Abstract
Molecular phylogeny indicates that metazoans (animals) emerged early in the Neoproterozoic era1, but physical evidence is lacking. The search for animal fossils from the Proterozoic eon is hampered by uncertainty about what physical characteristics to expect. Sponges are the most basic known animal type2,3; it is possible that body fossils of hitherto-undiscovered Proterozoic metazoans might resemble aspect(s) of Phanerozoic fossil sponges. Vermiform microstructure4,5, a complex petrographic feature in Phanerozoic reefal and microbial carbonates, is now known to be the body fossil of nonspicular keratosan demosponges6–10. This Article presents petrographically identical vermiform microstructure from approximately 890-million-year-old reefs. The millimetric-to-centimetric vermiform-microstructured organism lived only on, in and immediately beside reefs built by calcifying cyanobacteria (photosynthesizers), and occupied microniches in which these calcimicrobes could not live. If vermiform microstructure is in fact the fossilized tissue of keratose sponges, the material described here would represent the oldest body-fossil evidence of animals known to date, and would provide the first physical evidence that animals emerged before the Neoproterozoic oxygenation event and survived through the glacial episodes of the Cryogenian period.
Subject terms: Palaeontology, Marine biology
Vermiform microstructure in microbial reefs dating to approximately 890 million years ago resembles the body fossils of Phanerozoic demosponges, and may represent the earliest known physical evidence of animals.
Main
Benthic microbial structures (stromatolites and other microbialites) provide conspicuous evidence of pre-Phanerozoic life, but are difficult to understand because they rarely preserve recognizable evidence of the organisms involved. Stromatolitologists have struggled for over a century to decipher their microscopic laminae and clots, which are assumed to have been produced or influenced by in vivo and/or post-mortem biogeochemical activity, and to formalize the ‘taxonomy’ of their morphology and microstructure5,11.
The existence of metazoans by the Ediacaran period (the last period of the Neoproterozoic) is indicated by bilaterian ‘body’ and trace fossils12, and geochemical evidence (biomarkers)13 provides disputed14,15, indirect evidence for Cryogenian poriferans. The search for definitive physical evidence of pre-Cryogenian metazoans is confounded by uncertainty about what to look for, but preserved physical evidence should be small, subtle and possibly altogether unfamiliar. Given that sponges are the most basic of known animals2,3, physical evidence of Neoproterozoic sponges could be sought, but effort focused on the characteristics of mineralized sponge skeletons (siliceous or calcareous spicules)16–18 overlooks sponges with only proteinaceous (spongin or keratin19,20) skeletons. Early metazoan evidence might instead resemble taphonomic (preservational) products of sponge soft tissue21–23 rather than mineralized sponge skeletal components. Although molecular clock data suggest that sponges emerged in the early Neoproterozoic1, the oldest undisputed sponge body fossils are from the Cambrian period15.
Recent work6,7 has shown that vermiform microstructure4,5—an unusual microscopic feature in Phanerozoic reefs and stromatolites that was initially interpreted as being related to algae4 or protozoans24,25—is instead a keratose sponge body fossil comprising complexly anastomosing cement-filled microtubules enclosed in carbonate microspar. It is produced taphonomically6,10 in nonspicular keratose demosponges through post-mortem calcification of soft tissue to produce carbonate microspar (automicrite), which surrounds the tough spongin fibres of the ‘skeleton’ of the sponge. Decay of the spongin then produces a network of complexly anastomosing tubular moulds that eventually become passively filled with sparry calcite cement. Although questioned26, the association between vermiform microstructure and sponges has been confirmed in undisputed body fossils of Phanerozoic sponges7. Three-dimensional reconstruction of vermiform microstructure has shown that tubule shape and branching configuration are too consistent and complex to be abiogenic (for example, compacted peloids), do not resemble the branching style of other possible organism types (microbial or fungal)6 and are identical to the spongin meshworks of keratose sponges6. Although the existence of Proterozoic vermiform microstructure has been predicted6,10,27, published examples are rare28,29 and difficult to understand.
The calcification of decaying sponge soft tissue has been documented in modern sponges21,22, and produces sponge ‘mummies’22 as well as a range of subtle carbonate sedimentary textures (such as peloid clusters) in living and Phanerozoic fossil sponges7,9,21,22. Taphonomic sediment textures (polymuds) that may be poriferan-related have previously been identified in the reefs that are the subject of this Article23.
Background
This petrographic study presents possible evidence of sponge body fossils in thin sections (30-μm-thick rock slices, viewed microscopically in transmitted light) from the approximately 890-million-year-old (Ma)30,31 Little Dal reefs (Stone Knife Formation32, northwestern Canada) (Fig. 1a, b). These large (about 500 m in thickness, and kilometres in diameter) microbial reefs33–36 were built mainly by variably preserved calcimicrobes that have been interpreted as filamentous cyanobacteria (photosynthesizers)33,35,36, and developed palaeotopographic relief of up to about 100 m above the surrounding subphotic, level-bottom carbonate-mud seafloor. Reef framework, which is generally not discernible in natural exposures, was documented from slabbed hand samples and thin sections35.
Fig. 1. Geographic and stratigraphic location of the study material.
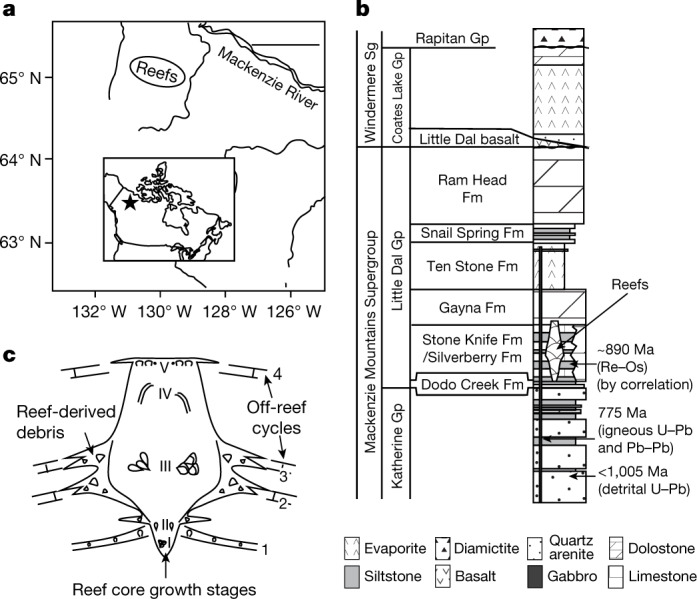
a, Location of Little Dal reefs in northwestern Canada. Scale bar, 100 km. b, Stratigraphic position of the Little Dal reefs in the Stone Knife Formation (Fm)32; depositional age is known through litho- and chemostratigraphic correlation with the 892 ± 13-Ma (Re–Os black shale)30 Boot Inlet Formation31 (Shaler Supergroup (Sg)), together with other geochronological constraints50,51. Gp, group. c, Reef growth stages34,35, simplified summary of framework morphologies35 and off-reef cycles34.
