Abstract
Purpose:
Delivery of radiation at ultrahigh dose rates, known as FLASH, has recently been shown to preferentially spare normal tissues from radiation damage compared to tumor tissues. However, the underlying mechanism of this phenomenon remains unknown, with one of the most widely considered hypothesis being that the effect is related to substantial oxygen depletion upon FLASH, thereby altering the radiochemical damage during irradiation, leading to different radiation responses of normal and tumor cells. Testing of this hypothesis would be advanced by direct measurement of tissue oxygen in vivo during and after FLASH irradiation.
Methods and Materials:
Oxygen measurements were performed in vitro and in vivo using the phosphorescence quenching method and a water-soluble molecular probe Oxyphor 2P. The changes in oxygen per unit dose (G-values) were quantified in response to irradiation by 10 MeV electron beam at either ultra-high dose rates (UHDR) reaching 300 Gy/s or conventional radiotherapy dose rates of 0.1 Gy/s.
Results:
In vitro experiments with 5% BSA solutions at 23°C resulted in G-values for oxygen consumption of 0.19–0.21 mmHg/Gy (0.34–0.37 μM/Gy) for conventional irradiation and 0.16–0.17 mmHg/Gy (0.28–0.30 μM/Gy) for UHDR irradiation. In vivo, the total decrease in oxygen after a single fraction of 20 Gy FLASH irradiation was 2.3±0.3 mmHg in normal tissue and 1.0±0.2 mmHg in tumor tissue with p-value < 0.00001, while no decrease in oxygen was observed from a single fraction of 20 Gy applied in conventional mode.
Conclusions:
Our observations suggest that oxygen depletion to radiologically relevant levels of hypoxia is unlikely to occur in bulk tissue under FLASH irradiation. For the same dose FLASH irradiation induces less oxygen consumption than conventional irradiation in vitro, which may be related to the FLASH sparing effect. However, the difference in oxygen depletion between FLASH and conventional irradiation could not be quantified in vivo, because measurements of oxygen depletion under conventional irradiation are hampered by resupply of oxygen from the blood.
Introduction
It is now widely accepted that irradiation delivered at high dose rates (>40 Gy/sec, termed a ‘FLASH’) results in reduction of normal tissue radiation damage, as compared to conventional radiotherapy dose rates (~0.03Gy/s) (1). While the respective area of research has advanced significantly in the last few years, most studies have been concerned with phenomenological observations using tissue-level functional assays rather than mechanistic measurements. There are several radiobiological hypotheses around the mechanisms underlying normal tissue sparing, however to date none are proven. Most proposed mechanisms are linked to oxygen depletion in tissues (2,3), which is expected to occur rapidly under FLASH dose rates via oxygen-derived radicals and their subsequent consumption in chemical reactions (4). While this is not the only existing hypothesis, few other mechanisms can alter the radiation damage as profoundly as reduction in the availability of oxygen (5). The overall data support the fact that high dose rates result in distinctly different radiobiological cell killing and/or repair, altering the survival curve for normal tissues, but not tumor tissues. Studies have shown reduction in damage in normal brain (6,7), colon (8), lung (9,10) and skin (11,12), while still preserving the equivalent therapeutic effect in tumors.
One factor limiting the understanding of the radiobiological effect of FLASH has been the lack of accurate information regarding oxygen changes that occur in tissue as well as in in vitro model systems, which could be used to compare FLASH and conventional radiation with respect to various models of radiobiological damage. Oxygen is an efficient radiosensitizer, and well-oxygenated cells are more sensitive to ionizing radiation than fully hypoxic cells by approximately a factor of 3, known as the oxygen enhancement ratio (OER). Radiolytic oxygen depletion and the induced sparing effect caused by radiochemical reactions during radiation at very high dose rates has been well-demonstrated in bacteria (13,14) and mammalian cell lines (15,16). Subsequently, several groups hypothesized that oxygen depletion might underpin the FLASH effect (17,18). However, oxygen depletion has only been seen in cell cultures (19–21), and no reports have documented oxygen depletion under FLASH irradiation in vivo.
Oxygen measurements by phosphorescence quenching are based on the ability of dioxygen (O2) to interact with a molecule of a phosphorescent probe in its excited triplet state, leading to shortening of the phosphorescence decay time. However, reactive oxygen species, such as superoxide anion or H2O2, potentially can also interact with triplet states and lead to their quenching via e.g. electron transfer reactions. The net effect of such reactions would also be shortening of the phosphorescence lifetime, making it difficult to distinguish which species, oxygen or another quencher molecule, led to quenching. Due to their slow response times, oxygen electrodes cannot be used for fast transient oxygen measurements, however they may be instrumental for cross-validation of phosphorescence-based measurements under steady state conditions. In a conventional oxygen electrode system, the electro-active metal surface (usually Pt) is protected by an inert plastic membrane, through which neutral oxygen molecules can diffuse, while H2O2 and other polar molecules cannot, making the electrode selective for molecular oxygen. In this study we used a commercial oxygen electrode system to cross-validate the phosphorescence decay measurements in vitro upon application of radiation.
Oxygen is transported by the hemoglobin in the red blood cells with subsequent diffusion from the blood vessels to the surrounding tissue (22,23). Consumption of oxygen in other processes, such as in production of oxygen radicals and their reactions, can limit oxygen available for respiration, while re-oxygenation of tissue by diffusion from the blood capillaries could take seconds-to-minutes, depending on the extent of perfusion and intercapillary distances, which vary between tissues (24). In this work we measured oxygen concentration in solution samples in vitro as well as in tissues at the time of application of 10 MeV electron FLASH irradiation pulses. Oxygen was quantified optically using the phosphorescence quenching method (25) and oxygen probe Oxyphor 2P (26). The oxygen levels were recorded in order to compare oxygen consumption by FLASH vs conventional radiation, using a range of solution samples, normal tissues and xenograft tumors.
