Abstract
Background:
A prominent therapeutic indication for alcohol use disorder (AUD) is reduction in chronic repetitive alcohol use. Glutamate α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (AMPARs) regulate chronic alcohol self-administration in preclinical models. Recent evidence indicates that the expression and function of AMPARs require the transmembrane AMPAR regulatory protein γ-8 (TARP γ-8). This study evaluated the preclinical efficacy of JNJ-55511118, a novel, selective, high-affinity inhibitor of TARP γ-8-bound AMPARs, in reducing chronic operant alcohol self-administration.
Methods:
Separate groups of male and female C57BL/6J mice (n = 8/sex/group) were trained to lever press for sweetened alcohol (9% v/v + sucrose 2% w/v) or sucrose only (2% w/v) in operant conditioning chambers using an FR-4 schedule of reinforcement. After a 40-day baseline, JNJ-55511118 (0, 1, and 10 mg/kg, p.o.) was administered in randomized order 1 h before self-administration sessions. Parameters of operant behavior including response rate, total reinforcers, and head entries in the drinking troughs were computer recorded.
Results:
During baseline, responding to alcohol, but not sucrose, was greater in female than male mice. In male mice, both doses of JNJ-55511118 decreased multiple parameters of alcohol self-administration but did not reduce behavior-matched sucrose-only self-administration. JNJ-55511118 had no effect on sweetened alcohol or sucrose self-administration in female mice. Subsequent tests of motor function showed that the lowest effective dose of JNJ-55511118 (1 mg/kg) had no effect on open-field activity in male mice.
Conclusions:
This study shows for the first time that TARP γ-8-bound AMPARs regulate a behavioral pathology associated with addiction. The preclinical efficacy of JNJ-55511118 in reducing alcohol self-administration in male mice suggests that inhibition of TARP γ-8-bound AMPARs is a novel and highly significant neural target for developing medications to treat AUD and other forms of addiction.
Keywords: alcohol self-administration, AMPA, behavior, JNJ-55511118, TARP γ-8
INTRODUCTION
The high prevalence of alcohol use disorders (AUDs) represents one of the most significant health problems in modern society. In the United States, over 14 million adults age 18+ (male = 64%, female = 36%) were reported to have AUD in 2018 (SAMHSA, 2019). The Centers for Disease Control estimate that approximately 95,000 people die each year from alcohol-related causes (CDC, 2019) resulting in an economic cost of $249 billion (Sacks et al., 2015). However, only 16% of individuals with AUD receive treatment (male = 63%; female = 37%; SAMHSA, 2019), which underscores the need for development of new effective therapeutic strategies to treat AUD and associated disorders.
A prominent therapeutic indication of AUD is reduction in chronic repeated alcohol use. It has been long known that neural mechanisms of reward and reinforcement underlie the repetitive nature of alcohol-seeking behavior (Hodge et al., 1992, 1995, 1997; Samson & Hodge, 1993; Samson et al., 1992) and promote the development and maintenance of AUD (Koob & Volkow, 2010; Wise & Koob, 2013). Thus, a goal for preclinical AUD medication development is to identify neural targets that drive reinforcement processes and validate compounds that inhibit, or modulate, this action (Anton et al., 1995; Faccidomo et al., 2018; Gorini et al., 2011; Heilig & Egli, 2006).
Alcohol gains enduring control over behavior by engaging glutamatergic α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor (AMPAR) mechanisms of synaptic plasticity in brain reward pathways (Loweth et al., 2014; Marty & Spigelman, 2012; McCool, 2011; Winder et al., 2002). In support of this concept, preclinical evidence indicates that AMPAR activity in the amygdala is both targeted by self-administered alcohol and required for the positive reinforcing effects of the drug (Sailing et al., 2016). Alternatively, activation of AMPARs, systemically or in amygdala, promotes escalated alcohol self-administration (Agoglia et al., 2015; Cannady et al., 2013; Cannady et al., 2016). Together, these and other findings suggest that alcohol use is associated with dysfunctional AMPAR activity within specific neural pathways and that selective inhibition of this system may provide a targeted strategy for treating behavioral pathologies in AUD (Holmes et al., 2013). However, inhibition of AMPAR activity is associated with adverse side effects, including motor impairment and memory disruption (Reuillon et al., 2017), which has complicated development of AMPAR-based pharmacotherapies for a variety of disorders.
One promising pharmacotherapeutic strategy for negatively modulating AMPAR activity was recently developed that involves targeting a subgroup of AMPARs that are bound to the transmembrane AMPAR regulatory protein gamma 8 (TARP γ-8; Maher et al., 2016). TARP γ-8 is enriched in the postsynaptic density (PSD) and is required for postsynaptic expression and activity of AMPARs, and LTP (Bissen et al., 2019; Maher et al., 2016; Patriarchi et al., 2018; Rouach et al., 2005). Anatomically, TARP γ-8 expression is highly restricted to corticolimbic brain regions such as the hippocampus (HPC), prefrontal cortex (PFC), and basolateral amygdala (Maher et al., 2016); all of which are known to be sensitive to alcohol exposure and regulate AUD-related behaviors and physiology (Faccidomo et al., 2016; Hodge et al., 1996; Hopf & Mangieri, 2018; Talani et al., 2014; White & Swartzwelder, 2004). Interestingly, these brain regions also send excitatory glutamatergic projections to the nucleus accumbens, which suggests that systemic inhibition of TARP γ-8-bound AMPARs may represent a selective pharmacotherapeutic strategy for modulating specific reward-related behaviors, such as chronic repetitive alcohol self-administration.
To test this hypothesis, we evaluated the preclinical efficacy of JNJ-55511118 (Figure 1A), a newly characterized high affinity and selective negative modulator of AMPARs containing TARP γ-8 (Maher et al., 2016) to reduce alcohol self-administration in C57BL/6J mice. JNJ-55511118 selectively inhibits this subclass of AMPARs by disrupting the interaction between TARP γ-8 and the pore-forming GluA subunits (Figure 1B). JNJ-55511118 is highly brain penetrant in both mice and rats and exhibits >80% receptor occupancy up to 6-hrs following an oral dosage of 10 mg/kg (Maher et al., 2016). The compound exhibits significant dose-dependent inhibition of AMPAR-mediated synaptic activity and shows strong anticonvulsant effects in rodent models with a minimal side-effect profile (Maher et al., 2016).
FIGURE 1.
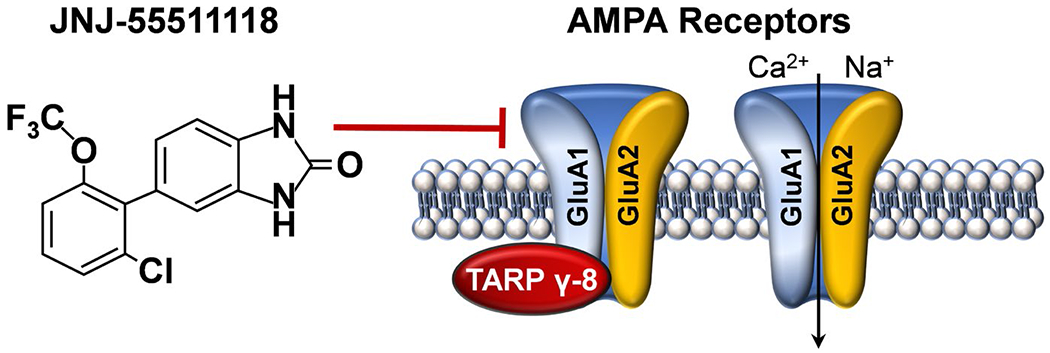
Chemical structure and mechanism of action of JNJ-55511118. Evidence indicates that JNJ-55511115 selectively inhibits glutamate AMPARs bound to TARP γ-8 (Maher et al., 2016)
In the experiments described here, JNJ-55511118 was administered to male and female C57BL/6J mice prior to operant self-administration sessions where lever-press responses were reinforced by presentation of sweetened alcohol (9% EtOH v/v + 2% sucrose w/v) or sucrose-only (2% w/v) as a behavior-matched nondrug control. This well-characterized method (Faccidomo et al., 2009, 2015, 2016, 2019; Salling et al., 2016, 2017) provides a parallel behavior-matched nondrug control, which is critical for evaluating the behavioral specificity of changes in alcohol self-administration; thus, altered sucrose-only self-administration as a control can reveal nonspecific motor, memory, or general reward-related disruption. In general, use of sweetened alcohol solutions in preclinical research also provides enhanced face validity to the binge-intoxication stage of alcohol addiction in humans where sweeteners are commonly added to alcohol (Crabbe et al., 2011). Moreover, QTL research with recombinant inbred mouse strains shows that 59% of the genetic variation in consumption of sweetened 10% alcohol and saccharin is held in common; however, there is no genetic correlation between unsweetened 10% alcohol and saccharin intake (Phillips et al., 1994). Thus, preclinical research that identifies neural mechanisms of sweetened alcohol as compared to sucrose-only self-administration provides enhanced face and mechanistic validity under rigorous behavioral control conditions.
