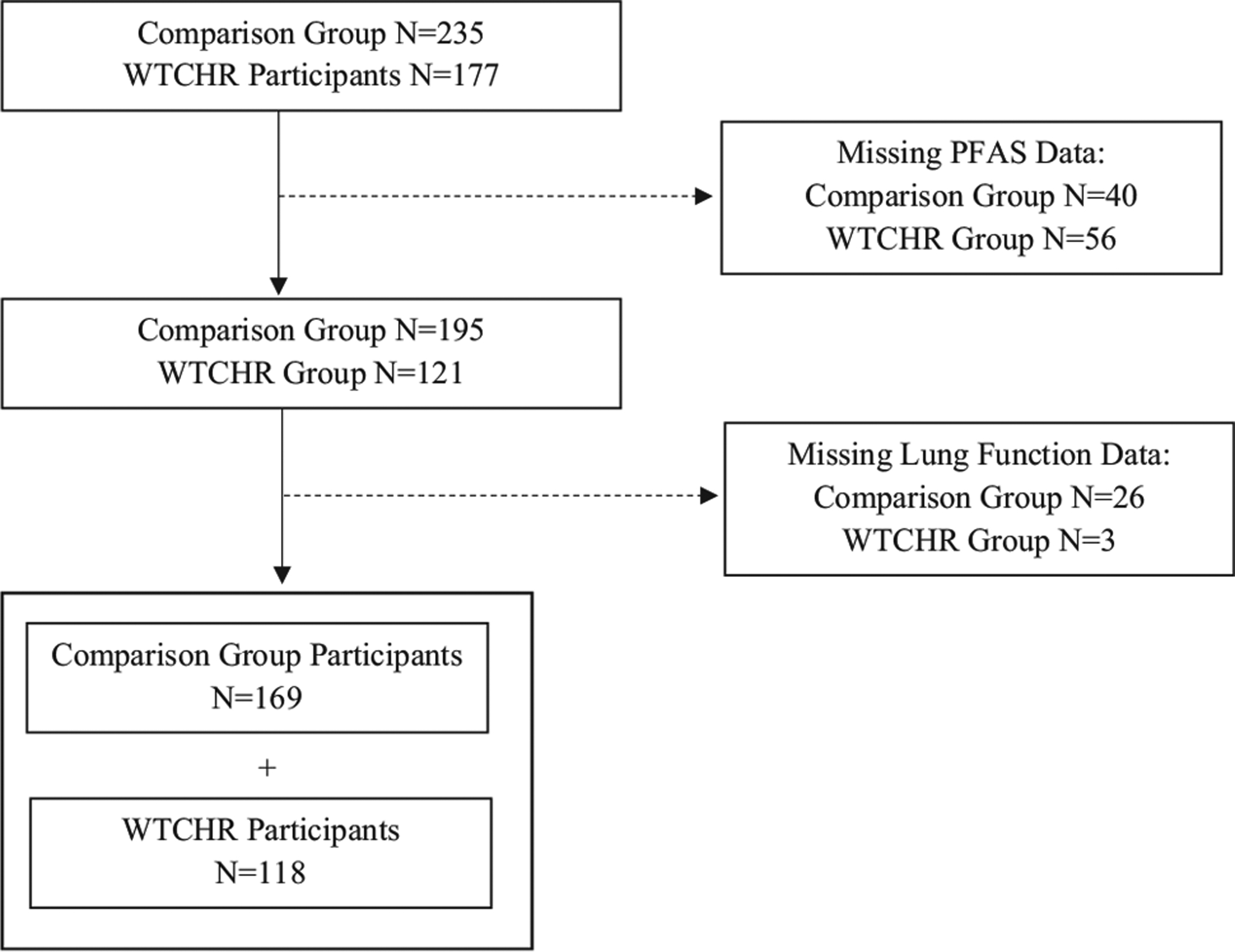
Study population flow chart.
The current analysis consisted of 118 WTCHR subjects and 169 subjects in the comparison group. Table 1 provides a breakdown of the participant population statistics in the WTCHR and comparison groups. The WTCHR group had a significantly higher proportion of males (p = 0.001) and a higher median daily caloric intake (p = 0.007) than the comparison group. The racial makeup of the two groups also differed significantly (p = 0.008). These differences may be a result of the geographic location of the two groups as well as the different population sizes. The WTCHR group had significantly higher exposure to dust cloud, home dust, and psychological trauma from the World Trade Center disaster (p < 0.0001). However, WTC home dust and trauma exposures were still present in the comparison group (4.9% and 22.8%, respectively). The two groups did not differ significantly in terms of age, income below $25,000 per year, BMI, tobacco exposure, or asthma diagnosis before 9/11/2001.
Table 1.
