Abstract
Background:
Air pollution may be associated with elevated dementia risk. Prior research has limitations that may affect reliability, and no studies have evaluated this question in a population-based cohort of men and women in the United States.
Objectives:
We evaluated the association between time-varying, 10-y average fine particulate matter () exposure and hazard of all-cause dementia. An additional goal was to understand how to adequately control for age and calendar-time-related confounding through choice of the time axis and covariate adjustment.
Methods:
Using the Adult Changes in Thought (ACT) population-based prospective cohort study in Seattle, we linked spatiotemporal model-based exposures to participant addresses from 1978 to 2018. Dementia diagnoses were made using high-quality, standardized, consensus-based protocols at biennial follow-ups. We conducted multivariable Cox proportional hazards regression to evaluate the association between time-varying, 10-y average exposure and time to event in a model with age as the time axis, stratified by apolipoprotein E (APOE) genotype, and adjusted for sex, education, race, neighborhood median household income, and calendar time. Alternative models used calendar time as the time axis.
Results:
We report 1,136 cases of incident dementia among 4,166 individuals with nonmissing APOE status. Mean [mean ± standard deviation (SD)] 10-y average was . Each increase in the moving average of 10-y was associated with a 16% greater hazard of all-cause dementia [1.16 (95% confidence interval: 1.03, 1.31)]. Results using calendar time as the time axis were similar.
Discussion:
In this prospective cohort study with extensive exposure data and consensus-based outcome ascertainment, elevated long-term exposure to was associated with increased hazard of all-cause dementia. We found that optimal control of age and time confounding could be achieved through use of either age or calendar time as the time axis in our study. Our results strengthen evidence on the neurodegenerative effects of . https://doi.org/10.1289/EHP9018
Introduction
Neurodegenerative diseases, including Alzheimer’s disease (AD) and related dementias (ADRD), pose a growing burden on our rapidly aging society (Livingston et al. 2020). In 2016, dementia was the fifth leading cause of death around the world (Nichols et al. 2019). Because no medication successfully alters the course of ADRD, there has been an increasing focus on prevention by addressing potentially modifiable risk factors.
Several prior observational epidemiological studies have evaluated links between fine particulate matter with aerodynamic diameter () and ADRD. This research suggests that elevated exposure to is associated with an increased hazard of ADRD, though studies have estimated a range of effects (Cacciottolo et al. 2017; Carey et al. 2018; Chen et al. 2017; Grande et al. 2020; Jung et al. 2015; Oudin et al. 2018; Smargiassi et al. 2020; Yuchi et al. 2020). Limitations in this current body of literature indicate the need for further study. Most previous studies evaluated only exposure periods of 5 y or less, which may not capture the etiologically relevant exposure window, given the extended timeframe likely required for the development of dementia (Jack et al. 2010). Additionally, many studies used administrative data to ascertain dementia status; misclassification is a concern when using this approach (Wilkinson et al. 2018). Finally, all but three prior analyses used calendar time as the time axis in the survival model, which may provide only incomplete adjustment for the confounding effects of age in comparison with using age as the primary time axis (Cologne et al. 2012; Korn et al. 1997; Thiébaut and Bénichou 2004). Questions remain regarding the appropriate time axis to use, particularly for exposures exhibiting strong temporal trends, like air pollution, and for outcomes strongly linked to age, such as ADRD.
To address these limitations and existing questions, we used a population-based prospective cohort study in the greater Seattle area of the Puget Sound region with high-quality, standardized, consensus-based outcome assessments to evaluate the association between time-varying, long-term average exposure to and incidence of all-cause dementia and AD. We hypothesized that elevated long-term exposure would be associated with a greater hazard of dementia. Given the ubiquity of air pollution, a better understanding of the potential impact of on ADRD could inform policies to reduce exposures across the population.
Methods
Study Design
The Adult Changes in Thought (ACT) study is a population-based prospective cohort study in the Puget Sound region of Washington State (Kukull et al. 2002). This cohort comprises an urban and suburban elderly () population from a well-established health maintenance organization (HMO) (originally Group Health, now Kaiser Permanente Washington). All HMO members without recorded dementia diagnosis and not living in a nursing home were eligible to be selected at random. After the initial invitation letter, individuals received a phone call for clinic or at-home cognitive assessment screening. After the screening visit, persons without dementia [defined as CASI (Cognitive Abilities Screening Instrument) score of or consensus diagnosis of “not demented” even after ] were invited to join the cohort. Enrollment began in 1994–96 (original cohort, ), with additional enrollments in 2000–2003 (expansion cohort, ) and 2005–2018 (continuous enrollment, to maintain 2,000 at-risk person-years per calendar year).
Follow-up visits (in person, either at home or at the clinic, with flexible scheduling to accommodate participants) occur every 2 y; possible changes in address are detected from HMO administrative records and quarterly newsletter mailings. Mortality is ascertained through death records search, obituary tracking, quarterly mailing contact, and medical records. All procedures—including recruitment, enrollment, and follow-up—have remained consistent across all phases of the study. As of September 2018, a total of 5,546 participants have been enrolled in this ongoing cohort.
Individuals are censored on death or if they are lost to follow-up despite multiple attempts to reestablish contact; if individuals leave the HMO or move out of the study region, they remain in the ACT study. Individuals with no informative follow-up after baseline () and no available data for any averaging period () were dropped. From a remaining cohort of 4,744 individuals, our final analytical sample included 4,166 individuals with nonmissing apolipoprotein E (APOE) status.
ACT participants signed forms indicating their informed consent to enroll in the study. Study procedures were approved by the University of Washington and Kaiser Permanente institutional review boards.
Exposure Assessment
Annual average concentrations linked to residential addresses [geocoded with ArcMap (version 10.5)] were calculated based on 2-wk average concentrations obtained from a newly developed hierarchical spatiotemporal prediction model that incorporates both land use regression (LUR) and geostatistical smoothing. This model was based on data from five types of monitors covering the years 1978–2019 across the Puget Sound region: 35 long-term () regulatory monitors at 29 sites, 52 from research studies conducted during the period 1999–2001 and 2012, and low-cost sensor measurements from 105 community and ACT participant home sites (collected during the period 2017–2019) with an additional 5 co-located with regulatory monitors. Model types, locations, and time frames covered are detailed in Table S1 and Figure S1 and discussed briefly in the following paragraphs.
Standard federal monitoring for began in 1998 with fixed-site instruments measuring on an hourly or daily basis. These monitors used the federal reference method (FRM) or the tapered element oscillating microbalance (TEOM), a federal equivalent method (FEM); both the FRM and TEOM use gravimetric methods to measure . To obtain data prior to 1998, we used information collected by nephelometers, which have been in place in the Puget Sound since 1968. Nephelometers use a light-scattering approach to measure ; this method is well-correlated with more direct measurement approaches (Liu et al. 2002; Puget Sound Clean Air Agency 2018). However, due to data gaps, we only included nephelometer data beginning in 1978 for our model. For all agency monitors, we used data from locations that covered a minimum of 52 2-wk periods to estimate the long-term trends in the spatiotemporal model.
To supplement agency monitors, we used data from three research monitoring campaigns. Liu et al. monitored using Harvard Personal Environmental Monitors (HPEM) and nephelometers at 38 homes from 1999 to 2001; this study is referred to as the “Seattle panel” study (Liu et al. 2003). In 2012, data were collected using HPEMs at 27 locations (homes, businesses, and public sites) as part of the Diesel Exhaust Exposure in the Duwamish Study (DEEDS) (Schulte et al. 2013).
