Abstract
Background
The interaction between COVID-19, non-communicable diseases, and chronic infectious diseases such as HIV and tuberculosis is unclear, particularly in low-income and middle-income countries in Africa. South Africa has a national HIV prevalence of 19% among people aged 15–49 years and a tuberculosis prevalence of 0·7% in people of all ages. Using a nationally representative hospital surveillance system in South Africa, we aimed to investigate the factors associated with in-hospital mortality among patients with COVID-19.
Methods
In this cohort study, we used data submitted to DATCOV, a national active hospital surveillance system for COVID-19 hospital admissions, for patients admitted to hospital with laboratory-confirmed SARS-CoV-2 infection between March 5, 2020, and March 27, 2021. Age, sex, race or ethnicity, and comorbidities (hypertension, diabetes, chronic cardiac disease, chronic pulmonary disease and asthma, chronic renal disease, malignancy in the past 5 years, HIV, and past and current tuberculosis) were considered as risk factors for COVID-19-related in-hospital mortality. COVID-19 in-hospital mortality, the main outcome, was defined as a death related to COVID-19 that occurred during the hospital stay and excluded deaths that occurred because of other causes or after discharge from hospital; therefore, only patients with a known in-hospital outcome (died or discharged alive) were included. Chained equation multiple imputation was used to account for missing data and random-effects multivariable logistic regression models were used to assess the role of HIV status and underlying comorbidities on COVID-19 in-hospital mortality.
Findings
Among the 219 265 individuals admitted to hospital with laboratory-confirmed SARS-CoV-2 infection and known in-hospital outcome data, 51 037 (23·3%) died. Most commonly observed comorbidities among individuals with available data were hypertension in 61 098 (37·4%) of 163 350, diabetes in 43 885 (27·4%) of 159 932, and HIV in 13 793 (9·1%) of 151 779. Tuberculosis was reported in 5282 (3·6%) of 146 381 individuals. Increasing age was the strongest predictor of COVID-19 in-hospital mortality. Other factors associated were HIV infection (adjusted odds ratio 1·34, 95% CI 1·27–1·43), past tuberculosis (1·26, 1·15–1·38), current tuberculosis (1·42, 1·22–1·64), and both past and current tuberculosis (1·48, 1·32–1·67) compared with never tuberculosis, as well as other described risk factors for COVID-19, such as male sex; non-White race; underlying hypertension, diabetes, chronic cardiac disease, chronic renal disease, and malignancy in the past 5 years; and treatment in the public health sector. After adjusting for other factors, people with HIV not on antiretroviral therapy (ART; adjusted odds ratio 1·45, 95% CI 1·22–1·72) were more likely to die in hospital than were people with HIV on ART. Among people with HIV, the prevalence of other comorbidities was 29·2% compared with 30·8% among HIV-uninfected individuals. Increasing number of comorbidities was associated with increased COVID-19 in-hospital mortality risk in both people with HIV and HIV-uninfected individuals.
Interpretation
Individuals identified as being at high risk of COVID-19 in-hospital mortality (older individuals and those with chronic comorbidities and people with HIV, particularly those not on ART) would benefit from COVID-19 prevention programmes such as vaccine prioritisation as well as early referral and treatment.
Funding
South African National Government.
Introduction
The first case of COVID-19 in South Africa was documented on March 5, 2020. By March 27, 2021, 1 545 431 cases had been reported and the cumulative incidence was 2592·0 cases per 100 000 people.1 Meta-analyses have reported in-hospital mortality rates between 15% and 24%, with substantial global and temporal heterogeneity.2, 3, 4 Worldwide, studies have linked COVID-19-related mortality to older age, male sex, and underlying medical conditions, including cardiac disease, diabetes, cancer, chronic pulmonary disease, obesity, and kidney disease.2, 3, 5, 6 Race or ethnicity and poverty were associated with increased risk of death in COVID-19 cases in large population cohorts and meta-analyses.7, 8, 9
Research in context.
Evidence before this study
We searched PubMed for research articles published between Dec 1, 2019, and May 5, 2021, without language restrictions, using three sets of search terms: “COVID-19”, “risk factors”, and “mortality”; “HIV”, “COVID-19”, and “mortality”; and “TB”, “COVID-19”, and “mortality”. Pooled together, we identified 2786 published papers. Additionally, we searched medRxiv for research articles published between April 25, 2020, and May 5, 2021, without language restrictions, using two sets of search terms: “HIV”, “COVID-19”, and “mortality”; and “TB”, “COVID-19”, and “mortality”. Pooled together, we identified 7744 preprints. Since the emergence of the COVID-19 pandemic, studies have identified older age, male sex, and presence of underlying comorbidities, including heart disease and diabetes, as risk factors for severe disease and death. Very few studies, however, have been carried out in low-income and middle-income countries in Africa, many of which have high poverty rates, insufficient access to health care, and high prevalence of chronic communicable diseases, such as HIV and tuberculosis. Data are also scarce from settings with limited access to HIV treatment programmes. Early small cohort studies, mainly from high-income countries, were not conclusive on whether HIV or tuberculosis are risk factors for disease severity and death in patients with COVID-19. Large population cohort studies from South Africa's Western Cape province and the UK have found people living with HIV to have a moderately increased risk of COVID-19-associated mortality. Of these studies, only the Western Cape study presented data on mortality risk associated with presence of high viral load or immunosuppression, and found similar levels of severity irrespective of these factors. Recent meta-analyses have confirmed the association of HIV with COVID-19 mortality. No studies reported on the interaction between HIV infection and non-communicable comorbidities on COVID-19-associated mortality
Added value of this study
Among a large national cohort of almost 220 000 individuals admitted to hospital with COVID-19 in a setting with 19% HIV prevalence among people aged 15–49 years and 0·7% tuberculosis prevalence, we found that along with age, sex, and non-communicable comorbidities, HIV and tuberculosis were associated with a moderately increased risk of COVID-19 in-hospital mortality. We found increasing risk of COVID-19 in-hospital mortality among people with HIV not on antiretroviral therapy compared with those on antiretroviral therapy. Among people with HIV, the prevalence of non-communicable comorbidities was high (29·2%) and the effect of increasing numbers of comorbidities on COVID-19 in-hospital mortality was similar in people with HIV and HIV-uninfected individuals. Our study included 13 793 people with HIV from all provinces in the country with varying levels of access to HIV treatment programmes.
Implications of all the available evidence
The evidence suggests that people with HIV and patients with tuberculosis should be prioritised for COVID-19 prevention and treatment programmes, particularly those with additional comorbidities. Increasing age and presence of chronic underlying illness are important additional factors associated with COVID-19 in-hospital mortality in a middle-income African setting. The completeness of data is a limitation of this national surveillance system, and additional data are needed to confirm these findings.
Characterisation of populations at increased risk of COVID-19 mortality is important for prioritisation of interventions, particularly in low-income and middle-income countries, where resources are limited. However, available data on risk factors for severe COVID-19, including mortality, are mostly from high-income countries. High poverty rates, insufficient access to health care, and high prevalence of chronic communicable diseases, such as HIV and tuberculosis, probably affect the burden of COVID-19 and disease severity in low-income and middle-income countries.10
South Africa is a middle-income country with coinciding epidemics of non-communicable diseases and chronic infectious diseases (HIV and tuberculosis). In 2020, the prevalence of HIV among people aged 15–49 years was 19%,11 and tuberculosis prevalence was 0·7% for all ages.12 In 2019, 7·5 million people were estimated to be living with HIV in South Africa, of whom 2·3 million (31%) were eligible for, but not receiving, treatment.13
An understanding of whether HIV and tuberculosis are associated with mortality among patients admitted to hospital with COVID-19 is of huge importance for South Africa, and other countries in the region with large HIV and tuberculosis epidemics, to guide public health action around prevention and treatment of COVID-19. Early single-centre cohort studies14, 15 and meta-analyses16, 17 from high-income countries with relatively small numbers of people living with HIV did not find HIV to be a risk factor for severe COVID-19. Larger population cohorts18, 19, 20 and more recent meta-analyses21, 22, 23 found people with HIV to have an increased population risk of COVID-19-associated mortality. The interaction between non-communicable comorbidities, HIV infection, and COVID-19 in-hospital mortality is not well described.
We aimed to examine the association between HIV infection, tuberculosis, non-communicable comorbidities, and in-hospital mortality among patients with laboratory-confirmed SARS-CoV-2 infection using data from a national surveillance programme in South Africa.
Methods
Study design and data sources
This cohort study was done in South Africa, which is administratively divided into nine provinces. South Africa has a dual health system, with a publicly funded district health system that serves approximately 84% of the population and a private health system largely funded by private health insurance schemes.24
In the absence of existing national COVID-19 hospital surveillance systems, the National Institute for Communicable Diseases established DATCOV as a national surveillance system for COVID-19 hospital admissions on April 1, 2020.25 DATCOV was adopted for national implementation by the South African Government on July 15, 2020, and by October, 2020, full coverage of all hospitals had been achieved. By March 27, 2021, 393 public-sector hospitals and 251 private-sector hospitals had reported COVID-19 admissions on DATCOV. Further details about the DATCOV surveillance system, including its implementation, data management, and data quality assurance, are described in the appendix (p 1).
The Human Research Ethics Committee (Medical) at the University of the Witwatersrand (Johannesburg, South Africa) approved the project protocol as part of a national surveillance programme (M160667). In South Africa, surveillance for notifiable medical conditions such as COVID-19 requires health facilities to submit data on all cases of emerging pathogens to national authorities. As such, individual consent for inclusion of their data to DATCOV is waivered. All personal identifying information was delinked for our analysis and stored in a secure server.
Potential risk factors and covariates
Age, sex, race or ethnicity, and comorbidities (hypertension, diabetes, chronic cardiac disease, chronic pulmonary disease and asthma, chronic renal disease, malignancy in the past 5 years, obesity, HIV, and past and current tuberculosis) were considered as potential risk factors for COVID-19 in-hospital mortality. Data on comorbidities (including HIV status, antiretroviral therapy (ART) status, and CD4 cell count and viral load in the past 12 months), obtained from patients' written and electronic hospital records, were submitted to DATCOV by the hospitals. The DATCOV team did not independently verify these data or access laboratory records to obtain these data. The date of the most recent CD4 cell count or viral load was not documented. The level of virological control or immunosuppression was assessed based on last available viral load or CD4 cell count result within the past year and categorised as virologically suppressed (HIV RNA <1000 copies per mL) or viraemic (HIV RNA ≥1000 copies per mL), and immune reconstituted (CD4 count ≥200 cells per μL) or immunosuppressed (CD4 count <200 cells per μL).26 CD4 cell count is no longer routinely measured in all patients, in accordance with South African HIV care guidelines.27
COVID-19 in-hospital mortality was defined as a death related to COVID-19 that occurred during the hospital stay and excluded deaths that occurred because of other causes or after discharge from hospital. Because the main outcome of the study was COVID-19 in-hospital mortality, all analyses included patients with laboratory-confirmed SARS-CoV-2 infection who had a known in-hospital outcome (ie, discharged alive or died) at the time of data extraction (March 27, 2021).
Statistical analysis
We analysed DATCOV data from patients admitted to hospital with SARS-CoV-2 infection between March 5, 2020, and March 27, 2021. For the main analysis, to account for incomplete or missing data on selected variables, we used multivariate imputation by chained equation (MICE) and generated ten complete imputed datasets that were used for subsequent analyses. Variables analysed by use of MICE included sex, race or ethnicity, month of admission, and the following comorbidities: HIV infection, tuberculosis infection, hypertension, diabetes, chronic pulmonary disease and asthma, malignancy in the past 5 years, chronic cardiac disease, and chronic renal disease. ART status, HIV viral load, and CD4 cell counts were also incomplete and were conditionally imputed only among HIV-positive patients (either with observed or imputed HIV status). Complete variables included in the imputation process were age, province, health sector (ie, public or private), and in-hospital outcome (ie, discharged alive or died). Descriptive statistics such as frequencies and percentages were used for categorical variables, and continuous variables were expressed as mean with SD or median and IQR on the imputed datasets.
We implemented three post-imputation random-effect (on admission facility) multivariable logistic regression models: first, to compare people with HIV and individuals who were HIV-uninfected; second, to determine the factors associated with COVID-19 in-hospital mortality, including ART status, HIV viral load, and CD4 cell count among HIV-positive individuals; and, third, to evaluate the combined effect of multiple non-HIV comorbidities on COVID-19 in-hospital mortality. A combined comorbidity variable categorised none, one, two, and three or more comorbidities among factors that were individually significantly associated with COVID-19 in-hospital mortality in the third model. We evaluated the effect of multiple comorbidities among all patients and among people with HIV and HIV-uninfected patients separately through stratification by HIV infection status. In addition, we assessed the potential differential effect (through the inclusion of an interaction term) of multiple or individual comorbidities on COVID-19 in-hospital mortality between people with HIV and HIV-uninfected individuals.
A random effect on admission facility was included for all analyses to account for potential differences in the service population and the quality of care at each facility. For each multivariable model, we assessed all variables that were significant at p values of less than 0·2 in the univariate analysis (to evaluate lack of significance in the univariate analysis after adjusting for potential confounders) and excluded non-significant factors (p≥0·05) with manual backward elimination. Pairwise interactions were assessed by inclusion of product terms for all variables remaining in the final multivariable additive model. We implemented several sensitivity analyses separately, analysing risk factors for COVID-19 in-hospital mortality in the public and private sectors using post-imputation random-effect multivariable logistic regression models. We also reported the univariate and multivariate association of all covariates evaluated in the analyses described previously with HIV infection and COVID-19 in-hospital mortality using non-imputed data. The statistical analysis was implemented using Stata (version 15). We followed STROBE guideline recommendations.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
From March 5, 2020, to March 27, 2021, 229 154 individuals were admitted to hospital with laboratory-confirmed SARS-CoV-2 infection and reported to DATCOV. Of these patients, 219 265 had a known in-hospital outcome and were included in analyses (appendix p 5). The percentage of missing data for the imputed variables varied and was highest for obesity (163 570 [74·6%]), race or ethnicity (73 389 [33·5%]), and for HIV-specific variables including HIV diagnosis (67 486 [30·8%]), being on ART (5715 [41·4%] of 13 793 known HIV-positive individuals), viral load (12 077 [87·6%] of 13 793), and CD4 cell count (11 023 [79·9%] of 13 793; appendix pp 6–7). Obesity was excluded from the analysis due to the high proportion of missing data.