This study is the first evaluation of the mechanistic role of TARP γ-8-containing AMPARs in regulating behavioral pathologies associated with AUD or other forms of addiction. Overall results indicate that JNJ-55511118 exhibits preclinical efficacy for the reduction in chronic repetitive alcohol self-administration. Inhibition of TARP γ-8-containing AMPARs represents a highly novel method for treating addiction and other neurological disorders associated with glutamatergic hyperactivity.
MATERIALS AND METHODS
Mice
Male and female C57BL/6J mice (The Jackson Laboratory) were group-housed in techniplast cages (28 × 17 × 14 cm). A PVC pipe (8 cm long × 5 cm wide) and cotton nestlet were placed in each cage to provide environmental enrichment. Food and water were available ad libidum except where indicated. The housing and separate behavioral testing rooms were maintained on a 12 h:12 h light/dark cycle (dark 7:00 a.m. to 7:00 p.m.) with temperature at 21 ± 1°C and humidity at 40 ± 2%. Experimental procedures involving mice were approved by the University of North Carolina at Chapel Hill Institutional Care and Use Committee and conducted as recommended by the Guide for the Care and Use of Laboratory Animals (NRC, 2011).
Operant alcohol or sucrose self-administration
Adaptation to alcohol and sucrose solutions
One week after arrival in the laboratory, mice were provided with two drinking bottles in the home cage for 2 weeks. The alcohol self-administration group voluntarily consumed a sweetened alcohol solution (alcohol 9% v/v + sucrose 2% w/v) or water. The control group consumed a sucrose-only solution (sucrose 2% w/v) or water. Our research has shown that prior home-cage exposure access to the alcohol solution is associated with reliable and consistent intake in subsequent operant self-administration procedures (Faccidomo et al., 2015).
Apparatus: operant conditioning chambers
After 2 weeks of home-cage alcohol and sucrose exposure, mice were trained to lever press for presentation of sweetened alcohol or sucrose-only reinforcement in computer-controlled operant conditioning chambers (Med Associates) as previously described (Faccidomo et al., 2009, 2015, 2016, 2019; Hodge et al., 2006; Salling et al., 2008). Each operant chamber was housed in a sound-attenuating cabinet that was ventilated by a 28 V fan, which attenuated external noise. A stainless steel ultrasensitive retractable response lever was located on the left side of the left and right walls. Responses on one lever (the “active” lever) were reinforced by pump-activated delivery of the alcohol or sucrose solution into a drinking trough located adjacent to the lever in the center of the wall. Lever press responses emitted on the alternate “inactive” lever were computer-recorded but produced no programmed consequence(s). Delivery of the alcohol or sucrose-only solution was accompanied by simultaneous illumination of a stimulus light (800 ms). Head entries (headpokes) into the drinking receptacle were recorded by interruption of a photobeam spanning the opening. Headpokes per reinforcer were then calculated as a measure of consummatory behavior. Responses that were emitted during reinforcer delivery did not count toward completion of the subsequent FR-4 response requirement. Operant chambers were connected to an interface and computer (Windows PC running MED-PC for Windows v.4.1) that recorded inputs (lever-press and headpoke responses in real time) and controlled outputs (light, pump activation). Fluid intake was confirmed by an experimenter at the end of each session by visual examination of the drinking trough. All fluids delivered were consumed during all sessions.
Operant self-administration training
Self-administration of sweetened alcohol and sucrose-only solutions was established using a well-characterized procedure (Faccidomo et al., 2009; Salling et al., 2008). For initial training, mice (N = 32) were water restricted for 23-hrs and then placed in an operant chamber for three 16-h sessions during which spontaneous exploratory behavior results in acquisition of the operant lever-press response. During the first 16-h session, responses on the active lever (counter-balanced across subjects) were reinforced on a fixed-ratio one (FR 1) schedule of reinforcement by delivery of sweetened alcohol (n = 16, 8 males, 8 females) or sucrose-only (n = 16, 8 males, 8 females) into the drinking trough (0.014 ml) after each response. During the second 16-h session, the reinforcement schedule increased from FR 1 to FR 2 and to a final value of FR-4 after completion of each successive 25 increments (e.g., after earning 25 reinforcers at each schedule value). During the third and final 16-h training session, the reinforcement schedule is set at FR-4 for 16 h. All subsequent self-administration sessions were 1 h in duration and the reinforcement schedule remained constant at FR-4. Sessions were conducted 5 days per week (M–F) during the dark phase of the light cycle (between 1200 and 1600 h). Our prior research has shown that this procedure results in pharmacologically relevant blood alcohol (BAC; Faccidomo et al., 2009). BAC was not measured in the present study to prevent behavioral disruption and potential stress-induced insertion of calcium-permeable AMPARs (Kuniishi et al., 2020), which are functionally regulated by TARP γ-8 (Bats et al., 2013).
Drugs
The sweetened alcohol solution was prepared by diluting 95% EtOH (Pharmco Products Inc.) in a sucrose solution to produce alcohol (9% v/v) + sucrose (2% w/v). Sucrose (2% w/v) was prepared in distilled water. The high affinity and selective negative modulator of AMPA receptors containing TARP γ-8, 5-[2-Chloro-6-(trifluoromethoxy)phenyl]-1,3-dihydro-2H-benzimidazol-2-one (JNJ-55511118) was obtained from Tocris. JNJ-55511118 (0, 1, or 10 mg/kg) was suspended in 10% (w/v) carboxymethyl-β-cyclodextrin sodium salt (CMC) and administered by oral gavage (p.o.) in a volume of 1 ml/100 g body weight. JNJ-55511118 (10 mg/kg, p.o.) reaches 80% receptor occupancy at 1-h post-oral administration and declines to 50% occupancy at 6 h after administration in mice (Maher et al., 2016). JNJ-55511118 failed to go into CMC suspension at concentrations higher than 1.0 mg/ml; thus, 10.0 mg/kg was the maximum dose tested.
Sweetened alcohol or sucrose-only self-administration: effects of JNJ-55511118
After a 40-day baseline of sweetened alcohol or sucrose-only reinforced, male and female mice were habituated to vehicle (CMC) administration by oral gavage (p.o., 1-h pretest) until levels of self-administration following vehicle administration were unchanged as compared to noninjection sessions (2 to 4 injections). Next, the selective JNJ-55511118 (0, 1, or 10 mg/kg, p.o.) was injected 1 h before a self-administration test session. The drug dose range was selected based on evidence that JNJ-55511118 reaches 80% receptor occupancy at 1 h after p.o. administration in mice (Maher et al., 2016). Vehicle and individual drug doses were administered according to a Latin square design with no more than two injections per week. Injections were performed only if responding returned to baseline levels during the interval since the prior injection.
