Abstract
Automated insulin delivery (AID) systems have proven safe and effective in improving glycemic outcomes in individuals with type 1 diabetes (T1D). Clinical evaluation of this technology has progressed to large randomized, controlled outpatient studies and recent commercial approval of AID systems for children and adults. However, several challenges remain in improving these systems for different subpopulations (e.g., young children, athletes, pregnant women, seniors and those with hypoglycemia unawareness). In this review, we highlight the requirements and challenges in AID design for selected subpopulations, and discuss current advances from recent clinical studies.
Keywords: Type 1 Diabetes, Automated Insulin Delivery, Subpopulations, Clinical Investigations
1. Introduction
Individuals with type 1 diabetes (T1D) require life-long replacement of insulin for maintaining glucose levels in a safe euglycemic range. Automated insulin delivery (AID) systems close the loop between a glucose sensing device and an insulin delivery device to compute and deliver insulin (typically every five minutes) to achieve a desired glucose level while reducing the risk of extreme glucose variations below (hypoglycemia) or above desired range (hyperglycemia) in individuals with T1D. There is increasing evidence that AID systems, even with limitations, such as requiring user-initiated meal and correction insulin boluses, improve outcomes over conventional open-loop therapy for adults and children [1, 2].
Clinical investigations of AID systems have primarily focused on adults and children ≥ 6 years of age, in low-risk groups using well-defined clinical ranges, such as those below a maximum HbA1c threshold (a measure of average glucose) and minimum total daily insulin (TDI). These exclusion criteria are used to limit or reduce the risk of extreme glucose variations. However, other challenges such as the difficulty tailoring and prioritizing features of AID systems for precise needs of different subpopulations still limit the reach of AID. As the technology matures, there is an increasing need to extend the reach of AID systems to broader criteria. In this review, we discuss recent progress over the last five years, challenges, and opportunities in AID systems with a focus on select subpopulations based on age and those who require systems tailored to a specific metabolic condition.
2. AID Technology: Devices, Algorithms, and their Taxonomy
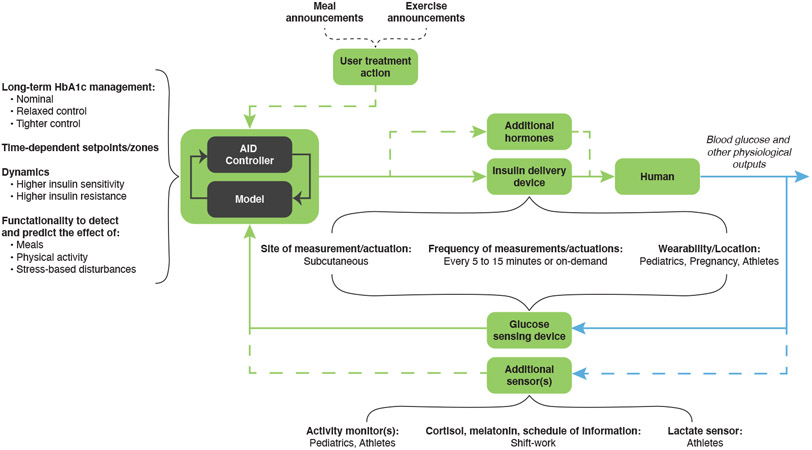
AID systems are feedback loops comprised of three primary components: the controller, the insulin delivery device, and the glucose sensing device, as illustrated in Figure 1. The AID systems have evolved from low glucose suspend (LGS) systems, which only suspend insulin delivery to prevent low glucose, to closed-loop systems where insulin can be both decreased and increased with hybrid features where users may also provide information such as meal and exercise announcements, to recent commercial approval of AID systems for children and adults [3]. The controller, implemented using algorithms such as model predictive control, fuzzy logic, optimal control, and proportional integral-derivative control [4] running on a dedicated device or a smartphone [5], incorporates design parameters to adjust how the controller will react to deviations in glucose from a desired concentration such as user-specific dynamic physiological information, such as insulin sensitivity, time-varying parameters, and functionalities to detect and predict the effect of meals, physical activity, stress, and other disturbances.
Figure 1:
Taxonomy of the automated insulin delivery (AID) system. The model-based AID controller, supplemented with user treatment action, regulates blood glucose through the primary feedback loop (solid lines) composed of insulin-glucose components, with optional feedback loops (dashed lines) composed of additional hormones (e.g., glucagon, for which a second pump would be required) and sensors (e.g., activity monitors). Components that pertain to specific subpopulations are emphasized. For long-term HbA1c management, nominal (i.e., HbA1c less than 7%) pertains largely to adults, pediatrics, shift workers, and athletes; relaxed control (i.e., HbA1c greater than 7%) pertains to pediatrics and seniors at hypoglycemia risk; tighter control (i.e.,HbA1c much less than 7%) pertains to pregnancy subpopulations. Time-dependent set-points and/or zones pertain primarily to shift workers. Higher insulin sensitivity pertains to young children, athletes, and early pregnancy, while higher insulin resistance pertains to adolescents, the second half of pregnancy, and shift workers. The green lines indicate signals or actions conducted during closed-loop operation, while blue lines distinguish physiological states or properties from measured or digital signals.