South Africa experienced a first wave of SARS-CoV-2 infection, peaking at 10 086 admissions per week in July, 2020, and a second wave peaking at 17 976 admissions per week in January, 2021 (appendix p 8).
The median age of patients admitted to hospital with SARS-CoV-2 infection was 54 years (IQR 40–66), and 121 937 (55·7%) of 218 827 patients were female (appendix p 6). Among 145 876 patients with available race information, 114 571 (78·5%) were Black (appendix p 6). The public sector accounted for 113 856 (51·9%) of 219 265 hospital admissions reported to DATCOV (appendix p 7). 176 272 (80·4%) of 219 265 hospital admissions were recorded by hospitals in four provinces (Western Cape, Gauteng, KwaZulu-Natal, and Eastern Cape; appendix p 7).
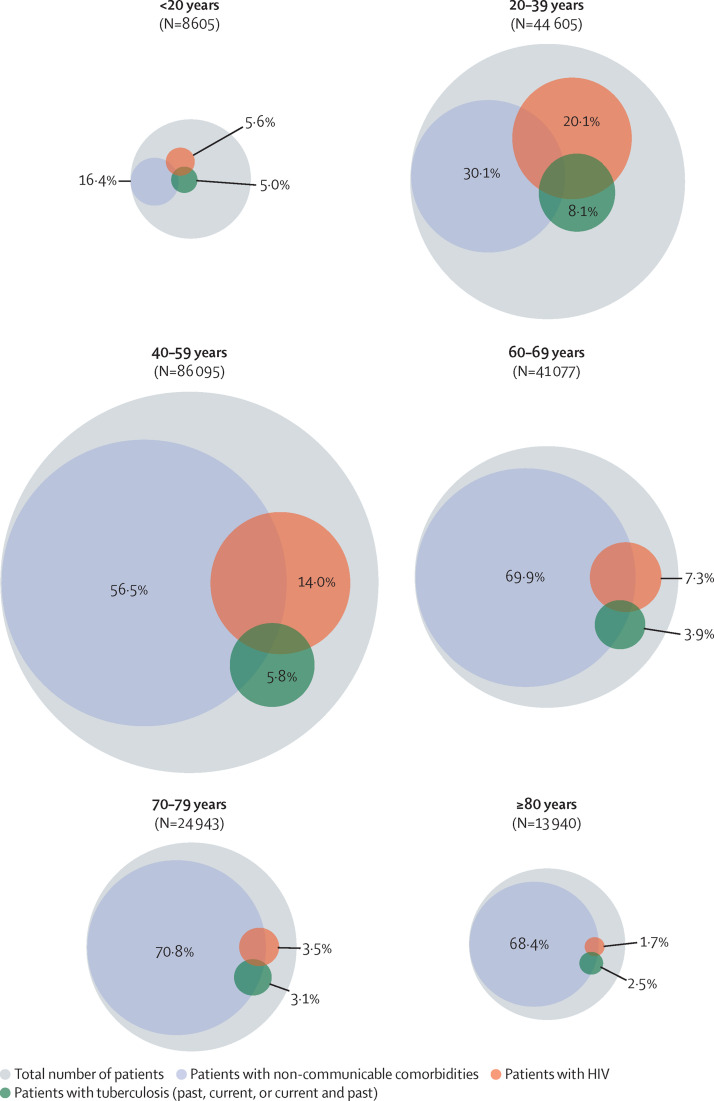
After multiple imputation, the estimated proportion of individuals reporting at least one comorbidity was 60·5% (95% CI 59·8–61·2). The most prevalent comorbidities were hypertension (38·6%), diabetes (29·0%), and HIV (11·7%). The prevalence of non-communicable comorbidities increased with age, whereas HIV and tuberculosis were most prevalent in individuals aged 20–59 years (figure 1 ).
Figure 1.
Estimated prevalence of comorbid conditions in patients admitted to hospital with SARS-CoV-2 infection by age
The percentage of patients within each age group is expressed over the total number of patients included in the analysis (n=219 265). Circle size is proportional to the number of patients represented. Inner circles represent a subpopulation (with prevalence expressed as percentage) of the outer circle. Overlapping circles represent co-infections and comorbidities.
Of 151 779 (69·2%) patients admitted to hospital with COVID-19 who had available data on HIV, 13 793 (9·1%) were HIV positive (appendix pp 6–7). HIV prevalence was 20·4% in the public sector and 2·2% in the private sector (table 1 ; age-specific HIV prevalence for public and private sector is presented in the appendix p 9). A subset of the 13 793 people with HIV had ART (8078 [58·6%]), viral load (1716 [12·4%]), and CD4 cell count (2770 [20·1%]) information available. 7484 (92·6%) of 8078 patients were receiving ART, and 443 (25·8%) of 1716 were viraemic (HIV RNA ≥1000 copies per mL), and 1080 (39·0%) of 2770 were immunosuppressed (CD4 count <200 cells per μL) based on the last available test.
Table 1.
Characteristics of people with HIV and HIV-uninfected people admitted to hospital with laboratory-confirmed SARS-CoV-2 infection, South Africa (n=219 265)
| People with HIV (n=13 793) | People without HIV (n=137 986) | Unadjusted OR (95% CI) unimputed | p value | Adjusted OR (95% CI) unimputed | p value | Unadjusted OR (95% CI) imputed | p value | Adjusted OR (95% CI) imputed | p value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||||
| Female | 8884/83 607 (10·6%) | 74 723/83 607 (89·4%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Male | 4893/68 090 (7·2%) | 63 197/68 090 (92·8%) | 0·75 (0·72–0·78) | <0·0001 | 0·75 (0·71–0·80) | <0·0001 | 0·78 (0·75–0·80) | <0·0001 | 0·73 (0·70–0·75) | <0·0001 |
| Age, years | ||||||||||
| <20 | 227/5067 (4·5%) | 4840/5067 (95·5%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| 20–39 | 4616/28 833 (16·0%) | 24 217/28 833 (84·0%) | 5·08 (4·35–5·92) | <0·0001 | 5·60 (4·42–7·09) | <0·0001 | 5·20 (4·32–6·26) | <0·0001 | 5·29 (4·39–6·38) | <0·0001 |
| 40–59 | 6851/62 529 (11·0%) | 55 678/62 529 (89·0%) | 4·03 (3·46–4·70) | <0·0001 | 6·88 (5·43–8·71) | <0·0001 | 4·26 (3·60–5·03) | <0·0001 | 5·42 (4·59–6·40) | <0·0001 |
| 60–69 | 1574/28 957 (5·4%) | 27 383/28 957 (94·6%) | 1·37 (1·17–1·61) | <0·0001 | 2·69 (2·10–3·45) | <0·0001 | 1·53 (1·29–1·81) | <0·0001 | 1·97 (1·65–2·35) | <0·0001 |
| 70–79 | 435/17 487 (2·5%) | 17 052/17 487 (97·5%) | 0·58 (0·48–0·69) | <0·0001 | 0·97 (0·73–1·28) | 0·82 | 0·66 (0·54–0·82) | 0·0005 | 0·80 (0·64–0·99) | 0·040 |
| ≥80 | 90/8906 (1·0%) | 8816/8906 (99·0%) | 0·26 (0·20–0·33) | <0·0001 | 0·41 (0·27–0·61) | <0·0001 | 0·30 (0·22–0·42) | <0·0001 | 0·33 (0·23–0·48) | <0·0001 |
| Race or ethnicity | ||||||||||
| White | 55/10 499 (0·5%) | 10 444/10 499 (99·5%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Black | 10 680/68 961 (15·5%) | 58 281/68 961 (84·5%) | 11·37 (8·61–15·02) | <0·0001 | 8·71 (6·19–12·27) | <0·0001 | 13·07 (9·96–17·17) | <0·0001 | 8·87 (6·80–11·58) | <0·0001 |
| Mixed | 210/5917 (3·5%) | 5707/5917 (96·5%) | 2·72 (1·98–3·73) | <0·0001 | 2·03 (1·38–2·99) | 0·0003 | 2·43 (1·73–3·42) | <0·0001 | 1·75 (1·26–2·44) | 0·0017 |
| Indian | 43/6588 (0·7%) | 6543/6588 (99·3%) | 0·60 (0·39–0·90) | 0·015 | 0·50 (0·30–0·83) | 0·0072 | 0·70 (0·45–1·09) | 0·11 | 0·54 (0·35–0·86) | 0·010 |
| Hypertension | ||||||||||
| No | 8042/98 473 (8·2%) | 90 431/98 473 (91·8%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 3857/50 770 (7·6%) | 46 913/50 770 (92·4%) | 0·51 (0·49–0·54) | <0·0001 | 0·75 (0·70–0·81) | <0·0001 | 0·58 (0·55–0·60) | <0·0001 | 0·87 (0·83–0·92) | <0·0001 |
| Diabetes | ||||||||||
| No | 8954/111 449 (8·0%) | 102 495/111 449 (92·0%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 2669/37 305 (7·2%) | 34 636/37 305 (92·8%) | 0·44 (0·42–0·47) | <0·0001 | 0·55 (0·51–0·59) | <0·0001 | 0·53 (0·50–0·55) | <0·0001 | 0·62 (0·58–0·66) | <0·0001 |
| Chronic cardiac disease | ||||||||||
| No | 10 284/142 253 (7·2%) | 131 969/142 253 (92·8%) | 1 (ref) | .. | .. | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 293/3374 (8·7%) | 3081/3374 (91·3%) | 0·79 (0·69–0·90) | <0·0001 | .. | .. | 1·12 (1·00–1·25) | 0·044 | 1·41 (1·24–1·60) | <0·0001 |
| Chronic pulmonary disease or asthma | ||||||||||
| No | 9943/137 862 (7·2%) | 127 919/137 862 (92·8%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 975/9626 (10·1%) | 8651/9626 (89·9%) | 0·79 (0·73–0·85) | <0·0001 | 0·85 (0·75–0·96) | 0·0093 | 0·93 (0·87–0·99) | 0·021 | 0·89 (0·82–0·97) | 0·0063 |
| Chronic renal disease | ||||||||||
| No | 9982/141 708 (7·0%) | 131 726/141 708 (93·0%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 541/3746 (14·4%) | 3205/3746 (85·6%) | 0·93 (0·84–1·02) | 0·13 | 1·72 (1·47–2·02) | <0·0001 | 1·26 (1·16–1·38) | <0·0001 | 1·88 (1·69–2·09) | <0·0001 |
| Malignancy in the past 5 years | ||||||||||
| No | 10 284/144 246 (7·1%) | 133 962/144 246 (92·9%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 154/985 (15·6%) | 831/985 (84·4%) | 1·78 (1·47–2·16) | <0·0001 | 1·35 (1·04–1·75) | 0·022 | 2·15 (1·86–2·48) | <0·0001 | 1·98 (1·59–2·46) | <0·0001 |
| Tuberculosis | ||||||||||
| Never | 7807/138 842 (5·6%) | 131 035/138 842 (94·4%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Past | 1207/2960 (40·8%) | 1753/2960 (59·2%) | 4·81 (4·41–5·24) | <0·0001 | 4·88 (4·22–5·64) | <0·0001 | 4·24 (3·92–4·59) | <0·0001 | 3·55 (3·20–3·95) | <0·0001 |
| Current | 360/954 (37·7%) | 594/954 (62·3%) | 9·44 (7·99–11·15) | <0·0001 | 7·35 (6·07–8·92) | <0·0001 | 6·42 (5·55–7·42) | <0·0001 | 5·55 (4·64–6·65) | <0·0001 |
| Current and past | 745/1259 (59·2%) | 514/1259 (40·8%) | 7·98 (6·97–9·12) | <0·0001 | 6·84 (5·39–8·70) | <0·0001 | 14·30 (13·05–15·66) | <0·0001 | 11·19 (9·98–12·54) | <0·0001 |
| Health sector | ||||||||||
| Private | 2057/94 167 (2·2%) | 92 110/94 167 (97·8%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Public | 11 736/57 612 (20·4%) | 45 876/57 612 (79·6%) | 21·86 (16·78–28·46) | <0·0001 | 6·51 (5·22–8·12) | <0·0001 | 17·89 (14·90–21·48) | <0·0001 | 13·59 (11·51–16·04) | <0·0001 |
| Province | ||||||||||
| Western Cape | 4178/34 530 (12·1%) | 30 352/34 530 (87·9%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Eastern Cape | 1898/20 468 (9·3%) | 18 570/20 468 (90·7%) | 1·74 (0·97–3·13) | 0·062 | 0·25 (0·18–0·36) | <0·0001 | 1·45 (0·89–2·35) | 0·13 | 0·65 (0·50–0·84) | 0·0010 |
| Free State | 866/10 453 (8·3%) | 9587/10 453 (91·7%) | 1·23 (0·61–2·50) | 0·56 | 0·32 (0·22–0·48) | <0·0001 | 1·10 (0·61–1·96) | 0·76 | 0·74 (0·55–1·01) | 0·059 |
| Gauteng | 1603/35 337 (4·5%) | 33 734/35 337 (95·5%) | 0·37 (0·21–0·66) | 0·0007 | 0·23 (0·16–0·33) | <0·0001 | 0·38 (0·24–0·61) | <0·0001 | 0·76 (0·59–0·98) | 0·034 |
| KwaZulu-Natal | 3321/30 459 (10·9%) | 27 138/30 459 (89·1%) | 1·76 (0·99–3·12) | 0·052 | 0·46 (0·33–0·63) | <0·0001 | 1·65 (1·04–2·64) | 0·035 | 1·50 (1·17–1·92) | 0·0014 |
| Limpopo | 469/5790 (8·1%) | 5321/5790 (91·9%) | 2·97 (1·45–6·09) | 0·0030 | 0·27 (0·18–0·42) | <0·0001 | 1·88 (1·04–3·39) | 0·036 | 0·74 (0·55–1·01) | 0·059 |
| Mpumalanga | 612/5173 (11·8%) | 4561/5173 (88·2%) | 5·79 (2·61–12·84) | <0·0001 | 0·25 (0·15–0·41) | <0·0001 | 3·27 (1·73–6·18) | <0·0001 | 1·64 (1·17–2·30) | 0·0039 |
| North West | 722/7196 (10·0%) | 6474/7196 (90·0%) | 1·11 (0·44–2·81) | 0·82 | 0·24 (0·14–0·43) | <0·0001 | 1·30 (0·62–2·71) | 0·49 | 1·07 (0·73–1·57) | 0·73 |
| Northern Cape | 124/2373 (5·2%) | 2249/2373 (94·8%) | 1·28 (0·43–3·77) | 0·66 | 0·26 (0·12–0·57) | 0·0007 | 0·88 (0·37–2·06) | 0·76 | 0·75 (0·48–1·20) | 0·23 |
| Outcome | ||||||||||
| Discharged alive | 10 386/117 675 (8·8%) | 107 289/117 675 (91·2%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Died in hospital | 3407/34 104 (10·0%) | 30 697/34 104 (90·0%) | 0·83 (0·79–0·87) | <0·0001 | 1·23 (1·15–1·33) | <0·0001 | 0·86 (0·82–0·90) | <0·0001 | 1·25 (1·18–1·33) | <0·0001 |
Data are n/N (%) unless otherwise stated. OR=odds ratio.