Data from our own study-specific monitoring campaign (ACT-AP) using low-cost monitors (LCM) at 105 participant and community resident homes from 2017 to 2019 were also included. (An additional five ACT-AP monitors were co-located with agency monitors.) We calibrated the LCM Plantower PMS 1003/3003 measurements according to the model described by Zusman et al. (Zusman et al. 2020). We used the same multiple linear regression approach to calibrate the nephelometer measurements, focusing on nonindustrial sites and following the same approach of leveraging co-located FRM and FEM measurements as described for the LCM calibration.
Due to the unbalanced nature of our monitoring data, we used a previously described hierarchical spatiotemporal model (Keller et al. 2015; Lindström et al. 2014; Sampson et al. 2011; Szpiro et al. 2009) to develop our predictions. Prior to modeling, we averaged the data to a 2-wk timescale and log-transformed them. We also modified the spatiotemporal modeling approach by removing a long-term trend fit by a locally weighted regression smoother, loess, with span = 0.5 to address the space–time confounding of monitor availability. The time trend was added back prior to obtaining the final exponentiated model predictions. The second smoothed time trend was allowed to vary over space, as described below.
The spatiotemporal model can be represented by the following formula:
where C(s,t) is the log-transformed detrended 2-wk average at location (s) and time (t), is the spatiotemporal mean surface, and v(s,t) is the space–time residual. In our final model, the spatiotemporal mean surface is a linear combination of spatially varying coefficients and trend functions written as:
where the trend coefficients, and , are modeled using a universal kriging model with a mean model composed of one partial least squares (PLS) component and the variance model using an exponential covariance. The temporal trend, , was estimated by smoothing the first singular vector of the singular value decomposition (SVD) of the data from all long-term monitors.
The spatially varying long-term mean and trend were fit using separate universal kriging models each with a mean that reflects land use characteristics that was estimated from more than 100 geographic covariates reduced to a single PLS score. Input geographic covariates included proximity variables (such as measured distance in meters to major roads, intersections, truck routes, railways, railyards, coastlines, airports, and ports) and buffer variables (such as those based on major road length, truck route length, land use category percentage, normalized difference vegetation index (NDVI), and the year 2000 population density). Kriging was captured using an exponential variogram. Predictions from the spatiotemporal model were exponentiated after adding back the long-term trend.
Model fitting was conducted using maximum likelihood estimation in the SpatioTemporal package (version 1.1.9.1) in R (version 3.6.1; R Development Core Team). We evaluated several specifications for model parameters, including the number of time trends (1 vs. 2), the degrees of freedom for smoothing time trends per year (4 vs. 8), the number of PLS scores per trend coefficient (1 vs. 2), and the covariance structure of the trend coefficients (spatial smoothing vs. no spatial smoothing). Our final model specifications were informed by Akaike information criterion (AIC), Bayesian information criterion (BIC), prediction maps, and cross-validation. The final model included one-time trend computed from singular value decomposition and smoothed using 8 degrees of freedom per year, one PLS score per trend coefficient, and spatial smoothing of the trend coefficients. We used the final model to predict long-term averages at participant homes from 1978 to 2018 and create individual-specific time-varying 10-y average exposures for each calendar year of observation. The final model had a cross-validated () of 0.87 and a root mean square error (RMSE) of for long-term averages at regulatory monitoring locations; these figures were , at low-cost measurement sites (Table S2).
To obtain individual-specific exposure estimates, we used geocoded participant address histories. High-quality participant address history from billing records was available starting in 1989; prior to that date, address information was available from archived Group Health/Kaiser Permanente administrative records, ACT study records, and a Lexis-Nexis search. If participants moved during the study period, updated addresses were incorporated when possible. We had exact geocoding matches for 97% of addresses across all person-years in this cohort. If participants moved out of the spatiotemporal modeling region, no estimates were available for that period, and therefore participants did not contribute person-years to that particular period of the survival analysis. We imputed missing address history information for two types of address coverage gaps: gaps prior to 1989 (when the bulk of the administrative address history data became available) and gaps after the first available address. We classified individuals with no missing address history information and those with a short gap in administrative address data (up to 2 y) with the same address before and after the gap as having a “complete” address history. Individuals with “nearly complete” address history had address gaps less than 2 y and a change of address during this time; they were assumed to have moved halfway through the time period. The remaining individuals had a “less complete” address history. When there was missing address information prior to the first recorded address, we projected the first address back in time and assigned a classification of “nearly complete” for the duration up to the time they were known to live at that address and a classification of “less complete” for any duration in excess.
Outcome Assessment
Outcome assessment for the ACT study follows best practices for neuropsychological evaluation. Participants had standardized cognitive assessments during in-person follow-up visits (either at home or at the clinic) conducted every 2 y (Kukull et al. 2002); there is no adjustment for practice effects. Individuals who scored 86 or higher on the CASI exam were classified as dementia-free. Individuals who scored lower than 86 or those referred due to staff and family concerns underwent standardized evaluations for dementia, including comprehensive neuropsychological tests as well as physical and neurological examinations. Diagnoses were made by consensus conference after thorough medical records review: Dementia diagnosis was based on criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM) IV (Guze 1995), whereas AD diagnosis (possible/probable) was based on McKhann et al. (1984) criteria (McKhann et al. 1984). To allow for consistency with noncases, we assigned the visit date that triggered the dementia diagnosis as the event onset date. For the analyses of AD or non-AD dementia, individuals were censored when they received an alternative dementia diagnosis.
Statistical Analysis
We used a Cox proportional hazards model to estimate a common hazard ratio (HR) across age for incident dementia or AD, stratified by APOE genotype (0 vs. copies of allele). Age was used as the time axis in the Cox model, which allows for nonparametric specification of the age effect. Additionally, using age as the time axis automatically adjusts for the confounding effect of age and therefore may be the preferred approach when evaluating a process such as dementia that is strongly related to aging (Cologne et al. 2012; Korn et al. 1997; Lamarca et al. 1998; Thiébaut and Bénichou 2004). This approach is aligned with prior survival analyses in the ACT cohort (Gray et al. 2011; Gray et al. 2015; Li et al. 2004).
Stratification by APOE genotype allows these groups to have separate baseline hazard functions and eliminates making a proportional hazards assumption for APOE groups. This is relevant to consider, given that APOE polymorphisms strongly influence AD pathology and disease risk (Corder et al. 1993; Liu et al. 2013; Verghese et al. 2011). Missingness in the APOE variable (), due to participant refusal of baseline blood draw or delay in blood sample processing, was addressed through inverse probability weighting (IPW). For IPW, we first took the pragmatic approach of imputing missing values of non-APOE covariates (median household income, degree, smoking status, body mass index (BMI) category, diabetes, heart disease, cardiovascular disease (CVD), hypertension) with the mean category or value of each to prevent individuals from dropping out of the subsequent selection model process. (Missingness was less than 2% for each of these covariates). Next, we modeled the probability of a nonmissing APOE status, using stepwise selection to obtain a final selection model using logistic regression. Starting variables for our stepwise selection (categorized as reflected in Table 1) included: ACT cohort, birth cohort, age at intake, sex, race, education/degree, median household income, smoking status, regular exercise, BMI, diabetes, CVD, heart disease, hypertension, and dementia status. Finally, we computed stabilized inverse probability (IP) weights (Cole and Hernán 2008), using a ratio of probabilities from the logistic regression models with sex in the numerator and the selected covariates as the denominator, to represent the IP of nonmissing APOE genotype. These values were used as weights in the stratified Cox proportional hazards model. The stepwise selection process produced a final selection model with the following covariates: ACT cohort, race, education/degree, sex, CVD, age at intake, and birth cohort. The of IP weights across the population was 1.15 (±0.56).
