Abstract
Context:
It is a known fact that periodontal tissue regeneration can be achieved by the use of periodontal ligament stem cells (PDLSCs). Current mainstay of periodontal treatment is focusing on stem cell tissue engineering as an effective therapy, making it important to isolate PDLSCs from periodontal tissues.
Aims:
The present research endeavor was undertaken to elucidate a technique for isolating PDLSCs for in vivo reconstructing the natural PDL tissue.
Settings and Design:
The study design involves In vitro prospective study.
Materials and Methods:
Premolar teeth were extracted from 12 patients who were under orthodontic treatment. PDL cells were scraped from their roots. Using 10 ml of Dulbecco's modified Eagle's medium with pH 7.2, the specimens of the periodontal tissue were transferred to laboratory where cell culture was done. Isolated stem cells were grown on 24-well microtiter plates-containing cover slips. They were incubated overnight at approximately 37°C in 95% air and 5% humidification. Anti-CD 45, CD73, CD90, CD105, and CD146 antibodies were used. After staining, cells were observed under phase-contrast microscopy and in inverted microscope.
Results:
The cells showed a marked growth and 90% confluence at day 6. Cells presented thin and long fibroblastic spindle morphology. Isolated PDLSCs showed colony-forming ability at the 14th day after seeding. Immunohistochemical staining of PDLSCs showed positive uptake for CD146, CD90, CD73, CD105, and negative uptake for CD45.
Conclusions:
The human PDLSCs can be clearly isolated and characterized by using CD90, CD73, CD146, and CD105 markers of stem cells.
Keywords: Periodontal, stem cells, tissue engineering
INTRODUCTION
There are myriad number diseases which affect the oral cavity. One of the foremost of them all is periodontitis. It's the prime reason which ends up in loss of teeth and its supporting structures. Dentists were historically unable to adequately reconstruct the damaged periodontal apparatus, as only the age-old conventional therapies were put to use. It had been indeed a challenging and daunting task. The science of tissue engineering has a rather unique goal; it strives to restore these lost cells and tissues of the body.[1] The human periodontal ligament (PDL) is in actuality a “well differentiated soft tissue that connected the tooth root surface with the alveolar bone socket,” Its composition includes a myriad number of cells like fiber-forming cells, cells of the endothelium, epithelial cells, bone-forming osteoblasts, cementum-forming cementoblasts, and the cell rests of Malassez.[2] It is also a long determined fact that the periodontium comprises numerous stem cells. It is noteworthy that these are a type of undifferentiated mesenchymal cells. They are also referred to as multipotent stem cells. These cells have a remarkable characteristic attribute that they can modify or convert into connective tissue linages, including PDL-derived from the cranial neural crest.[3]
In the beginning, stem cells of mesenchymal origin were identified from the PDL. They were aptly termed as PDL stem cells (PDLSCs). They were cells which were identified and located. These cells developed a specified and highly specialized tooth supporting attachment apparatus, and complexes similar to the PDL in animal studies done on rats. This was a major finding, which lead to an array of myriad animal and human clinical researches.[4] It now believed that these PDLSCs play a pivotal role in regenerative therapies, which are capable of restoration of in periodontal tissue in animals as well as humans.[5] The periodontal regeneration techniques use the PDLSCs as vegetative cell source.[6] Compared with other populations of the stem cells, PDLSCs possess certain degrees of special properties. These include their readiness to form support structures such as cementum, surrounding alveolar bone, and Sharpey's fibers. They also are capable of undergoing the process of self-renewal.[7] PDLSCs transplantation was found to be an outstanding solution to the restrictions of PDLSCs autologous transplantation.[8] It is also well known that PDLSCs and alveolar periosteal cells are related. The periosteal cells can simulate periodontal regeneration. Acceptable regeneration of the periodontium is possible by the means of tissue engineering. In this regard, a mixture of different tissue scaffolds and these stem cells and multipotent mesenchymal stem cells (MSCs) are utilized in clinical trials.[9,10] It may appear very promising, but the isolation of stem cells from a meager amount of tissue called the PDL has its own share of technicalities. Numerous difficulties and complications are encountered with extraction and use of PDLSCs.[7,10] The foremost and most dangerous of all is development of tumors from the PDLSCs. Then comes the problem of contamination and vulnerability of vegetative cell condition because of donor attributes like quality.[11] Finally is the relatively small amount of tissue availability which leads to an even smaller cell numbers, an average being 1250 cells per yield from the primary cultures. Quite assuring, that is not a good enough to urge the cells and form a sheet for PDL.[12,13] The current paper is aimed to elucidate a technique for isolating PDLSCs for in vivo reconstructing the natural PDL tissue.
