Abstract
Cigarette smoking is a known cause of many cancers, yet epidemiological studies have found protective associations with the risk of four “estrogen-related” malignancies: endometrial cancer, endometrioid and clear cell ovarian cancers, and thyroid cancer. This review considers epidemiological and biological aspects of these associations, focusing particularly on estrogen signaling, and contrasts them with those for breast cancer, another estrogen-related malignancy. The observational findings regarding the inverse associations are consistent and remain after adjustment for possible confounding factors. In general, women who smoke do not have lower circulating estrogen levels than non-smokers, eliminating one possible explanation for reduced risks of these malignancies. For endometrial and endometrioid ovarian cancer, the negative associations could plausibly be explained by interference with signaling through the estrogen receptor alpha. However, this is unlikely to explain the lower risks of thyroid and clear cell ovarian cancers. For thyroid cancer, an anti-inflammatory effect of nicotine and reduced TSH levels from smoking have been proposed explanations for the inverse association, but both lack convincing evidence. While the overall impact of cigarette smoking is overwhelmingly negative, protective associations such as those discussed here can provide potential clues to disease etiology, treatment and prevention.
Keywords: endometrial cancer, ovarian cancer, thyroid cancer, cigarette smoking, estrogens
Cigarette smoking is a well-known serious health hazard, increasing the incidence and mortality of a host of chronic diseases (1). It causes cancer at many sites, most prominently those with direct contact with cigarette smoke such as the oropharynx, larynx, and lung (2). Cigarette smoking is also associated with increased risks of cancer in some organs that lack direct smoke contact such as those in the urinary tract and pancreas (2). In contrast, smoking has been associated with a reduced risk of a few cancers in organs lacking smoke contact: endometrial cancer (3,4), endometrioid and clear cell ovarian cancer (5) and thyroid cancer (6). Is there reason to believe that these protective associations are real?
One thread connecting these malignancies is their relationship to sex: all endometrial and ovarian cancers and most thyroid cancers develop in women. Not surprisingly, these cancers are often thought to be “estrogen-related.” Here, we summarize the associations of cigarette smoking and estrogens with these malignancies and consider possible explanations for reduced risks in smokers. We also consider another estrogen-related malignancy - breast cancer – for which smokers do not have a reduced risk.
Cancer of the endometrium
Cigarette smoking has a clear inverse association with risk of endometrial cancer. A combined analysis of data from cohort and case-control studies show that current smokers had a lower risk for all histological types investigated except the rare clear cell tumors (4). The association were seen in both case-control and cohort studies. For the most common histologies (those reported as endometriod, “type 1” or simply adenocarcinoma) there was a 35-40% reduction in risk. Odds ratios for former smokers were intermediate between those for current and never smokers. A meta-analysis showed that the smoking association was limited to post-menopausal women and may be stronger among those who used menopausal estrogens (3). In two cohort studies, the duration of premenopausal smoking was unrelated to risk, while the duration of smoking after menopause was inversely associated (7,8).
Cigarette smoking also has an impact on the non-neoplastic endometrium. In a cross-sectional analysis of a “representative sample of healthy postmenopausal women,” the endometria of smokers were more atrophic than that of non-smokers (9). Two case-control analyses of women who underwent endometrial sampling reported that smokers have a lower risk of endometrial hyperplasia (10,11).
An important question is whether the apparent protective association of smoking with endometrial cancer is due to the smoking itself, or to other characteristics of smokers that confer protective effects. Of particular interest are factors associated with both smoking and estrogenic stimulation, as endometrial cancer risk is greatly increased by exposure to estrogens acting through the estrogen receptor α (ERα) (12,13) (Table 1). Large cohort studies document that pre-diagnostic circulating estrogen levels are strongly associated with endometrial cancer incidence in post-menopausal women (see e.g. (14-16)), and a meta-analysis documents high risk with use of unopposed menopausal estrogens (17). Collaborative analysis of case-control and cohort studies (4) as well as meta-analyses (18,19) document that in post-menopausal women high body mass index (BMI) is a strong risk factor, reflecting estrogens generated in adipose tissue by metabolism of adrenal androgens (20). In addition, there are several reproductive factors that have been associated with endometrial cancer. Collaborative analysis and meta-analyses document that early age at menarche, late age at menopause, and low parity/nulliparity are risk factors (4,21-23). These are thought to act through the greater cumulative estrogenic stimulation generated by the larger number of normal menstrual cycles experienced by women with these risk factors (24).
Table 1.
Estrogen-related associations of endometrial cancer, endometrioid and clear cell ovarian cancers, breast cancer and thyroid cancer
| Endometrial cancer |
Endometrioid ovarian cancer |
Clear cell ovarian cancer |
Breast cancer | Thyroid Cancer |
|
|---|---|---|---|---|---|
| Unopposed exogenous estrogen | ↑ ↑ (17) | ↑ (33) | 0 or ↓ (33,39) | ↑? (74,75) | 0? (58,63-66) |
| Age at menopause | ↑ (22) | ↑ (34) | ↑ (34) | ↑ (70) | ? (48,51-53) |
| Age at menarche | ↓ (4,23) | 0 (34) | ↓ (34) | ↓ (70) | ↑? (48,50) |
| Parity | ↓ (4,21) | ↓ (34) | ↓ (34) | ↓ (72,73) | ↑ (48,49) |
| Post-menopausal BMI | ↑ (4,18,19) | ↑(34-36) | 0? (34-36) | ↑ (71) | ↑? (56-62) |
↑↑ greatly increased risk; ↑ increased risk; 0 no association; ↓ decreased risk; ? unclear; 0? Suggestion of no association; ↑? Suggestion of increased risk
Several of these risk factors are also associated with cigarette smoking, raising the possibility that they could confound the smoking/endometrial cancer association. Meta-analyses show that current smokers have an earlier menopause than non-smokers (25,26) and the adverse effects of smoking on fertility and fecundity are well documented (2,27). Cross-sectional population surveys show that current smokers have a lower body weight than never or former smokers (see, for example (28-31)). However, the protective endometrial cancer associations described above are seen after adjustment for these factors, and have been reported in studies of various designs conducted in a variety of populations. Given the strength of this evidence, the associations do not appear to be due to chance, bias, or confounding.
Cancers of the ovary
Although cigarette smoking is not associated with the overall risk of epithelial ovarian cancer (5), this is a heterogeneous malignancy, comprising different phenotypes with distinct molecular and clinical characteristics (32). Only mucinous cancers are derived from ovarian tissue itself. Serous tumors originate in the fallopian tubes, and endometrioid and clear cell ovarian cancers are thought to be derived from the endometrium, probably through retrograde menstruation. Recent combined analyses of individual level data from case-control and cohort studies have provided detailed risk factor data for ovarian cancer according to tumor histology. Endometrioid ovarian cancer has many of the same estrogenic risk factors as endometrial cancer: increasing risk with use of unopposed menopausal estrogens (33), lower parity and late age at menopause (34) (Table 1). Higher BMI has also been associated with increased risks of endometrioid ovarian cancer in collaborative analyses and meta-analyses (34-36), though the association in postmenopausal women specifically is less clear in the few studies that have investigated it (37,38). Clear cell cancers share some estrogenic risk factors, but there is at most a weak association with BMI (34-36) (even among postmenopausal women (38)) and no association or a reduced risk with use of unopposed estrogens (Table 1) (33,39). ERα is commonly expressed in endometrioid ovarian cancers, but not in clear cell tumors (40,41).
Collaborative analysis of case-control (5,42) and cohort studies (5) show that the associations of ovarian cancer with cigarette smoking differ according to the histology of the tumors. Smoking is not associated with serous ovarian cancer, and confers an increased risk of mucinous tumors. In contrast, the relationship of smoking with risk of endometrioid ovarian cancer resembles that for smoking and endometrial cancer. There is a reduction in risk of about 20% among current smokers that is attenuated in former smokers, and the associations are not confounded by factors such as BMI, use of hormone replacement therapy, oral contraceptive use, or reproductive history. There are no interactions of smoking status with BMI, alcohol use, parity, oral contraceptive use, menopausal hormone use, or family history of ovarian or breast cancer. Unlike endometrial cancer, the association with current smoking appears to be similar in pre- and post-menopausal women. Population-based case-control studies and cohort studies showed similar findings. There are less data available regarding smoking and clear cell ovarian cancer, but the inverse association for this malignancy is similar to that for endometrioid ovarian cancer.