Population descriptive statistics.
| Comparison (n = 169) | WTCHR (n = 118) | ||
|---|---|---|---|
| n (%) | n (%) | p value | |
| Sex | |||
| Male | 65 (38.5) | 68 (57.6) | 0.001 |
| Female | 104 (61.5) | 50 (42.4) | |
| Income < $25,000a | 41 (24.3) | 19 (17.0) | 0.422 |
| Race/Ethnicityb | |||
| Asian non-Hispanic | 33 (19.5) | 30 (25.6) | 0.008 |
| Black non-Hispanic | 19 (11.2) | 15 (12.8) | |
| Hispanic | 40 (23.7) | 14 (12.0) | |
| White non-Hispanic | 72 (42.6) | 45 (38.5) | |
| Other | 5 (3.0) | 13 (11.1) | |
| Body Mass Index | |||
| Normal | 121 (71.6) | 93 (78.8) | 0.346 |
| Overweight | 37 (21.9) | 18 (15.3) | |
| Obese | 11 (6.5) | 7 (5.9) | |
| Tobacco Exposureb | |||
| Low | 50 (39.8) | 37 (31.4) | 0.161 |
| Medium | 99 (58.9) | 59 (50.0) | |
| High | 19 (11.3) | 22 (18.6) | |
| Asthma Before 9/11/01c | 14 (8.3) | 12 (10.3) | 0.559 |
| Dust Cloud Exposureb | 2 (1.2) | 22 (18.6) | < 0.0001 |
| Home Dust Exposured | 8 (4.8) | 44 (39.3) | < 0.0001 |
| Age | 16 (15, 19) | 17 (15, 19) | 0.190 |
n = 281.
n = 286.
n = 284.
n = 279.
Table 2 provides the levels of selected PFASs in blood serum in the comparison and WTCHR groups. PFHxS serum concentrations ranged from 0.09 to 28.66 ng/mL, PFOS from 0.47 to 27.76 ng/mL, PFOA from 0.34 to 8.12 ng/mL, PFNA from 0.11 to 2.40, PFDA from LOD to 0.87 ng/mL, and PFUnDA from LOD to 0.85 ng/mL. All mean PFAS levels were significantly higher in the WTCHR group than in the comparison group.
Table 2.
Serum PFAS concentrations in WTCHR and comparison groups.
| Comparison (n = 169) | WTCHR (n = 118) | p-value | ||||
|---|---|---|---|---|---|---|
| % < LOD | Mean (SD) | Median (min, max; IQR) | Mean (SD) | Median (min, max; IQR) | Wilcoxon | |
| Serum perfluoroalkyl substances (PFASs) (ng/mL) | ||||||
| PFHxS | 0 | 0.73 (0.75) | 0.53 (0.09, 5.26; 0.43) | 1.28 (2.00) | 0.67 (0.19, 15.5; 0.68) | 0.0002* |
| PFOS | 0 | 3.45 (3.30) | 2.75 (0.60, 27.8; 2.20) | 4.26 (2.51) | 3.72 (1.01, 14.2; 2.82) | < 0.0001* |
| PFOA | 0 | 1.53 (0.65) | 1.38 (0.36, 4.28; 0.69) | 1.91 (0.80) | 1.80 (0.56, 5.03; 0.90) | < 0.0001* |
| PFNA | 0 | 0.56 (0.30) | 0.49 (0.15, 2.40; 0.33) | 0.67 (0.30) | 0.61 (0.16, 1.97; 0.36) | < 0.0001* |
| PFDA | 24.0 | 0.13 (0.13) | 0.11 (0.01, 0.87; 0.15) | 0.17 (0.12) | 0.14 (0.01, 0.70; 0.12) | < 0.0001* |
| PFUnDA | 44.3 | 0.10 (0.13) | 0.01 (0.01, 0.85; 0.15) | 0.15 (0.15) | 0.12 (0.01, 0.76; 0.21) | < 0.0001* |
| Total PFAS | - | 6.50 (4.00) | 5.66 (1.39, 31.1; 3.45) | 8.44 (4.39) | 7.26 (2.15, 27.0; 5.25) | < 0.0001* |
LODs: PFHxS, PFNA, PFDA, PFUnDA = 0.02 ng/mL; PFOS = 0.03 ng/mL; PFOA = 0.05 ng/mL.
Significant at the p < 0.05 level.
Unit changes for the respiratory measure and absolute eosinophil count and odds ratios for asthma diagnosis after the WTC disaster are shown in Table 3. Interestingly, we saw significant decreases in R5, R20, and R5-R20 associated with several of the measured PFASs. PFHxS was associated with decreased forced expiratory volume in 1 s to forced vital capacity ratio (FEV1/FVC). TLC and FRC were both increased by all PFASs except for PFDA and PFUnDA. There were no statistically significant effects of PFASs on the odds of asthma diagnosis or changes in absolute eosinophil count.