MATERIALS AND METHODS
Premolar teeth, which were extracted from patients undergoing orthodontic treatment, were collected from 12 patients, 15–19 years old, at our institution's Oral and Maxillofacial Surgery Department. Only those Patients were selected for the study, who had willingness and the thought process of participating in the study and consented for the same in written. All the patients had a good general health and suffered from no diseases. Before the elective surgery procedures, approval and guidelines of the Institutional Review Board with Ethical no (MMCRL/IAEC/2017-18/04) were obtained.
The study individuals were given a povidone-iodine solution of 10% concentration of before collecting the tissue sample. They were all asked to rinse with the same for a period of one minute. Following atraumatic extraction, PDL cells were scraped from their roots. Using approximately 10 ml of Dulbecco's modified Eagle's medium (F-12): Gibco, New York, USA; pH 7.2) the specimens of the periodontal tissue were carefully transferred to the laboratory where the cell culture was to be done. This medium was supplemented with 100 Units/ml of the antibiotic penicillin, 100 μg/ml of antibiotic streptomycin and 0.5% antifungal amphotericin B. It also contained 10% heat-inactivated fetal bovine serum. Using strict sterilization and asepsis, the tissue culture was incorporated inside a flow hood. The next step comprised of a thorough washing of the tissue specimens. After the wash was concluded properly, the tissues were disinfected. It was achieved by application of 10% povidone-iodine solution and phosphate-buffered solution. Later, it was given a final wash with the medium. The samples of the tissue material so procured were then spliced into around 1 ml pieces which was an appropriate size, and then they were placed within the 15 ml centrifuge containing collagenase type 1 (1 mg/ml) and dispase (2 mg/ml). Centrifuge tube was incubated for 45 min to 1 h. An incubator at 37° centigrade was used for the culture. The plate was kept in a humidified atmosphere, which had around 95% of atmospheric air and 5% of Carbon dioxide mixture. The culture-containing plates were meticulously evaluated once every 24 h. An inverted microscope was used for the same.
Isolated stem cells were grown on a 24 well microtiter plate containing cover slips. It was incubated at 37°C during the presence of 95% air and 5% humidification for an overnight period. Then, fixation was done through with 4% paraformaldehyde for 10 min. Polyexcel HRP/3,3' diaminobenzen (DAB) detection system (PathnSitu-Cat N0-PEH2-50 ml) was used for immunocytochemistry. The immunocytochemistry steps were as follows-first, peroxide block was added for 5 min with H2O2. Then, it was incubated with primary antibodies cluster of differentiation (CD) 45-(PathnSitu-Cat No-PR023), CD-73 (Biogenex-Cat No-AM904), CD-90 (Biogenex Cat No-AN733), CD105(PathnSitu-Cat No-PR188), and CD146(Biogenex-Cat No-AN 716), for 45 min at room temperature. CD73 and CD105 antibodies were Mouse Monoclonal antibodies, CD45, CD90, and CD146 were Rabbit monoclonal antibodies. The immunocytochemistry steps were as follows-first, peroxide block was added for 5 min with H2O2. Then, it was incubated with primary antibodies CD 45, CD-73, CD-90, CD105, and CD146 for 45 min at room temperature. CD73 and CD105 antibodies were Mouse Monoclonal antibodies, CD45, CD90, and CD146 were Rabbit monoclonal antibodies. After that, the tissue sections were covered with target binder and incubated for 10 min at room temperature. Again, Poly-Horseradish Peroxidase on tissue sections was added and incubated for 10 min. Anti-mouse/anti rabbit IgG conjugated to HRP was used as secondary antibodies and human dental pulp stem cells and (HDP-SC)-T25 were used positive control. Then, a working concentration of DAB was added and incubated for 5 min at room temperature. Hematoxylin was added as a nucleus stain and incubated for an appropriate time at room temperature. Then, cells were observed under phase contrast microscopy and under inverted microscope.