Table 1.
Descriptive statistics on Puget Sound area (ACT) cohort based on baseline information (1994–2018) for individuals with nonmissing APOE; total and stratified by above/below mean-centered baseline .a Continuous variables reported as mean (± SD); categorical variables reported as n (%)b.
| Total () | Mean-centered baseline 10-y average | ||
|---|---|---|---|
| () | () | ||
| Intake age (y) | 75 () | 73 () | 76 () |
| Sex | |||
| Male | 1,748 (42 %) | 627 (44 %) | 1,121 (41 %) |
| Female | 2,418 (58 %) | 786 (56 %) | 1,632 (59 %) |
| ACT cohort | |||
| Original (1994–1996) | 2,135 (51 %) | 1 (0 %) | 2,134 (78 %) |
| Expansion (2000–2003) | 651 (16 %) | 48 (3 %) | 603 (22 %) |
| Replacement (2005–2018) | 1,380 (33 %) | 1,364 (97 %) | 16 (1 %) |
| Birth cohort | |||
| 162 (4 %) | 0 (0 %) | 162 (6 %) | |
| 325 (8 %) | 6 (0 %) | 319 (12 %) | |
| 627 (15 %) | 30 (2 %) | 597 (22 %) | |
| 890 (21 %) | 79 (6 %) | 811 (29 %) | |
| 790 (19 %) | 126 (9 %) | 664 (24 %) | |
| 425 (10 %) | 245 (17 %) | 180 (7 %) | |
| 947 (23 %) | 927 (66 %) | 20 (1 %) | |
| APOE allele | |||
| Yes | 1,103 (26 %) | 403 (29 %) | 700 (25 %) |
| No | 3,063 (74 %) | 1,010 (71 %) | 2,053 (75 %) |
| Race | |||
| White | 3,760 (90 %) | 1,259 (89 %) | 2,501 (91 %) |
| Non-White | 406 (10 %) | 154 (11 %) | 252 (9 %) |
| Year 2000 census tract median household income ($USD) | |||
| 383 (9 %) | 111 (8 %) | 272 (10 %) | |
| 1,292 (31 %) | 387 (27 %) | 905 (33 %) | |
| 2,048 (49 %) | 730 (52 %) | 1,318 (48 %) | |
| 443 (11 %) | 185 (13 %) | 258 (9 %) | |
| Degree | |||
| None | 345 (8 %) | 26 (2 %) | 319 (12 %) |
| GED/HS | 1,623 (39 %) | 314 (22 %) | 1,309 (48 %) |
| Bachelor’s | 966 (23 %) | 387 (27 %) | 579 (21 %) |
| Master’s | 614 (15 %) | 367 (26 %) | 247 (9 %) |
| Doctorate | 244 (6 %) | 129 (9 %) | 115 (4 %) |
| Other | 374 (9 %) | 190 (13 %) | 184 (7 %) |
| Smoking status | |||
| Never | 2,019 (48 %) | 712 (50 %) | 1,307 (47 %) |
| Past | 1,947 (47 %) | 667 (47 %) | 1,280 (46 %) |
| Current | 200 (5 %) | 34 (2 %) | 166 (6 %) |
| Regular exercisec | |||
| Yes | 3,021 (73 %) | 1,040 (74 %) | 1,981 (72 %) |
| No | 1,145 (27 %) | 373 (26 %) | 772 (28 %) |
| BMI | |||
| Underweight | 35 (1 %) | 8 (1 %) | 27 (1 %) |
| Normal | 1,304 (31 %) | 427 (30 %) | 877 (32 %) |
| Overweight | 1,764 (42 %) | 601 (43 %) | 1,163 (42 %) |
| Obese | 1,063 (26 %) | 377 (27 %) | 686 (25 %) |
| Diabetes | |||
| Yes | 436 (10 %) | 167 (12 %) | 269 (10 %) |
| No | 3,730 (90 %) | 1,246 (88 %) | 2,484 (90 %) |
| Heart disease | |||
| Yes | 690 (17 %) | 147 (10 %) | 543 (20 %) |
| No | 3,476 (83 %) | 1,266 (90 %) | 2,210 (80 %) |
| CVD | |||
| Yes | 360 (9 %) | 89 (6 %) | 271 (10 %) |
| No | 3,806 (91 %) | 1,324 (94 %) | 2,482 (90 %) |
| Hypertension | |||
| Yes | 1,680 (40 %) | 622 (44 %) | 1,058 (38 %) |
| No | 2,486 (60 %) | 791 (56 %) | 1,695 (62 %) |
| CASI IRT (Crane et al. 2008) score | 0.35 () | 0.54 () | 0.26 () |
| Moved during ACT follow-up | |||
| Yes | 2,350 (56 %) | 422 (30 %) | 1,928 (70 %) |
| No | 1,806 (43 %) | 988 (70 %) | 818 (30 %) |
| Missing | 10 (0.2%) | 3 (0.2%) | 7 (0.3%) |
| Dementia diagnosisd | |||
| Yes | 1,138 (27 %) | 175 (12 %) | 963 (35 %) |
| No | 3,028 (73 %) | 1,238 (88 %) | 1,790 (65 %) |
| Alzheimer’s disease diagnosisd | |||
| Yes | 921 (22 %) | 144 (10 %) | 777 (28 %) |
| No | 3,245 (78 %) | 1,269 (90 %) | 1,976 (72 %) |
Note: ACT, Adult Changes in Thought; AD, Alzheimer’s disease; APOE, apolipoprotein E; BMI, body mass index; CASI IRT, Cognitive Assessment Screening Instrument Item Response Theory; CVD, cardiovascular disease; GED, general equivalency diploma; HS, high school; USD, U.S. dollars.
Stratification by year-specific mean-centered exposure averages removes the influence of the strong temporal variation and focuses on within-year population comparisons. To calculate the mean-centered values, we subtracted the year-specific [(i.e., time-varying, based on baseline (entry) year] mean across 10-y average exposures from each 10-y average exposure.
Missingness reported for “Never moved during ACT follow-up.” Other variables had missingness of less than in the original data set. As described in the text, we imputed missing values of non-APOE covariates (median household income, degree, smoking status, BMI category, diabetes, heart disease, CVD, hypertension) with the mean category or value of each to prevent individuals from dropping out of the subsequent inverse probability weighting selection model process.
Regular exercise refers to self-reported exercise for at least 15 minutes three times per week.
Dementia and AD-subtype dementia case numbers reflect diagnoses across the entire study population. Case numbers may vary in specific analyses based on inclusion criteria; e.g., the primary analysis based on 10-y average exposure has two fewer dementia cases due to missing 10-y average exposure data.