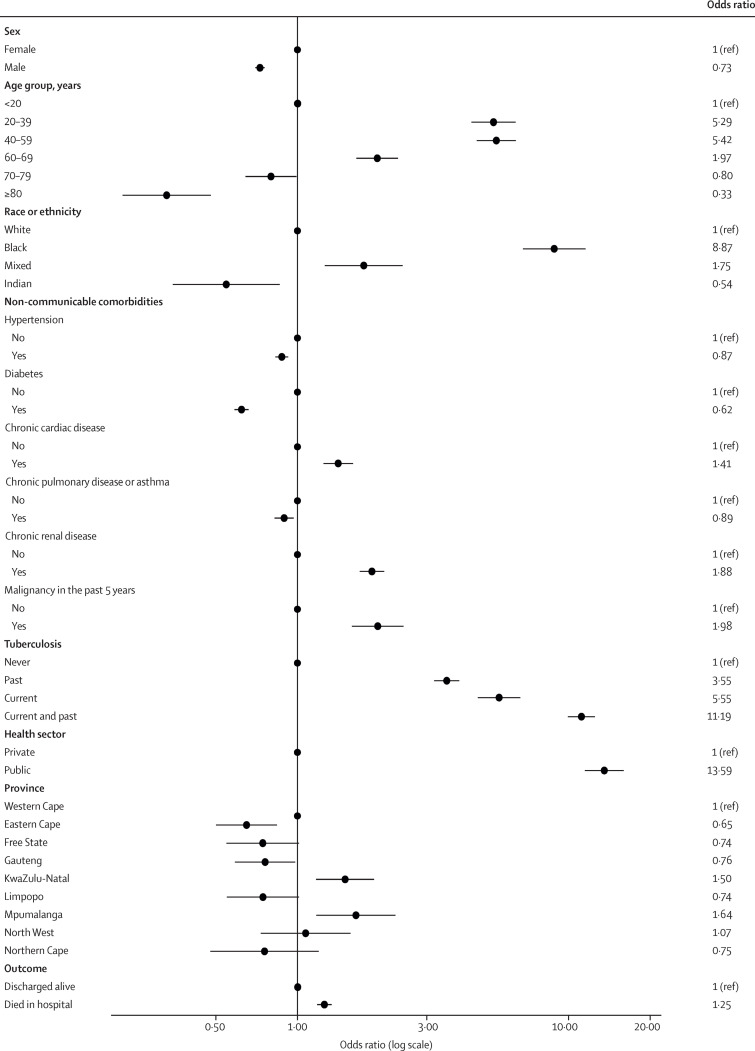
Compared with HIV-uninfected patients admitted to hospital with SARS-CoV-2 infection, those with HIV were more likely to be aged 20–39 years, 40–59 years, and 60–69 years compared with younger than 20 years; be Black or mixed race compared with White race; be admitted in the public health sector; have comorbid chronic cardiac disease, chronic renal disease, malignancy in the past 5 years, current tuberculosis, past tuberculosis, and past and current tuberculosis; and die in hospital. People with HIV were less likely to be aged 70–79 years and 80 years or older; be male; be of Indian ancestry; and to have comorbid hypertension, diabetes, and chronic pulmonary disease or asthma (table 1; figure 2 ). HIV prevalence among patients with COVID-19 varied, with lower prevalence in the Eastern Cape and Gauteng provinces and higher prevalence in the KwaZulu-Natal and Mpumalanga provinces (table 1).
Figure 2.
Multivariable analysis of factors associated with HIV infection
Dots represent adjusted odds ratios and error bars represent 95% CIs. See table 1 for 95% CI values.
Of the 219 265 patients with recorded outcomes, 168 228 (76·7%) were discharged alive and 51 037 (23·3%) died. The unadjusted in-hospital case fatality ratio for people with HIV was 3407 (24·7%) of 13 793 compared with 30 697 (22·2%) of 137 986 for HIV-uninfected individuals.
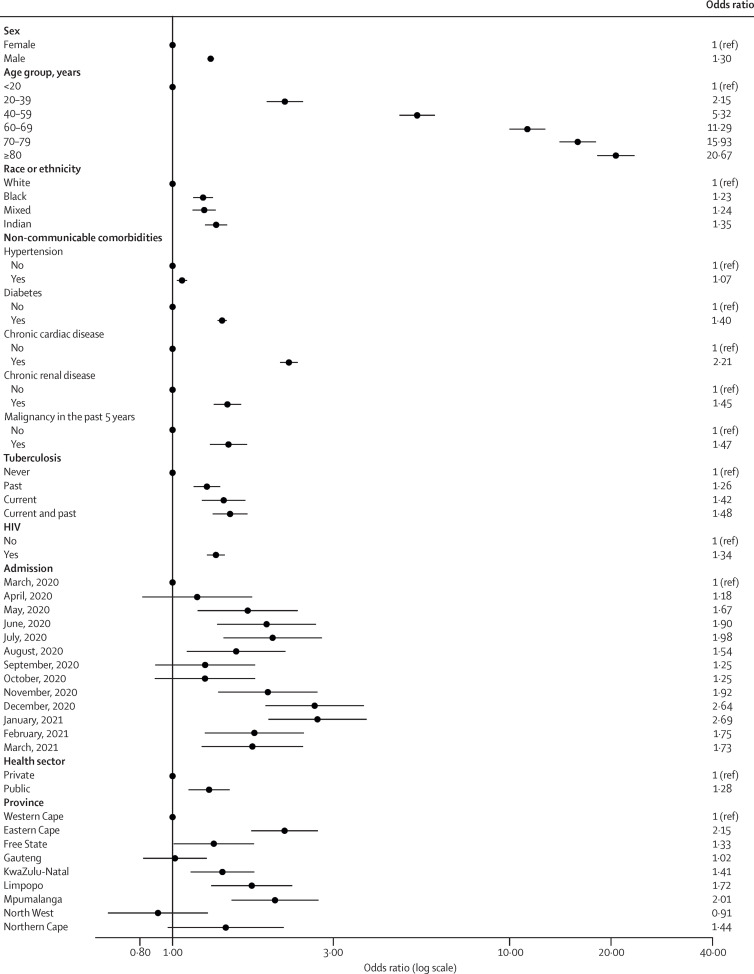
Factors statistically associated with COVID-19 in-hospital mortality were increasing age 20–39 years, 40–59 years, 60–69 years, 70–79 years, and 80 years or older, compared with younger than 20 years; male sex; Black, mixed race, and Indian ancestry compared with White race; having comorbid hypertension, diabetes, chronic cardiac disease, chronic renal disease, malignancy in the past 5 years, HIV, past tuberculosis, current tuberculosis or both past and current tuberculosis; and being admitted in the public health sector. COVID-19 in-hospital mortality increased each month of the epidemic to the peak of the first wave in July, 2020, then decreased between waves and increased again to the peak of the second wave in January, 2021. COVID-19 in-hospital mortality was significantly higher in Eastern Cape, Free State, KwaZulu-Natal, Limpopo, and Mpumalanga, compared with Western Cape (table 2 ; figure 3 ). The unimputed multivariate analysis showed similar findings (table 2).
Table 2.
Factors associated with COVID-19 in-hospital mortality among patients admitted to hospital with laboratory-confirmed SARS-CoV-2 infection, South Africa (n=219 265)
| Case fatality ratio | Unadjusted OR (95% CI) unimputed | p value | Adjusted OR (95% CI) unimputed | p value | Unadjusted OR (95% CI) imputed | p value | Adjusted OR (95% CI) imputed* | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Female | 26 466/121 937 (21·7%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Male | 24 490/96 890 (25·3%) | 1·32 (1·29–1·34) | <0·0001 | 1·32 (1·27–1·37) | <0·0001 | 1·32 (1·29–1·34) | <0·0001 | 1·30 (1·27–1·33) | <0·0001 |
| Age, years | |||||||||
| <20 | 300/8605 (3·5%) | 1 (ref) | <0·0001 | 1 (ref) | <0·0001 | 1 (ref) | .. | 1 (ref) | .. |
| 20–39 | 3516/44 605 (7·9%) | 2·38 (2·11–2·70) | <0·0001 | 2·60 (2·02–3·28) | <0·0001 | 2·38 (2·11–2·70) | <0·0001 | 2·15 (1·90–2·44) | <0·0001 |
| 40–59 | 16 285/86 095 (18·9%) | 6·88 (6·11–7·76) | <0·0001 | 6·90 (5·45–8·74) | <0·0001 | 6·88 (6·11–7·76) | <0·0001 | 5·32 (4·71–6·00) | <0·0001 |
| 60–69 | 14 412/41 077 (35·1%) | 15·08 (13·38–17·01) | <0·0001 | 14·41 (11·36–18·28) | <0·0001 | 15·08 (13·38–17·01) | <0·0001 | 11·29 (9·99–12·76) | <0·0001 |
| 70–79 | 10 509/24 943 (42·1%) | 20·75 (18·38–23·42) | <0·0001 | 20·85 (16·40–26·49) | <0·0001 | 20·75 (18·38–23·42) | <0·0001 | 15·93 (14·06–18·04) | <0·0001 |
| ≥80 | 6015/13 940 (43·1%) | 24·85 (21·96–28·12) | <0·0001 | 28·89 (22·62–36·89) | <0·0001 | 24·85 (21·96–28·12) | <0·0001 | 20·67 (18·19–23·48) | <0·0001 |
| Race or ethnicity | |||||||||
| White | 2689/12 661 (21·2%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Black | 28 602/114 571 (25·0%) | 0·77 (0·73–0·81) | <0·0001 | 1·21 (1·13–1·30) | <0·0001 | 0·79 (0·74–0·84) | <0·0001 | 1·23 (1·15–1·32) | <0·0001 |
| Mixed | 2310/10 053 (23·0%) | 0·92 (0·85–0·99) | 0·022 | 1·31 (1·18–1·44) | <0·0001 | 0·89 (0·83–0·96) | 0·0037 | 1·24 (1·15–1·34) | <0·0001 |
| Indian | 2014/8591 (23·4%) | 1·08 (1·00–1·17) | 0·046 | 1·32 (1·20–1·46) | <0·0001 | 1·07 (0·99–1·14) | 0·078 | 1·35 (1·25–1·45) | <0·0001 |
| Hypertension | |||||||||
| No | 19 123/102 252 (18·7%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 19 668/61 098 (32·2%) | 1·92 (1·87–1·97) | <0·0001 | 1·16 (1·11–1·21) | <0·0001 | 2·03 (1·97–2·08) | <0·0001 | 1·07 (1·03–1·10) | 0·0004 |
| Diabetes | |||||||||
| No | 22 742/116 047 (19·6%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 14 707/43 885 (33·5%) | 1·92 (1·87–1·98) | <0·0001 | 1·42 (1·36–1·48) | <0·0001 | 2·01 (1·96–2·06) | <0·0001 | 1·40 (1·36–1·45) | <0·0001 |
| Chronic cardiac disease | |||||||||
| No | 32 498/145 854 (22·3%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | ..ç |
| Yes | 1564/4178 (37·4%) | 1·95 (1·82–2·09) | <0·0001 | 1·22 (1·11–1·34) | <0·0001 | 3·47 (3·28–3·66) | <0·0001 | 2·21 (2·08–2·35) | <0·0001 |
| Chronic renal disease | |||||||||
| No | 32 009/145 095 (22·1%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 1785/4121 (43·3%) | 2·56 (2·39–2·74) | <0·0001 | 1·68 (1·50–1·89) | <0·0001 | 2·68 (2·51–2·87) | <0·0001 | 1·45 (1·33–1·60) | <0·0001 |
| Malignancy in the past 5 years | |||||||||
| No | 33 136/147 659 (22·4%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 439/1143 (38·4%) | 2·04 (1·80–2·32) | <0·0001 | 1·75 (1·48–2·08) | <0·0001 | 2·24 (2·02–2·49) | <0·0001 | 1·47 (1·29–1·66) | <0·0001 |
| Tuberculosis | |||||||||
| Never | 31 276/141 099 (22·2%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Past | 759/3001 (25·3%) | 0·93 (0·85–1·02) | 0·12 | 1·12 (0·95–1·30) | 0·17 | 1·16 (1·07–1·26) | 0·0006 | 1·26 (1·15–1·38) | <0·0001 |
| Current | 258/993 (26·0%) | 1·08 (0·93–1·25) | 0·33 | 1·58 (1·30–1·92) | <0·0001 | 1·04 (0·90–1·20) | 0·59 | 1·42 (1·22–1·64) | <0·0001 |
| Current and past | 307/1288 (23·8%) | 0·98 (0·85–1·14) | 0·80 | 2·05 (1·59–2·64) | <0·0001 | 1·14 (1·03–1·27) | 0·013 | 1·48 (1·32–1·67) | <0·0001 |
| HIV | |||||||||
| No | 30 697/137 986 (22·2%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Yes | 3407/13 793 (24·7%) | 0·84 (0·80–0·88) | <0·0001 | 1·29 (1·20–1·39) | <0·0001 | 0·86 (0·82–0·90) | <0·0001 | 1·34 (1·27–1·43) | <0·0001 |
| Admission | |||||||||
| March, 2020 | 45/400 (11·3%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| April, 2020 | 185/1449 (12·8%) | 1·26 (0·88–1·79) | 0·21 | 0·83 (0·48–1·43) | 0·51 | 1·26 (0·88–1·79) | 0·21 | 1·18 (0·81–1·72) | 0·38 |
| May, 2020 | 1072/5787 (18·5%) | 1·89 (1·37–2·61) | 0·0001 | 1·12 (0·68–1·84) | 0·66 | 1·89 (1·37–2·61) | 0·0001 | 1·67 (1·19–2·35) | 0·0033 |
| June, 2020 | 3698/18 209 (20·3%) | 2·22 (1·62–3·06) | <0·0001 | 1·39 (0·85–2·26) | 0·19 | 2·22 (1·62–3·05) | <0·0001 | 1·90 (1·36–2·66) | 0·0002 |
| July, 2020 | 8335/38 226 (21·8%) | 2·43 (1·77–3·34) | <0·0001 | 1·45 (0·89–2·37) | 0·13 | 2·43 (1·77–3·34) | <0·0001 | 1·98 (1·42–2·77) | 0·0001 |
| August, 2020 | 3707/19 671 (18·8%) | 2·00 (1·46–2·75) | <0·0001 | 1·19 (0·73–1·95) | 0·49 | 2·00 (1·45–2·75) | <0·0001 | 1·54 (1·10–2·16) | 0·012 |
| September, 2020 | 1314/8851 (14·8%) | 1·51 (1·09–2·08) | 0·013 | 0·94 (0·57–1·55) | 0·80 | 1·51 (1·09–2·08) | 0·013 | 1·25 (0·89–1·76) | 0·20 |
| October, 2020 | 1167/7735 (15·1%) | 1·45 (1·05–2·00) | 0·024 | 0·95 (0·57–1·57) | 0·84 | 1·45 (1·05–2·00) | 0·024 | 1·25 (0·89–1·76) | 0·20 |
| November, 2020 | 2506/11 110 (22·6%) | 2·17 (1·58–2·99) | <0·0001 | 1·63 (0·99–2·68) | 0·053 | 2·17 (1·58–2·99) | <0·0001 | 1·92 (1·37–2·69) | 0·0002 |
| December, 2020 | 10 621/39 582 (26·8%) | 2·98 (2·17–4·09) | <0·0001 | 1·94 (1·19–3·17) | 0·0081 | 2·98 (2·17–4·09) | <0·0001 | 2·64 (1·89–3·69) | <0·0001 |
| January, 2021 | 15 264/52 019 (29·3%) | 3·33 (2·42–4·57) | <0·0001 | 1·86 (1·14–3·04) | 0·013 | 3·32 (2·42–4·56) | <0·0001 | 2·69 (1·92–3·76) | <0·0001 |
| February, 2021 | 2380/11 940 (19·9%) | 2·00 (1·45–2·75) | <0·0001 | 1·34 (0·81–2·20) | 0·25 | 2·00 (1·45–2·75) | <0·0001 | 1·75 (1·25–2·45) | 0·0012 |
| March, 2021 | 737/4235 (17·4%) | 1·71 (1·23–2·37) | 0·0013 | 1·47 (0·87–2·46) | 0·15 | 1·71 (1·23–2·36) | 0·0014 | 1·73 (1·22–2·43) | 0·0020 |
