ABSTRACT
Anabolic steroids are well-known to cause liver injury, which may manifest with jaundice and elevated liver enzymes. Selective androgen receptor modulators (SARMs) have been developed to enhance muscle bulk without the side effects associated with exogenous androgen steroids. We report a case of significant cholestatic liver injury associated with a SARM, ostarine (enobosarm), similar to that associated with anabolic steroids. Liver injury from SARMs has not been reported frequently, and we speculate that this may be seen more often as the consumption of SARMs increases in the athletic market.
INTRODUCTION
Selective androgen receptor modulators (SARMs) have been heavily marketed as alternatives to androgenic anabolic steroids (AASs) for muscle gain and physical performance because of their perceived superior side-effect profile. We report a case of significant drug-induced liver injury (DILI) attributed to use of ostarine (enobosarm), a SARM. To date, only 1 recent article in the literature has described DILI in 2 patients associated with SARM use.1 This report builds on this association and is the first biopsy-proven case of liver injury caused by a SARM.
CASE REPORT
A previously healthy man in his early 40s presented with new-onset jaundice, anorexia, weight loss, lethargy, and diarrhea. He had been using enobosarm for weight training and muscle bulk. He was taking the medication without medical supervision for 2 months before presentation. His regular medications included finasteride for male pattern baldness and zopiclone as a sleep aid, which he took for several months before starting ostarine without any adverse effects. He denied alcohol or intravenous drug use.
Examination revealed normal vital signs but significant jaundice and scleral icterus. There was no stigmata of chronic liver disease or hepatosplenomegaly. Laboratory work included a hemoglobin of 15.6 g/dL, platelets of 313 × 109/L, sodium 139 mmol/L, and creatinine of 0.105 mmol/L. His liver biochemistry was gamma-glutamyl transferase (GGT) 49 U/L, alkaline phosphatase 268 U/L, alanine aminotransferase (ALT) 112 U/L, aspartate aminotransferase 69 U/L, total bilirubin 0.34 mmol/L, and direct bilirubin 0.25 mmol/L, and international normalized ratio 1.3. His R-factor was 0.8, which indicated a primary cholestatic liver injury. An ultrasound and computed tomography were negative for any ductal dilatation, cirrhosis, hepatomegaly, or intraabdominal venous thromboembolism. A confirmatory magnetic resonance cholangiopancreatography showed normal hepatic and biliary anatomy.
A complete medical workup was ordered for the etiology of liver damage. His hepatitis A, B, C, and E and human immunodeficiency virus serologies were negative. His Epstein-Barr virus and cytomegalovirus immunoglobulin M were negative, and immunoglobulin G were positive, suggesting previous but no current infection. Immunoglobulins were normal, and his antinuclear antibody test, antismooth muscle antibody, antimitochondrial antibodies were negative. His ceruloplasmin, ferritin, and alpha 1 antitrypsin levels were normal. With serial blood work, his total bilirubin continued to rise with a peak of 0.735 mmol/L while the liver enzymes continued to improve (Figure 1).
Figure 1.
The trend of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin from time of admission to postdischarge follow-up.
Given the progressive jaundice and negative diagnostic testing, a liver biopsy was ordered. His biopsy results showed mild bile ductular reaction with very mild duct damage and minimal inflammation. There was moderate to severe cholestasis consistent with drug-induced cholestatic liver injury (Figure 2). The patient was discharged, and follow-up with serial blood work for monitoring of his liver enzymes, which showed gradual improvement over several months after discharge.
Figure 2.
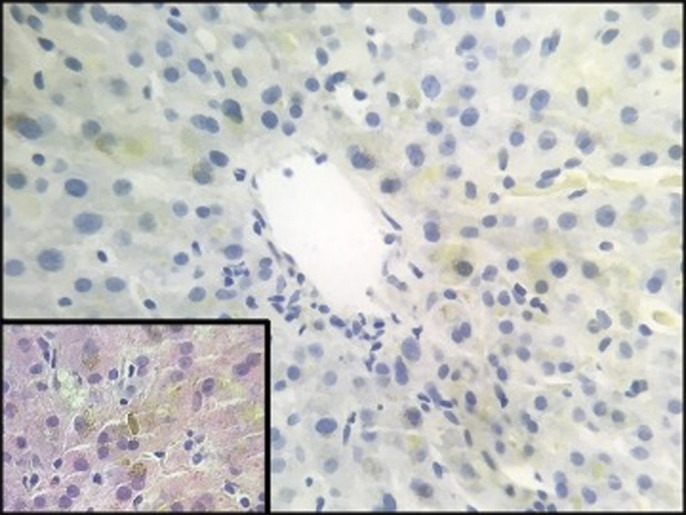
The liver biopsy revealed centrilobular cholestasis with yellow-green bile in hepatocytes and canaliculi (rhodanine stain, 400× magnification). Inset: histopathology of canalicular bile plug (hematoxylin and eosin stain, 400× magnification).