Table 3.
Univariable regression analysis of relevant pulmonary function outcomes, eosinophil count, and asthma by serum PFASs.
| PFAS (log-transformed) Respiratory Outcomes | PFHxS Unit change (95% CI) |
PFOS Unit change (95% CI) |
PFOA Unit change (95% CI) |
PFNA Unit change (95% CI) |
PFDA Unit change (95% CI) |
PFUnDA Unit change (95% CI) |
PFAS Total Unit change (95% CI) |
|---|---|---|---|---|---|---|---|
| R5 | −0.21 (−0.37, −0.06)* | −0.42 (−0.62, −0.23)* | −0.48 (−0.75, −0.21)* | −0.46 (−0.70, −0.21)* | −0.10 (−0.20, 0.01) | −0.06 (−0.15, 0.03) | −0.49 (−0.72, −0.26)* |
| R5–20 | −0.02 (−0.12, 0.08) | −0.25 (−0.38, −0.13)* | −0.26 (−0.44, −0.08)* | −0.30 (−0.46, −0.14)* | −0.09 (−0.16, −0.03)* | −0.07 (−0.12, −0.01)* | −0.27 (−0.42, −0.12)* |
| R20 | −0.19 (−0.30, −0.09)* | −0.17 (−0.31, −0.03)* | −0.22 (−0.41, −0.03)* | −0.15 (−0.33, 0.02) | −0.01 (−0.08, 0.07) | 0.004 (−0.06, 0.07) | −0.22 (−0.38, −0.06)* |
| FVC | 0.35 (0.22, 0.48)* | 0.27 (0.11, 0.43)* | 0.46 (0.23, 0.69)* | 0.32 (0.12, 0.52)* | −0.02 (−0.11, 0.07) | −0.09 (−0.16, −0.01)* | 0.41 (0.22, 0.60)* |
| FEV1 | 0.24 (0.14, 0.34)* | 0.21 (0.08, 0.34)* | 0.33 (0.15, 0.52)* | 0.26 (0.10, 0.42)* | −0.01 (−0.08, 0.07) | −0.06 (−0.12, −0.001)* | 0.30 (0.15, 0.46)* |
| FEV1/FVC | −1.40 (−2.63, −0.17)* | −0.60 (−2.15, 0.95) | −1.53 (−3.73, 0.67) | −0.31 (−2.23, 1.60) | 0.25 (−0.58, 1.07) | 0.38 (−0.33, 1.08) | −1.04 (−2.87, 0.80) |
| TLC | 0.35 (0.17, 0.54)* | 0.35 (0.10, 0.59)* | 0.56 (0.23, 0.90)* | 0.65 (0.35, 0.95)* | −0.04 (−0.18, 0.09) | −0.07 (−0.18, 0.05) | 0.51 (0.23, 0.79)* |
| RV | 0.05 (−0.09, 0.18) | 0.10 (−0.08, 0.27) | 0.12 (−0.13, 0.36) | 0.33 (0.12, 0.55)* | −0.01 (−0.10, 0.09) | 0.03 (−0.05, 0.11) | 0.14 (−0.06, 0.35) |
| FRC | 0.17 (0.02, 0.32)* | 0.25 (0.06, 0.44)* | 0.33 (0.06, 0.59)* | 0.53 (0.30, 0.77)* | 0.01 (−0.10, 0.11) | 0.01 (−0.08, 0.10) | 0.35 (0.13, 0.57)* |
| Eosinophil count (log-transformed) | −0.06 (−0.25, 0.12) | 0.03 (−0.22, 0.28) | −0.01 (−0.35, 0.33) | 0.04 (−0.26, 0.34) | 0.03 (−0.10, 0.16) | 0.004 (−0.11, 0.11) | 0.01 (−0.29, 0.30) |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Asthma diagnosis after 9/11/01 | 0.96 (0.65, 1.44) | 0.66 (0.39, 1.13) | 1.00 (0.49, 2.03) | 1.20 (0.63, 2.27) | 0.90 (0.69, 1.17) | 0.93 (0.74, 1.18) | 0.77 (0.42, 1.40) |
R5: Resistance at an oscillation frequency of 5 Hz; R5–20: Resistance at an oscillation frequency of 5 Hz minus resistance at 20 Hz; R20: Resistance at an oscillation frequency of 20 Hz; FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in 1 s; TLC: Total Lung Capacity; RV: Residual Volume; FRC: Functional Residual Capacity.
Significant at the p < 0.05 level.