Cancer of the Thyroid
The association of smoking with risk of thyroid cancer is also broadly similar to that for endometrial cancer: a meta-analysis (6) and a pooled analysis of cohort studies (43) found that current smokers (but not former smokers) have about a 25-30% lower risk than never smokers. This pattern is evident in women as well as in men (6). A combined analysis of cohort studies and the large Women’s Health Initiative cohort study document that the association remains after adjustment for alcohol intake and BMI (and for reproductive factors in women) (43,44). In addition, the combined cohort analyses reported that there is no apparent interaction of current smoking with age, sex, education, or BMI (43). The reduction in risk among current smokers is seen in both medullary and papillary thyroid cancer, though reductions in risk may be slightly larger for tumors with papillary histology (which comprise about 90% of thyroid cancers overall) (6,43),
An estrogen dependence of thyroid cancer is clearly evident in rodent models and cell culture studies (45,46), and ERα is expressed in this malignancy (46). Nonetheless, the epidemiological characteristics of an estrogen dependence are relatively weak (Table 1). Though incidence is higher among women than among men, mortality rates in the US are very similar (47). Meta-analysis and a pooled analysis of case-control studies show an increased risk among parous versus nulliparous women (though without a dose-response trend) (48,49) and there is a suggested increased risk with later age of menarche (48,50). Whether a late age of menopause is associated with an increased risk is not clear: a meta-analysis of cohort studies and the pooled case-control analysis (48,51) suggest that it is not, though two meta-analyses suggest that it is (52,53). In any case, the pooled analysis of case-control studies (48) and the Women’s Health Initiative cohort (54) show that women who have a surgical menopause have an increased risk. Meta-analysis (55) makes clear that thyroid cancer risk is positively associated with BMI in women. Studies that have assessed this in older or post-menopausal women in particular are generally consistent with a modest in increase in risk (56-62). Case-control (63) and cohort analyses (58,64-66) have not reported clear associations with unopposed menopausal estrogen therapy. Thus – at least epidemiologically -- thyroid cancer does not display the characteristics of an estrogen-sensitive malignancy.
A complicating factor in thyroid cancer epidemiology is the overdiagnosis of subclinical lesions, which has led to a dramatic increase in recorded incidence over the past several decades (47). Thus, differential surveillance might play a role in the higher incidence in women. However, it is unlikely to underlie the inverse association with smoking: the utilization of outpatient care by current smokers has been variously observed to be less than (67), greater than (68), or about the same as that of non-smokers (69).
Breast Cancer
Collaborative analysis of case-control and cohort studies of breast cancer show that the hormone receptor positive phenotypes share much of the estrogenic risk factor profile seen in endometrial cancer: increased risks associated with early menarche, late menopause, low parity, and high post-menopausal BMI (70-73) (Table 1). However, the effect of unopposed menopausal estrogens is not clear: observational studies have found users to have modestly increased risk (74), but the Women’s Health Initiative clinical trial found reduced risks (75) (Table 1).
In contrast to the other cancers considered here, cigarette smoking is not associated with a reduced risk of breast cancer. A pooled analysis of 14 cohort studies reported a small (7%) overall increase in risk among current smokers. There was a statistically significant interaction with alcohol drinking, with no increased risk among women who did not currently drink. Even the most estrogen-sensitive phenotypes, those that are ER positive or that have a luminal expression profile, do not display an inverse association with smoking: a pooled analysis of cohort studies reported a small increase in risk for ER positive breast cancer (76) and similar findings were reported for luminal phenotypes in population-based case-control studies (76-78).
An interesting detail is that the relationship between smoking and breast cancer seems to differ depending on the ages when the smoking occurs. A pooled analysis of cohort studies documents that smoking initiation early in life has been consistently associated with a small increase in risk, particularly among women who started smoking before their first term birth (76). On the other hand, three large cohort studies found that the duration of post-menopausal smoking was inversely associated with risk while that for premenopausal smoking tended to increase risk (79-81). This mirrors the pattern observed for endometrial cancer, in which post-menopausal smoking – but not premenopausal smoking - is associated with reduced risks (7,8).
Although smoking is not inversely associated with breast cancer risk, women who currently smoke have a lower radiographic breast density than former or never smokers (see, for example (82,83)). A recent comprehensive study from a health insurance plan included over 23,0000 women and provides the most precise estimates (83). The lower breast density was seen in both premenopausal and postmenopausal current smokers, and was largely due to an increase in the non-dense breast area rather than a reduction in the dense areas.
Cigarette Smoking and Circulating Estrogen Levels
One explanation for the inverse association of three estrogen-related cancers with cigarette smoking would be that smoking lowers concentrations of circulating estrogens. However, serum estrogen levels are not lower among women who smoke. Among premenopausal women, circulating estradiol has variously been found to be slightly higher in current smokers than in non-smokers (e.g. (84)) or similar (e.g. (85)). There are more data for post-menopausal women, conveniently summarized in a collaborative analysis. For them as well, current smokers have similar or slightly higher circulating estradiol and estrone than their non-smoking peers (86). Estimated (86) and measured (87) free estradiol levels show the same pattern. Clearly, differences in circulating estrogen levels cannot explain an inverse association of smoking with the estrogen-related cancers being considered here.
Post-menopausal women who both smoke and use oral estrogens do have lower serum estrogen levels than non-smokers, exhibiting as much as 50% lower circulating estradiol and estrone (88,89). This is due to hepatic first pass metabolism of the oral hormones induced by constituents of cigarette smoke, as discussed below. No differences are seen in women using parenteral (88) or percutaneous (89) estrogens. The lower achieved estrogen levels in women who smoke while using oral estrogens result in smaller improvements in bone mineral density and serum lipoprotein levels than for non-smoking postmenopausal women (89). There is as well a reduced estrogen-induced trophic effect on the endometrium (9). These findings could contribute to the reduced risk of estrogen-related cancers among women who actively smoke while taking menopausal estrogens. Nonetheless, in a large cohort study (90) and a hospital-based case-control study (90) the reduced risk of endometrial cancer among smokers was still found among those who used menopausal hormone therapy. (This issue has not been specifically investigated for endometrioid and clear cell ovarian cancers or thyroid cancer).
Cigarette Smoking, Nicotine and Aromatase
How could cigarette smoking impede estrogen-related carcinogenesis without reducing circulating estrogen levels? One possibility is through the reduction of local synthesis of estrogens in the organs at cancer risk (Table 2). In peripheral tissues, cytochrome P450-19 (CYP450-19); aromatase) metabolizes the androgens androstenedione and testosterone to estrone and estradiol, respectively (91). This is important for local estrogen signaling: in the breast and ovary, for example, tissue levels of estrogens are an order of magnitude higher than would be predicted from circulating levels (92). The enzyme is present in the proliferative endometrium, the ovary and the thyroid, and is expressed in breast, endometrial and thyroid cancers as well as in endometriosis and endometrioid ovarian cancer (93-96). Nicotine and other constituents of tobacco and tobacco smoke inhibit the enzyme in-vitro (97,98), and smokers have lower expression in the brain (99) and in the placentas of pregnant women at term (100). Smoking may also inhibit aromatase in breast cancer tissue (101). The effect of smoking on aromatase in endometrial, ovarian and thyroid cancers has not been investigated, but important effects on endometrial cancer are unlikely since aromatase expression is low in that malignancy (102).
Table 2.
Possible Mechanisms Underlying Reduced Risk of Estrogen-Related Cancers by Cigarette Smoking
| Cigarette Smoke Constituent | ||
|---|---|---|
| Nicotine | AhR agonists | |
| Endometrial Cancer | Aromatase inhibition? | AhR/ER cross talk |
| Endometrioid Ovarian Cancer | Anti-inflammatory effects? Aromatase inhibition |
ER/AhR cross talk |
| Clear Cell Ovarian Cancer | Anti-inflammatory effects? | ER/AhR cross talk? |
| Thyroid Cancer | Anti-inflammatory effects? Reduction of TSH levels? |
ER/AhR cross talk? |
? Entry an unlikely or uncertain contributor to an inverse association with cigarette smoking
In general, nicotine is not thought to have anti-neoplastic properties (103), though it can have anti-inflammatory effects, due at least in part to stimulation of α7 cholinergic receptors on immune cells (104). This can suppress macrophage secretion of inflammatory mediators such as IL-1β, TNFα, and IL-6; inhibit dendritic cell activity; and modulate T-cell differentiation (105,106). Observed consequences are an inhibition of Th1 and Th17 inflammation, and an increase in T-regulatory cell activity (107). Consistent with these effects, transdermal nicotine can reduce skin inflammation caused by UV light exposure (108) and induce remission in ulcerative colitis (109). Current smoking has been inversely associated with some inflammatory disorders: ulcerative colitis (110), pemphigus (111), and sarcoidosis (112), for example. But it certainly does not have a general anti-inflammatory impact, as it is positively associated with inflammatory diseases such as rheumatoid arthritis (113), systemic lupus erythematosus (114), and Crohn’s disease (110).