Open-field locomotor activity: effects of JNJ-55511118
Apparatus
After completion of the dose–response curves on self-administration, male mice were evaluated in the open-field activity test for potential locomotor effects of the lowest effective dose of JNJ-55511118 (0 or 1 mg/kg). Open-field locomotor activity (distance traveled in cm) was measured in computer-controlled activity chambers (27.9 cm2; ENV-510, Med Associates) as previously described (Agoglia et al., 2016; Faccidomo et al., 2020; Stevenson et al., 2008). Horizontal distance (cm) traveled in the open field was calculated by Activity Monitor Software (Med Associates, v.7.) that counted the number of infrared photobeam breaks (grid pattern, 2.54-cm intervals).
Procedure
Mice were first habituated by being placed in the locomotor test chamber for a single 2-h session. After 1 week, half of the male mice with a history of alcohol or sucrose self-administration were administered JNJ-55511118 (0 or 1 mg/kg, p.o.). Mice were then placed in the center of the locomotor chamber 1 h after injection in accordance with the inject-to-test interval in operant self-administration studies. One week later, the alternate treatment (vehicle or drug) was administered in a counterbalanced order.
Statistical analysis
All statistical comparisons and graphing were conducted with Prism (Version 9.0, GraphPad Software). Parameters of operant behavior collected were total response on the active and inactive levers, reinforcers earned, alcohol g/kg consumed, sucrose ml/kg consumed, and headpokes into the drinking trough during each session. Male and female baseline data were compared by between-subjects t-test. Due to the significant sex-dependent differences in baseline alcohol and sucrose self-administration, male and female JNJ-55511118 data were analyzed separately. JNJ-55511118 drug dose-effect (0, 1, 10 mg/kg) curves were analyzed within sex by one-way repeated measures analysis of variance (RM ANOVA). Significant drug dose effects were followed by comparison to vehicle by Holm–Šídák’s multiple comparisons test. Operant response rate (cumulative responses/5-min intervals) was analyzed by two-way RM ANOVA (DOSE × TIME) to assess dose- and time-dependent effects of JNJ-55511118. Locomotor-dependent measures were assessed with a paired t-test (0 vs 1.0 JNJ-5551118). The α-level was set at 0.05 for all statistical tests.
RESULTS
Baseline alcohol and sucrose self-administration
Alcohol
Results showed that female mice self-administered significantly more sweetened alcohol than male mice during baseline (Figure 2A–E). When averaged over the course of the 40-day baseline period, female mice exhibited significantly more responses on the alcohol-reinforced lever (t = 2.032, df = 14, p = 0.03; Figure 2A). However, there was not a sex difference in responding on the inactive lever (t = 1.116, df = 14, p = 0.142, ns; Figure 2B). Increased responses by female mice on the active lever resulted in a significant increase both in number of reinforcers (t = 2.280, df = 14, p = 0.02; Figure 2C) and EtOH intake (g/kg/1 h; t = 3.761, df = 14, p = 0.001; Figure 2D). There was no sex-dependent difference in the percentage of responses emitted on the active versus inactive lever, with both male (MEAN ± SEM; 76.5 ± 4.3%) and female mice (MEAN ± SEM; 76.6 ± 4.4) exhibiting similar lever discrimination (t = 0.009, df = 14, p = 0.5, ns; data not shown). The number of headpokes per reinforcer was not different between male and female mice (t = 0.6704, df = 14, p = 0.26, ns; Figure 2E) suggesting that there was no sex-dependent difference general consummatory behavior.
FIGURE 2.
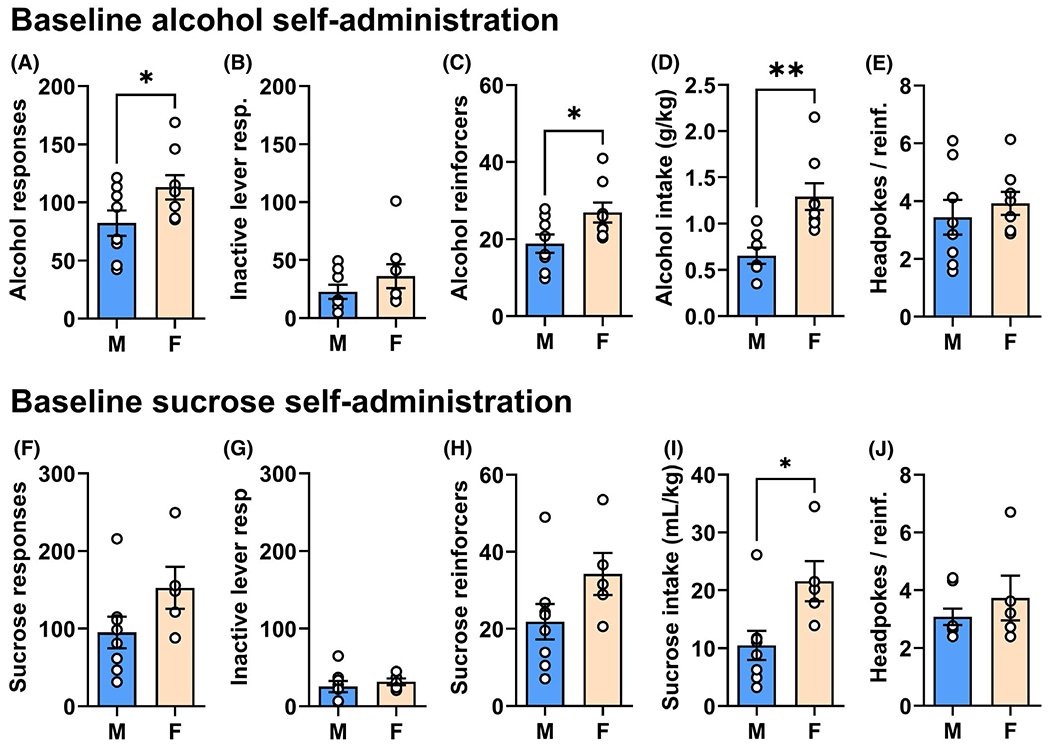
Operant self-administration behavior by male (M) and female (F) mice during baseline. Baseline measures of operant sweetened alcohol self-administration shown as (A) total alcohol lever-press responses, (B) inactive lever responses, (C) number of reinforcers earned, (D) intake, and (E) headpokes / reinforcer. Bars represent MEAN ± SEM performance by male (n = 8) and female (n = 8) mice over the 40-day baseline period. And baseline measures of sucrose-only self-administration shown as (F) total alcohol lever-press responses, (G) inactive lever responses, (H) number of reinforcers earned, (I) intake, and (J) headpokes/reinforcer. Bars represent MEAN ± SEM performance by male (n = 8) and female (n = 5). * indicates significant difference between male and female, t-test, p ≤ 0.05
Sucrose
There were no statistically significant differences between male and female mice on measures of sucrose-reinforced active lever responding (t = 1.724, df = 11, p = 0.056, ns; Figure 2F) or inactive lever responding (t = 0.626, df = 11, p = 272, ns; Figure 2G). Although there was no sex-dependent difference in the number of sucrose reinforcers earned (t = 1.709, df = 11, p = 0.057, ns; Figure 2H), there was an increase in overall sucrose intake by female mice (t = 2.653, df = 11, p = 0.01; Figure 2I). No sex-dependent differences were observed in the percentage of responses emitted on the sucrose-associated lever compared with the inactive lever for both male (MEAN ± SEM; 78.1 ± 4.8) and female (78.6 ± 5.0) mice (t = 0.07927, df = 11, p = 0.47, ns; data not shown). Finally, there was no difference in headpokes per reinforcer (t = 0.9312, df = 11, p = 0.19, ns; Figure 2J), which suggests that there were no sex-dependent differences in sucrose preference or general consummatory behavior.
To determine whether the 2% sucrose-only reinforcement condition served as a behavior-matched control for the sweetened alcohol condition (9% EtOH/2% sucrose), active lever responding for each solution was compared in male and female mice. No differences in active lever responding were observed for male (t = 0.563, df = 14, p = 0.291, ns) or female mice (t = 1.595, df = 11, p = 0.07, ns). This suggests the sucrose-only condition is an acceptable parallel behavior-matched control for both male and female mice.