The reefs grew in five stages (Fig. 1c), each with different microbialite morphologies: anastomosing millimetre-to-centimetre-scale masses with no consistent shape (stages I–III); centimetre-scale anastomosing columns and digits (stages II, III and V); and steep sheet-like masses at a scale of decimetres to 10 m (stage IV). Stage V includes cement-rich to micritic, domical, turbinate and columnar stromatolites that generally lack calcimicrobes, with associated ooids and stromaclasts. Microbialites of stage I to IV grew predominantly in moderate-energy, illuminated palaeoenvironments33,35,36, but stage V records a shallow-water, high-energy environment. The microstructure that forms most of the reefal microbialites (especially in stages III and IV) comprises filaments that are about 10 μm in diameter, separated by 10–100-μm masses of marine cement that probably represents the calcified sheath polysaccharide of cyanobacteria33,36 that was permineralized during microbialite growth. The reef framework, consisting predominantly of this microstructure and its taphonomically degraded equivalents36, defines primary void networks (millimetres to centimetres in size) that are commonly floored with geopetal carbonate mud and lined by isopachous, fibrous marine calcite cement. The relative timing of void-filling by marine cement precipitation versus geopetal sediment accumulation is variable, attesting to the very early timing of marine cement precipitation.
Results
Vermiform microstructure in samples from stages II, III and V of the Little Dal reefs (Fig. 1c) is identifiable only in rare thin sections, in which it forms millimetre-to-centimetre-scale masses of anastomosing tubes that are filled with calcite spar and surrounded by calcite microspar groundmass (Fig. 2a, b). The approximately 20–30-μm-wide tubules have complex, divergent branching and rejoining at a spacing of about 30–100 μm, form very irregular three-dimensional polygonal meshworks, are defined by enclosing microspar, lack walls, and are filled with clear, equant calcite crystals up to 20 μm wide (Fig. 2b). The homogeneous microspar groundmass that encloses the tubules comprises cloudy, equant, interlocking calcite crystals of about 2–8 μm wide, and differs texturally and compositionally from other fine-grained reefal carbonate in its uniform crystal size, lack of sedimentary texture, and dearth of detrital silicate impurities. Vermiform microstructure preservation is good to barely discernible.
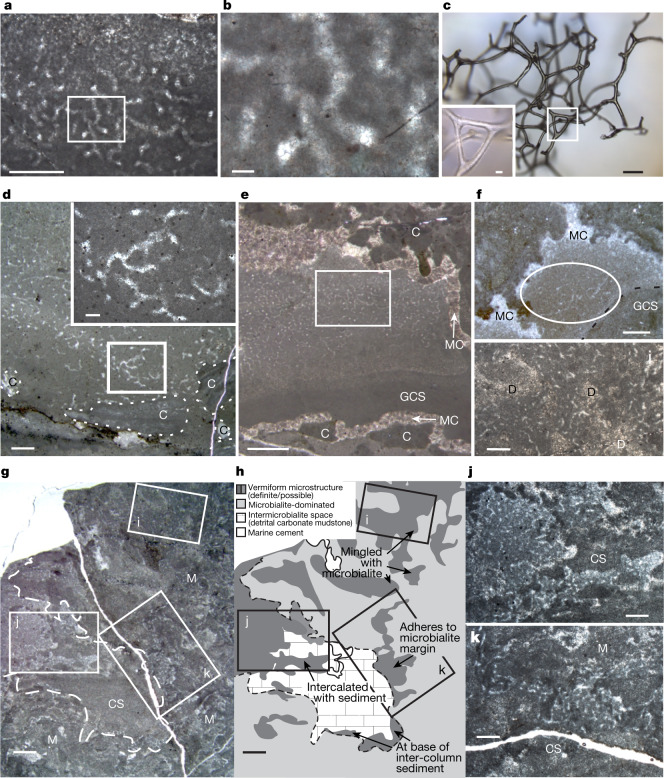
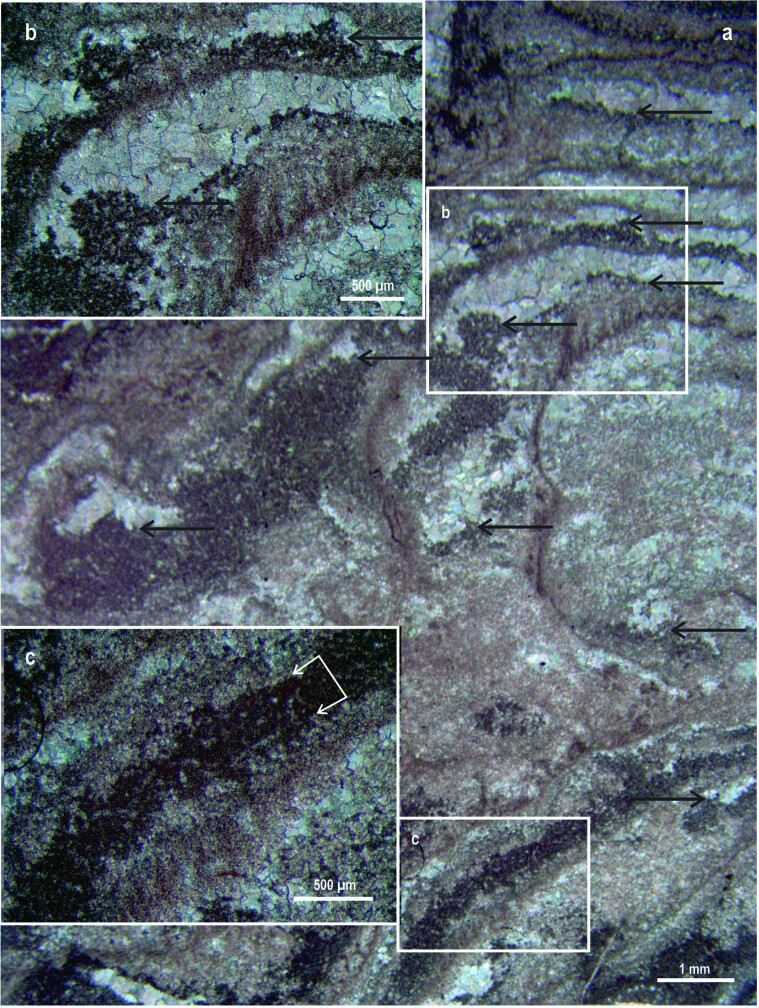
Fig. 2. Characteristics and distribution of Little Dal vermiform microstructure in stratigraphically oriented 30-μm-thick thin sections.
