Abstract
Background:
Serum DDTs during or just after pregnancy were associated with breast cancer in mothers (F0), and with breast cancer, mammographic density and obesity in adult daughters (F1) in the Child Health and Development Studies multi-generational cohort in prior publications. Here we investigate F0 perinatal serum DDT associations with granddaughters’(F2) measured obesity at a median age of 26 and self-reported age at menarche.
Methods:
F2 weight, height and waist circumference were measured by trained examiners. o,p’-DDT, p,p’-DDT and p,p’-DDE were measured in archived F0 perinatal serum. F0 DDT associations with F2 outcomes, accounting for F1 characteristics, were estimated in log-linear models adjusted for F0 and F1 body mass index (BMI), race, and menarche timing (N=258 triads for obesity; N=235 triads for early menarche). Interactions between F0 BMI and DDTs were estimated.
Results:
F0 o,p’-DDT was associated with F2 obesity (Odds ratio, OR, 2.6; 95% Confidence Interval (CI), 1.3, 6.7, tertile 3 vs. 1), among normal weight F0 (70%), but not among overweight and obese F0 (p-interaction=0.03), independent of other DDTs. F0 o,p’-DDT was also associated with F2 early menarche (OR, 2.1; 95% CI, 1.1, 3.9, tertile 3 vs. 1) and this association was not modified by F0 BMI.
Conclusions:
Ancestral exposure to environmental chemicals, banned decades ago, may influence the development of earlier menarche and obesity, which are established risk factors for breast cancer and cardiometabolic diseases.
Impact:
Discovery of actionable biomarkers of response to ancestral environmental exposures in young women may provide opportunities for breast cancer prevention.
Keywords: DDT; o,p’-DDT; Developmental Origins of Health and Disease; Early Age at Menarche; Obesity; Breast Cancer Risk Factors; Multi-generational; Child Health and Development Studies; CHDS; Prospective; cohort; women’s health; environmental chemicals; cardiometabolic risk; pregnancy
INTRODUCTION
Persistent organic pollutants (POPs) were ubiquitous, world-wide chemical exposures during the 1960s (1–3). As such these legacy chemicals, including DDT, remain highly relevant to the health of people born in the 1960s who were exposed during development and to their children born in the 1980s and 1990s who were exposed when they were in ovum.
The commercial product known as “DDT” was composed primarily of p,p’-DDT, the active insecticide, but o,p’-DDT was an additional low level contaminant (about 15%) of commercial DDT (4). The primary metabolite of p,p’-DDT, p,p’-DDE, is the most environmentally persistent of these DDT compounds (https://www.cdc.gov/biomonitoring/DDT_BiomonitoringSummary.html). In contrast, o,p’-DDT is more quickly metabolized than either p,p’-DDT or p,p’-DDE and exhibits considerable inter-individual differences in metabolism (5). Importantly, the different chemical structures of these DDTs results in different biological activities. For example, o,p’-DDT is known as more estrogenic, with p,p’-DDE as largely anti-androgenic (6). During active use of DDT, as during the 1960’s when bloods were collected in the current study, these three compounds could all be found in human serum (7–11). However, consistent with more rapid metabolism of o,p’-DDT, after DDT was banned in the U.S. in 1972, serum levels of o,p’-DDT were detected in only about 1 percent of NHANES II (1976–1980) samples (12). In contrast, exposure to p,p’-DDE, the primary metabolite of p,p’-DDT, is ongoing worldwide due to its ubiquitous contamination of the food supply (13). These secular changes are reflected in a series of breast cancer studies in human populations conducted with blood samples from 1960s to early 2000s which demonstrate dramatic declines in p,p’-DDT that were steeper than for p,p’-DDE (7,8). It is important to note that DDT has not been universally banned, thus exposure to DDT remains ongoing among people living where DDT is or has been recently manufactured or used, as well as among migrants from these countries (14–16).