RESULTS
The growth of the PDL cells is shown in Figure 1. Figure 1 shows the growth of the cells at day 2 [Figure 1a], day 6 [Figure 1b], and day 9 [Figure 1c]. As it is evident from the figure, the cells show a marked growth and 90% confluence was reached at day 6. In general, the cells presented thin and long fibroblastic spindle morphology.
Figure 1.
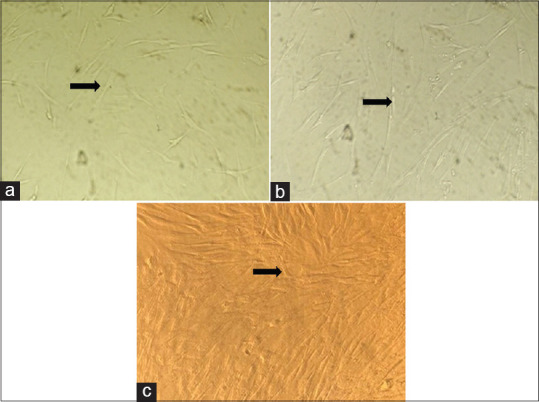
(a) Growth of PDL cells at day 1; (b) Growth of PDL cells at day 6; (c) Growth of PDL cells at day 9. PDL – Periodontal ligament
One of the prime indicator characteristics of the undifferentiated stem cells is their specific clonogenic activity. It has been prudently established that it is a rather sensitive indicator of these cells. In the present study, isolated PDLSCs indeed showed a colony-forming ability. The Colonies of PDLSCs were formed from isolated PDLSCs at 7 days [Figure 2a] after the cells were plated at low density. The result was quite evident at 14 days [Figure 2b] with the increase in the volume of the colony.
Figure 2.
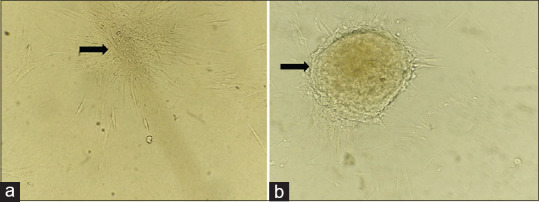
(a) Clonogenic cells at day 7; (b) Clonogenic cells at day 14
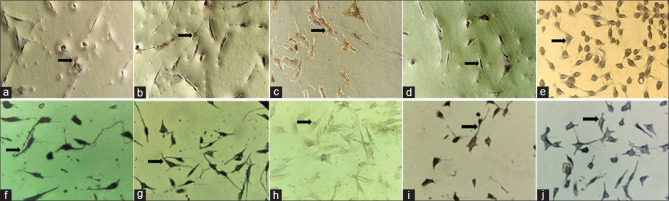
Immunocytochemistry revealed that cells had taken up adequate staining, which indicated overall positivity of the cells. The cells in the tissue samples had taken up a positive stain for the primary antibodies CD73, CD90, CD105, and CD146 under × 20 as shown in Figure 3a–d. It showed a negative staining for CD45 as shown in Figure 3e. HDP-SC-T25 (HI media Cat No-CL008) cells were used as positive control in this study. Similarly, positive control cells showed positive stain for the primary antibodies CD73, CD90, CD105, and CD146 as shown in Figures 3f–i. It also showed a negative staining for CD45 as shown in Figure 3j. Thus, we may say that CD73, CD90, CD105, and CD146 can be used successfully to identify MSCs from dental tissues such as the PDL.