Relevant covariates for our survival analyses were identified based on prior literature and the use of a directed acyclic graph (DAG). We used a prespecified tiered model approach to understand the importance of potential confounding in our inferential analyses by considering the data collected at or based on in-person baseline study visits (unless otherwise noted) with occasional missingness () filled in as described for variables other than APOE genotype in IPW modeling above: model 1 (M1): APOE status (obtained via genotyping from blood sample) stratification only; model 2 (M2) (a priori): (self-report), educational degree category (self-report: none; general equivalency diploma (GED)/high school; bachelor’s; master’s; doctorate; other) year 2000 neighborhood median household income in U.S. Dollars (from census tract data: ; ; ; ), race (self-report: White; non-White; collapsed from following original categories due to low numbers among non-White categories: White, Black, Asian, American Indian/Native Alaskan, Native Hawaiian or Pacific Islander, other/mixed), time-varying calendar year categories (2-y categories, except for a 3-y category covering the most recent years 2016–2018); model 3 (M3): (self-report: current/former/never; threshold for former ), regular physical activity (self-report: includes walking, hiking, bicycling, aerobics or calisthenics, swimming, water aerobics, weight training or stretching, or other exercise at least 15 min three times per week); model 4 (M4): (hypertension, diabetes, CVD, heart disease from HMO medical records) and BMI category (calculated from measured height and weight at study visit: underweight; normal; overweight; obese). The rationale for using baseline values of covariates is that our primary goal with covariate adjustment is to unconfound the exposure–outcome relationship; later values of time-varying covariates may have been affected by the exposure. Neighborhood median household income was obtained from the year 2000, based on the assumption that neighborhood rank has remained consistent over time. We selected M2 as the a priori model based on prior literature, the DAG, and expert consultation to identify the most relevant confounders for this analysis.
The time-varying exposure at each age is defined as the average level over the 10 calendar years prior to an event onset date for everyone in the risk set at that time. We allowed these exposures to change once per year on the 1st of January; each participant has a corresponding continuous age on this date. For example, a 10-y average estimate linked to an event age in the year 2010 would be calculated from during the years 2000–2009. If fewer than 10 years of data were available, the exposure average was calculated for the time period available and assigned as the 10-y average. Exposures outside the modeling region were not included in the averages. Based on which exposure data were available, individuals were able to be included in some exposure averaging periods but not others. The partial likelihood used for inference on regression coefficients in the Cox models considered was based on risk sets adjusting for delayed entry (left truncation).
Our primary analysis focused on the association between 10-y average and incidence of all-cause dementia. In secondary analyses, we evaluated the association between 10-y average and incidence of AD, as well as alternative exposure averaging periods (1-y; 5-y; 20-y; 10-y with 5-y lag; 10-y with 10-y lag) for both all-cause dementia and AD to understand different windows of susceptibility. In sensitivity analyses, we used an alternate approach to adjust for calendar time [5-y calendar time categories or 5- to 20-y birth cohort categories developed to create balance across categories (1800–1909, 1910–1914, 1915–1919, 1920–1924, 1925–1929, 1930–1934, and 1935–1954)] when using age as the time axis; an alternative approach for classifying onset date (as midpoint between the last two visits for cases, the standard ACT study onset date classification); dropped the APOE IP weights from the model; restricted to individuals with a complete address history (as described above); dropped individuals employed at baseline, evaluated non-AD dementia as the outcome; and used calendar time as the time axis (instead of age) with either intake age or birth cohort adjustment.
We also conducted exploratory analyses to consider potential effect modification by APOE genotype, sex, binary BMI category (underweight/normal vs. overweight/obese), binary education category (none/GED/high school/other vs. bachelor’s/master’s/doctorate degrees), and binary age at enrollment category ( vs. ) by incorporating a single product interaction term for each of these variables, separately, with in the Cox model and evaluating whether this parameter was significant. As noted above, APOE strongly influences AD pathology and disease (Corder et al. 1993; Liu et al. 2013; Verghese et al. 2011), and some prior studies of air pollution and cognitive decline have identified effect modification by genotype (Cacciottolo et al. 2017; Schikowski et al. 2015). APOE influences antioxidant capacity and inflammatory response. If contributes to dementia through the pathways of oxidative stress and inflammation (Dose et al. 2016; Jofre‐Monseny et al. 2008), then individuals with one or more copies of the risk allele may be more susceptible to the neurodegenerative effects of these exposures. Similarly, differences in inflammation by sex (Hanamsagar and Bilbo 2016), age (Godbout and Johnson 2009), and BMI (Miller and Spencer 2014) may affect susceptibility to neurodegeneration and cerebrovascular injury. Education was evaluated given hypotheses regarding cognitive reserve and dementia risk (Meng and D’Arcy 2012; Stern 2012).
All analyses were conducted using R (version 3.6.3; R Development Core Team).
Results
Exposure and Participant Characteristics
Figures S2–S5 describe exposure coverage and address history quality for all participants. Although there were some gaps in exposure coverage due to participants living outside the spatiotemporal modeling region, mean coverage across all weeks of the study period was 99.8% (Figure S2). Some address history imputation was needed prior to the year 2000; after that time, the average number of weeks requiring address imputation was nearly zero (Figure S3). Correspondingly, address history quality based on the criteria provided in the “Methods” section above was rated as “complete” for nearly all participants starting in the year 2000. The proportion of time with exact geocoding quality fluctuated above 75% prior to 2005 but was nearly 100% after that time. We had nearly complete coverage of 10-y average , the exposure period for our primary analysis, at the person-level: Across all individuals and years, we were missing only 0.097% person-years of exposure data. (Ten-year average data were missing for 40 individuals; these individuals were not included in analyses for this averaging period but could still be included in secondary analyses exploring other exposure averaging periods.)
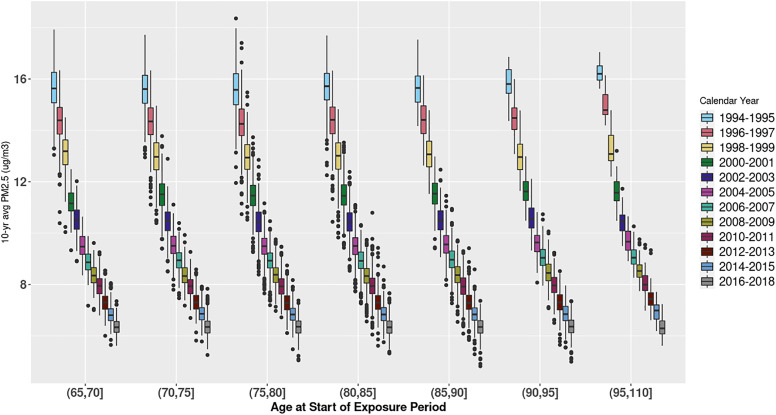
Mean 10-y average exposure across all follow-up years among those with nonmissing APOE status was . This simple summary statistic masks important temporal trends: the 1994 mean was 16.0 (±0.8), whereas it was 6.2 (±0.3) in 2018. Figure 1 depicts 10-y average exposure by age and calendar time with one observation per person year, illustrating the strong influence of calendar year in exposure to within age groups. Year-specific summary statistics are provided in Table S3. Figure 2 depicts 10-y average exposure predictions for 2000–2009—a middle range of the study period—and jittered participant residences across the Puget Sound study region; this figure highlights the refined exposure contrasts obtained through the newly developed spatiotemporal model. Overall, in our data, between-year variation of (SD: 3.0) was much higher than average within-year variation (SD: 0.5).
Figure 1.
Ten-year average by age at start of exposure period and calendar year. X-axis indicates age by 5-y age groups. Color coding indicates calendar year category of exposure. In each box plot, the middle line represents the median value; the edges of the box represent the 25th and 75th percentiles, and the whiskers extended up to 1.5 times the interquartile range (IQR). Points represent outlier observations outside this range.
Figure 2.
Ten-year average exposure predictions based on 2000–2009 data, and jittered Adult Changes in Thought participant residences (as indicated with shaded circles). The map is for example visualization purposes only and therefore shows smoothened predictions. The exposure predictions used in this analysis are at point locations with greater variability (see Table S3).
Baseline population characteristics stratified by mean-centered 10-y average exposure at entry are shown in Table 1. Stratification by mean-centered exposure averages removes the influence of the strong temporal variation that we see in this population and allows us to focus on within-year population comparisons. To calculate the mean-centered values, we subtracted the year-specific mean of 10-y average exposures from each 10-y average exposure.