Inflammation appears to promote endometrial carcinogenesis independently of estrogens (115,116) so any anti-inflammatory effect of smoking could contribute to an anti-neoplastic impact on endometrial cancer. Nicotine’s anti-inflammatory effect could also be relevant for ovarian cancers derived from endometriotic tissue since endometriosis is clearly an inflammatory disorder and nicotine has been found to ameliorate it in experimental models (Table 2) (117). But translation of this finding to human ovarian cancer is not straightforward: some studies have reported lower endometriosis prevalence among women who smoke, but – as summarized in a meta-analysis -- overall there does not appear to be a substantial association (118).
Cigarette Smoking, Polycyclic Aromatic Hydrocarbons, and the Aryl Hydrocarbon Receptor (AhR)
In addition to nicotine, cigarette smoke contains close to 4000 chemicals, including tobacco-specific nitrosamines and over 500 polycyclic aromatic hydrocarbons (PAHs). Many of these are well known carcinogens (1). However, PAHs and other compounds in cigarette smoke have other important biological effects as ligands for the aryl hydrocarbon receptor (AhR) (119). In its classical function, the liganded AhR, together with its partner protein, the AhR nuclear translocator (ARNT), binds to specific binding sites in the promotor regions of target genes (the xenobiotic responsive element (XRE) containing a core GCTGC sequence). This initiates transcription of a broad spectrum of well-characterized genes and activation of pathways such as phase I and II xenobiotic metabolism (120,121). Cigarette smoke is rich in AhR ligands, many formed during the incomplete combustion of tobacco (119). The AhR receptor plays a role both in maintaining cellular homeostasis and in pathophysiology, and is widely expressed in malignancies, including all those discussed here (122,123).
The importance of AhR ligands in estrogen-sensitive tissues extends beyond metabolism of carcinogens, since there is well-established cross-talk between AhR and ERα signaling (Table 2, Figure). Some PAHs – or their metabolites – can act in vitro as weak selective estrogen receptor modulators (SERMs) (124), activating ERα and ERβ with a potency many orders of magnitude weaker than that of estradiol. These effects vary across tissues depending on expression levels of the receptors and cell context (124,125). But in general, AhR ligands, including 2,3,7,8-tetrachlorodibenzodioxin (TCDD, “dioxin”) and PAHs such as benzo[a]pyrene, benz[a]anthracene, and methylcholanthrene (3-MC) exhibit clear anti-estrogenic effects. In breast cancer cell lines that express ERα, they counter the effects of estradiol on proliferation, epidermal growth factor receptor (EGFR) levels and cyclin D expression, and downregulate ERα expression (126,127). Similar anti-estrogenic effects of AhR ligands are seen in endometrial cancer cell lines (128-130).
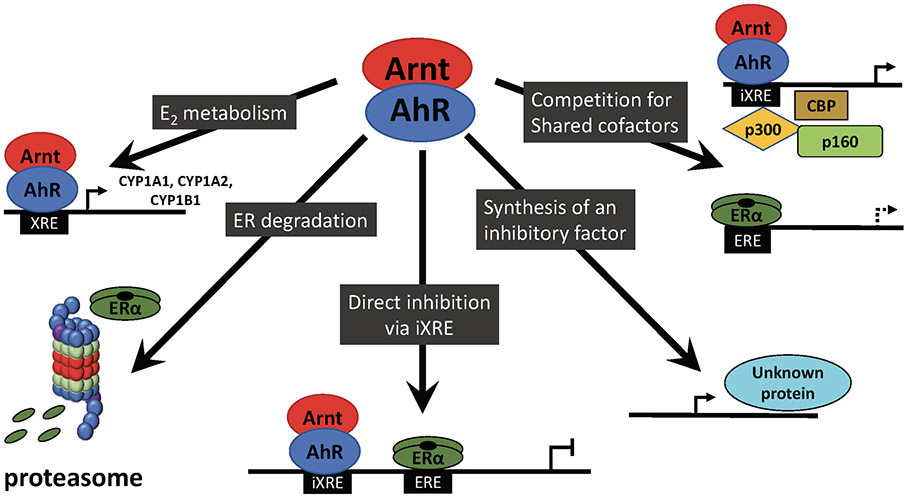
Figure 1.
Possible Mechanisms of AhR:ER crosstalk
Arnt, Aryl hydrocarbon nuclear translator; AhR, Aryl hydrocarbon receptor; XRE xenobiotic response element; iXRE incomplete xenobiotic response element; EREh estrogen response element; ERα, estrogen receptor α
The anti-estrogenic cross talk occurs through several pathways (Figure) (127,131). AhR signaling induces CYP1A1, CYP1A2, and CYP1B1, enzymes that metabolize the major estrogens estrone and estradiol to less active metabolites, thus reducing the local concentration of the most potent ERα agonists (132). CYP1A1 is responsible for the first pass metabolism of oral estrogens in the liver. Liganded AhR also promotes proteasomal degradation of ERα and competes with ERα for shared nuclear cofactors and coactivators. Further, AhR signaling has the potential to directly interfere with the transcription of ERα-regulated genes: binding of the liganded AhR complex to an inhibitory XRE in some of these genes disrupts the interactions of coactivators needed for ERα transcription. Finally, induction of a protein that inhibits estrogen signaling has also been observed (though not identified).
In-vivo, an anti-estrogenic impact of AhR ligands is evident. In ovariectomized rats, 3-MC attenuated expression of the majority of estradiol-induced genes in the mammary gland and endometrium, and reduced the development of mammary terminal end buds (133,134). AhR ligands also inhibited expression of the ERα and the progesterone receptor in the uteri of estradiol-treated rats (135-137) and reduced estrogen-induced rodent uterine growth (136,138,139). In a 2-year study of dioxin in rats, exposed animals had a reduced incidence of “uterine changes,” including endometrial hyperplasia, cyst formation and adenomatous polyps (140). In another study, an endogenous AhR agonist, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), suppressed endometrial cancer xenografts (130).
Given these observations, it is not surprising that cigarette smoke itself has shown anti-neoplastic effects in preclinical models of estrogen-related malignancies. Despite the fact that constituents of cigarette smoke, including some PAHs, are recognized mammary carcinogens (141), cigarette smoke reduces mammary tumor incidence in rats (142,143) (as does nicotine-dosed feeding with smokeless tobacco (144)). In one long term study, rats chronically exposed to cigarette smoke had fewer uterine and ovarian tumors than those unexposed (142).
Cigarette Smoking, Inflammation, Cell Proliferation and Thyroid Cancer
Smoking is associated with several thyroid disorders, including an increased prevalence of goiter in iodine-deficient areas, and an increased risk of Graves hyperthyroidism (145,146). However, these findings would not explain an apparent protective effect of smoking on thyroid cancer. An anti-estrogenic effect of cigarette smoking has been proposed as an explanation, but the lack of epidemiological markers of estrogen responsiveness for thyroid cancer etiology reduces the plausibility of this argument.
Chronic inflammation has a well-recognized carcinogenic impact (147) that may be relevant for thyroid cancer. Autoimmune (Hashimoto’s) thyroiditis is a chronic inflammation of the gland that has been associated with thyroid cancer in a series of cross-sectional studies (see, for example (148-150)) and may also be inversely associated with smoking. In population surveys in Western countries (151,152), current smokers have a lower prevalence of the autoantibodies associated with autoimmune thyroiditis (151,152), though this association may depend on iodine status (152,153) and findings in Asia have been mixed (see e.g. (153-156)). Data regarding the association of smoking with thyroiditis itself are conflicting. An early meta-analysis of two studies found increased risks in smokers (145). Subsequent investigations reported reduced risks (157,158) or null findings (for example, (159-161)). Interpretation of all these studies is hampered by differences in criteria for autoimmune thyroiditis, and the fact that many of the investigations suffer from an ill-defined study base, investigation of prevalent cases, small sample sizes, and/or unadjusted analyses. Furthermore, evidence for an association between thyroiditis and thyroid cancer is largely derived from cross-sectional studies that were prone to selection biases in which high risk patients were more likely to be confirmed as cancer cases (148,162). Given these uncertainties, an anti-inflammatory effect of smoking is also not a convincing explanation for the inverse association of smoking with thyroid cancer.
Another proposed explanation for a reduced risk of thyroid cancer in smokers is the lower levels of thyroid stimulating hormone (TSH, thyrotrophin) that has been documented in current smokers in population surveys ((152,156,163,164)). TSH is a growth factor for thyrocytes, and suppression of TSH secretion is used in the treatment of thyroid cancer (165). In cross-sectional studies of patients investigated for thyroid disease, those with thyroid cancer have higher TSH levels than those without (166), but in nested cohort analyses pre-diagnostic levels are not higher in future cases than in controls (167,168) and in a large Korean cohort study, adjustment for serum TSH only slightly attenuated the observed smoking association (169). TSH-supported proliferation may be needed for the progression of transformed cells, but TSH stimulation alone is not thought to lead to thyroid carcinogenesis (170,171). Thus, whether lower TSH levels explain the reduced thyroid cancer risk in smokers is uncertain.