The results from the multivariable regression analyses are shown in Table 4. All regressions were adjusted for sex, race/ethnicity, BMI, age, and tobacco exposure. Most of the statistically significant associations seen in the univariable regression analyses disappeared after correcting for covariates. PFHxS had a significant inverse effect on R20 (unit change = −0.12, 95% CI = −0.23, −0.003). RV and FRC were significantly increased with PFNA exposure (unit change = 0.24, 95% CI = 0.003, 0.48) and total PFAS (unit change = 0.31, 95% CI = 0.07, 0.55). However, these associations were not statistically significant after adjusting for multiple comparisons using the Bonferroni-Šídák method (p = 0.28, p = 0.28, p = 0.07, respectively).
Table 4.
Multivariable regression analysis of relevant pulmonary function outcomes, eosinophil count, and asthma by serum PFASs.
| PFAS (log-transformed) Respiratory Outcomes | PFHxS Unit change (95% CI) |
PFOS Unit change (95% CI) |
PFOA Unit change (95% CI) |
PFNA Unit change (95% CI) |
PFDA Unit change (95% CI) |
PFUnDA Unit change (95% CI) |
PFAS Total Unit change (95% CI) |
|---|---|---|---|---|---|---|---|
| R5 | −0.09 (−0.25, 0.07) | −0.10 (−0.32, 0.12) | −0.11 (−0.39, 0.19) | −0.11 (−0.36, 0.14) | 0.03 (−0.08, 0.15) | 0.004 (−0.09, 0.10) | −0.15 (−0.37, 0.15) |
| R5–20 | 0.03 (−0.07, 0.13) | −0.09 (−0.23, 0.04) | −0.05 (−0.23, 0.13) | −0.10 (−0.26, 0.05) | −0.003 (−0.07, 0.07) | −0.004 (−0.06, 0.05) | 0.08 (−0.24, 0.08) |
| R20 | −0.12 (−0.23,−0.003)* | −0.006 (−0.17, 0.15) | −0.05 (−0.27, 0.16) | −0.005 (−0.19, 0.18) | 0.04 (−0.04, 0.12) | 0.01 (−0.06, 0.08) | −0.35 (−0.22, 0.16) |
| FVC | 0.03 (−0.08, 0.13) | 0.01 (−0.12, 0.15) | −0.05 (−0.23, 0.13) | 0.02 (−0.14, 0.17) | −0.03 (−0.10, 0.04) | −0.04 (−0.10, 0.02) | 0.01 (−0.16, 0.17) |
| FEV1 | −0.01 (−0.10, 0.08) | −0.02 (−0.13, 0.10) | −0.02 (−0.24, 0.07) | 0.01 (−0.12, 0.14) | −0.03 (−0.09, 0.03) | −0.03 (−0.08, 0.02) | −0.03 (−0.17, 0.11) |
| FEV1/FVC | −0.90 (−2.29, 0.48) | −0.63 (−2.45, 1.20) | −1.02 (−3.45, 1.40) | −0.18 (−2.25, 1.90) | −0.15 (−1.11, 0.81) | 0.09 (−0.68, 0.86) | −0.88 (−3.05, 1.27) |
| TLC | 0.001 (−0.18, 0.18) | −0.02 (−0.27, 0.23) | −0.12 (−0.45, 0.21) | 0.27 (−0.01, 0.55) | −0.10 (−0.22, 0.03) | −0.04 (−0.15, 0.06) | −0.01 (−0.30, 0.28) |
| RV | 0.01 (−0.15, 0.16) | 0.08 (−0.12, 0.28) | −0.04 (−0.32, 0.23) | 0.24 (0.003, 0.48)* | −0.06 (−0.17, 0.05) | 0.01 (−0.08, 0.10) | 0.02 (−0.22, 0.27) |
| FRC | 0.03 (−0.13, 0.18) | 0.01 (−0.21, 0.23) | −0.02 (−0.30, 0.27) | 0.31 (0.07, 0.55)* | −0.06 (−0.17, 0.05) | −0.02 (−0.11, 0.08) | 0.06 (−0.19, 0.32) |
| Eosinophil count (log-transformed) | −0.09 (−0.30, 0.19) | 0.01 (−0.29, 0.31) | −0.14 (−0.53, 0.25) | −0.05 (−0.38, 0.29) | −0.01 (−0.16, 0.14) | −0.02 (−0.15, 0.10) | −0.05 (−0.40, 0.30) |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Asthma diagnosis after 9/11/01 | 1.09 (0.68, 1.74) | 0.89 (0.45, 1.76) | 1.34 (0.55, 3.29) | 1.74 (0.81, 3.73) | 1.03 (0.74, 1.43) | 1.02 (0.77, 1.34) | 1.13 (0.51, 2.50) |
All analyses are adjusted for sex, race/ethnicity, age, BMI, and tobacco smoke exposure.
R5: Resistance at an oscillation frequency of 5 Hz; R5–20: Resistance at an oscillation frequency of 5 Hz minus resistance at 20 Hz; R20: Resistance at an oscillation frequency of 20 Hz; FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in 1 s; TLC: Total Lung Capacity; RV: Residual Volume; FRC: Functional Residual Capacity.