Sweetened alcohol self-administration: effects of JNJ-55511118
Male
Oral administration of JNJ-55511118 significantly reduced alcohol-reinforced responding in male mice (Figure 3A–E). Two-way RM ANOVA showed that JNJ-55511118 (0 to 10 mg/kg, p.o.) produced a significant dose-dependent, F (2, 14) = 5.677, p = 0.02, and time-dependent, F (12, 84) = 44.93, p < 0.001, decrease in alcohol-reinforced response rate in male mice (Figure 3A). There was also a significant dose × time interaction, F (24, 168) = 2.21, p = 0.002, indicating that the effects of dose depended on time. Holm–Šídák’s multiple comparisons test showed that both doses of JNJ-55511118 (1 and 10 mg/kg, p.o.) reduced operant alcohol-reinforced response rate from 10 to 60 min as compared to CMC vehicle at each corresponding time point (Figure 3A). Aggregate analyses followed by Holm–Šídák’s multiple comparisons tests showed that both doses of JNJ-55511118 (1 & 10 mg/kg, p.o.) reduced the total number of alcohol active lever presses as compared to vehicle control, F (2, 14) = 4.734, p = 0.027 (Figure 3B). Accordingly, the number of alcohol reinforcers, F (2, 14) = 4.807, p = 0.026 (Figure 3D) and alcohol intake in g/kg, F (2, 14) = 4.706, p = 0.027 (Figure 3E) were also reduced. The percentage of responses emitted on the active lever was also significantly reduced, F (2, 14) = 9.002, p = 0.003, from 92.4 ± 1.4 (vehicle MEAN ± SEM) to 76.5 ± 5 and 75.7 ± 3.5 by JNJ-55511118 (1 and 10 mg/kg), respectively. However, JNJ-55511118 (10 mg/kg, p.o.) increased responding on the inactive lever, F (2, 14) = 5.971, p = 0.026. The number of headpokes in the drinking trough per reinforcer was not altered, F (2, 14) = 3.564, p = 0.056, which is consistent with lack of nonspecific effects on consummatory, exploratory, or motor behavior (data not shown).
FIGURE 3.
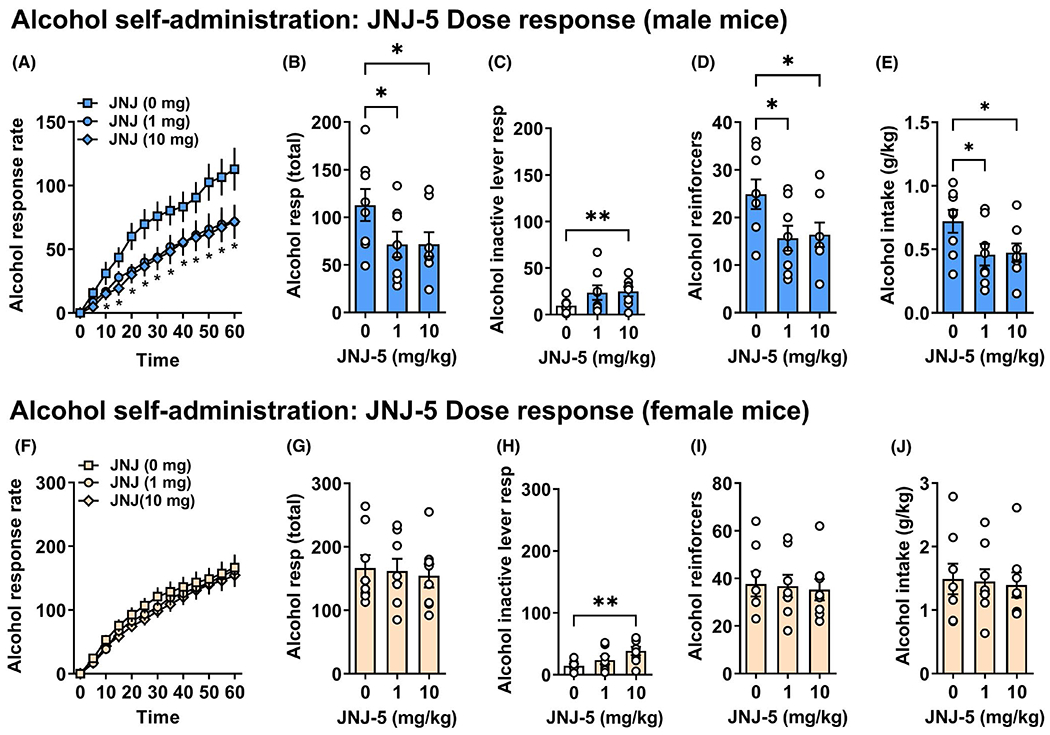
Inhibition of TARP γ-8-bound AMPAR activity reduced alcohol self-administration by male mice. Panels show the effects of JNJ-55511118 (0, 1, and 10 mg/kg, p.o.) in male (n = 8) or female (n = 8) C57BL/6J mice on operant self-administration of sweetened alcohol. In male mice, (A) alcohol-reinforced response rate is shown as a cumulative rate plot (cumulative responses/5-min). (B) Total alcohol responses, (C) inactive lever responses (D) alcohol reinforcers, and (E) intake (g/kg/1 h) are plotted as a function of drug dose. For alcohol response rate in male mice, * indicates both the 1 and 10 mg/kg dose of JNJ-55511118 were significantly different from vehicle at the corresponding time points; significant two-way RM ANOVA interaction followed by Holm–Šídák’s multiple comparisons test, p < 0.05. For male bar graphs, asterisks indicate significantly different from vehicle at the specific dose of JNJ-55511118; significant one-way RM ANOVA followed by Holm–Šídák’s multiple comparisons test (*p ≤ 0.05; **p ≤ 0.01). In female mice, (F) alcohol-reinforced response rate is shown as a cumulative rate plot (cumulative responses/5-min). (G) Total alcohol responses, (H) inactive lever responses, (I) alcohol reinforcers, and (J) intake (g/kg/1 h) are plotted as a function of drug dose. For female bar graph, asterisks indicate significant difference from vehicle at the specific dose of JNJ-55511118 (10 mg/kg) on inactive lever responses; one-way RM ANOVA followed by Holm–Šídák’s multiple comparisons test, ***p ≤ 0.001. All data are shown as MEAN ± SEM
Female
JNJ-55511118 (0 to 10 mg/kg p.o.) produced no effect on alcohol-reinforced response rate in female mice (Figure 3F). Accordingly, there was no change in total active responding, F (2, 14) = 0.247, p = 0.784, ns (Figure 3G), reinforcers, F (2, 14) = 0.174, p = 0.842, ns (Figure 3I), or intake in g/kg, F (2, 14) = 0.166, p = 0.849, ns (Figure 3J). However, there was a dose-dependent reduction in the percentage of responses on the alcohol-reinforced lever, F (2, 14) = 10.90, p = 0.001, that was attributable to a reduction following the highest does of JNJ-55511118 (vehicle = 92 ± 1.8 vs 10 mg/kg = 79.9 ± 3.7; Holm–Šídák’s multiple comparison test, p = 0.0009). This reduction in percent responding on the alcohol-associated lever was influenced by an increase in responses on the inactive lever, F (2, 14) = 9.599, p = 0.004 (Figure 3H). While JNJ-55511118 did not elicit a reduction in active lever responding for female mice, the decrease in relative responding suggests a reduction in lever discrimination. The number of headpokes in the drinking trough per reinforcer was not altered, F (2, 14) = 1.337, p = 0.289 (data not shown), which is consistent with lack of nonspecific effects on consummatory, exploratory, or motor behavior.
