Abstract
Objective:
The overall goal of this study was to compare verbal and visuo-spatial working memory in children with normal hearing (NH) and with cochlear implants (CI). The main questions addressed by this study were: 1) Does auditory deprivation result in global or domain-specific deficits in working memory in children with CIs compared to their NH age-mates? 2) Does the potential for verbal recoding affect performance on measures of reasoning ability in children with CIs relative to their NH age-mates? 3) Is performance on verbal and visuo-spatial working memory tasks related to spoken receptive language level achieved by children with CIs?
Design:
A total of 54 children ranging in age from 5–9 years participated; 25 children with CIs and 29 children with NH. Participants were tested on both simple and complex measures of verbal and visuo-spatial working memory. Vocabulary was assessed with the Peabody Picture Vocabulary Test (PPVT) and reasoning abilities with two subtests of the WISC-IV: Picture Concepts (verbally mediated) and Matrix Reasoning (visuo-spatial task). Groups were compared on all measures using analysis of variance (ANOVA) after controlling for age and maternal education.
Results:
Children with CIs scored significantly lower than children with NH on measures of working memory, after accounting for age and maternal education. Differences between the groups were more apparent for verbal working memory compared to visuo-spatial working memory. For reasoning and vocabulary, the CI group scored significantly lower than the NH group for PPVT and Picture Concepts, but similar to NH age mates on Matrix Reasoning.
Conclusions:
Results from this study suggest that children with CIs have deficits in working memory related to storing and processing verbal information in working memory. These deficits extend to receptive vocabulary and verbal reasoning and remain even after controlling for the higher maternal education level of the NH group. Their ability to store and process visuo-spatial information in WM and complete reasoning tasks that minimize verbal labeling of stimuli more closely approaches performance of NH age mates
INTRODUCTION
Pisoni and colleagues have long advocated using information processing models developed by cognitive psychologists to explain variability in spoken language outcomes in pediatric cochlear implant (CI) recipients (Pisoni et al. 2016). Under these models, human behaviors are assumed to involve a hierarchical sequencing of processing including sensation, perception, attention, learning and memory. This type of model views the human nervous system as an information processing system that encodes, stores and manipulates various types of symbolic representations (Lachman et al. 1979). Specifically, the ability to process and store information in immediate memory contributes to development of both verbal and visuo-spatial reasoning abilities and ultimately language and academic performance (Alloway et al. 2006). This ability may be impaired in children born with severe to profound hearing loss who experience auditory deprivation during early developmental years critical for development of skills that are dependent on the processing of verbal information.The sensory deficit imposed by hearing loss in these children (even when remediated with CIs) typically results in delays in spoken language (Osberger 1986; Svirsky et al. 2004; Bingham et al. 2009; Geers et al. 2009). Children who receive CIs in early childhood represent a group of children who gain partial access to auditory speech information at some point during this critical developmental period. Whether this altered sensory input affects only verbal processing and language or whether a more general deficit affects both verbal and visuo-spatial memory/reasoning has been the subject of some debate in the literature, particularly with regard to the process known as working memory.
Working memory refers to an individual’s capacity to store and manipulate information for short periods of time (Alloway et al. 2006). A widely accepted model of working memory developed by Baddeley and Hitch (1974) proposes that working memory is under the control of a domain general attention system referred to as the central executive. The central executive is responsible for coordinating activity of two subsystems for short-term storage of verbal and visuo-spatial information (phonological loop and visuo-spatial sketchpad, respectively).
The tasks designed to assess working memory skills may be classified as simple vs. complex; simple tasks primarily require storage (i.e. serial recall of digits or words) while complex tasks require coordination of both storage and processing (e.g. adding a task that requires the participant to identify and verbally name colors of digits that are to be serially recalled) (Engle et al. 1999; Hale et al. 2011; Ead et al. 2013). Tasks have been developed to measure both simple and complex memory processes in the verbal and visuo-spatial domains, depending on the nature of the stimuli (Baddeley & Logie 1999; Cocchini et al. 2002).
Assessment Considerations
The verbal working memory system is responsible for storing information that is expressed in spoken language (e.g. numbers, words, sentences). An important consideration for assessing verbal working memory is ensuring that the verbal items to be remembered are encoded (i.e., perceived) accurately. One way to guarantee that a participant has correctly encoded each item in a verbal series is to have them repeat each item out loud.
The visuo-spatial working memory system is responsible for storing information related to locations or sequences of objects or images. An important consideration for assessing visuo-spatial working memory is ensuring the task has eliminated the possibility of verbal mediation. Thus, it is important that no verbalization be permitted and that the reporting of the responses is spatial. Tasks that use sequencing of colors or objects may offer opportunities for verbal recoding of stimuli (Archibald & Gathercole 2006). That is, children may use learning strategies (i.e. verbal rehearsal) to help maintain items in working memory and aid in their recall. If opportunities for verbal recoding are present, assessment of visuo-spatial working memory as separate from verbal working memory may be compromised (Poppen et al. 1969; Wyke & Asso 1979; Montgomery 1993).
Verbal working memory in children with CIs
Consistent with the hypothesis that an impoverished auditory sensory input disrupts accurate encoding of verbal information necessary for phonological processing and memory, several studies have found that children with CIs exhibit poorer performance than their normal hearing (NH) age mates on tests of both simple and, in some cases, complex verbal working memory (Pisoni & Geers 2000; Pisoni & Cleary 2003; Watson et al. 2007; Kronenberger et al. 2013). For example, this result is seen with forward and backward digit span tasks, widely used for evaluating short term (simple forward span task) and working (complex backward span task) memory in NH children (Pisoni & Geers 2000; Burkholder & Pisoni 2003; Watson et al. 2007; Pisoni et al. 2011; Harris et al. 2013; Kronenberger et al. 2013). Accurate encoding of verbal stimuli may be an issue for children with hearing loss, especially when stimuli are presented auditorily. Lower performance by children with CIs compared to age mates with NH may be related to diminished audibility and/or poorer frequency resolution. Moreover, speech production difficulties of children with CIs may prohibit clear and accurate verbal responses. In order to address these issues, AuBuchon and colleagues designed tasks that were likely to minimize the effects of poor audibility/frequency resolution and speech production issues for children with CIs (AuBuchon et al. 2015). The digit span tasks were administered using both visual and auditory presentation of stimuli and both verbal (i.e., spoken recall) and non-verbal (i.e., touch screen) responses. They found that the NH group performed better than the CI group on forward digit span regardless of presentation mode or response modality. Both children with NH and children with CIs performed more poorly on backward digit span than forward. However, the differences between the groups on backward span were not statistically different. The authors suggest that the lack of a statistically significant difference between the two groups for complex span may be related to lack of statistical power to detect true differences. They also suggest that reversing the order of digits makes the task more difficult for both groups, however this may be “particularly detrimental for the normal-hearing controls because it also interferes with their ability to initiate lexical processing strategies”. They subsequently conclude that deficits in verbal working memory abilities are not due to reduced audibility or speech production capabilities, rather children with CIs are less efficient at using phonological and linguistic strategies to maintain and process verbal information.
Other studies reporting similar deficits in verbal working memory for CI recipients have used serial recall of non-words or non-word word repetition to estimate storage (simple task) and sentence completion and recall to estimate storage and processing (complex task) (Lyxell et al. 2008; Wass et al. 2008). The majority of these presented the stimuli so that the participant could both hear the talker and see their face and the participants’ responses were verbal. In a departure from the tasks described previously, Nittrouer and colleagues (2013, 2017) used a single serial recall task to obtain separate estimates of storage and processing (i.e. simple and complex) in verbal working memory. Pediatric CI recipients were required to listen to closed sets of rhyming and non-rhyming nouns and adjectives and respond by tapping pictures arranged on a computer screen in the same order as they were presented. They hypothesized that non-rhyming words would be recalled with greater accuracy since they are more phonologically distinct and would have more robust representations in storage. Thus, differences in accuracy of recall of rhyming and non-rhyming words would assess storage capacity. They suggest that nouns require less of a processing load than adjectives because nouns can be readily represented with an actual item or picture whereas adjectives must be inferred from a picture. They hypothesized that rate of recall would index processing with the expectation that adjectives would have longer response rates due to greater processing demands. Using two separate experiments on adults and children respectively, they found that accuracy was poorest for the rhyming words and recall was much slower for the adjectives. They also reported that correlations between accuracy and response time were not significant, confirming that these measures represent different abilities. The authors concluded that storage and processing (simple and complex working memory) could be assessed separately using a single task. A group of CI recipients and age mates with NH were tested at second grade (Nittrouer et al. 2013) and again two years later at fourth grade (Nittrouer et al. 2017). At the second grade test session, the CI recipients performed significantly worse than age mates with NH on accuracy, but similar on rate of responding (on accurately recalled items). However, at the fourth grade test session the CI recipients performed more poorly on accuracy (fewer items recalled correctly) and they exhibited slower reaction times. The authors concluded that children with CIs exhibited verbal working memory deficits related to both storage and processing. Furthermore, at the second test session they found the major source of variability for verbal working memory abilities differed for the NH and CI groups. The major source of variability for verbal working memory was phonological awareness for the NH group and vocabulary for the CI group.