2.1. Subpopulation Characterization
We define a subpopulation as set of individuals who have distinct diabetes care requirements including glycemic disturbances and targets and who would benefit from tailoring of the AID design, including the pipeline from simulation tools to the regulatory approval. The differences due to subpopulation classification is separate from differences due to individual factors. Several subpopulations based on age (pediatrics, young adults and seniors) and specific metabolic conditions (pregnant women, shift-workers, and athletes) were selected to highlight advances and challenges in design and clinical evaluation of AID systems.
2.1.1. Aged-based Subpopulations: Pediatrics, Young Adults, and Seniors
For this review, we broadly group the pediatric subpopulation as young children, typically aged 2 – 6 years, school-aged children, typically aged 6 – 12 years (before the onset of puberty), and adolescents, aged 12 – 18 years (undergoing puberty), or as developmentally appropriate [6]. In the pediatric subpopulation, insulin dosages have to be continuously adjusted with age and pubertal stage to balance short term risks, such as overnight hypoglycemia, as well as long-term risks of glycemic variability [7]. The young adults subpopulation consists of adults transitioning to full adulthood, typically aged 18 – 25 years.
The senior subpopulation consists of individuals, typically older than 65 years, who live with T1D. Aging and long-term duration of diabetes result in impaired counter-regulatory responses, leading to a higher risk of hypoglycemia [8]. Seniors have often been excluded from clinical studies due to higher risks such as hypoglycemia unawareness resulting in them not being aware of symptoms, and thus may not be intervening as others would with carbohydrate intake, as well as other cognitive and metabolic challenges [8].
2.1.2. Subpopulations with Specific Metabolic Conditions: Pregnant Women, Shift-workers, and Athletes
Pregnancy in T1D has unique dietary, insulin use, and target glycemic control requirements [9]. Due to the changes in insulin requirements throughout gestation, pregnant women with T1D are at a higher risk of hypoglycemia in early pregnancy and a higher risk of hyperglycemia for the remainder of their pregnancy [10]. The challenges of conducting clinical trials for AID systems in this subpopulation include lack of data on safety and effectiveness of CGM use during pregnancy, and the risks involving both mother and fetus.
Shift workers engage in work outside of the usual daytime hours of 6 am to 6 pm. Common shift-work schedules include evening, night, morning, rotating, and irregular shifts. Circadian misalignment causes a disruption of the glucose-insulin regulation system resulting in impaired glucose tolerance and reduced insulin sensitivity [11, 12]. Finally, we consider competitive athletes with T1D who train and professionally compete in sports [13]. Since athletes exercise and compete regularly, effective glucose management surrounding physical activity becomes essential for overall glucose control, as well as for harnessing enhanced athletic performance [14].
3. Differing Goals and Challenges for Selected Subpopulations
Table 1 provides a comparison of three main disturbances that affect glucose regulation:
Table 1:
Comparison of meals, physical activities, and stress disturbances on glucose, as well as design of AID system using glucose targets, behavioral considerations, and AID controller features for subpopulations based on age and metabolic conditions. The subpopulations are ordered by the quality of clinical validation starting with the adolescents and young adult subpopulation. Studies involving children, pregnant women and seniors subpopulations have only recently been initiated. In particular, AID systems for shift-workers and athletes subpopulations require clinical validation.
| Sub- population |
Disturbances | Design | ||||
|---|---|---|---|---|---|---|
| Meals | Physical activities |
Stress | Glucose targets | Behavioral Considerations | Controller Features | |
| Adolescents (pubertal, age 12 – 18); Young Adults (age 18 – 25) | Typically large meals | Activities with peers, moderate to vigorous intensity | Peer pressure, school performance, changes in lifestyle | HbA1c < 7% | Less diligence in diabetes care, sedentary lifestyle | Prioritize extended hyperglycemia prevention; high absolute basal, insulin resistance and large TDI |
| School-Age Children (pre-pubertal, age 6 – 12) | Small and frequent meals | School activities, moderate to vigorous intensity | Related to friends/peers and siblings | Limited autonomy in diabetes care | Prioritize hypoglycemia and hyperglycemia prevention; low absolute basal, high insulin sensitivity and small TDI, activity detection and announcement | |
| Young Children (age 2 – 6) | Irregular meals | Active in short bursts | Related to friends/peers and siblings | Completely dependent on others for diabetes care, challenges in communicating hypoglycemia symptoms | Prioritize glycemic variability and hypoglycemia prevention; very low absolute basal, larger portion of TDI for bolus, high insulin sensitivity with small TDI, activity detection and announcement | |
| Pregnant women (Pregnancy with pre-existing T1D) | Moderately low carbohydrate intake | Moderate intensity | Pregnancy-specific stressors | HbA1c < 6% fasting: 95 mg/dL, 1-h postprandial: < 140 mg/dL, 2-h postprandial: < 120 mg/dL | Early pregnancy: decreased food intake; Mid- to late pregnancy: increased food intake. Declined physical activity due to maternal fatigue and other discomfort | Adaptive to changing insulin requirements through pregnancy. Assertive postprandial control |
| Seniors (age ≥ 65 years) | Gradual decrease in appetite | Decline in muscle mass and strength | Depression due to grief, loneliness, failing health, lack of mobility | HbA1c < 7 – 7.5%; Healthy target: 90 – 150 mg/dL; Severe chronic illness target: 100 – 180 mg/dL | Unidentified cognitive impairment and dementia leading to difficulties in self-monitoring and use of diabetes technology | Prioritize minimization of hypoglycemia and severe hyperglycemia; reduction of overall risk from hypoglycemia unawareness |
| Shift-workers (People working outside typical 6 am to 6 pm schedule) | Change in timing and frequency, increased consumption of snacks | Decreased opportunities for physical activity, altered responses to exercise | Altered social life resulting in psychological stress and psychosomatic disorders | HbA1c < 7% | Disruption of circadian rhythms and sleep deficits | Adapt timing and dosing to frequent changes in routine, incorporate circadian and impaired glucose dynamics |
| Athletes (People who train and compete in sports) | Training- and competition-specific dietary requirements | Highly active, schedules depend on training and competition goals | Increased risk of stress-induced hyperglycemia around competitions | HbA1c < 7%; Training: TIR > 70%; Competition: TIR > 75% | Location and type of the wearable device preference based on sport type and convenience | Exercise-informed control with announcement or detection of exercise. Multi-hormone systems for better prevention against exercise induced glycemic variations. |
Meal size and the macronutrient composition (e.g., carbohydrate, fat, and protein) causes an increase in glucose over the span of four to six hours, which is primarily rejected through insulin-dependent glucose uptake [15].