a, Well-preserved vermiform microstructure exhibits a polygonal meshwork of anastomosing, slightly curved, approximately 30-μm-diameter tubules embedded in calcite microspar (KEC25). Scale bar, 500 μm. b, Enlarged rectangle from a, showing branching tubules forming three-dimensional polygons intersected at various angles by the thin section; clear calcite crystals, about 10–20 μm in width, fill tubules in groundmass of more finely crystalline calcite (dark grey). Scale bar, 50 μm. c, Three-dimensional fragment of spongin skeleton from a modern keratosan sponge, illustrating its branching and anastomosing network of fibres (incident light). Scale bars, 100 μm (main panel), 20 μm (inset). d, Vermiform microstructure in debris that includes calcimicrobialite and other reef-derived clasts (C) flanking reef stage III (MV63). Scale bars, 1 mm (main panel), 100 μm (inset). e, Vermiform microstructure in shelter pore beneath microbialite clast, in detrital sediment occupying a reef-top depression; pore is thinly lined with marine calcite cement (MC) (indicated with an arrow), and partly filled with geopetal carbonate sediment (GCS) (KEC25; stage-III reef core). Rectangle is enlarged in a. Scale bar, 1 mm. f, Vermiform microstructure in a microbialite (M) framework void is overlain by pore-occluding marine calcite cement; circled area indicates moderately well-preserved tubule meshwork (DL32a; reef stage II; detailed characteristics depicted in Extended Data Fig. 1). Scale bar, 1 mm. g, Patches of vermiform microstructure in various relationships with micritic microbialite masses (white dashed outline) and detrital carbonate sediment (CS) (KES23; resedimented stage-II reef clast). Rectangles are enlarged in i–k. Scale bar, 1 mm. h, Simplified depiction of relationships among vermiform microstructure, microbialite masses and detrital carbonate sediment in g. Scale bar, 1 mm. i, Vermiform microstructure mingled with microbial micrite within a microbialite digit (enlarged from g). Scale bar, 500 μm. j, Vermiform-microstructured mass within sediment between microbialite digits; also contains diagenetic dolomite patches (D) (enlarged from g). Scale bar, 500 μm. k, Vermiform-microstructured mass adhering to the margin of microbialite digit (enlarged from g). Scale bar, 500 μm. All images except c are in plane-polarized transmitted light. Samples from resedimented reef debris are depicted in depositional orientation based on geopetal structures. Reef locations and abbreviations (such as KEC) are described in a previous publication35. Larger versions of vermiform microstructure photomicrographs are provided in Extended Data Figs. 1–5.
Vermiform microstructure is present in three microfacies (i, ii and iii (the last divided into iiia and iiib subsets)), representing three palaeoenvironments (Figs. 2, 3). It is not present in calcimicrobe-dominated stromatolites or level-bottom carbonate mudstone that is distal to reefs.
Fig. 3. Palaeoenvironments occupied by the Little Dal vermiform microstructure interpreted as possible body fossils of keratose sponges.
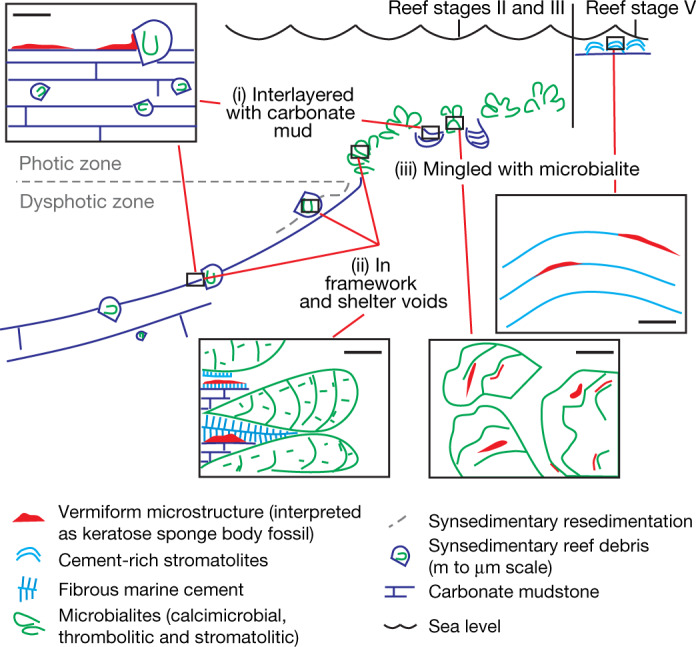
The organism lived (i) on poorly illuminated to non-illuminated carbonate mud surfaces in depressions on the reef surface and on debris aprons mantling reef flanks; (ii) in voids produced by the growth of the complex microbial framework of the reef; and (iii) interlayered with non-calcimicrobial microbialites (cement-rich and muddy-laminated stromatolites in high-energy reef-capping phase V; irregularly muddy-laminated to clotted microbialites in moderate-energy environments of reef stages II and III). Scale bars, 5 mm.
In microfacies i (Fig. 2d, e, g, j, Extended Data Figs. 1, 2), vermiform microstructure is intercalated with carbonate mud (with or without larger reef-derived clasts and terrigenous impurities) in (1) synsedimentary debris flanking reef stages II and III, and (2) millimetre-to-metre-scale palaeodepressions on reef growth surfaces (stages II and III). It locally encrusts sides of reef-framework clasts in sediment, extends into crevices, and occupies shelter porosity under clasts (Fig. 2e).