The fetal origins of disease model introduced by Barker (17) and further developed as the Developmental Origins of Health and Disease concept by Gluckman and Hanson (18) extends to chemical exposures (19,20). A meta-analysis of 7 prospective cohort studies across North American, Europe and Asia found that prenatal or early life p,p’-DDE exposure was associated with obesity in people up to twenty years old (13). Since the publication of that meta-analysis, it was shown in the Child Health and Development Studies (CHDS) cohort that perinatal o,p’-DDT exposure in the 1960s was associated with increased risk of obesity in middle-aged (fifties) daughters (11). Experimental evidence supports these findings; for example mice dosed with a mixture of p,p’-DDT and o,p’-DDT (replicating their commercial mixture) during pregnancy and early lactation at exposure doses that produced circulating DDT and DDE levels in the mouse mothers (F0) within the range of the CHDS F0 mothers had obese ‘daughter’( F1) mice (21). Multiple generations of rats were also vulnerable to p,p’-DDT-induced obesity and ovarian diseases, such as polycystic ovarian syndrome and primary ovarian insufficiency (22–24). The multi-generational obesogenic and ovarian effects of environmental DDT and DDE isoform exposures that have been observed experimentally (22–24) remain to be investigated or demonstrated in humans.
Because p,p’-DDT commercial formulas were contaminated with o,p’-DDT and both are metabolized in living creatures to their DDE isoforms, these chemicals frequently co-exist in human observation and rodent experimental studies. We suspect because o,p’-DDT is shorter lived among these POPs, that it serves as the most sensitive biomarker of uniquely perinatal exposures and associated susceptibilities and thus may be the best biomarker for perinatal exposure among these related compounds in humans. This assumption is supported by findings in the CHDS. F0 perinatal o,p’-DDT has been shown to be the relevant DDT compound in association with daughters’ (F1) midlife obesity (11), breast cancer (9), breast density (10) and DNA methylation in breast cancer related genes (25). Hence, we focused on F0 perinatal o,p’-DDT as the primary potential predictor of granddaughter outcomes given that the granddaughters are exposed in the egg during their mothers’ development in utero.
In the present paper we test the hypothesis that perinatal o,p’-DDT exposure in the F0 (grandmother) generation of the CHDS cohort is associated with age at menarche and also with obesity and adiposity at age 26 in the F2 (granddaughter) generation of the CHDS cohort. This is the first paper to report on human associations of F0 exposures to POPs with F2 health outcomes.
MATERIALS AND METHODS
Study Population
The CHDS is a population-based, multi-generational cohort with ongoing follow-up for more than 60-years, beginning in the 1960s (26). Archived serum samples drawn during pregnancy and the early postpartum from the original generation (F0) make it possible to examine associations of environmental chemical levels with health outcomes in 3 generations: founding generation of women exposed during pregnancy (F0), the offspring generation exposed in utero during development (F1), and the grandchild generation exposed in the egg (F2). The timing of the CHDS F0 pregnancies, in the early 1960s, coincides with high use of legacy pesticides and industrial chemicals (e.g. DDTs, PCBs and PFAS compounds) (7,27,28). The CHDS F0 are now in their late 70s, their offspring (F1) are in their late 50s, and the grandchildren (F2) are in their mid-20s. Thus, the CHDS represents a unique opportunity to test the concept that ancestral exposures during these critical windows impact the health of current human populations. The CHDS recruited more than 98% of women seeking obstetric care at the Kaiser Foundation Health Plan in the San Francisco East Bay Area from 1959–1967 (26). These founding grandmothers (F0) were interviewed in person early in pregnancy, and provided permission to access medical records for themselves and their offspring (F1) and gave blood samples at each trimester and one in the near post-partum, generally within 3 days of delivery.
The current study is based on the daughters (F1) and granddaughters (F2) who participated in the Three Generations of Breast Cancer (3Gs) Study conducted from 2010 to 2013. Daughter offspring (F1) born into the CHDS who were surviving at the time of study recruitment and were phone locatable were eligible to participate in the telephone interview phase of the 3Gs Study (N=5,003). Daughters known to be incarcerated, institutionalized, to have a severe mental illness, or to have requested “not to be contacted”; and, who were already eligible for another adult follow-up study in progress were excluded from eligibility. Sixty percent of eligible subjects completed the phone interview (N=3,003). Due to budget constraints, a subset were targeted for participation in a home visit from the following three groups: daughters of mothers with breast cancer, daughters who had participated in an earlier breast density study and a random sample of daughters (29). Of the 1,879 daughters who were eligible, 1,194 completed a home visit (64%). Daughters (F1) were asked to invite their daughters (granddaughters, F2) to participate in the home visit along with them, using the technique of snowball recruitment. In total, 729 F2 granddaughters participated in the home visit and 356 F2 completed an adult questionnaire designed for participants ages ≥18 years. The distribution of baseline grandmaternal characteristics by participation type for F1 and F2 samples was highly comparable, suggesting that participation bias is unlikely (Supplementary Table 1).