Significant at the p < 0.05 level.
All other regression outcomes from the multivariable models were not significant at the p < 0.05 level even before adjusting for multiple comparisons. Increasing odds of asthma were seen with exposure to all PFASs except for PFOS (OR = 0.89, 95% CI = 0.45, 1.76). The greatest increases in odds of asthma resulted from PFNA exposure (OR = 1.74, 95% CI = 0.81, 3.73) and PFOS exposure (OR = 1.34, 95% CI = 0.55, 3.29). Almost all PFAS exposures were associated with decreased levels of absolute eosinophil count. PFOS was the only PFAS associated with an increased absolute eosinophil count, although this change was miniscule (unit change = 0.01, 95% CI = −0.29, 0.31).
4. Discussion
In the present study, we found no statistically significant associations between the measured serum PFAS concentrations and lung function parameters (R5, R5–R20, R20, FVC, FEV1, FEV1/FVC, TLC, RV, FRC), asthma diagnosis, or eosinophil count in children ages 13–22 enrolled in the WTCHR and a matched control group after adjusting for multiple comparisons. The high frequency of null results suggests that exposure to PFASs in childhood does not adversely affect lung function, asthma incidence, or eosinophil count. However, the levels of serum PFASs in this study were lower than we expected. Despite exposure to WTC dust, the mean levels of PFHxS, PFOS, and PFOA observed in the WTCHR group were only slightly higher than those seen in the most recent (2013–2014) NHANES report in children ages 12–19 (1.27 ng/mL, 3.45 ng/mL, and 1.66 ng/mL, respectively) (Fourth, 2018).
The PFAS levels reported in our study were also lower than those seen in other studies that have established associations between lung function and/or asthma. The association between asthma outcomes and PFOA exposure in a 2014 study using NHANES data from children ages 12–19 reported median serum PFOA concentrations around 4.0 ng/mL (Humblet et al., 2014), while we observed a median PFOA level of 1.5 ng/mL. Qin et al. (2017) also observed much higher median concentrations of PFOS in a Taiwanese cohort of 300 asthmatics (31.5 ng/mL) and non-asthmatics (28.8 ng/mL) than was seen in this study (3.0 ng/mL for both asthmatics and non-asthmatics). Alternatively, Dong et al. (2013) did observe similarly low median concentrations of PFOS (0.5–1.2 ng/mL) associated with increased odds of asthma, but this association was seen in a larger cohort of children (n = 456) in Taiwan).
One possible explanation for the low PFAS concentrations seen in this study is that home dust exposure was higher in excluded participants, so we may not have captured the true PFAS profile of those exposed to the WTC disaster. We must also consider that both PFAS exposure and asthma prevalence differ across and within countries, which may speak to the differing conclusions described here (Ericson et al., 2009; Qin et al., 2017).
There are several limitations to the current analysis. Unlike previous studies of WTC dust on lung function (Berger et al., 2016), we were unable to measure bronchodilation or bronchoprovocation by albuterol or methacholine, as these methods were judged to be significantly more than minimal risk by the NYU School of Medicine Institutional Review Board. Without a clear picture of airway obstruction and recovery, asthma diagnosis may be under-diagnosed in the study population (Horak et al., 2016). The methods of lung function assessment that were used in this study (spirometry, plethysmography, oscillometry, and eosinophil count) were only measured at one time point after the WTC disaster. We, therefore, cannot explore how the lung function parameters and eosinophil levels changed before and after the WTC disaster. Additionally, the sample size may have been too small in this study to see a meaningful change in lung function parameters or asthma incidence after the WTC disaster. This issue of sample size also limited our ability to stratify the analysis by asthma status or sex, as seen in other epidemiological studies (Zhu et al., 2016; Qin et al., 2017).
We did not have information on other risk factors for asthma and lung function that are common in New York City, such as living near a high-traffic area or frequent exposure to mold in the home (Idris et al., 2016; Mendell et al., 2011). Since individual PFAS exposure is related to exposures of other PFASs, there may be confounding of each PFAS by the other PFASs in the analyses. The estimates from the analyses of total PFAS exposure may, therefore, be the most reliable in the current study. We also did not include the timing of blood draw as a covariate in our models, which could confound the hypothesized relationships, although the level of confounding would need to be high in order to meaningfully affect these results. Finally, we did not measure other toxic chemicals in children’s blood that were present in WTC dust that may contribute to asthma (Horii et al., 2010).