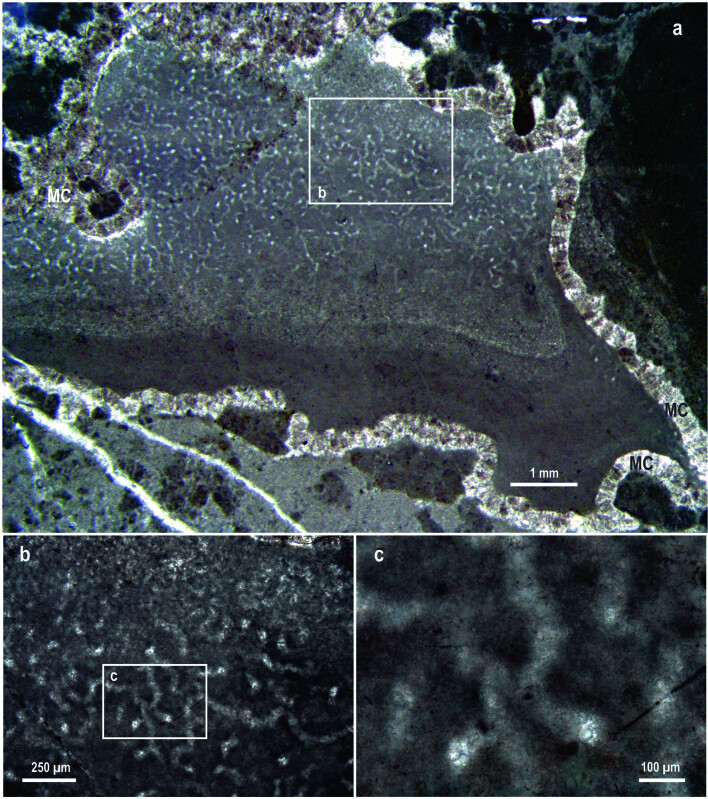
Extended Data Fig. 1. Vermiform microstructure of microfacies i.
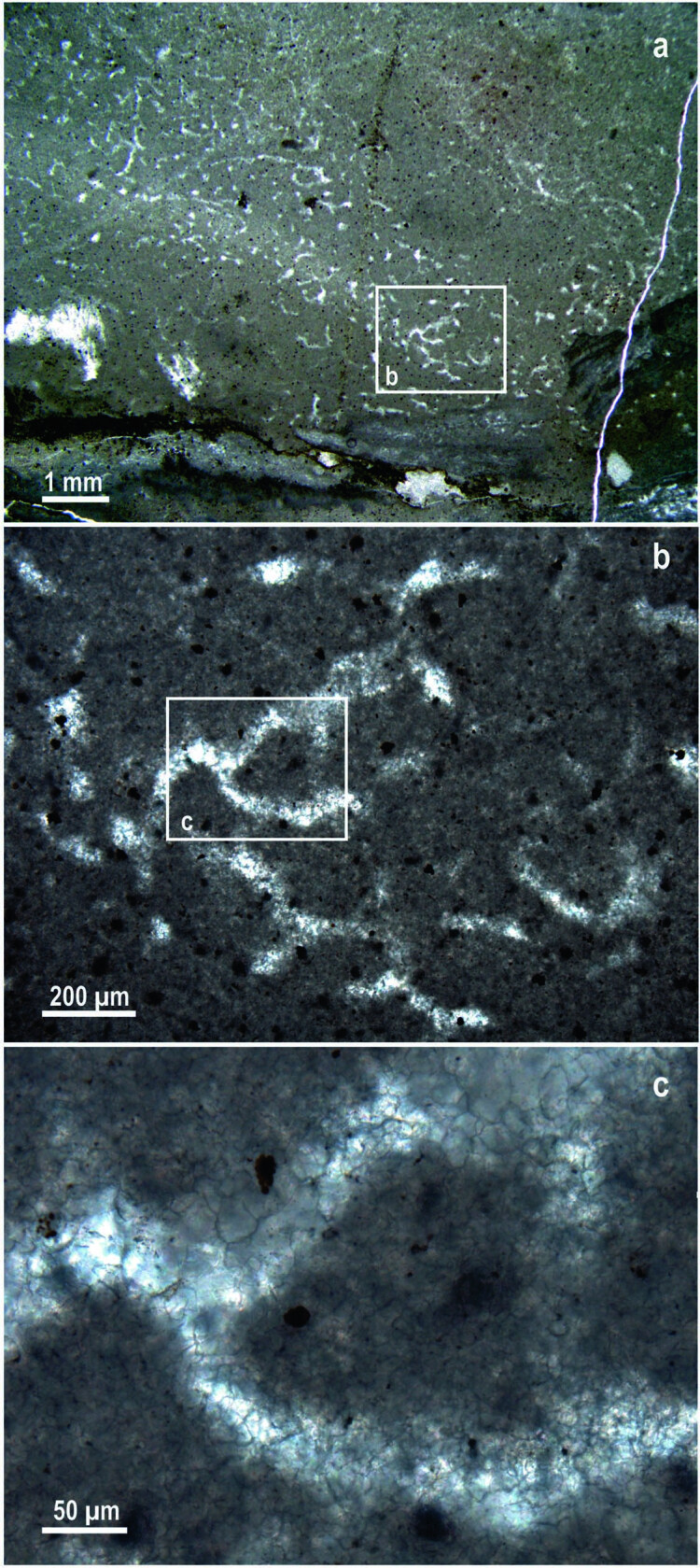
Elaboration of Fig. 2d. a, Vermiform microstructure is intercalated with detrital sediment (lower 1/3 of image) that includes calcimicrobialite clasts. b, Three-dimensional meshwork of vermiform microstructure transected by the plane of the 30-μm-thick thin section shows anastomosing tubule system; enlarged from a. c, Anastomosing tubules occluded by clear blocky calcite enclosed by cloudy calcite groundmass of slightly smaller crystals; enlarged from b. The tubules have no constructional walls and are defined by the dark enclosing groundmass. Oriented sample MV63 in plane-polarized transmitted light.
Extended Data Fig. 2. Vermiform microstructure of microfacies i.
Elaboration of Fig. 2e. a, Vermiform microstructure in a shelter void beneath an irregular microbialite clast and floored by angular reef clasts in carbonate mud, from a reef-top depression. Fibrous isopachous marine calcite cement lined the pore before its occupation by the vermiform mass. b, Three-dimensional meshwork of vermiform microstructure transected by the plane of the 30-μm-thick thin section shows anastomosing tubule system; enlarged from a. c, Anastomosing tubules occluded by clear blocky calcite enclosed by cloudy calcite groundmass of slightly smaller crystals; enlarged from b. The tubules have no constructional walls and are defined by the dark enclosing groundmass. Oriented sample KEC25 in plane-polarized transmitted light.