Materials and Methods
Electron FLASH Delivery
The electron FLASH beam used in this study was derived from a Varian Clinac 2100 C/D (Palo Alto, CA) after reversible conversion procedures developed in our previous work (27). Briefly, the LINAC was converted to deliver 10 MeV electron FLASH beam by selecting 10 MV photon beam energy in the treatment console, retracting the x-ray target from the beam’s path and positioning the carousel on an empty port. For the in vitro experiments, a 10 MeV electron FLASH beam with a wide field size of 20 × 20 cm2 covered the whole solution sample, and the dose rate was 300 Gy/s at the isocenter. For the in vivo experiments, a 6 × 6 cm2 electron applicator with a circular diaphragm (1.5 cm in diameter) was used to project the electron FLASH beam on tissue at a specific position on each mouse. The average dose rate was 270 Gy/s at the beam isocenter. Table S1 summarizes the dosimetric parameters as used. The central axis percentage depth dose (PDD) and 2D surface dose distributions are shown in Fig. S1, as measured by Gafchromic Film EBT-XD (Ashland Inc., Covington, KY).
Oxyphor 2P
The oxygen probe used in this study was Oxyphor 2P, which is a dendritic phosphorescent probe having Pt tetraarylphthalimido-porphyrin as a core (26). The phosphorescence quantum yield of Oxyphor 2P in deoxygenated aqueous solutions at 22°C is 0.23, making it an exceptionally bright phosphorescent sensor. The molecular weight of Oxyphor 2P is ~75 kDa, and the approximate diameter of the molecule in aqueous medium is ~7 nm. The probe has two absorption bands, near 440 nm and 630 nm, and it phosphoresces near 760 nm, which is within the near-infrared window of tissue. Oxyphor 2P exhibits nearly unlimited aqueous solubility and can be used even at high concentrations (millimolar range). In the experiments reported herein Oxyphor 2P was used in <1 μM final concentration. The phosphorescence lifetime of Oxyphor 2P changes with oxygen concentration from 9 μs on air to 38 μs under fully anaerobic conditions at physiological temperature (36.7°C). The response of the phosphorescence lifetime of Oxyphor 2P to oxygen levels was calibrated as described previously (26).
Oximetry
Using Oxyphor 2P and an OxyLED phosphorometer (Oxygen Enterprises Ltd) partial pressures of oxygen (pO2) can be measured in solution samples as well as in biological tissues in vivo over the entire range of physiological pO2 values. OxyLED phosphorometer is a fiber optic-based system, whereby the excitation light (635 nm) is delivered to the tissue surface by a fiber (4 mm in diameter), while the emitted phosphorescence is collected and carried to the detector (an avalanche photodiode) by another similar fiber. The fiber tips were positioned next to one another ~1–2 mm away from the tissue surface. As a result, the illuminated spot on the surface was ~7 mm in diameter. Considering average tissue absorption/scattering parameters, the depth of tissue penetration (i.e. the depth at which the incident light intensity is attenuated by 1/e) for the combination of 635 nm excitation and 770 nm emission (the phosphorescence maximum of Oxyphor 2P) was ~3–4 mm. Therefore, the sampled volume was approximately 100–150 mm3. The phosphorescent probe was distributed throughout the sampled volume. As a result, the measured phosphorescence decay comprised a superposition of multiple decays, each with its own phosphorescence lifetime corresponding to the microscopic local oxygen concentration and the pre-exponential factor defined by the distributions of the probe and excitation intensity. Single-exponential fitting of such measured decay led to the “volume-averaged” phosphorescence lifetime, representing the average concentration of oxygen in the sample. In parallel, measurements of oxygen in vitro were performed using an oxygen electrode system (FiveGo F4, Mettler Instruments, Switzerland). This electrode system operates in the range of 0%−200% O2 (pO2 0–320 mmHg), having a resolution of 0.1% O2 (pO2 0.16 mmHg), and was appropriate for sampling the oxygen present in aqueous solutions, but not in tissue.
Sample preparation
Bovine Serum Albumin (BSA) was used in our experiments as a scavenger of reactive oxygen species (ROS) produced during irradiation. The normal levels of albumin in the blood are 34–54 g/L, which is 3–5 % w/w. For the in vitro experiments we used Oxyphor 2P (~0.5–1 μM) in a standard phosphate buffer saline (PBS) solution containing BSA (5%). A solution containing Oxyphor 2P and BSA was deoxygenated by slow nitrogen bubbling, avoiding formation of foam, until near-zero oxygen levels were reached, as indicated by the phosphorescence lifetime of Oxyphor 2P. Prior to experiments different aliquots of the same but aerated solution were added to glass vials with deoxygenated solutions, giving samples with different oxygen levels. The vials were sealed with screw caps made of ~1.5 mm-thick high-density resin with low gas permeability. An excess liquid was squeezed out as the cap was screwed onto each vial leaving no air bubbles inside. The final pO2 levels in the vials were measured by phosphorescence.
Animal preparation
All animals were cared for and handled in accordance with National Institutes of Health guidelines for the care and use of experimental animals and study protocol. Nude mice at an age of 6 weeks (Charles River Labs, Wilmington, MA) were injected with 106 MDA-MB 231 cells under the skin on the flank. Oxygen measurements in tumors were performed when the tumors were approximately 8 mm in diameter. Oxyphor 2P (200 μM,20 μL) was injected into the tail vein about 24 h before the measurements and irradiation. Mice were kept under general anesthesia administered as inhaled isofluorane at 1.5%, via flowing air through a nose cone during all treatment and measurement procedures. A heating pad was used to maintain the normal physiological body temperature.
