SUMMARY
Physical activity (PA) is widely considered to improve sleep, but a comprehensive review of the research on this topic has not been performed. In this umbrella review, conducted initially for the 2018 Physical Activity Guidelines for Americans Advisory Committee and updated to reflect more recent research, we examined whether PA enhances sleep outcomes across the lifespan as well as among individuals with sleep disorders. Systematic reviews and meta-analyses were utilized to assess the evidence. We also examined dose-response considerations and whether the association between PA and sleep was moderated by various factors (e.g., timing, sociodemographic characteristics). We found strong evidence that both acute bouts of PA and regular PA improved sleep outcomes. Moderate evidence indicated that longer bouts of PA (both acute and regular) improved sleep, and that the effects of PA on sleep outcomes were generally preserved across adult age groups and sex. Finally, moderate evidence demonstrated that PA improved sleep in adults with insomnia symptoms or obstructive sleep apnea. Several important areas in need of future research were also identified. Overall, the review supported the claim that PA improves sleep, but highlighted gaps that need to be addressed to facilitate more widespread utilization of PA for improving sleep.
Keywords: exercise, insomnia, obstructive sleep apnea, physical activity, sedentary behavior, sleep
INTRODUCTION
Sleep is an essential behavior that is important for a broad range of functions including neural development, learning, memory, emotional regulation, immune function, and cardiovascular and metabolic health [1,2]. As such, sleep is a key determinant of health and well-being across the lifespan, with a multitude of health consequences associated with insufficient or poor sleep (e.g., impaired daytime function, cardiovascular disease, obesity) [3].
Despite its biological necessity, insufficient and/or poor sleep is widespread. Over 50% of children and adolescents [4] and 35% of adults [5] report obtaining too little sleep on a regular basis compared to age-specific recommendations. Approximately 20% of children and adults report sleep complaints that occur often or ≥ 3 nights/week, respectively [6,7]. Moreover, sleep disorders are observed from childhood through older adulthood, with insomnia and obstructive sleep apnea (OSA) the most prevalent [8–10]. With its high prevalence and associated adverse health consequences, poor sleep constitutes a high public health burden [11]; thus, it is important to identify and promote ways to improve sleep that are safe and easily accessible.
Physical activity (PA) is often endorsed as a non-pharmacologic way to improve sleep [12–14], and multiple reviews have recently summarized the literature examining the impact of PA on specific sleep problems (e.g., insomnia, OSA) [15,16] or its impact on groups among whom disturbed sleep is prevalent (e.g., older adults, cancer patients) [17,18]. However, a review providing an overview of the current evidence on the relationships between PA and sleep across the lifespan, including individuals with and without sleep complaints, remains needed. This information is essential to inform public health recommendations, guide future research, and identify how PA may be optimally prescribed to improve sleep.
To support the development of the 2018 Physical Activity Guidelines for Americans [19], the Physical Activity Guidelines Advisory Committee (PAGAC) reviewed the scientific literature regarding the impact of PA on sleep [20]. This review serves to update the review conducted by the PAGAC. In addition to examining the evidence base regarding the associations between PA and sleep, the current review aims to address the following questions: 1) Is there a dose-response relationship for either acute bouts of PA or regular PA? If yes, what is the shape of the relationship?; 2) Does the relationship between PA and sleep vary by age, sex, race/ethnicity, socioeconomic status, or weight status?; and 3) Does the relationship between PA and sleep exist for individuals with impaired sleep behaviors or disorders? If yes, for which sleep disorders? [20].
METHODS
This umbrella review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [21]. The protocol for this review was prospectively registered at PROSPERO (#CRD42018096732).
Search Strategy and Inclusion Criteria
An overview of the methods used to conduct the systematic reviews that informed the PAGAC Scientific Report have been described in detail elsewhere [20,22]. Briefly, a literature search was conducted using the Cochrane, Cumulative Index of Nursing and Allied Health Literature (CINAHL), and PubMed electronic databases. The original search included papers published from 2006 to July 24, 2017 (i.e., for informing the PAGAC report); the search was expanded to include literature published through May 6, 2020, for this updated review.
Inclusion criteria were predefined and papers were considered potentially eligible if: 1) they were systematic reviews, meta-analyses, or pooled analyses of experimental or observational studies; 2) were published in English; 3) reported on studies with PA as the intervention/exposure and sleep as the outcome. The PA intervention/exposure could be any type or intensity of PA and either acute or regular PA. A variety of sleep variables, including sleep quality, sleep architecture (e.g., slow-wave sleep), and sleep disorder symptoms (e.g., apnea-hypopnea index for OSA), were included as outcomes. Healthy people, people with psychiatric disorders or cognitive impairment, and people with impaired sleep or sleep disorders of all ages were included. Reviews focused on people with chronic conditions (other than psychiatric conditions or cognitive impairment), people living in long-term care, hospitalized patients, athletes, or animals were excluded. The full search strategy is provided in Table S1.
Article Selection Process
The titles and abstracts of papers identified in the original and updated literature searches were independently screened by two reviewers (CHH, KEP, RFM, or CEK). Full texts of relevant articles were reviewed to identify papers meeting the inclusion criteria. Disagreement between reviewers was resolved by discussion or by a third person (KIE). Figure 1 depicts the search strategy and article review process for the original and updated searches. Table S2 provides a list of articles excluded following abstract screening or full-text review.
Figure 1. Flow diagram of literature search strategy and article selection.
The original literature search was conducted on July 24, 2017; the updated search was conducted on May 6, 2020. CINAHL: Cumulative Index of Nursing and Allied Health Literature.
Data Extraction and Methodological Study Quality Assessment
Study characteristics from the selected articles (e.g., number of studies, types of studies included, whether dose-response relationships were addressed, effect sizes) were extracted by trained abstractors. The quality of the selected articles was assessed using a tailored version of A Measurement Tool to Assess Systematic Reviews (AMSTAR) [23] previously used to evaluate the methodological quality of meta-analyses focused on exercise and blood pressure (AMSTARExBP) [24]. Table S3 provides a quality assessment of each included study.
Grading of Evidence
Following the original literature search and data extraction, the PAGAC evaluated the evidence. Given the diversity of topics considered by the PAGAC and the diverse bodies and types of literature available for the many topics, the PAGAC chose not to develop a specific point system for the evaluation process. Instead, PAGAC members considered the following criteria to determine the general strength of the evidence, guided by a standardized rubric (see Table 1 of [22]): a) applicability of the study sample, exposures, and outcomes to the research question, b) generalizability to the population of interest, c) risk of bias/study limitations, d) quantity and consistency of findings across studies, and e) magnitude and precision of the effect. The evidence was examined and discussed first by, in this case, three members of the PAGAC Brain Health Subcommittee, then by the full Subcommittee (5 members, 2 consultants), and, finally, by the full 17-member PAGAC. Through these discussions, final evidence grades and conclusion statements for each research question were developed. Consensus by the full PAGAC was required, and all concerns were resolved by discussion. For the updated search, the new information was reviewed by the entire authorship team to determine whether the evidence grades and conclusions made in the 2018 PAGAC report should be revised.
Table 1.
Descriptive summary of articles selected for inclusion in the umbrella review.