Figure 3.
(a) Positive uptake for CD 73; (b) Positive uptake for CD 90; (c) Positive uptake for CD 105; (d) Positive uptake for CD 146; (e) Negative uptake for CD 45; (f) Immunohistochemistry control for CD 73; (g) Immunohistochemistry control for CD 90; (h) Immunohistochemistry control for CD 105; (i) Immunohistochemistry control for CD 146; (j) Immunohistochemistry control for CD 45. CD – Cluster of differentiation
DISCUSSION
Tissue-engineering methods are recently developed. They can rather be thought as novel substitutions to current regenerative periodontal treatments. They utilize different varieties of biomaterial scaffolds. They use vitro-cultured cells which can replace irreparably harmed tissues. They are introduced into diseased areas, sites and used for regeneration of lost tissues.[14] PDLSCs have numerous advantages. These include a limited but sure availability with ease. They are amenable to manipulation, have a rather low risk of cancer formation or expression and possess skills to undergo amenable transformation into numerous kinds of cells. Thus, they can be effectively be used quite successfully in clinical trials.[15]
The periodontium is also vital in the proper growth and maturation of the alveolar bone. As a consequence, it is a dynamically adapting and lifelong remodeling tissue, as per the need. Researches conducted in the present era have suggested that the MSCs in the periodontium space are able to differentiate into cementum progenitor cells and bone forming cells. They are also capable of transformation into specialized cells producing fibroblasts. They eventually differentiate also within the PDL space.[8,16,17]
The PDLSCs are located in a specific zone or area in the periodontal tissue. They are notable only in the perivascular space of the periodontium. They possess certain favorable attributes of MSCs. Due to these special characteristics, they afford themselves as a valuable tool for various periodontal regenerative processes. In the present times, quite a commendable advancement and progression has been done in the realm of autologous transplantation of PDLSCs. The principle focus of the current researchers and scientific investigators is to make the most of the proliferative capacity of the PDLSCs. At the same instance, they are also trying to keep them in an undifferentiated form, which is highly beneficial.[18] For the purpose of this study, PDL samples were obtained by scratching the root's surface, a process which was previously used by Navabazam et al.,[19] Park et al.,[20] and Silvério et al.,[21] to sample the PDL.
In the present research, the authors faced a peculiar, but a very important issue. That was the avoidance of any form of microbial contamination. The tissue samples can easily be contaminated in any of the following three stages, (i) during removal of the tissue from the patient, (ii) during transportation to the laboratory where the research is to be carried out, and (iii) during media preparation and the processing of the tissues.[5,13] In a study by Wanichpakorn and Kedjarune-Laggat[22] it was suggested that the tissue sample dimensions may correlate with the microbial contamination. To overcome contamination in our study, we used 10% povidone-iodine. For approximately 2 min, the tissue was placed it in the specified strength of povidone-iodine solution. Another very effective way of dealing with contamination, which was meticulously followed by us, was the addition of antimycotics and antibiotics in the media. This is a well-established fact and is in accordance with several other studies. 100 units/mL of penicillin was added to prevent the growth of Gram-positive bacteria. Similarly, 100 μg/L of streptomycin was incorporated to inhibit the growth of Gram-negative bacteria. The drug Amphotericin B acted as an effective antifungal agent.
The ability of one particular cell to proliferate and form a large colony is termed as clonogenic expansion. This is, as stated above, one of the most important characteristics of the stem cells. The quantitative technique to examine this growth is termed as a Clonogenic assay. It is a very widely used in vitro quantitative technique in a lot of researches pertaining to all types of stem cells.[22] The present study aptly demonstrated this characteristic colony-forming capacity of the PDLSCs.