Conclusions
The inverse associations between cigarette smoking and risk of endometrial cancer, endometrioid and clear cell ovarian cancers and thyroid cancer are reasonably well-documented. Studies of various designs in different populations have reported the findings, and confounding variables or obvious study biases do not readily explain the associations. The epidemiology is consistent with causal associations. In contrast, another estrogen-related cancer, breast cancer, does not display similar smoking associations.
Although women who smoke cigarettes do not have lower circulating estrogen levels than non-smokers, the hormonal milieu within a tissue is likely to be more relevant for estrogen-related carcinogenesis. Inhibition of aromatase and the anti-estrogenic AhR effects detailed above clearly have the potential to interfere with local effects of estrogens. For endometrial cancer, this AhR interference with estrogen signaling is a plausible explanation for a causal protective effect of smoking. Inhibition of aromatase and consequent interference with local estrogen production seems less relevant, given the low aromatase expression in that malignancy, but a possible anti-inflammatory effect of smoking could support an anti-neoplastic effect (Table 2).
Inhibition of aromatase and AhR interference with estrogen signaling both seem relevant as explanations for the reduced risk of endometrioid ovarian cancer in smokers. But for clear cell ovarian cancer, the lack of expression of ERα and the weaker epidemiological suggestions of an estrogen dependence make estrogen-related mechanisms much less compelling (Table 2). The possible anti-inflammatory effects of smoking could theoretically contribute to an anti-neoplastic effect of both endometrioid and clear cell ovarian cancers, but the lack of a clear association of smoking with endometriosis renders this uncertain.
Proposed explanations for the inverse association of smoking with risk of thyroid cancer are also unsatisfactory (Table 2). Since the epidemiology of this malignancy does not suggest a marked estrogen dependence, an anti-estrogenic effect of smoking is not a strong candidate. An anti-inflammatory impact of nicotine on thyroid cancer seems uncertain in light of the inconsistent data regarding the associations of smoking with thyroiditis and thyroiditis with thyroid cancer. Cigarette smoking does lower TSH levels, but it is not clear if that would be sufficient to lower risk of thyroid cancer. In summary, none of the proposed mechanisms for a protective effect of smoking on thyroid cancer risk are supported by compelling evidence (Table 2).
In contrast to the other estrogen-related cancers considered here, ER positive breast cancer is not inversely associated with cigarette smoking. Nonetheless, there are indications that post-menopausal smoking may temper any increased risks associated with premenopausal exposure. Together with the conflicting signals from preclinical studies, these findings suggest that smoking could have counterbalancing effects on breast carcinogenesis: a direct carcinogenic impact and a mitigating postmenopausal anti-estrogenic effect.
It is conceivable that cigarette smoking could impede carcinogenesis through pathways other than interference with estrogen signaling, hormonal regulation, or inflammation. The AhR is a “promiscuous” receptor that can respond to a wide variety of environmental (and endogenous) compounds drawn from many classes of substances (172). Recent research has shown that the AhR is involved in a range of biological processes relevant to carcinogenesis: cell cycle progression, cell adhesion, proliferation, and immune response, for example (173,174). Often this enhances carcinogenesis, but sometimes it can be anti-neoplastic (175,176). The extent to which these mechanisms explain the inverse associations of cigarette smoking with the cancers considered here have not been studied in any detail.
This reinforces an important point, that the effects of various AhR ligands and other constituents of cigarette smoke can differ. Moreover, AhR/ERα crosstalk is known to be context-dependent, varying across tissues with the expression of the AhR, ERα, ERβ, coactivators and suppressors of these receptors, and the various enzymes induced by the AhR (122). The fact that ERβ signaling often counteracts the effects of ERα (177) adds another element of complexity. ERβ is expressed in all the cancers discussed here (46,177,178), and may be particularly important for endometrioid and clear cell ovarian cancer because of its role in supporting the progression of endometriosis, which can evolve into these malignancies (94,179).
High BMI is a risk factor for endometrial cancer, endometrioid and clear cell ovarian cancers and thyroid cancer, but the impact of smoking on these malignancies cannot be interpreted as an “anti-obesity” effect of some sort. As noted above, current smokers do tend to have a lower BMI than non-smokers. But they also tend to have an abdominal distribution of adipose tissue, as reflected in a higher waist-to-hip ratio (180,181). This confers many of the adverse consequences of high BMI itself – including increased risks of endometrial, postmenopausal breast and thyroid cancers (55,182); an increased risk of diabetes (183); and decreased circulating adiponectin levels (184).
Although there are plausible mechanisms to explain smoking’s effect on endometrial cancer and endometrioid ovarian cancer, additional research will be required to more fully understand the inverse associations of smoking with clear cell ovarian cancer and thyroid cancer. Understanding what factors lead endometriosis to predispose to clear cell versus endometrioid ovarian cancer, with rather different estrogen associations, would contribute to the understanding of smoking’s association with the former. Clarification of the associations of thyroiditis with smoking on the one hand and thyroiditis with thyroid cancer on the other is needed to understand the role of inflammation in thyroid carcinogenesis and the impact of smoking on thyroid cancer incidence.
Whatever the possible beneficial effects of smoking described here, the overall impact of cigarettes on health is undeniably extremely negative. The value of the unexpected protective associations discussed here is that they can provide clues to disease etiology, treatment and prevention, as has been the case for smoking’s associations with ulcerative colitis and Parkinson’s disease (185). The fact that smoking appears to exert an anti-estrogenic effect through AhR signaling suggests that this pathway would be a productive avenue for research regarding prevention or treatment for estrogen-related malignancies. Indeed, use of selective AhR ligands for treatment of breast cancer, and perhaps other malignancies, is an active line of research (122) and the AhR-active drug aminoflavone (186,187) has been in clinical trials for treatment of breast and other cancers.
Acknowledgements:
The authors thank Dr. E. Robert Greenberg for helpful comments.
Financial support:
Dr. Safe was supported by P30-ES029067 from the National Institute of Environmental Health Sciences.
Footnotes
The authors declare no conflicts of interest.
References
- 1.US Deptartment of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease : a report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon Genera. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3.Zhou B, Yang L, Sun Q, Cong R, Gu H, Tang N, et al. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. Am J Med 2008;121(6):501–8 e3. [DOI] [PubMed] [Google Scholar]
- 4.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013;31(20):2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaborative Group on Epidemiological Studies of Ovarian Cancer, Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, et al. Ovarian cancer and smoking: individual participant meta-analysis including 28,114 women with ovarian cancer from 51 epidemiological studies. Lancet Oncol 2012;13(9):946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YA, Kim J. Thyroid cancer risk and smoking status: a meta-analysis. Cancer Causes Control 2014;25(9):1187–95. [DOI] [PubMed] [Google Scholar]
- 7.Weiderpass E, Baron JA. Cigarette smoking, alcohol consumption, and endometrial cancer risk: a population-based study in Sweden. Cancer Causes Control 2001;12(3):239–47. [DOI] [PubMed] [Google Scholar]
- 8.Al-Zoughool M, Dossus L, Kaaks R, Clavel-Chapelon F, Tjonneland A, Olsen A, et al. Risk of endometrial cancer in relationship to cigarette smoking: results from the EPIC study. Int J Cancer 2007;121(12):2741–7. [DOI] [PubMed] [Google Scholar]
- 9.Byrjalsen I, Haarbo J, Christiansen C. Role of cigarette smoking on the postmenopausal endometrium during sequential estrogen and progestogen therapy. Obstet Gynecol 1993;81(6):1016–21. [PubMed] [Google Scholar]