Results
In vitro experiments
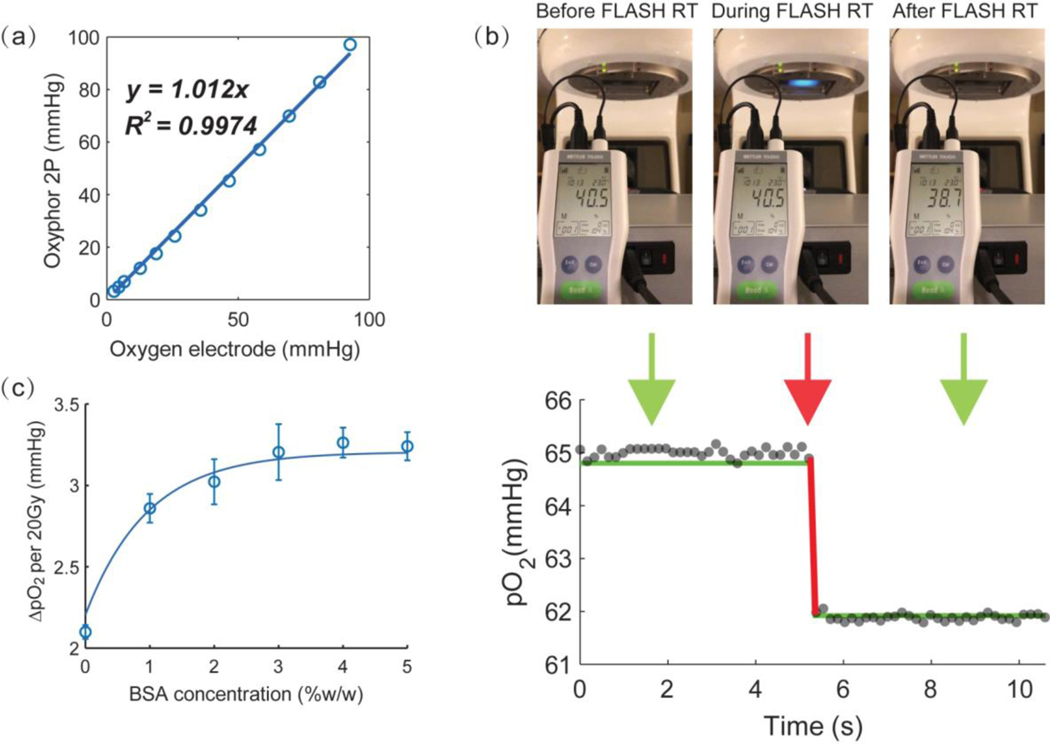
In the control experiments oxygen in aqueous solutions was measured by the OxyLED phosphorometer as well as using an oxygen electrode. The electrode was submerged in the solution, while the optical fibers were positioned next to the vial. These experiments confirmed that pO2 levels measured by phosphorescence and the electrode system were the same (Fig. 1a).
Fig. 1.
Measurements of oxygen in vitro by phosphorescence quenching and by oxygen electrode. Oxygen measurements in solutions having different pO2 levels (a) and in a solution upon application of FLASH irradiation (b), with the instantaneous change (red line) occurring in both phosphorescence (grey dots) and electrode (green lines) coincident with the time of the linac pulse. The unit displayed on the oxygen electrode panel is “% of air saturation”. The oxygen depletion rate in solutions with different scavenger concentrations after application of 20 Gy irradiation in FLASH mode (c)
A total of 20 Gy dose was delivered at a rate of 300 Gy/s, exposing the entire vial volume to the radiation pulse. As the electron beam hit the glass cover, Cherenkov emission was detected as a blue glow (central image in Fig. 1b). The maximal sampling rate possible in the reported experiments was ~7 Hz, i.e. ~150 ms per measurement. The delivery time for 20 Gy FLASH at the dose rate of 270 Gy/s was ~74 ms. Therefore, the sampling rate was inadequate to observe changes in oxygen during FLASH. Furthermore, strong scintillation from the optical fibers during FLASH prevented reliable measurements. Hence, the data points immediately before and immediately after the FLASH were discarded, so that the “blind” time was ~450 ms and it fully encompassed the FLASH pulse (red line) (Fig. 1b). The pO2 values before and after FLASH irradiation detected by the OxyLED phosphorometer were close to the readings obtained by the oxygen electrode (green line) (Fig. 1b), suggesting that at least prior to and after the action of FLASH the primary quenching species in solutions were oxygen molecules as opposed to other transient species (e.g. oxygen-derived radicals). This conclusion is contingent upon the assumption that the electrode is selective for oxygen; and if so, it confirms that Oxyphor 2P, and presumably other dendritic phosphors, can be used as selective oxygen sensors under the conditions of our measurements. The dependence of oxygen depletion on the scavenger (BSA) concentration is shown in Fig.1c. Oxygen depletion increased with the BSA concentration, and the effect saturated at ~3% BSA.