A recent study by Impinen et al. (2018) examined the effects of prenatal exposure to PFASs on multiple respiratory outcomes at 2 and 10 years of age. In this study, prenatal PFAS exposure was not associated with allergy and asthma in childhood but was associated with risk of common cold and lower respiratory tract infections (Impinen et al., 2018). These findings suggest that PFAS exposure may suppress rather than upregulate immune function. In fact, PFAS concentrations collected from blood may not be representative of concentrations in the lung and airway tissue (i.e., target tissues), where we would expect them to have their greatest effect on lung function. Perez et al. (2013) investigated the localization of different PFASs in the body and found that the lung contained high concentrations of pentafluorobenzoic acid (PFBA) and moderate concentrations of perfluorohexanoic acid (PFHxA), but other PFAS concentrations were low or virtually non-existent. According to a study by Ericson et al. (2009) drinking water is most likely the major source of PFAS exposure. Despite potential inhalation of WTC dust, children were more likely to be exposed through contaminated water and food. Though exposure via ingestion would not cause direct contact with the lungs, it could pose a greater risk to immune system suppression. Further exploration of the effects of PFASs on immune-related biomarkers and diseases may yield more promising results than studies on lung function outcomes alone.
Although this study did not find strong associations between PFAS exposure and lung function, we recommend continued follow-up in addition to other longitudinal studies in larger cohorts to elucidate the long-term effects of childhood PFAS exposure on lung function. Such studies should consider lung diseases that occur later in adulthood. The results from the current analysis highlight the need to investigate the longitudinal respiratory health effects of chemicals other than PFASs that were present in the WTC dust.
5. Conclusions
In this study, we found no statistically significant associations between the measured serum PFAS concentrations and lung function parameters (R5, R5–R20, R20, FVC, FEV1, FEV1/FVC, TLC, RV, FRC), asthma diagnosis, or eosinophil count in children ages 13–22. These findings suggest that the levels of PFASs seen in this cohort are not a significant risk factor for asthma or poor lung function. There may be unmeasured chemicals to which WTCHR children were exposed that do increase the likelihood of developing asthma.
Supplementary Material
Acknowledgements
The authors are grateful to staff, coinvestigators, participants and volunteers for their time and support.
Funding source
This research was supported by the Centers for Disease Control and Prevention/National Institute of Occupational Safety and Health, through cooperative agreements U01OH01394 and U01OH01714. The funding organizations had no roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- WTC
World Trade Center
- WTCHR
World Trade Center Health Registry
- PFAS
Perfluoroalkyl substance
- FVC
Forced vital capacity
- FEV1
Forced expiratory volume in 1 s
- FRC
Functional residual capacity
- TLC
Total lung capacity
- RV
Residual volume
- FOT
Forced oscillation technique
- R5
Resistance frequency at 5 Hz
- R20
Resistance frequency at 20 Hz)
- FDR
Frequency dependence of resistance
- NHANES
National Health and Nutrition Examination Survey
- BMI
Body Mass Index
- PFHxS
Perfluorohexanesulfonic acid
- N-meFOSAA
N-methyl perfluorooctanesulfonamido acetic acid
- PFOSA
Perfluorooctane sulfonamide
- PFOS
Perfluorooctanesulfonate
- PFDS
Perfluorodecanesulfonate
- PFHpA
Perfluoroheptanoic acid
- PFOA
Perfluorooctanoic acid
- PFNA
Perfluorononanoic acid
- PFDA
Perfluorodecanoic acid
- PFUnDA
Perfluoroundecanoic acid
- PFDoDA
Perfluorododecanoic acid
- PFBA
Pentafluorobenzoic acid
- PFHxA
Perfluorohexanoic acid
Footnotes
Trial registration
Declarations of interest
None.
Potential conflicts of interest
The authors have no conflicts of interest relevant to this article to disclose.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2019.02.024.
References
- Akinbami LJ, Schoendorf KC, Parker J, 2003. US childhood asthma prevalence estimates: the Impact of the 1997 National Health Interview Survey redesign. Am. J. Epidemiol 158 (2), 99–104 (published Online First: 2003/07/10). [DOI] [PubMed] [Google Scholar]
- Berger KI, Kalish S, Shao Y, et al. , 2016. Isolated small airway reactivity during bronchoprovocation as a mechanism for respiratory symptoms in WTC dust-exposed community members. Am. J. Ind. Med 59 (9), 767–776. 10.1002/ajim.22639. (published Online First: 2016/09/02). [DOI] [PubMed] [Google Scholar]
- Beydon N, 2006. Assessment of bronchial responsiveness in preschool children. Paediatr. Respir. Rev 7 (Suppl 1), S23–S25. 10.1016/j.prrv.2006.04.016. (published Online First: 2006/06/27). [DOI] [PubMed] [Google Scholar]
- CDC, 2017. Healthy Weight: U.S. Department of Health and Human Services: Centers for Disease Control and Prevention. [Available from: <https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html>.