Physical activity increases insulin sensitivity over the following hours, but the immediate glycemic impact depends on the modality of activity (e.g., aerobic, anaerobic, or mixed) [16].
Psychological and physiological stress could lead to changes in stress hormones and insulin action [17].
3.1. Meals
Meal requirements, based on a balanced diet, can significantly vary based on age, pubertal stage, and activity levels [6, 18]. The meal size and frequency can vary more in younger children, while meal sizes are relatively larger in adolescence. In seniors, a decline in food intake and loss of motivation to eat are common. Disease-related inflammation, illnesses, medication, impaired abilities, as well as dietary restrictions can contribute to loss of appetite [8].
For pregnant women with T1D, a moderately low carbohydrate diet with protein and fiber consumption is recommended [9]. Shift workers typically do not significantly modify their total energy intake; they change the timing and frequency of eating, the content of meals, and a greater proportion of snacks are consumed [19, 20]. For athletes, nutrition is a crucial pillar of athletic performance and effective nutrition strategies differ by exercise modality [21]. As a result, athletes have individualized training- and competition-specific dietary requirements.
3.2. Physical Activity
Compared to adults, children and adolescents require greater regimentation in terms of exercise and daily scheduled activities, including moderate to vigorous intensity aerobic physical activity [22]. Additionally, young children may be more active than adolescents. In seniors, muscle mass and strength decline with age. These decrements may be exacerbated by comorbidities and periods of hospitalization leading to a degradation in glucose regulation.
For pregnant women, moderate intensity exercise is recommended, although there is a shortage of research on the effects of exercise during pregnancy with T1D [23]. The already reduced glucose target range may increase the risk of exercise-related hypoglycemia in this subpopulation. Shift work generally decreases opportunities for physical activity, but subjective and physiological responses can be altered; for example, if exercise occurs at unusual times of day or if the shift worker is sleep-deprived [24]. In athletes, general guidelines about exercise-related glucose management need to be individually customized to avoid hypoglycemia. Glycemic response to exercise is affected by the timing, intensity, and duration of exercise, as well as the starting levels of glucose, ingested meals, and insulin [25].
3.3. Stress
The primary sources of stress in pediatrics arise from siblings and peer pressure, as well as from school performance [6, 26, 27]. In seniors, stresses come from ageing, loneliness, failing health, and lack of mobility.
Pregnant women with T1D exhibit higher stress compared to pregnant women without diabetes [28]. Some of the pregnancy-specific stressors are concerns related to the baby’s health, physical discomforts due to pregnancy, and childbirth [29]. Shift-workers experience stress from sleep debt and reduced participation in regular social life, resulting in psychological stress and psychosomatic disorders [19]. For athletes, stress from competition may lead to an increased risk of hyperglycemia [30].
3.4. Glucose Targets
The primary goal of AID systems is to regulate glucose by rejecting previously noted disturbances on glucose to maintain an average glucose approximated by an HbA1c < 7% for adults and pediatrics [18], with a percent time in range (TIR) of 70 – 180 mg/dL > 70%, percent time below (TB) 70 mg/dL < 4%, and percent time above (TA) 180 mg/dL < 25% [31]. In pediatrics, a more relaxed HbA1c < 7.5% is recommended for those with hypoglycemia unawareness and other special conditions.
For seniors, depending on coexisting chronic illnesses and cognitive function, HbA1c < 8% and TIR > 50% is recommended. Further, the recommended fasting glucose range for seniors is 90 – 150 mg/dL for those with few complications, or 100 – 180 mg/dL for those with poor health [8].
Glucose targets for pregnancy are tighter at HbA1c < 6% with TIR using 63 – 140 mg/dL, TB using 63 mg/dL, and TA using 140 mg/dL [31]. Additionally, recommended glycemic targets for pregnancy are fasting plasma glucose levels below 95 mg/dL and either one-hour postprandial glucose below 140 mg/dL or two-hour postprandial glucose below 120 mg/dL [9].
For shift-workers, the recommendations are generally similar to adults. For athletes, the recommendation is to have TIR > 70% during training and TIR > 75% during competitions [30].
3.5. Behavioral Considerations and Controller Features
While all subpopulations share the larger goal of safe and tight glucose regulation, differences in nominal magnitude and frequency of disturbances, as well as behavioral considerations, motivates the design of tailored AID systems. These systems must prioritize and find a balance between several controller features and posed glycemic risk for each subpopulation, as discussed in this section and in Table 1.