In microfacies ii (Fig. 2f, Extended Data Fig. 3), vermiform microstructure occupies millimetre-to-centimetre-scale framework voids of reef stages II and III. Void-filling vermiform microstructure of microfacies i and ii either underlies (Fig. 2f) or overlies (Fig. 2e) isopachous void-lining marine cement.
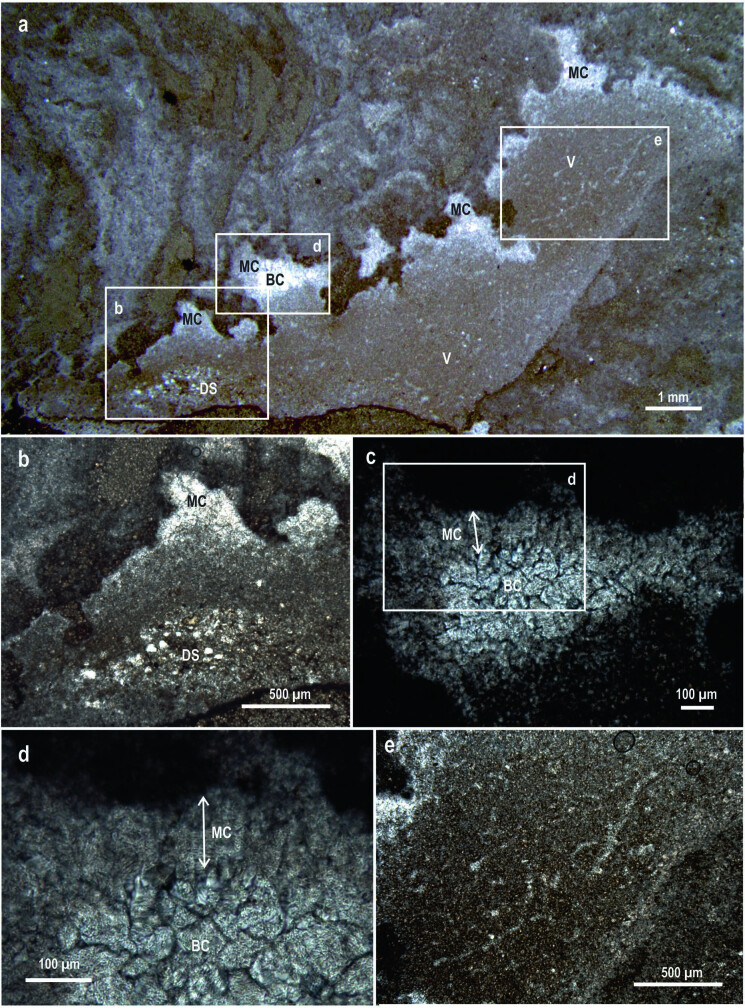
Extended Data Fig. 3. Spatial–temporal relationships among vermiform microstructure, geopetal detrital sediment and marine cement that collectively fill reef framework voids of microfacies ii.
Elaboration of Fig. 2f. a, Reef framework void among non-calcimicrobial stromatolites of reef stage II. Detrital sediment (DS) occupies lowest part of the void. Much of the void is occupied by vermiform microstructure (V); uppermost parts of the void are occupied by marine cement (MC) and local burial cement (BC). b, Detrital sediment accumulation, which includes quartz silt (transparent white particles); enlarged from a. c, Upper part of void is lined by isopachous cloudy marine calcite cement (arrow) (MC) and the remaining porosity occluded by burial calcite cement (pale) (BC); enlarged from a. d, Enlargement from c to demonstrate cloudy, isopachous, fibrous nature of the marine calcite cement, versus more transparent, equant, blocky shape of burial cement that occupies the small amount of pore space remaining after accumulation of geopetal sediment, growth of vermiform microstructure and precipitation of marine cement. e, Moderately preserved vermiform microstructure; enlarged from a. Oriented sample DL32a from reef stage II, in plane-polarized transmitted light.
In rare microfacies iiia (Fig. 2g–k, Extended Data Fig. 4), vermiform microstructure encrusts non-calcimicrobial microbialite columns and mingles with irregular muddy microbialite microstructure of reef stages II and III. In rare microfacies iiib (Extended Data Fig. 5), vermiform microstructure is sub-millimetrically interlayered with stage-V non-calcimicrobial stromatolites where it locally passes laterally to geopetal peloid accumulations in lenticular voids.
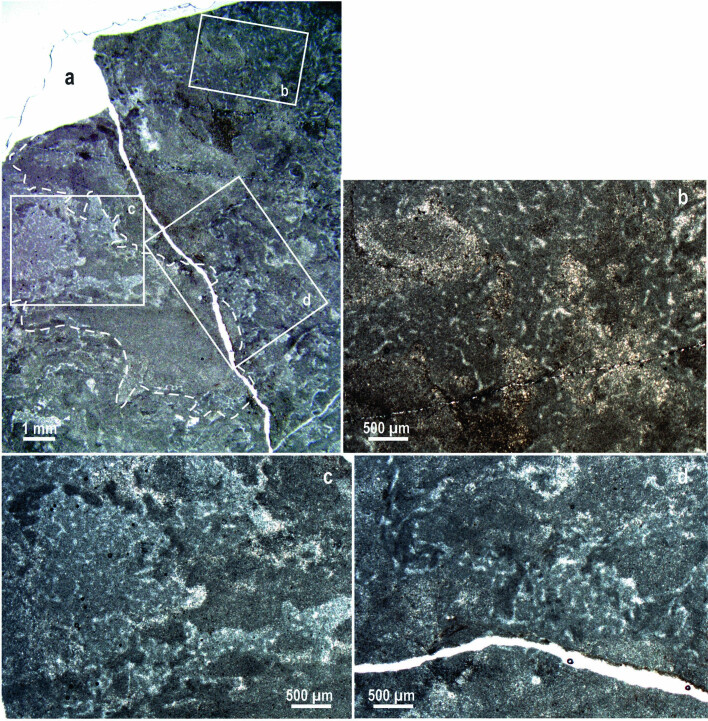
Extended Data Fig. 4. Associations of vermiform microstructure, micritic microbialite columns and detrital sediment in microfacies i and iiia.