- Comer JS, Fan B, Duarte CS, et al. , 2010. Attack-related life disruption and child psychopathology in New York City public schoolchildren 6-months post-9/11. J. Clin. Child Adolesc. Psychol.: Off. J. Soc. Clin. Child Adolesc. Psychol. Am. Psychol. Assoc. Div 53 460–469. 10.1080/15374416.2010.486314. (published Online First: 2010/07/01). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E, Sangiovanni E, Avogadro A, et al. , 2012. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicol. Appl. Pharmacol 258 (2), 248–255. 10.1016/j.taap.2011.11.004. (published Online First: 2011/11/29). [DOI] [PubMed] [Google Scholar]
- Dewitt JC, Copeland CB, Strynar MJ, et al. , 2008. Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ. Health Perspect 116 (5), 644–650. 10.1289/ehp.10896. (published Online First: 2008/05/13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong GH, Tung KY, Tsai CH, et al. , 2013. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ. Health Perspect 121 (4), 507–513. 10.1289/ehp.1205351. (published Online First: 2013/01/12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elshout FJ, van Herwaarden CL, Folgering HT, 1994. Oscillatory respiratory impedance and lung tissue compliance. Respir. Med 88 (5), 343–347 (published Online First: 1994/05/01). [DOI] [PubMed] [Google Scholar]
- Ericson I, Domingo JL, Nadal M, et al. , 2009. Levels of perfluorinated chemicals in municipal drinking water from Catalonia, Spain: public health implications. Arch. Environ. Contam. Toxicol 631–638. 10.1007/s00244-009-9375-y. (published Online First: 2009/08/18). [DOI] [PubMed] [Google Scholar]
- Fairley KJ, Purdy R, Kearns S, et al. , 2007. Exposure to the immunosuppressant, perfluorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol. Sci.: Off. J. Soc. Toxicol 97 (2), 375–383. 10.1093/toxsci/kfm053. (published Online First: 2007/03/21). [DOI] [PubMed] [Google Scholar]
- Fireman P, 2003. Understanding asthma pathophysiology. Allergy Asthma Proc 24 (2),79–83 (published Online First: 2003/06/05). [PubMed] [Google Scholar]
- Fourth, C.D.C., 2018. National Report on Human Exposure to Environmental Chemicals: U.S. Department of Health and Human Services [Google Scholar]
- Helsel DR, 2012. Statistics for Censored Environmental Data Using Minitab and R, 2 ed. Wiley, Hoboken. [Google Scholar]
- Horak F, Doberer D, Eber E, et al. , 2016. Diagnosis and management of asthma – statement on the 2015 GINA guidelines. Wien. Klin. Wochenschr 128 (15), 541–554. 10.1007/s00508-016-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Y, Jiang Q, Hanari N, et al. , 2010. Polychlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls, and naphthalenes in plasma of workers deployed at the World trade Center after the collapse. Environ. Sci. Technol 44 (13), 5188–5194. 10.1021/es100282d. (published Online First: 2010/05/12). [DOI] [PubMed] [Google Scholar]
- Humblet O, Diaz-Ramirez LG, Balmes JR, et al. , 2014. Perfluoroalkyl chemicals and asthma among children 12–19 years of age: NHANES (1999–2008). Environ. Health Perspect 122 (10), 1129–1133. 10.1289/ehp.1306606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris IB, Ghazi HF, Zhie KH, et al. , 2016. Environmental air pollutants as risk factors for asthma Among children seen in pediatric clinics in UKMMC, Kuala Lumpur. Ann. Glob. Health 82 (1), 202–208. 10.1016/j.aogh.2016.01.021. (published Online First: 2016/06/22). [DOI] [PubMed] [Google Scholar]
- Impinen A, Nygaard UC, Lødrup Carlsen KC, et al. , 2018. Prenatal exposure to perfluoralkyl substances (PFASs) associated with respiratory tract infections but not allergy- and asthma-related health outcomes in childhood. Environ. Res 160, 518–523. 10.1016/j.envres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Jones LL, Hashim A, McKeever T, et al. , 2011. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir. Res 12, 5. 10.1186/1465-9921-12-5. (published Online First: 2011/01/12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, et al. , 2004. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol 38(17), 4489–4495. 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Koshy TT, Attina TM, Ghassabian A, et al. , 2017. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ. Int 109, 128–135. 10.1016/j.envint.2017.08.003. (published Online First: 2017/09/12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell MJ, Mirer AG, Cheung K, et al. , 2011. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ. Health Perspect 119 (6), 748–756. 10.1289/ehp.1002410. (published Online First: 2011/01/29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg M, Jaakkola MS, Woodward A, et al. , 2011. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 377 (9760), 139–146. 10.1016/s0140-6736(10)61388-8. (published Online First: 2010/11/30). [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, et al. , 2002. Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 National Center for health statistics version. Pediatrics 109 (1), 45–60 (published Online First: 2002/01/05). [DOI] [PubMed] [Google Scholar]