| Health sector | |||||||||
| Private | 19 763/105 409 (18·7%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Public | 31 274/113 856 (27·5%) | 1·99 (1·70–2·33) | <0·0001 | 1·50 (1·28–1·75) | <0·0001 | 1·99 (1·70–2·33) | <0·0001 | 1·28 (1·12–1·48) | 0·0005 |
| Province | |||||||||
| Western Cape | 9739/45 341 (21·5%) | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Eastern Cape | 9334/28 671 (32·6%) | 2·32 (1·79–3·01) | <0·0001 | 1·94 (1·51–2·49) | <0·0001 | 2·32 (1·79–3·01) | <0·0001 | 2·15 (1·71–2·70) | <0·0001 |
| Free State | 2627/11 759 (22·3%) | 1·14 (0·83–1·56) | 0·43 | 1·19 (0·89–1·59) | 0·24 | 1·14 (0·83–1·56) | 0·43 | 1·33 (1·01–1·74) | 0·044 |
| Gauteng | 11 938/59 548 (20·0%) | 0·76 (0·60–0·98) | 0·033 | 1·15 (0·90–1·47) | 0·28 | 0·76 (0·60–0·98) | 0·033 | 1·02 (0·82–1·26) | 0·88 |
| KwaZulu-Natal | 10 411/42 712 (24·4%) | 1·45 (1·13–1·86) | 0·0040 | 1·41 (1·11–1·80) | 0·0056 | 1·45 (1·13–1·86) | 0·0040 | 1·41 (1·13–1·75) | 0·0021 |
| Limpopo | 2431/8110 (30·0%) | 1·98 (1·44–2·72) | <0·0001 | 1·32 (0·95–1·82) | 0·095 | 1·98 (1·44–2·72) | <0·0001 | 1·72 (1·30–2·27) | 0·0001 |
| Mpumalanga | 2209/8287 (26·7%) | 2·27 (1·62–3·20) | <0·0001 | 1·43 (0·99–2·06) | 0·060 | 2·27 (1·62–3·20) | <0·0001 | 2·01 (1·50–2·71) | <0·0001 |
| North West | 1651/11 343 (14·6%) | 0·73 (0·49–1·09) | 0·12 | 1·18 (0·79–1·76) | 0·43 | 0·73 (0·49–1·09) | 0·12 | 0·91 (0·64–1·27) | 0·57 |
| Northern Cape | 697/3494 (19·9%) | 1·32 (0·84–2·08) | 0·23 | 1·43 (0·85–2·40) | 0·18 | 1·32 (0·84–2·08) | 0·23 | 1·44 (0·97–2·14) | 0·073 |
OR=odds ratio.
Chronic pulmonary disease and asthma variable excluded in the final multivariate model due to being not significant.
Figure 3.
Multivariable analysis of factors associated with COVID-19 in-hospital mortality
Dots represent adjusted odds ratios and error bars represent 95% CIs. See table 2 for 95% CI values.
A sensitivity analysis of risk factors for COVID-19 in-hospital mortality in the public and private sectors (appendix pp 10–11) confirmed increased association of COVID-19 in-hospital mortality with age, male sex, race, and comorbidities. Divergent findings in the health sector analysis included hypertension being associated with COVID-19 in-hospital mortality in the private but not the public sector; a stronger effect of age, sex, race, and comorbidities in the private sector; and a stronger effect of province and month of admission in the public sector, with a significant difference in COVID-19 in-hospital mortality observed only at the peak of the second wave in the private sector.
When adjusting for age, sex, race or ethnicity, health sector, province, month of admission, non-communicable diseases, and past or current tuberculosis, compared with HIV-negative individuals, people with HIV not on ART were more likely to die in hospital than those on ART; people with HIV with a history of immune suppression (CD4 count <200 cells per μL) were more likely to die in hospital than those with CD4 counts of 200 cells or more per μL; and people with HIV with a history of a viral load of 1000 copies per mL or more were more likely to die in hospital than those with a viral load of less than 1000 copies per mL (table 3 ).
Table 3.
Effect of ART, CD4 cell count, and HIV viral load on COVID-19 in-hospital mortality among people with HIV admitted to hospital with laboratory-confirmed SARS-CoV-2 infection, South Africa
| Case fatality ratio unimputed | Case fatality ratio (95% CI) imputed | Unadjusted OR (95% CI) imputed | p value | Adjusted OR (95% CI) imputed* | p value | Adjusted OR (95% CI) imputed* | p value | |
|---|---|---|---|---|---|---|---|---|
| ART status | ||||||||
| HIV negative | 30 697/137 986 (22·2%) | 23·1% (22·9–23·3) | 1 (ref) | .. | 1 (ref) | .. | 0·77 (0·72–0·82) | <0·0001 |
| HIV positive on ART | 2046/7484 (27·3%) | 24·6% (23·8–25·4) | 0·85 (0·80–0·90) | <0·0001 | 1·30 (1·22–1·39) | <0·0001 | 1 (ref) | .. |
| HIV positive not on ART | 192/594 (32·3%) | 28·1% (25·2–31·1) | 0·99 (0·86–1·15) | 0·98 | 1·89 (1·60–2·23) | <0·0001 | 1·45 (1·22–1·72) | <0·0001 |
| CD4 count | ||||||||
| HIV negative | 30 697/137 986 (22·2%) | 23·1% (22·9–23·3) | 1 (ref) | .. | 1 (ref) | .. | 1·06 (0·93–1·20) | 0·37 |
| HIV positive CD4 count, ≥200 cells per μL | 368/1690 (21·8%) | 19·7% (17·7–21·7) | 0·64 (0·57–0·73) | <0·0001 | 0·95 (0·83–1·08) | 0·37 | 1 (ref) | .. |
| HIV positive CD4 count, <200 cells per μL | 380/1080 (35·2%) | 32·2% (29·9–34·5) | 1·23 (1·09–1·38) | 0·0023 | 2·19 (1·92–2·49) | <0·0001 | 2·31 (1·82–2·93) | <0·0001 |
| Viral load | ||||||||
| HIV negative | 30 697/137 986 (22·2%) | 23·1% (22·9–23·3) | 1 (ref) | .. | 1 (ref) | .. | 0·83 (0·76–0·90) | 0·0002 |
| HIV positive viral load, <1000 HIV RNA copies per mL | 316/1273 (24·8%) | 24·7% (23·0–26·4) | 0·86 (0·78–0·94) | 0·0029 | 1·21 (1·11–1·32) | 0·0002 | 1 (ref) | .. |
| HIV positive viral load, ≥1000 HIV RNA copies per mL | 128/443 (28·9%) | 25·2% (21·0–29·4) | 0·85 (0·69–1·05) | 0·13 | 1·88 (1·53–2·31) | <0·0001 | 1·55 (1·20–2·01) | 0·0029 |
Model adjusted for age, sex, race or ethnicity, health sector, province, month of admission, non-communicable comorbidities, and past or current tuberculosis. ART=antiretroviral therapy. OR=odds ratio.
Output from the same model but with different reference categories to assess the effect of the predictors compared with HIV-uninfected individuals (model 1) or individuals on ART, with high CD4 count, or with low viral load (model 2).
Among people living with HIV, the prevalence of other comorbidities was 29·2% compared with 30·8% among HIV-uninfected individuals. In a multivariable model adjusting for age, sex, race or ethnicity, HIV (for the non-stratified model on HIV status only), health sector, province, and month of admission, there were increasing odds of COVID-19 in-hospital mortality for individuals with multiple non-HIV comorbidities, irrespective of HIV status (table 4 ). There was no statistical evidence of interaction between the presence of other multiple comorbidities and HIV infection status on COVID-19 in-hospital mortality (table 4). No interaction was found for individual comorbidities and HIV infection status on COVID-19 in-hospital mortality (appendix p 12).
Table 4.
Effect of single or multiple non-communicable comorbidities on COVID-19 in-hospital mortality among patients admitted to hospital with laboratory-confirmed SARS-CoV-2 infection, South Africa
| Case fatality ratio unimputed | Case fatality ratio (95% CI) imputed | Adjusted OR (95% CI) imputed* | p value | ||
|---|---|---|---|---|---|
| Any individual | |||||
| Comorbid condition | |||||
| No comorbidity | 2507/15 469 (16·2%) | 15·6% (15·3–15·9) | 1 (ref) | .. | |
| One comorbidity | 3764/14 402 (26·1%) | 24·1% (23·7–24·6) | 1·34 (1·28–1·39) | <0·0001 | |
| Two comorbidities | 3477/10 137 (34·3%) | 31·7% (31·2–32·2) | 1·66 (1·59–1·74) | <0·0001 | |
| Three or more comorbidities | 1353/3317 (40·8%) | 41·5% (40·7–42·3) | 2·40 (2·28–2·53) | <0·0001 | |
| HIV-negative individuals | |||||
| Comorbid condition | |||||
| No comorbidity | 2074/12 993 (16·0%) | 15·6% (15·3–15·9) | 1 (ref) | .. | |
| One comorbidity | 3277/12 586 (26·0%) | 24·3% (23·8–24·8) | 1·34 (1·28–1·40) | <0·0001 | |
| Two comorbidities | 3082/8962 (34·4%) | 32·0% (31·4–32·6) | 1·66 (1·58–1·74) | <0·0001 | |
| Three or more comorbidities | 1159/2858 (40·6%) | 41·5% (40·4–42·5) | 2·39 (2·24–2·54) | <0·0001 | |
| HIV-positive individuals | |||||
| Comorbid condition | |||||
| No comorbidity | 341/2073 (16·4%) | 15·6% (14·6–16·6) | 1 (ref) | .. | |
| One comorbidity | 384/1524 (25·2%) | 23·2% (21·9–24·5) | 1·34 (1·20–1·49) | <0·0001 | |
| Two comorbidities | 292/932 (31·3%) | 29·4% (27·7–31·0) | 1·67 (1·48–1·90) | <0·0001 | |
| Three or more comorbidities | 143/352 (40·6%) | 41·9% (40·0–43·8) | 2·46 (2·22–2·73) | <0·0001 | |
| Interaction effect of multiple comorbidities with HIV infection (HIV-negative is the reference group) | |||||
| Comorbid condition | |||||
| No comorbidity | NA | NA | 1 (ref) | .. | |
| One comorbidity | NA | NA | 1·00 (0·89–1·12) | 0·98 | |
| Two comorbidities | NA | NA | 1·01 (0·89–1·15) | 0·88 | |
| Three or more comorbidities | NA | NA | 1·03 (0·90–1·17) | 0·65 | |
Comorbid conditions included were a combination of hypertension, chronic cardiac or renal disease, diabetes, malignancy in the past 5 years, and past or current tuberculosis. NA=not applicable.