Visuo-spatial Working Memory in Children with CIs
Assessment of visuo-spatial working memory may be confounded by verbal demands of the experimental task (Archibald & Gathercole 2006). This was apparent in an early study that found that pediatric CI recipients had shorter spans for locations of color sequences presented through visual cues alone than was observed in age mates with NH sensitivity (Cleary et al. 2001). Subsequent studies addressing visuo-spatial working memory in children with CIs have predominantly used tasks to assess simple working memory that are not subject to verbal encoding (Lyxell et al. 2008; Wass et al. 2008; Conway et al. 2011). These studies used various versions of matrix pattern or dot location tasks that require the subject to view patterns of filled matrices or dot locations on a computer screen for a short period of time and then replicate the pattern or locations on an empty matrix. In some cases, the number of locations or patterns to be recalled increases until the subject reaches a ceiling performance level. The majority of these tasks do not require items to be recalled in a specific order (i.e. serial recall). In a departure from typical visuo-spatial tasks, an earlier study of pediatric CI recipients used a task that required subjects to replicate alternating hand movements (fist vs. palm) in the order they were presented (Dawson et al. 2002). In the aforementioned studies, children with CIs performed on par with their age mates with normal hearing sensitivity.
The communication backgrounds of children from these studies have varied with some reporting exposure to spoken language exclusively (Dawson et al. 2002), and others reporting some exposure to sign language prior to receiving a CI (Lyxell et al. 2008; Wass et al. 2008; Conway et al. 2011). Interestingly, reliance on sign language has been associated with superior performance, compared to reliance on spoken language, on tasks requiring visuo-spatial memory (Marschark 2006). A subsequent study, however failed to demonstrate a similar advantage for sign language users (Marschark et al. 2015).
Auditory Deprivation and Sequential Processing
Some researchers (e.g., Conway et al. 2011) propose that experience with the temporal nature of sound signals affects how individuals learn and process serial input. Lack of auditory experience by children with hearing impairment may disrupt temporal processing from both auditory and visual modalities (e.g., visual sequence learning), and may result in global changes in neurocognitive functions (Pisoni et al. 2016). These researchers propose a domain-general delay on working memory tasks that require sequential processing, whether stimuli are presented in visual or auditory modalities. Khan and colleagues found that children with hearing aids (HAs) exhibited poorer performance than children with NH and children with CIs on tasks that required sequential ordering of visual stimuli (Khan et al. 2005). Another study found that children with CIs performed significantly below published norms on a task that required visual monitoring of sequentially presented visual stimuli (Horn et al. 2005). More recent studies of adult and pediatric CI recipients report delays in both visual and motor sequence-learning or processing skills (Lévesque et al. 2014; Ulanet et al. 2014; Bharadwaj & Mehtra 2016). Yet in other studies in which stimuli were carefully designed to preclude the use of overlearned labels (e.g., colors) that could be verbally rehearsed, CI recipients and normal hearing age-mates exhibited similar sequential-learning abilities (Torkildsen et al. 2018; Hall et al. 2017). Moreover, some studies of children with CIs have found that performance on nonverbal and visual tasks requiring sequential processing of stimuli is associated with spoken language skills (Dawson et al. 2002; Edwards & Anderson 2014; Ulanet et al. 2014). Differences in general trends across studies may be due, in part, to different methodologies (i.e., learning accuracy vs. reaction times) and to the extent to which non-verbal stimuli may be verbally mediated.
Importance of Working Memory for Language Development
Reaching developmental milestones in speech and language at a rate commensurate with typically developing children with normal hearing sensitivity is supported by verbal working memory abilities (Gathercole et al. 1997; Adams & Gathercole 2000; Alloway et al. 2009). Moreover, several studies with pediatric CI recipients have demonstrated that verbal working memory abilities are correlated with spoken language outcomes (Pisoni & Geers 2000; Pisoni & Cleary 2003; Ibertsson et al. 2009). More recently, Marschark et al. (2015) demonstrated that spatial processing in deaf individuals is more highly associated with language ability than in a NH population, and this relation is independent of the child’s preferred mode of communication (sign or speech). These findings suggest that strong working memory skills may serve an important function in language acquisition in children, but visuo-spatial memory may be more critical in the case of children with hearing loss.
Verbal and Visuo-spatial Reasoning Abilities in Children with CIs
The accurate assessment of cognitive abilities in children with hearing loss is dependent on minimizing the effects of auditory and language deficits (Phillips et al. 2014) by using visual-motor and visuo-spatial (i.e., non-verbal) tasks. Tasks that require a child to solve problems by drawing upon visual-motor and visuo-spatial skills are thought to provide an overall estimate of general cognitive abilities in children with normal hearing (Cattell 1987) and children with hearing loss perform similarly to age mates with NH on such tasks (Krivitski et al. 2004; Khan et al. 2005; Zekveld et al. 2007). Cognitive assessments that have not controlled for the effects of auditory and verbal skills may underestimate non-verbal cognitive skills of children with hearing loss (Braden 1992; Van Boxtel et al. 2000; Phillips et al. 2014). Castellanos and colleagues compared visual concept formation skills of a group of 57 long-term CI users to 71 NH peers (Castellanos et al. 2015). The CI participants ranged in age from 7–27 years old, had received a CI prior to age 7 and had used their CI for at least 7 years. The majority (~63%) of participants used a unilateral CI only (i.e. no device at the non-implanted ear). The communication mode for these CI participants varied from mostly sign to auditory verbal only. Concept formation tasks were administered to assess the ability to respond to a common set of features to classify objects or events. Study participants were asked to identify and verbalize the underlying rule for grouping objects. Rules for grouping could be related to size, quantity, shape or color and were distributed across three categories; single comparison and single rule, multiple comparisons and single rule and multiple comparisons and multiple rules. They found that CI recipients performed significantly poorer on concept formation tasks, especially when multiple comparisons and rules were involved. They also found that concept formation skills were more strongly related to vocabulary skills for the CI group compared to the NH group. The authors concluded that early auditory deprivation combined with an impoverished auditory signal from a CI affect higher order cognitive skills including conceptual reasoning. While the stimuli in the aforementioned study were non-verbal, the participants were required to verbalize their responses. Thus, to the degree that these tasks require some degree of verbal mediation, children with hearing loss may score lower than age mates with normal hearing.
Objectives of the Current Investigation
The principal goal of this study was to compare working memory in children using CIs with their NH age-mates, using both simple and complex tasks in both verbal and visuo-spatial domains. This study only included subjects who relied on spoken communication to control for possible influence of sign language proficiency on visuo-spatial working memory. The main questions addressed by this study were: 1) Does auditory deprivation result in global or domain-specific deficits in working memory in children with CIs compared to their NH age-mates? 2) Does the potential for verbal recoding affect performance on measures of reasoning ability in children with CIs relative to their NH age-mates? 3) Is performance on verbal and visuo-spatial working memory tasks related to spoken receptive language level achieved by CI children?
To address these questions children with NH and CIs were tested on both simple and complex measures of verbal and visuo-spatial working memory. A receptive vocabulary test and two tests of perceptual reasoning abilities were also administered.
METHOD
Fifty-four children ranging in age from 5–9 years participated: 25 children with CIs and 29 children with NH. There were 10 females in the CI group and 14 females in the NH group. All children were recruited from the local metropolitan area in St. Louis, Missouri (USA). Children with CIs were recruited through local CI surgical centers and a private oral school for the deaf. Children with NH were recruited from local grade schools. All 25 children with CIs had parents with normal hearing sensitivity and received educational services via spoken language (English) in either a general public education setting or an auditory-oral private school for the deaf. None of the children were reported to have any delays in cognitive development. Maternal education level was used as a socio-demographic variable and calculated in total years of education through college or beyond. The average educational level was 16.8 years (SD= 1.9) for the NH group and 15 years (SD= 2.6) for the CI group. The average age at test was 7.9 years (SD = 1.6) for the CI group and 7.4 years (SD = 1.7) for the NH group. Over 80% of both the CI and NH groups were Caucasian. The only significant difference in demographic variables between the CI and NH groups was mother’s education level, F (1, 52) = 8.21, p < .01.