- Oppenheimer BW, Goldring RM, Berger KI, 2009. Distal airway function assessed by oscillometry at varying respiratory rate: comparison with dynamic compliance. Copd 6 (3), 162–170 (published Online First: 2009/10/09). [DOI] [PubMed] [Google Scholar]
- Oppenheimer BW, Berger KI, Segal LN, et al. , 2014. Airway dysfunction in obesity: response to voluntary restoration of end expiratory lung volume. PLoS One 9 (2), e88015. 10.1371/journal.pone.0088015. (published Online First: 2014/02/08). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Nadal M, Navarro-Ortega A, et al. , 2013. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int 59, 354–362. 10.1016/j.envint.2013.06.004. (published Online First: 2013/07/31). [DOI] [PubMed] [Google Scholar]
- Qin XD, Qian ZM, Dharmage SC, et al. , 2017. Association of perfluoroalkyl substances exposure with impaired lung function in children. Environ. Res 155, 15–21. 10.1016/j.envres.2017.01.025. (published Online First: 2017/02/09). [DOI] [PubMed] [Google Scholar]
- Ryu MH, Jha A, Ojo OO, et al. , 2014. Chronic exposure to perfluorinated compounds: impact on airway hyperresponsiveness and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol 307 (10), L765–L774. 10.1152/ajplung.00100.2014. (published Online First: 2014/09/14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Garcia D, Sjodin M, Hellstrandh M, et al. , 2018. Cellular accumulation and lipid binding of perfluorinated alkylated substances (PFASs) - a comparison with lysosomotropic drugs. Chem.-Biol. Interactions 281, 1–10. 10.1016/j.cbi.2017.12.021. (published Online First: 2017/12/19). [DOI] [PubMed] [Google Scholar]
- Silkoff PE, Strambu I, Laviolette M, et al. , 2015. Asthma characteristics and biomarkers from the Airways Disease Endotyping for Personalized Therapeutics (ADEPT) longitudinal profiling study. Respir. Res 16, 142. 10.1186/s12931-015-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh TS, Lee S, Kim HH, et al. , 2012. Perfluorooctanoic acid induces mast cell-mediated allergic inflammation by the release of histamine and inflammatory mediators. Toxicol. Lett 210 (1), 64–70. 10.1016/j.toxlet.2012.01.014. (published Online First: 2012/02/11). [DOI] [PubMed] [Google Scholar]
- Stein CR, McGovern KJ, Pajak AM, et al. , 2016. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National health and Nutrition Examination Survey. Pediatr. Res 79 (2), 348–357. 10.1038/pr.2015.213. (published Online First: 2015/10/23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyasu S, Kannan K, So MK, et al. , 2005. Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J. Chromatogr. A 1093 (1), 89–97. 10.1016/j.chroma.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Trasande L, Koshy TT, Gilbert J, et al. , 2017. Serum perfluoroalkyl substances in children exposed to the world trade center disaster. Environ. Res 154, 212–221. 10.1016/j.envres.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trye A, Berger K, Naidu M, et al. , 2018. Respiratory health and lung function in children exposed to the world trade center disaster. J. Pediatr [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kant KD, Paredi P, Meah S, et al. , 2016. The effect of body weight on distal airway function and airway inflammation. Obes. Res. Clin. Pract 10 (5), 564–573. 10.1016/j.orcp.2015.10.005. (published Online First: 2015/12/02). [DOI] [PubMed] [Google Scholar]
- WHO, 2019. BMI-for-age (15–19 years) [Available from: <https://www.who.int/growthref/who2007_bmi_for_age/en/>.
- Yao Y, Zhao Y, Sun H, et al. , 2018. Per- and polyfluoroalkyl substances (PFASs) in indoor air and dust from homes and various microenvironments in China: implications for human exposure. Environ. Sci. Technol 52 (5), 3156–3166. 10.1021/acs.est.7b04971. (published Online First: 2018/02/09). [DOI] [PubMed] [Google Scholar]
- Zheng L, Dong GH, Zhang YH, et al. , 2011. type 1 and type 2 cytokines imbalance in adult male C57BL/6 mice following a 7-day oral exposure to perfluorooctanesulfonate (PFOS). J. Immunotoxicol 8 (1), 30–38. 10.3109/1547691x.2010.537287. (published Online First: 2011/02/09). [DOI] [PubMed] [Google Scholar]
- Zhu Y, Qin XD, Zeng XW, et al. , 2016. Associations of serum perfluoroalkyl acid levels with T-helper cell-specific cytokines in children: by gender and asthma status. Sci. Total Environ 559, 166–173. 10.1016/j.scitotenv.2016.03.187. (published Online First: 2016/04/10). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