| Article | Review type/ Studies reviewed | Sample/ ages studied | Total/ relevant studies1 | PA exposure | Sleep outcomes | D-R? | Effect modifiers? |
|---|---|---|---|---|---|---|---|
| Aiello et al., 2016 [25] * | SR, MA: Experimental (RCT, single-group pre-posttest) | Adults with OSA (mean ages 32–54 y) | 8 / 8 | Exercise programs (≥ 2 mo duration; aerobic, resistance, and/or oropharyngeal) | AHI, ESS | No | No |
| Alessi & Vitiello, 2015 [26] * | SR: RCT | Older adults (≥ 60 y) with insomnia | 14 / 2 | Moderate-intensity exercise (e.g., tai chi, walking; ≥ 3 times/wk, ≥ 16 wk) | Self-reported sleep quality (PSQI) | No | No |
| Antczak et al., 2020 [27] | SR, MA: Observational (cross-sectional, longitudinal) and experimental | Healthy children (3–13 y) | 47 / 47 | Observational: self-reported or accelerometer-assessed PA; experimental: acute moderate- to vigorous-intensity exercise | Self-reported (e.g., diary, questionnaire) or objective (accelerometry, PSG) sleep parameters | Yes | Gender; age |
| Banno et al., 2018 [28] | SR, MA: RCT | Adults with sleep complaints or insomnia (mean ages 18–65 y) | 9 / 9 | Acute exercise bout or regular exercise programs (any intensity, duration, or frequency were included) | Self-reported sleep quality (PSQI), insomnia severity (ISI); self-reported or objective (accelerometry, PSG) SE, SOL, TST | Yes | Gender; insomnia diagnosis; study quality |
| Bartel et al., 2015 [29] * | MA: Observational (cross-sectional) | Adolescents (mean ages 12–18 y) | 41 / 11 | General PA, exercise, or sports participation | Self-reported or objective (accelerometry, PSG) bedtime, SOL, TST | No | No |
| Bin et al., 2019 [30] | SR: Observational (longitudinal) and experimental (pre-posttest, RCT) | Adults undergoing transmeridian travel (mean ages 24–47 y) | 13 / 3 | Pre-travel or post-travel aerobic exercise | Self-reported (e.g., diary) and objective (accelerometry, PSG) sleep measures; jet lag symptoms | No | No |
| Bollens et al., 2018 [31] | SR: Experimental (pre-posttest, RCT) | Adults with OSA (mean ages 32–81 y) | 8 / 8 | Exercise therapy programs (aerobic, resistance exercise; 1–12 wk) | AHI, ODI, SaO2 nadir, PSG-assessed TST, self-reported sleepiness and sleep quality | No | No |
| Booker et al., 2018 [32] | SR: Observational (cross-sectional, longitudinal) and experimental | Adult shift workers (ages not reported) | 58 / 5 | Self-reported PA habits or objectively measured fitness | Self-reported (e.g., diary, ESS, PSQI) sleep parameters | No | No |
| Costigan et al., 2013 [33] * | SR: Observational (cross-sectional, longitudinal) and experimental | Adolescent females (12–18 y) | 33 / 2 | Leisure-time SED (e.g., TV, video gaming) assessed via self-report, observation, or accelerometry | Self-reported sleep problems | No | No |
| Dolezal et al., 2017 [34] * | SR: Observational (cross-sectional, longitudinal) and experimental | Adolescents through older adults (ages unspecified) | 34 / 34 | Observational: PA participation; experimental: interventions with specific prescriptions (e.g., intensity) | Self-reported (e.g., PSQI) and objective (accelerometry, PSG) sleep measures | No | Age |
| Edwards et al., 2019 [35] | SR, MA: RCT | Adults with OSA (mean ages 39–61 y) | 10 / 4 | Exercise programs with prescribed intensity and duration (supervised aerobic and/or resistance exercise) | AHI | No | No |
| Flahr et al., 2018 [36] | SR: RCT | Adult shift workers (mean ages 20–58 y) | 7 / 2 | PA interventions with specific prescriptions (e.g., frequency, intensity) lasting 4 wk to 6 mo | Self-reported sleep quality, duration | No | No |
| Gao et al., 2019 [37] | SR, MA: RCT | Adults with OSA (ages ≥ 18 y) | 89 / 4 | Physical exercise (otherwise unspecified) | AHI, ESS | No | No |
| Harrison et al., 2019 [38] | SR: RCT | Adults with RLS (mean ages 44–64 y) | 11 / 2 | Exercise intervention (aerobic and resistance exercise training, yoga) | IRLS, PSQI, self-reported sleep duration | No | No |
| Iftikhar et al., 2014 [39] * | MA: Experimental (RCT, single-group pre-posttest) | Adults with OSA (mean ages 42–54 y) | 5 / 5 | Exercise interventions (≥ 12 wk; aerobic and/or resistance) | AHI, ESS, SE | No | No |
| Iftikhar et al., 2017 [40] * | MA: RCT | Adults with OSA (ages unspecified) | 80 / 5 | Supervised aerobic exercise training | AHI, ESS, ODI, SE, SaO2 nadir | No | No |
| Janssen et al., 2020 [41] | SR, MA: Observational and experimental | Children ages 0–4 y | 31 / 31 | Screen time or any movement behavior (SED, PA), sports participation | Parent-reported bedtime, sleep duration, SOL, awakenings, sleep quality, daytime napping | Yes | No |
| Kovacevic et al., 2018 [42] | SR: RCT | Adults (mean ages 21–79 y) | 13 / 13 | Supervised acute (< 4 bouts) or chronic (≥ 4 bouts) resistance exercise | Self-reported (e.g., diaries, ISI, PSQI) and objective (PSG, accelerometry) sleep parameters | Yes | No |
| Kredlow et al., 2015 [43] * | MA: Experimental (RCT, single-group pre-posttest) | Adults (mean ages 18–88 y) | 63 / 63 | Acute (< 1 wk) and regular (≥ 2 wk) exercise | Self-reported and objective (accelerometry, PSG) sleep quality, SE, SOL, TST, WASO, sleep stages | Yes | Age; sex; PA level; sleep complaints |
| Lambert et al., 2016 [44] * | SR: Experimental (RCT, single-group pre-posttest) | Adult family caregivers (mean ages 41–74 y) | 14 / 2 | Variety of PA (e.g., walking, strength training, yoga, meditation, tai chi, lifestyle [e.g., gardening, stair climbing, dancing]) | Self-reported sleep quality | No | No |
| Lang et al., 2016 [45] * | SR, MA: Observational (cross-sectional) | Adolescents and young adults (ages 14–24 y) | 21 (MA: 12) / 21 | Self-reported and objective (i.e., accelerometer, pedometer) PA | Self-reported sleep quality, quantity, insomnia; PSG sleep parameters | No | PA and sleep measurement types |
| Lederman et al., 2019 [46] | SR, MA: RCT | Adults with severe mental illness (mean ages 23–72 y) | 8 / 8 | Exercise interventions (e.g., aerobic activity, movement-based yoga, tai chi), lifestyle interventions if exercise was ≥ 50% of intervention | Self-reported (e.g., diaries, ISI, PSQI) sleep parameters | Yes | Mental health diagnosis; study setting (inpatient, community) |
| Lins-Filho et al., 2020 [47] | SR, MA: Experimental (RCT, single-group pre-posttest) | Adults with OSA (mean ages 45–58 y) | 7 / 7 | Exercise training programs (≥ 2 times/wk, ≥ 2 wk) | AHI; self-reported sleep quality, daytime sleepiness | No | No |
| Lowe et al., 2019 [15] | SR: RCT | Adults with insomnia symptoms or disorder (mean ages 44–62 y) | 11 / 11 | Exercise programs (multiple sessions of > 30 min/day; aerobic, resistance, or mind-body exercise) | Self-reported (e.g., diaries, ISI, PSQI) and objective (e.g., accelerometry, PSG) sleep | No | PA type (mind-body, exercise); insomnia (symptoms, disorder) |
| Mendelson et al., 2018 [16] | SR, MA: RCT | Adults with OSA (mean ages 45–63 y) | 6 / 6 | Exercise training programs lasting ≥ 3 wk | AHI, ESS | No | No |
| Passos et al., 2012 [48] * | SR: Experimental (RCT, single-group pre-posttest) | Adults with insomnia or sleep complaints (ages unspecified) | 5 / 5 | Moderate-intensity aerobic exercise interventions lasting ≥ 4 wk | PSG SE, SOL, TST, WASO; self-reported sleep quality (PSQI) | No | No |
| Rubio-Arias et al., 2017 [49] * | SR, MA: RCT | Middle-aged women (mean ages 48–55 y) | 5 / 5 | PA/exercise programs (≥ 8 wk; otherwise unspecified) | Self-reported sleep quality (PSQI), insomnia severity (ISI) | Yes | No |
| Smagula et al., 2016 [50] * | SR: Longitudinal observational | Older adults (> 50 y) | 21 / 5 | PA (otherwise unspecified) | Self-reported sleep complaints, sleep quality; objective sleep parameters | No | No |
| Stutz et al., 2019 [51] | SR, MA: Experimental | Adults (≥ 18 y) with normal sleep | 23 / 23 | Acute exercise performed in the evening (e.g., < 4 h prior to bedtime) | Self-reported and objective (accelerometry, PSG) sleep parameters | Yes | Participant PA; stress; body temp at bedtime; end time before bedtime |
| Vanderlinden et al., 2020 [17] | SR: Experimental (RCT, single-group pre-posttest) | Older adults (≥ 60 y) | 14 / 14 | Interventions that included regular (≥ 2 wk) PA | Self-reported sleep quality, objective (accelerometry, PSG) sleep parameters | No | No |
| Xu et al., 2018 [52] | SR: RCT | Adults with primary RLS (ages not given) | 12 / 2 | Aerobic/resistance exercise training, yoga | IRLS, PSQI, self-reported sleep duration | No | No |
| Yang et al., 2012 [53] * | SR, MA: RCT | Middle-aged and older adults (> 40 y) with sleep problems | 6 / 6 | Aerobic (walking, tai chi) or resistance exercise training program | Self-reported sleep quality (e.g., PSQI) or PSG sleep parameters | No | No |
| Yang et al., 2017 [54] * | SR, MA: Observational (cross-sectional, longitudinal), RCT | Adults (range 18–100 y) | 16 / 16 | SED assessed via self-report (e.g., TV, computer) or accelerometry | Self-reported sleep quality, sleep disturbance, insomnia severity, sleepiness, OSA risk | No | SED domain, measure; age |
| Yang et al., 2020 [55] | SR, MA: RCT | Pregnant women (mean ages 26–32 y) | 7 / 7 | Exercise of any type (e.g., aerobic exercise, stretching, yoga, tai chi) | Self-reported sleep quality, insomnia severity | No | No |
Article included in 2018 PAGAC Report;
Relevant studies are those that included PA as an exposure. AHI: apnea-hypopnea index; D-R: dose-response; ESS: Epworth Sleepiness Scale; IRLS: International Restless Legs Scale; ISI: Insomnia Severity Index; MA: meta-analysis; ODI: oxygen desaturation index; OSA: obstructive sleep apnea; PA: physical activity; PSG: polysomnography; PSQI: Pittsburgh Sleep Quality Index; RCT: randomized controlled trial; RLS: restless legs syndrome; SaO2: oxygen saturation; SE: sleep efficiency; SED: sedentary behavior; SOL: sleep onset latency; SR: systematic review; TST: total sleep time; TV: television; WASO: wake after sleep onset.
RESULTS
Description of the Evidence
Overview.