Mean follow-up time was 9.9 (±5.5) y (minimum: 1 y; maximum: 25 y; median: 9 y). Our analytical cohort included 4,166 individuals with nonmissing APOE genotype. Mean age at entry across the entire cohort was 75 y. Most individuals in the cohort were female (58%), White (90%), and had no APOE alleles (74%). Population characteristics were generally similar across categories of mean-centered baseline ; however, a higher percentage of individuals with elevated mean-centered baseline had only a GED/high school education and were more likely to be born in earlier birth cohort years. Table S4 contains descriptive statistics for the total cohort (; including those with missing APOE genotype) and those dropped from the population due to lack of informative follow-up after baseline ().
Analytical Results
Our primary analysis of all-cause dementia was based on 1,136 events over 41,329 person years from individuals with nonmissing APOE status. When comparing participants with a difference in exposure, adjusting for the a priori (M2) covariates listed above, a higher 10-y average was associated with a 16% greater [1.16 (1.03, 1.31)] hazard of all-cause dementia onset. In our secondary analysis of AD dementia, we estimated that each increment in 10-y average was associated with an 11% greater [1.11 (0.97, 1.27)] hazard of AD diagnosis, after adjusting for the same covariates (Table S5).
Table S6 illustrates that results for all-cause dementia in a secondary analysis using a 20-y exposure period were slightly attenuated in comparison with the primary analysis. Shorter term exposures (1-y, 5-y) as well as the 10-y exposure with a 10-y lag suggested elevated HRs but had confidence intervals consistent with a range of results. Similar patterns are observed for AD dementia (Table S7).
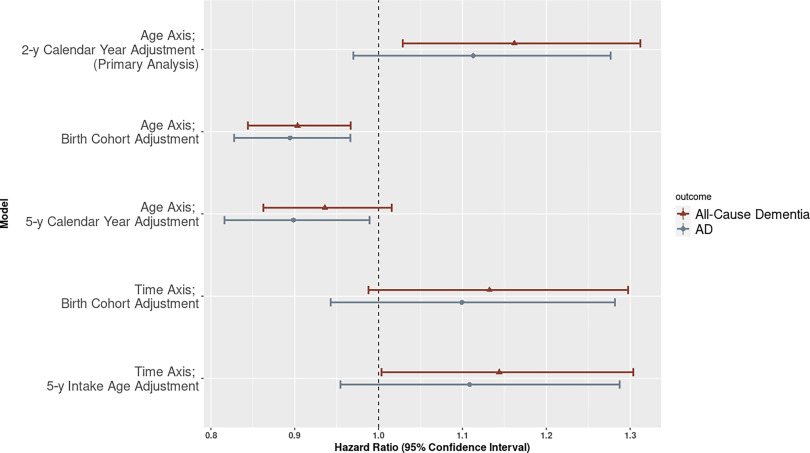
We observed sensitivity to insufficient adjustment for calendar time when using age as the time axis in the Cox model. More specifically, using crude categories to adjust for calendar time (birth cohort categories or 5-y calendar year categories) attenuated the results and indicated an inverse association between and dementia onset [age axis, birth cohort adjustment: 0.90 (0.84, 0.97); age axis, 5-y calendar year adjustment: 0.94 (0.86, 1.02)] (Figure 3; Table S5). Results were also attenuated for AD (age axis, birth cohort adjustment: 0.89 (0.83, 0.97); age axis, 5-y calendar year adjustment: 0.90 (0.82, 0.99) (Figure 3; Table S5). Use of the alternative time axis of calendar time provided estimates that were similar to the primary model for AD [time axis, birth cohort adjustment: 1.10 (0.94, 1.28); time axis, 5-y intake age adjustment: 1.11 (0.95, 1.29)] but slightly attenuated, with the confidence intervals just overlapping the null, for dementia [time axis, birth cohort adjustment: 1.13 (0.99, 1.30); time axis, 5-y intake age adjustment: 1.14 (1.00, 1.30)] Additional sensitivity analyses, including analyses without the use of IPW, were aligned with primary results (Table S5), with the exception that we observed sensitivity (i.e., substantial changes in the effect estimates) to insufficient adjustment for covariates in the crude (unadjusted) model (Tables S6 and S7). Results were strengthened for both non-AD dementia [1.35 (1.04, 1.75)] and after dropping individuals employed at baseline for dementia: [1.24 (1.09, 1.41)] and AD [1.19 (1.03, 1.37)] (Table S5).
Figure 3.
Hazard ratios (95% CI) for a increase in 10-y and all-cause dementia or AD. All models use APOE stratification and adjustment for a priori covariates: sex, educational degree, race, and neighborhood median household income. Choice of time scale and adjustment for calendar time, intake age or birth cohort are indicated in the figure. Corresponding numeric data can be found in Table S5. Note: AD, Alzheimer disease; APOE, apolipoprotein E; CI, confidence interval.
In exploratory interaction analyses, we identified effect modification by sex [male: 1.23 (1.08, 1.39); female: 1.13 (1.00, 1.28); ] but not APOE [: 1.20 (1.06, 1.36); : 1.14 (1.01, 1.29); )], BMI [underweight/normal: 1.17 (1.04, 1.32); overweight/obese: 1.14 (1.00, 1.29); ], education [none/GED/high school/other: 1.17 (1.03, 1.33); bachelor’s/master’s/doctorate: 1.16 (1.03, 1.31); ], or age at intake [: 1.15 (1.02, 1.30); : 1.16 (1.03, 1.31); ] in our analysis of all-cause dementia (Table S8). We did not observe effect modification by sex, APOE, BMI, education, or age at intake for AD (Table S8).
Discussion
In our population-based prospective cohort study with four decades of exposure data and high-quality, standardized, consensus-based outcome ascertainment, we report that each increment in the moving 10-y average was associated with a 16% greater hazard of all-cause dementia diagnosis [1.16 (1.03, 1.31)]. The association with AD dementia indicated an 11% greater hazard [1.11 (0.97, 1.27)]. We observed effect modification by sex in the analysis of all-cause dementia, with a larger estimated HR for males compared with females. We did not observe effect modification by APOE status.
Several prior studies have evaluated the association between and incident ADRD (Excel Table S1). Here, we review these results with HRs rescaled to a increment to facilitate comparison with our work. Using a primary care practice database for older adults in London () with mean () of , a increase in annual average was associated with a 1.03 (0.96, 1.12) increase in the hazard of dementia and a 1.11 (1.02, 1.20) increase in the hazard of AD (Carey et al. 2018). In a study of administrative data for Ontario, Canada (), where mean () was —similar to the average exposure over time in our cohort—a increase in a 2-y lagged 5-y moving average of was associated with a 1.008 (1.006, 1.010) increase in the hazard of dementia (Chen et al. 2017). Using a female-only research cohort in the United States () with a mean () of , Cacciottolo et al. estimated that a increase in 3-y average was associated with 1.07 (1.03, 1.12) increase in the hazard of all-cause dementia (Cacciottolo et al. 2017). Recent studies in Canada using administrative databases based in Vancouver (; mean () for non-AD dementia ) and Quebec (; mean ) reported that increases in over the 4-y exposure period for 1-y average exposures were associated with a 1.01 (0.99, 1.03) increase and 1.004 (1.00, 1.007) increase in the hazard of dementia, respectively (Smargiassi et al. 2020; Yuchi et al. 2020). Our hazard ratios are higher than those from the studies above, perhaps due to our high-quality exposure and outcome ascertainment or the older age distribution of the cohort, further discussed below. Two studies have reported higher hazard ratios than ours for at least one time period evaluated (Grande et al. 2020; Jung et al. 2015), yet concerns about possible biases with regard to their methodological choices suggest caution with interpretation and comparison. (Ilango and Shaffer 2020; Power et al. 2016). For example, our interpretation of the Jung et al. study is that follow-up time differed by disease status (Jung et al. 2015), which could lead to bias given the expected secular trends in exposure. In the recent Grande et al. study, use of dispersion modeling may result in exposure misclassification compared to our use of spatiotemporal models informed by ground monitoring data. Additionally, hazard ratios reported for different exposure time periods are conflicting and neither was designated as the a priori focus for the analysis, which provides challenges for interpretation because it is unclear which period is most appropriate for inference [5 y preceding event: 1.63 (1.38, 1.92); 6–11 y preceding event: 0.81 (0.68, 1.01)]. Dementia diagnosis may also have been problematic: outcome assessment occurred only every 3 or 6 y, depending on participant age; those who died also had dementia diagnosis included based on medical records. Ultimately the limited methodological details leave many unanswered questions.