During the home visit, anthropometric and blood pressure measurements were taken from F1 and F2, using standardized protocols. The F1 and adult F2 questionnaires included questions about menarche, menstruation and pregnancy, health behavior and body image.
Analysis Sample.
The present analysis sample is based on the F2 who completed an adult questionnaire as part of the 3Gs Study (n=365), and also participated in a home visit, had available DDT measures from grandmothers’ serum, and had available information on body mass index (BMI) in all three generations (n=258). The analysis sample for age at menarche associations was slightly smaller, requiring information on age at menarche in all three generations (n=235).
The institutional review board of the Public Health Institute approved the study protocols for this research. At enrollment CHDS F0 gave informed oral consent, as was customary in the 1960’s, for themselves and their children (F1). F1 and F2 who participated in the 3Gs Study from 2010–2013 gave full informed verbal consent before completing the study surveys and written consent before participating in the home visits.
Granddaughter (F2) Outcomes.
Weight and height of the F2 were measured during the 3Gs home visit using a detailed, standardized protocol. Examiners were trained to strictly implement the protocol. Standing height, weight and waist circumference was measured using a standardized protocol (11). Only measured F2 height and weight were used as outcomes in this study. Body mass index (BMI) was calculated from weight (kg) divided by height (m2), measured during the home visit. Age at menarche was assessed from self-report via the F2 adult survey completed on hard copy or online. Other socio-demographic information such as F2 age, race and education were assessed from the adult survey. To create outcome variables based on clinically relevant categories, BMI was classified as ≥30 vs. <30 kg/m2 to represent obesity in the F2 (30) and waist circumference was classified as ≥80 cm vs. <80 cm (31). Age at menarche was classified as ≤11 vs. >11 years to represent early menarche. Age 11 was selected as the threshold for early menarche because it was the 25th percentile cut-point for the distribution of age at menarche in the F2.
Grandmother (F0) Exposures and Covariates.
We measured o,p’-DDT, p,p’-DDT, p,p’-DDE, and lipids in non-fasting grandmother (F0) perinatal serum samples collected during 1959–1967 preferentially collected in the early postpartum (1–3 days after delivery) as previously described (9,32). Some subjects lacked postpartum samples so alternative samples were used. F0 serum drawn in the early post-partum accounts for 86% of DDT assays in this study, serum from the 3rd trimester was used for 12%, and serum from the 2nd trimester was used for 2% of assays. Adding trimester of draw as a covariate to models did not affect associations observed for F2 obesity and early menarche, nor was trimester of draw, itself, associated with these outcomes.
Previous research has demonstrated measurement reliability over time and correspondence in levels across gestation for these chemicals, supporting the assumption that timing of specimen collection during the perinatal period is representative of pregnancy exposures (33). Samples were measured using gas chromatography with electron capture detection (GC/ECD) fit with a capillary column (18%), GC/ECD fit with dual columns (45%), or GC triple quadrupole mass spectrometry (37%). Companion assays for total cholesterol and triglycerides were measured at the Clinical and Epidemiologic Research Laboratory (CERLab) at Boston Children’s Hospital, using methods previously described (34).
DDTs were characterized as continuous variables, as log-transformed variables and as tertiles using 2 dummy variables representing tertile 2 and tertile 3, versus tertile 1 as the reference category. Tertile variables were used to test for patterns of dose response.
Demographics and health-related behavior were collected from grandmothers during in-person interviews at enrollment, including age, race and education. Clinical measures were abstracted from medical records beginning 6 months prior to pregnancy through labor and delivery and are the source of data on baseline weight of grandmothers. Grandmothers’ BMI was calculated from weight (kg) divided by height (m) squared, measured or reported at interview or first prenatal visit. Weight was adjusted to compensate for variation in the timing of measurement by regressing weight on gestational age using the locally weighted scatterplot smoothing technique (35). Adjusted weight was then imputed as the fitted mean weight at day 101 of gestation (median value for day of interview) plus the residual from the regression procedure. F0 BMI was assessed as a continuous variable and as a binary variable, dichotomized as overweight, BMI ≥25 vs. <25 kg/m2. Grandmothers’ age at menarche was collected from both baseline interview and medical record.