- 10.Weir HK, Sloan M, Kreiger N. The relationship between cigarette smoking and the risk of endometrial neoplasms. International journal of epidemiology 1994;23(2):261–6. [DOI] [PubMed] [Google Scholar]
- 11.Clarke MA, Long BJ, Sherman ME, Lemens MA, Podratz KC, Hopkins MR, et al. A prospective clinical cohort study of women at increased risk for endometrial cancer. Gynecol Oncol 2020;156(1):169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felix AS, Yang HP, Bell DW, Sherman ME. Epidemiology of Endometrial Carcinoma: Etiologic Importance of Hormonal and Metabolic Influences. Adv Exp Med Biol 2017;943:3–46. [DOI] [PubMed] [Google Scholar]
- 13.Kamal A, Tempest N, Parkes C, Alnafakh R, Makrydima S, Adishesh M, et al. Hormones and endometrial carcinogenesis. Horm Mol Biol Clin Investig 2016;25(2):129–48. [DOI] [PubMed] [Google Scholar]
- 14.Brinton LA, Trabert B, Anderson GL, Falk RT, Felix AS, Fuhrman BJ, et al. Serum Estrogens and Estrogen Metabolites and Endometrial Cancer Risk among Postmenopausal Women. Cancer Epidemiol Biomarkers Prev 2016;25(7):1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2010;127(2):442–51. [DOI] [PubMed] [Google Scholar]
- 16.Michels KA, Brinton LA, Wentzensen N, Pan K, Chen C, Anderson GL, et al. Postmenopausal Androgen Metabolism and Endometrial Cancer Risk in the Women's Health Initiative Observational Study. JNCI Cancer Spectr 2019;3(3):pkz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol 1995;85(2):304–13. [DOI] [PubMed] [Google Scholar]
- 18.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19(12):3119–30. [DOI] [PubMed] [Google Scholar]
- 19.Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol 2015;26(8):1635–48. [DOI] [PubMed] [Google Scholar]
- 20.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 2015;15(8):484–98. [DOI] [PubMed] [Google Scholar]
- 21.Wu QJ, Li YY, Tu C, Zhu J, Qian KQ, Feng TB, et al. Parity and endometrial cancer risk: a meta-analysis of epidemiological studies. Sci Rep 2015;5:14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Sun W, Liu H, Zhang D. Age at Menopause and Risk of Developing Endometrial Cancer: A Meta-Analysis. Biomed Res Int 2019;2019:8584130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong TT, Wang YL, Ma XX. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Sci Rep 2015;5:14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Key TJ, Pike MC. The dose-effect relationship between 'unopposed' oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 1988;57(2):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Midgette AS, Baron JA. Cigarette smoking and the risk of natural menopause. Epidemiology 1990;1(6):474–80. [DOI] [PubMed] [Google Scholar]
- 26.Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. International journal of epidemiology 2014;43(5):1542–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, et al. Effects of cigarette smoking on reproduction. Hum Reprod Update 2011;17(1):76–95. [DOI] [PubMed] [Google Scholar]
- 28.Audrain-McGovern J, Benowitz NL. Cigarette Smoking, Nicotine, and Body Weight. Clinical pharmacology & Therapeutics 2017;90(1):164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plurphanswat N, Rodu B. The association of smoking and demographic characteristics on body mass index and obesity among adults in the U.S., 1999–2012. BMC Obes 2014;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe T, Tsujino I, Konno S, Ito YM, Takashina C, Sato T, et al. Association between Smoking Status and Obesity in a Nationwide Survey of Japanese Adults. PLoS One 2016;11(3):e0148926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molarius A, Seidell JC, Kuulasmaa K, Dobson AJ, Sans S. Smoking and relative body weight: an international perspective from the WHO MONICA Project. J Epidemiol Community Health 1997;51(3):252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol 2011;42(7):918–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collaborative Group On Epidemiological Studies Of Ovarian C, Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, et al. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet 2015;385(9980):1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol 2016;34(24):2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer 2013;20(2):251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collaborative Group on Epidemiological Studies of Ovarian C. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med 2012;9(4):e1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer 2007;43(4):690–709. [DOI] [PubMed] [Google Scholar]
- 38.Yang HP, Trabert B, Murphy MA, Sherman ME, Sampson JN, Brinton LA, et al. Ovarian cancer risk factors by histologic subtypes in the NIH-AARP Diet and Health Study. Int J Cancer 2012;131(4):938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koskela-Niska V, Pukkala E, Lyytinen H, Ylikorkala O, Dyba T. Effect of various forms of postmenopausal hormone therapy on the risk of ovarian cancer--a population-based case control study from Finland. Int J Cancer 2013;133(7):1680–8. [DOI] [PubMed] [Google Scholar]
- 40.Shafrir AL, Rice MS, Gupta M, Terry KL, Rosner BA, Tamimi RM, et al. The association between reproductive and hormonal factors and ovarian cancer by estrogen-alpha and progesterone receptor status. Gynecol Oncol 2016;143(3):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen F, Zhang X, Zhang Y, Ding J, Chen Q. Hormone receptors expression in ovarian cancer taking into account menopausal status: a retrospective study in Chinese population. Oncotarget 2017;8(48):84019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faber MT, Kjaer SK, Dehlendorff C, Chang-Claude J, Andersen KK, Hogdall E, et al. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes Control 2013;24(5):989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitahara CM, Linet MS, Beane Freeman LE, Check DP, Church TR, Park Y, et al. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control 2012;23(10):1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabat GC, Kim MY, Wactawski-Wende J, Rohan TE. Smoking and alcohol consumption in relation to risk of thyroid cancer in postmenopausal women. Cancer Epidemiol 2012;36(4):335–40. [DOI] [PubMed] [Google Scholar]
- 45.Rajoria S, Suriano R, George AL, Shanmugam A, Jussim C, Shin EJ, et al. Estrogen activity as a preventive and therapeutic target in thyroid cancer. Biomed Pharmacother 2012;66(2):151–8. [DOI] [PubMed] [Google Scholar]
- 46.Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer 2014;21(5):T273–83. [DOI] [PubMed] [Google Scholar]
- 47.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140(4):317–22. [DOI] [PubMed] [Google Scholar]
- 48.Negri E, Dal Maso L, Ron E, La Vecchia C, Mark SD, Preston-Martin S, et al. A pooled analysis of case-control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control 1999;10(2):143–55. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J, Zhu X, Tu C, Li YY, Qian KQ, Jiang C, et al. Parity and thyroid cancer risk: a meta-analysis of epidemiological studies. Cancer Med 2016;5(4):739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mannathazhathu AS, George PS, Sudhakaran S, Vasudevan D, Krishna Km J, Booth C, et al. Reproductive factors and thyroid cancer risk: Meta-analysis. Head Neck 2019 [DOI] [PubMed] [Google Scholar]
- 51.Caini S, Gibelli B, Palli D, Saieva C, Ruscica M, Gandini S. Menstrual and reproductive history and use of exogenous sex hormones and risk of thyroid cancer among women: a meta-analysis of prospective studies. Cancer Causes Control 2015;26(4):511–8. [DOI] [PubMed] [Google Scholar]
- 52.Cao Y, Wang Z, Gu J, Hu F, Qi Y, Yin Q, et al. Reproductive Factors but Not Hormonal Factors Associated with Thyroid Cancer Risk: A Systematic Review and Meta-Analysis. Biomed Res Int 2015;2015:103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang P, Lv L, Qi F, Qiu F. Increased risk of papillary thyroid cancer related to hormonal factors in women. Tumour Biol 2015;36(7):5127–32. [DOI] [PubMed] [Google Scholar]
- 54.Luo J, Hendryx M, Manson JE, Liang X, Margolis KL. Hysterectomy, Oophorectomy, and Risk of Thyroid Cancer. J Clin Endocrinol Metab 2016;101(10):3812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev 2015;16(12):1042–54. [DOI] [PubMed] [Google Scholar]
- 56.Son H, Lee H, Kang K, Lee I. The risk of thyroid cancer and obesity: A nationwide population-based study using the Korea National Health Insurance Corporation cohort database. Surg Oncol 2018;27(2):166–71. [DOI] [PubMed] [Google Scholar]
- 57.Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guenel P. Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. Am J Epidemiol 2007;166(10):1140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabat GC, Kim MY, Wactawski-Wende J, Lane D, Wassertheil-Smoller S, Rohan TE. Menstrual and reproductive factors, exogenous hormone use, and risk of thyroid carcinoma in postmenopausal women. Cancer Causes Control 2012;23(12):2031–40. [DOI] [PubMed] [Google Scholar]