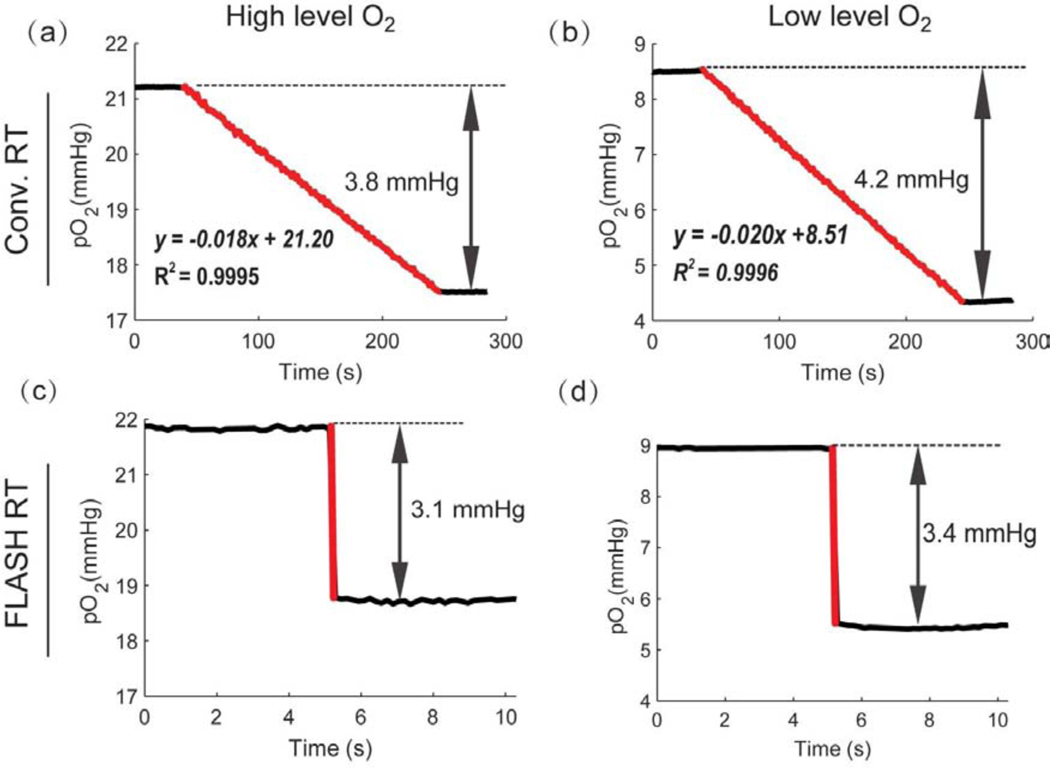
Oxygen consumption upon delivery of 20 Gy in the FLASH vs conventional mode in solutions with two different starting oxygen concentrations, 8–9 mmHg and 21–22 mmHg, was evaluated using Oxyphor 2P. The dose rates were 300 Gy/s and 0.1 Gy/s for the FLASH and conventional irradiation modes, respectively, where the delivery times were 74 ms and 200 s, resulting in equal total doses delivered to the samples. In the case of conventional irradiation (Figs 2a, 2b), pO2 decreased linearly with time at the rates of 0.18 mmHg/s and 0.20 mmHg/s for the samples with lower and higher starting oxygen concentrations. The oxygen consumption G-values were 0.19 mmHg/Gy and 0.21 mmHg/Gy (0.34 μM/Gy and 0.37 μM/Gy), respectively, indicating that the starting oxygen levels did not play a significant role in defining the consumption rate, although for higher starting oxygen concentration the depletion rate was slightly higher. In the FLASH experiments, pO2 values could be obtained reliably only before and after application of the pulses. From the differences in the pO2 values before and after the FLASH, the G-values were estimated to be 0.16 mmHg/Gy and 0.17 mmHg/Gy (0.28 μM/Gy and 0.30 μM/Gy) for the samples with low and high pO2, respectively (Figs 2c, 2d). Therefore, it appears that there was a systematic difference in oxygen consumption between FLASH and conventional modes of irradiation, comprising approximately 18±2 % difference between the conventional and FLASH mode.
Fig. 2.
Comparison of oxygen depletion in solutions treated with conventional vs FLASH irradiation. Oxygen depletion in solutions containing BSA (5%) induced by conventional (a, b) and FLASH (c, d) irradiation for different starting oxygen concentrations. Red lines mark the times of the radiation delivery.
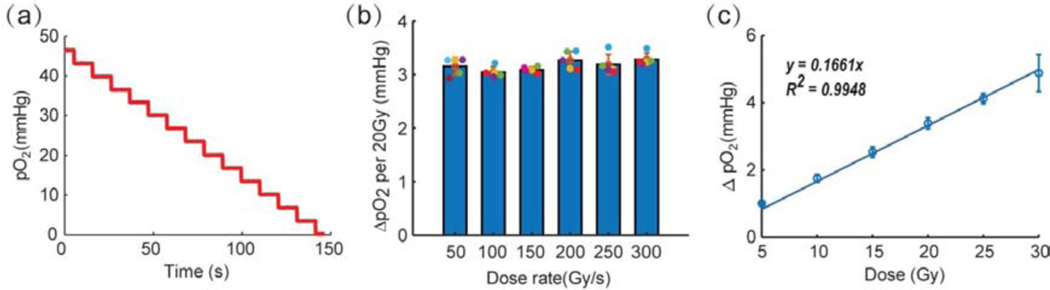
Next, we determined how many FLASH exposures were needed to fully deplete oxygen in solution starting with the initial oxygen pressure of ~46 mmHg. Doses of 20 Gy were delivered in the FLASH mode repeatedly 14 times, and pO2 in solution decreased linearly as a function of the number of exposures (Fig. 3a). Overall, more than 280 Gy was necessary to fully deplete oxygen in the sample. The oxygen depletion per 20 Gy FLASH was 3.3±0.1 mmHg (0.29 μM/Gy), and it was constant throughout the experiment, thus further confirming that the extent of depletion is independent of the starting oxygen level in solution. Furthermore, the total depletion per 20 Gy FLASH was shown to be independent of the FLASH dose rate in the range of 50 Gy/s-300 Gy/s (Fig. 3b). However, it proved to change linearly with the total delivered radiation dose (Fig. 3c), as expected.
Fig. 3.