A total of 34 articles were included in this review; 15 of these were included in the 2018 PAGAC report, and additional 19 were identified in the updated search. Table 1 presents a summary of the characteristics of the articles. The evidence base comprised 19 meta-analyses [16,25,27–29,35,37,39–41,43,45–47,49,51,53–55] and 15 systematic reviews [15,17,26,30–34,36,38,42,44,48,50,52]. Most reviews included adult samples (n = 23) [15,16,25,28,30–32,35–40,42–44,46–48,51,52,54,55]; however, some focused on children (n = 2) [27,41], adolescents and young adults (n = 3) [29,33,45], middle-aged adults (n = 2) [49,53], older adults (n = 3) [17,26,50], or all ages (n = 1) [34]. The majority of reviews focused on sleep without regard to a specific disorder, but others focused on samples with OSA (n = 8) [16,25,31,35,37,39,40,47], insomnia or sleep complaints (n = 5) [15,26,28,48,53], restless legs syndrome (n = 2) [38,52], shift workers (n = 2) [32,36], or jet lag (n = 1) [30]. Most reviews included only experimental studies (n = 25); others included both observational (i.e., cross-sectional and longitudinal) and experimental studies (n = 7) [27,30,32–34,41,54] or only observational studies (n = 3) [29,45,50] in their reviews. The number of studies in each review that were relevant (i.e., with PA as the exposure and sleep as the outcome) ranged from two to 63, though most reviews included between five and 15 studies (median number of studies = 6). The 34 reviews included a total of 295 unique studies; 46 of these studies were cited in two reviews, seven were cited in three reviews, and nine were cited in four or more reviews. In particular, three experimental studies focused on exercise and OSA were cited across six or more reviews.
Exposures.
Most articles focused on PA; only three examined sedentary behavior [33,41,54]. Reviews of experimental studies often included studies that featured supervised moderate-to-vigorous intensity aerobic and/or resistance exercise; only one review focused exclusively on resistance exercise [42]. Most reviews of experimental studies only considered regular PA interventions (i.e., ≥ 2 wk); only five reviews examined the impact of acute (< 1 wk) exercise [28,34,42,43,51], with one exclusively focused on acute evening exercise (e.g., < 4 h before bedtime) [51]. Reviews of studies that included observational data often utilized self-reported or accelerometer-based measures of PA or sedentary behavior.
Outcomes.
The majority of reviews included self-reported measures of sleep as outcomes, using diary-based reports of various sleep parameters (e.g., sleep duration) or a wide variety of locally derived or validated questionnaires [15,17,27,29,30,32–34,36,41–46,48,50,51,53–55]. In particular, questionnaires were commonly used to assess sleep quality (e.g., Pittsburgh Sleep Quality Index [PSQI]) [15,17,26,28,32,34,36,42,44,46,48,49,53–55], daytime sleepiness (e.g., Epworth Sleepiness Scale [ESS]) [32,34,54], insomnia severity (Insomnia Severity Index [ISI]) [15,28,32,42,46,49,54,55], and restless legs symptoms (International Restless Legs Scale [IRLS]) [38,52]. Many of the reviews also included studies that utilized accelerometry [15,17,27–30,34,42,43,48,51,54]. Fewer reviews included studies that used polysomnography (PSG) [15,17,27–29,34,42,43,45,48,51,53], which permitted evaluation of sleep stages. Each of the eight reviews focused on OSA included the apnea-hypopnea index (AHI) as an outcome, with most of these reviews also assessing other measures of OSA severity (e.g., oxygen desaturation index) [31,40], PSG-assessed sleep parameters (e.g., sleep efficiency) [31,39,40], and self-reported daytime sleepiness (via the ESS) [16,25,31,37,39,40,47].
Review of the Evidence: Sleep Outcomes Across Age Groups
Overall, there was strong evidence that acute bouts of PA and regular PA improve sleep outcomes among adults. However, evidence was somewhat mixed among older adults, and there was insufficient evidence available to examine relationships between PA and sleep in children and adolescents (Table 2).
Table 2.
Committee-assigned grades for the effects of physical activity on sleep outcomes.
| Outcome | Grade |
|---|---|
| Acute bouts of physical activity and regular physical activity improve sleep outcomes | Strong |
| Longer duration acute bouts of physical activity and regular physical activity improve sleep outcomes, with the positive effects being independent of exercise intensity | Moderate |
| The effects of physical activity on sleep outcomes in adults are preserved across age and sex, with the exception of sleep onset latency, which declines with age | Moderate |
| Insufficient evidence to examine relationships between physical activity and sleep in children and adolescents and whether the relationships vary according to race/ethnicity, socioeconomic status, or weight status | Not assignable |
| Greater amounts of moderate-to-vigorous physical activity improves sleep in adults who report sleep problems, primarily symptoms of insomnia, and for obstructive sleep apnea | Moderate |
Adults.
Of the 26 reviews focused on adults or without regard to age group, 10 of these reviews focused on common sleep outcomes (i.e., not in relation to a specific sleep complaint or disorder) [34,42–44,46,49,51,53–55]. In general, these reviews all reported beneficial effects of PA on one or more aspect of sleep. The strongest evidence comes from analyses of 66 controlled intervention studies involving 2863 community-dwelling adults ranging from 18 to 88 y of age, with a wide range of PA modes and intensities represented [43]. The findings from this meta-analysis indicated consistently small-to-moderate sized benefits of both acute bouts of PA and regular PA on multiple sleep outcomes (Figure 2), including total sleep time (TST; both acute and regular), sleep efficiency (both acute and regular), sleep onset latency (SOL; both acute and regular), and sleep quality (regular; insufficient information regarding acute). In addition, because many of the studies that investigated the effect of acute exercise on sleep utilized PSG, acute exercise was found to reduce time spent in stage 1 non-REM (NREM) sleep (d = −0.35; 95% confidence interval [CI]: −0.52, −0.18) and increase slow-wave sleep (SWS) (d = 0.19; 95% CI: 0.02, 0.35) [43]. Acute exercise also was found to reduce rapid eye movement (REM) sleep (d = −0.27; 95% CI: −0.45, −0.08), an effect with uncertain physiological significance [43]. Although the magnitude of these effects may be viewed as small as a whole, the vast majority (89%) of the studies in the meta-analysis did not recruit individuals with sleep complaints; as a result, many participants presumably had little room for improvement in their sleep [56]. With that in mind, these results also suggest that PA does not disturb sleep among normal sleepers.
Figure 2. The effect of acute and regular exercise on sleep outcomes in adults.
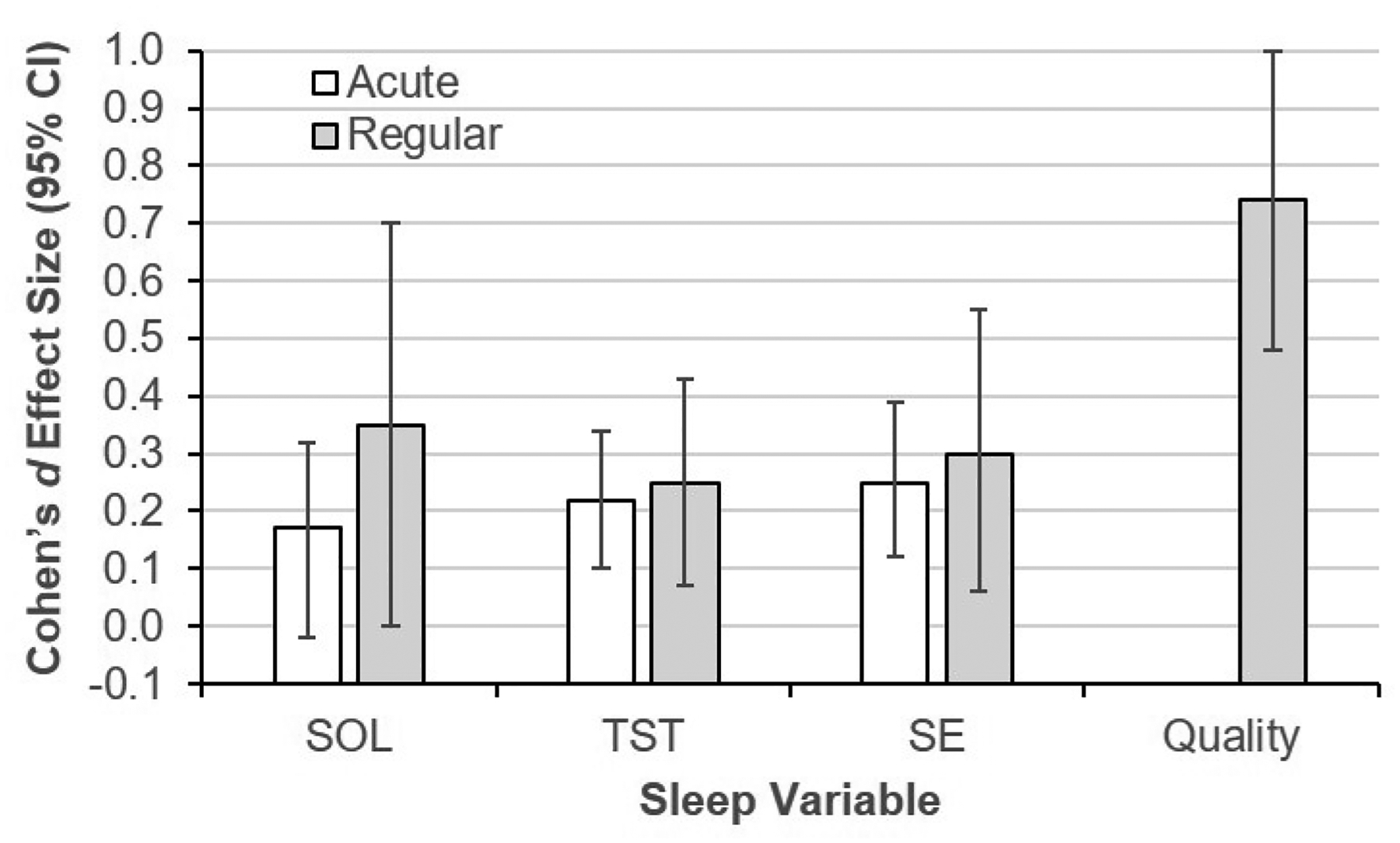
CI: confidence interval; SE: sleep efficiency; SOL: sleep onset latency; TST: total sleep time. Effect sizes are presented so that positive values represent an improvement in the specific sleep variable. The effect size defines the strength of the relationship, with d = 0.20 indicating a small, d = 0.50 a medium, and d = 0.80 a large magnitude of effect. Data are adapted from Kredlow et al. [43], with permission from Springer Nature.