Phenotypically, MSCs express several non-specfic markers such as CD73 (5′-ribonucleotide phosphohydrolase), CD90 (Thy-1; thymocyte differentiation antigen 1), CD105 (endoglin) and CD146 (MelCAM/MUC18). The MSCs are identifiable by certain classical markers. CD73 is one such important marker with good specificity. It is used to delineate the MSC population. CD73 or NTSE (ecto-5'-neuclotidase) is a glycophosphatidylinositol (GPI) anchored enzyme that generates extracellular adenosine. One aspect worth mentioning here is that a uniform pattern of CD73 expression is not noted. This non-uniform pattern of CD73 expression in MSCs could be associated with their diverse reparative property. Our results very clearly revealed good amount of CD73 in the PDL cells.
CD90 is a membrane glycoprotein which is also referred to as Thy 1 because it is discovered as a thymocyte antigen.[23] It is a 25–35 kDa heavily N-glycosylated GPI anchored conserved cell surface protein. It is one of the most common stem cell markers. Our results show that the expression of CD90 in the PDL cells. CD105 is a hypoxia inducible protein, which is rather abundantly expressed in angiogenic endothelial cells, and quite naturally, it plays an important role in angiogenesis.[24] Our results show that the expression of CD105 in the PDL cells was well noted.
PDLSCs positive for CD146 have greater colony-forming efficiency and osteogenic potential than negative cells.[23,25] CD146 is a transmembrane glycoprotein, that is also referred to as S-Endo 1–associated antigen.[26,27] It is commonly known by two different names, namely the melanoma cell adhesion molecule and the cell surface glycoprotein MUC18. In current study, we were able to isolate CD146 +ve human PDLSCs and the proportion of which was similar to the previous studies.[20,28] Therefore, this makes our study comparable with the other studies on human PDLSCs. It noteworthy that CD146, along with STRO-1, are two important primarily identifiable and easily distinguishable MSC markers.[29,30]
The current study had a limitation that we did not use STRO-1, even though STRO-1 is a cell marker present on all clonogenic stromal precursors. It is a noted and documented marker of MSCs,[18,31,32] but there are certain ambiguities over the positivity's and negativity's of use of STRO-1 for immunohistochemistry. Conflicting data exist whether a particular MSC population is STRO-1 positive or negative.[33,34,35] To substantiate this, a research by Hung et al. showed that “size sieved stem cells,” which are a specialized population of human bone marrow-derived stem cells, are consistently negative for STRO-1.[36] Therefore, the use of STRO-1 antibody as MSC marker is marred with controversies and needs critical re-evaluation. This is the main reason we chose to eliminate STRO-1 from our research. Another point worth mentioning here is about the negative uptake of CD45. CD45 are hematopoietic stem cell markers. It is involved in the regulation of signal transduction in hematopoiesis. Our results revealed the absence of CD45 markers in PDL cells and that conformed to the previous studies.[37,38]
CONCLUSIONS
With effective minimally invasive procedures, the progenitor cells in periodontal tissue can be expanded in vitro, providing a singular reservoir of stem cells. They are a viable alternative to bone marrow aspiration, as procurement of PDLSCs from premolars is less invasive and morbid procedure with minimal complications. The present study findings suggest that PDLSCs can be isolated and cultured from PDL cells of extracted adult premolars by using CD45, CD73, CD90, CD105, and CD146 markers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong J, Gronthos S, Bartold PM. Role of the epithelial cell rests of Malassez in the development, maintenance and regeneration of periodontal ligament tissues. Periodontol 2000. 2013;63:217–33. doi: 10.1111/prd.12023. [DOI] [PubMed] [Google Scholar]