Oxygen depletion upon FLASH irradiation of aqueous solutions (containing BSA 5%w/w). (a) Changes in pO2 upon application of a series of FLASH exposures, each delivering 20 Gy. (b) Reduction in oxygen upon application of FLASH at different dose rates at a fixed total dose of 20 Gy. No statistically significant difference was found between the dose rates used here, using ANOVA, with Tukeýs multiple comparison test, p-value< 0.05, for n=5. (c) Reduction in oxygen as a function of the total dose at a fixed dose rate (300 Gy/s).
In vivo experiments
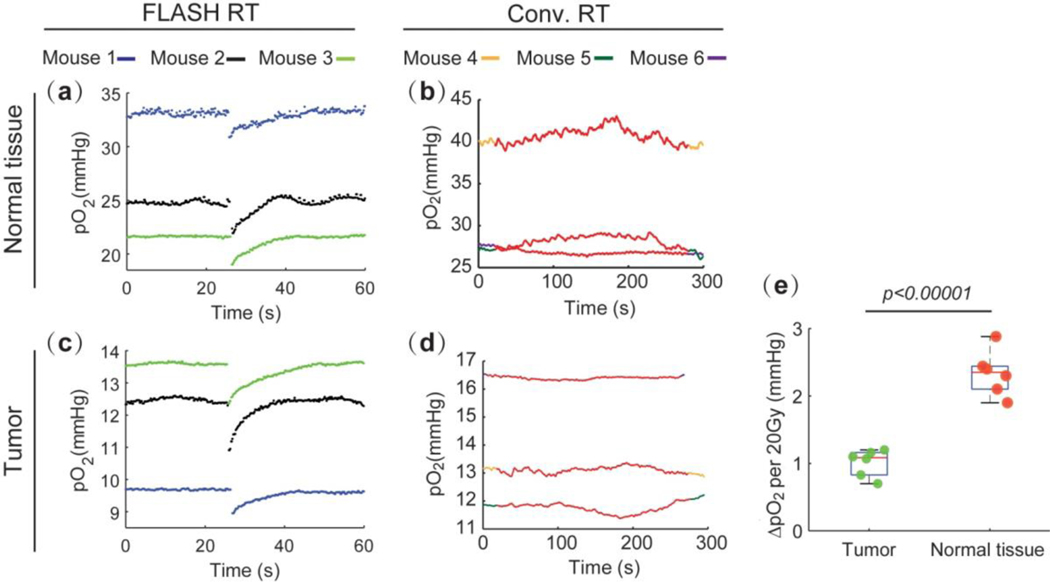
Nine mice with subcutaneous MDA-MB-231 tumors were treated with FLASH (6 mice) or alternatively with conventional (3 mice) radiation, where the total dose of 20 Gy was deposited either to the tumors or to contralateral normal tissues 24 h after intravenous injection of Oxyphor 2P. The pO2 was monitored continuously as radiation was applied. All mice were sacrificed immediately after the experiment. For clarity we show the oxygen depletion profiles only for 3 mice in the FLASH-treated group, although other groups showed similar results. Figs. 4(a) and (b) show the pO2 traces in normal tissues treated by FLASH vs conventional radiation. The pO2 clearly decreased upon application of FLASH, but no apparent changes were seen in the case of conventional irradiation. Similar results were obtained for tumors (Figs. 4(c, d)). The gap of ~450 ms in oxygen sampling during FLASH prevented us from quantifying the actual extent of oxygen depletion by FLASH, since oxygen could partially recover after the FLASH, but before the following reliable data point was recorded. However, the measured oxygen depletion in vivo is very close to that observed in our in vitro experiments, which were conducted in a closed system with no possibility for re-oxygenation. Furthermore, based on the temporal profiles of the recovery phase following the FLASH, we can estimate that during ~450 ms (the time between two data points encompassing FLASH) oxygen could increase by no more than 0.06 mmHg. Thus, the observed oxygen depletion is likely to be very close to the actual depletion by fast FLASH pulses.
Fig. 4.
Oxygen measurements in vivo. Oxygen transients in normal tissue upon application of FLASH (a) and conventional radiation (b). Oxygen transients in tumors upon application of FLASH (c) and conventional radiation (d). (e) Quantification of oxygen depletion in normal tissues and tumors upon FLASH irradiation (20 Gy). Statistical analysis was performed using two-sample student’s t-test, for n = 6.
FLASH irradiation was able to partially deplete oxygen, after which pO2 gradually recovered to the initial level. Interestingly, oxygen was found to be depleted more in normal tissues (higher average pO2) than in tumors (lower average pO2) (Fig. 4e). This finding might be important for the interpretation of the FLASH effect and requires further confirmation.
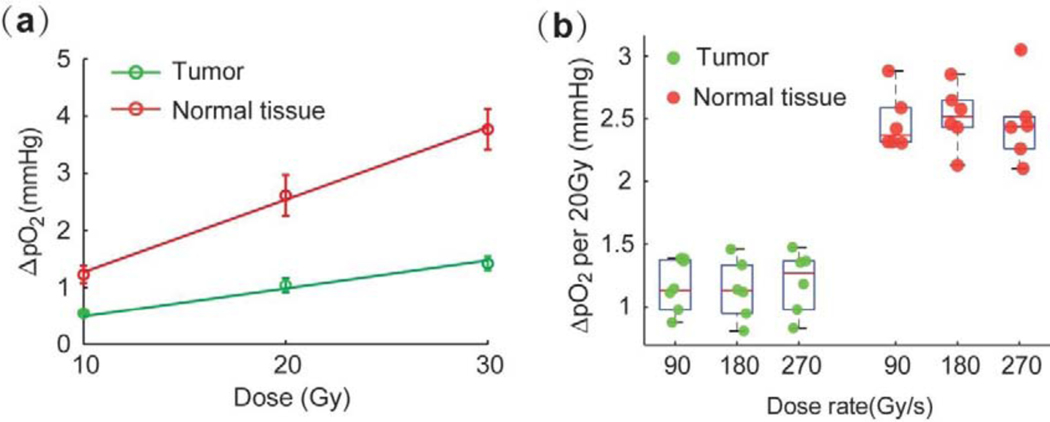
Additional experiments using 12 mice with subcutaneous MDA-MB-231 tumors were performed to determine whether pO2 levels in vivo are dependent on the total dose and/or dose rate of FLASH. To determine the effect of the total dose, 6 mice were exposed to 10Gy, 20Gy and 30Gy FLASH radiation doses at the same dose rate of 270 Gy/s. In each mouse, the radiation was applied to a tumor and to the contralateral normal leg tissue. To examine the effect of dose rate, 6 mice were treated with the total dose of 20Gy but at different dose rates. The rates were adjusted by varying the repetition rates of the electron pulses comprising the FLASH (120 pulse/sec, 240 pulse/sec and 360 pulse/sec) to achieve the average dose rates of 90Gy/s, 180Gy/s, and 270Gy/s. As in the in vitro experiments, these measurements revealed that the amount of depleted oxygen increases with the total radiation dose for both normal tissues and tumors (Fig. 5a). However, we could not detect a dependence on the dose rate for the high rates tested in these experiments, likely because the trend was buried in noise (Fig. 5b).