Model adjusted for age, sex, race or ethnicity, HIV (for the non-stratified model on HIV status only), health sector, province, and month of admission.
Discussion
Among a large cohort of patients admitted to hospital with SARS-CoV-2 infection in a high HIV and tuberculosis prevalence setting, although age was the strongest predictor of COVID-19 in-hospital mortality, we found that HIV and tuberculosis were associated with a moderately increased risk of COVID-19 in-hospital mortality, similar to the increased risk associated with other underlying conditions such as diabetes, chronic renal disease, and malignancy in the past 5 years.
This cohort included 13 793 people with HIV and 5282 patients with tuberculosis, and in addition 2312 patients co-infected with SARS-CoV-2, HIV, and tuberculosis. Describing mortality risk among these groups is important because studies have shown that among patients with SARS-CoV-2 infection, those who also had HIV and tuberculosis had altered T-cell functions and were at risk of more severe disease.28 Large population cohorts from the Western Cape province of South Africa18 and the UK19, 20 and more recent meta-analyses21, 22, 23 found people with HIV to have an increased risk of COVID-19 mortality, with increased in-hospital mortality reported in the Western Cape18 and UK19 studies. Our study, however, could not conclude that HIV is a risk factor at the population level.
We describe an increased risk of COVID-19 in-hospital mortality in people with HIV not on ART, with increasing HIV-associated immunosuppression, and with higher viral load, although missing data limited inference. Few studies have explored the effect of the association between ART status, increased immunosuppression, and viraemia on COVID-19 mortality among people with HIV. A Western Cape public sector study found no association with the presence of high viral load or immunosuppression, using latest CD4 cell count and viral load assessment within the past 18 months through linkage with laboratory information systems. This finding could be explained by the fact that our study used a larger dataset over a longer time period, including data from the private sector, and from other provinces with different levels of access to care and treatment and different HIV and tuberculosis burden.
The HIV prevalence in our study was 2·2% in the private sector and 20·4% in the public sector, due to socioeconomic differences in the populations served by each sector. The lower prevalence of HIV (11·7%) in individuals admitted to hospital with SARS-CoV-2 infection in our study, than in the population among adults (19%), is probably because of the over-representation of private sector COVID-19 admissions and possible under-reporting of comorbidities in the public sector.
The prevalence of other comorbidities, including tuberculosis, malignancy in the past 5 years, and chronic cardiac and renal disease, was high among people with HIV included in our analysis. This high prevalence could be due to antiretroviral drugs. For example, tenofovir disoproxil fumarate, which is part of the first-line ART regimen in South Africa, has new or worsening renal failure as one of its side-effects29 and other antiretroviral drugs have side-effects that include hyperlipidaemia, cardiac disease, diabetes, and liver disease.30 Additionally, because people with HIV who are on ART live longer, the risk of developing non-communicable diseases increases with age. HIV-positive people with immunosuppression are also more likely to develop tuberculosis and HIV-related malignancies.31 Increasing numbers of comorbidities were associated with increased risk of COVID-19 mortality, possibly because of poorer overall health status, more compromised immunity, and presence of chronic inflammatory state, which could create a pathway for severe COVID-19.31 The effect was similar in people with HIV and HIV-uninfected individuals.
Age was the strongest predictor of COVID-19 in-hospital mortality, as is reported in meta-analyses.32, 33 In our cohort, 67% of patients who died were younger than 70 years. A review of 27 countries showed a greater proportion of deaths in low-income and middle-income countries occur at younger age, with people younger than 70 years constituting 63% of deaths attributed to COVID-19 in low-income and middle-income countries on average, versus 13% in high-income countries.34 Lower recovery rates in middle-aged people are thought to be driven by high prevalence of pre-existing conditions in younger people in low-income and middle-income countries, and limited access to hospitals and intensive care.34, 35 In low-income and middle-income countries, younger people with comorbidities should also be prioritised for vaccination along with older people.
Although increasing age was the strongest predictor, male sex, non-White race, and chronic underlying illness were associated with increased COVID-19 in-hospital mortality in our study population, as reported in meta-analyses.2, 3, 5, 6 For non-communicable diseases, immune function impairment, severe hypoxaemia, inflammatory activation, and hypercoagulability might be contributory mechanisms for increased mortality.36 Increased mortality associated with non-White race could be related to burden of infection and prevalence of comorbidities in non-White races. Race as a potential proxy for poverty has been shown in other studies as an additional risk factor for higher COVID-19 in-hospital mortality7, 8, 9 and has been suggested to be related to structural discrimination towards marginalised populations, translating to inequities in the delivery of care and barriers to accessing care.7
The COVID-19 in-hospital mortality rate of 23·3% observed among patients in our analysis was at the upper bounds of the range of 15% and 24% reported in meta-analyses.2, 3, 4 Observed variation in case fatality ratios at different times during the epidemic and between regions and health sectors might be a result of population demographics and prevalence of comorbidities, varying population levels of COVID-19, changes in admission practices, the severity of illness in admitted cases, limited access to care, higher numbers of admissions overwhelming services, improved care and treatment options as the pandemic progressed, health services' effectiveness, and completeness of death reporting.8, 37, 38
The main strengths of this analysis are that DATCOV is nationally representative across all provinces, has 100% coverage of hospitals in the public and private health sector in South Africa, and contains close to 220 000 hospital admissions for SARS-CoV-2 infection.
There were several limitations related to the study population and data completeness. Patients with SARS-CoV-2 infection reported to DATCOV had COVID-19 symptoms, were admitted for isolation, developed nosocomial COVID-19, or tested positive for SARS-CoV-2 incidentally during admission for unrelated reasons. We were not able to exclude patients admitted for isolation and those with incidental findings because of incomplete data on reason for admission to DATCOV, and this might have introduced bias. Although probably a result of high pressure for hospital beds, such individuals would have made up a small proportion of included cases.
Hospitals reported a small number of deaths (219; appendix p 5) from causes other than COVID-19; however, we have little information on the process used by the hospital for classification of cause of death other than that it is done by the attending clinician. It is possible that other deaths reported to DATCOV have not undergone similar review and might have been misclassified as COVID-19 deaths. It is also possible that some COVID-19 admissions have not been reported to the surveillance system.
Patients who were transferred to other hospitals and had no further records of admission, and those still in hospital at the time of the analysis were excluded. We do not know whether the case fatality ratio among excluded patients would differ from that of included patients, possibly introducing biases. However, given the small number of the excluded patients (1·8%), the effect of biases would be limited.
Private-sector hospital admissions are over-represented because of a lower threshold for admission to private hospitals, resulting in similar proportions of patients with COVID-19 admitted to hospitals in the public and private sector even though they serve 84% and 16% of the population, respectively. This might explain why the HIV prevalence in the overall cohort (11·7%) was lower than the population prevalence of HIV in South Africa (14%).11 However, HIV prevalence among patients aged 20–59 years admitted to hospitals in the public sector was similar to the population HIV prevalence in this age group (appendix p 9).
Data quality in a surveillance system is dependent on the information submitted by health-care institutions. Data on comorbidities were submitted to DATCOV by the hospital based on information contained in the patient's written or electronic hospital record, and were not independently verified because there are no systematic information systems that would verify pre-existing comorbidities. We used multiple imputation to address missing data; however, the validity of the imputed data relies on the assumption that data were missing at random. Fields with the highest proportion of incomplete data included race (33·5%) and comorbidities (25·5–32·2%). The level of control of non-communicable diseases such as diabetes, using objective measures such as glycated haemoglobin, was not consistently reported. Other data have shown that individuals with poorly controlled non-communicable diseases were at greater risk of COVID-19 mortality.39
The most recent CD4 cell count and viral load results in the past year were submitted by the hospital based on information contained in the patients' hospital records and not obtained from laboratory information systems. These fields had a high degree of missing data, which is an important limitation given that this analysis focuses on outcomes in people with HIV. Moreover, the hospital did not record the date of the CD4 cell count and viral load tests, so we are not able to calculate the median time between the test and COVID-19 hospital admission, or to be certain that the status of immunosuppression or viraemia had not changed, which might also have introduced measurement bias. It must be noted that DATCOV is a new surveillance system that has not yet been developed to link to other data sources and allow, for example, linkage of laboratory records to the hospital record.
This study confirmed age as the strongest predictor of COVID-19 in-hospital mortality in South Africa, as well as sex, race, and comorbid disease. The demonstration of modest increases in COVID-19 in-hospital mortality for individuals with HIV and tuberculosis (particularly those who were not on ART) are important, given the high prevalence of these diseases in South Africa. People with HIV, particularly those with additional comorbidities, would benefit from COVID-19 prevention programmes such as vaccine prioritisation, as well as early referral and treatment programmes that include prioritising linkage and retention to HIV care, ART adherence, virological suppression, and subsequent immune restoration. The increased case fatality ratio in the public sector, in certain provinces, and during the peaks of the epidemic require further interrogation for resources and support to be directed where they are found to be required, ahead of a possible resurgence of cases.
For the DATCOV author group see https://www.nicd.ac.za/diseases-a-z-index/covid-19/surveillance-reports/daily-hospital-surveillance-datcov-report/
Data sharing
Data used in this manuscript are available upon reasonable request to the corresponding author. Individual participant data that underlie the results reported in this Article, after de-identification (text, tables, figures, and appendices), will be shared with investigators whose proposed use of the data has been approved by an independent review committee (learned intermediary) identified for this purpose. To gain access, data requestors will need to sign a data access agreement. The request will have to be approved by the South African National Department of Health.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
DATCOV, as a national surveillance system, is funded by the South African National Institute for Communicable Diseases (NICD) and the South African National Government. No additional funding was obtained towards the completion of this analysis and the development of this manuscript. We acknowledge the support and contribution from the following organisations: NICD, the South African National Department of Health, the nine provincial departments of health, the Hospital Association of Southern Africa, and the private hospital groups and public sector hospitals that submitted data to DATCOV. Health professionals who submitted data are acknowledged and are listed as the DATCOV author group. We also acknowledge the DATCOV team (Rebone Kai, Simphiwe Dyasi, Tracy Arendse, Beverley Cowper, Kholofelo Skhosana, Felicia Malomane, Monwabisi Blom, Akhona Mzoneli, Salaminah Mhlanga, Bracha Chiger, Busisiwe Ali, Siphamandla Mzobe, Linamandla Qekeleshe, Caroline Magongwa, Caroline Mudara, Lovelyn Nnaji, Richard Welch, Noel Mfongeh, Pinky Manana, Yongama Mangwane, Mami Mokgosana, Thobile Buthelezi, Portia Makwene, and Murray Dryden); the Partners in Performance team (Christo Greyling, David Spade, Winrie Kruger, Supriya Soorju, and Ryan Hudson); and the ComUnity team (Gareth Jane, David Prosser, Tina Hay, Kelello Moloi, and George Ulloa). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Contributors
DS, SG, and WJ contributed to the literature search. WJ, MM, and CC contributed to the study design and data collection. WJ, PM, and ST contributed to data analysis, and creation of tables and figures. WJ, ST, PM, and MM verified all the underlying data in the study. WJ, ST, CC, TK, SW, and LB contributed to data interpretation and writing. WJ drafted the manuscript and all other authors contributed scientific inputs equally to drafts of the manuscript. The corresponding author had final responsibility for the decision to submit for publication.