- 3.Kawanabe N, Murata S, Murakami K, Ishihara Y, Hayano S, Kurosaka H, et al. Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen-4. Differentiation. 2010;79:74–83. doi: 10.1016/j.diff.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Pryce BA, Schweitzer R, Ryder MI, Ho SP. Differentiating zones at periodontal ligament-bone and periodontal ligament-cementum entheses. J Periodontal Res. 2015;50:870–80. doi: 10.1111/jre.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández-Monjaraz B, Santiago-Osorio E, Monroy-García A, Ledesma-Martínez E, Mendoza-Núñez VM. MSCs of dental origin for inducing tissue regeneration in periodontitis: A mini-review. Int J Mol Sci. 2018;19:944. doi: 10.3390/ijms19040944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu XY, Li X, Wang J, He XT, Sun HH, Chen FM, et al. Concise review: Periodontal tissue regeneration using stem cells: Strategies and translational considerations. Stem Cells Transl Med. 2019;8:392–403. doi: 10.1002/sctm.18-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menicanin D, Mrozik KM, Wada N, Marino V, Shi S, Bartold PM, et al. Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev. 2014;23:1001–11. doi: 10.1089/scd.2013.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki K, Akazawa K, Nagata M, Komaki M, Honda I, Morioka C, et al. The fate of transplanted periodontal ligament stem cells in surgically created periodontal defects in rats. Int J Mol Sci. 2019;20:192. doi: 10.3390/ijms20010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Kim KH, Seo BM, Koo KT, Kim TI, Seol YJ, et al. Alveolar bone regeneration by transplantation of PDLSCs and bone marrow stem cells in a canine peri-implant defect model: A pilot study. J Periodontol. 2009;80:1815–23. doi: 10.1902/jop.2009.090249. [DOI] [PubMed] [Google Scholar]
- 10.Tomokiyo A, Wada N, Maeda H. Periodontal ligament stem cells: Regenerative potency in periodontium. Stem Cells Dev. 2019;28:974–85. doi: 10.1089/scd.2019.0031. [DOI] [PubMed] [Google Scholar]
- 11.Tomokiyo A, Yoshida S, Hamano S, Hasegawa D, Sugii H, Maeda H, et al. Detection, characterization, and clinical application of mesenchymal stem cells in periodontal ligament tissue. Stem Cells Int. 2018;2018:5450768. doi: 10.1155/2018/5450768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Athanassiou-Papaefthymiou M, Papagerakis P, Papagerakis S. Isolation and characterization of human adult epithelial stem cells from the periodontal ligament. J Dent Res. 2015;94:1591–600. doi: 10.1177/0022034515606401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassir SH, Wisitrasameewong W, Raanan J, Ghaffarigarakani S, Chung J, Freire M, et al. Potential for stem cell-based periodontal therapy. J Cell Physio. 2016;231:50–61. doi: 10.1002/jcp.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong T, Heng BC, Lo EC, Zhang C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells Int. 2016;2016:9204574. doi: 10.1155/2016/9204574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iviglia G, Kargozar S, Baino F. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J Funct Biomater. 2019;10:3. doi: 10.3390/jfb10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata T, Yamato M, Washio K, Yoshida T, Tsumanuma Y, Yamada A, et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets - A safety and efficacy study in ten patients. Regen Ther. 2018;9:38–44. doi: 10.1016/j.reth.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan XZ, Yang F, Jansen JA, de Vries RB, van den Beucken JJ. Cell-based approaches in periodontal regeneration: A Systematic review and meta-analysis of periodontal defect models in animal experimental work. Tissue Eng Part B Rev. 2015;21:411–26. doi: 10.1089/ten.TEB.2015.0049. [DOI] [PubMed] [Google Scholar]
- 18.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 19.Navabazam AR, Sadeghian Nodoshan F, Sheikhha MH, Miresmaeili SM, Soleimani M, Fesahat F. Characterization of MSCs from human dental pulp, preapical follicle and periodontal ligament. Iran J Reprod Med. 2013;11:235–42. [PMC free article] [PubMed] [Google Scholar]