Sucrose-only self-administration: effect of JNJ-55511118
Male
JNJ-55511118 (0 to 10 mg/kg, p.o.) produced a significant dose × time interaction on the rate of sucrose-reinforced responding in male mice, F (2.671,18.70) = 4.539, p = 0.02. However, follow-up analysis with Holm–Šídák’s multiple comparisons test did not identify significant differences between doses of JNJ-55511118 at any time point (Figure 4A). There were trends for an increase in sucrose-reinforced response rate at several points late in the session following the 10 mg/kg dose; however, none of these data points were significantly different from vehicle. Aggregate analyses also showed no effect of JNJ-55511118 on total number of sucrose responses, F (2, 14) = 2.674, p = 0.103, ns (Figure 4B) or inactive lever responses, F (2, 14) = 2.502, p = 0.118, ns (Figure 4C). Accordingly, there were no changes in sucrose reinforcers, F (2, 14) = 2.243, p = 0.143, ns (Figure 4D), sucrose intake, F (2, 14) = 3.155, p = 0.0825, ns (Figure 4E) or rate of relative responding for sucrose, F (2, 12) = 0.496, p = 0.607, ns. Finally, the number of headpokes in the drinking trough per reinforcer was not altered, F (2, 12) = 0.3518, p = 0.648, which is consistent with lack of nonspecific effects on consummatory, exploratory, or motor behavior (data not shown).
FIGURE 4.
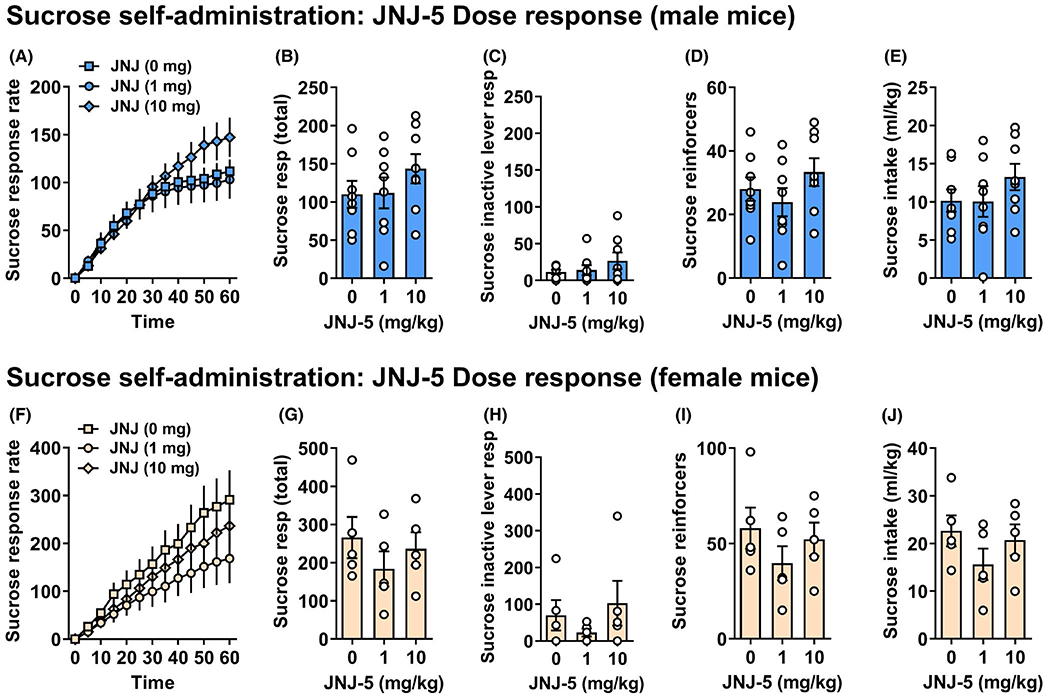
Effects of JNJ-55511118 on sucrose self-administration. Panels show the effects of JNJ-55511118 (0, 1, and 10 mg/kg, p.o.) in male (n = 8) or female (n = 5) C57BL/6J mice on operant self-administration of sucrose. In male mice, (A) sucrose-reinforced response rate is shown as a cumulative rate plot (cumulative responses/5-min). (B) Total sucrose responses, (C) inactive lever presses, (D) reinforcers, and (E) intake (ml/kg/1 h) are plotted as a function of drug dose. In female mice, (F) alcohol-reinforced response rate is shown as a cumulative rate plot (cumulative responses/5-min). (G) Total alcohol responses, (H) inactive lever responses (I) alcohol reinforcers, and (J) intake (ml/kg/1 h) are plotted as a function of drug dose. Although RM ANOVA detected a significant dose × time interaction on sucrose-reinforced response rate by male mice, none of the individual data points were different from control; Holm–Šídák’s multiple comparisons test (p > 0.05). There were no statistically significant effects of JNJ-55511118 on other parameters of sucrose-reinforced responding in male mice. JNJ-55511118 produced no dose-dependent changes in operant sucrose-reinforced responding in female mice. All data are shown as MEAN ± SEM
Female
Although trends were visually apparent, JNJ-55511118 (0 to 10 mg/kg) produced no statistically significant effect on the rate of sucrose self-administration in female mice (Figure 4F). JNJ-55511118 also produced no significant change in the total number of sucrose responses, F (2, 8) = 2.157, p = 0.178, ns (Figure 4G) or inactive lever responses, F (2, 8) = 1.319, p = 0.320, ns (Figure 4H). Accordingly, there were no changes in sucrose reinforcers, F (2, 8) = 2.122, p = 0.198, ns (Figure 4I), sucrose intake, F (2, 8) = 1.965, p = 0.213, ns (Figure 4J), or relative responding for sucrose following JNJ-55511118, F (2, 8) = 1.469, p = 0.286 (data not shown). Further, headpokes per reinforcer were not altered, F (2, 8) = 4.664, p = 0.057 (data not shown).
Open-field locomotor activity: effects of JNJ-55511118 in male mice
To determine whether significant effects of JNJ-55511118 on alcohol self-administration were associated with alterations in general locomotor behavior, male mice were pretreated with the lowest effective dose of JNJ-55511118 (0 or 1 mg/kg, p.o.) that reduced alcohol self-administration 1 h prior to a 1-h open-field locomotor behavior test. JNJ-55511118 had no effect on ambulatory distance by alcohol (t = 1.633, df = 7, p = 0.0733, ns), or sucrose (t = 0.6074, df = 7, p = 0.281, ns)-exposed mice (Figure 5).
FIGURE 5.
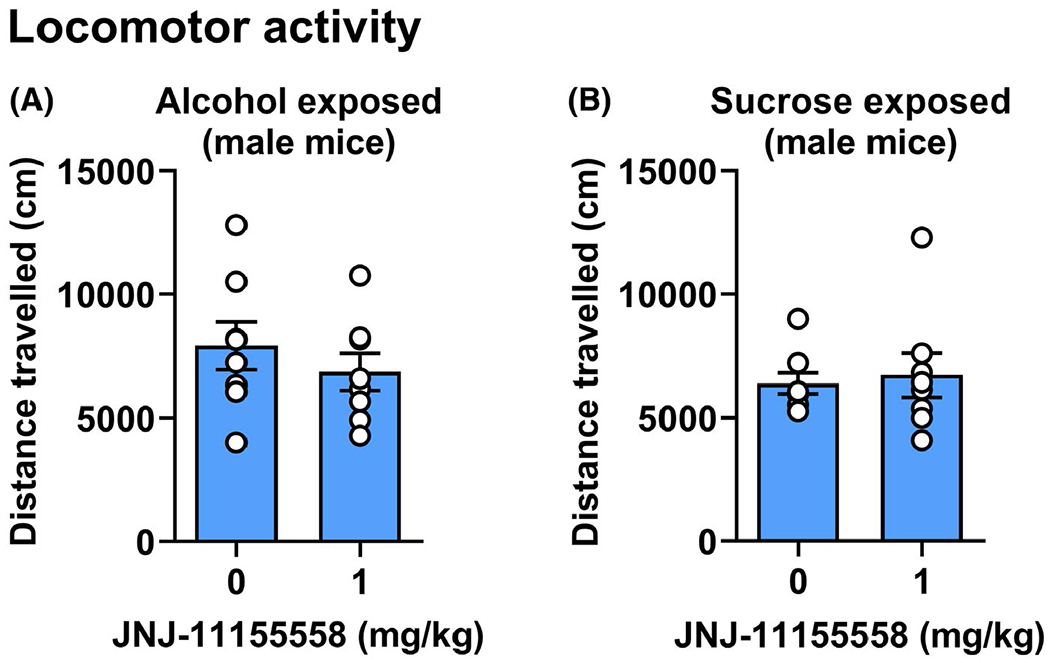
No effect of JNJ-55511118 on locomotor activity in male mice. Horizontal distance traveled (cm/1 h) in an open-field test plotted as a function of JNJ-55511118 dosage in (A) alcohol exposed and (B) sucrose-exposed male mice. Locomotor tests were conducted to evaluate potential nonspecific motor effects of the lowest effective dose of JNJ- = 55511118 that reduced alcohol self-administration in male mice. Female mice were not tested since JNJ-55511118 had no effect on operant behavior. All data are shown as MEAN ± SEM
DISCUSSION
The development and progression of AUD are associated with a hyperglutamatergic state, which represents a challenging but promising area for medications development (Holmes et al., 2013). This study shows for the first time that glutamatergic AMPARs bound to the accessory protein TARP γ-8 regulate alcohol self-administration and suggests that recently developed compounds that target this subgroup of AMPARs (e.g., Kato et al., 2016; Maher et al., 2016, 2017) may be useful in the medical management of AUD and other addictions.