- 59.Leitzmann MF, Brenner A, Moore SC, Koebnick C, Park Y, Hollenbeck A, et al. Prospective study of body mass index, physical activity and thyroid cancer. Int J Cancer 2010;126(12):2947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin HY, Jee YH, Cho ER. Body mass index and incidence of thyroid cancer in Korea: the Korean Cancer Prevention Study-II. J Cancer Res Clin Oncol 2017;143(1):143–9. [DOI] [PubMed] [Google Scholar]
- 61.Sado J, Kitamura T, Sobue T, Sawada N, Iwasaki M, Sasazuki S, et al. Risk of thyroid cancer in relation to height, weight, and body mass index in Japanese individuals: a population-based cohort study. Cancer Med 2018;7(5):2200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song YM, Sung J, Ha M. Obesity and risk of cancer in postmenopausal Korean women. J Clin Oncol 2008;26(20):3395–402. [DOI] [PubMed] [Google Scholar]
- 63.Rossing MA, Voigt LF, Wicklund KG, Williams M, Daling JR. Use of exogenous hormones hormones and risk of papillary thyroid cancer (Washington, United States). Cancer Causes Control 1998;9(3):341–9. [DOI] [PubMed] [Google Scholar]
- 64.Horn-Ross PL, Canchola AJ, Ma H, Reynolds P, Bernstein L. Hormonal factors and the risk of papillary thyroid cancer in the California Teachers Study cohort. Cancer Epidemiol Biomarkers Prev 2011;20(8):1751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schonfeld SJ, Ron E, Kitahara CM, Brenner A, Park Y, Sigurdson AJ, et al. Hormonal and reproductive factors and risk of postmenopausal thyroid cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol 2011;35(6):e85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simin J, Tamimi R, Lagergren J, Adami HO, Brusselaers N. Menopausal hormone therapy and cancer risk: An overestimated risk? Eur J Cancer 2017;84:60–8. [DOI] [PubMed] [Google Scholar]
- 67.Miller EA, Pinsky PF. Healthcare Access, Utilization, and Preventive Health Behaviors by Eligibility for Lung Cancer Screening. J Cancer Educ 2019 [DOI] [PubMed] [Google Scholar]
- 68.Wacker M, Holle R, Heinrich J, Ladwig KH, Peters A, Leidl R, et al. The association of smoking status with healthcare utilisation, productivity loss and resulting costs: results from the population-based KORA F4 study. BMC Health Serv Res 2013;13:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kahende JW, Adhikari B, Maurice E, Rock V, Malarcher A. Disparities in health care utilization by smoking status--NHANES 1999-2004. Int J Environ Res Public Health 2009;6(3):1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collaborative Group on Hormonal Factors in Breast C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012;13(11):1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Proejct Expert Report 2018. Diet, nutrition, physical activity and breast cancer 2018. [Google Scholar]
- 72.Lambertini M, Santoro L, Del Mastro L, Nguyen B, Livraghi L, Ugolini D, et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer treatment reviews 2016;49:65–76. [DOI] [PubMed] [Google Scholar]
- 73.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res 2006;8(4):R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collaborative Group on Hormonal Factors in Breast Cancer. Type and Timing of Menopausal Hormone Therapy and Breast Cancer Risk: Individual Participant Meta-Analysis of the Worldwide Epidemiological Evidence. Lancet 2019;394(10204):1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, et al. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women's Health Initiative Randomized Clinical Trials. Jama 2020;324(4):369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaudet MM, Carter BD, Brinton LA, Falk RT, Gram IT, Luo J, et al. Pooled analysis of active cigarette smoking and invasive breast cancer risk in 14 cohort studies. International journal of epidemiology 2017;46(3):881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellingjord-Dale M, Vos L, Hjerkind KV, Hjartaker A, Russnes HG, Tretli S, et al. Alcohol, Physical Activity, Smoking, and Breast Cancer Subtypes in a Large, Nested Case-Control Study from the Norwegian Breast Cancer Screening Program. Cancer Epidemiol Biomarkers Prev 2017;26(12):1736–44. [DOI] [PubMed] [Google Scholar]
- 78.Butler EN, Tse CK, Bell ME, Conway K, Olshan AF, Troester MA. Active smoking and risk of Luminal and Basal-like breast cancer subtypes in the Carolina Breast Cancer Study. Cancer Causes Control 2016;27(6):775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dossus L, Boutron-Ruault MC, Kaaks R, Gram IT, Vilier A, Fervers B, et al. Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int J Cancer 2014;134(8):1871–88. [DOI] [PubMed] [Google Scholar]
- 80.Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med 2011;171(2):125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van den Brandt PA. A possible dual effect of cigarette smoking on the risk of postmenopausal breast cancer. European journal of epidemiology 2017;32(8):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacobsen KK, Lynge E, Vejborg I, Tjonneland A, von Euler-Chelpin M, Andersen ZJ. Cigarette smoking and mammographic density in the Danish Diet, Cancer and Health cohort. Cancer Causes Control 2016;27(2):271–80. [DOI] [PubMed] [Google Scholar]
- 83.McBride RB, Fei K, Rothstein JH, Alexeeff SE, Song X, Sakoda LC, et al. Alcohol and Tobacco Use in Relation to Mammographic Density in 23,456 Women. Cancer Epidemiol Biomarkers Prev 2020;29(5):1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verkasalo PK, Thomas HV, Appleby PN, Davey GK, Key TJ. Circulating levels of sex hormones and their relation to risk factors for breast cancer: a cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom). Cancer Causes Control 2001;12(1):47–59. [DOI] [PubMed] [Google Scholar]
- 85.Berta L, Frairia R, Fortunati N, Fazzari A, Gaidano G. Smoking effects on the hormonal balance of fertile women. Hormone research 1992;37(1-2):45–8. [DOI] [PubMed] [Google Scholar]
- 86.Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Roddam AW, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer 2011;105(5):709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brand JS, Chan MF, Dowsett M, Folkerd E, Wareham NJ, Luben RN, et al. Cigarette smoking and endogenous sex hormones in postmenopausal women. J Clin Endocrinol Metab 2011;96(10):3184–92. [DOI] [PubMed] [Google Scholar]
- 88.Geisler J, Omsjo IH, Helle SI, Ekse D, Silsand T, Lonning PE. Plasma oestrogen fractions in postmenopausal women receiving hormone replacement therapy: influence of route of administration and cigarette smoking. The Journal of endocrinology 1999;162(2):265–70. [DOI] [PubMed] [Google Scholar]
- 89.Jensen J, Christiansen C. Effects of smoking on serum lipoproteins and bone mineral content during postmenopausal hormone replacement therapy. Am J Obstet Gynecol 1988;159(4):820–5. [DOI] [PubMed] [Google Scholar]
- 90.Polesel J, Serraino D, Zucchetto A, Lucenteforte E, Dal Maso L, Levi F, et al. Cigarette smoking and endometrial cancer risk: the modifying effect of obesity. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation 2009;18(6):476–81. [DOI] [PubMed] [Google Scholar]
- 91.Simpson ER. Sources of estrogen and their importance. The Journal of steroid biochemistry and molecular biology 2003;86(3-5):225–30. [DOI] [PubMed] [Google Scholar]
- 92.Sissung TM, Price DK, Sparreboom A, Figg WD. Pharmacogenetics and regulation of human cytochrome P450 1B1: implications in hormone-mediated tumor metabolism and a novel target for therapeutic intervention. Mol Cancer Res 2006;4(3):135–50. [DOI] [PubMed] [Google Scholar]
- 93.Bulun SE, Simpson ER. Aromatase expression in women's cancers. Adv Exp Med Biol 2008;630:112–32. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Nicholes K, Shih IM. The Origin and Pathogenesis of Endometriosis. Annu Rev Pathol 2020;15:71–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dalla Valle L, Ramina A, Vianello S, Fassina A, Belvedere P, Colombo L. Potential for estrogen synthesis and action in human normal and neoplastic thyroid tissues. J Clin Endocrinol Metab 1998;83(10):3702–9. [DOI] [PubMed] [Google Scholar]
- 96.Kuhnel R, Delemarre JF, Rao BR, Stolk JG. Correlation of aromatase activity and steroid receptors in human ovarian carcinoma. Anticancer Res 1986;6(5):889–92. [PubMed] [Google Scholar]
- 97.Biegon A In vivo visualization of aromatase in animals and humans. Front Neuroendocrinol 2016;40:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barbieri RL, Gochberg J, Ryan KJ. Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. The Journal of clinical investigation 1986;77(6):1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Biegon A, Alexoff DL, Kim SW, Logan J, Pareto D, Schlyer D, et al. Aromatase imaging with [N-methyl-11C]vorozole PET in healthy men and women. J Nucl Med 2015;56(4):580–5. [DOI] [PubMed] [Google Scholar]
