Abstract
The recent global pandemic of coronavirus disease 2019 (COVID-19) has led to vaccination in many parts of the world for herd immunity, and as vaccination has progressed, several rare adverse events have been reported. Immune thrombocytopenia (ITP) has been reported to be one of the rare adverse events caused by vaccination with MMR (measles-mumps-rubella) vaccine and influenza vaccine. In addition, ITP has been reported to occur in a small number of cases associated with the COVID-19 messenger ribonucleic acid (mRNA) vaccine. However, there are few reports on the details of the treatment and clinical course; optimal treatment has not yet been established. We report the case of a 20-year-old woman who developed ITP after receiving Pfizer-BioNTech’s BNT162b2 vaccine. She had generalized subcutaneous hemorrhage, 14 days after vaccination. At the time of our visit, she had marked thrombocytopenia and intraoral bleeding; she was diagnosed with ITP. Treatment with oral steroids was started and the platelet count promptly improved after 4 days of treatment. Since the response to treatment was very good, we tapered off the steroids. As these vaccines will be increasingly used in the future, it is important to recognize ITP as a possible adverse event.
Keywords: COVID-19 vaccine, Immune thrombocytopenia, BNT162b, Steroids
Introduction
Immune thrombocytopenia (ITP) is an immune-mediated disorder that causes thrombocytopenia and bleeding. There are two types of ITP: primary and secondary. Secondary ITP is sometimes caused by infectious diseases, autoimmune diseases, vaccines, and other drugs [1]. In recent years, the global epidemic of coronavirus disease 2019 (COVID-19) has caused many deaths worldwide, and in response, vaccinations are being administered worldwide to provide herd immunity. In particular, messenger ribonucleic acid (mRNA) vaccines such as Pfizer-BioNTech’s BNT162b2 and Moderna’s mRNA-1273 have shown very positive results in clinical trials and are already being administered in many countries around the world [2,3]. However, several adverse events that were not reported in the clinical trials have been recorded. Here, we report a case of secondary ITP that occurred after the first dose of the BNT162b2 mRNA vaccine.
Case report
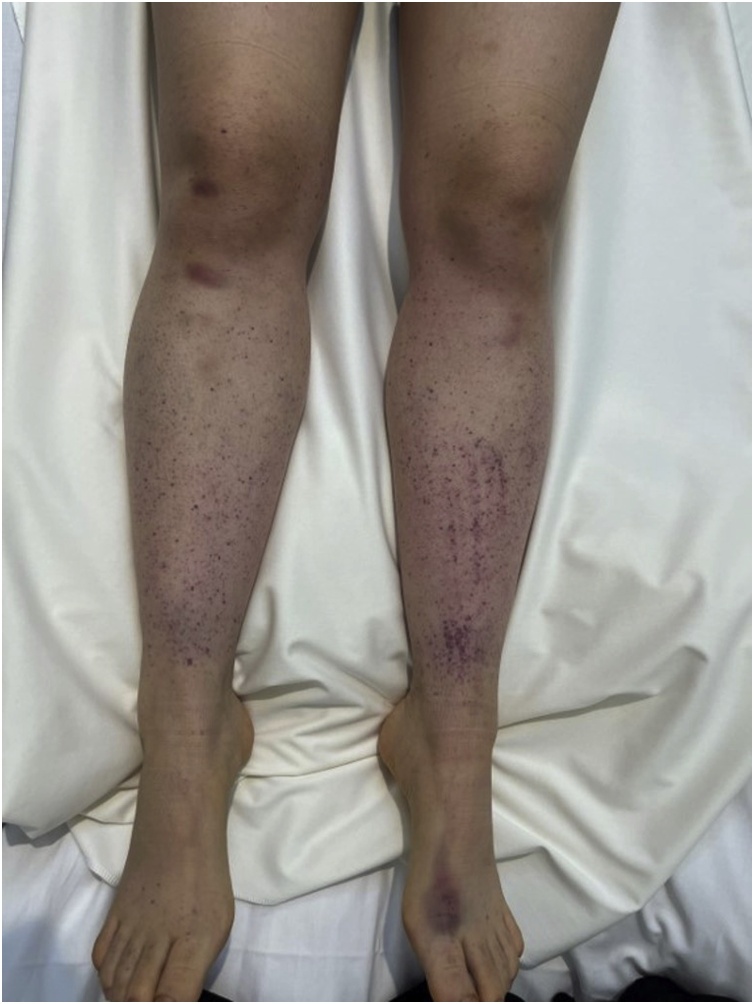
A 20-year-old Japanese woman presented with subcutaneous hemorrhage in her extremities and trunk, 12 days after receiving the BNT162b2 mRNA vaccine. Seventeen days after the vaccination, she developed oral bleeding and was admitted to the Yodogawa Christian Hospital in Osaka, Japan. Her vital signs were normal. Physical examination revealed petechial hemorrhage and subcutaneous hematoma in the extremities and trunk (Fig. 1). Blood tests showed that her white blood cell count and hemoglobin levels were within the reference values, while her platelet count was low (16,000/μL) and her immature platelet fraction was elevated to 11.9 % (normal range 1.1–6.1 %). No blasts appeared in the peripheral blood, and no significant findings were observed in other biochemical and