Our previous work has shown that glutamate AMAPR activity in reward-related brain regions is a target of alcohol self-administration that, in turn, mechanistically regulates chronic self-administration of the drug (Agoglia et al., 2015; Cannady et al., 2013, 2016; Faccidomo et al., 2020; Salling et al., 2016). Emerging evidence indicates that TARP γ-8 is a critically important auxiliary protein that regulates AMPAR expression, trafficking, activity, pharmacology, and plasticity (Bissen et al., 2019; Jackson & Nicoll, 2011; Nicoll et al., 2006; Rouach et al., 2005; Tomita et al., 2003). Given these highly significant functional properties of TARP γ-8, interest is growing in pharmacologically targeting TARP γ-8-bound AMPARs as a therapeutic strategy for neurological and neuropsychiatric disorders (Gill & Bredt, 2011; Maher et al., 2017). However, no studies to date have evaluated the potential role of TARP γ-8 in AUD or other forms of addiction.
To address this gap in knowledge, this preclinical study sought to determine whether TARP γ-8-bound AMPARs regulate voluntary operant alcohol self-administration by male and female C57BL/6J mice. Results showed that acute oral administration of JNJ-55511118, a novel brain-penetrant, high affinity, and selective negative modulator of AMPA receptors bound to TARP γ-8 (Maher et al., 2016), significantly decreased operant self-administration of sweetened alcohol by male C57BL/6J mice. These data indicate that TARP γ-8-bound AMPARs regulate the reinforcing effects of sweetened alcohol in male mice, which demonstrates preclinical efficacy of JNJ-55511118 as a potential method for medicinally managing chronic repetitive drinking associated with AUD. However, it is noteworthy that JNJ-55511118 had no effect in females that self-administered significantly higher doses of alcohol than male mice. Thus, additional studies are needed to determine whether female mice are less sensitive to JNJ-55511118 or whether the inhibitor has diminished efficacy under conditions of heavy alcohol intake.
To evaluate potential reinforcer specificity, we examined the effects of JNJ-55511118 on sucrose-only self-administration as a parallel behavior-matched nondrug control. JNJ-55511118 did not decrease any measure of sucrose-reinforced responding in male or female mice, suggesting that the rate-reducing effect of the AMPAR-negative modulator was specific to alcohol-reinforced responding. Lack of reductions in the parallel behavior-matched sucrose control condition also indicates the absence of nonspecific disruption of motor activity, memory, or general consummatory behavior that might have altered alcohol self-administration in male mice. It is not entirely clear from the present results, however, if TARP γ-8 regulates sucrose reinforcement or other reward functions. Rate of sucrose-reinforced responding showed a significant interaction between JNJ-55511118 dose and session time due to an upward trend following the 10 mg/kg dose late in the session; however, none of the individual points were different from control suggesting a minimal increase in response rate, which contrasts with the rate-reducing effects of JNJ-55511118 on alcohol self-administration. Prior research has shown that sucrose-reinforced responding is less sensitive than alcohol to disruption by neuropharmacological manipulations (Hodge et al., 1996; Hodge et al., 1993a). Thus, further work is needed that evaluates a higher dose range of JNJ-55511118 on sucrose or other nondrug reinforcement. Overall, the findings of these sucrose control experiments indicate that JNJ-55511118 shows efficacy for reducing alcohol self-administration at doses that have no off-target rate-reducing effects on other reward-related behavior.
A secondary behavioral effect was observed in both male and female mice. At the highest dose tested, JNJ-55511118 (10 mg/kg) produced an increase in non-reinforced inactive lever responding. This dose-dependent increase was not accompanied by increases in other behaviors such as headpokes/reinforcer, or responding on the active lever, which indicates that it was not a function of generalized motor activation. Interestingly, however, this effect of JNJ-55511118 on inactive lever pressing was not observed under conditions of sucrose-only self-administration. This suggests that inhibition of TARP γ-8-bound AMPARS disrupted the operant response-reinforcer contingency in an alcohol-dependent manner in both sexes. This hypothesis could be explored further using second-order schedules of reinforcement, such as a concurrent schedule of alcohol and sucrose reinforcement that facilitate within-subject evaluation of response-reinforcer relations (Hodge et al., 1993b; Slawecki et al., 1997a; Slawecki et al., 1997b).
JNJ-55511118 had minimal effects alcohol self-administration in female mice and no effects on sucrose self-administration. Although the mechanism(s) of this null finding was not pursued here, several potential contributing factors merit discussion. First, female rodents are well-known to consume more alcohol than males in a variety of experimental preparations (Becker & Koob, 2016; Hwa et al., 2011). We observed significantly higher levels of operant sweetened-alcohol self-administration by female C57BL/6J mice. Thus, one potential explanation for lack of effect of JNJ-55511118 on alcohol self-administration by females is that the higher rate of responding reflects enhanced reinforcement function, which could predict greater resistance to change (Nevin, 2012); however, this is not always observed with pharmacological disruption of operant behavior (c.f., Pinkston et al., 2009). In a related manner, it is possible that greater baseline self-administration of sweetened alcohol by female mice was driven by sucrose content. Since JNJ-55511118 had no effect on sucrose-only self-administration, this may explain the lack of effect on sweetened alcohol self-administration by females. Future studies could address this possibility by evaluating effects of JNJ-55511118 on self-administration of unsweetened alcohol (10%), which does not induce a sex difference (Sneddon et al., 2020). Second, these data suggest that female mice are less sensitive to the pharmacological effect of JNJ-55511118 on operant alcohol self-administration as compared to male mice. Reduced sensitivity in female mice could derive from differential pharmacokinetics; however, this is unknown since the original pharmacokinetic profile of JNJ-55511118 was determined in male C57BL/6J mice (Maher et al., 2016). Fully testing this hypothesis requires evaluating higher dose(s) in female mice, which was precluded in the present experiment due to solubility/suspension difficulties with higher concentrations. Third, it is possible that female mice exhibit differential TARP γ-8 expression or function as compared to male mice, which might alter behavioral responses to an antagonist. Fourth, female rodents show differential alcohol self-administration during the estrus/diestrus versus proestrus phases of the reproductive cycle (Ford et al., 2002; Forger & Morin, 1982; Roberts et al., 1998). Although estrous cycle was not measured, operant self-administration by female mice was stable during the baseline and drug testing phases of this experiment, which is consistent with prior work examining freely cycling mice (Satta et al., 2018). Future studies could evaluate these and other potential sex-linked mechanisms that might underlie response to JNJ-55511118.