- 20.Park JC, Kim JM, Jung IH, Kim JC, Choi SH, Cho KS, et al. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: In vitro and in vivo evaluations. J Clin Periodontol. 2011;38:721–31. doi: 10.1111/j.1600-051X.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 21.Silvério KG, Rodrigues TL, Coletta RD, Benevides L, Da Silva JS, Casati MZ, et al. Mesenchymal stem cell properties of periodontal ligament cells from deciduous and permanent teeth. J Periodontol. 2010;81:1207–15. doi: 10.1902/jop.2010.090729. [DOI] [PubMed] [Google Scholar]
- 22.Wanichpakorn S, Kedjarune-Laggat U. Primary cell culture from human oral tissue: Gingival keratinocytes, gingival fibroblasts and periodontal ligament fibroblasts. Songklanakarin J Sci Technol. 2010;32:327–31. [Google Scholar]
- 23.Rajendran V, Jain MV. In vitro tumorigenic assay: Colony forming assay for cancer stem cells. Methods Mol Biol. 2018;1692:89–95. doi: 10.1007/978-1-4939-7401-6_8. [DOI] [PubMed] [Google Scholar]
- 24.Xiong J, Menicanin D, Zilm PS, Marino V, Bartold PM, Gronthos S. Investigation of the cell surface proteome of human PDLSCs. Stem Cells Int. 2016;2016:1947157. doi: 10.1155/2016/1947157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadkhoda Z, Rafiei SC, Azizi B, Khoshzaban A. Assessment of surface markers derived from human PDLSCs: An in vitro Study. J Dent (Tehran) 2016;13:325–32. [PMC free article] [PubMed] [Google Scholar]
- 26.Chopra H, Liao C, Zhang CF, Pow EHN. Lapine periodontal ligament stem cells for musculoskeletal research in preclinical animal trials. J Transl Med. 2018;16:174. doi: 10.1186/s12967-018-1551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stalin J, Nollet M, Dignat-George F, Bardin N, Blot-Chabaud M. Therapeutic and diagnostic antibodies to CD146: Thirty years of research on its potential for detection and treatment of tumors. Antibodies (Basel) 2017;6:17. doi: 10.3390/antib6040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu W, Tan Y, Qiu Q, Li X, Huang Z, Fu Y, et al. Comparison of the properties of human CD146 + and CD146- periodontal ligament cells in response to stimulation with tumour necrosis factor α. Arch Oral Biol. 2013;58:1791–803. doi: 10.1016/j.archoralbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Dehghani Nazhvani A, Hosseini SM, Tahoori B, Tavangar MS, Attar A. Identification of mesenchymal stem cell marker STRO-1 in oral reactive lesions by immunofluorescence method. J Dent (Shiraz) 2015;16:246–50. [PMC free article] [PubMed] [Google Scholar]
- 30.Shwetha HR, Kotrashetti VS, Babu NC, Kumbar V, Bhat K, Reddy R, et al. Ex vivo culture of oral keratinocytes using direct explant cell culture technique. J Oral Maxillofac Pathol. 2019;23:243–7. doi: 10.4103/jomfp.JOMFP_105_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oyajobi BO, Lomri A, Hott M, Marie PJ. Isolation and characterization of human clonogenic osteoblast progenitors immunoselected from fetal bone marrow stroma using STRO-1 monoclonal antibody. J Bone Miner Res. 1999;14:351–61. doi: 10.1359/jbmr.1999.14.3.351. [DOI] [PubMed] [Google Scholar]
- 32.Shi S, Gronthos S. Perivascular niche of postnatal MSCs in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 33.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–63. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF, et al. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–15. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 36.Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH, et al. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–58. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, et al. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials. 2013;34:7033–47. doi: 10.1016/j.biomaterials.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Tang R, Wei F, Wei L, Wang S, Ding G. Osteogenic differentiated periodontal ligament stem cells maintain their immunomodulatory capacity. J Tissue Eng Regen Med. 2014;8:226–32. doi: 10.1002/term.1516. [DOI] [PubMed] [Google Scholar]