Fig. 5.
Influence of total dose and dose rate on oxygen depletion due to application of radiation in vivo. Oxygen depletion in normal tissues and in tumors upon FLASH irradiation at different total dose levels (a) and dose rates (b). No statistically significant difference was found between the dose rates, analyzed by ANOVA, with Tukeýs multiple comparison test (p-value < 0.05, for n=6).
Discussion
This work presents the first direct measurements of oxygen depletion in vivo by 10 MeV electron beam operating in FLASH mode. The measurements were performed using oxygen-dependent quenching of phosphorescence and the probe Oxyphor 2P (26). Our measurements show that application of UHDR irradiation with a clinically relevant single fraction dose (20 Gy) leads to oxygen depletion by 1–3 mmHg in intact tissue. The magnitude of this change is much smaller than would be required to achieve global tissue hypoxia in normal tissues that are well oxygenated. While the observed oxygen depletion could in principle result in radioprotective benefits of FLASH to the cell populations that are already at or near hypoxia (pO2~2–5 mmHg) (28,29), the previous studies that reported the tissue sparing effect of FLASH did not quantify the oxygen tension in the irradiation sites. Hence there seem to be no experimental evidence for attributing the FLASH effect to oxygen depletion (10). If there are microscopic niches in which pO2 is already very low, they may be exceptionally challenging to observe without resorting to microscopic methods. It is also worth noting that oxygen consumption by conventional irradiation is nearly impossible to measure in vivo because at low dose rates oxygen depletion is compensated by the continuous re-supply of oxygen by the blood. Thus, if oxygen depletion is the phenomenon that underpins the FLASH effect, the connection will require further proof and investigation.
As shown in Fig.2, G-values for oxygen depletion in aqueous solutions in vitro were found to be 0.19–0.21 mmHg/Gy (0.34–0.37 μM/Gy) for conventional irradiation, that is 18 ±2% higher than 0.16–0.17 mmHg/Gy (0.28–0.30 μM/Gy) for FLASH irradiation. Similar results were reported by Weiss et al (19), where the G-values for E.coli B/r bacterial suspensions were 0.35 mmHg/Gy for conventional irradiation using a 60Co source (0.2 Gy/s) and 0.14 mmHg/Gy for FLASH irradiation by a 3 ns electron pulses (2×1012 Gy/s) at 450 KeV. The differences between the g-values measured in our study and reported by Weiss et al could be due to the differences in the radiation sources (10 MeV electron beam at dose rate 0.1 Gy/s used here) as well as due to the differences between the measurement media. For example, G-values of 0.24 mmHg/Gy (21), 0.19 mmHg/Gy and 0.29 mmHg/Gy (20) were reported for pure water, phosphate buffer saline and cell growth media, respectively, irradiated by the same 60Co source. It is possible that the observed 18% decrease in oxygen consumption rate in FLASH vs conventional irradiation could be caused by self-quenching of the radical species produced by FLASH at high local instantaneous concentrations. As a result, for the same dose level FLASH potentially could inflict less radiobiological damage in vivo than conventional irradiation. In the future it will be interesting to explore whether the reduced oxygen depletion by FLASH may lead to information about the FLASH normal tissue-sparing effect.
Both FLASH and conventional radiation lead to consumption of oxygen in solutions in the presence of biological molecules (e.g. BSA) (Figs. 2, 3). The amount of depleted oxygen changed linearly with the total radiation dose for both irradiation types in the closed system in vitro. However, upon applying conventional irradiation in vivo, no oxygen depletion could be detected. This fact is consistent with the much lower rate of oxygen depletion by conventional irradiation than the rate at which oxygen is replenished in the irradiated volume by diffusion from the blood. In contrast, application of FLASH at a rate of >100 Gy/s results in fast local oxygen depletion, followed by a recovery period.