Contributor Information
DATCOV author group:
Shaina Abdullah, Fiona Abrahams, Vincentius Adams, Fhima Adnane, Sonia Adoni, Dieketso Melitta Adoons, Veronique Africa, D Aguinaga, Susan Akach, Prisha Alakram Khelawon, George Aldrich, Olatunde Alesinloye, Mathale Biniki Aletta, Mametja Alice, Tebogo Aphane, Moherndran Archary, Felicity Arends, Shireen Arends, Munonde Aser, T Asmal, Mohammed Asvat, Theunis Avenant, Muvhali Avhazwivhoni, Magnolia Azuike, Johanna Baartman, Dlava Babalwa, Johan Badenhorst, Miranda Badenhorst, Bianca Badripersad, Lalihla Badul, M Bagananeng, Mncedisi Bahle, Liezl Balfour, Liezl Balfour, T C Baloyi, S Baloyi, Tinyiko Baloyi, Tshepo Mpho Baloyi, Thokozani Banda, Shimon Barit, Nicole Bartsch, Junaid Bayat, Siyabulela Bazana, Marlene Beetge, Marlene Beetge, Nosindiso Bekapezulu, Rammala Belebele, Phala Bella, Zanenkululeko Belot, Lindi Gladys Bembe, Sonja Bensch, Gishma Beukes, Karla Bezuidenhout, Themba Bhembe, N A Bikisha, Ben Bilenge, Leesa Bishop, Baphamandla Biyela, Cyntheola Blaauw, Mark Blaylock, Nicola Bodley, Power Bogale, Sibongile Bokolo, Stefan Bolon, Mary Booysen, Eldereze Booysen, Lia Boretti, Paula Borges, Millicent Boshoga, Natasha Bosman, Lucinda Bosvark, Nicky Botes, Adele Botha, Chantall Botha, Jana Botha, Chantall botha, Mandlakayise Irvin Botha, Alet Botha, Janet Bradbury, Zandisile Breakfast, Maria Breed, Molele Brenda, Moshito Brice, Jolene Britz, Amanda Brown, T Buchanan, Thozama Bucwa, Crystelle Burger, Ziyanda Busakwe, Nosiviwe Bushula, Zinhle Buthelezi, Dumsile Buthelezi, Thubelihle Buthelezi, Mpumelelo Basil Buthelezi, Fundiswa Lidwina Buthelezi, Nadia Bux, Christoff Buys, Anneline Buys, Ernestina Caka, Armando Sanchez Canal, Sithole Caroline, Monrick Casper, Shannon Cawood, Oratile Cebisa, Nothando Cele, Sboniso Cele, Sthembile Goodness Cele, Mkhacani Chauke, Pinkie Chauke, Nevil Chelin, Xiaohui Chen, Venmalla Chetty, Kerisse Chetty, Christinah Cheu, Vindana Chibabhai, Takudzwa Chirima, Mantwa ChisaleMabotja, Charity Chivenge, Ngoasheng Choene, Mbali Nosisa Choko, Martin Choshi, Sabbir Chowdhury, Anastacia Christoforou, S L S Chuene, T S Chueu, Dale Cilliers, Vanessa Cilliers, Marcel Claassen, Jeané Cloete, Chantelle Coelho, Chantelle Coelho, Carol Coetzee, Hans Jurgens Coetzee, Christine Coetzee, Marelize Coetzee, Dane Coetzer, Sizwe Coka, M Colane, Herkulaas Combrink, Songezo Conjwa, Colleen Contrad, Faith Cornelissen, Leezelle Cronje, Christine Crouse, Tshidi Dabi, Ziyanda Dandala, Ziyaad Dangor, Gildenhuys Daniel, Ngwana Daniel, Alfred Daumas, Madelein Dauth, Mongalo David, Wayne Davids, Nozuko Daweti, Halima Dawood, Wandisa Dayile, B De Bruin, Karin De Klerk, Tanya De la Rosa, Marice de Nysschen, Marie De vos, Darien De Wet, Mohith Debising, Darshan Deenadayalu, Babalwa Dekeda, Mofokeng Desiree, Annelise Deysel, Abram Dhlamini, Makgethwa Dhlala Diala, Mathapelo Diale, Bella Diketane, Nosisa Dingani, Siyabonga Diniso, Lesego Diphatse, Anele Diya, Zihloniphile Dladla, Nompumelelo Dladla, Mlungisi Dladla, Patience Dladla, Baphilie Dlamini, Nonhlanhla Dlamini, Linda Dlamini, Nonzwakazi Dlamini, Wendy Dlamini, Ncomeka Dlamini, Siyabonga Dlamini, Nicodemus Dlamini, Lebohang Dlamini, Motshedise Dlamini, Babalwa Christine Dlava, Phikiwe Dlova, Lindiwe Dlozi, Maenetja Doreen, Vumile Doyi, Athini Doyi, Belinda Du Plessis, Johanna Aletta du Plessis, Eddie du Plessis, Nicolette du Plessis, Karin du Plessis, Briette du Toit, Narissa du Toit, Jabulile Dube, Athayanda Dubula, Msomi Duduzile, Sechaba Duiker, Unati Bongile Duma, Kholiwe Duma, Kella Dunne, Kholeka Dyantyi, Avile Dyantyi, Simphiwe Dyasi, Chauke Dyondzo, Phelisa Dyubhele, B J Dywili, Letitia Edwards, Madie Eksteen, Tersia Ellis, Tia Ellis, Glenda Emmerson, Theusia Enslin, Odimula Epule, Lana Erasmus, Mathonsi Erick, Lerato Etsane, Shimange Eunice, Zanele Fani, Mariette Ferreira, K L Finger-Motsepe, Fabion Floris, Tseko Fobo, Keresemetse Fokotsane, Duduzile Emmelda Fokwana, Genevieve Marion Fords, Juanita Fortein, Christine Fouche, Rulandi Fourie, Andrew Frean, Ludwig Fredericks, Wandile Funda, kabelo funjwa, Martha Futhane, Amanda Futuse, Dora Gabaediwe, Nonhlanhla Gabuza, Janycke Galant, Zanele Gama, Thobile Gano, Emma Cora Gardiner, Henri Gastrow, Kelly Gate, Ben Gaunt, Rikhotso Gavaza, Thapelo Gayi, Nkosinathi Gcakasi, Nomusa Gcobo, Leon Geffen, S Geldenhuys, Jenny George, Martha Gerber, Zolisa Getyengana, Nkululo Gigi, Radha Gihwala, Mitchell Gilliland, Zandile Gloria, Elitia Glover, Ellen Gokailemang, Suseth Goosen, Maria Gopane, Thandazile Gosa-Lufuta, Bernadett Gosnell, Sharleen Gouws, Christina Govender, Raksha Govender, Pearl Govender, Sally Govender, Christina Govender, Roxanne Govender, K Govender, Savie Govender, Rashika Govinden, Luphumlo Gqabuza, Nomthandazo Gqaji, Maneo Gqetywa, Caroline Green, Nathan Green, Neera Green, Hendrik Grobler, Pamela Groenwald, Daniel Grootboom, Beatrice Gumede, Nomonde Gumede, Simphiwe Gumede, Simphiwe Gumede, Slindile Gumede, Ntombikayise Gumede, Zenande Gumede, Thandiswa Gxotiwe, Makhubela H L, Nonhlanhla Hadebe, Skhumbuzo Hadebe, Christos Halkas, Ansie Hamer, Ebrahim Hamida, Juan Hammond, Sumayia Haniff, Annelise Hare, lorinda Hattingh, Thenjiwe Hendricks, Philip-George Henecke, Brends Henly-Smith, Glynis Herselman, Ansie Heymans, Chantel Heyns, Golekane Hlabahlaba, Lucky Hlabangwane, Simango Hlamarisa, Ntokozo Hlanzi, Hlengiwe Hlela, Katlego Hlokwe, Thembinkosi Hlongwa, Anele Hlongwana, Themba Hlubi, Tozama Hobo, Nare Nathaniel Hopane, Mariska House, Catharina Hudson, Marinda Huysamen, Jezreen Indheren, Samantha Ingle, Gavin Isaacs, T S Thekiso Isaacs, Maringa Itumeleng, Karien J van Rensburg, Saloshni Jackson, Neziswa Jacob, Burton Jacobs, Tshireletso Jacobs, Gugulethu Jacobs, Mesadi Jaftha, Zimkhitha Jaji, Sibusiso Jali, Gcobisa James, Gillian January, Andiswa Jeke, Laurent Jeremiah, L S Jeremiah, Mubeen Jhetam, Maureen John, Chuene John, Thandiwe Jola, Yolande Jonas, Anovick Jonas, Amilcar Juggernath, Eileen Kaba, Venetia Kabo, Disebo Kadi, Karabo Kaizer, Moshaya Peter Kambule, Lorraine Kapp, Tshepo Kau, Nchabeleng Keneth, O Kgabi, Tebogo Audrey Kgafela, Vincent Kgakgadi, Isabella Kgaswe, Tsholofelo Kgathlane, Vuyelwa Julia Kgetha, Mmaselloane Kgomojoo, B Kgoro, Christinah Kgosiemang, Gloria Kgosiencho, Stephen Khambula, Ariffa Khan, Refemetswe Khanare, Ncamsile Khanyase, Nokwethemba Khanyile, Fillip Kharatsi, Simangele Khawula, Themba Khohlakala, Letitia Khomo, Isabel Khoza, Sinethemba Khoza, Nombulelo Khukule, Busisiwe Khumalo, Tracy Khumalo, Zinhle khumalo, Vuyelwa Khumalo, Delisile Khumalo, Lebohang Khumalo, Boitumelo Khumalo, Thuli Khumalo, Gugu Khumalo, Bongiwe Khuzwayo, Thembhelihle Khuzwayo, Hennie Kidson, Jesne Kistan, Gugu Klaas, Marilyn Klassen, Josehine Koeberg, Marizel Koen, Simphiwe Koena, Ina Kok, Imraan Kola, Karabo Kolokoto, Ramachandra Konar, Dr Kotsedi, Jaline Kotze, Martins Koupis, Helen Kritzinger, Marlize Kruger, Henk Kruger, Tlangelani Kubayi, Thabisile Kubeka, Nonjabulo Kubheka, Melusi Kubheka, Sibusiso Clifford Kubheka, Erol Kubheka, Monica Kumalo, Thulani Kunene, Siphilile Candy Kunene, Yvette Kunneke, R P Kupa, Rachel Kutama, Nompumelelo Kwakwazi, Lwanele Kweyama, Maureen Labuschagne, Marina Labuschagne, Prabha Lakshman, Prabha Lakshman, Lungelo Lamani, Thembela Lamani, Naomi Langa, Khangelani Langeni, Aphelele Langeni, Nwabisa Hazel Langeni, Gena Langeveldt, Anchen Laubscher, Laetitia Le Roux, Magagane Leah, Collen Lebea, Sello Lebea, Viyella Phumla Cynthia Lebenya, Lorraine Lebogang, P K Leboho, Chantel Lee, Kelebogile Rejoice Lefakane, Zandile Legoabe, Patrick Lekala, Motsitsi Lekhoaba, Tanki Shadrack Lekunutu, Galaletsang Lerefolo, N Letebele, Tsepo Patric Lethoba, Emission Letlalo, Ofentse Letlhage, D S V Letshufi, Dineo Fiona Letsoalo, Seleka Jones Letsoalo, Pennelope Letsoalo, Getrude Letwaba, Sobekwa Linda, Katleho Lipholo, Sabata Litabe, Harsha Lochan, Linda Lomax, Francina Lombaard, Elmarie Loots, Ariana Lourens, Ariana Lourens, Celeste Louw, Rianna Louw, Zikhona Lubambo, Msebenzi Moises Lubambo, Gregory Ludada, Michael Lukas, Thembela Lungu, Nomvume Lupindo, Emmah Lusenga, Happiness Luthuli, Zoleka Sylvia Luvuno, Gwangwa M H, Mustafa Maarman, Buyisiwe Mabaso, Cynthia Mabaso, Morena Mabitle, Grace Mabogoane, Kgakgamatso Mabone, Rueben Mabuza, Velaphi Mabuza, Mogantla Madiseng, Thobile Madlala, Mashooase Madolo, Thabiso Madonsela, Lesetsa Madubanya, Amukelani Maepa, Namhla Mafumana, Caroline Mafumo, Pumeza Magadla, Viscah Magale, Nompumelelo Magaqa, Oberholzer Magda, Rakgoale Magdeline, Tswai Maggie, Bongeka Maginxa, Cathrine Maite Magoba, Caroline Magongwa, Agretia Magubane, Agretia Ntombizodwa Magubane, R Magwai, D I Mahabane, Padmini Mahabeer, Elsie Mahadulula, Lungiswa Mahanjana, Amy Maharaj, Qedusiza Mahlambi, Yvonne Mahlangu, Lerato Mahlangu, Ntombifikile Mahlangu, Makhosazana Mahlangu, Mahlatsi Mahlangu, Penelope Mahlasela, Thosago Mahlatse, Regina Mahlobo, Dikhing Mahole, Adam Mahomed, Mapeu Debora Mahubane, Peter Mahume, Lehlogonolo Maifo, Vincent Maimane, Petunia Maimele, Phakoe Maine, Patricia Senyanyathi Mainongwane, Nomalungisa Majamani, Amahle Majozini, Noluthando Makalima, Nomfundo Makam, Khanyisa Makamba, R Makan, Mashiane Makarapa, Malesela Makgahlela, Mogoiwa David Makgisa, Makgoba Makgomo, M A Makgopa, Mabone Makhalema, Lindokuhle Lizo Makhanya, Philile Valentia Makhanya, Tolerance Makharaedzha, Nathi Makhathini, Elizabeth Makhesi, Cinile Makhubela, Nkululeko Freedom Makhunga, Nomalinge Makhupula, R R Makhura, Rangwato Makola, Zingisa Makuba, Asanda Makubalo, Lonwabo Makumsha, George Makuya, Levy Mmachuene Malaka, Themba Malangeni, M L Malatji, Pelonomi Malebana-Metsing, Malek Malek, Luthando Malevu, Luthando Malevu, Juanita Malgas, Dimakatso Malgas, Paul Makgasane Malope, Monyeki Malose, Katekani Maluleke, Kato Mambane, Nthabiseng Mamorobela, Kukami Manamela, Tshepo Manana, Sathiel Maneto, Aron Kabelo Manganye, Pheto Mangena, Anna Mangoale, Tinotenda Florence Mangozho, Pariva Manickchund, Zandisile Mankayi, Arthur Manning, Kelebogile Manyaapelo Manyaapelo, Tabea Manyane, Zoliswa Manzana, Milton Manzini, Busisiwe Mapasa-Dube, Siboniso Maphumulo, Ntombifuthi Maphumulo, Sindy Maponya, Khomotso Mumsy Maponya, Napjadi Maponya, Lami Maqubela, Lizeka Maqubela, Vuyo Maqungo, Marisa Marais, Chantal Marais, Nondumiso Maramba, Annelize Mare, Madumetsa Maredi, Afikile Martins, Johanna Marule, Refilwe Marumo, N N Masakona, Kedibone Vincentia Masehla, Eric Maseko, Tshilidzi Maselesele, Mojalefa Maselo, M Maseloa, M E Masemola, Thembi Masemola, Bella Mashaba, James Mashangwane, Mantebele Mashao, Shalom Mashego, Lerato Mashele, Ester Mashiane, Joyce Mashibini, J Mashilo, Tumi Mashiloane, Charity Mashishi, Ngazibini Mashiyi, Khomola Mashudu, Aluwani Masindi, Caroline Maslo, Nduduzo Masondo, Dumisile Masuku, Cry Matamela, Mirriam Matandela, Nontokozo Mathabela, T Mathabi, Keitumetse Mathe, Mathabo Mathebula, Catherine Mathebula, Mdungazi Andres Mathebula, Nqobizwe Mathenjwa, Jane Mathibe, Lebohang Mathibela, Makwela Mathilda, Khakhu Mathiva, Mokgadi