Daughter (F1) Exposures and Covariates.
Weight and height at age 30 years, and age at menarche, as well as age and other socio-demographic information were assessed via self-report during a computer-assisted telephone interview. F1 BMI at age 30 was calculated from reported weight and height at age 30. F1 BMI at age 30 and F1 age at menarche were implemented as continuous variables.
Statistical Methods.
We implemented log-linear models (PROC GENMOD) executed in SAS 9.4 to estimate associations using the repeated option to adjust the covariance matrix for correlation within family clusters. Models included all three DDTs: o,p’-DDT, p,p’-DDT and p,p’-DDE. Although the DDTs are correlated, each compound represents different exposure sources, metabolic response, and bioactivity. We have recently examined this empirically in a metabolomics analysis that identified different correlated metabolomic pathways for these compounds (36). The decision to mutually adjust for these compounds is based on biological considerations as previously reviewed (8). The three DDTs are different exposures that vary in proportion in individuals and have different toxicities and consequences. p,p’-DDE is acquired independently of its parent compound, p,p’-DDT, via environmental sources due to extreme persistence and via individual metabolism during active exposure. The relative amount of o,p’-DDT compared to p,p’-DDT is an indication of recency of exposure to commercial DDT because o,p’-DDT is metabolized and excreted more rapidly than p,p’-DDT. The biological activities of these compounds differ as well because of their different chemical structures (6). So, one of these compounds cannot be a proxy for the others (8). This is partly empirically revealed by finding that associations can be in opposing directions for these compounds or observed only for one of the three—very likely for complex reasons including metabolic rate, and exposure source and timing. It is essential to consider the compounds together to exclude mutual confounding. In the CHDS, we have previously observed different relationships for these different DDTs with various outcomes in jointly adjusted models (7–9,36–38). We have also observed that individuals in our population have widely varying relative proportions of these compounds – consistent with differences in exposure timing, exposure sources and metabolism that is particular to the active use and intake of commercial DDT during the 1960’s (39). These variations suggest that these compounds should be considered in models together to determine differential effects. As noted in the introduction, in this paper we focus on o,p’-DDT associations as a marker for recent perinatal DDT exposure and because in utero exposure to o,p’-DDT has been associated with F1 outcomes in the CHDS. However, in supplementary tables we present estimated coefficients for all three DDT compounds for completeness.
Models were stratified by whether F0 had a BMI ≥25 kg/m2 vs. <25 kg/m2 at pregnancy interview. We applied a lower BMI threshold for F0 (≥25 kg/m2) than for F2 (≥30 kg/m2) owing to the different distributions in the two samples. Since only 9% of F0 met the definition of obesity (>30kg/m2) compared to 32% of F2, we combined the overweight and obese categories to examine BMI in F0. Our rationale for stratifying by grandmothers’ (F0) BMI is that p,p’-DDT and p,p’-DDE are highly lipophilic and grandmothers’ BMI could serve to moderate exposures during gestation. Stratified models that estimated F0 o,p’-DDT associations with F2 obesity (BMI ≥30 kg/m2) included: F0 pregnancy p,p’-DDT (continuous), o,p’-DDT (continuous), p,p’-DDE (continuous), race and self-reported F1 BMI at age 30 years. To support the decision to stratify models by F0 overweight we created a product term between F0 BMI (continuous) X o,p’-DDT (continuous) to test whether associations estimated from stratified models were statistically different. A similar strategy was used for models estimating early menarche (≤11 vs. >11 years) which included continuous terms for p,p’-DDT, o,p’-DDT and p,p’-DDE, and were adjusted for F0 age at menarche and race, and F1 age at menarche and self-reported weight at age 30. In a set of secondary analyses designed to evaluate adiposity as distinct from BMI, we also examined F0 o,p’-DDT associations with large F2 waist circumference (≥80 cm vs. <80 cm) in models as described for F2 obesity. We estimated odds ratios for the difference between high F0 o,p’-DDT (median of 3rd tertile) levels vs. low levels (median of 1st tertile) using contrasts calculated from models for each outcome. We chose tertiles based on our prior work which also used this classification (7,9).We identified outlier o,p’-DDT values using the exploratory data approach (EDA) described by Tukey (40) and conducted a sensitivity analysis to determine whether results were impacted by extreme values.