Children have limited ability to communicate hypoglycemia symptoms, count carbohydrates, and manage diabetes care independently. Challenges with adherence to diabetes care practices are seen in adolescents and young adults [32]. Additionally, in young children, user-announced boluses may increase the risk of hypoglycemia if the meal is not completed or if emesis occurs. Compared to the adult population, insulin sensitivities are higher for children requiring smaller TDI, and lower (i.e., increased insulin resistance) for adolescents and young adults requiring large TDI. Varying stages of puberty and the confounding effects of other hormones also factor into the changing insulin requirements. Children have relatively low absolute basal rate, whereas these are higher for adolescents and young adults. In children, the bolus insulin forms a larger portion of TDI [33].
Consequently, the controller has to cover a wide range of insulin requirements while having limited margin of error, especially in young children. For children, the controller should prioritize glycemic variability, hypoglycemia and hyperglycemia prevention; for adolescents, the controller should prioritize extended hyperglycemia prevention. Additionally, the controller may require exercise announcements and detection with predictive modulation of insulin delivery to prevent hypoglycemia.
For pregnancy, controllers need to adapt to the changes in insulin-glucose metabolism during gestation, increased risk of postprandial hyperglycemia during late gestation, and changing TDI requirements throughout pregnancy [34]. Design considerations for wearable glucose sensors and insulin injection devices must include pregnancy-related anatomic and physiologic changes (e.g., potential discomfort with these devices around the abdomen). Meal habits are likely affected by food cravings and aversion during pregnancy [35]. Similar to young children, pregnant women are at an additional risk of meal bolus-induced hypoglycemia as vomiting is a symptom during early pregnancy [36].
For seniors, higher rates of unidentified cognitive impairment and dementia lead to difficulties in adhering to complex diabetes self-care activities. Thus, the treatment regimens must focus on minimizing hypoglycemia and severe hyperglycemia, as well as reducing the overall risk of hypoglycemia unawareness by using a higher threshold for attenuation of insulin.
Current clinical studies are investigating use of AID systems in individuals prone to hypoglycemia NCT04266379, because they are not aware of symptoms and may not be intervening as others would with carbohydrate intake.
For shift-workers, glycemic control with rotating shift patterns, varied eating habits and times, alterations in physical activity, as well as fluctuations in hormone levels present challenges to optimal insulin dosing. The controller design should incorporate circadian dynamics and changes in routine to determine insulin requirements.
For athletes, the need for individualized and adaptive AID systems stems from training related changes in energy metabolism, frequent physical activity engagement, increased risk for developing hypoglycemia unawareness, and compromised counter-regulation due to prolonged exercise [37]. Exercise informed glucose control systems that can integrate the time, duration, and modality of exercise as well as training and competition schedules would improve glucose control in this population. Multi-hormone systems can also be used for improved hypoglycemia protection [38]. Design considerations must include convenience and degree of user interaction during active competition [39].
4. Recent Advances in Clinical Trials
We analyzed original investigations of AID systems for T1D published in journals over the 5 years between 2017 and 2021. Studies reporting separate results for a mixed population or different AID configurations were included separately. Publications were excluded if the duration of investigation was less than one day, if they reported retrospective analysis, if they used multi-hormonal or LGS systems, or if they did not report all clinical outcomes described below. Data from the conventional therapy or control arm of the studies are not included.
4.1. AID Investigations in 2017-2021
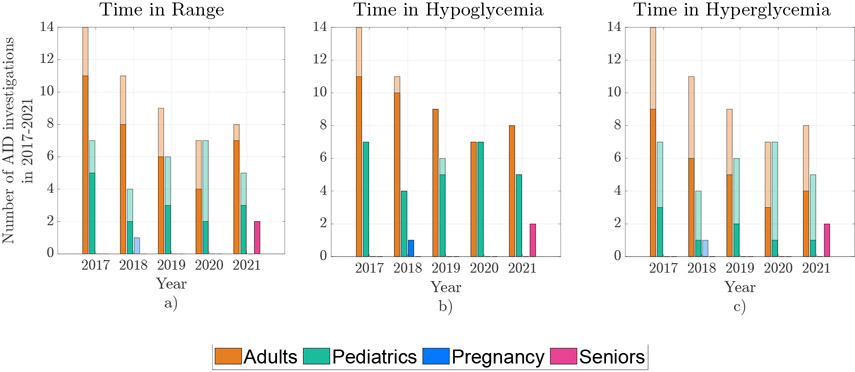
Figure 2 compares the TIR, TB, and TA from eligible studies and Figure 3 compares the number of studies over each of the five years. The eligible investigations (78) included adults (49), pediatrics (29), pregnant women (1) and seniors (2) subpopulations in various settings. Studies ranged from single-arm early feasibility studies [5] to large, randomized-crossover, multicenter, outpatient studies in adults, adolescents and children [40, 41, 42, 43], pregnant women [44] and seniors [45, 46], and to studies on subjects with hypoglycemia unawareness [47]. The number of participants in the AID arm ranged from 4 to 882 and duration ranged from 1 to 182 days, the combination of the number of participants and the duration ranged from 7.5 to 56, 448 subject-days. Complete list of included studies is provided in Supplementary Table 1 2.