Alinah Mathobela, Fikile Pearl Mathonsi, K P Mathonsi, Katlego Mathosa, Noluvo Matiwane, Emma Matjeke, Bella Matjiane, Thabang Matjila, Chidi Matlala, Petlo Matome, Nolusindiso Matoti, C Matseliso, Dineo Matsemela, Phumeza Matsha, Gaalebale Prudence Matshediso, Motsumi Matshediso, Esther Matshela, Bongeka Mavuma, Pearl Mavundla, Nomthandazo Mavuso, Lovender Mawasha, Rebecca Mawelela, Nelisiwe Mazibuko, Phumlani Mazibuko, Lindiwe Mazubane, Bavumile Mbanjwa, Ayanda Mbasa, Nosimilo Mbatha, Zanele Mbatha, Rudolph Zenzele Mbatha, Gift Mbedzi, Tatenda Trevor Mbizi, Khumbulani Mbonambi, Nondumiso Mboniswa, Nomfanelo Mbonisweni, Jody Mbuilu, Siyabonga Mbulawa, Zama Mbutho, Natasha Mbuzi, Nonkululeko Mchunu, Cyprian Mchunu, Nokuzola Mchunu, Masesi Thandeka Mchunu, Vuyokazi Mciteka, Solly Mdaka, Neho Mdakane, Siyabonga Mdediswa, Melusi Mdima, Nozipho Mdima Masondo, Siviwe Mdindana, Ntombizikhona Mdleleni, Sibusiso Mdletshe, Gcobisa Precious Mdoda, Ntombi Mdolo, Anele Mdontsane, Ruchikas Mehta, Philile Rittah Memela, Masande Methuse, Keatlaretse Metshile, Pheliswa Metuse, Anton Meyer, Gavin Meyer, Cameron Meyer, Sisonke Mfazwe, Andiswa Mfecane, Bongeka Mfecane, Nelisiwe Mfeka, Busisiwe Mgaga, Thandiwe Portia Mgauli, Thembekile Mgedezi, Vuyokazi Mgedezi, Kalipile Mgevane, Bongni Mgiba, Babalwa Mgoduka, Patrick Mhlaba, Zeldah Mhlaba, Ntombizodwa Mhlanga, Vangile Mhlinza, Nokuthula Mhlongo, sibongiseni Mhlongo, Unamandla Mhlotshana, Mabaso Mikateko, Helena Minnie, Karen Mintoor, Bongi Miyeni, Mabelane M J, Rosy Mjethu, Gloria Mkhize, Mvuselelo Mkhize, Ntokozo Siyabonga Mkhize, Victoria Mkhize, Nomkhosi Mkhize, Nokuthula Mkhize, Mathini Mkhwanazi, Nolwandle Mkile, Kholofelo Mkise, Nokwandiso Mkiya, Pearl Mkongi, Pearl Mkongi, Mnonopheli Mkungeka, Hlomile Mlahleki, Nolukholo Mlibali, Sakhumzi Mlungwana, Jonas Mmachele, Mashatole Mmateka, Molebatsi Mmokwa, Thembisa Mmutlane, Zanele Olive Mndebele, Nonhlanhla Mngomezulu, Noluthando Millicent Mnguni, Pumza Mngunyana, Nomxolisi Mngunyana, Ntombebongo Mngxekeza, Zenzele Mnisi, Hlengiwe Precious Mnqayi, Phumzile Mnqayi, Thabiso Mntungwa, Siya Mnyaka, Ntombikayise Mnyakeni, Vuyani Mnyamana, Nomzingisi Mnyipika, Koena Moabelo, Mmakgoshi Alseria Moatshe, Jennifer Mochaki-Senoge, Sharon Moche, Tebello Mocwagae, Koeikantse Modibane, Tebogo godfrey Modimoeng, Obakeng Modisa, Itumeleng Modisane, Olebogeng Modise, Makaepeaa Flovia Modjadji, Sharon Modupe, Maja Moeketsi, Ntswaki Moeketsi, Kereditse Kingsley Moeng, Naledi Nthabiseng Mofamere, Samuel Mofokeng, Thabo Mofokeng, Jonas Mofomme, Vicky Mogakane, Lehlohonolo Mogale, Audrey Mogapi, Thomas Mogashoa, Mphaka James Mogatla, Kgaladi Mogoale, Dikeledi Maggie Mohajane, Nkuba Mohapi, Mthoamihla Mohatsela, Irene Mohlala, Daphney Mohlala, Mpho Mohlamonyane, Bonolo Millord Mohutsiwa, Selemela Moipone, Tshepang Moisi, Nelly Mojalefa, Vuyo Moji, Buhle Mokangwana, Matloa Mokgabo, Manaka Mokgaetji, Jane Mokgaotsi, Neo Theodore Mokgoro, Thalitha Mokhatla, Lerato Lovedalia Mokhele, Sheila Mokhema, Mamoya Mokoena, Mojalefa Mokoena, Lleka Mokome, Cynthia Mokone, Ipeleng Mokono, Thabiso Mokonyama, Josiah Mokori, Dolores Mokuena, Danny Mokumo, Oddy Mokwena, Kgaogelo Mokwena, Kgantshi Sam Mokwena, Lebogang Mokwene, Thato Elliott Molate, Ditoche Molebalwa, Boingotlo Molefe, Kgopa Stanley Molehe, Kgomotso Moleme, Sarah Moliane, Fanyana Moloi, Retshepile Joseph Molorane, Glenda Tsholanang Molotsi, Lerato Molukanele, Joy Monareng, Thapelo Moncho, Modiadie Monica, Refilwe Monnane, Andile Monqo, Neo Montewa, Kgalalelo Montsioa, Reitumetse Monyaki, Masekhobe Jeanett Monyane, Lipson Monyela, Yudeshan Moodley, Kriesen Moodley, Kaira Moodley, Boitumelo Donald Mooka, Prea Moonsamy, Simmi Moopanar, David Moore, Lineo Mophethe, Tshegohatso Moremedi, Kealeboga Moremong, Nthangeni Morgan, Egma Moripa, Lulamile Morris, Me. A.M. Mosala, Thabo Mosana, Alice Mosase, Yolanda Mose, Maponya Mosehlo, Mothusi Moseki, Mojalefa David Moshabe, D A Moshai, Mbulelo Moshani, Pelisa Moshani, Ledwaba Mosima, Ezrom Mosima, M P Mosoma, Lebohang Motaung, Mokete Motaung, Thozama Charmain Motaung Xhama, Purine Khethiwe Motha, Lerato Motimele, Boitumelo Motimeng, Shirley Motladiile, Otsile Motlhabane, Joshua Motlhamme, Mandla Motloba, Kagiso Motse, Sophia Motshegoa, Edward Moutlana, Irma Mouton, Zanele Moya, Nomonde Moyake, Maja M P, Jenny Mpete, Luamba Meltha Mpfuni, Seputule Mphahlele Mphahlele, Mashadi Mphake, Ephraim Letlhogonolo Mphanya, Mashudu Mphaphuli, Tebogo Chwene Mphela, MS Mpontshane, Thabile Mqotyana, Babalwa Mqungquthu, Noluthando Busane Msane, Malusi Mseleku, Sibusiso Msibi, Mancele Msibi, Thulisile Msibi, Siyabonga Linda Msibi, Clement Nhlanhla Msiza, Lungelo Msomi, Mandlenkosi Mtatambi, Thembisa Mthathambi, December Mthembu, Nhlahla Mthembu, Fezile Mbali Mthembu, Lungiswa Mthembu, Nompumelelo Petunia Mthethwa, Khulekani Mthimkhulu, Lungani Percival Mthuli, Ashley Mthunzi, Xolani Sydney Mtolo, Nomonde Precious Mtolo, Linda Mtshali, Neliswa Mtwa, Fezeka Mtyobile, Kanyisa Mtyobile, Mpfariseni Mudau, Magwabeni Muemeleli, Isaac Mulaudzi, Rebecca Mulaudzi, Mhlelekedzeni Mulaudzi, Dakalo Rejoyce Muligwe, Blessing Muponda, Mmbangiseni Stella Mushadi, M Mushid, Konanani Muthaphuli, J Muthavhine, Mpho Muthika, Samkelisiwe Mvelase, Vusi Mvelase, Laurent Kayumba Mwehu, Thabile Myaka, Magriet myburgh, Zimkhitha Mzamo, Fezeka Mzawuziwa, Mfundo Lunga Mzini, Oscar Mzizana, Ntokozo Mzobe, Thokozile Mzobe, Zamaswazi Mzobe, Mtimkulu Mzwandile, Fathima Naby, Keshnee Naicker, Pregashnie Naicker, Saroja Naicker, Pershen Naicker, Saiyen Virgil Naicker, Ria Naidoo, Sam Naidoo, Mergan Naidoo, Kamalambal Naidoo, Aroomugam Naidoo, Sivuyile Naku, Firdose Nakwa, Masoga Nancy, Rita Nathan, Maritsa Naude, Gcobisa Ncaza, Aviwe Ncaza, Relebohile Ncha, Yanelisa Ncoyini, Snothile Ncube, Mrs Ndaba, Vusumuzi Ndaba, Mmapula Ndaba, Siziwe Ndawonde, Ziphozihle Ndevu, Nonhlanhla Faith Ndhlovu, Simphiwe Ndima, Sindisiwe Ndlela, Thobsile P Ndlela, Nobuhle Ndlovu, Nwabisa Ndlovu, Virginia Dipuo Ndlovu, Sombekhaya Ndlumbini, Khululiwe Nduli, Priscilla Nontokozo Nduli, Michael Ndwambi, Jeremy Nel, Rina Nel, Lizelle Nel, Ntsundeni florah Nemanashi, Usinkhangwe Nyaphophi Nemudivhiso, Joyce Nemutavhanani Nemutavhanani, Jabu Nene, Xolani Nene, David Netshilonga, Rendani Netsianda, Charmaine Newton, Vuyo Leroy Ngalo, Ncumisa Ngani, Thabisa Monica Ngcakaza, Thamela Ngcobo, Trulove Nonhlanhla Ngcobo, Richards Ngcobo, Gcinile Ngcobo, Guguletu Ngcobo, Thozama Ngetu, Pinkie Ngewu, Tshepo Ngobeni, Providence Ngobeni, Khanyisile Ngobeni, Prudence Ngobeni, Thembisile Ngobese, Tracy Ngomane, Nolusindiso Ngondo, Nokukhanya Ngubane, Sithembiso Ngubane, Ntombizodwa Praxedise Nguse, Tholakele Ngwane, Elizabeth Ngwasheng, Siphamandla Ngwenya, Gugu Ngwenya, Nomthandazo Ngwenya, Themba Ngwenya, Eva Ngwenya, Zintlanu Ngxola, Tshegofatso Nhabe, Jabulile Nhlabathi, Ishmael Nhlangwana, Sithembile Nhlapo, Matlala Nick, Vicky Niemand, Carina Nienaber, Louise Nix, Chumisa Njikelana, Masiza Njomi, Lucia Nkabinde, M NKABINDE, Boitumelo Nkabiti, Gugu Nkabule, Mankopodi Nkadimeng, Nonkanyiso Nkanjeni, Palesa Portia Nkatlo, Bongani Nkewana, Audrey Nkhwashu, Ngokoana Nkoana, Mmathapelo Nkoane, M Nkogatse, Fezile Nkomo, Ntando Nkomo, Nontobeko Nkonyane, Sydney Nkosi, Ntombikayise Nkosi, Phumzile Nkosi, Ntombifuthi Nkosi, TINTSWALO NKOSI, ML Nkosi, Godfrey Nkosi, Amukelani Nkosi, Fikile Vinoliah Nkosi, Mbali Nkosi, Nomcebo Lucia Nkosi, Siphokazi Nkosi, Amanda Nkuhlu, Phumzile Nkumane, Malebo Nkuna, Wendy Nkwakwha, Sesi Noge, Elizabeth Nolte, Peko Nomawabo, Malibongwe Nombita, Nandipha Nophale, Jeanetta Nothnagel, Bongiwe Novokoza, Zanele Nqaphi, Thobekile Nqondo, Siphokazi Nqwelo, Nkoana N S, Sindiswa Ntabeni, Mr Ntabeni, mawethu Ntampula, Mthutuzeli Ntebe, Mokwabo Ntela, Hezekiah Ntimbane, Xolisa Ntintsilana, Patrick Ntleki, Zanele Ntobela, Bandile Ntombela, Bandile Ntombela, Zamaswazi Ntombela, Khonelihle Zandile Ntombela, Praisegod Samkelo Thobani Ntombela, Lindiwe Ntonintshi, Dipuo Ntseane, Thobeka Ntseane, Xolelwa Ntsham, Mbalenhle Ntshele, Amanda Ntshewula, Zinzi Ntsoko, Athini Ntsoto, Nomsa Ntuli, Nokwazi Ntuli, Nomvula Ntuli, Andrew Diffar Ntuli, Faith Ntuli, Margrit Nurnberger, Ntsikelelo Nxala, Sithandiwe Nxasane, Thanda Nxumalo, Xolani Nyathi, Nontobeko Nyawula, Nhlakanipho Nzama, Maila Nkuneng Obed, Florence Ogwal, Maureen Olifant, B Oliphant, Monota Olive, Kagisho Olyn, Raymond Omoighe, Phumeza One, Ratombo Oscar, Nkuna Owen, Mailula P, Nalini Padayachee, Vasaily Padayachy, Ntombizakhe Pakade, Mosiuoa Palime, Jane Palisa, Arifa Parker, Lesenyeho Parkies, Andy Parrish, Nilesh Patel, Anastasia Pather, Mkhombo Tsakani Patience, Marisa Patzke, Akhumzi Pawuli, Ntandokazi Pelako, Phaswana Sibasa Penrose, Litha Peppeta, Santosh Pershad, Makheda Pertunia, Nkuna Pertunia, Dane Perumal, Mongameli Peter, Justin Peters, Vatiswa Petlane, Harideen Petrus, Kgomotso Phahladira, Matebesi John Phakisa, R Phale, Livhuwani Phathela, Sekate Daniel Phillip, Beverly Phiri, Mapule Precious Phiri, Thapelo Phokane, Frank Phokoane, Moele Pholosho, Sekoro Phooko, Sekodi Geoffrey Phooko, Maponya Phutiane, Faiza Pillay, Melanie Pillay, Sayuri Pillay, C R Pillay, Zikhona Plaatjie, James Pootona, Samantha Potgieter, Marius Potgieter, Mulaudzi Mulatedzi Precious, Paul Janus Pretorius, Hans Prozesky, Mokhethi Pule, Jayshina Punwasi, Dot Putzier, Lutho Qankqiso, Siphokazi Qebedu, Phozisa Qhola, Ntombesithathu Qotoyi, Sipho Victor Qotso, Zanele Qwabe, Helena Rabie, Phoebe Rabothata, Christina Rachoene, Mteteleli Radana, Maria Radebe, Dr. Valentino Radebe, Nonkululeko Radebe, Ella Radinne, Sherly Raduvha, Shamintha Raghunath, Claudine Rajagopaul, Mary Rakgwale, Malumbete Michael Ralethe, Kenneth Ralimo, Motlalepule Ramafoko, Maduvhahafani Ramagoma, Charlotte Raman, Dr Ramavhuya, Molly Rambally, Nivasha Ramdeen, Tanusha Ramdin, Sharita Rameshwarnath, Yeishna Ramkillawan, Ramotlou, Faith Rampedi, Vijayluxmi Rampersad, Avhashoni Ramuima, Noluthando Ranone, Mabohlale Portia Rapasa, Mpharoane rapelang, Nika Raphaely, Lesiba Rashokeng, Caroline Rashopola, Tebogo Ratau, M Ratau, Mpfariseni David Ratshili, Elmari Rautenbach, Rofhiwa Ravele, Johannes Reachable, Peta Mmalahla Rebecca, Kessendri Reddy, Andrew Redfern, Robertha Reed, Mumsy Rees, Dr Reji, Gary Reubenson, Veena Rewthinarain, Paul Rheeder, Nkonayani Rhulani, Mufamadi Richard, J S Rikhotso, Shatimone Beverley Rikhotso, Lavhelani Ndivhaleni Robert, Noncedo Roto, Gideon Ruder, Kapil Rugnath, Lizette Ruiters, Mina Ruiters, Sue Russell, Lynn Ruwiza, Molokoane R Y, Mandy Saaiman, Emmanuel Sabela, Lerato Sadiq, Litha Saki, Hyppolite Salambwa, Menitha Samjowan, Nazlee Samodien, Rakgolele Samuel, Fakudze Sandile, Cekuse Sanelisiwe, Mandlankosi Sani, Simangele Sawuka, Lelani Schoeman, Magriet Scholts, Ronel Schroder, Mamotetekoane Sebalabala, Selwalenkwe Collet Sebati, Jacoline Seboko, Wilheminah Sebuthoma, Annah Segami, Ruth Segokotlo, MR Sehloho, Khutjo Seisa, Antony Sekgobela, Monica Sekhosana, John Sekonyela, Mpho Sekoto, Naledi Sekulisa, Mokgadi Vanessa Sekwadi, Lebogo