A total of 18 children used bilateral CIs (CI at each ear) and 7 used bimodal devices (CI with a HA at the non-implanted ear). The audiologic data for the total group and for the bilateral and bimodal groups separately are shown in Table 1. Note on Table 1 that data for age at HA was unavailable for one participant and unaided pre-CI pure-tone average (PTA) for another participant. The only significant difference between the bilateral and bimodal CI groups was age at 1st CI, F (1, 23) = 8.06, p < .01. The bilateral group received their 1st CI at an earlier age than the bimodal group. There were no significant differences between the two CI groups for age at test, age at 1st HA or any of the calculated unaided pure-tone averages (uPTAs). The combined CI group had their loss first identified at age 10 months (SD=10), although for five children the age at identification was unknown. They received their first HAs at an average age of 16.8 months (SD = 9.4) and their first CI at 30 months of age (SD = 15.7). Approximately half (52%) of the participants reported the etiology of the hearing loss as unknown and the other half were nearly equally distributed across Connexin, enlarged vestibular aqueduct (EVA) and syndromic.
Table 1.
Audiological characteristics of participants, listed first for the full sample, then followed by means for each device group, bilateral and bimodal.
| Mean | SD | N | |
|---|---|---|---|
| Age First HA (months) | 16.8 | 9.4 | 24 |
| Bilateral Cochlear Implants (2 CIs) | 16.2 | 8.2 | 17 |
| Bimodal Devices (1 HA and 1 CI) | 18.1 | 12.3 | 7 |
| Age at First CI (months) | 30.1 | 15.7 | 25 |
| Bilateral Cochlear Implants (2 CIs) | 25.2 | 13.5 | 18 |
| Bimodal Devices (1 HA and 1 CI) | 42.7 | 14.8 | 7 |
| Pre-CI unaided PTA (dB HL) 1st CI ear | 107.1 | 11.1 | 24 |
| Bilateral Cochlear Implants (2 CIs) | 107.1 | 11.8 | 17 |
| Bimodal Devices (1 HA and 1 CI) | 107.2 | 10.2 | 7 |
| Pre-CI unaided PTA (dB HL) 2nd ear | 94.5 | 18.3 | 24 |
| Bilateral Cochlear Implants (2 CIs) | 98.1 | 18.4 | 17 |
| Bimodal Devices (1 HA and 1 CI) | 85.8 | 15.8 | 7 |
All children with CIs had open set word recognition and aided threshold testing completed as part of their audiology care within 15 months of the test date for the study. Open set speech perception tests were administered at a conversational level of 60–65 dB SPL using one of the following word lists: the Lexical Neighborhood Test (LNT) (Kirk et al. 1995) the Consonant Nucleus Consonant (CNC) word lists (Peterson & Lehiste 1962) or the Phonetically Balanced Kindergarten Wordlist (PBK) (Haskins 1949). The majority of the children had received the LNT (n=19), followed by the CNC (n=5) and the PBK (n=1). The average speech perception score across all tests was 77% (SD=16) correct. The average LNT score was 75% (SD= 12), the CNC score was 81% (SD=12) and the PBK score was 96%. All children had aided threshold PTAs (@ .5, 1 and 2 kHz) ≤30 dB HL. Before testing in the current study, all devices were checked for proper functioning by a certified pediatric audiologist. Testing was conducted in a quiet testing suite in the research lab and all testing was conducted by a single examiner under the supervision of a certified cognitive psychologist.
Test Measures
Verbal working memory and visuo-spatial working memory were assessed using both simple and complex versions of the tasks. Simple tasks required serial recall of visually presented items while complex tasks required serial recall of items while performing a secondary task (i.e. counting geometric shapes or recalling location of different shape on grid). A pictorial representation of each task is shown in Figure 1.
Figure 1.
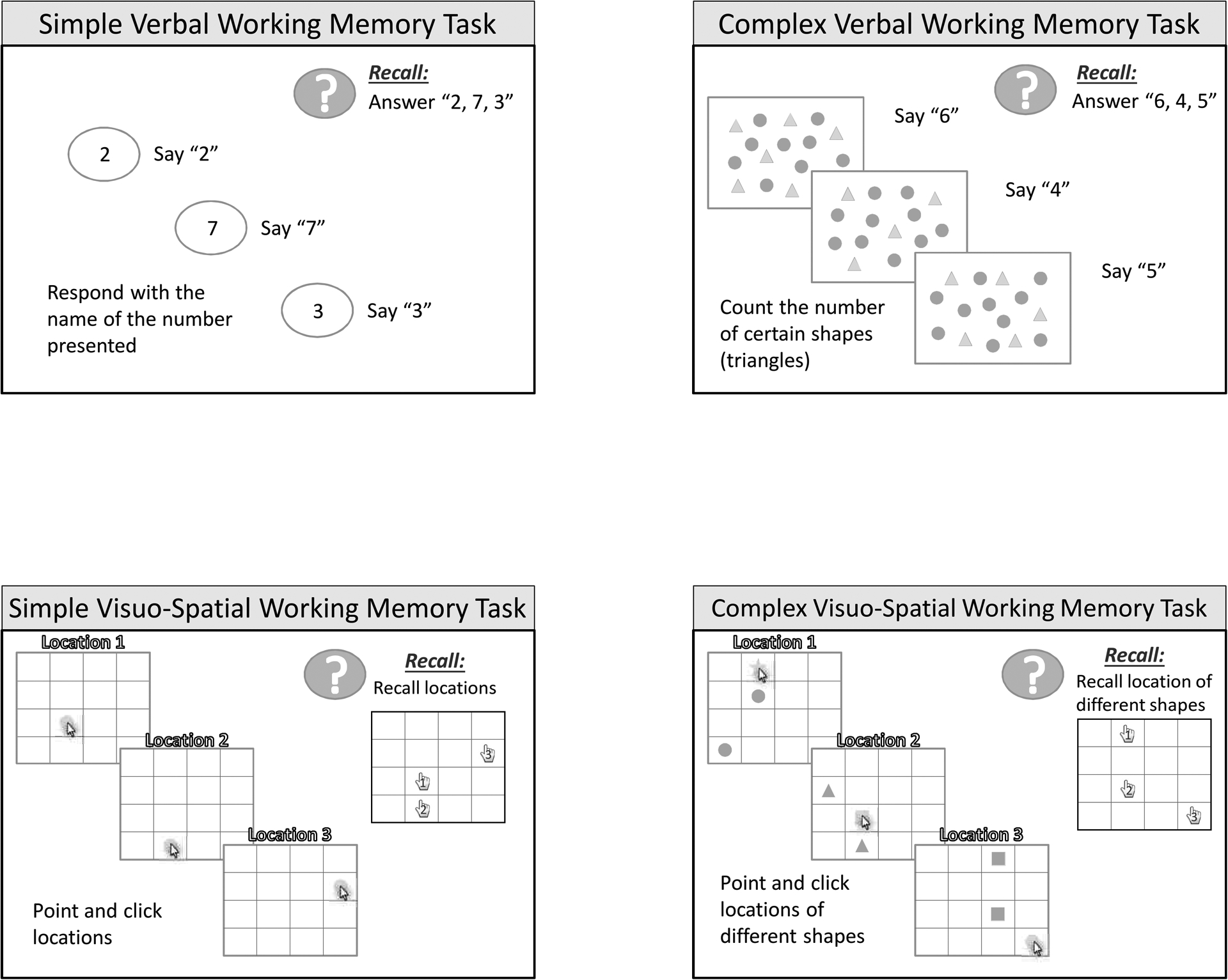
Pictorial representations of the tasks for a) simple verbal working memory, b) complex verbal working memory, c) simple visuo-spatial working memory, d) complex visuo-spatial working memory
Simple Verbal Working Memory - (digit span)
For this task, participants watched a series of 2 to 7 digits presented on a computer screen. Each digit of a given series was presented individually and participants were instructed to repeat each digit out loud when it appeared on the screen. Digits were on the screen for 2000 ms with an inter-stimulus interval of 500 ms. A green box was illuminated at the completion of each digit series to signal the participant to repeat the digits out loud in the order they appeared. Four trials were presented for each digit series; correct serial recall of 3 out of the 4 trials was required to proceed to the next series length (e.g., from 2 to 3 digits). A practice trial of a 3-digit series was given before the test was initiated. Memory span was calculated by taking the longest digit span at which the participant correctly recalled 3 out of 4 trials.
Complex Verbal Working Memory - (counting span)
For this task, participants watched a computer screen with an array of geometric shapes that consisted of triangles and circles. The primary task was to remember the total counts associated with each screen and the secondary task was to count the triangles out loud. Participants watched individual screens with numbers of circles ranging from 2–7 and were told to count the shapes out loud and repeat (out loud) the total number counted for each screen. When signaled by the green go box, they were told to repeat the numbers counted per screen out loud, in the order they appeared. Four trials were presented for each counting series (2–7); correct serial recall of 3 out of the 4 trials was required to proceed to the next higher counting series. A practice trial of 3 counting series was given before the test was initiated. Memory span was calculated by taking the longest counting span at which the participant correctly recalled 3 out of 4 trials.