Elaboration of Fig. 2g–k. a, Low-magnification image of two irregular microbialite columns and inter-column sediment (outlined by white dashed line). b, Vermiform microstructure is intermingled with muddy microbialite in column interior; enlarged from a. c, Vermiform microstructure intercalated with inter-column sediment; enlarged from a. d, Vermiform microstructure encrusting the margin of a microbialite column; enlarged from a. Sample KES23, from reef stage II, in plane-polarized transmitted light; sample is from a resedimented reef clast and is oriented on the basis of geopetal structures out of the field of view.
Extended Data Fig. 5. Spatial and textural association of vermiform microstructure and geopetal peloids in microfacies iiib.
This association suggests that geopetal peloid accumulations are a taphonomic product derived through poor preservation of vermiform microstructure. a, Poorly preserved vermiform microstructure (arrowed; inset c) adjacent to laminar to lenticular voids containing geopetal peloid accumulations (arrowed; inset b) in cement-rich non-calcimicrobial stromatolites of reef stage V. Oriented IR drillcore sample RT A19-495.4′ in plane-polarized transmitted light.
Discussion
The shape, size, branching style and polygonal meshworks of the Little Dal vermiform tubules closely resemble both spongin fibre networks of modern keratosan sponges (Fig. 2a–c) and vermiform microstructure either demonstrated or interpreted to be sponge-derived in diverse Phanerozoic microbial, reefal and non-reefal carbonate rocks6–8,10,24,25,27,37. The compositional and textural homogeneity of the microspar groundmass supports an origin through permineralization of a pre-existing biological substance9, rather than incremental accumulation of detrital sediment or microbial carbonate that passively incorporated complexly anastomosing tubular microfossils. Variable preservation and association with geopetal peloid accumulations are familiar aspects of Phanerozoic sponge taphonomy9,21,22,38. In previous work, detailed comparison of the three-dimensional characteristics of vermiform microstructure with branching cylindrical organism types yielded no convincing alternative to the sponge interpretation6.
The preference of Little Dal vermiform microstructure for environments that were not inhabited by photosynthetic calcimicrobes (reef flanks, depressions on active reef growth surface, and framework and shelter voids), versus its absence from filamentous calcimicrobial reef-framework components, suggests that (1) illumination may not have been a requirement and (2) the organism may have been unable to compete with reef-building photosynthesizers that grew and/or calcified rapidly. The interlayering of vermiform microstructure with calcimicrobe-free microbialite (microfacies iiib) in the high-energy, well-illuminated reef surfaces of reef stage V supports the interpretation that the vermiform-microstructured organism was not capable of competing with reef-building filamentous cyanobacteria, but instead occupied niches in which the filamentous calcimicrobes did not live owing to (1) poor illumination or (2) high hydrodynamic energy. The occupation of cryptic microniches (shelter and reef framework voids) by sponges (for example, microfacies i and ii), is well known in the Phanerozoic21,37,39,40.
The obligatory spatial association of vermiform microstructure with reefs built by oxygen-producing cyanobacteria may indirectly support a metazoan interpretation. Prior to the Neoproterozoic oxygenation event, marine dissolved oxygen was probably low41 except perhaps in the vicinity of photosynthesizing microbial communities; the metabolic requirements of metazoans may have limited early animals to localized, comparatively well-oxygenated (for the time) environments (oxygen ‘oases’). Given the approximately 890-Ma depositional age30,31, the vermiform-microstructured Little Dal organism may been tolerant of ‘low’ oxygen (that is, relative to modern levels), which is a characteristic of some modern and fossil sponges42.
If the vermiform-microstructured masses in the Little Dal reefs are accepted as early sponge body fossils, their approximately 890-Ma age would imply that (1) the evolutionary emergence of metazoans was decoupled from the Neoproterozoic oxygenation event41–45 and (2) early animal life was not catastrophically affected by the Neoproterozoic glacial episodes. If the Little Dal objects are truly sponge body fossils, they are older than the next-youngest undisputed sponge body fossils (Cambrian)15 by approximately 350 million years.
It would not be surprising to find that the earliest sponges were reef-dwellers; the history of Phanerozoic reefs is rich with reef-building and reef-dwelling sponges46. If the masses of vermiform microstructure in the Little Dal reefs were to be accepted as an early Neoproterozoic expression of sponge tissue preservation, their age and proposed identity would be compatible with (1) evidence that the opisthokont (animal + fungus) clade was already established by the time of the Mesoproterozoic–Neoproterozoic transition47,48, (2) possible evidence of 1-billion-year-old multicellular holozoans49, (3) molecular clock estimates for the emergence of the Porifera in the early Neoproterozoic1 and (4) a revised taxonomy of nonspiculate keratose sponges showing that they are a sister group to other demosponges19. The Little Dal vermiform microstructure is perhaps exactly what should be expected of the earliest metazoan body fossils: preservation through post-mortem calcification of sponge-grade soft tissue in the decaying bodies of small, shapeless, sessile, epibenthic and cryptic animals most closely affiliated with keratose sponges.
Methods
Field work was done on foot from two-person, backpacking-style camps placed at sites that are accessible only by helicopter. Samples were collected at various times between 1992 and 2018, under all required permits. Recording sample locations using GPS is not possible for most sites owing to the extreme topography of the exposures’ cliffs, pinnacles and canyons, and so sample location was documented using photographs and sketches. Several samples are from a mineral-exploration drill-core stored on-site in the field. Owing to the homogeneous grey weathering of reef surfaces, lithofacies cannot be identified in the field. Instead, hand samples were collected and later slabbed and thin-sectioned. Vermiform microstructure was identified in a small proportion of the samples collected. Repeat visits focused primarily on resampling the rare areas in which vermiform microstructure had been identified.
Standard 30-μm-thick thin sections were examined in plane-polarized transmitted light using a Nikon C-Pol binocular microscope fitted with digital camera and Luminera Infinity Analyze software (for lower-magnification images) and an Olympus BX-51 petrographic microscope equipped with Q-Imaging digital capture system (for higher-magnification images).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-021-03773-z.
Supplementary information
Acknowledgements
Field work and sample preparation were supported by Natural Sciences and Engineering Research Council (NSERC) Discovery Grants (E.C.T.), and the Agouron Institute (2016), based on locations initially discovered during post-graduate work on microbialites funded through the NSERC grants of G. M. Narbonne and N.P. James.