DISCUSSION
AASs are commonly used for their beneficial effects in improving lean body mass and muscle strength. Unfortunately, they are also well-known to cause DILI, with case reports dating back to the 1950s.2 Indeed, in a recent 2018 survey of 3,312 DILI cases, anabolic steroids were the 10th highest-ranking drug class causing liver injury worldwide.3 In 2014, the Drug-Induced Liver Injury Network reported the most common histopathologic patterns of injury as inflammation, cholestasis, or both, emphasizing the importance of histological analysis.4
While a large proportion of AAS-induced liver injuries recover with time, there is considerable morbidity, impact on the quality of life, and productivity in what is often a young, economically active patient cohort. In addition, serious medical complications can and do occur. There are reports in the literature of significant bile acid nephropathy and intractable pruritis requiring treatment with plasmapheresis.5
Although there are few current medical indications for anabolic steroids, these drugs continue to have a persistent market, off-prescription, in the world of physical performance. A survey from 2006 in the United States estimated that up to 2.9% of young adults had taken AAS at least once in their lives. Among weight trainers attending gyms and health clubs, it was 15-30%.6 Another report estimated 3%–12% of male and 0.5%–2% of female adolescents use AAS in the United States. Nearly 80% of these were “nonathletes” looking to improve their physical appearance.7 The limiting factor with anabolic steroids is the inability to separate desired anabolic effects on muscle and bone from unwanted side effects. This, in turn, has led to the development of SARMs, namely, nonsteroidal, tissue-selective anabolic agents, with a greatly reduced androgenic side-effect profile. SARMs also allow for oral dosing, which is a clear preference to the user over the transdermal or intramuscular administrations of AASs.
The culprit drug in our case report, ostarine (marketed as enobosarm) is a tissue-selective SARM. It was developed by GTx, previously under Merck. In a 12-week double-blind, placebo-controlled phase II trial,8 ostarine was found to have statistically significant, dose-dependent increases in total lean body mass. Therefore, it was claimed to be useful in prevention and treatment of muscle wasting, sarcopenia, and cancer cachexia in elderly men and women.8 Interestingly, although no increased rate of adverse events was found compared with placebo, 1 subject discontinued the trial due to rise in ALT to 4× upper limit of normal. Seven other subjects experienced a smaller ALT rise, which settled while still on the drug. No significant increases in total bilirubin, GGT, or alkaline phosphatase were reported.8 Despite a lack of reported adverse events, the trial did demonstrate dose-dependent hepatotoxic effects of ostarine in participants who received the drug.
The patient in our case report was using ostarine as an aid to physical development. He reported having acquired it online as a group purchase for a larger number of gym users, suggesting a broader potential for misuse of this drug class in sport and recreation. The histopathological pattern was of a moderate-to-severe centrilobular cholestasis with sparse portal inflammation, classic for AAS-induced liver injuries. The almost complete absence of bile duct damage explains the normal GGT and has been described previously in steroid-induced liver injuries.9 We speculate that ostarine resulted in cholestasis to endogenous androgens. Given their increasing popularity, awareness of this risk to consumers and health care providers is necessary.
DISCLOSURES
Author contributions: Harjot Bedi, MD, MSc, MHSc, and Carl Hammond, MBChB MRCP(Lond), contributed equally to this manuscript. H. Bedi and C. Hammond wrote the manuscript and approved the final manuscript. D. Sanders, H-M Yang, and EM Yoshida revised the manuscript for intellectual content and approved the final manuscript. EM Yoshida is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Harjot Bedi, Email: harjbedi@gmail.com.
Carl Hammond, Email: carlhammond21@hotmail.com.
David Sanders, Email: sandersubc@gmail.com.
Hui-Min Yang, Email: huimin.yang@vch.ca.
REFERENCES
- 1.Flores JE, Chitturi S, Walker S. Drug‐induced liver injury by selective androgenic receptor modulators. Hepatol Commun. 2020;4(3):450–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaffner F, Popper H, Chesrow E. Cholestasis produced by the administration of norethandrolone. Am J Med. 1959;26(2):249–54. [DOI] [PubMed] [Google Scholar]
- 3.Teschke R. Top-ranking drugs out of 3312 drug-induced liver injury cases evaluated by the Roussel Uclaf Causality Assessment Method. Expert Opin Drug Metab Toxicol. 2018;14(11):1169–87. [DOI] [PubMed] [Google Scholar]
- 4.Cabb E, Baltar S, Powers David W, Mohan K, Martinez A, Pitts E. The diagnosis and manifestations of liver injury secondary to off-label androgenic anabolic steroid use. Case Rep Gastroenterol. 2016;10(2):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores A, Nustas R, Nguyen HL, Rahimi RS. Severe cholestasis and bile acid nephropathy from anabolic steroids successfully treated with plasmapheresis. ACG Case Rep J. 2016;3(2):133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkinson AB, Evans NA. Anabolic androgenic steroids: A survey of 500 users. Med Sci Sports Exerc. 2006;38(4):644–51. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez‐Osorio M, Duarte‐Rojo A, Martínez‐Benítez B, Torre A, Uribe M. Anabolic–androgenic steroids and liver injury. Liver Int. 2008;28(2):278–82. [DOI] [PubMed] [Google Scholar]
- 8.Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: Results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2(3):153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Sulllvan AJ, Kennedy MC, Day RO, Casey JH, Wodak AD, Corrigan B. Anabolic‐androgenic steroids: Medical assessment of present, past and potential users. Med J Aust. 2000;173(6):323–7. [DOI] [PubMed] [Google Scholar]