Simple Visuo-spatial Working Memory - (location span)
For this task, participants watched a computer screen with a 4×4 grid and touched the location on the grid with a red circle. Upon touching the location, the circle disappeared and another screen with a circle in a different grid location was presented. Participants were told to remember the location of the circle for each screen and when the green go box was illuminated to touch the locations of the circles on the grid in the order that they appeared. The number of locations to be remembered ranged from 2–7. Four trials were presented for each location series length; correct serial recall of 3 out of the 4 trials was required to proceed to the next series length. A practice trial of 3 location series was given before the test was initiated. Memory span was calculated by taking the longest location span at which the participant correctly recalled 3 out of 4 trials.
Complex Visuo-spatial Working Memory - (odd-one-out location span)
For this task, participants watched a computer screen with various geometric shapes placed in a 4×4 grid. Each to be remembered location appeared on the screen as a unique shape accompanied by two irrelevant locations filled with two identical shapes (different from the target). The participant was instructed to identify (and touch) the unique shape and then recall, in serial order, the touched locations only when the green go box was illuminated. The primary task is to remember the location of the “touched items” and the secondary task is to “find and touch” the odd-one-out. The number of locations to be remembered ranged from 2–7. Four trials were presented for each location series; correct serial recall of 3 out of the 4 trials was required to proceed to the next higher level. A practice trial of 3 location series was given before the test was initiated. Memory span was calculated by taking the longest location span at which the participant correctly recalled 3 out of 4 trials.
The Picture Concepts and Matrix Reasoning Subtests from the Wechsler Intelligence Scale for Children, 4th edition (Wechsler 2003) were used to assess overall perceptual (fluid) reasoning abilities. The Picture Concepts and Matrix Reasoning tasks were chosen to represent tasks that differ in the extent to which verbal skills may be required to solve visual and visuo-spatial problems. Picture Concepts measures perceptual organization of nonverbal concepts (i.e. organize and categorize common features of nonverbal concepts). For this subtest, the participants were presented with two to three rows of pictures and asked to select one picture from each row that related conceptually to one another. For example, the child sees one row with a picture of a car and a picture of a book, and the second row shows a picture of the sun and a picture of a bus. The child should point to the car and the bus since they are both modes of transportation. The number of items in each row and the numbers of rows increase as the test progresses. For example, the child is presented with three rows with three pictures in each row. The child should choose (point to) the bathtub, fire-hydrant and water hose, since all use water. Although stimulus presentation and response demands are non-verbal, accurate responses require verbal reasoning. The child receives 1 point for each correct answer. Testing stops after 5 consecutive incorrect answers. The Matrix Reasoning subtest measures a child’s skill at grasping shapes, designs and visuo-spatial patterns in order to identify or correct missing aspects of these concepts, and complete or correct them. For this subtest, the child is presented with a series of incomplete matrices, each of which is a series of abstract patterns and designs. The child is directed to select the best from five item choices in order to complete the matrix. For example, the child is shown colored matrices or visual patterns with something missing and asked to select the missing piece from a range of options. The child receives 1 point for each correct answer. Testing stops after 4 consecutive incorrect answers.
The Peabody Picture Vocabulary Test- III (PPVT)
The PPVT (Dunn & Dunn 1997) was used as a measure of receptive vocabulary for children in both the CI and NH groups. The test was administered face to face. The child was instructed to select the picture that best represented the spoken target word from four alternatives. The raw score corresponds to the number of items below a basal level (7 consecutive correct responses) plus the number of items correctly selected.
RESULTS
Statistical Analyses:
Analysis of variance (ANOVA) was used to compare group differences on all outcome measures (working memory, PPVT, WISC Picture Concepts and Matrix Reasoning). Correlational analyses were used to examine associations between possible device and demographic variables and all outcome variables, as well as working memory variables and vocabulary and verbal reasoning outcomes.
Selection of Predictor variables:
Within the CI group, correlations between audiological variables (uPTAs, age at 1st HA and age at CI) and all outcome measures (verbal working memory, visuo-spatial working memory, PPVT, WISC-Picture Concepts and Matrix Reasoning) were not significant (r-value range,−.33 to .17; p-values > .05), so these variables were not included in further analyses. There were no significant differences between data for the bimodal and bilateral CI users on any outcome measure (verbal working memory, visuo-spatial working memory, PPVT, WISC Picture Concepts and Matrix Reasoning). Based on these results, further analyses comparing outcome measures in the NH and CI groups were conducted with the two CI groups combined.
Age at test was significantly correlated with all outcome measures (r –value range, .43 to .71, p values <.01) and was used as a covariate for all group comparisons. Correlations between mother’s education and all outcome measures were not significant except for PPVT (r = .47; p<.01). However, due to the significant difference between CI and NH groups in this variable, group comparisons were conducted with maternal education controlled.
Conversion to Standardized Scores
In order to compare across WM measures with different distributions, raw scores were converted to z scores based on the distribution of scores in the CI and NH groups combined. Age norms were not available on the WISC (Picture Concepts and Matrix Reasoning) for the youngest children in this study so for comparable age correction, raw scores were converted to z-scores for these subtests as well. Table 2 includes the mean and standard deviations for the raw scores and z-scores for all tasks; verbal and visuo-spatial working memory (simple and complex), PPVT and Picture Concepts and Matrix Reasoning from the WISC.
Table 2:
Group Mean and Standard Deviation (SD) values for all measures
| Simple Verbal Span | Simple Visuo-Spatial Span | Complex Verbal Span | Complex Visuo-Spatial Span | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 3.0 | 0.8 | 2.8 | 1.1 | 2.2 | 0.9 | 1.9 | 1.1 | |||||
| NH | 29 | 3.9 | 1.5 | 3.1 | 1.3 | 2.8 | 1.1 | 2.2 | 1.0 | ||||
| z scores | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| CI | 25 | −0.39 | 0.64 | −0.12 | 0.89 | −0.32 | 0.85 | −0.15 | 1.07 | ||||
| NH | 29 | 0.34 | 1.14 | 0.10 | 1.09 | 0.28 | 1.05 | 0.13 | 0.94 | ||||
| WISC-PC | WISC-MR | PPVT | |||||||||||
| Raw scores | Mean | SD | Mean | SD | Mean | SD | |||||||
| CI | 25 | 13.4 | 3.3 | 16.6 | 5.9 | 104.1 | 24.8 | ||||||
| NH | 29 | 15.1 | 4.4 | 17.3 | 5.9 | 140.1 | 20.2 | ||||||
| z scores | Mean | SD | Mean | SD | Mean | SD | |||||||
| CI | 25 | −0.23 | 0.8 | −0.06 | 1.0 | −0.67 | 0.9 | ||||||
| NH | 29 | 0.19 | 1.1 | 0.05 | 1.0 | 0.58 | 0.7 | ||||||
WISC-PC is Wechsler Intelligence Scale for Children, Picture Concepts test, WISC-MR is Wechsler Intelligence Scale for Children, Matrix Reasoning test and PPVT is the Peabody Picture Vocabulary Test.
Analysis of Covariance
The z-scores for the working memory tasks were analyzed using a mixed model analysis of variance with age at test and maternal education entered as a covariates (ANCOVA) to adjust for the difference in maternal education between the CI and NH group and age-related differences across the participants. A significant effect for age was found (F (1, 50) = 50.74, p <.001), however there was no significant effect for maternal education (F (1, 50) = 1.06, p > .05. Group (NH vs. CI) was entered as a between subjects’ variable and domain (verbal vs. visuo-spatial) and task difficulty (simple vs. complex) were entered as within subject variables. Results revealed a main effect for group (F (1, 50) = 10.03, p < .01). Cohen’s d effect size was .90. There were no significant interactions. To test the validity of the normality assumption for data, the residuals from the ANCOVA model were examined to control for systematic between-groups variability. Due to multiple error terms (i.e., multiple within-subjects linear combinations), several residual terms were checked for normality. Results from the Shapiro-Wilk test (p values > .05) revealed that all residuals (domain, difficulty & difficulty X domain) were normally distributed.
The z-scores for the PPVT, Matrix Reasoning and Picture Concepts were each compared between the two groups (CI and NH) using an ANCOVA with age at test and maternal education entered as a covariates. Results from the Shapiro-Wilk test (p values > .05) revealed that the PPVT, WISC Picture Concept and Matrix Reasoning data were normally distributed.