Some of these studies also investigated potential effect modification by sex and APOE, with overall inconsistent results across the existing limited evidence base. No effect modification by sex was identified in a studies of administrative cohorts in Ontario or Vancouver (Chen et al. 2017; Yuchi et al. 2020), but stratification by sex suggested a higher risk among men in a research cohort in Sweden (Oudin et al. 2018). Women in a research cohort in the United States carrying the APOE risk allele exhibited higher risk (Cacciottolo et al. 2017), but in a research cohort of both men and women in Sweden, there were no meaningful differences when stratifying by the presence of the APOE risk allele (Grande et al. 2020). Our exploratory interaction analyses suggest effect modification by sex for all-cause dementia [male: 1.23 (1.08, 1.39); female: 1.13 (1.00, 1.28); ], but other interaction term p-values were not significant for other subgroup analyses. Although, in general, subgroup analyses should be interpreted with caution (Weiss 2008), we encourage future work in this area, given the plausible biological rationale for differential susceptibility (Dose et al. 2016; Hanamsagar and Bilbo 2016; Jofre‐Monseny et al. 2008).
An important difference between our study and many prior studies is that we used age as the time axis for our primary survival analysis. Some authors have argued that using calendar time as the time axis may lead to bias when age is an important confounder (Cologne et al. 2012; Thiébaut and Bénichou 2004). Because of the strong effect of age on risk of dementia, we initially believed that using age as the time axis would be the most appropriate approach for these analyses; this also allows for straightforward interpretation of the hazard function as the age-specific incidence function (Thiébaut and Bénichou 2004). Yet, it should be noted that using age as the time axis creates a situation where comparisons are made between individuals of the same age, regardless of the calendar time when exposure occurred.
Our exposure of interest—time-varying —demonstrates clear and very strong secular trends (Figure 1), and therefore our Cox model compares individuals of the same age but who lived at different times and experienced different exposures. To adjust for these important secular trends, we determined that it was necessary to include a rich adjustment for calendar time in the risk model. Although we initially planned to adjust for birth cohort categories with age as the time axis, we realized that this categorization was too coarse: To account for small group numbers, some of our categories spanned 20 y, which did not provide adequate control of the strong temporal confounding present in this dataset. We saw similar results with crude adjustment for 5-y categories of calendar time using age as the time axis (Figure 3; Table S5). Thus, our primary analysis uses adjustment for more refined 2-y calendar year categories. Given the very strong decreasing trends in air pollution over time (Figure 1), and the year duration of the ACT cohort, we believe this approach provides the best control of substantial temporal confounding when using age as the time axis in our Cox model. Yet, our decision to finely control for temporal confounding and focus on within-year variation comes at the expense of removing much of the exposure contrast in the inferential analysis: As described above, between year variance was much higher than within-year variance.
Although prior studies have taken varying approaches to adjusting for calendar time when using age as the time scale (Chen et al. 2017; Grande et al. 2020; Smargiassi et al. 2020), the longest follow-up period in previous research () was approximately half the time our study (). Thus, strong secular trends in exposure were less likely, leading to fewer potential concerns with temporal confounding when using age as the time axis. By contrast, as noted above, our extended follow-up period and strong secular trends made it essential to ensure tight control of temporal confounding in our analysis.
Reassuringly, our results using this model are aligned with sensitivity results using calendar time as the time axis, where control for temporal confounding is inherent in the choice of time axis (Figure 3; Table S5). Our experience suggests that models with differing time axes—either age or calendar time—will provide consistent results when there is adequate control of confounding; our disparate results using the age axis with an adjustment for crude categories of either birth cohort or calendar year suggest that coarse groupings of these covariates do not properly control for the strong temporal confounding present in our data set. Future survival analyses of air pollution and dementia should carefully consider the strong trends in both the exposure and outcome when selecting the time axis and covariates (including their functional form) for the Cox model.
A central strength of this study is that it draws on 25 y of high quality, consistently implemented, biennial evaluations of cognitive status and ADRD in the ACT study (Kukull et al. 2002). For prior studies of and ADRD that have used administrative data for outcome ascertainment (Excel Table S1), misclassification is a key concern (Wilkinson et al. 2018).
Using a newly developed spatiotemporal exposure prediction model specifically for the Puget Sound region, we were able to estimate residence-based for 40 y (1978–2018). Prior to our study, the longest exposure model, which was developed based on dispersion modeling, covered 22 y (Grande et al. 2020). We complemented these exposure data with address histories available through Group Health/Kaiser Permanente of Washington records, with nearly complete histories since 1989 for the entire cohort and reasonably good coverage prior to 1989. Overall, we were able to estimate 10-y average exposures using known address history for 91% of the person-years.
Evaluating a long exposure averaging period is crucial for this research question, given the extended period of disease development in ADRD (Jack et al. 2010). In fact, in our data set, hazard ratios from shorter term averaging periods (1-y, 5-y) were attenuated, suggesting that these exposure windows may be inadequate to capture the true effects of . Most prior studies have focused on exposure averaging periods of 5 y or less for the Cox model (Excel Table S1). Thus, our study is unique in the ability to estimate 10-y exposures with high coverage and quality across the cohort and over time.
A challenge for our extended exposure coverage over time is that there were limited monitoring sites across the region in the early years; as such, we cannot rule out the possibility of higher exposure measurement error in early time periods. However, many of the features that predict spatial contrasts in exposure, such as heavy industry, shipping, and the road network, have been in place for the entire time period, suggesting that it is appropriate to leverage more recent spatial information to predict historical spatial contrasts. Prior work on patterns of woodsmoke in the Seattle area provides evidence to support this assumption of consistent spatial contrasts over time in this region (Su et al. 2008).
We were only able to include estimates of in our models because spatiotemporal models for other pollutants were not available for such extended follow-up periods. Other common air pollutants, such as ozone, nitrogen oxides, and ultrafine particles, may also play a role in ADRD and related neurodegeneration (Peters et al. 2019). Focusing on the single pollutant effect does not fully capture the impact of real-world, multipollutant exposures.
A common challenge in cohort studies—particularly those of elderly populations—is selection bias, which occurs with differential enrollment or attrition of study participants. However, the ACT study has an exceptional Completeness of Follow-up Index (CFI) (Clark et al. 2002), which reflects the proportion of planned visits for which data are available; the ACT CFI of 95.6% minimizes our concern with bias from selective attrition. Still, differential enrollment is a potential concern: Individuals had to have health insurance, survive, and be free of dementia to enter the cohort. Yet, eligibility began at age 65 y, which reduces our concern with selection bias due to insurance coverage because of Medicare eligibility. Membership in Group Health/Kaiser Permanente for individuals of age is representative of the analogous Medicare population in the regional catchment area, and the ACT cohort draws a random sample from the Group Health/KP system (Kukull et al. 2002).