Selaelo, Johannes Selatlha, Kgomotso Selekolo, William Selfridge, Lucy Semenya, Ivy Sengakane, Masabata Sengata, Petronella Sentle, Malebo Seoketsa, Pratheesha Seonandan, Thomas Mambushi Serumula, Nkululeko Setheni, Refiloe Setlale, Tumediso Setlhodi, Barbara Setlhodi, Robert Setloghele, Aarthi Sewpersad, Ryan Sewpersadh, Phumlile Shabalala, Owen Shabangu, Kungesihe Shabangu, Harriet Sbonangaye Shabangu, Thokozani Shabangu, Clifford Shadi, Hasifa Shaik, Tseliso Shale, Qedani Shandu, Nomvelo Shandu, Ntswaki Marcia Shange, Abongile Shenxane, A Sherriff, Sebenzile Shezi, Thenjiwe Shezi, Scally Shihangule, Cheyeza Shikwambana, Lungisani Shoba, Kamogelo shokane, Nora Sibande, Lydia Sibeko, Xolani Sibeko, Zanele Sibiya, Mncedisi Sibiya, Sphamandla Sibuta, Thembakazi Sifumba, Sipho Sigcau, Lutho Sigila, Kayakazi Sihentshe, Bongani Sihlangu, Daisy Sikhakhane, Shaun Nhlanhla Sikhakhane, Mbali Siko, Sipho Sikonje, Khumbulekile Simanga, Nomsa Simango, Thulisile Simela, Ntombikayise Simelane, Sashah Singh, Marjorie Singh, Ragani Singh, Shash Singh, Anita Singh, Hitekani Sithole, Senzekile Sithole, Ntokozo Danielle Sithole, Koketso Maxwell Sithole, Jonnie Situma, Annie Sivraman, Katekani Siwela, Nonqubela Siyewuyewu, Maweya Sizeka, Nonceba Siziba, Andrew Skhosana, Khanyisile Skhosana, Rorisang Skhosana, Tandiwe Skoko, Sunet Slabbert, Ntombela Smangaliso, Christine Smedley, Lydia Smit, Natassia Smit, Lizelle Smit, Michelle Smit, Fasie Smith, Lizzie Smith, Sunell Smith, Cassius Smith, Stefan Smuts, Ayanda Sofe, Khobane Solomon, L J Solomon, chauke Sombani, Richard Songca, Anga Sontamo, Supriya Soorju, Zubenathi Sopazi, Brian Soqasha, Bongiwe Sosibo, Ntsika Sotsaka, Mandy Soula, Simon Spoor, Sarah Stacey, Asanda Stali, Mutele Mmboniseni Stephina, Myra Steup, Sinoxolo Steven, AW Stevens, Vincent Stevens, Dewald Steyn, Bianca Steyn, Pat Stocks, Henk Stolk, Alida Stoltz, Renate Strehlau, Anneke Stroebel, Loraine Strydom, Jean-Marie Strydom, Anton Strydom, Ursula Strydom, Midhu Sunnyraj, Nwabisa Swana, Winnie Swanepoel, Suzan Swanepoel, Elsie Swartbooi, Estley Swartz Swartz, Casandra Syce, Shihambi T E, Joyce Tabane, N E Tabane, Mrs Tawana, Ntene Tebello, Siphosetu Wiseman Tembe, Samantha Terblanche, Ntombifuthi Thabede, Nkhumeleni Thabelo, Sibusiso Thabethe, Lekhanya Thabo George, Keorapetse Thare, Makofane Thebogo, Lerato Thekiso, Lloyd Theko, Celimphilo Zandi Themba, Danie Theron, Henda Theron, Ilze Theron, Thandiwe Thingathinga, M M Thlabadira, Dikeledi Thoka, Zanele Thokwana, Gustav Thom, Mamphot Joel Thubakgale, Theodora Thwala, P Thys, Monethi Tieho, Matodzi Timothy, Ndlovu Tintswalo, Babalwa Tivana, Molefi Tladi, Bongiwe Tokota, Simthandile Toni, Ariel Torres, Mande Toubkin, Marinda Tsatsi, Khanyisile Tshabalala, Nozibele Tshamase, Gontse Tshefu, Makgoga Tshegofatjo, Given Tshikomba, Thapelo Tshilo, Lerato Tshira, S T Tshirado, Maipfi Tshisikule, G Tsoke, N TSOKE, Alatha Tsoko, Mosele Tsotetsi, Sandeva Tsubella, Noxolo Tuswa, Maipato Tutse, Nomayenzeke Tutu, Sphephelo Twala, Nhlanhla Twala, Simphiwe Twala, John Ubisi, Tefo Unathi, A Van Aswegen, Marietjie van der Merwe, Trudie van der Merwe, Patience van der Plank, Elmarie van der Spuy, Linda Van Der Westhuizen, Adele Van Der Westhuizen, Talana van der Westhuizen, Mene van der Westhuyzen, Thea Van Dyk, Ingrid van Heerden, Ryno van Jaarsveld, Ryno van Jaarsveld, M Van Lill, Heidi van Niekerk, Ben van Niekerk, Amanda van Rensburg, Judy van Schallwyk, Zeitschke Yarnrich Van Sensie, Magda van Vuuren, Cloete van Vuuren, Olga Funiswa Vandu, Mandisa Vane, Lucia VanZyl, Ebrahim Variava, Mariam Veerus, Nokhwezi Velapi, Sebina Veleko, Z Velezantsi, Retha Venter, Corlia Vergottini, Corlia Vergottini, Inga Vermeulen, Liabara Lufuluvhi Vidah, Bongani Vilakazi, Treasure N Vilakazi, Mbalenhle Precious Vilakazi, Karen Viljoen, Werner Viljoen, Karen Viljoen, Zuretha Volschenk, Angelo Vos, Matlala V V, Jacques Walters, Kate Webb, John Welsh, D Wessels, Judy Wheller, Fundile White, Priscilla White, Carmen Whyte, Ansie Willemse, Sape William, Daniel Williams, Kamielah Williams, Mercia Williams, Anne Williamson, Cherade Wilson, Boipelo Wolff, Michelle Wray, Ntombizonke B Xaba, Thabang Jabulani Xaba, Thanks Xiniwe, Mtshali Xoliswa, Funokwakhe Xulu, Gibson Xulu, Sandlakazi Yam, NM Zakhura, Mashela Zareloa, Sive Zinto, Dyibeni Zinziswa, Lulamile Ziselo, Zakhele Zitha, Emmanuel Zitha, Anele Zokufa, Innocent Zondi, Sikhumbuzo Bernard Zondi, Sbuyi Zondi, Thulani Zondi, Wandiswa Zongola, Liesl Zühlke, Zandile Zulu, Lungelo Zulu, Thandeka Zulu, Slindili Zulu, Nkosinathi Zulu, Angel Zuma, Precious Zungu, Pamela Zungu, Melusi Zungu, Priscilla Zungu, Bongo Lihle Zwakala, Antonia Zwane, Promise Zwane, Muziwendoda Zwane, Hlengiwe Priscila Zwane, and Nomgcobo Zwane
Supplementary Material
References
- 1.National Institute for Communicable Diseases COVID-19 weekly epidemiology brief. South Africa, Week 12 2021. https://www.nicd.ac.za/wp-content/uploads/2021/04/COVID-19-Weekly-Epidemiology-Brief-week-12-2021.pdf
- 2.Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noor FM, Islam MM. Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis. J Community Health. 2020;45:1270–1282. doi: 10.1007/s10900-020-00920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macedo A, Goncalves N, Febra C. COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis. Ann Epidemiol. 2021;57:14–21. doi: 10.1016/j.annepidem.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chidambaram V, Tun NL, Haque WZ, et al. Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29–30 doi: 10.1016/j.eclinm.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9:407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker PGT, Whittaker C, Watson OJ, et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020;369:413–422. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Statistics South Africa Mid-year population estimates, 2020. July 9, 2020. http://www.statssa.gov.za/publications/P0302/P03022020.pdf
- 12.South Africa National Department of Health The first national TB prevalence survey. South Africa 2018. Short report. https://www.knowledgehub.org.za/system/files/elibdownloads/2021-02/A4_SA_TPS%20Short%20Report_10June20_Final_highres.pdf
- 13.UNAIDS UNAIDS South Africa. Country factsheets. https://www.unaids.org/en/regionscountries/countries/southafrica
- 14.Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–e564. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigel K, Swartz T, Golden E, et al. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin Infect Dis. 2020;71:2933–2938. doi: 10.1093/cid/ciaa880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KW, Yap SF, Ngeow YF, Lye MS. COVID-19 in people living with HIV: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18073554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper TJ, Woodward BL, Alom S, Harky A. Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: a systematic review. HIV Med. 2020;21:567–577. doi: 10.1111/hiv.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulle AA, Davies M, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1198. published online Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Xie N, Nie J, Marly G, Tang W. The interaction between HIV infection and COVID-19: a systematic review and meta-analysis. SSRN. 2020 doi: 10.2139/ssrn.3716873. published online Oct 23. (preprint). [DOI] [Google Scholar]
- 22.Mellor MM, Bast AC, Jones NR, et al. Risk of adverse coronavirus disease 2019 outcomes for people living with HIV. AIDS. 2021;35:F1–10. doi: 10.1097/QAD.0000000000002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesoriero JM, Swain CE, Pierce JL, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maluleke R. Inequality trends in South Africa. A multidimensional diagnostic of inequality. 2019. http://www.statssa.gov.za/publications/Report-03-10-19/Report-03-10-192017.pdf
- 25.Jassat W, Cohen C, Kufa T, et al. COVID-19 special public health surveillance bulletin. June 10, 2020. https://www.nicd.ac.za/wp-content/uploads/2020/06/COVID-19-Special-Public-Health-Surveillance-Bulletin-10-June-2020-005.pdf
- 26.WHO WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2007. https://www.who.int/hiv/pub/guidelines/WHO%20HIV%20Staging.pdf
- 27.Nel J, Dlamini S, Meintjes G, et al. Southern African HIV Clinicians Society guidelines for antiretroviral therapy in adults: 2020 update. South Afr J HIV Med. 2020;21 doi: 10.4102/sajhivmed.v21i1.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riou C, Du Bruyn E, Stek C, et al. Profile of SARS-CoV-2-specific CD4 T cell response: relationship with disease severity and impact of HIV-1 and active Mycobacterium tuberculosis co-infection. medRxiv. 2021 doi: 10.1101/2021.02.16.21251838. published online Feb 20. (preprint). [DOI] [Google Scholar]
- 29.United States Department of Health and Human Services Tenofovir disoproxil fumarate. April 27, 2020. https://aidsinfo.nih.gov/drugs/290/tenofovir-disoproxil-fumarate/0/patient
- 30.United States Department of Health and Human Services Side effects of HIV medicines. Sept 24, 2020. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-medicines-and-side-effects
- 31.WHO TB/HIV: a clinical manual. 2021. https://apps.who.int/iris/bitstream/handle/10665/42830/9241546344.pdf?sequence=1&isAllowed=y
- 32.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35:1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 34.Demombynes G. COVID-19 age-mortality curves are flatter in developing countries. Policy Research Working Paper 9313. July, 2020. https://openknowledge.worldbank.org/bitstream/handle/10986/34028/COVID-19-Age-Mortality-Curves-Are-Flatter-in-Developing-Countries.pdf?sequence=4&isAllowed=y
- 35.Chauvin JP, Fowler A, Herrera LN. The younger age profile of COVID-19 deaths in developing countries. November, 2020. [DOI]
- 36.Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13:1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goel S, Jain T, Hooda A, et al. Clinical characteristics and in-hospital mortality for COVID-19 across the globe. Cardiol Ther. 2020;9:553–559. doi: 10.1007/s40119-020-00189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okonji EF, Okonji OC, Mukumbang FC, Van Wyk B. Understanding varying COVID-19 mortality rates reported in Africa compared to Europe, Americas and Asia. Trop Med Int Health. 2021 doi: 10.1111/tmi.13575. https://doi/10.1111/tmi.13575 published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this manuscript are available upon reasonable request to the corresponding author. Individual participant data that underlie the results reported in this Article, after de-identification (text, tables, figures, and appendices), will be shared with investigators whose proposed use of the data has been approved by an independent review committee (learned intermediary) identified for this purpose. To gain access, data requestors will need to sign a data access agreement. The request will have to be approved by the South African National Department of Health.