Reviews that focused on specific subgroups reported similar sleep-promoting benefits of PA. For instance, a meta-analysis of eight randomized controlled trials (RCTs) conducted in adults with mental illness found that PA led to a moderate-sized improvement in sleep quality (Hedges’ g = 0.73; 95% CI: 0.18, 1.28) [46]. Similarly, PA significantly improved the sleep quality of pregnant women (based on seven RCTs) [55], middle-aged women (based on five RCTs) [49], and family caregivers (based on two experimental studies) [44]. Every individual study (n = 21) included in these reviews focused on self-reported sleep quality as the outcome.
Other reviews focused on the relationship between specific modes or domains of PA and sleep. Noting that most research (and reviews) have focused on aerobic exercise, Kovacevic and colleagues reviewed 13 trials that included resistance exercise [42]. They concluded that regular resistance exercise was effective at improving self-reported sleep outcomes, with greater effects on sleep quality than sleep duration; the effects of an acute bout of resistance exercise remained unclear [42]. In particular, regular resistance exercise that was higher intensity and performed ≥ 3 times per week seemed to be most effective at improving sleep. Finally, while other reviews focused exclusively on moderate-to-vigorous PA, Yang and colleagues examined the association between sedentary behavior and sleep [54]. In a meta-analysis of 12 cross-sectional and four cohort studies with sample sizes ranging from 300 to 7880 adults per study, they found greater amounts of sedentary behavior were associated with increased risk of sleep disturbance (odds ratio = 1.38; 95% CI: 1.28, 1.49). However, most of these studies relied upon self-reported sedentary behavior, with unclear findings as to whether specific domains of sedentary behavior (e.g., television viewing) were associated with greater risk for poor sleep. Because it did not examine PA, the meta-analysis was unable to consider the potential interaction of PA and sedentary behavior on sleep [54].
Older Adults.
Due to the high prevalence of sleep disturbance among older adults (i.e., age ≥ 65 y) [57], two reviews focused exclusively on the relationship between PA and sleep in this age group. A review of five prospective observational studies found mixed evidence that PA was a protective factor against the development of sleep disturbance in older adults [50]. In contrast, a review of 14 experimental studies reported that exercise programs significantly improved multiple sleep outcomes among healthy older adults (e.g., self-reported sleep duration, difficulty falling back to sleep), with PSQI-assessed sleep quality the most commonly improved outcome [17]. Specifically, Vanderlinden and colleagues concluded that studies which featured moderate-intensity exercise programs performed 3 times/week for ≥ 12 weeks were most likely to report significant improvements in sleep outcomes [17].
Children/Adolescents.
In contrast to the reviews focused on adults and older adults, the five reviews focused on the relationship between PA and sleep in children and adolescents included mostly cross-sectional studies, with few experimental or longitudinal observational studies included [27,29,33,41,45]. Thus, while the available evidence suggests a more equivocal association between PA and sleep in children and adolescents compared to adults, the evidence was deemed insufficient due to a lack of experimental research in this age group.
A review of 31 studies focused on children ages 0 to 4 y found a consistent association between sedentary behavior and poor sleep; specifically, higher levels of total daily screen time, evening screen time, and total sedentary time were each associated with unfavorable sleep outcomes (e.g., shorter duration, later bedtime, greater night awakenings) [41]. However, evidence was mixed regarding an association between PA and sleep; some studies suggested that outdoor play and moderate-to-vigorous PA were associated with better sleep in toddlers (1–2 y) and preschoolers (3–4 y), while other studies found null or even negative associations [41]. Similarly, in a review of 47 studies of children ages 3 to 13 y, Antczak and colleagues found a negligible association between PA and sleep (r = 0.02; 95% CI: −0.03, 0.07); sleep duration was the sleep outcome examined most often. In moderator analyses, they found that the association between moderate-to-vigorous PA and sleep strengthened with increasing age of the sample [27].
The relationship between PA and sleep seems to be more robust among adolescents than children, albeit based on observational data. In a meta-analysis of 12 cross-sectional studies of 12,604 individuals ages 14 to 24 y, Lang and colleagues reported a large overall effect of PA on sleep (d = 0.89; 95% CI: 0.48, 1.31) when individual PA and sleep outcomes were aggregated. Notably, they observed medium- to large-sized effects regardless of the measurement approach (i.e., device-based, self-report) for either PA or sleep; unfortunately, the analyses did not examine which dimensions of PA and sleep had the most robust associations [45]. Another meta-analysis of 11 cross-sectional studies with (ages 12–18 y) reported a relationship between greater PA and earlier bedtime (r = −0.14; 95% CI: −0.26, −0.02), with weaker (and nonsignificant) associations observed with SOL (r = −0.08; 95% CI: −0.29, 0.13) and TST (r = 0.12; 95% CI: −0.07, 0.30) [29]. Similarly, a review of observational studies focused on adolescent females reported that greater screen-based sedentary time was associated with greater sleep problems [33].
Review of the Evidence: Sleep Disorders
Overall, there was moderate evidence that greater amounts of moderate-to-vigorous PA improves sleep in adults who report sleep problems, though this applies primarily for adults with insomnia or obstructive sleep apnea (OSA) (Table 2).
Insomnia.
Moderate evidence indicates a beneficial impact of PA on sleep parameters among adults with insomnia. These conclusions were based on five reviews, encompassing a total of 17 individual studies, that focused on the impact of PA on sleep among individuals with insomnia symptoms or diagnosed with insomnia disorder. The PA interventions in these studies were primarily supervised programs in which the participants accumulated approximately 120–150 min/wk of moderate-intensity aerobic PA, yoga, or tai chi.
Two systematic reviews of RCTs concluded that exercise training programs led to significant improvements in sleep parameters among adults with insomnia, including SOL and sleep quality [15,48]; results were most prominent for self-reported sleep outcomes, though some studies also observed improvements in objective sleep parameters [15]. Passos and colleagues noted that the improvements in sleep following exercise training among adults with insomnia compare favorably to those observed following hypnotic medication use [48], whereas Lowe and colleagues found that the effects of exercise on sleep were stronger for adults with insomnia symptoms compared to those with insomnia disorder [15].
Two meta-analyses provided similar conclusions regarding self-reported sleep outcomes. In a meta-analysis of six RCTs (N = 305) that focused on middle- and older-aged adults with insomnia symptoms, Yang and colleagues observed improvements in overall sleep quality (utilizing the PSQI; standardized mean difference [SMD] = 0.47; 95% CI: 0.08, 0.86) and the PSQI subscales of sleep latency (SMD = 0.58; 95% CI: 0.08, 1.08) and sleep medications (SMD = 0.44; 95% CI: 0.14, 0.74) but no change in other PSQI subscales (e.g., sleep duration, daytime functioning; each SMD ≤ 0.35) [53]. Similarly, in a meta-analysis of nine RCTs (N = 557) of adults with insomnia symptoms or insomnia disorder, exercise programs improved sleep quality (i.e., 2.9-point PSQI reduction [95% CI: −3.9, −1.8]) and reduced insomnia severity (i.e., 3.2-point ISI reduction [95% CI: −5.4, −1.1]), but did not significantly impact objectively-assessed sleep efficiency [28].
Each of the above-noted reviews focused on adult samples, with most studies focusing on middle- to older-aged adults. None of the reviews reported on insomnia symptoms among children or adolescents. Only one systematic review focused on insomnia in older adults. On the basis of two individual studies, Alessi and Vitiello concluded that exercise may improve sleep in older adults with insomnia; however, the evidence was deemed weak [26].
Multiple reviews noted limitations of the existing evidence [15,28,53]. Many studies had methodological issues (e.g., small sample sizes, inconsistent operationalization of insomnia, infrequent reporting of adverse events), while none explored the impact of different exercise parameters (e.g., mode, duration, intensity) on sleep among adults with insomnia. Despite the many methodological limitations of the studies described in this section, the consistency of associations and conclusions across reviews provide moderate evidence for PA to improve sleep in adults with insomnia.
Obstructive sleep apnea.
Moderate evidence indicates that PA is associated with a significant improvement in the severity of obstructive sleep apnea (OSA), typically assessed using the apnea-hypopnea index (AHI; calculated as the mean number of apneic plus hypopneic events per hour of sleep). In addition, PA leads to reduced daytime sleepiness and improved sleep efficiency among adults with OSA. These conclusions were based on eight reviews, encompassing a total of 17 unique studies, that focused on the impact of PA on OSA severity and/or sleep outcomes of adults with OSA. The PA interventions in these studies were primarily supervised exercise training programs in which the participants accumulated approximately 150 min/wk of moderate-intensity PA.