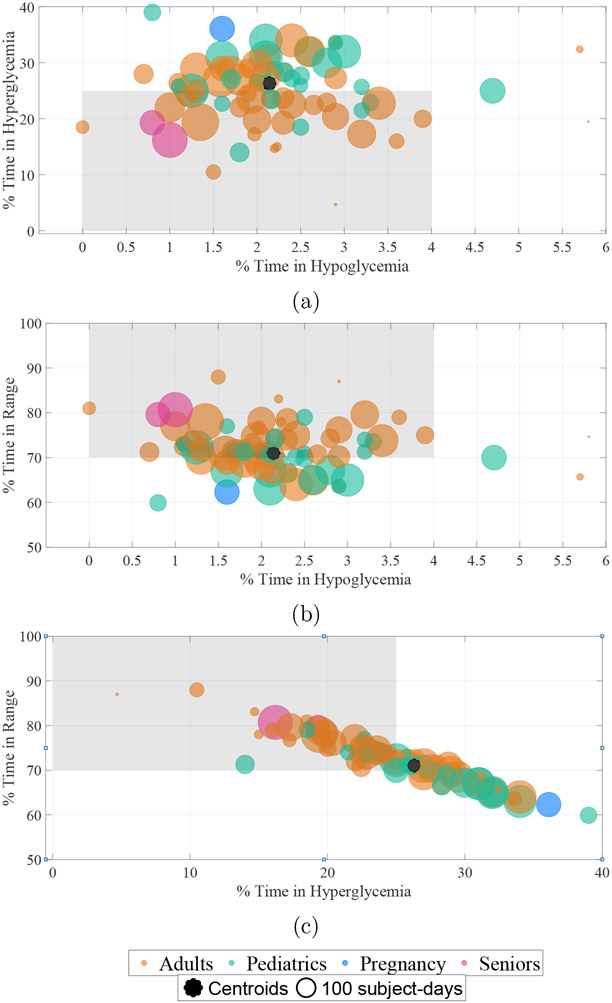
Figure 2:
Comparison of percent time spent in range (TIR), percent time below (TB), and percent time above (TA) from investigations conducted in 2017-2021. Comparison of TA and TB is shown in (a), TIR and TB in (b), and TIR and TA in (c). Each data point represents a measure of central tendency reported in the study; the size of the bubble is proportional to the number of participants in the AID arm times the length of the study. The centroid or the weighted average of percent time metrics is shown by an asterisk. The clinical targets of TIR > 70%, TB < 4%, and TA < 25% for assessment of glucose control are shown by the shaded region, where TIR, TB and TA are calculated using subpopulation specific ranges and thresholds described in Section 3.4.
Figure 3:
Year-by-year comparison of the number of AID investigations grouped by four subpopulations, with overlapping filled bars representing the number of investigations whose outcomes satisfied clinical targets of: (a) TIR > 70%, (b) TB < 4%, and (c) TA < 25%.
4.2. Time in Range
For all subjects, the TIR centroid was 71%. The TIR centroid in adults was 72.5%, in pediatrics was 67.5%, in pregnancy was 62.3% and in seniors was 80.3%. A larger proportion of adult studies (36/49, or 73.5%) achieved the TIR target compared to that of pediatric studies (15/29, or 51.7%), highlighting the challenge of glucose control in children and adolescents.
4.3. Time in Hypoglycemia
For all subjects, the TB centroid was 2.1%. The TB centroid in adults was 2.1%, in pediatrics was 2.3%, in pregnancy was 1.6%, and in seniors was 0.9%. The plots reveal that AID systems reached the clinical target of TB consistently over the years and across the four subpopulations in 73/78, or 93.5% of study data points conducted in different settings. This result underscores the effectiveness of AID systems in reducing the primary risk associated with intensive insulin therapy [48].
4.4. Time in Hyperglycemia
For all subjects, the TA centroid was 26.3%. The TA centroid in adults was 24.9%, in pediatrics was 29.6%, in pregnancy was 36.1%, and in seniors was 17.2%. As with TIR, a larger proportion of adult studies (27/49, or 55.1%) achieved the TA target compared to pediatric studies (8/29, or 27.5%). The proportion of studies satisfying the TA target was lower than the TIR and TB targets, thus highlighting the challenges in postprandial glucose management even with meal announcements [49]. As shown in Figure 2, TIR and TA were negatively correlated (ρ = −0.96, p < 0.001), suggesting that reduction in TA could lead to direct improvements in TIR, whereas no correlations were observed between TIR and TB (ρ = −0.1, p = 0.37), nor between TA and TB (ρ = −0.01, p = 0.90).
5. Conclusions and Future Work
Recent advances in AID system development have led to improved clinical outcomes, including increased time in range and significant reduction in time in hypoglycemia. However, large barriers remain for reducing time in hyperglycemia. In this review, we emphasized age- and specific metabolic condition-based subpopulations to discuss tailoring and prioritizing controller design to reject disturbances and achieve glucose regulation, and highlighted areas for further research. Additional subpopulations and conditions not discussed in this review include individuals who are hospitalized, those with poor glycemic control, and during menstrual cycles, as well as aspects on use, access, and cost of AID systems. Future goals for AID algorithms, simulation tools, and device design, as well as its clinical validation, include the consideration of different requirements and use cases to bridge the care gap for all subpopulations with T1D.
Supplementary Material
Highlights.
People with Type 1 Diabetes need insulin injections to keep glycemia in a safe range.