Because of the population-based sampling approach, mean age of entry in our cohort was 75 y, with a range of 65–101. Our cohort is older than most cohorts in prior analogous studies, which may contribute to differences in effect estimates. For example, it is possible that older individuals are more susceptible to the adverse effects of , contributing to the higher HRs observed in our study compared to most prior studies. On the other hand, older enrollees had to survive free of dementia for a longer period, and characteristics that contributed to their healthy survival might create bias in the other direction. In the case of smoking and AD, it has been hypothesized that selection bias may account for the apparent “protective” effect of smoking: due to premature mortality and/or early ADRD diagnosis, smokers are eliminated from the eligible population (Hernán et al. 2008). It is possible that a similar situation could arise with exposure, given the well-established link to premature mortality (Liu et al. 2019) and the growing link to ADRD. In this scenario, our effect estimate would likely be biased to the null. This may also explain our null estimates regarding effect modification for most selected variables. There is also possible bias in our effect estimates because the traditional Cox model approach does not account for competing risk of death. Finally, it should also be noted that our cohort was fairly healthy at baseline, with relatively low rates of comorbidities such as CVD and diabetes. Our results might not be generalizable to a population with higher rates of comorbidities.
Overall, there is biological plausibility for the effects observed in our study. The central nervous system (CNS) effects of may be mediated through direct and/or indirect pathways leading to oxidative stress and inflammation (Block and Calderón-Garcidueñas 2009; Heusinkveld et al. 2016; Jayaraj et al. 2017). The direct pathway involves the translocation of particulate matter and associated toxic constituents to the brain, where they may trigger local inflammatory and oxidative stress reactions (Block and Calderón-Garcidueñas 2009; Jayaraj et al. 2017). Plausible routes through which this particle transfer could occur include transport through the olfactory, trigeminal, or vagal nerves, or passage from the peripheral circulation through the blood brain barrier (Lucchini et al. 2012). In contrast, in the indirect pathway scenario, PM components do not physically enter the brain but instead trigger systemic oxidative stress and inflammatory reactions that may spill over into the CNS (Jayaraj et al. 2017). There are well-established linkages between air pollution and vascular disease (Brook et al. 2010) as well as a growing understanding of the vascular contributions to dementia (Gorelick et al. 2011). The fact that our results were attenuated when focusing on AD-subtype dementia may suggest the importance of vascular injury—and the indirect, peripheral pathway—in mediating the association between air pollution and dementia (Ilango et al. 2020).
Conclusion
In this population-based prospective cohort study with region-specific exposure data covering four decades and high quality, standardized, consensus-based outcome ascertainment, we report that a increase in 10-yr average was associated with a higher hazard of all-cause dementia [1.16 (1.03, 1.31)]. These results add to a growing body of both epidemiological and toxicological evidence on the neurodegenerative effects of air pollution and suggest that reducing exposures across the population could contribute to reducing the burden of dementia (Livingston et al. 2020). Additionally, our findings suggest that when conducting a survival analysis with an outcome that is age-related and an exposure that has strong temporal trends, use of either age or calendar time as the time axis in the Cox model can be appropriate, as long as there is adequate control of the alternate confounding factor; these observations can help inform and strengthen future related epidemiological research.
Supplementary Material
Acknowledgments
The authors thank C. Schumacher (previously at the University of Washington) and A. Gassett for their work assembling data for and developing the fine particulate matter exposure model; B. High, A. Kritchevsky, and C. Lanfear for their programming and data analysis support; R. Pardee for his data management; and ACT study participants, staff, and family members for their essential contributions to this research.
R.M.S., M.N.B., G.L., and L.S. conceptualized and designed the analysis. R.M.S. conducted data analysis and led manuscript preparation, drafting, and revisions. S.D.A., M.C., A.A.S., J.D.K., and T.V.L. advised on key analytical issues. E.B.L. and P.K.C. manage the ACT study and provided guidance during the analysis and write-up. All authors reviewed, edited, and approved the final manuscript.
R.M.S. was supported by National Institute for Environmental Health Sciences (NIEHS) F31ES030972-02, NIEHS T32ES015459, National Institute on Aging (NIA) T32AG052354, the University of Washington Retirement Association Aging Fellowship, and the Seattle Chapter of the Achievement Rewards for College Scientists (ARCS) Foundation. M.N.B. was supported by NIEHS T32ES015459 and the Seattle ARCS foundation. G.L., M.C., A.A.S., J.D.K., T.V.L., E.B.L., P.K.C., and L.S. were supported by NIEHS and NIA R01ES026187. E.B.L. and P.K.C. were supported by U01 AG006781. J.D.K. was supported by NIEHS P30ES007033. Additional sources of support for study maintenance and data collection include NIEHS ES026187, NIA AG05136, R01 AG056711 and U01 NS091272.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516, PMID: 19716187, 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, et al. 2017. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 7(1):e1022, PMID: 28140404, 10.1038/tp.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey IM, Anderson HR, Atkinson RW, Beevers SD, Cook DG, Strachan DP, et al. 2018. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 8(9):e022404, PMID: 30206085, 10.1136/bmjopen-2018-022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Hystad P, van Donkelaar A, Tu K, et al. 2017. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int 108:271–277, PMID: 28917207, 10.1016/j.envint.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Clark TG, Altman DG, De Stavola BL. 2002. Quantification of the completeness of follow-up. Lancet 359(9314):1309–1310, PMID: 11965278, 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernán MA. 2008. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168(6):656–664, PMID: 18682488, 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cologne J, Hsu W-L, Abbott RD, Ohishi W, Grant EJ, Fujiwara S, et al. 2012. Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiology 23(4):565–573, PMID: 22517300, 10.1097/EDE.0b013e318253e418. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small G, et al. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123):921–923, PMID: 8346443, 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crane PK, Narasimhalu K, Gibbons LE, Mungas DM, Haneuse S, Larson EB, et al. 2008. Item response theory facilitated cocalibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol 61(10):1018–1027. e1019, PMID: 18455909, 10.1016/j.jclinepi.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dose J, Huebbe P, Nebel A, Rimbach G. 2016. APOE genotype and stress response-a mini review. Lipids Health Dis 15(1):1–15, PMID: 27457486, 10.1186/s12944-016-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. 2009. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol Allergy Clin North Am 29(2):321–337, PMID: 19389585, 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. 2011. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42(9):2672–2713, PMID: 21778438, 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G, Ljungman PLS, Eneroth K, Bellander T, Rizzuto D. 2020. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol 77(7):801, PMID: 32227140, 10.1001/jamaneurol.2019.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. 2015. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 175(3):401–407, PMID: 25621434, 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Walker R, Dublin S, Haneuse S, Crane PK, Breitner JCS, et al. 2011. Histamine‐2 receptor antagonist use and incident dementia in an older cohort. J Am Geriatr Soc 59(2):251–257, PMID: 21314645, 10.1111/j.1532-5415.2010.03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guze SB. 1995. Diagnostic and statistical manual of mental disorders, 4th ed. (DSM-IV). Am J Psychiatry 152(8):1228–1228, 10.1176/ajp.152.8.1228. [DOI] [Google Scholar]