The degree of oxygen depletion by FLASH in normal tissue, quantified by G-values, was different in vitro vs in vivo, i.e. 0.16–0.17 mmHg/Gy (0.28–0.30 μM/Gy) vs 0.10–0.14 mmHg/Gy (0.14–0.20 μM/Gy). It is important to note that the extent of oxygen depletion in a medium containing organic molecules is dependent not only on the concentration of these molecules, but also on their composition. In this regard, BSA solutions can serve only as a rough approximation of the environment in vivo. For example, extravascular environment does not contain albumin, but contains other proteins and small organic molecules that can react with oxygen-derived species. Therefore, the apparent difference between oxygen scavenging in vitro and in vivo could be caused in part by different chemical environments. In addition, measurements in vitro and in vivo necessarily involved different measurement geometries. In the in vitro experiments the fibers were positioned near the location/depth within the vial where the deposition of the radiation was maximal. In the in vivo experiments the fibers were positioned near the tissue surface close to where the beam was projected onto the tissue. However, deposition of radiation is a function of depth, and it is possible that a larger fraction of the dose was deposited in deeper tissue layers. The contribution of that deeper tissue volume, in which oxygen depletion was maximal, to the overall phosphorescence decay, was mixed with contributions of the better oxygenated surface layers, and consequently it was smaller than in the in vitro experiment, where the depletion throughout the volume was more uniform. Thus, while our measurements showed consistently that the G-values for oxygen depletion by FLASH in vitro and in vivo were different, chemical heterogeneity of the environment in vivo combined with signal averaging effects makes it difficult to quantitatively compare in vitro and in vivo measurements.
Fig. 4 (e) shows that the total oxygen depletion by FLASH in tumors is only a half of that in normal tissues. However, our in vitro experiments suggest that these differences are not caused by the differences in the initial oxygen levels between tumors and normal tissues. One possible explanation is the existence of necrotic areas inside tumors, where the oxygen levels are already close to zero. The change (decrease) in oxygen upon irradiation of these volumes is therefore small, and it results in a smaller overall change in the signal averaged over the entire tumor volume. More in vivo experiments with different tumor sizes and tumor lines will be required to further confirm and interpret this effect. It is important to note that large variations exist in pO2 levels between different mice as shown in Fig.4, while the oxygen depletion changes were similar in all mice. The main reasons for the baseline differences are likely to be differences of the anesthesia levels, body temperature and other physiological differences between individual animals. Nevertheless, on average normal tissues were characterized by significantly higher pO2 values (20–40 mmHg) compared to tumors (5–15 mmHg).
When interpreting the pO2 values obtained in the reported experiments it is important to consider the distribution of Oxyphor 2P in tissue. Upon intravascular injection of the probe, oxygen measurements initially represent the vascular compartment. However, in tissues with leaky vasculature, such as tumors, over time the probe diffuses out from the vessels into the interstitial space. In the previously published work with dendritic oxygen probes (30), which had nearly identical chemical compositions to the one of Oxyphor 2P, e.g. Oxyphor PtG4 (31), we have shown than these probes accumulate in the interstitial space, presumably via the enhanced permeability and retention (EPR) effect, and remain there for days. Oxyphor 2P behaves in the same way, since its chemical composition and molecular size are similar to those of PtG4 and other dendritic sensors.
It is important to emphasize that all oxygen measurements reported in this study were extracellular oxygen measurements. When fully PEGylated dendritic oxygen probes such as Oxyphor 2P are free in solution, the immediate environment of the chromophore is defined by the encapsulating dendrimer, while the PEG residues make the probe chemically inert and prevent its binding to proteins, membranes, etc. The calibration constants of such probes are completely sustained in biological environments and oxygen measurements are quantitative. However, because of the PEGylated shell, Oxyphor 2P is unable to diffuse across biological membranes.
Under normal physiological conditions oxygen gradients across cellular membranes are negligible even under very high metabolic loads. However, the chemical composition of the intracellular milieu is different from that of the extracellular (interstitial) fluid. Therefore, one cannot rule out that upon irradiation at extremely high dose rates oxygen depletion inside cells, e.g. near nuclei where the DNA damage may occur, could be more pronounced than outside cells, and steeper than normal trans-membrane oxygen gradients could be temporarily created. Whether such gradients exist and if they are relevant to the FLASH mechanism would require additional investigation. However, it is likely that even if such gradients are formed, the diffusion of oxygen from the extracellular space into cells would erase these gradients within milliseconds after FLASH.
Conclusions
In summary, we reported the first direct measurements of oxygen transients in vivo induced by an UHDR electron beam. Global changes in the average tissue pO2 were found to be in the range of 1–3 mmHg upon deposition of 20 Gy dose at the rate of 270 Gy/s. These small oxygen changes appear to be insufficient to produce bulk tissue hypoxia for normally oxygenated tissues, although we cannot refute that this might contribute to radioprotective benefits for cell populations existing at very low oxygen levels, (<5 mmHg). The connection between oxygen depletion and FLASH effect will require further proof and investigation to explain the previous studies that reported the tissue sparing effect of FLASH.
Interestingly, for the same deposited dose in vitro oxygen consumption by FLASH appears to be lower by ~18% than consumption by conventional irradiation. This effect might be attributed to radical self-quenching at high radical concentrations instantaneously produced by FLASH. It is conceivable that partial self-annihilation of radical species and consequently lesser radiobiological tissue damage are related to the FLASH tissue-sparing effect, although further studies are required to demonstrate the connection.
Supplementary Material
Acknowledgments
Funding statement
This work was supported by the grants R01 EB024498 (BWP), U24 EB028941 and R21 EB027397 (S.A.V.) from the National Institutes of Health as well as by shared irradiation resources from the Norris Cotton Cancer Center (funded by P30 CA023108 (B.W.P.)).
Footnotes
Data sharing statement
All data generated and analyzed during this study are included in this published article.