Automated insulin delivery (AID) improves outcomes over conventional therapy.
Subpopulations require tailoring systems although excluded from studies due to risks.
Advances and challenges in AID systems are highlighted for selected subpopulations.
Original investigations of AID systems published over the last 5 years are analyzed.
Acknowledgments
The authors acknowledge funding from the National Institutes of Health (R01DK120358, DP3DK104057, DP3DK113511 and UC4DK108483), from the William K. Bowes Jr. Foundation (WKB-2020-38415), from The Leona M. and Harry B. Helmsley Charitable Trust (T1-DEXI).
E.D. reports receiving grants from JDRF, NIH, and Helmsley Charitable Trust, personal fees from Roche and Eli Lilly, patents on artificial pancreas technology, and product support from Dexcom, Insulet, Tandem, and Roche. E.D. is currently an employee and shareholder of Eli Lilly and Company. The work presented in this manuscript was performed as part of his academic appointment and is independent of his employment with Eli Lilly and Company. J.E.P reports receiving grant support, provided to his institution, and consulting fees and speaker fees from Tandem Diabetes Care; grant support, provided to his institution, and advisory board fees from Medtronic; grant support, provided to his institution, and consulting fees from Eli Lilly; grant support and supplies, provided to his institution, from Insulet; and supplies, provided to his institution, from Dexcom. F.J.D. reports equity, licensed IP and is a member of the Scientific Advisory Board of Mode AGC. All other authors report no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Readers can retrieve all published clinical studies of the AID systems by accessing the Artificial Pancreas Clinical Trial Database at https://www.thedoylegroup.org/apdatabase. This tool allows researchers to perform queries and comparisons of clinical trial details.
References
- [1].Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, Haidich A-B, Hovorka R, Tsapas A, Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis, BMJ 361 (2018) k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karageorgiou V, Papaioannou TG, Bellos I, Alexandraki K, Tentolouris N, Stefanadis C, Chrousos GP, Tousoulis D, Effectiveness of artificial pancreas in the non-adult population: A systematic review and network meta-analysis, Metabolism 90 (2019) 20–30. [DOI] [PubMed] [Google Scholar]
- [3].Boughton CK, Hovorka R, New closed-loop insulin systems, Diabetologia 64 (2021) 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shi D, Deshpande S, Dassau E, Doyle FJ III, Feedback control algorithms for automated glucose management in T1DM: The state of the art, in: Peña RSS, Cherñavvsky DR (Eds.), The Artificial Pancreas, Elsevier, 2019, pp. 1–27. [Google Scholar]
- [5].Deshpande S, Pinsker JE, Zavitsanou S, Shi D, Tompot R, Church MM, Andre C, Doyle FJ III, Dassau E, Design and clinical evaluation of the interoperable artificial pancreas system (iAPS) smartphone app: Interoperable components with modular design for progressive artificial pancreas research and development, Diabetes Technol Ther 21 (2019) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chiang JL, Maahs DM, Garvey KC, Hood KK, Laffel LM, Weinzimer SA, Wolfsdorf JI, Schatz D, Type 1 diabetes in children and adolescents: A position statement by the American Diabetes Association, Diabetes Care 41 (2018) 2026–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cameron FJ, Northam EA, Ryan CM, The effect of type 1 diabetes on the developing brain, Lancet Child Adolesc Health 3 (2019) 427–436. [DOI] [PubMed] [Google Scholar]
- [8].12. older adults: Standards of medical care in diabetes2021, Diabetes Care 44 (2021) S168–S179. [DOI] [PubMed] [Google Scholar]
- [9].14. management of diabetes in pregnancy: Standards of medical care in diabetes2021, Diabetes Care 44 (2021) S200–S210. [DOI] [PubMed] [Google Scholar]
- [10].García-Patterson A, Gich I, Amini S, Catalano PM, De Leiva A, Corcoy R, Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction, Diabetologia 53 (2010) 446–451. [DOI] [PubMed] [Google Scholar]
- [11].Leong I, Shift work causes insulin resistance, Nat Rev Endocrinol 14 (2018) 503–503. [DOI] [PubMed] [Google Scholar]
- [12].Sharma A, Laurenti MC, Dalla Man C, Varghese RT, Cobelli C, Rizza RA, Matveyenko A, Vella A, Glucose metabolism during rotational shift-work in healthcare workers, Diabetologia 60 (2017) 1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, Dimeff R, Douglas PS, Glover DW, Hutter AM, Krauss MD, Maron MS, Mitten MJ, Roberts WO, Puffer JC, Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update, Circulation 115 (2007) 1643–1655. [DOI] [PubMed] [Google Scholar]