Extended data figures and tables
Author contributions
E.C.T. conducted all aspects of the study.
Data availability
All relevant data are contained with the Article and its Supplementary Information, or are available from the author upon reasonable request.
Competing interests
The author declares no competing interests.
Footnotes
Peer review information Nature thanks Jeong-Hyun Lee, Joachim Reitner, Robert Riding and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41586-021-03773-z.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-021-03773-z.
References
- 1.Dohrmann M, Wörheide G. Dating early animal evolution using phylogenomic data. Sci. Rep. 2017;7:3599. doi: 10.1038/s41598-017-03791-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feuda R, et al. Improved modeling of compositional heterogeneity supports sponges as sister to all other animals. Curr. Biol. 2017;27:3864–3870.e4. doi: 10.1016/j.cub.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Simion P, et al. A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr. Biol. 2017;27:958–967. doi: 10.1016/j.cub.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Walter, M. R. Stromatolites and the Biostratigraphy of the Australian Precambrian and Cambrian (Special Papers in Palaeontology 11) (The Palaeontological Association, 1972).
- 5.Bertrand-Sarfati, J. in Stromatolites (Developments in Sedimentology 20) (ed. Walter, M. R.) 251–259 (Elsevier, 1976).
- 6.Luo C, Reitner J. First report of fossil “keratose” demosponges in Phanerozoic carbonates: preservation and 3-D reconstruction. Naturwissenschaften. 2014;101:467–477. doi: 10.1007/s00114-014-1176-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee J-H, et al. Furongian (Late Cambrian) sponge-microbial maze-like reefs in the north China platform. Palaios. 2014;29:27–37. doi: 10.2110/palo.2013.050. [DOI] [Google Scholar]
- 8.Luo C, Reitner J. ‘Stromatolites’ built by sponges and microbes - a new type of Phanerozoic bioconstruction. Lethaia. 2016;49:555–570. doi: 10.1111/let.12166. [DOI] [Google Scholar]
- 9.Shen Y, Neuweiler F. Questioning the microbial origin of automicrite in Ordovician calathid-demosponge carbonate mounds. Sedimentology. 2018;65:303–333. doi: 10.1111/sed.12394. [DOI] [Google Scholar]
- 10.Lee J-H, Riding R. Keratolite – stromatolite consortia mimic domical and branched columnar stromatolites. Palaeogeog. Palaeoclimatol. Palaeogeog. 2021;571:110288. doi: 10.1016/j.palaeo.2021.110288. [DOI] [Google Scholar]
- 11.Grey, K. & Awramik, S. M. Handbook for the Study and Description of Microbialites (Geological Survey of Western Australia Bulletin 147) (Geological Survey of Western Australia, 2020)
- 12.Chen Z, Zhou C, Yuan X, Xiao S. Death march of a segmented and trilobate bilaterian elucidates early animal evolution. Nature. 2019;573:412–415. doi: 10.1038/s41586-019-1522-7. [DOI] [PubMed] [Google Scholar]
- 13.Love GD, et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature. 2009;457:718–721. doi: 10.1038/nature07673. [DOI] [PubMed] [Google Scholar]
- 14.Antcliffe JB. Questioning the evidence of organic compounds called sponge biomarkers. Palaeontology. 2013;56:917–925. [Google Scholar]
- 15.Antcliffe JB, Callow RH, Brasier MD. Giving the early fossil record of sponges a squeeze. Biol. Rev. Camb. Philos. Soc. 2014;89:972–1004. doi: 10.1111/brv.12090. [DOI] [PubMed] [Google Scholar]
- 16.Muscente AD, Michel FM, Dale J, Ziao S. Assessing the veracity of Precambrian ‘sponge’ fossils using in situ nanoscale analytical techniques. Precambr. Res. 2015;263:142–156. doi: 10.1016/j.precamres.2015.03.010. [DOI] [Google Scholar]
- 17.Ma J-Y, Yang Q. Early divergence dates of demosponges based on mitogenomics and evaluated fossil calibrations. Palaeoworld. 2016;25:292–302. doi: 10.1016/j.palwor.2015.03.004. [DOI] [Google Scholar]
- 18.Botting JP, Muir LA. Early sponge evolution: a review and phylogenetic framework. Palaeoworld. 2018;27:1–29. doi: 10.1016/j.palwor.2017.07.001. [DOI] [Google Scholar]
- 19.Erpenbeck D, et al. Horny sponges and their affairs: on the phylogenetic relationships of keratose sponges. Mol. Phylogenet. Evol. 2012;63:809–816. doi: 10.1016/j.ympev.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich H, et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013;3:3497. doi: 10.1038/srep03497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reitner J. Modern cryptic microbialite/metazoan facies from Lizard Island (Great Barrier Reef, Australia) – formation and concepts. Facies. 1993;29:3–39. doi: 10.1007/BF02536915. [DOI] [Google Scholar]
- 22.Neuweiler F, Daoust I, Bourque P-A, Burdige DJ. Degradative calcification of a modern siliceous sponge from the Great Bahama Bank, The Bahamas: a guide for interpretation of ancient sponge-bearing limestones. J. Sediment. Res. 2007;77:552–563. doi: 10.2110/jsr.2007.058. [DOI] [Google Scholar]
- 23.Neuweiler F, Turner EC, Burdige D. Early Neoproterozoic origin of the metazoan clade recorded in carbonate rock texture. Geology. 2009;37:475–478. doi: 10.1130/G25621A.1. [DOI] [Google Scholar]
- 24.Gürich G. Les spongiostromides du Viséen de la Province de Namur. Mém. Musée R. d’Hist. Nat. Belg. 1906;3:1–55. [Google Scholar]
- 25.Gürich G. Spongiostromidae – eine neue Familie krustenbildender Organismen aus dem Kohlenkalk von Belgien. Neues Jahrb. Min. Geol. Paläont. 1907;1:131–138. [Google Scholar]
- 26.Kershaw S, Li Q, Li Y. Addressing Phanerozoic carbonate facies conundrum – sponges or clotted micrite? Evidence from Early Silurian reefs, South China Block. The Sed. Record (Washington) 2021;19:3–10. [Google Scholar]
- 27.Lee J-H, Riding R. The ‘classic stromatolite’ Cryptozoön is a keratose sponge-microbial consortium. Geobiol. 2021;19:189–198. doi: 10.1111/gbi.12422. [DOI] [PubMed] [Google Scholar]
- 28.Vologdin, A. G. The Oldest Algae of the USSR (in Russian) (USSR Academy of Sciences, 1962).