Older children outperformed younger children on all three outcomes: (PPVT, F (1, 50) = 33.9, p < .001; WISC Picture Concepts, F (1, 50) = 41.1, p < .001; WISC Matrix Reasoning F (1, 50) = 62.8, p < .001). Maternal education was only significant for the PPVT outcome (F (1, 50) = 9.99, p < .01). The NH group outperformed the CI group for PPVT F (1, 50) = 44.61, p < .001, and for Picture Concepts F (1, 50) = 4.53, p < .05, however there were no significant differences for Matrix Reasoning F (1, 50) =.078, p >.05. Cohen’s d effects sizes were 1.58 and .43 for the PPVT and WISC Picture Concepts, respectively.
Comparing Verbal and Visuo-spatial Domains
Results of z-score comparisons indicated that CI users exhibited significantly lower performance relative to NH peers on tasks that required some degree of verbal processing (verbal working memory, WISC-Picture Concepts and PPVT) and exhibited similar performance on visuo-spatial processing tasks (visuo-spatial working memory, WISC-Matrix Reasoning). Figure 2 (Panel a) summarizes the mean z-scores and 95% confidence intervals for the CI and NH groups for verbal processing tasks and Figure 2 (Panel b) for visuo-spatial tasks. These scores have been adjusted for age and maternal education. Overlapping distributions for CI and NH children are apparent for visuo-spatial tasks compared to the relative separation of distributions for the verbal tasks.
Figure 2.
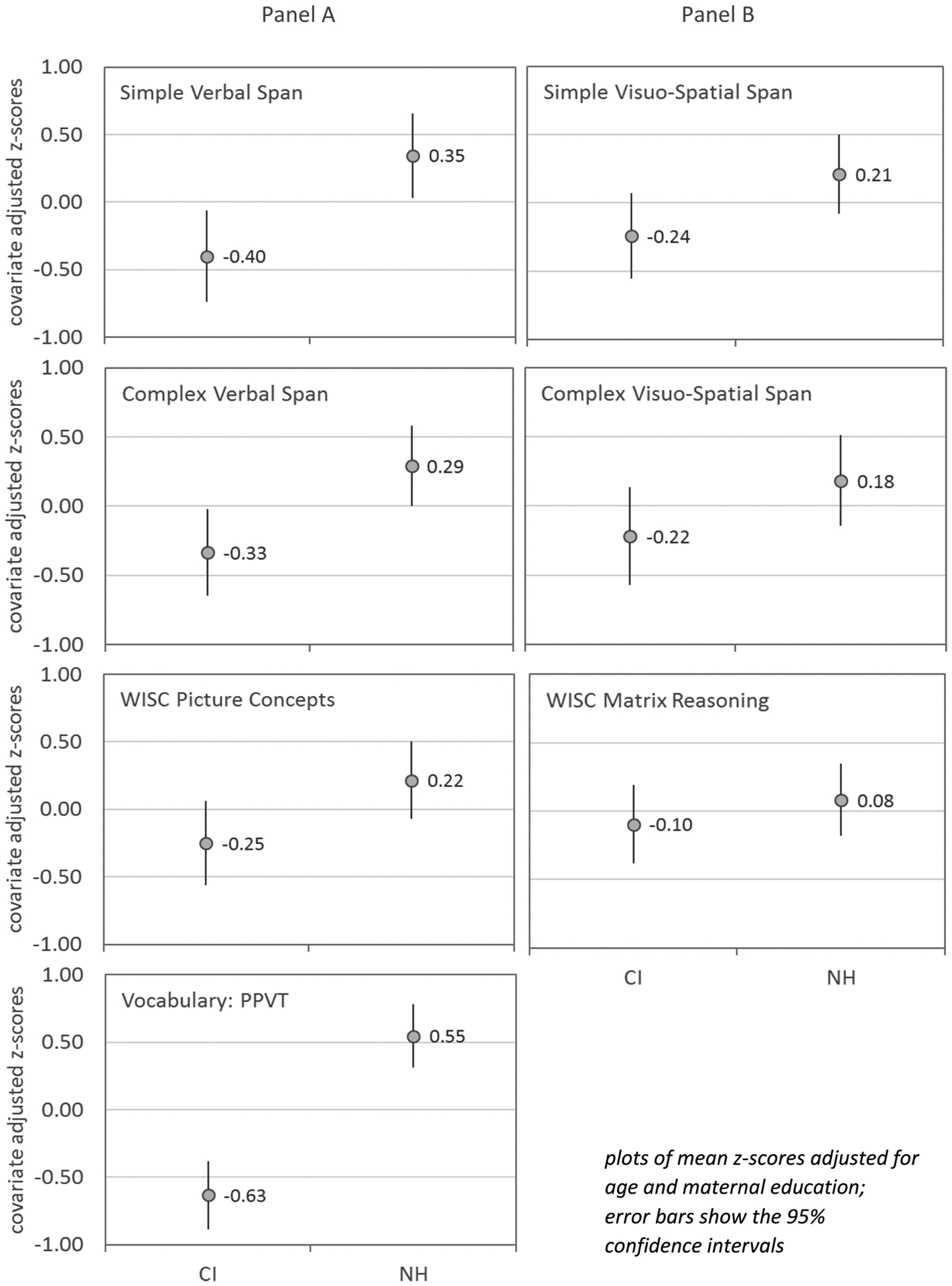
Means and 95% confidence intervals for z-scores for NH and CI groups for simple verbal working memory longest span, complex verbal working memory longest span, WISC Picture Concepts and PPVT (Panel A left column) and simple visuo-spatial working memory longest span, complex visuo-spatial working memory longest span, WISC Matrix Reasoning (panel B right column)
Correlations
Table 3 shows partial correlations between working memory spans and outcome measures controlling for age at test and maternal education. Bonferroni corrections for 12 comparisons required a p-value of <.0042 for significance. Correlations were calculated between measures of working memory and measures of vocabulary and perceptual reasoning (PPVT, Picture Concepts & Matrix Reasoning) to examine whether the contribution of memory span (verbal and visuo-spatial) varied based on the extent of verbal task demands. Simple verbal working memory scores showed positive significant correlations with the PPVT (partial correlation r = .39, p< .0041) however the correlations for WISC Picture Concepts were not statistically significant. Simple visuo-spatial working memory scores were positively correlated with WISC Matrix Reasoning (partial correlation .48, p< .0041), but not with PPVT scores nor WISC Picture Concepts scores. None of the correlations between the complex measures of working memory (visuo-spatial and verbal) and vocabulary or reasoning outcomes (PPVT, WISC Picture Concepts and Matrix Reasoning) were statistically significant.
Table 3:
Table shows partial correlations (controlling for age and maternal education) between working memory spans and outcome measures. Bonferroni corrections for 12 comparisons require a p-value of <.0042 for significance. All scores have been converted to z-scores to allow for direct comparison in the same units.
| Partial Correlations | ||||||
|---|---|---|---|---|---|---|
| Control Variables | SVspan.z | SVSspan.z | CVspan.z | CVSspan.z | ||
| Age & Maternal | PPVT.z | Correlation | 0.39 | 0.37 | 0.37 | 0.218 |
| Education | Sig (2-tailed) | 0.0041 | 0.007 | 0.007 | 0.120 | |
| df | 50 | 50 | 50 | 50 | ||
| WISC.MR.z | Correlation | 0.267 | 0.48 | 0.304 | 0.334 | |
| Sig (2-tailed) | 0.056 | 0.000 | 0.028 | 0.015 | ||
| df | 50 | 50 | 50 | 50 | ||
| WISC.PC.z | Correlation | 0.369 | 0.260 | 0.349 | 0.301 | |
| Sig (2-tailed) | 0.007 | 0.063 | 0.011 | 0.030 | ||
| df | 50 | 50 | 50 | 50 | ||
SV span z is the z-score for Simple Verbal longest span, SVS span z is Simple Visuo-Spatial longest span, CV span z is Complex Verbal longest span and CVS span z is Complex Visuo-Spatial longest span. PPVT is the z-score on the Peabody Picture Vocabulary Test, WISC-PC is the z-score on the Wechsler Intelligence Scale for Children, Picture Concepts test and WISC-MR is the z-score on the Wechsler Intelligence Scale for Children, Matrix Reasoning test.
DISCUSSION
The principal goal of this study was to examine the effects of early auditory deprivation on cognitive function in the verbal and visuo-spatial domains by addressing three questions.
1). Does early auditory deprivation result in global or domain-specific deficits in working memory in children with CIs compared to age-mates who have NH?
The results from this study suggest that working memory deficits in children with CIs are domain specific. That is, they are related to deficits in encoding, storing and manipulating spoken verbal information. These deficits are at least partially due to the spectrally degraded auditory signal provided by the cochlear implant. Overall the children with CIs scored significantly lower on both simple and complex verbal working memory tasks compared to their age mates with NH, however their performance on measures of visuo-spatial working memory tasks was similar to NH peers.