- [14].Baldi JC, Cassuto NA, Foxx-Lupo WT, Wheatley CM, Snyder EM, Glycemic status affects cardiopulmonary exercise response in athletes with type 1 diabetes., Med Sci Sports Exerc 42 (2010) 1454–1459. [DOI] [PubMed] [Google Scholar]
- [15].Bell KJ, Smart CE, Steil GM, Brand-Miller JC, King B, Wolpert HA, Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era, Diabetes care 38 (2015) 1008–1015. [DOI] [PubMed] [Google Scholar]
- [16].Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, Kowalski A, Rabasa-Lhoret R, McCrimmon RJ, Hume C, Annan F, Fournier PA, Graham C, Bode B, Galassetti P, Jones TW, Milln IS, Heise T, Peters AL, Petz A, Laffel LM, Exercise management in type 1 diabetes: a consensus statement, Lancet Diabetes Endocrinol 5 (2017) 377–390. [DOI] [PubMed] [Google Scholar]
- [17].Wiesli P, Schmid C, Kerwer O, Nigg-Koch C, Klaghofer R, Seifert B, Spinas GA, Schwegler K, Acute psychological stress affects glucose concentrations in patients with type 1 diabetes following food intake but not in the fasting state, Diabetes Care 28 (2005) 1910–1915. [DOI] [PubMed] [Google Scholar]
- [18].13. children and adolescents: Standards of medical care in diabetes—2021, Diabetes Care 44 (2021) S180–S199. [DOI] [PubMed] [Google Scholar]
- [19].Gupta CC, Coates AM, Dorrian J, Banks S, The factors influencing the eating behaviour of shiftworkers: What, when, where and why, Ind Health 57 (2019) 419–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qian J, Dalla Man C, Morris CJ, Cobelli C, Scheer FA, Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans, Diabetes Obes Metab 20 (2018) 2481–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burke LM, Castell LM, Casa DJ, Close GL, Costa RJS, Desbrow B, Halson SL, Lis DM, Melin AK, Peeling P, Saunders PU, Slater GJ, Sygo J, Witard OC, Bermon S, Stellingwerff T, International Association of Athletics Federations consensus statement 2019: nutrition for athletics, Int J Sport Nutr Exerc Metab 29 (2019) 73–84. [DOI] [PubMed] [Google Scholar]
- [22].Adolfsson P, Riddell MC, Taplin CE, Davis EA, Fournier PA, Annan F, Scaramuzza AE, Hasnani D, Hofer SE, ISPAD clinical practice consensus guidelines 2018: Exercise in children and adolescents with diabetes, Pediatr Diabetes 19 (2018) 205–226. [DOI] [PubMed] [Google Scholar]
- [23].Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF, Physical activity/exercise and diabetes: a position statement of the American Diabetes Association, Diabetes care 39 (2016) 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Atkinson G, Fullick S, Grindey C, Maclaren D, Exercise, energy balance and the shift worker, Sports Med 38 (2008) 671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McGaugh SM, Zaharieva DP, Pooni R, DSouza NC, Vienneau T, Ly TT, Riddell MC, Carbohydrate requirements for prolonged, fasted exercise with and without basal rate reductions in adults with type 1 diabetes on continuous subcutaneous insulin infusion, Diabetes care 44 (2021) 610–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Delamater AM, de Wit M, McDarby V, Malik JA, Hilliard ME, Northam E, Acerini CL, ISPAD clinical practice consensus guidelines 2018: Psychological care of children and adolescents with type 1 diabetes, Pediatr Diabetes 19 (2018) 237–249. [DOI] [PubMed] [Google Scholar]
- [27].Cameron FJ, Garvey K, Hood KK, Acerini CL, Codner E, ISPAD clinical practice consensus guidelines 2018: Diabetes in adolescence, Pediatr Diabetes 19 (2018) 250–261. [DOI] [PubMed] [Google Scholar]
- [28].Egan AM, Dunne FP, Lydon K, Conneely S, Sarma K, McGuire BE, Diabetes in pregnancy: worse medical outcomes in type 1 diabetes but worse psychological outcomes in gestational diabetes, QJM 110 (2017) 721–727. [DOI] [PubMed] [Google Scholar]
- [29].DiPietro JA, Ghera MM, Costigan K, Hawkins M, Measuring the ups and downs of pregnancy stress, J Psychosom Obstet Gynecol 25 (2004) 189–201. [DOI] [PubMed] [Google Scholar]
- [30].Riddell MC, Scott SN, Fournier PA, Colberg SR, Gallen IW, Moser O, Stettler C, Yardley JE, Zaharieva DP, Adolfsson P, Bracken RM, The competitive athlete with type 1 diabetes, Diabetologia 63 (2020) 1475–1490.∗ This article provides a perspective on challenges and therapeutic approaches for competitive athletes with T1D.
- [31].Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C, Dassau E, DeVries JH, Donaghue KC, Dovc K, Doyle FJ, Garg S, Grunberger G, Heller S, Heinemann L, Hirsch IB, Hovorka R, Jia W, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Levine B, Mayorov A, Mathieu C, Murphy HR, Nimri R, Nørgaard K, Parkin CG, Renard E, Rodbard D, Saboo B, Schatz B, Stoner K, Urakami T, Weinzimer SA, Phillip M, Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range, Diabetes care 42 (2019) 1593–1603.∗ This article provides an international consensus on time in range glycemic targets using continuous glucose monitoring for various subpopulations with T1D.