- 29.Shapovalova, I. G. Stratigraphy and Stromatolites of the Riphean of the Northern Part of the Judomian –Maisk Trough (in Russian) (Nauka, 1974).
- 30.van Acken D, Thomson D, Rainbird RH, Creaser RA. Constraining the depositional history of the Neoproterozoic Shaler Supergroup, Amundsen Basin, NW Canada: Rhenium–osmium dating of black shales from the Wynniatt and Boot Inlet Formations. Precambr. Res. 2013;236:124–131. doi: 10.1016/j.precamres.2013.07.012. [DOI] [Google Scholar]
- 31.Greenman W, Rainbird RH, Turner EC. High-resolution correlation between contrasting early Tonian carbonate successions in NW Canada highlights pronounced global carbon isotope variations. Precambr. Res. 2020;346:105816. doi: 10.1016/j.precamres.2020.105816. [DOI] [Google Scholar]
- 32.Turner, E. C. & Long, D. G. F. Formal definition of the Neoproterozoic Mackenzie Mountains Supergroup (NWT), and formal stratigraphic nomenclature for its carbonate and evaporite formations (Geological Survey of Canada. Open File 7112) (Geological Survey of Canada, 2012)
- 33.Turner EC, Narbonne GM, James NP. Neoproterozoic reef microstructures from the Little Dal Group, northwestern Canada. Geology. 1993;21:259–262. doi: 10.1130/0091-7613(1993)021<0259:NRMFTL>2.3.CO;2. [DOI] [Google Scholar]
- 34.Turner EC, James NP, Narbonne GM. Growth dynamics of Neoproterozoic calcimicrobial reefs, Mackenzie Mountains, northwest Canada. J. Sediment. Res. 1997;67:437–450. [Google Scholar]
- 35.Turner, E. C., Narbonne, G. M. & James, N. P. Framework composition of early Neoproterozoic calcimicrobial reefs and associated microbialites, Mackenzie Mountains, N.W.T. In Carbonate Sedimentation and Diagenesis in the Evolving Precambrian World (SEPM Special Publication 67) (ed. Grotzinger, J. P. & James, N. P.) 179–205 (SEPM, 2000).
- 36.Turner EC, James NP, Narbonne GM. Taphonomic control on microstructure in early Neoproterozoic reefal stromatolites and thrombolites. Palaios. 2000;15:87–111. doi: 10.1669/0883-1351(2000)015<0087:TCOMIE>2.0.CO;2. [DOI] [Google Scholar]
- 37.Park J, et al. Crouching shells, hidden sponges: unusual Late Ordovician cavities containing sponges. Sedim. Geol. 2017;347:1–9. doi: 10.1016/j.sedgeo.2016.11.003. [DOI] [Google Scholar]
- 38.Lee J-H, Hong J, Lee D-J, Choh S-J. A new Middle Ordovician bivalve–siliceous sponge–microbe reef-building consortium from north China. Palaeogeog. Palaeoclim. Palaeoecol. 2016;457:23–30. doi: 10.1016/j.palaeo.2016.05.034. [DOI] [Google Scholar]
- 39.Kobluk DR, James NP. Cavity-dwelling organisms in Lower Cambrian patch reefs from southern Labrador. Lethaia. 1979;12:193–218. doi: 10.1111/j.1502-3931.1979.tb00997.x. [DOI] [Google Scholar]
- 40.Hong J, Choh S-J, Lee D-J. Tales from the crypt: early adaptation of cryptobiontic sessile metazoans. Palaios. 2014;29:95–100. doi: 10.2110/palo.2014.076. [DOI] [Google Scholar]
- 41.Och LM, Shields-Zhou GA. The Neoproterozoic oxygenation event: environmental perturbations and biogeochemical cycling. Earth Sci. Rev. 2012;110:26–57. doi: 10.1016/j.earscirev.2011.09.004. [DOI] [Google Scholar]
- 42.Mills DB, et al. Oxygen requirements of the earliest animals. Proc. Natl Acad. Sci. USA. 2014;111:4168–4172. doi: 10.1073/pnas.1400547111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner EC, Bekker A. Thick sulfate evaporite accumulations marking a mid-Neoproterozoic oxygenation event (Ten Stone Formation, NWT, Canada) Geol. Soc. Am. Bull. 2016;128:203–222. [Google Scholar]
- 44.Lenton TM, Boyle RA, Poulton SW, Shields-Zhou GA, Butterfield NJ. Coevolution of eukaryotes and ocean oxygenation in the Neoproterozoic. Nat. Geosci. 2014;7:257–265. doi: 10.1038/ngeo2108. [DOI] [Google Scholar]
- 45.Butterfield NJ. The Neoproterozoic. Curr. Biol. 2015;25:R859–R863. doi: 10.1016/j.cub.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Wood, R. Reef Evolution (Oxford Univ. Press, 1999).
- 47.Loron CC, Rainbird RH, Turner EC, Greenman WJ, Javaux EJ. Organic-walled microfossils from the late Meso- to early Neoproterozoic lower Shaler Supergroup (Arctic Canada): diversity and biostratigraphic significance. Precambr. Res. 2019;321:349–374. doi: 10.1016/j.precamres.2018.12.024. [DOI] [Google Scholar]
- 48.Loron CC, et al. Early fungi from the Proterozoic era in Arctic Canada. Nature. 2019;570:232–235. doi: 10.1038/s41586-019-1217-0. [DOI] [PubMed] [Google Scholar]
- 49.Strother PK, et al. A possible billion-year-old holozoan with differentiated multicellularity. Curr. Biol. 2021;31:2658–2665.e2. doi: 10.1016/j.cub.2021.03.051. [DOI] [PubMed] [Google Scholar]
- 50.Leslie, C. D. Detrital Zircon Geochronology and Rift Related Magmatism: Central Mackenzie Mountains, Northwest Territories. Unpublished MSc thesis, Univ. of British Columbia (2009).
- 51.Milton JE, Hickey KA, Gleeson SA, Friedman RM. New U-Pb constraints on the age of the Little Dal basalts and Gunbarrel-related volcanism in Rodinia. Precambr. Res. 2017;296:168–180. doi: 10.1016/j.precamres.2017.04.030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are contained with the Article and its Supplementary Information, or are available from the author upon reasonable request.