- Hanamsagar R, Bilbo SD. 2016. Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol 160:127–133, PMID: 26435451, 10.1016/j.jsbmb.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Alonso A, Logroscino G. 2008. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology 19(3):448–450, PMID: 18414087, 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- Heusinkveld HJ, Wahle T, Campbell A, Westerink RHS, Tran L, Johnston H, et al. 2016. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 56:94–106, PMID: 27448464, 10.1016/j.neuro.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Ilango SD, Chen H, Hystad P, van Donkelaar A, Kwong JC, Tu K, et al. 2020. The role of cardiovascular disease in the relationship between air pollution and incident dementia: a population-based cohort study. Int J Epidemiol 49(1):36–44, PMID: 31347651, 10.1093/ije/dyz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango SD, Shaffer RM. 2020. Air pollution, cardiovascular disease, and dementia. JAMA Neurol 77(12):1581, PMID: 33165534, 10.1001/jamaneurol.2020.4309. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. 2010. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9(1):119–128, PMID: 20083042, 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj RL, Rodriguez EA, Wang Y, Block ML. 2017. Outdoor ambient air pollution and neurodegenerative diseases: the neuroinflammation hypothesis. Curr Environ Health Rep 4(2):166–179, PMID: 28444645, 10.1007/s40572-017-0142-3. [DOI] [PubMed] [Google Scholar]
- Jofre‐Monseny L, Minihane AM, Rimbach G. 2008. Impact of APOE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res 52(1):131–145, PMID: 18203129, 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- Jung CR, Lin YT, Hwang BF. 2015. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimers Dis 44(2):573–584, PMID: 25310992, 10.3233/JAD-140855. [DOI] [PubMed] [Google Scholar]
- Keller JP, Olives C, Kim S-J, Sheppard L, Sampson PD, Szpiro AA, et al. 2015. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the multi-ethnic study of atherosclerosis and air pollution. Environ Health Perspect 123(4):301–309, PMID: 25398188, 10.1289/ehp.1408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn EL, Graubard BI, Midthune D. 1997. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 145(1):72–80, PMID: 8982025, 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. 2002. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 59(11):1737–1746, PMID: 12433261, 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- Lamarca R, Alonso J, Gomez G, Muñoz Á. 1998. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci 53(5):M337–M343, PMID: 9754138, 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- Li G, Higdon R, Kukull WA, Peskind E, Moore KVV, Tsuang D, et al. 2004. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology 63(9):1624–1628, PMID: 15534246, 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al. 2019. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 381(8):705–715, PMID: 31433918, 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Liu C-C, Kanekiyo T, Xu H, Bu G. 2013. Apolipoprotein e and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9(2):106–118, PMID: 23296339, 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LJ, Box M, Kalman D, Kaufman J, Koenig J, Larson T, et al. 2003. Exposure assessment of particulate matter for susceptible populations in Seattle. Environ Health Perspect 111(7):909–918, PMID: 12782491, 10.1289/ehp.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L-JS, James CS, Timothy VL. 2002. Comparison of light scattering devices and impactors for particulate measurements in indoor, outdoor, and personal environments. Environ Sci Technol 36(13):2977–2986, PMID: 12144275, 10.1021/es0112644. [DOI] [PubMed] [Google Scholar]
- Lindström J, Szpiro AA, Sampson PD, Oran AP, Richards M, Larson TV. 2014. A flexible spatio-temporal model for air pollution with spatial and spatio-temporal covariates. Environ Ecol Stat 21(3):411–433, PMID: 25264424, 10.1007/s10651-013-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. 2020. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396(10248):413–446, PMID: 32738937, 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, Veronesi B. 2012. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 33(4):838–841, PMID: 22178536, 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. 1984. Clinical diagnosis of Alzheimer’s disease. Neurology 34(7):939–944, PMID: 6610841, 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meng X, D’Arcy C. 2012. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 7(6):e38268, PMID: 22675535, 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ. 2014. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun 42:10–21, PMID: 24727365, 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, et al. 2019. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(1):88–106, PMID: 30497964, 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Segersson D, Adolfsson R, Forsberg B. 2018. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS One 13(6):e0198283, PMID: 29897947, 10.1371/journal.pone.0198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. 2019. Air pollution and dementia: a systematic review. J Alzheimers Dis 70(s1):S145–S163, PMID: 30775976, 10.3233/JAD-180631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Adar SD, Yanosky JD, Weuve J. 2016. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology 56:235–253, PMID: 27328897, 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puget Sound Clean Air Agency. 2018. 2017 Air Quality Data Summary. https://pscleanair.gov/DocumentCenter/View/3337/Air-Quality-Data-Summary-2017?bidId= [accessed 15 July 2021]. [Google Scholar]
- Sampson PD, Szpiro AA, Sheppard L, Lindström J, Kaufman JD. 2011. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ 45(36):6593–6606, 10.1016/j.atmosenv.2011.04.073. [DOI] [Google Scholar]
- Schikowski T, Vossoughi M, Vierkötter A, Schulte T, Teichert T, Sugiri D, et al. 2015. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res 142:10–16, PMID: 26092807, 10.1016/j.envres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Schulte JK, Magzamen S, Oron AP, Beaudet N, Kaufman JD, Larson TV, et al. 2013. Diesel Exhaust Exposure in the Duwamish Study: Technical Report. Seattle, WA: University of Washington School of Public Health, Department of Environmental Health Sciences. http://www.duwamishdiesel.org/home/technical-report. [Google Scholar]
- Smargiassi A, Sidi EAL, Robert L-E, Plante C, Haddad M, Gamache P, et al. 2020. Exposure to ambient air pollutants and the onset of dementia in Quebec, Canada. Environ Res 190:109870, PMID: 32739624, 10.1016/j.envres.2020.109870. [DOI] [PubMed] [Google Scholar]
- Stern Y. 2012. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11(11):1006–1012, PMID: 23079557, 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JG, Buzzelli M, Brauer M, Gould T, Larson TV. 2008. Modeling spatial variability of airborne levoglucosan in Seattle, Washington. Atmos Environ 42(22):5519–5525, 10.1016/j.atmosenv.2008.03.023. [DOI] [Google Scholar]
- Szpiro AA, Sampson PD, Sheppard L, Lumley T, Adar SD, Kaufman J. 2009. Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics 21(6):606–631, PMID: 24860253, 10.1002/env.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiébaut ACM, Bénichou J. 2004. Choice of time‐scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med 23(24):3803–3820, PMID: 15580597, 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. 2011. Apolipoprotein e in Alzheimer’s disease and other neurological disorders. Lancet Neurol 10(3):241–252, PMID: 21349439, 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NS. 2008. Subgroup-specific associations in the face of overall null results: should we rush in or fear to tread? Cancer Epidemiol Biomarkers Prev 17(6):1297–1299, PMID: 18559544, 10.1158/1055-9965.EPI-08-0144. [DOI] [PubMed] [Google Scholar]
- Wilkinson T, Ly A, Schnier C, Rannikmäe K, Bush K, Brayne C, et al. 2018. Identifying dementia cases with routinely collected health data: a systematic review. Alzheimers Dement 14(8):1038–1051, PMID: 29621480, 10.1016/j.jalz.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuchi W, Sbihi H, Davies H, Tamburic L, Brauer M. 2020. Road proximity, air pollution, noise, green space and neurologic disease incidence: a population-based cohort study. Environ Health 19(1):8, PMID: 31964412, 10.1186/s12940-020-0565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman M, Schumacher CS, Gassett AJ, Spalt EW, Austin E, Larson TV, et al. 2020. Calibration of low-cost particulate matter sensors: model development for a multi-city epidemiological study. Environ Int 134:105329, PMID: 31783241, 10.1016/j.envint.2019.105329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.