coagulation tests, as shown in Table 1. The Helicobacter pylori stool antigen test was negative. She had no drinking or smoking habits and had no medical or family history nor medications of note. The platelet count was within the reference level at the checkup performed 1 year and 6 months as well as 11 months prior, and no other abnormal findings were noted. Bone marrow aspiration revealed normocellular marrow with an increased number of megakaryocytes. We diagnosed ITP and administered prednisolone 50 mg/body weight (1 mg/kg). On day 4 after the start of treatment, the patient's platelet count improved to 210,000/μL, and the subcutaneous petechiae tended to disappear. On day 8, the platelet count was still within the reference value (153,000–343,000/μL) and the dose of prednisolone was reduced to 30 mg/body weight. Thereafter, the dose of prednisolone was tapered to 20 mg/body weight after 13 days, and the patient responded without relapse.
Fig. 1.
Purpuric lesions on the patient’s lower legs.
Table 1.
Patient laboratory data on administration.
| <Complete blood count> | ||
|---|---|---|
| White blood cells | 5900 | /μL |
| Red blood cells | 451 × 104 | /μL |
| Hemoglobin | 12.8 | g/dL |
| Hematocrit | 38.1 | % |
| Platelet | 1.6 × 104 | /μL |
| <Biochemistry> | ||
|---|---|---|
| Total protein | 7.1 | g/dL |
| Total bilirubin | 0.71 | mg/dL |
| Albumin | 4.8 | g/dL |
| AST | 19 | IU/L |
| ALT | 10 | IU/L |
| γ-GTP | 270 | IU/L |
| LDH | 349 | IU/L |
| ALP | 161 | IU/L |
| Blood urea nitrogen | 13.7 | mg/dL |
| Creatinine | 0.6 | mg/dL |
| C-reactive protein | 0.2 | mg/dL |
| Vitamin B12 | 370 | pg/mL |
| Folate | 6.3 | ng/mL |
| Iron | 76 | μg/dL |
| Haptoglobin | 15 | mg/dL |
| Ferritin | 8 | ng/mL |
| Anti-nuclear antibody | negative | |
| <Infection> | |
|---|---|
| HBs Ag | negative |
| HBs Ab | negative |
| HBc Ab | negative |
| HCV Ab | negative |
| HIVAg/Ab | negative |
Abbreviations: Ab, antibody; Ag, antigen; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBc, hepatitis B core; HBs, hepatitis B surface; HCV, hepatitis C virus; HIV, Human Immunodeficiency Virus; LDH, lactate dehydrogenase; γ-GTP, γ-glutamyl transpeptidase.
Discussion
As of June 24, 2021, a total of 23 cases of newly diagnosed ITP have been reported after administration of the COVID-19 Pfizer-BioNTech’s BNT162b2 and Moderna’s mRNA-1273 vaccines [[4], [5], [6], [7], [8], [9], [10]]. It is impossible to strictly distinguish between vaccine-induced secondary ITP and incidental primary ITP that occurred soon after vaccination. However, of the cases identified so far, 22 occurred after the first vaccination and only one after the second vaccination. Since the frequencies of occurrence are highly uneven, there is likely to be a causal relationship between the COVID-19 vaccine and the development of ITP. Lee et al. also reported that symptoms of bleeding occurred between 1 and 23 days (median 5 days) after vaccine administration [8]. In the present case, bleeding symptoms occurred 14 days after the first dose of the vaccine, which is consistent with a previously reported course.