Multiple reviews concluded that exercise training results in moderate-sized reductions in OSA severity [16,25,31,35,39,47]. Across five meta-analyses that examined the impact of exercise training on AHI, 14 experimental studies were included; however, each meta-analysis included between four and eight trials based on their publication date and inclusion/exclusion criteria. Four of these meta-analyses reported the impact of exercise on AHI in terms of absolute AHI reduction, ranging from −6.3 events/h (95% CI: −8.5, −4.0) to −11.4 events/h (95% CI: −13.4, −9.4) following exercise training [16,35,39,47]; these equated to a 28–32% reduction in AHI from baseline [16,39]. The remaining meta-analysis found that exercise training led to a medium-sized AHI reduction (SMD = −0.54; 95% CI: −0.87, −0.21) relative to control [25]. Although the magnitude of AHI reduction from exercise training across these meta-analyses would likely be characterized as modest, each meta-analysis noted that the AHI reduction was achieved without a significant reduction in body mass index (BMI) [16,25,35,39].
Some meta-analyses directly compared exercise to common OSA treatments, such as weight loss, mandibular advancement devices (MAD), and positive airway pressure (PAP), for its ability to reduce OSA severity. Edwards and colleagues compared lifestyle interventions that included only exercise, only diet, or diet and exercise [35]. They found similar reductions in AHI across the three different types of interventions: −8.1 events/h (95% CI: −15.7, −0.4) for exercise-only, −8.6 events/h (95% CI: −15.9, −1.3) for diet-only, and −8.2 events/h (95% CI: −12.1, −4.2) for diet and exercise. However, the similar AHI reduction was achieved despite substantially lower BMI reduction in the exercise-only trials (diet-only: −4.0 kg/m2 [95% CI: −5.8, −2.2]; diet and exercise: −2.3 kg/m2 [95% CI: −4.8, 0.3]; exercise-only: −0.5 kg/m2 [95% CI: −1.0, 0.0) [35]. Iftikhar and colleagues performed a network meta-analysis that included 80 RCTs (N = 4325) in which they compared aerobic exercise training (five RCTs; n = 72) with diet-induced weight loss, MAD, and PAP [40]. The AHI reduction for exercise training (−17.2 events/h [95% CI: −25.8, −8.6]) was not significantly different from the AHI reductions observed for diet-induced weight loss (−12.3 events/h [95% CI: −18.8, −5.8]), MAD (−15.2 events/h [95% CI: −19.5, −10.9]), or PAP (−25.3 events/h [95% CI: −28.5, −22.0]). In contrast, another network meta-analysis of 89 RCTs (N = 6346) that compared 18 different types of OSA interventions found that exercise training (four RCTs; n = 110) reduced AHI to a lesser extent than MAD or PAP (−5.6 events/h [95% CI: −13.1, 2.0] vs −13.3 events/h [95% CI: −17.7, −8.9] and −23.3 events/h [95% CI: −27.4, −19.2], respectively) when compared against no treatment [37].
Additionally, several reviews examined the impact of PA on sleep outcomes and daytime sleepiness among adults with OSA, as OSA often worsens sleep quality and daytime function [58]. A meta-analysis of eight experimental trials reported significantly improved sleep quality, as indicated by a mean 2.0-point reduction (95% CI: −3.6, −0.5) on the PSQI global score, and significantly reduced daytime sleepiness, as indicated by a mean 3.7-point reduction (95% CI: −6.1, −1.2) on the ESS, following exercise training relative to baseline [47]. Other meta-analyses observed a similar magnitude of ESS reduction among patients with OSA following exercise training [16,39], while another reported a large-sized reduction in ESS score following exercise training (SMD = −1.25; 95% CI: −2.40, −0.10) [25]. A meta-analysis of five trials found that exercise training increased sleep efficiency by 5.8% (95% CI: 2.5, 9.0) in adults with OSA [39]. Finally, in a network analysis conducted by Iftikhar and colleagues, the magnitude of reduction in daytime sleepiness following exercise training (mean 3.1-point reduction on the ESS [95% CI: −5.5, −0.7]) was similar to that observed for weight loss (−2.1 points [95% CI: −4.2, −0.0]), PAP (−2.4 points [95% CI: −3.0, −1.9]), and MAD (−2.7 points [95% CI: −3.6, −1.8]) [40]. Likewise, exercise led to an improvement in sleep efficiency (4.8% improvement [95% CI: 1.4, 8.1]) similar to MAD (+4.3% [95% CI: 2.2, 6.4]) and PAP (+3.0% [95% CI: 2.0, 3.9]) [40].
Collectively, these findings provide moderate strength evidence for a consistent relationship between greater PA and clinically significant improvements in OSA severity and sleep outcomes for adults with OSA. However, multiple reviews stressed that their findings were based on small (e.g., N < 50) and relatively brief (e.g., ≤ 12 wk) exercise trials; larger studies with longer intervention duration and exploration into the influence of duration, intensity, and mode on OSA severity and sleep outcomes are needed [25,39,47].
Other Sleep Disorders.
Beyond OSA and insomnia, there is insufficient evidence regarding relationships between PA and sleep for other sleep and circadian disorders. Across two reviews of non-pharmacological therapies for restless legs syndrome (RLS) [38,52], only two trials involving PA were able to be reviewed. While increased PA reduced RLS severity and improved sleep quality in these individual studies, both reviews emphasized the need for additional research to verify these preliminary findings [38,52].
Similarly, one review focused on non-pharmacological strategies to alleviate jet lag [30], a condition characterized by fatigue and daytime impairment due to transient circadian misalignment following travel across multiple time zones. They found minimal evidence that PA reduces jet lag severity based on the five studies included in the review [30]. Finally, although shift work per se is not a sleep disorder, it frequently leads to circadian misalignment and significant sleep disturbance. Across two reviews focused on this topic [32,36], a total of seven studies examined the association between PA and sleep among shift workers. Based on this limited evidence, the reviews cited preliminary support for the use of PA to improve sleep and reduce sleep-related impairment in shift workers [32,36].
Evidence of a Dose-Response Relationship
Moderate evidence indicates a dose-response relationship between the length in minutes (i.e., duration), but not the intensity, of an acute bout of PA and sleep outcomes (Table 2). In adults, this evidence is primarily supported by analyses from Kredlow and colleagues [43], as no other reviews examined dose-response relations between PA and sleep outcomes. In their meta-analysis (59 controlled studies, N = 2863 participants), they found that a longer duration of an acute bout of PA led to greater decreases in SOL and REM sleep and greater increases in TST and SWS [43]. However, exercise intensity (light, moderate, or vigorous) did not impact the effect of an acute bout of PA on any sleep outcomes [43].
In terms of regular PA, limited but concordant evidence (again from Kredlow and colleagues) suggests that longer individual bouts of moderate-to-vigorous PA lead to greater decreases in SOL, but have no impact on other sleep outcomes (Table 2) [43]. Regarding other PA dose parameters, Kredlow and colleagues found that the frequency of regular PA (i.e., number of days per week) did not differentially impact sleep. However, increased duration (i.e., number of weeks) of the PA intervention led to a slightly smaller improvement in TST, but had no impact on other sleep outcomes [43].
Evidence on Moderating Factors
Few reviews have examined the potential impact of various moderating factors (e.g., age, exercise mode) on the relationship between PA and sleep, likely because few individual studies have probed these factors. The most comprehensive examination of moderating factors was undertaken in the meta-analysis by Kredlow and colleagues [43], with significant findings highlighted below. However, it is noteworthy that moderating factors did not influence the relationship between PA and the majority of sleep outcomes [43].
Age.
In adults, moderate evidence indicates that relationships between PA and sleep outcomes are consistent in their effects across young, middle-aged, and older adults [17,34,43,45,53]. The notable exception may be among children and adolescents, as reviews provided mixed evidence on the association between PA and sleep (Table 2) [27,41]. Meta-analytic evidence from Kredlow and colleagues indicated a reduced beneficial effect of regular exercise on SOL with aging, consisting of a 0.15 standard deviation lower benefit for every 10-y increase in mean age in the studies they evaluated, in which the age range was 18 – 88 y [43]. In contrast, age did not moderate the relationship between acute PA and sleep or between regular PA and any other sleep outcomes [43].
Other demographic factors and weight status.
Limited evidence suggests that an acute bout of PA provides a nominally greater benefit for men than women on a few sleep outcomes (i.e., stage 1 NREM sleep, wake time after sleep onset), but the relationship between PA and the majority of sleep outcomes is not significantly different for men and women [43]. Insufficient data were available to determine whether the relationship between PA varied by race/ethnicity, socioeconomic factors, or body weight.
Physical activity habits/fitness level.
According to analyses by Kredlow and colleagues, regular PA levels influence the response to an acute bout of PA on SWS.[43] Among individuals with high baseline PA, an acute bout of PA significantly increased SWS the next night, whereas an acute bout of PA did not increase SWS among those with low baseline PA levels [43]. However, the amount of regular PA did not alter the effect of an acute bout on other sleep outcomes [43]; thus, most of the beneficial effects of acute bouts of PA on sleep seem to be similar regardless of their regular PA.
Time of day.