Prior work has shown that systemic administration of the competitive AMPAR antagonist NBQX reduced operant self-administration of alcohol, sucrose, and saccharin but only at doses that also reduced locomotor activity, indicating reduction in operant performance by nonspecific motor effects (Stephens & Brown, 1999). To determine whether JNJ-55511118 altered alcohol self-administration by male mice via nonspecific motor disruption, the lowest effective dosage was administered alcohol- and sucrose-exposed prior to open-field locomotor tests. Potential locomotor effects of JNJ-55511118 were not tested in female mice since we observed no effect of the drug on operant behavior. JNJ-55511118 (1 mg/kg) had no effect on locomotor activity in male alcohol or sucrose-exposed mice, suggesting that the rate-reducing effect of the drug on operant sweetened-alcohol self-administration in male mice was not due to nonspecific motor effects.
At the mechanistic level, results of the present study are consistent with growing evidence indicating that glutamate signaling through AMPAR molecular pathways regulates alcohol self-administration. Alcohol increases glutamate levels in the amygdala after chronic exposure (Roberto et al., 2004). Elevated glutamate promotes Ca2+ influx via NMDA receptors or L-type channels. Calmodulin binds Ca2+ and the complex causes rapid phosphorylation of CaMKII-T286 (Soderling et al., 2001). pCaMKII-T286 translocates to the membrane and/or PSD where it phosphorylates AMPAR GluA1-S831, which increases and sustains channel activity (Hayashi et al., 2000). We have shown that alcohol self-administration increases both pCaMKII-T286 and pGluA1-S831, and that this is associated with upregulated AMPAR-mediated synaptic transmission in the amygdala (Salling et al., 2016). Moreover, activation of AMPARs in the amygdala promotes escalated alcohol self-administration in a CaMKII-dependent manner (Cannady et al., 2016). Activated (e.g., phosphorylated) CaMKII also phosphorylates AMPAR-bound TARP γ-8, which promotes binding of its C terminus to PSD-95 and trapping of AMPARs in the plasma membrane (Patriarchi et al., 2018). CaMKII phosphorylation of TARP γ-8 is required for AMPAR-mediated synaptic transmission, and LTP and memory formation are impaired in mice lacking CaMKII phosphorylation sites on TARP γ-8 (Park et al., 2016). Together with the present results, these findings suggest that activity of the CaMKII-TARP γ-8-AMPAR molecular cell signaling pathway mediates chronic alcohol self-administration and, therefore, underlie behavioral pathologies associated with AUD.
In conclusion, use of AMPAR-negative modulators is appealing for pharmacological treatment of CNS disorders involving increased neuronal excitability, such as AUD. However, general inhibition of AMPARs produces undesirable side effects including sedation and memory disruption. Given the restricted anatomical expression of TARP γ-8 (e.g., HPC, frontal cortex, amygdala), this AMPAR auxiliary protein has been proposed as a novel target for inhibition of specific brain regions via systemic treatment (Maher et al., 2017). Based on results of the present study, we propose that inhibition of TARP γ-8-bound AMPARs by JNJ-55511118, or related compounds, is a viable pharmacotherapeutic strategy for reducing chronic drinking associated with AUD. Further research addressing TARP γ-8 in various models of addiction will also enhance understanding of how alcohol hijacks fundamental mechanisms of plasticity to drive the development and maintenance of behavioral pathologies.
ACKNOWLEDGMENTS
Research reported in this publication was supported by awards from the National Institute on Alcohol Abuse and Alcoholism (R37 AA014983, R01 AA028782 and P60 AA011605 to CWH) and (F32 AA028993 to JLH), and by the Bowles Center for Alcohol Studies at The University of North Carolina at Chapel Hill.
Funding information
National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: AA011605, AA014983 and AA028993
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Agoglia AE, Holstein SE, Reid G & Hodge CW (2015) CaMKIIalpha-GluA1 activity underlies vulnerability to adolescent binge alcohol drinking. Alcoholism, Clinical and Experimental Research, 39, 1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoglia AE, Holstein SE, Eastman VR & Hodge CW (2016) Cannabinoid CB1 receptor inhibition blunts adolescent-typical increased binge alcohol and sucrose consumption in male C57BL/6J mice. Pharmacology, Biochemistry, and Behavior, 143, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Kranzler HR & Meyer RE (1995) Neurobehavioral aspects of the pharmacotherapy of alcohol dependence. Clinical Neuroscience, 3, 145–154. [PubMed] [Google Scholar]
- Bats C, Farrant M & Cull-Candy SG (2013) A role of TARPs in the expression and plasticity of calcium-permeable AMPARs: evidence from cerebellar neurons and glia. Neuropharmacology, 74, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB & Koob GF (2016) Sex differences in animal models: focus on addiction. Pharmacological Reviews, 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissen D, Foss F & Acker-Palmer A (2019) AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking. Cellular and Molecular Life Sciences, 76, 2133–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Durant B, Besheer J & Hodge CW (2013) Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addiction Biology, 18, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Graham C, Crayle J, Besheer J & Hodge CW (2016) Potentiation of amygdala AMPA receptor activity selectively promotes escalated alcohol self-administration in a CaMKII-dependent manner. Addiction Biology, 22, 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2019) Alcohol Related Disease Impact (ARDI) application. Available at: www.cdc.gov/ARDI [Accessed 13 January 2021].
- Crabbe JC, Harris RA & Koob GF (2011) Preclinical studies of alcohol binge drinking. Annals of the New York Academy of Sciences, 1216, 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Besheer J, Stanford PC & Hodge CW (2009) Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl), 204, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Holstein SE, Santanam TS, Saunders BL, Swaim KS, Reid GT et al. (2020) Pharmacological inhibition of glycogen synthase kinase 3 increases operant alcohol self-administration in a manner associated with altered pGSK-3beta, protein interacting with C kinase and GluA2 protein expression in the reward pathway of male C57BL/6J mice. Behavioural Pharmacology, 31(1), 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Reid GT, Agoglia AE, Ademola SA & Hodge CW (2016) CaMKII inhibition in the prefrontal cortex specifically increases the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Behavioural Brain Research, 298, 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Salling MC, Galunas C & Hodge CW (2015) Operant ethanol self-administration increases extracellular-signal regulated protein kinase (ERK) phosphorylation in reward-related brain regions: selective regulation of positive reinforcement in the prefrontal cortex of C57BL/6J mice. Psychopharmacology (Berl), 232, 3417–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Swaim KS, Saunders BL, Santanam TS, Taylor SM, Kim M et al. (2018) Mining the nucleus accumbens proteome for novel targets of alcohol self-administration in male C57BL/6J mice. Psychopharmacology (Berl), 235, 1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC & Samson HH (2002) Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcoholism, Clinical and Experimental Research, 26, 635–643. [PubMed] [Google Scholar]
- Forger NG & Morin LP (1982) Reproductive state modulates ethanol intake in rats: effects of ovariectomy, ethanol concentration, estrous cycle and pregnancy. Pharmacology, Biochemistry and Behavior, 17, 323–331. [DOI] [PubMed] [Google Scholar]
- Gill MB & Bredt DS (2011) An emerging role for TARPs in neuropsychiatric disorders. Neuropsychopharmacology, 36, 362–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini G, Bell RL & Mayfield RD (2011) Molecular targets of alcohol action: translational research for pharmacotherapy development and screening. Progress in Molecular Biology and Translational Science, 98, 293–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC & Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science, 287, 2262–2267. [DOI] [PubMed] [Google Scholar]