ITP has been reported to be associated with various vaccinations such as the MMR vaccine, influenza vaccine, and polio vaccine [11]. The pathogenesis is presumed to be immune-mediated and is thought to be related to the increased B-cell function seen in primary ITP [12,13]. Since mRNA vaccines have a different mode of action from conventional vaccines, it is unclear what mechanism causes the immune-mediated reaction. However, many patients, including the patient in this report, responded well to immunosuppressive therapy, suggesting that an immune-mediated mechanism may be involved.
In this case, we administered 1 mg/kg of prednisolone as initial treatment as with primary ITP. The platelet count recovered to the reference level within a short period of 4 days. We assumed that this was vaccine-induced secondary ITP and rapidly reduced the dose. We avoided long-term steroid administration, as recommended for primary ITP. The platelet count remained normal after dose reduction.
We encountered an extremely rare case of secondary ITP presumed to have occurred after BNT162b2 vaccination. Secondary ITP being a very rare complication, the optimal treatment has not yet been determined, and further case series will be necessary. On the other hand, the incidence of symptomatic thrombocytopenia after vaccination is much lower than the risk of death and morbidity due to SARS-CoV-2 infection, as stated in the Medical Advisory Board statement on the Platelet Disorder Support Association website [14]. The purpose of this case report is not to diminish the usefulness of vaccination or the well-documented safety profile of Pfizer-BioNTech’s BNT16B2b2 mRNA vaccine.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Written informed consent was obtained from the patient for submission of the case report and an accompanying image. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
HA and SK wrote the manuscript with support from all other authors. JR, HM, DS, YK and NI treated the patient and provided a clinical history. All authors critically revised and approved the final version of the manuscript.
References
- 1.Cines D.B., Bussel J.B., Liebman H.A., Luning Prak E.T. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fueyo-Rodriguez O., Valente-Acosta B., Jimenez-Soto R., Neme-Yunes Y., Inclán-Alarcón S.I., Trejo-Gonzalez R. Secondary immune thrombocytopenia supposedly attributable to COVID-19 vaccination. BMJ Case Rep. 2021;14:14. doi: 10.1136/bcr-2021-242220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganzel C., Ben-Chetrit E. Immune thrombocytopenia following the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Isr Med Assoc J. 2021;23:341. [PubMed] [Google Scholar]
- 6.Idogun P.O., Ward M.C., Teklie Y., Wiese-Rometsch W. Baker J. Newly diagnosed idiopathic thrombocytopenia post COVID-19 vaccine administration. Cureus. 2021;13:E14853. doi: 10.7759/cureus.14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Julian J.A., Mathern D.R., Fernando D. Idiopathic thrombocytopenic purpura and the moderna Covid-19 vaccine. Ann Emerg Med. 2021;77:654–656. doi: 10.1016/j.annemergmed.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasin F., Calabrese A., Pelagatti L. Immune thrombocytopenia following COVID-19 mRNA vaccine: casuality or causality? Intern Emerg Med. 2021:1–3. doi: 10.1007/s11739-021-02778-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarawneh O., Tarawneh H. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am J Hematol. 2021;96:E133–134. doi: 10.1002/ajh.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perricone C., Ceccarelli F., Nesher G., Borella E., Odeh Q., Conti F. Immune thrombocytopenic purpura (ITP) associated with vaccinations: a review of reported cases. Immunol Res. 2014;60:226–235. doi: 10.1007/s12026-014-8597-x. [DOI] [PubMed] [Google Scholar]
- 12.Giordano P., Cascioli S., Lassandro G., Marcellini V., Cardinale F., Valente F. B-cell hyperfunction in children with immune thrombocytopenic purpura persists after splenectomy. Pediatr Res. 2016;79:262–270. doi: 10.1038/pr.2015.211. [DOI] [PubMed] [Google Scholar]
- 13.Yokomichi H., Tanaka-Taya K., Koshida R., Nakano T., Yasui Y., Mori M. Immune thrombocytopenic purpura risk by live, inactivated and simultaneous vaccinations among Japanese adults, children and infants: a matched case-control study. Int J Hematol. 2020;112:105–114. doi: 10.1007/s12185-020-02866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platelet Disorder Support Association . 2021. COVID-19 + ITP.https://pdsa.org/patients-caregivers/disease-information/covid-19.html Accessed June 24, 2021. [Google Scholar]