It is often speculated as to whether there is an optimal timing of PA to improve sleep and, conversely, whether PA close to bedtime is harmful to sleep. However, the time of day at which an acute bout of moderate-to-vigorous PA is performed appears unrelated to most aspects of sleep. In the meta-analysis by Kredlow and colleagues, they compared the effect of acute bouts of moderate-to-vigorous PA performed > 8 h before bedtime, 3–8 h before bedtime, and < 3 h before bedtime on subsequent sleep; they found no detectable difference on SOL, TST, sleep efficiency, SWS, NREM stage 2 sleep, or REM sleep latency [43]. In fact, PA bouts performed < 3 h before bedtime were associated with reduced wake time after sleep onset and stage 1 NREM sleep, suggesting slightly deeper and more consolidated sleep following evening exercise. In contrast, PA bouts performed 3–8 h before bedtime were associated with reduced REM sleep [43]. A more recent meta-analysis focused on 23 experimental studies that examined the effect of evening exercise (i.e., < 4 h before bedtime) on sleep [51]. Evening exercise decreased stage 1 NREM sleep (−0.9%; 95% CI: −1.5, −0.4%) and increased SWS (+1.3%; 95% CI: 0.1, 2.6%) compared to a non-exercise control condition, suggesting a modest increase in sleep depth following evening exercise. However, subgroup analyses suggested that vigorous-intensity exercise ending ≤ 1 h prior to bedtime might impair sleep by increasing SOL [51]. Thus, available research indicates there is unlikely an optimal time of day to perform PA to improve sleep, and that evening exercise generally does not impair sleep. An important limitation regarding the evidence base on exercise timing and sleep is that very few studies have directly compared multiple times of day of exercise against each other; in particular, we are unaware of any studies that have compared more than two times of day (e.g., morning vs. afternoon). Another key limitation is that available research has only included healthy adults who were good sleepers [43,51]; whether these results are similar for sleep-disturbed adults is unknown. For instance, it is plausible that adults with insomnia may be more reactive to late-night exercise than those without sleep complaints [51].
Mode.
Most of the existing research on PA has utilized aerobic modes of PA, and few studies have directly compared modes of PA for their impact on sleep. Nevertheless, the available evidence suggests that the effect of moderate-to-vigorous PA on sleep outcomes does not seem to differ much across different types of PA. In the meta-analysis by Kredlow and colleagues, no differences in sleep outcomes were found between an acute bout of aerobic or anaerobic PA or between regular mind-body exercise (e.g., tai chi, yoga) and traditional PA [43]. In contrast, Rubio-Arias found that studies utilizing aerobic exercise led to larger improvements in sleep quality in middle-aged women than studies utilizing yoga, though this difference could be attributed to differences in intensity [49]. Although Kredlow and colleagues found that an acute bout of cycling increased SWS to a greater extent than running, the specific exercise type did not impact any other sleep parameters [43]. Similarly, upon reviewing studies focused on resistance exercise, Kovacevic and colleagues reported that the effect of resistance exercise on sleep quality was similar to that commonly observed for aerobic exercise [42].
Future Research Directions
This review identified several areas of research in need of future inquiry, which are described in greater detail below. Many of these future research needs were originally identified under the broader umbrella of ‘brain health’ (i.e., cognitive development, quality of life, anxiety and depression, sleep) in the 2018 PAGAC Scientific Report [20]; these needs have been updated for the purpose of this review.
-
Conduct high-quality RCTs of moderate-to-vigorous PA in multiple age groups, especially youth, to better understand its effects on multiple dimensions of sleep.
A large amount of research has focused on the importance of PA on sleep, especially in adults; however, the quality of this research has often been suboptimal. Multiple reviews commented on the low quality, small sample sizes, and/or limited sleep measures of the studies included in their reviews, and recommended more rigorously conducted and large-scale RCTs to provide more definitive conclusions [15,17,25,39,42,46,47,49,51,55].
-
Conduct RCTs that manipulate the timing and/or dose of PA in a systematic fashion to improve the understanding of the time-of-day and dose-response relationships between PA and sleep. These studies should be performed in multiple age groups (i.e., healthy children, adults, and older adults) and among those with mild or subclinical sleep complaints and in sleep-disordered samples.
To date, little evidence exists from empirical studies to draw definitive conclusions about the optimal timing, intensity, duration, and frequency of PA to improve sleep. This work is critically needed to better inform the public, clinicians, and policymakers about how PA can be utilized to improve sleep outcomes among healthy individuals with subclinical sleep complaints and in individuals with sleep disorders.
Conduct RCTs of both light and moderate-to-vigorous PA in individuals with sleep disorders to better understand its effects on sleep and daytime impairment in these conditions. Further, conduct observational and experimental studies with individuals at different levels of severity or impairment, including studies in individuals with physical (e.g., obesity, pregnancy) and/or mental (e.g., anxiety, depression) health conditions that increase risk for sleep problems, to examine whether PA delays or prevents sleep disorder onset and progression. In addition, conduct RCTs to better understand how PA compares against, interacts with, and augments common treatments used by individuals with sleep disorders.
-
Conduct RCTs of PA in multiple age groups and across the spectrum of sleep complaints that examine participant characteristics (e.g., brain imaging, blood biomarkers) and plausible mechanisms underlying the link between PA and sleep in order to identify individuals whose sleep might benefit the most from PA and better understand how PA improves sleep.
Minimal research has attempted to identify participant characteristics or attributes that are associated with a greater improvement in sleep with PA [59]. Moreover, although multiple pathways have been postulated (e.g., improved thermoregulation, increased brain-derived neurotrophic factor, increased parasympathetic activity) [60–62], there has been little systematic research conducted in humans regarding the mechanisms by which PA improves sleep. Complicating matters further is the likelihood that these underlying mechanisms may differ for acute versus regular PA [60].
-
Conduct observational studies to examine the association between sedentary behavior and sleep and conduct RCTs that systematically reduce sedentary behavior to improve the understanding of the impact of varying contexts, patterns, and durations of sedentary behavior (including its potential interaction with PA) on sleep outcomes in multiple age groups.
Minimal research has focused on the impact of sedentary behavior on sleep [54]; however, as with other health outcomes (e.g., mortality) [63], sedentary behavior may also moderate how PA is associated with sleep and vice versa. In addition, current research has focused on whether the duration of sedentary behavior is related to sleep, but the pattern of sedentary behavior (e.g., duration in prolonged bouts, frequency of sit-to-stand transitions) may be important.
Conduct appropriate analyses to examine effect modification by demographic factors. Such analytical approaches require studies that include large samples and substantial variation in sample characteristics (e.g., race, ethnicity, socioeconomic status).
Conduct more rigorously designed and longer-term RCTs and prospective observational studies that will improve understanding of the latency, time course, and persistence of the improvements in sleep following both acute and regular PA. These studies should be accompanied by larger sample sizes, longer follow-up periods, and a broader range of sleep assessment methods and measures of daytime impairment. To extend our knowledge past the highly controlled settings of most of the current experimental research, studies are also needed that attempt to disseminate this research into more generalizable settings.
Conduct RCTs and prospective observational research on the impact of muscle-strengthening exercise (e.g., resistance training), mind-body activity (e.g., yoga, tai chi, qigong), and other modes of activity on sleep outcomes. In addition, research is needed to examine the impact of different domains of PA (e.g., leisure-time, occupational, household) on sleep, as most research described herein focused on leisure-time PA.
DISCUSSION
The original scientific report accompanying the Physical Activity Guidelines for Americans, first published in 2008 and citing 31 individual studies, concluded that “a small number of observational, population-based studies provides initial evidence supporting a positive association of regular participation in physical activity with lower odds of disrupted or insufficient sleep, including sleep apnea” [64]. The report also concluded that “a small number of RCTs supports the conclusion that regular participation in physical activity has favorable effects on sleep quality and is a useful component of good sleep hygiene” [64]. The current review, which includes and updates the 2018 PAGAC Report, considerably expands these findings by including a significantly larger body of evidence (i.e., 31 studies in 2008 compared with 295 unique studies across 34 reviews for the current review), the results of which indicate that consistent evidence now shows positive effects of both acute and regular PA on numerous sleep outcomes. This review also extends the 2008 PAGAC findings by providing a more explicit focus on the evidence base regarding PA and the sleep disorders of OSA and insomnia.
This umbrella review provides, for the first time, a comprehensive evaluation of the body of evidence on the association between PA and sleep using available systematic reviews and meta-analyses. This is especially relevant given the heightened interest and activity in this area of research over the past 10 years, likely driven by an increased appreciation of sleep as an important health outcome [3]. In a recent bibliometric analysis of research focusing on exercise and sleep from 1979 through 2018, the authors found that > 80% of the papers had been published in 2009 or later [65]. Indeed, this is highlighted by the articles selected for this umbrella review: 19 of the 34 articles included in the umbrella review were not included in the 2018 PAGAC scientific report [20], as they were published between mid-2017 and mid-2020.
Our strongest conclusions concerned the general relationship between PA and sleep among healthy individuals. Most prominently, there was strong evidence that both acute bouts of PA and regular PA improved sleep outcomes (Table 2). This reflected our appraisal that the evidence base was large, consistent in its findings, generalizable, and with low risk of bias [22]. Although this conclusion was based heavily on the findings from the meta-analysis led by Kredlow and colleagues [43], other reviews reported similar findings [17,34,42,49]. In addition, there was moderate evidence to support each of the following statements: 1) that longer duration acute bouts of PA and regular PA improve sleep outcomes, with these effects being independent of PA; and 2) that the effects of PA on sleep outcomes in adults are preserved across age and sex, with the exception of SOL. These conclusions provide important insight into moderators of the relationship between PA and sleep and suggest that, despite somewhat limited evidence, the impact of PA on sleep is similar across a variety of potential moderators.
We also concluded that there was moderate evidence that PA improves sleep in adults who report symptoms of insomnia or who have OSA (Table 2). Given the high prevalence of these two sleep disorders [8,9] and the significant limitations (e.g., side effects, availability) associated with their primary treatment options [66–68], this conclusion suggests that PA could potentially play a prominent role in the management of these disorders. Currently, though, PA is rarely considered as a stand-alone or adjunct treatment option for these disorders [69,70]. In particular, while PA is recommended as a means to achieve weight loss for the treatment of OSA [71], multiple reviews described herein indicated that the effects of PA on OSA are independent of weight change [16,25,35,39]. Future research should address the prominent limitations in this area of research so that PA can be formally evaluated for its appropriateness as a treatment option for insomnia and OSA.