- 100.Huuskonen P, Amezaga MR, Bellingham M, Jones LH, Storvik M, Hakkinen M, et al. The human placental proteome is affected by maternal smoking. Reprod Toxicol 2016;63:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berstein LM, Larionov AA, Chernitsa OI, Kolesnik OS, Manikhas AG. [Aromatase activity and its gene expression in tumor tissue of smokers and non-smokers with breast cancer]. Vopr Onkol 1998;44(6):680–2. [PubMed] [Google Scholar]
- 102.Cornel KMC, Bongers MY, Kruitwagen R, Romano A. Local estrogen metabolism (intracrinology) in endometrial cancer: A systematic review. Mol Cell Endocrinol 2019;489:45–65. [DOI] [PubMed] [Google Scholar]
- 103.Grando SA. Connections of nicotine to cancer. Nat Rev Cancer 2014;14(6):419–29. [DOI] [PubMed] [Google Scholar]
- 104.Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol 2009;183(10):6681–8. [DOI] [PubMed] [Google Scholar]
- 105.Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, et al. Physiological functions of the cholinergic system in immune cells. J Pharmacol Sci 2017;134(1):1–21. [DOI] [PubMed] [Google Scholar]
- 106.Hoover DB. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Ther 2017;179:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang DW, Zhou RB, Yao YM, Zhu XM, Yin YM, Zhao GJ, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther 2010;335(3):553–61. [DOI] [PubMed] [Google Scholar]
- 108.Mills CM, Hill SA, Marks R. Transdermal nicotine suppresses cutaneous inflammation. Arch Dermatol 1997;133(7):823–5. [PubMed] [Google Scholar]
- 109.McGrath J, McDonald JW, Macdonald JK. Transdermal nicotine for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2004(4):CD004722. [DOI] [PubMed] [Google Scholar]
- 110.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc 2006;81(11):1462–71. [DOI] [PubMed] [Google Scholar]
- 111.Lai O, Recke A, Zillikens D, Kasperkiewicz M. Influence of cigarette smoking on pemphigus - a systematic review and pooled analysis of the literature. J Eur Acad Dermatol Venereol 2018;32(8):1256–62. [DOI] [PubMed] [Google Scholar]
- 112.Gomes JP, Watad A, Shoenfeld Y. Nicotine and autoimmunity: The lotus' flower in tobacco. Pharmacol Res 2018;128:101–9. [DOI] [PubMed] [Google Scholar]
- 113.Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther 2014;16(2):R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang F, Li S, Jia C. Smoking and the risk of systemic lupus erythematosus: an updated systematic review and cumulative meta-analysis. Clin Rheumatol 2015;34(11):1885–92. [DOI] [PubMed] [Google Scholar]
- 115.Kubyshkin AV, Aliev LL, Fomochkina II, Kovalenko YP, Litvinova SV, Filonenko TG, et al. Endometrial hyperplasia-related inflammation: its role in the development and progression of endometrial hyperplasia. Inflamm Res 2016;65(10):785–94. [DOI] [PubMed] [Google Scholar]
- 116.Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev 2005;14(12):2840–7. [DOI] [PubMed] [Google Scholar]
- 117.Yamada-Nomoto K, Yoshino O, Akiyama I, Ushijima A, Ono Y, Shima T, et al. Alpha-7 nicotinic acetylcholine receptor (nAChR) agonist inhibits the development of endometriosis by regulating inflammation. Am J Reprod Immunol 2016;76(6):491–8. [DOI] [PubMed] [Google Scholar]
- 118.Bravi F, Parazzini F, Cipriani S, Chiaffarino F, Ricci E, Chiantera V, et al. Tobacco smoking and risk of endometriosis: a systematic review and meta-analysis. BMJ open 2014;4(12):e006325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kitamura M, Kasai A. Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett 2007;252(2):184–94. [DOI] [PubMed] [Google Scholar]
- 120.Dertinger SD, Nazarenko DA, Silverstone AE, Gasiewicz TA. Aryl hydrocarbon receptor signaling plays a significant role in mediating benzo[a]pyrene- and cigarette smoke condensate-induced cytogenetic damage in vivo. Carcinogenesis 2001;22(1):171–7. [DOI] [PubMed] [Google Scholar]
- 121.Gebremichael A, Tullis K, Denison MS, Cheek JM, Pinkerton KE. Ah-receptor-dependent modulation of gene expression by aged and diluted sidestream cigarette smoke. Toxicol Appl Pharmacol 1996;141(1):76–83. [DOI] [PubMed] [Google Scholar]
- 122.Safe S, Lee SO, Jin UH. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicological sciences : an official journal of the Society of Toxicology 2013;135(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deuster E, Mayr D, Hester A, Kolben T, Zeder-Goss C, Burges A, et al. Correlation of the Aryl Hydrocarbon Receptor with FSHR in Ovarian Cancer Patients. Int J Mol Sci 2019;20(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu S, Abdelrahim M, Khan S, Ariazi E, Jordan VC, Safe S. Aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha in MCF-7 breast cancer cells. Biological chemistry 2006;387(9):1209–13. [DOI] [PubMed] [Google Scholar]
- 125.Swedenborg E, Pongratz I. AhR and ARNT modulate ER signaling. Toxicology 2010;268(3):132–8. [DOI] [PubMed] [Google Scholar]
- 126.Chaloupka K, Krishnan V, Safe S. Polynuclear aromatic hydrocarbon carcinogens as antiestrogens in MCF-7 human breast cancer cells: role of the Ah receptor. Carcinogenesis 1992;13(12):2233–9. [DOI] [PubMed] [Google Scholar]
- 127.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol 2003;16(7):807–16. [DOI] [PubMed] [Google Scholar]
- 128.Wormke M, Castro-Rivera E, Chen I, Safe S. Estrogen and aryl hydrocarbon receptor expression and crosstalk in human Ishikawa endometrial cancer cells. The Journal of steroid biochemistry and molecular biology 2000;72(5):197–207. [DOI] [PubMed] [Google Scholar]
- 129.Castro-Rivera E, Wormke M, Safe S. Estrogen and aryl hydrocarbon responsiveness of ECC-1 endometrial cancer cells. Mol Cell Endocrinol 1999;150(1-2):11–21. [DOI] [PubMed] [Google Scholar]
- 130.Bian Y, Li Y, Shrestha G, Wen X, Cai B, Wang K, et al. ITE, an endogenous aryl hydrocarbon receptor ligand, suppresses endometrial cancer cell proliferation and migration. Toxicology 2019;421:1–8. [DOI] [PubMed] [Google Scholar]
- 131.Tarnow P, Tralau T, Luch A. Chemical activation of estrogen and aryl hydrocarbon receptor signaling pathways and their interaction in toxicology and metabolism. Expert Opin Drug Metab Toxicol 2019;15(3):219–29. [DOI] [PubMed] [Google Scholar]
- 132.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 1998;19(1):1–27. [DOI] [PubMed] [Google Scholar]
- 133.Helle J, Keiler AM, Zierau O, Dorfelt P, Vollmer G, Lehmann L, et al. Effects of the aryl hydrocarbon receptor agonist 3-methylcholanthrene on the 17beta-estradiol regulated mRNA transcriptome of the rat uterus. The Journal of steroid biochemistry and molecular biology 2017 [DOI] [PubMed] [Google Scholar]
- 134.Helle J, Bader MI, Keiler AM, Zierau O, Vollmer G, Chittur SV, et al. Cross-Talk in the Female Rat Mammary Gland: Influence of Aryl Hydrocarbon Receptor on Estrogen Receptor Signaling. Environ Health Perspect 2016;124(5):601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Romkes M, Safe S. Comparative activities of 2,3,7,8-tetrachlorodibenzo-p-dioxin and progesterone as antiestrogens in the female rat uterus. Toxicol Appl Pharmacol 1988;92(3):368–80. [DOI] [PubMed] [Google Scholar]
- 136.Astroff B, Safe S. Comparative antiestrogenic activities of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 6-methyl-1,3,8-trichlorodibenzofuran in the female rat. Toxicol Appl Pharmacol 1988;95(3):435–43. [DOI] [PubMed] [Google Scholar]
- 137.McDougal A, Gupta MS, Morrow D, Ramamoorthy K, Lee JE, Safe SH. Methyl-substituted diindolylmethanes as inhibitors of estrogen-induced growth of T47D cells and mammary tumors in rats. Breast Cancer Res Treat 2001;66(2):147–57. [DOI] [PubMed] [Google Scholar]
- 138.Ohta R, Takagi A, Ohmukai H, Marumo H, Ono A, Matsushima Y, et al. Ovariectomized mouse uterotrophic assay of 36 chemicals. J Toxicol Sci 2012;37(5):879–89. [DOI] [PubMed] [Google Scholar]
- 139.Newman WC, Moon RC. Anti-uterotrophic response of immature mice to 3-methylcholanthrene. Nature 1969;221(5175):89. [DOI] [PubMed] [Google Scholar]
- 140.Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, et al. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol 1978;46(2):279–303. [DOI] [PubMed] [Google Scholar]
- 141.Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ Mol Mutagen 2002;39(2-3):119–26. [DOI] [PubMed] [Google Scholar]
- 142.Dalbey WE, Nettesheim P, Griesemer R, Caton JE, Guerin MR. Lifetime Exposures of Rats to Cigarette Tobacco Smoke. In: Sanders CL, Cross FT, Dagle GE, Mahaffey JA, editors. Pulmonary Toxicology of Respirable Particles Proceedings of the Nineteenth Annual Hanford Life Sciences Symposium; October 22–24, 1979; Springfield (VA): U.S. Department of Energy; 1980. p 522–35. [Google Scholar]