Verbal working memory deficits for this CI group persist even with good audibility (aided thresholds ≤ 30 dB HL). Aided detection thresholds for CI users that are ≤ 30 dB HL or better from 250–4000 Hz have been shown to be associated with better speech perception scores and spoken language outcomes for both adult and pediatric CI recipients (Donaldson & Allen 2003; Davidson et al. 2010; Holden et al. 2013; Davidson et al. 2014). Additionally, good open-set speech recognition had been documented for this group (mean score of 77% correct). Children in the current study repeated the numbers out loud after the presentation of each individual digit (simple task) and they counted the number of shapes out loud (from each series) and repeated the total number counted before being prompted to repeat the entire series. This was done to ensure that the younger children, especially those with CIs, had identified the digits correctly and counted the shapes accurately. Thus, the CI participants’ performance may have been affected by less efficient production skills compared to the NH children. Although, recall that when AuBuchon et al (2015) used tasks that minimized the effects of both audibility and speech production on forward digit span they reported that the NH group performed better than the CI group regardless of presentation mode or response modality. Alternatively, having the children repeat digits out loud may have allowed all children an added opportunity for articulatory rehearsal of digits for more efficient storage and retrieval from short term memory. Despite this opportunity for the CI group to overtly rehearse the digits, their performance still remained poorer than their NH age mates.
The NH group outperformed the CI group on both simple and complex tasks of verbal working memory, supporting deficits in both storage and processing of stimuli in the verbal domain. This finding is consistent with other studies reporting similar deficits in both storage and processing in the verbal domain (Pisoni & Geers 2000; Burkholder & Pisoni, 2003; Watson et al 2007; Kronenberger et al. 2013; Pisoni et al. 2011; Harris et al. 2013) and more recent results from Nittrouer et al. (2017).
In contrast to verbal working memory, the children with CIs in the present study were similar to their NH age mates on visuo-spatial working memory tasks, both simple and complex. The results of the current study are similar to other studies that have shown CI recipients perform similarly to NH age mates on tasks that primarily assess storage for visuo-spatial working memory (Lyxell et al. 2008; Wass et al. 2008; Conway et al. 2011). Furthermore, even though the visuo-spatial memory tasks required serial recall of locations, the performance for the CI group was on par with NH age mates. Recall that some propose that lack of early auditory experience may affect how individuals learn and process serial input, in both the auditory and visual domains (Conway et al. 2011). Thus, children diagnosed with early hearing loss may show deficits on tasks that require sequential processing, whether stimuli are presented in visual or auditory modalities (Cleary et al. 2001; Horn et al. 2005; Ulanet et al. 2014). This finding may have resulted from using tasks that allowed for verbal mediation even though the presentation was visual and the response was nonverbal (e.g., presenting sequences of colored lights for recall when colors are subject to verbal encoding, and may advantage NH participants). In contrast, the stimuli and response requirements for the visuo-spatial tasks used in the current study were carefully constructed to minimize the likelihood of verbal encoding of the visual stimuli. For example, the tasks required that the children point to the location on a 4×4 grid with 16 cells, therefore the ability to verbally label the locations was minimized. This is an important issue to address as the type of task and the stimuli may influence the strategies participants use to encode, store and retrieve items in working memory. Thus the children with CIs in this study were able to perform on par with their age mates when the visuo-spatial task was designed in such a way that minimized any opportunity to verbally encode or label stimuli.
2). Does the potential for verbal recoding affect performance on measures of reasoning ability in children with CIs relative to their NH age-mates?
Comparisons of group scores on the PPVT support that children with CIs have deficits related to the verbal domain. These verbal deficits were apparent even after controlling for the higher level of maternal education of the NH group. Higher levels of maternal education have been positively associated with language development in typically developing children as well as children with CIs and HAs (Dollaghan et al.1999; Geers et al. 2009; Niparko et al. 2010; Ching et al. 2017). Both the quantity and quality of early linguistic and communicative input provided to the child are thought to be at least partly responsible for the positive influence on spoken language and academic outcomes (Carney & Moeller 1998; DesJardin & Eisenberg 2007). The group effect remained significant in the working memory analysis showing that overall the group with NH outperformed the group with CIs.
As expected and consistent with other studies in the literature, the CI group had significantly lower scores on receptive vocabulary compared to NH age mates, even after controlling for group differences in maternal education (Blamey et al. 2001; Fagan et al. 2007; Geers et al. 2016).
While tests of receptive vocabulary are clearly verbal in nature, the WISC Picture Concepts and Matrix Reasoning tasks differ in their reliance on verbal encoding. Both are designed to assess overall perceptual reasoning abilities by drawing primarily upon visual and visuo-spatial skills, however Picture Concepts is more likely to draw upon verbal mediation skills than Matrix Reasoning. Comparison of CI and NH scores for the Matrix Reasoning subtest reveals if the opportunity to verbally recode stimuli is minimized, children with CIs perform similarly to their NH age mates. However, on the Picture Concepts subtest the children with CIs scored significantly lower than their age mates with NH, even though their responses were nonverbal (i.e. they pointed to objects from each row that shared similar attributes). These results align with those obtained by Castanellos et al. (2015) who found that long term pediatric CI recipients performed more poorly than NH age mates on a visual concept task that required participants to verbalize the underlying rule for grouping objects. Thus, even though the children in this study were not required to verbalize results, they still exhibited deficits in visual reasoning abilities. Therefore, results for reasoning measures were consistent with those obtained with working memory tasks, indicating domain-specific deficits in verbal processing associated with early auditory deprivation.
3). Is performance on verbal and visuo-spatial working memory tasks related to spoken receptive language level achieved by CI children?
After controlling for age and maternal education, only simple verbal span was significantly correlated with vocabulary scores. Complex verbal span was not significantly correlated with vocabulary, however many of the youngest participants performed poorly on this task, therefore floor effects may be partially responsible for these results. Furthermore, simple and complex visuo-spatial working memory spans failed to correlate significantly with verbally-mediated outcomes (i.e. PPVT and Picture Concepts) although simple visuo-spatial span scores were significantly correlated with a reasoning task that is considered non-verbal (i.e. Matrix Reasoning). Thus, visuo-spatial working memory is not as closely tied to language outcomes as verbal working memory.
One major limitation to the current study was that the small sample size prohibited a comprehensive analysis of the unique contributions of verbal and visuo-spatial working memory to both verbal and nonverbal skills for children with CIs. Several studies have examined how various working memory tasks (verbal and visuo-spatial) and cognitive measures relate to spoken language skills, communication skills, and reading for children with CIs (Lyxell et al. 2008; Ibertsson et al. 2009; Lyxell et al. 2011; Pisoni et al. 2011; Edwards & Anderson 2014). One focus of future research may be to determine the unique contribution of verbal and visuo-spatial working memory/cognitive skills to spoken language outcomes in children with CIs. This may provide insight for targeting children with CIs who are at risk for deficits that extend beyond processing verbal information. Thus, children with CIs who fail to make significant progress in spoken language despite early intervention services and devices may have processing deficits that extend to the visuo-spatial domain.
In summary, the results from this study support that children with CIs have domain specific deficits related to storing and processing verbal information in working memory. These deficits extend to receptive vocabulary and perceptual reasoning. That is, children with CIs exhibited vocabulary and verbal working memory deficits compared to NH age mates as well as lower scores on a visual perceptual reasoning task when verbal encoding was required for optimal performance. In contrast, their ability to store and process visuo-spatial information in working memory seems to be intact and on par with their NH age mates. Additionally, no difference between CI and NH groups was observed on a reasoning task that required purely visuo-spatial skills.
The extent that memory delays in CI users are specific to the verbal domain or generalized to both verbal and visuo-spatial domains may have importance for determining appropriate habilitation strategies. To the degree that diminished sensory input results in a domain-specific verbal deficit, it may be habilitated with improved and earlier sensory (i.e., auditory) input provided by improved CI processing strategies that offer greater fidelity of the speech signal. Language intervention strategies that are designed to expand memory for auditory-verbal information may also be considered. Moreover, the contribution of maternal education to vocabulary outcomes highlights the importance of early linguistic input for language skills. This is particularly relevant for children with CIs who must also contend with early auditory deprivation as well as a spectrally impoverished signal from the CI. Early intervention strategies that target improving the quantity and quality of early linguistic input in the home environments may be valuable. Some children may also benefit from habilitation efforts focused on the use of top-down processing (Nittrouer et al. 2013) and effective communication strategies (Lyxell et al. 2008). Identifying the unique effects of both sensory and cognitive abilities on spoken language function in children with hearing loss will make it possible to identify future directions for habilitation and education.