While this umbrella review was able to make several conclusions about PA and sleep, it also pointed out several needs for future research to advance our current understanding of this relationship. While several prominent research gaps were identified, most were related to the need to more accurately characterize how various types, intensities, doses, and patterns of PA and sedentary behavior impact multiple sleep dimensions across a range of samples (e.g., in multiple age groups, among sleep-disordered individuals) and in more generalizable settings. In particular, there is a need to better understand how PA impacts sleep among those who are at especially high risk for sleep problems (e.g., pregnant women, individuals with anxiety or depression). Due to a scarcity of high-quality research in this area, we were unable to make many conclusions regarding these specific parameters and populations. However, this information is greatly needed for us to understand how PA may be optimally prescribed to improve sleep.
This review has several significant advantages, including a transparent literature search process involving three electronic databases, broad inclusion criteria to allow for a comprehensive examination of the relationship between PA and sleep, an updated search to reflect the most recent findings, and a focused description of existing knowledge gaps. However, several limitations should be noted. The biggest limitation is the reliance on existing systematic reviews and meta-analyses. The PAGAC did not conduct its own review of individual studies and did not perform its own meta-analysis; as a result, we were reliant upon published reviews. As a result, this made it more difficult to make conclusions on specific parameters of interest (e.g., dose-response issues, moderators) when available reviews did not focus on these parameters. In addition, the heterogeneity of PA exposures and sleep outcomes (including their measurement) across different reviews complicated our ability to make conclusions about how these factors influenced the association between PA and sleep. Finally, given that many reviews cited poor quality evidence as a key factor that limited their conclusions [15,17,25,39,42,46,47,49,51,55], our umbrella review is likewise limited by the current body of evidence.
In summary, our review found that PA exerts a positive influence on multiple parameters of sleep. We were able to make several conclusions regarding the influence of both acute and regular PA with sleep, with these effects generally preserved across adulthood. We also observed accumulating evidence regarding the impact of PA on insomnia and obstructive sleep apnea, two prominent sleep disorders. In general, though, the evidence base needs higher quality research that incorporates larger sample sizes, a wider variety of modes, intensities, and patterns of PA and sedentary behavior, and more rigorous assessment of sleep outcomes. There is a strong need for these studies to be conducted in child/adolescent samples and among sleep-disordered individuals. Our hope is that these findings and identification of research gaps will facilitate additional research that leads to greater utilization of PA as a behavioral approach to improve sleep and, thereby, the health of society.
Supplementary Material
PRACTICE POINTS.
Both acute bouts of physical activity and regular physical activity improve a variety of sleep outcomes in healthy adults, with the effects typically small to moderate in magnitude.
Physical activity reduces the severity of insomnia and obstructive sleep apnea.
The effects of physical activity on sleep outcomes are generally consistent across age and sex.
While most physical activity characteristics (e.g., mode, intensity) do not seem to differentially impact sleep, a longer duration of physical activity (both acute and regular) leads to greater improvements in sleep.
RESEARCH AGENDA.
Future research should strive to:
Conduct high-quality randomized trials of physical activity to examine its impact on multiple dimensions of sleep, in samples of healthy individuals and those with sleep disorders, and across multiple age groups (i.e., children/adolescents, adults, older adults).
Perform randomized trials that examine the impact of the timing and/or dose of physical activity on sleep outcomes.
Examine plausible physiological mechanisms to better understand how physical activity improves sleep and analyze demographic factors to identify whether the effect of physical activity on sleep is generalizable.
Conduct studies that examine the impact of sedentary behavior on sleep.
Examine the influence of muscle-strengthening exercise, mind-body exercise, and other domains of physical activity (e.g., occupational, household) on sleep.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Work completed by the Physical Activity Guidelines Advisory Committee was supported by the US Department of Health and Human Services. Committee members were reimbursed for travel and per diem expenses for the five public meetings. Committee members volunteered their time. HHS staff provided general administrative support to the Committee and assured that the Committee adhered to the requirements for Federal Advisory Committees. HHS also contracted with ICF, a global consulting services company, to provide technical support for the literature searches conducted by the Committee. HHS and ICF staff collaborated with the Committee in the design and conduct of the searches by assisting with the development of the analytical frameworks, inclusion/exclusion criteria, and search terms for each primary question; using those parameters, ICF performed the literature searches.
Support for CEK in conducting the updated search was provided by K23HL118318.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AMSTARExBP
A Measurement Tool to Assess Systematic Reviews (Exercise and Blood Pressure)
- BMI
body mass index
- CI
confidence interval
- CINAHL
Cumulative Index of Nursing and Allied Health Literature
- ESS
Epworth Sleepiness Scale
- IRLS
International Restless Legs Scale
- ISI
Insomnia Severity Index
- MAD
mandibular advancement devices
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PA
physical activity
- PAP
positive airway pressure
- PAGAC
Physical Activity Guidelines Advisory Committee
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- RCT
randomized controlled trial
- REM
rapid eye movement
- RLS
restless legs syndrome
- SMD
standardized mean difference
- SWS
slow-wave sleep
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Authors report no conflicts of interest.
REFERENCES
* The most important references are denoted by an asterisk.
- [1.].Mukherjee S, Patel SR, Kales SN, Ayas NT, Strohl KP, Gozal D, et al. An official American Thoracic Society statement: the importance of healthy sleep. Recommendations and future priorities. Am J Respir Crit Care Med 2015; 191: 1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2.].Zielinski MR, McKenna JT, McCarley RW. Functions and mechanisms of sleep. AIMS Neurosci 2016; 3: 67–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3.].Luyster FS, Strollo PJ Jr., Zee PC, Walsh JK. Sleep: a health imperative. Sleep 2012; 35: 727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4.].Wheaton AG, Jones SE, Cooper AC, Croft JB. Short sleep duration among middle school and high school students - United States, 2015. MMWR Morb Mortal Wkly Rep 2018; 67: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5.].Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults--United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 65: 137–41. [DOI] [PubMed] [Google Scholar]
- [6.].Calhoun SL, Fernandez-Mendoza J, Vgontzas AN, Liao D, Bixler EO. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Med 2014; 15: 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7.].Grandner MA, Martin JL, Patel NP, Jackson NJ, Gehrman PR, Pien G, et al. Age and sleep disturbances among American men and women: data from the U.S. Behavioral Risk Factor Surveillance System. Sleep 2012; 35: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8.].Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9.].Roth T, Coulouvrat C, Hajak G, Lakoma MD, Sampson NA, Shahly V, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry 2011; 69: 592–600. [DOI] [PubMed] [Google Scholar]
- [10.].Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics 2010; 125: e1410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11.].Institute of Medicine. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- [12.].Buman MP, King AC. Exercise as a treatment to enhance sleep. Am J Lifestyle Med 2010; 4: 500–14. [Google Scholar]
- [13.].Kline CE. The bidirectional relationship between exercise and sleep: implications for exercise adherence and sleep improvement. Am J Lifestyle Med 2014; 8: 375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14.].Youngstedt SD. Effects of exercise on sleep. Clin Sports Med 2005; 24: 355–65, xi. [DOI] [PubMed] [Google Scholar]
- *[15.].Lowe H, Haddock G, Mulligan LD, Gregg L, Fuzellier-Hart A, Carter LA, et al. Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal. Clin Psychol Rev 2019; 68: 1–12. [DOI] [PubMed] [Google Scholar]
- *[16.].Mendelson M, Bailly S, Marillier M, Flore P, Borel JC, Vivodtzev I, et al. Obstructive sleep apnea syndrome, objectively measured physical activity and exercise training interventions: a systematic review and meta-analysis. Front Neurol 2018; 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[17.].Vanderlinden J, Boen F, van Uffelen JGZ. Effects of physical activity programs on sleep outcomes in older adults: a systematic review. Int J Behav Nutr Phys Act 2020; 17: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18.].Mercier J, Savard J, Bernard P. Exercise interventions to improve sleep in cancer patients: a systematic review and meta-analysis. Sleep Med Rev 2017; 36: 43–56. [DOI] [PubMed] [Google Scholar]
- [19.].Department of Health and Human Services. Physical Activity Guidelines for Americans. Washington, DC: Department of Health and Human Services; 2018. [Google Scholar]
- [20.].Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: Department of Health and Human Services; 2018. [Google Scholar]