- [32].Berget C, Messer LH, Vigers T, Frohnert BI, Pyle L, Wadwa RP, Driscoll KA, Forlenza GP, Six months of hybrid closed loop in the real-world: An evaluation of children and young adults using the 670G system, Pediatr Diabetes 21 (2020) 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cemeroglu AP, Thomas JP, Zande LTV, Nguyen NT, Wood MA, Kleis L, Davis AT, Basal and bolus insulin requirements in children, adolescents, and young adults with type 1 diabetes mellitus on continuous subcutaneous insulin infusion (CSII): effects of age and puberty, Endocr Pract 19 (2013) 805–811. [DOI] [PubMed] [Google Scholar]
- [34].Murphy HR, Elleri D, Allen JM, Harris J, Simmons D, Rayman G, Temple RC, Umpleby AM, Dunger DB, Haidar A, Nodale M, Wilinska ME, Hovorka R, Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy, Diabetologia 55 (2012) 282–293. [DOI] [PubMed] [Google Scholar]
- [35].Bayley TM, Dye L, Jones S, DeBono M, Hill AJ, Food cravings and aversions during pregnancy: relationships with nausea and vomiting, Appetite 38 (2002) 45–51. [DOI] [PubMed] [Google Scholar]
- [36].Lacroix R, Eason E, Melzack R, Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change, Am J Obstet Gynecol 182 (2000) 931–937. [DOI] [PubMed] [Google Scholar]
- [37].Sandoval DA, Guy DLA, Richardson MA, Ertl AC, Davis SN, Effects of low and moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes, Diabetes 53 (2004) 1798–1806. [DOI] [PubMed] [Google Scholar]
- [38].Rickels MR, DuBose SN, Toschi E, Beck RW, Verdejo AS, Wolpert H, Cummins MJ, Newswanger B, Riddell MC, Mini-dose glucagon as a novel approach to prevent exercise-induced hypoglycemia in type 1 diabetes, Diabetes Care 41 (2018) 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Seereiner S, Neeser K, Weber C, Schreiber K, Habacher W, Rakovac I, Beck P, Schmidt L, Pieber TR, Attitudes towards insulin pump therapy among adolescents and young people, Diabetes Technol Ther 12 (2010) 89–94. [DOI] [PubMed] [Google Scholar]
- [40].Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, Laffel LM, Levy CJ, Pinsker JE, Wadwa RP, Dassau E, Doyle FJ III, Anderson SM, Church MM, Dadlani V, Ekhlaspour L, Forlenza GP, Isganaitis E, Lam DW, Kollman C, Beck RW, iDCL Trial Research Group, Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes, N Engl J Med 381 (2019) 1707–1717.∗ This article reports outcomes from a pivotal study involving adolescents and young adults with T1D.
- [41].Tauschmann M, Allen JM, Nagl K, Fritsch M, Yong J, Metcalfe E, Schaeffer D, Fichelle M, Schierloh U, Thiele AG, Abt D, Kojzar H, Mader JK, Slegtenhorst S, Barber N, Wilinska ME, Boughton C, Musolino G, Sibayan J, Cohen N, Kollman C, Hofer SE, Frohlich-Reiterer E, Kapellen TM, Acerini CL, de Beaufort C, Campbell F, Rami-Merhar B, Hovorka R, Kids APC, Home use of day-and-night hybrid closed-loop insulin delivery in very young children: A multicenter, 3-week, randomized trial, Diabetes Care 42 (2019) 594–600. [DOI] [PubMed] [Google Scholar]
- [42].Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, Schoelwer M, Ruedy KJ, Jost E, Carria L, Emory E, Hsu LJ, Oliveri M, Kollman CC, Dokken BB, Weinzimer SA, DeBoer MD, Buckingham BA, Chernavvsky D, Wadwa RP, iDCL Trial Research Group, A randomized trial of closed-loop control in children with type 1 diabetes, N Engl J Med 383 (2020) 836–845.∗ This article reports outcomes from a pivotal study involving children aged 6 – 13 with T1D.
- [43].Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, Battelino T, Danne T, Weinzimer SA, Sibayan J, Johnson ML, Bailey RJ, Calhoun P, Carlson A, Isganaitis E, Bello R, Albanese-O’Neill A, Dovc K, Biester T, Weyman K, Hood K, Phillip M, A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial, Lancet 397 (2021) 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stewart ZA, Wilinska ME, Hartnell S, ONeil LK, Rayman G, Scott EM, Barnard K, Farrington C, Hovorka R, Murphy HR, Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial, Diabetes Care 41 (2018) 1391–1399. [DOI] [PubMed] [Google Scholar]
- [45].Pinsker JE, Mller L, Constantin A, Leas S, Manning M, McElwee Malloy M, Singh H, Habif S, Real-world patient-reported outcomes and glycemic results with initiation of Control-IQ technology, Diabetes Technol Ther 23 (2021) 120–127.∗∗ This article provides real-world evidence of improved glycemic outcomes on a large sample of age-based subpopulations.
- [46].Bisio A, Gonder-Frederick L, McFadden R, Cheravvsky D, Voelmle M, Pajewski M, Yu P, Bonner H, Brown SA, The impact of a recently approved automated insulin delivery system on glycemic, sleep, and psychosocial outcomes in older adults with type 1 diabetes: A pilot study, J Diabetes Sci Technol (2021).∗ This article provides preliminary evidence of improved glycemic outcomes in seniors with T1D.
- [47].Anderson SM, Buckingham BA, Breton MD, Robic JL, Barnett CL, Wakeman CA, Oliveri MC, Brown SA, Ly TT, Clinton PK, Hsu LJ, Kingman RS, Norlander LM, Loebner SE, Reuschel-DiVirglio S, Kovatchev BP, Hybrid closed-loop control is safe and effective for people with type 1 diabetes who are at moderate to high risk for hypoglycemia, Diabetes Technol Ther 21 (2019) 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus, N Engl J Med 329 (1993) 977–986. [DOI] [PubMed] [Google Scholar]
- [49].Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R, The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes, Diabetes Obes Metab 20 (2018) 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.