ACKNOWLEDGMENTS
This research was supported by the McDonnell Neuroscience Foundation at Washington University School of Medicine in St. Louis, MO and NIDCD grant RO1 DC012778 . Appreciation is expressed to the 54 students and their parents who graciously gave their time and effort to participate in this study, and to Sarah Fessenden for conducting testing. This research was approved by the Human Studies Committee at Washington University School of Medicine (#201107375).
REFERENCES
- Adams AM, & Gathercole SE (2000). Limitations in working memory: Implications for language development. Int J Lang Commun Disord, 35(1), 95–116. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Kirkwood H, & Elliott J (2009). The Cognitive and Behavioral Characteristics of Children With Low Working Memory. Child Dev, 80(2), 606–621. doi: 10.1111/j.1467-8624.2009.01282.x [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, & Pickering SJ (2006). Verbal and visuo-spatial short-term and working memory in children: Are they separable? Child Development, 77(6), 1698–1716. doi: 10.1111/j.1467-8624.2006.00968.x [DOI] [PubMed] [Google Scholar]
- Archibald LM, & Gathercole SE (2006). Visuo-spatial immediate memory in specific language impairment. J Speech Lang Hear Res, 49(2), 265–277. doi: 10.1044/1092-4388(2006/022) [DOI] [PubMed] [Google Scholar]
- AuBuchon AM, Pisoni DB, & Kronenberger WG (2015). Short-Term and Working Memory Impairments in Early-Implanted, Long-Term Cochlear Implant Users Are Independent of Audibility and Speech Production. Ear and Hearing, 36(6), 733–737. doi: 10.1097/Aud.0000000000000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, & Logie LH (1999). Working memory: The multiple component model. In Shah AMP (Ed.), Models of working memory (pp. 28–61). New York: Cambridge University Press. [Google Scholar]
- Baddeley AD, & Hitch G (1974). Working Memory. In Gordon HB (Ed.), Psychology of Learning and Motivation (Vol. Volume 8, pp. 47–89): Academic Press. [Google Scholar]
- Bharadwaj SV, & Mehta JA (2016). An exploratory study of visual sequential processing in children with cochlear implants. International Journal of Pediatric Otorhinolaryngology, 85, 158–165. [DOI] [PubMed] [Google Scholar]
- Bingham K, Jenstad LM, & Shahnaz N (2009). Longitudinal Changes in Real-Ear to Coupler Difference Measurements in Infants. J Am Acad Audiol, 20(9), 11p. doi: 10.3766/jaaa.20.9.4 [DOI] [PubMed] [Google Scholar]
- Blamey PJ, Sarant JZ, Paatsch LE, Barry JG, Bow CP, Wales RJ, … Tooher R (2001). Relationships Among Speech Perception, Production, Language, Hearing Loss, and Age in Children With Impaired Hearing. Journal of Speech, Language, and Hearing Research, 44(2), 264–285. doi: 10.1044/1092-4388(2001/022) [DOI] [PubMed] [Google Scholar]
- Braden JP (1992). Intellectual assessment of deaf and hard-of-hearing people: A quantitative and qualitative research synthesis. School Psychology Review, 21(1), 82–94. [Google Scholar]
- Burkholder RA, & Pisoni DB (2003). Speech timing and working memory in profoundly deaf children after cochlear implantation. J Exp Child Psychol, 85(1), 63–88. doi: 10.1016/S0022-0965(03)00033-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney A & Moeller MP (1998). Treatment efficacy in Aural Rehabilitation. JSHR Supplement 41(1), S61–S84. [DOI] [PubMed] [Google Scholar]
- Castellanos I, Kronenberger WG, Beer J, Colson BG, Henning SC, Ditmars A, & Pisoni DB (2015). Concept Formation Skills in Long-Term Cochlear Implant Users. J Deaf Stud Deaf Educ, 20(1), 27–40. doi: 10.1093/deafed/enu039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB (1987). Intelligence: Its structure, growth and action (Vol. 35): Elsevier. [Google Scholar]
- Ching TYC, Dillon H, Leigh G, & Cupples L (2017). Learning from the Longitudinal Outcomes of Children with Hearing Impairment (LOCHI) study: Summary of 5-year findings and implications. International Journal of Audiology, DOI: 10.1080/14992027.2017.1385865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M, Pisoni DB, & Geers AE (2001). Some measures of verbal and spatial working memory in eight- and nine-year-old hearing-impaired children with cochlear implants. Ear Hear, 22(5), 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchini G, Logie RH, Della Sala S, MacPherson SE, & Baddeley AD (2002). Concurrent performance of two memory tasks: evidence for domain-specific working memory systems. Mem Cognit, 30(7), 1086–1095. [DOI] [PubMed] [Google Scholar]
- Conway CM, Karpicke J, Anaya EM, Henning SC, Kronenberger WG, & Pisoni DB (2011). Nonverbal cognition in deaf children following cochlear implantation: Motor sequencing disturbances mediate language delays. Dev Neuropsychol, 36(2), 237–254. doi: 10.1080/87565641.2010.549869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LS, Geers AE, & Brenner C (2010). Cochlear Implant Characteristics and Speech Perception Skills of Adolescents With Long-Term Device Use. Otol Neurotol. doi: 10.1097/MAO.0b013e3181eb320c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LS, Geers AE, & Nicholas JG (2014). The effects of audibility and novel word learning ability on vocabulary level in children with cochlear implants. Cochlear Implants Int, 15(4), 211–221. doi: 10.1179/1754762813Y.0000000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PW, Busby PA, McKay CM, & Clark GM (2002). Short-term auditory memory in children using cochlear implants and its relevance to receptive language. J Speech Lang Hear Res, 45(4), 789–801. [DOI] [PubMed] [Google Scholar]
- DesJardin JL, & Eisenberg LS (2007). Maternal Contributions: Supporting Language Development in Young Children with Cochlear Implants. Ear Hear, 28(4), 456–469. [DOI] [PubMed] [Google Scholar]
- Dollaghan CA, Campbell TF, Paradise JL, Feldman HM, Janosky JE, Pitcairn DN, & Kurs-Lasky M (1999). Maternal Education and Measures of Early Speech and Language. J Speech Lang Hear Res, 42(6), 1432–1443. doi: 10.1044/jslhr.4206.1432. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, & Allen SL (2003). Effects of presentation level on phoneme and sentence recognition in quiet by cochlear implant listeners. Ear Hear, 24(5), 392–405. doi: 10.1097/01.AUD.0000090340.09847.39 [DOI] [PubMed] [Google Scholar]
- Dunn LM, & Dunn LM (1997). PPVT-III: Peabody picture vocabulary test: American Guidance Service.