- 143.Davis BR, Whitehead JK, Gill ME, Lee PN, Butterworth AD, Roe FJ. Response of rat lung to inhaled tobacco smoke with or without prior exposure to 3,4-benzpyrene (BP) given by intratracheal instillation. Br J Cancer 1975;31(4):469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Theophilus EH, Hayes JR, Ayres PH, Morgan WT, Potts RJ, Garner CD, et al. Toxicological evaluation of smokeless tobacco: 2-year chronic toxicity and carcinogenicity feeding study in Wistar Han rats. Exp Toxicol Pathol 2015;67(10):539–50. [DOI] [PubMed] [Google Scholar]
- 145.Vestergaard P Smoking and thyroid disorders--a meta-analysis. Eur J Endocrinol 2002;146(2):153–61. [DOI] [PubMed] [Google Scholar]
- 146.Knudsen N, Brix TH. Genetic and non-iodine-related factors in the aetiology of nodular goitre. Best Pract Res Clin Endocrinol Metab 2014;28(4):495–506. [DOI] [PubMed] [Google Scholar]
- 147.Todoric J, Antonucci L, Karin M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev Res (Phila) 2016;9(12):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab 2013;98(2):474–82. [DOI] [PubMed] [Google Scholar]
- 149.Lai X, Xia Y, Zhang B, Li J, Jiang Y. A meta-analysis of Hashimoto's thyroiditis and papillary thyroid carcinoma risk. Oncotarget 2017;8(37):62414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kitahara CM, KRF D, Jorgensen JOL, Cronin-Fenton D, Sorensen HT. Benign Thyroid Diseases and Risk of Thyroid Cancer: A Nationwide Cohort Study. J Clin Endocrinol Metab 2018;103(6):2216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pedersen IB, Laurberg P, Knudsen N, Jorgensen T, Perrild H, Ovesen L, et al. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol 2008;158(3):367–73. [DOI] [PubMed] [Google Scholar]
- 152.Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2004;89(12):6077–86. [DOI] [PubMed] [Google Scholar]
- 153.Jia M, Shi X, Gu X, Guan H, Teng X, Teng D, et al. Smoking Is Positively Associated with Antithyroperoxidase Antibodies and Antithyroglobulin Antibodies in Populations with Mildly Deficient Iodine Intake. Biol Trace Elem Res 2019;187(2):383–91. [DOI] [PubMed] [Google Scholar]
- 154.Cho NH, Choi HS, Kim KW, Kim HL, Lee SY, Choi SH, et al. Interaction between cigarette smoking and iodine intake and their impact on thyroid function. Clin Endocrinol (Oxf) 2010;73(2):264–70. [DOI] [PubMed] [Google Scholar]
- 155.Kim SJ, Kim MJ, Yoon SG, Myong JP, Yu HW, Chai YJ, et al. Impact of smoking on thyroid gland: dose-related effect of urinary cotinine levels on thyroid function and thyroid autoimmunity. Sci Rep 2019;9(1):4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Y, Shi L, Zhang Q, Peng N, Chen L, Lian X, et al. The association between cigarette smoking and serum thyroid stimulating hormone, thyroid peroxidase antibodies and thyroglobulin antibodies levels in Chinese residents: A cross-sectional study in 10 cities. PLoS One 2019;14(11):e0225435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Galanti MR, Cnattingius S, Granath F, Ekbom-Schnell A, Ekbom A. Smoking and environmental iodine as risk factors for thyroiditis among parous women. European journal of epidemiology 2007;22(7):467–72. [DOI] [PubMed] [Google Scholar]
- 158.Effraimidis G, Strieder TG, Tijssen JG, Wiersinga WM. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. Eur J Endocrinol 2011;164(1):107–13. [DOI] [PubMed] [Google Scholar]
- 159.Carle A, Bulow Pedersen I, Knudsen N, Perrild H, Ovesen L, Banke Rasmussen L, et al. Smoking cessation is followed by a sharp but transient rise in the incidence of overt autoimmune hypothyroidism - a population-based, case-control study. Clin Endocrinol (Oxf) 2012;77(5):764–72. [DOI] [PubMed] [Google Scholar]
- 160.Prummel MF, Wiersinga WM. Smoking and risk of Graves' disease. Jama 1993;269(4):479–82. [PubMed] [Google Scholar]
- 161.Rendina D, De Palma D, De Filippo G, De Pascale F, Muscariello R, Ippolito R, et al. Prevalence of simple nodular goiter and Hashimoto's thyroiditis in current, previous, and never smokers in a geographical area with mild iodine deficiency. Horm Metab Res 2015;47(3):214–9. [DOI] [PubMed] [Google Scholar]
- 162.Castagna MG, Belardini V, Memmo S, Maino F, Di Santo A, Toti P, et al. Nodules in autoimmune thyroiditis are associated with increased risk of thyroid cancer in surgical series but not in cytological series: evidence for selection bias. J Clin Endocrinol Metab 2014;99(9):3193–8. [DOI] [PubMed] [Google Scholar]
- 163.Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med 2007;167(13):1428–32. [DOI] [PubMed] [Google Scholar]
- 164.Jorde R, Sundsfjord J. Serum TSH levels in smokers and non-smokers. The 5th Tromso study. Exp Clin Endocrinol Diabetes 2006;114(7):343–7. [DOI] [PubMed] [Google Scholar]
- 165.Nieto H, Boelaert K. WOMEN IN CANCER THEMATIC REVIEW: Thyroid-stimulating hormone in thyroid cancer: does it matter? Endocr Relat Cancer 2016;23(11):T109–T21. [DOI] [PubMed] [Google Scholar]
- 166.Su A, Zhao W, Wu W, Wei T, Ruan M, Li Z, et al. The association of preoperative thyroid-stimulating hormone level and the risk of differentiated thyroid cancer in patients with thyroid nodules: A systematic review and meta-analysis. Am J Surg 2020 [DOI] [PubMed] [Google Scholar]
- 167.Huang H, Rusiecki J, Zhao N, Chen Y, Ma S, Yu H, et al. Thyroid-Stimulating Hormone, Thyroid Hormones, and Risk of Papillary Thyroid Cancer: A Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev 2017;26(8):1209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rinaldi S, Plummer M, Biessy C, Tsilidis KK, Ostergaard JN, Overvad K, et al. Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. Journal of the National Cancer Institute 2014;106(6):dju097. [DOI] [PubMed] [Google Scholar]
- 169.Cho A, Chang Y, Ahn J, Shin H, Ryu S. Cigarette smoking and thyroid cancer risk: a cohort study. Br J Cancer 2018;119(5):638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xing M Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013;13(3):184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Williams ED. Mechanisms and pathogenesis of thyroid cancer in animals and man. Mutat Res 1995;333(1-2):123–9. [DOI] [PubMed] [Google Scholar]
- 172.Denison MS, Faber SC. And Now for Something Completely Different: Diversity in Ligand-Dependent Activation of Ah Receptor Responses. Curr Opin Toxicol 2017;2:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicological sciences : an official journal of the Society of Toxicology 2011;124(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 2019;19(3):184–97. [DOI] [PubMed] [Google Scholar]
- 175.Kolluri SK, Jin UH, Safe S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Archives of toxicology 2017;91(7):2497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Feng S, Cao Z, Wang X. Role of aryl hydrocarbon receptor in cancer. Biochim Biophys Acta 2013;1836(2):197–210. [DOI] [PubMed] [Google Scholar]
- 177.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Archives of toxicology 2012;86(10):1491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chan KKL, Siu MKY, Jiang YX, Wang JJ, Wang Y, Leung THY, et al. Differential expression of estrogen receptor subtypes and variants in ovarian cancer: effects on cell invasion, proliferation and prognosis. BMC Cancer 2017;17(1):606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Andersen CL, Boisen MM, Sikora MJ, Ma T, Tseng G, Suryawanshi S, et al. The Evolution of Estrogen Receptor Signaling in the Progression of Endometriosis to Endometriosis-Associated Ovarian Cancer. Horm Cancer 2018;9(6):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes Res 2005;13(8):1466–75. [DOI] [PubMed] [Google Scholar]
- 181.Morris RW, Taylor AE, Fluharty ME, Bjorngaard JH, Asvold BO, Elvestad Gabrielsen M, et al. Heavier smoking may lead to a relative increase in waist circumference: evidence for a causal relationship from a Mendelian randomisation meta-analysis. The CARTA consortium. BMJ open 2015;5(8):e008808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Research. WCRFAIfC. Continuous Update Project Expert Report 2018. Body fatness and weight gain and the risk of cancer. 2018. [Google Scholar]
- 183.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama 2007;298(22):2654–64. [DOI] [PubMed] [Google Scholar]
- 184.Kotani K, Hazama A, Hagimoto A, Saika K, Shigeta M, Katanoda K, et al. Adiponectin and smoking status: a systematic review. J Atheroscler Thromb 2012;19(9):787–94. [DOI] [PubMed] [Google Scholar]
- 185.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 2016;15(12):1257–72. [DOI] [PubMed] [Google Scholar]
- 186.Itkin B, Breen A, Turyanska L, Sandes EO, Bradshaw TD, Loaiza-Perez AI. New Treatments in Renal Cancer: The AhR Ligands. Int J Mol Sci 2020;21(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baker JR, Sakoff JA, McCluskey A. The aryl hydrocarbon receptor (AhR) as a breast cancer drug target. Med Res Rev 2020;40(3):972–1001. [DOI] [PubMed] [Google Scholar]