- [21.].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22.].Torres A, Tennant B, Ribeiro-Lucas I, Vaux-Bjerke A, Piercy K, Bloodgood B. Umbrella and systematic review methodology to support the 2018 Physical Activity Guidelines Advisory Committee. J Phys Act Health 2018; 15: 805–10. [DOI] [PubMed] [Google Scholar]
- [23.].Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24.].Johnson BT, MacDonald HV, Bruneau ML Jr., Goldsby TU, Brown JC, Huedo-Medina TB, et al. Methodological quality of meta-analyses on the blood pressure response to exercise: a review. J Hypertens 2014; 32: 706–23. [DOI] [PubMed] [Google Scholar]
- [25.].Aiello KD, Caughey WG, Nelluri B, Sharma A, Mookadam F, Mookadam M. Effect of exercise training on sleep apnea: a systematic review and meta-analysis. Respir Med 2016; 116: 85–92. [DOI] [PubMed] [Google Scholar]
- [26.].Alessi C, Vitiello MV. Insomnia (primary) in older people: non-drug treatments. BMJ Clin Evid 2015; 2015: 2302. [PMC free article] [PubMed] [Google Scholar]
- [27.].Antczak D, Lonsdale C, Lee J, Hilland T, Duncan MJ, Del Pozo Cruz B, et al. Physical activity and sleep are inconsistently related in healthy children: a systematic review and meta-analysis. Sleep Med Rev 2020; 51: 101278. [DOI] [PubMed] [Google Scholar]
- [28.].Banno M, Harada Y, Taniguchi M, Tobita R, Tsujimoto H, Tsujimoto Y, et al. Exercise can improve sleep quality: a systematic review and meta-analysis. PeerJ 2018; 6: e5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29.].Bartel KA, Gradisar M, Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev 2015; 21: 72–85. [DOI] [PubMed] [Google Scholar]
- [30.].Bin YS, Postnova S, Cistulli PA. What works for jetlag? A systematic review of non-pharmacological interventions. Sleep Med Rev 2019; 43: 47–59. [DOI] [PubMed] [Google Scholar]
- [31.].Bollens B, Reychler G. Efficacy of exercise as a treatment for obstructive sleep apnea syndrome: a systematic review. Complement Ther Med 2018; 41: 208–14. [DOI] [PubMed] [Google Scholar]
- [32.].Booker LA, Magee M, Rajaratnam SMW, Sletten TL, Howard ME. Individual vulnerability to insomnia, excessive sleepiness and shift work disorder amongst healthcare shift workers: a systematic review. Sleep Med Rev 2018; 41: 220–33. [DOI] [PubMed] [Google Scholar]
- [33.].Costigan SA, Barnett L, Plotnikoff RC, Lubans DR. The health indicators associated with screen-based sedentary behavior among adolescent girls: a systematic review. J Adolesc Health 2013; 52: 382–92. [DOI] [PubMed] [Google Scholar]
- [34.].Dolezal BA, Neufeld EV, Boland DM, Martin JL, Cooper CB. Interrelationship between sleep and exercise: a systematic review. Adv Prev Med 2017; 2017: 1364387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[35.].Edwards BA, Bristow C, O’Driscoll DM, Wong AM, Ghazi L, Davidson ZE, et al. Assessing the impact of diet, exercise and the combination of the two as a treatment for OSA: a systematic review and meta-analysis. Respirology 2019; 24: 740–51. [DOI] [PubMed] [Google Scholar]
- [36.].Flahr H, Brown WJ, Kolbe-Alexander TL. A systematic review of physical activity-based interventions in shift workers. Prev Med Rep 2018; 10: 323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37.].Gao YN, Wu YC, Lin SY, Chang JZ, Tu YK. Short-term efficacy of minimally invasive treatments for adult obstructive sleep apnea: a systematic review and network meta-analysis of randomized controlled trials. J Formos Med Assoc 2019; 118: 750–65. [DOI] [PubMed] [Google Scholar]
- [38.].Harrison EG, Keating JL, Morgan PE. Non-pharmacological interventions for restless legs syndrome: a systematic review of randomised controlled trials. Disabil Rehabil 2019; 41: 2006–14. [DOI] [PubMed] [Google Scholar]
- [39.].Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014; 192: 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40.].Iftikhar IH, Bittencourt L, Youngstedt SD, Ayas N, Cistulli P, Schwab R, et al. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med 2017; 30: 7–14. [DOI] [PubMed] [Google Scholar]
- [41.].Janssen X, Martin A, Hughes AR, Hill CM, Kotronoulas G, Hesketh KR. Associations of screen time, sedentary time and physical activity with sleep in under 5s: a systematic review and meta-analysis. Sleep Med Rev 2020; 49: 101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[42.].Kovacevic A, Mavros Y, Heisz JJ, Fiatarone Singh MA. The effect of resistance exercise on sleep: a systematic review of randomized controlled trials. Sleep Med Rev 2018; 39: 52–68. [DOI] [PubMed] [Google Scholar]
- *[43.].Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med 2015; 38: 427–49. [DOI] [PubMed] [Google Scholar]
- [44.].Lambert SD, Duncan LR, Kapellas S, Bruson AM, Myrand M, Santa Mina D, et al. A descriptive systematic review of physical activity interventions for caregivers: effects on caregivers’ and care recipients’ psychosocial outcomes, physical activity levels, and physical health. Ann Behav Med 2016; 50: 907–19. [DOI] [PubMed] [Google Scholar]
- *[45.].Lang C, Kalak N, Brand S, Holsboer-Trachsler E, Puhse U, Gerber M. The relationship between physical activity and sleep from mid adolescence to early adulthood: a systematic review of methodological approaches and meta-analysis. Sleep Med Rev 2016; 28: 32–45. [DOI] [PubMed] [Google Scholar]
- [46.].Lederman O, Ward PB, Firth J, Maloney C, Carney R, Vancampfort D, et al. Does exercise improve sleep quality in individuals with mental illness? A systematic review and meta-analysis. J Psychiatr Res 2019; 109: 96–106. [DOI] [PubMed] [Google Scholar]
- [47.].Lins-Filho OL, Pedrosa RP, Gomes JML, Dantas Moraes SL, Vasconcelos BCE, Lemos CAA, et al. Effect of exercise training on subjective parameters in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med 2020; 69: 1–7. [DOI] [PubMed] [Google Scholar]
- *[48.].Passos GS, Poyares DL, Santana MG, Tufik S, de Mello MT. Is exercise an alternative treatment for chronic insomnia? Clinics 2012; 67: 653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49.].Rubio-Arias JA, Marin-Cascales E, Ramos-Campo DJ, Hernandez AV, Perez-Lopez FR. Effect of exercise on sleep quality and insomnia in middle-aged women: a systematic review and meta-analysis of randomized controlled trials. Maturitas 2017; 100: 49–56. [DOI] [PubMed] [Google Scholar]
- [50.].Smagula SF, Stone KL, Fabio A, Cauley JA. Risk factors for sleep disturbances in older adults: evidence from prospective studies. Sleep Med Rev 2016; 25: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51.].Stutz J, Eiholzer R, Spengler CM. Effects of evening exercise on sleep in healthy participants: a systematic review and meta-analysis. Sports Med 2019; 49: 269–87. [DOI] [PubMed] [Google Scholar]
- [52.].Xu XM, Liu Y, Jia SY, Dong MX, Cao D, Wei YD. Complementary and alternative therapies for restless legs syndrome: an evidence-based systematic review. Sleep Med Rev 2018; 38: 158–67. [DOI] [PubMed] [Google Scholar]
- [53.].Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother 2012; 58: 157–63. [DOI] [PubMed] [Google Scholar]
- [54.].Yang Y, Shin JC, Li D, An R. Sedentary behavior and sleep problems: a systematic review and meta-analysis. Int J Behav Med 2017; 24: 481–92. [DOI] [PubMed] [Google Scholar]
- [55.].Yang SY, Lan SJ, Yen YY, Hsieh YP, Kung PT, Lan SH. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res 2020; 14: 1–10. [DOI] [PubMed] [Google Scholar]
- [56.].Youngstedt SD. Ceiling and floor effects in sleep research. Sleep Med Rev 2003; 7: 351–65. [DOI] [PubMed] [Google Scholar]
- [57.].Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin 2017; 12: 31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58.].Bjorvatn B, Pallesen S, Grønli J, Sivertsen B, Lehmann S. Prevalence and correlates of insomnia and excessive sleepiness in adults with obstructive sleep apnea symptoms. Percept Mot Skills 2014; 118: 571–86. [DOI] [PubMed] [Google Scholar]
- [59.].Buman MP, Hekler EB, Bliwise DL, King AC. Moderators and mediators of exercise-induced objective sleep improvements in midlife and older adults with sleep complaints. Health Psychol 2011; 30: 579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[60.].Chennaoui M, Arnal PJ, Sauvet F, Léger D. Sleep and exercise: a reciprocal issue? Sleep Med Rev 2015; 20: 59–72. [DOI] [PubMed] [Google Scholar]
- *[61.].Tan X, van Egmond L, Cedernaes J, Benedict C. The role of exercise-induced peripheral factors in sleep regulation. Mol Metab 2020; 42: 101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62.].Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiol Behav 2007; 90: 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63.].Ekelund U, Brown WJ, Steene-Johannessen J, Fagerland MW, Owen N, Powell KE, et al. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br J Sports Med 2019; 53: 886–94. [DOI] [PubMed] [Google Scholar]
- [64.].Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- [65.].Memon AR, Vandelanotte C, Olds T, Duncan MJ, Vincent GE. Research combining physical activity and sleep: a bibliometric analysis. Percept Mot Skills 2020; 127: 154–81. [DOI] [PubMed] [Google Scholar]
- [66.].Wilt TJ, MacDonald R, Brasure M, Olson CM, Carlyle M, Fuchs E, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016; 165: 103–12. [DOI] [PubMed] [Google Scholar]
- [67.].Thomas A, Grandner M, Nowakowski S, Nesom G, Corbitt C, Perlis ML. Where are the behavioral sleep medicine providers and where are they needed? A geographic assessment. Behav Sleep Med 2016; 14: 687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68.].Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 2011; 15: 343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69.].Epstein LJ, Kristo D, Strollo PJ Jr., Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5: 263–76. [PMC free article] [PubMed] [Google Scholar]
- [70.].Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016; 165: 125–33. [DOI] [PubMed] [Google Scholar]
- [71.].Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, et al. The role of weight management in the treatment of adult obstructive sleep apnea: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e70–e87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