- Heilig M & Egli M (2006) Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacology & Therapeutics, 111, 855–876. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM & Samson HH (1995) GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcoholism, Clinical and Experimental Research, 19, 1486–1493. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM & Samson HH (1996) Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcoholism, Clinical and Experimental Research, 20, 1631–1638. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H & Samson HH (1993a) Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcoholism, Clinical and Experimental Research, 17, 370–375. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V et al. (2006) The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl), 183, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Samson HH & Haraguchi M (1992) Microinjections of dopamine agonists in the nucleus accumbens increase ethanol-reinforced responding. Pharmacology, Biochemistry and Behavior, 43, 249–254. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Lewis RS & Erickson HL (1993b) Specific decreases in ethanol- but not water-reinforced responding produced by the 5-HT3 antagonist ICS 205-930. Alcohol, 10,191–196. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH & Chappelle AM (1997) Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcoholism, Clinical and Experimental Research, 21, 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R & Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl), 229, 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW & Mangieri RA (2018) Do alcohol-related AMPA-type glutamate receptor adaptations promote intake? In: Grant HE (Ed.) Series do alcohol-related AMPA-type glutamate receptor adaptations promote intake?, vol. 248, the neuropharmacology of alcohol. Alcohol. Handb Exp Pharmacol. Springer, pp. 157–186. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF & Miczek KA (2011) Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism, Clinical and Experimental Research, 35, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC & Nicoll RA (2011) The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron, 70, 178–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Burris KD, Gardinier KM, Gernert DL, Porter WJ, Reel J et al. (2016) Forebrain-selective AMPA-receptor antagonism guided by TARP gamma-8 as an antiepileptic mechanism. Nature Medicine, 22, 1496–1501. [DOI] [PubMed] [Google Scholar]
- Koob GF & Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology, 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniishi H, Yamada D, Wada K, Yamada M & Sekiguchi M (2020) Stress induces insertion of calcium-permeable AMPA receptors in the OFC-BLA synapse and modulates emotional behaviours in mice. Translational Psychiatry, 10, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY & Wolf ME (2014) Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology, 76, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MP, Matta JA, Gu S, Seierstad M & Bredt DS (2017) Getting a handle on neuropharmacology by targeting receptor-associated proteins. Neuron, 96, 989–1001. [DOI] [PubMed] [Google Scholar]
- Maher MP, Wu N, Ravula S, Ameriks MK, Savall BM, Liu C et al. (2016) Discovery and characterization of AMPA receptor modulators selective for TARP-gamma8. Journal of Pharmacology and Experimental Therapeutics, 357, 394–414. [DOI] [PubMed] [Google Scholar]
- Marty VN & Spigelman I (2012) Long-lasting alterations in membrane properties, k(+) currents, and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Frontiers in Neuroscience, 6, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA (2011) Ethanol modulation of synaptic plasticity. Neuropharmacology, 61, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA (2012) Resistance to extinction and behavioral momentum. Behavioural Processes, 90, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S & Bredt DS (2006) Auxiliary subunits assist AMPA-type glutamate receptors. Science, 311, 1253–1256. [DOI] [PubMed] [Google Scholar]
- NRC.(2011) National Research Council (US) Committee for the update of the guide for the care and use of laboratory animals. Guide for the care and use of laboratory animals, 8th edition. Washington, D.C.: National Academies Press. [Google Scholar]
- Park J, Chavez AE, Mineur YS, Morimoto-Tomita M, Lutzu S, Kim KS et al. (2016) CaMKII phosphorylation of TARPgamma-8 is a mediator of LTP and learning and memory. Neuron, 92, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T, Buonarati OR, Hell JW (2018) Postsynaptic localization and regulation of AMPA receptors and Cav1.2 by β2 adrenergic receptor/PKA and Ca 2+/CaMKII signaling. The EMBO Journal, 37(20). 10.15252/embj.201899771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P & Belknap JK (1994) Localization of genes affecting alcohol drinking in mice. Alcoholism, Clinical and Experimental Research, 18, 931–941. [DOI] [PubMed] [Google Scholar]
- Pinkston JW, Ginsburg BC & Lamb RJ (2009) Examination of reinforcement magnitude on the pharmacological disruption of fixed-ratio performance. Experimental and Clinical Psychopharmacology, 17, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuillon T, Ward SE & Beswick P (2017) Modulating AMPA receptors for the treatment of CNS disorders. In: Chackalamannil S & Ward SE (Eds.) Comprehensive Medicinal Chemistry III, comprehensive medicinal chemistry III. Elsevier, pp. 447–480. [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH & Siggins GR (2004) Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. Journal of Neuroscience, 24, 1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C & Koob GF (1998) Estrous cycle effects on operant responding for ethanol in female rats. Alcoholism, Clinical and Experimental Research, 22, 1564–1569. [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S et al. (2005) TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nature Neuroscience, 8, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE & Brewer RD (2015) 2010 National and state costs of excessive alcohol consumption. American Journal of Preventive Medicine, 49, e73–e79. [DOI] [PubMed] [Google Scholar]
- Salling MC, Faccidomo S & Hodge CW (2008) Nonselective suppression of operant ethanol and sucrose self-administration by the mGluR7 positive allosteric modulator AMN082. Pharmacology, Biochemistry and Behavior, 91, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M et al. (2016) Moderate alcohol drinking and the amygdala proteome: identification and validation of calcium/calmodulin dependent kinase II and AMPA receptor activity as novel molecular mechanisms of the positive reinforcing effects of alcohol. Biological Psychiatry, 79, 430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Hodge CJ, Psilos KE, Eastman VR, Faccidomo SP & Hodge CW (2017) Cue-induced reinstatement of alcohol-seeking behavior is associated with increased CaMKII T286 phosphorylation in the reward pathway of mice. Pharmacology, Biochemistry, and Behavior, 163, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. (2019) Results from the 2018 National Survey on Drug Use and Health: detailed tables. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- Samson HH & Hodge CW (1993) The role of the mesoaccumbens dopamine system in ethanol reinforcement: studies using the techniques of microinjection and voltammetry. Alcohol and Alcoholism, 2, 469–474. [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Haraguchi M & Hodge CW (1992) Alcohol self-administration: role of mesolimbic dopamine. Annals of the New York Academy of Sciences, 654, 242–253. [DOI] [PubMed] [Google Scholar]
- Satta R, Hilderbrand ER & Lasek AW (2018) Ovarian hormones contribute to high levels of binge-like drinking by female mice. Alcoholism, Clinical and Experimental Research, 42, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Hodge CW & Samson HH (1997a) Dopaminergic and opiate agonists and antagonists differentially decrease multiple schedule responding maintained by sucrose/ethanol and sucrose. Alcohol, 14, 281–294. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Samson HH & Hodge CW (1997b) Differential changes in sucrose/ethanol and sucrose maintained responding by independently altering ethanol or sucrose concentration. Alcoholism, Clinical and Experimental Research, 21, 250–260. [PubMed] [Google Scholar]
- Sneddon EA, Ramsey OR, Thomas A & Radke AK (2020) Increased responding for alcohol and resistance to aversion in female mice. Alcoholism, Clinical and Experimental Research, 44, 1400–1409. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Chang B & Brickey D (2001) Cellular signaling through multifunctional Ca2+/calmodulin-dependent protein kinase II. Journal of Biological Chemistry, 276, 3719–3722. [DOI] [PubMed] [Google Scholar]
- Stephens DN & Brown G (1999) Disruption of operant oral self-administration of ethanol, sucrose, and saccharin by the AMPA/kainate antagonist, NBQX, but not the AMPA antagonist, GYKI 52466. Alcoholism, Clinical and Experimental Research, 23, 1914–1920. [PubMed] [Google Scholar]
- Stevenson RA, Besheer J & Hodge CW (2008) Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology (Berl), 197, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talani G, Licheri V, Masala N, Follesa P, Mostallino MC, Biggio G et al. (2014) Increased voluntary ethanol consumption and changes in hippocampal synaptic plasticity in isolated C57BL/6J mice. Neurochemical Research, 39, 997–1004. [DOI] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA et al. (2003) Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. Journal of Cell Biology, 161, 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM & Swartzwelder HS (2004) Hippocampal function during adolescence: a unique target of ethanol effects. Annals of the New York Academy of Sciences, 1021, 206–220. [DOI] [PubMed] [Google Scholar]
- Winder DG, Egli RE, Schramm NL & Matthews RT (2002) Synaptic plasticity in drug reward circuitry. Current Molecular Medicine, 2, 667–676. [DOI] [PubMed] [Google Scholar]
- Wise RA & Koob GF (2013) The development and maintenance of drug addiction. Neuropsychopharmacology, 39, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]