Conflict of interest
B.W.P. is a founder and president of DoseOptics LLC, which develops camera systems and software for radiotherapy imaging of Cherenkov light for dosimetry. S.A.V. has partial ownership of Oxygen Enterprises Ltd, which owns the intellectual property for dendritic phosphorescent oxygen probes technology (US Pat. No. 9,556,213; US, 2017/0137449 A1). All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vozenin MC, Hendry JH, Limoli CL. Biological benefits of ultra-high dose rate flash radiotherapy: Sleeping beauty awoken. Clin Oncol (R Coll Radiol) 2019;31:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adrian G, Konradsson E, Lempart M, et al. The flash effect depends on oxygen concentration. Br J Radiol 2020;93:20190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jay-Gerin JP. Ultra-high dose-rate (flash) radiotherapy: Generation of early, transient, strongly acidic spikes in the irradiated tumor environment. Cancer Radiother 2020. [DOI] [PubMed] [Google Scholar]
- 4.Spitz DR, Buettner GR, Petronek MS, et al. An integrated physico-chemical approach for explaining the differential impact of flash versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol 2019;139:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimes DR, Partridge M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed Phys Eng Express 2015;1:045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100gy/s. Radiother Oncol 2017;124:365–369. [DOI] [PubMed] [Google Scholar]
- 7.Favaudon V, Fouillade C, Vozenin M. Ultrahigh dose-rate,” flash” irradiation minimizes the side-effects of radiotherapy. Cancer radiotherapie: journal de la Societe francaise de radiotherapie oncologique 2015;19:526–531. [DOI] [PubMed] [Google Scholar]
- 8.Diffenderfer ES, Verginadis II, Kim MM, et al. Design, implementation, and in vivo validation of a novel proton flash radiation therapy system. Int J Radiat Oncol Biol Phys 2020;106:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouillade C, Curras-Alonso S, Giuranno L, et al. Flash irradiation spares lung progenitor cells and limits the incidence of radio-induced senescence. Clin Cancer Res 2019. [DOI] [PubMed] [Google Scholar]
- 10.Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate flash irradiation increases the differential response between normal and tumor tissue in mice. Science translational medicine 2014;6:245ra93–245ra93. [DOI] [PubMed] [Google Scholar]
- 11.Vozenin MC, De Fornel P, Petersson K, et al. The advantage of flash radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res 2019;25:35–42. [DOI] [PubMed] [Google Scholar]
- 12.Soto LA, Casey KM, Wang J, et al. Flash irradiation results in reduced severe skin toxicity compared to conventional-dose-rate irradiation. Radiat Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewey D, Boag J. Modification of the oxygen effect when bacteria are given large pulses of radiation. Nature 1959;183:1450–1451. [DOI] [PubMed] [Google Scholar]
- 14.Epp ER, Weiss H, Santomasso A. The oxygen effect in bacterial cells irradiated with high-intensity pulsed electrons. Radiation research 1968;34:320–325. [PubMed] [Google Scholar]
- 15.Town C Effect of high dose rates on survival of mammalian cells. Nature 1967;215:847–848. [DOI] [PubMed] [Google Scholar]
- 16.Michaels HB, Epp ER, Ling CC, et al. Oxygen sensitization of cho cells at ultrahigh dose rates: Prelude to oxygen diffusion studies. Radiation research 1978;76:510–521. [PubMed] [Google Scholar]
- 17.Pratx G, Kapp DS. Ultra-high-dose-rate flash irradiation may spare hypoxic stem cell niches in normal tissues. International Journal of Radiation Oncology• Biology• Physics 2019;105:190–192. [DOI] [PubMed] [Google Scholar]
- 18.Petersson K, Adrian G, Butterworth K, et al. A quantitative analysis of the role of oxygen tension in flash radiotherapy. International Journal of Radiation Oncology• Biology• Physics 2020. [DOI] [PubMed] [Google Scholar]
- 19.Weiss H, Epp E, Heslin J, et al. Oxygen depletion in cells irradiated at ultra-high dose-rates and at conventional dose-rates. International Journal of Radiation Biology and Related Studies in Physics, Chemistry and Medicine 1974;26:17–29. [DOI] [PubMed] [Google Scholar]
- 20.Whillans D, Rauth A. An experimental and analytical study of oxygen depletion in stirred cell suspensions. Radiation Research 1980;84:97–114. [PubMed] [Google Scholar]
- 21.Michaels HB. Oxygen depletion in irradiated aqueous solutions containing electron affinic hypoxic cell radiosensitizers. International Journal of Radiation Oncology• Biology• Physics 1986;12:1055–1058. [DOI] [PubMed] [Google Scholar]
- 22.Popel AS. Theory of oxygen transport to tissue. Critical reviews in biomedical engineering 1989;17:257. [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman D Theoretical models of microvascular oxygen transport to tissue. Microcirculation 2008;15:795–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlier P, Bertoldi D, Baligand C, et al. Muscle blood flow and oxygenation measured by nmr imaging and spectroscopy. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo 2006;19:954–967. [DOI] [PubMed] [Google Scholar]
- 25.Vanderkooi JM, Maniara G, Green TJ, et al. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. Journal of Biological Chemistry 1987;262:5476–5482. [PubMed] [Google Scholar]
- 26.Esipova TV, Barrett MJ, Erlebach E, et al. Oxyphor 2p: A high-performance probe for deep-tissue longitudinal oxygen imaging. Cell Metabolism 2019;29:736–744. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman M, Ashraf MR, Zhang R, et al. Electron FLASH delivery at treatment room isocenter for efficient reversible conversion of a clinical LINAC. Int J Radiat Oncol Biol Phys., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Field S, Bewley D. Effects of dose-rate on the radiation response of rat skin. International Journal of Radiation Biology and Related Studies in Physics, Chemistry and Medicine 1974;26:259–267. [DOI] [PubMed] [Google Scholar]
- 29.Hendry JH, Moore JV, Hodgson B, et al. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiation Research 1982;92:172–181. [PubMed] [Google Scholar]
- 30.Lebedev AY, Cheprakov AV, Sakadzic S, et al. Dendritic phosphorescent probes for oxygen imaging in biological systems. ACS applied materials & interfaces 2009;1:1292–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X, Allu SR, Jiang S, et al. Tissue pO 2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy. Nat commun 2020;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.