- Ead B, Hale S, DeAlwis D, & Lieu JEC (2013). Pilot study of cognition in children with unilateral hearing loss. Int J Ped Otorhinolaryngol, 77(11), 1856–1860. doi: 10.1016/j.ijporl.2013.08.028 [DOI] [PubMed] [Google Scholar]
- Edwards L, & Anderson S (2014). The Association Between Visual, Nonverbal Cognitive Abilities and Speech, Phonological Processing, Vocabulary and Reading Outcomes in Children With Cochlear Implants. Ear and Hearing, 35(3), 366–374. doi: 10.1097/Aud.0000000000000012 [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, & Conway ARA (1999). Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology-General, 128(3), 309–331. doi: 10.1037/0096-3445.128.3.309 [DOI] [PubMed] [Google Scholar]
- Fagan MK, Pisoni DB, Horn DL, & Dillon CM (2007). Neuropsychological Correlates of Vocabulary, Reading, and Working Memory in Deaf Children With Cochlear Implants. Journal of deaf studies and deaf education, 12(4), 461–471. doi: 10.1093/deafed/enm023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Hitch GJ, Service E, & Martin AJ (1997). Phonological short-term memory and new word learning in children. Dev Psychol, 33(6), 966–979. [DOI] [PubMed] [Google Scholar]
- Geers AE, Moog JS, Biedenstein J, Brenner C, & Hayes H (2009). Spoken language scores of children using cochlear implants compared to hearing age-mates at school entry. J Deaf Stud Deaf Educ, 14(3), 371–385. doi: 10.1093/deafed/enn046 [DOI] [PubMed] [Google Scholar]
- Geers AE, Nicholas J, Tobey E, & Davidson L (2016). Persistent Language Delay Versus Late Language Emergence in Children With Early Cochlear Implantation. Journal of Speech, Language, and Hearing Research, 59(1), 155–170. doi: 10.1044/2015_JSLHR-H-14-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale S, Rose NS, Myerson J, Strube MJ, Sommers M, Tye-Murray N, & Spehar B (2011). The Structure of Working Memory Abilities Across the Adult Life Span. Psychology and Aging, 26(1), 92–110. doi: 10.1037/a0021483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ML, Eigsti I-M, Bortfeld H, & Lillo-Martin D (2017). Auditory access, language access, and implicit sequence learning in deaf children. Dev Sci, e12575–n/a. doi: 10.1111/desc.12575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MS, Kronenberger WG, Gao S, Hoen HM, Miyamoto RT, & Pisoni DB (2013). Verbal short-term memory development and spoken language outcomes in deaf children with cochlear implants. Ear Hear, 34(2), 179–192. doi: 10.1097/AUD.0b013e318269ce50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins H (1949). A phonetically balanced test of speech discrimination for children. Unpublished Master’s Thesis. Northwestern University. Evanston, IL. [Google Scholar]
- Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, … Skinner MW (2013). Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear, 34(3), 342–360. doi: 10.1097/AUD.0b013e3182741aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DL, Davis RA, Pisoni DB, & Miyamoto RT (2005). Development of visual attention skills in prelingually deaf children who use cochlear implants. Ear Hear, 26(4), 389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibertsson T, Hansson K, Asker-Àrnason L, & Sahlén B (2009). Speech recognition, working memory and conversation in children with cochlear implants. Deafness & Education International, 11(3), 132–151. doi: 10.1002/dei.261 [DOI] [Google Scholar]
- Khan S, Edwards L, & Langdon D (2005). The Cognition and Behaviour of Children with Cochlear Implants, Children with Hearing Aids and Their Hearing Peers: A Comparison. Audiology and Neurotology, 10(2), 117–126. [DOI] [PubMed] [Google Scholar]
- Kirk KI, Pisoni DB, & Osberger MJ (1995). Lexical effects on spoken word recognition by pediatric cochlear implant users. Ear Hear, 16(5), 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivitski EC, McIntosh DE, Rothlisberg B, & Finch H (2004). Profile analysis of deaf children using the universal nonverbal intelligence test. Journal of Psychoeducational Assessment, 22(4), 338–350. [Google Scholar]
- Kronenberger WG, Pisoni DB, Harris MS, Hoen HM, Xu H, & Miyamoto RT (2013). Profiles of verbal working memory growth predict speech and language development in children with cochlear implants. J Speech Lang Hear Res, 56(3), 805–825. doi: 10.1044/1092-4388(2012/11-0356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman R, Lachman JL, & Butterfield E (1979). Cognitive psychology and information processing: An introduction. Hillsdale, N.J.: Lawrence Erlbaum Associates. [Google Scholar]
- Lévesque J, Théoret H, & Champoux F (2014). Reduced procedural motor learning in deaf individuals. Front Hum Neurosci, 8, 343. doi: 10.3389/fnhum.2014.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyxell B, Sahlen B, Wass M, Ibertsson T, Larsby B, Hallgren M, & Maki-Torkko E (2008). Cognitive development in children with cochlear implants: relations to reading and communication. Int J Audiol, 47 Suppl 2, S47–52. doi: 10.1080/14992020802307370 [DOI] [PubMed] [Google Scholar]
- Lyxell B, Wass M, Sahlén B, Uhlén I, Samuelsson C, Asker-Árnason L, … Hällgren M (2011). Development of cognitive and reading skills in deaf children with CIs. Cochlear Implants International, 12(sup1), S100–S198. doi: 10.1179/146701011X13001035752688 [DOI] [PubMed] [Google Scholar]
- Marschark M (2006). Intellectual functioning of deaf adults and children: Answers and questions. Eur J Cogn Psychol, 18(1), 70–89. doi: 10.1080/09541440500216028 [DOI] [Google Scholar]
- Marschark M, Spencer LJ, Durkin A, Borgna G, Convertino C, Machmer E, … Trani A (2015). Understanding Language, Hearing Status, and Visual-Spatial Skills. J Deaf Stud Deaf Educ, 20(4), 310–330. doi: 10.1093/deafed/env02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JW (1993). Haptic Recognition of Children With Specific Language Impairment: Effects of Response Modality. J Speech Lang Hear Res, 36(1), 98–104. doi: 10.1044/jshr.3601.98 [DOI] [PubMed] [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang N, Quittner AL, Fink NE, CDaCI Investigative Team FT. Spoken Language Development in Children Following Cochlear Implantation. JAMA. 2010;303(15):1498–1506. doi: 10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S, Caldwell-Tarr A, Low KE, & Lowenstein JH (2017). Verbal Working Memory in Children With Cochlear Implants. J Speech Lang Hear Res, 60(11), 3342–3364. doi: 10.1044/2017_JSLHR-H-16-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S, Caldwell-Tarr A, & Lowenstein JH (2013). Working memory in children with cochlear implants: Problems are in storage, not processing. Int J Pediatr Otorhinolaryngol, 77(11), 1886–1898. doi: 10.1016/j.ijporl.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osberger MJ (1986). Language and learning skills of hearing-impaired students. Introduction. ASHA Monogr(23), 3–5. [PubMed] [Google Scholar]
- Peterson GE, & Lehiste I (1962). Revised CNC Lists for Auditory Tests. Journal of Speech and Hearing Disorders, 27(1), 62–70. doi: 10.1044/jshd.2701.62 [DOI] [PubMed] [Google Scholar]
- Phillips J, Wiley S, Barnard H, & Meinzen-Derr J (2014). Comparison of two nonverbal intelligence tests among children who are deaf or hard-of-hearing. Res Dev Disabil, 35(2), 463–471. doi: 10.1016/j.ridd.2013.11.020 [DOI] [PubMed] [Google Scholar]
- Pisoni DB, & Cleary M (2003). Measures of working memory span and verbal rehearsal speed in deaf children after cochlear implantation. Ear Hear, 24(1 Suppl), 106S–120S. doi: 10.1097/01.AUD.0000051692.05140.8E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, & Geers AE (2000). Working memory in deaf children with cochlear implants: correlations between digit span and measures of spoken language processing. Ann Otol Rhinol Laryngol Suppl, 185, 92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Kronenberger WG, Chandramouli SH, & Conway CM (2016). Learning and Memory Processes Following Cochlear Implantation: The Missing Piece of the Puzzle. Front Psychol, 7, 1–19. doi: 10.3389/fpsyg.2016.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni DB, Kronenberger WG, Roman AS, & Geers AE (2011). Measures of digit span and verbal rehearsal speed in deaf children after more than 10 years of cochlear implantation. Ear Hear, 32(1 Suppl), 60s–74s. doi: 10.1097/AUD.0b013e3181ffd58e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppen R, Stark J, Eisenson J, Forrest T, & Wertheim G (1969). Visual sequencing performance of aphasic children. J Speech Hear Res, 12(2), 288–300. [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, & Neuburger H (2004). Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol, 9(4), 224–233. doi: 10.1159/000078392 [DOI] [PubMed] [Google Scholar]
- Torkildsen J. v. K., Arciuli J, Haukedal CL, & Wie OB (2018). Does a lack of auditory experience affect sequential learning? Cognition, 170, 123–129. doi: 10.1016/j.cognition.2017.09.017 [DOI] [PubMed] [Google Scholar]
- Ulanet PG, Carson CM, Mellon NK, Niparko JK, & Ouellette M (2014). Correlation of neurocognitive processing subtypes with language performance in young children with cochlear implants. Cochlear Implants Int, 15(4), 230–240. doi: 10.1179/1754762814Y.0000000077 [DOI] [PubMed] [Google Scholar]
- Van Boxtel MPJ, Van Beijsterveldt CEM, Houx PJ, Anteunis LJC, Metsemakers JFM, & Jolles J (2000). Mild hearing impairment can reduce verbal memory performance in a healthy adult population. Journal of Clinical and Experimental Neuropsychology, 22(1), 147–154. [DOI] [PubMed] [Google Scholar]
- Wass M, Ibertsson T, Lyxell B, Sahlen B, Hallgren M, Larsby B, & Maki-Torkko E (2008). Cognitive and linguistic skills in Swedish children with cochlear implants - measures of accuracy and latency as indicators of development. Scand J Psychol, 49(6), 559–576. doi: 10.1111/j.1467-9450.2008.00680.x [DOI] [PubMed] [Google Scholar]
- Watson DR, Titterington J, Henry A, & Toner JG (2007). Auditory sensory memory and working memory processes in children with normal hearing and cochlear implants. Audiol Neurootol, 12(2), 65–76. doi: 10.1159/000097793 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV). San Antonio, TX: Pearson. [Google Scholar]
- Wyke MA, & Asso D (1979). Perception and memory for spatial relations in children with developmental dysphasia. Neuropsychologia, 17(2), 231–239. [DOI] [PubMed] [Google Scholar]
- Zekveld AA, Deijen JB, Goverts ST, & Kramer SE (2007). The relationship between nonverbal cognitive functions and hearing loss. J Speech Lang Hear Res, 50(1), 74–82. doi: 10.1044/1092-4388(2007/006) [DOI] [PubMed] [Google Scholar]