RESULTS
Table 1 provides distributions of study variables for three generations, grandmother (F0), daughter (F1) and granddaughter (F2). Birth cohorts for the three generations span more than 60 years, with an approximate 25-year span between the median ages at data collection for successive generations. This study observed grandmothers at a median age of 26 years in 1962 (median year of interview), daughters at a median age of 49 years and granddaughters at a median age of 26 years, the same median age as when their grandmothers donated biospecimens during pregnancy. Consistent with secular changes observed nationally and worldwide (41), weight and BMI increased in consecutive generations. Age at menarche also shows an expected downward trajectory between the F1 and F2 generations, also consistent with international patterns (42–45).
Table 1.
Distribution of study characteristics among three CHDS generations: grandmothers (F0), daughters (F1) and granddaughters (F2)
| Grandmothers (F0), n=258 | Daughters (F1), n=258 | Granddaughters (F2), n=258 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentile | Percentile | Percentile | ||||||||||
| Study variable | Mean (SD) | 25th | 50th | 75th | Mean (SD) | 25th | 50th | 75th | Mean (SD) | 25th | 50th | 75th |
| Year of Birth | 1936 (6.6) | 1931 | 1937 | 1941 | 1962 (1.8) | 1961 | 1962 | 1964 | 1989 (3.6) | 1987 | 1990 | 1993 |
| Year of Interview | 1962 (1.8) | 1961 | 1962 | 1964 | 2011 (0.7) | 2011 | 2012 | 2012 | 2013 (0.0) | 2013 | 2013 | 2013 |
| Age (years) | 26 (6.0) | 22 | 25 | 31 | 49 (2.0) | 48 | 49 | 51 | 26 (4.4) | 22 | 26 | 29 |
| Weight (kg) | 62.9 (14.0) | 53.7 | 59.3 | 69.0 | 70.8 (16.4) | 59.0 | 65.8 | 77.1 | 76.9 (23.4) | 61.0 | 70.05 | 89.0 |
| Height (m) | 1.62 (0.07) | 1.57 | 1.63 | 1.68 | 1.66 (0.07) | 1.60 | 1.65 | 1.70 | 1.66 (0.07) | 1.62 | 1.66 | 1.71 |
| Body Mass Index (kg/m2) | 23.9 (5.1) | 20.9 | 22.5 | 25.6 | 25.8 (5.6) | 22.0 | 23.9 | 29.2 | 28.0 (8.4) | 21.8 | 25.1 | 32.9 |
| Age at menarche (years) | 12.7 (1.4) | 12 | 13 | 13 | 12.8 (1.8) | 12 | 13 | 14 | 12.4 (1.6) | 11 | 12 | 13 |
| Parity | 1.8 (2.0) | 0 | 1 | 3 | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| Year of blood draw | 1962 (1.8) | 1961 | 1962 | 1964 | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| p,p’-DDT (ng/mL) | 14.5 (10.4) | 7.1 | 11.6 | 19.8 | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| o,p’-DDT (ng/mL) | 0.66 (0.67) | 0.24 | 0.45 | 0.86 | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| p,p’-DDE (ng/mL) | 48.5 (23.8) | 31.6 | 43.4 | 60.4 | ----- | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| N (%) | N (%) | N (%) | ||||||||||
| Race | ||||||||||||
| African American | 78 (30%) | 84 (33%) | 73 (33%)1 | |||||||||
| Asian | 6 ( 3%) | 4 ( 2%) | 12 ( 5%)1 | |||||||||
| Hispanic | 13 ( 5%) | 17 ( 6%) | 17 ( 8%)1 | |||||||||
| White | 153 (59%) | 146 (56%) | 117 (53%)1 | |||||||||
| Other/Mixed | 8 ( 3%) | 7 ( 3%) | 3 ( 1%)1 | |||||||||
| Education | ||||||||||||
| <High School | 53 (22%)2 | 12 ( 4%) | 13 ( 6%)3 | |||||||||
| High School | 89 (37%)2 | 31 (12%) | 48 (22%)3 | |||||||||
| Some College | 52 (22%)2 | 105 (41%) | 115 (54%)3 | |||||||||
| ≥College | 44 (19%)2 | 110 (43%) | 38 (18%)3 | |||||||||
| BMI | ||||||||||||
| <25kg/m2 | 182 (71%) | 153 (59%) | 129 (50%) | |||||||||
| ≥25 --<30kg/m2 | 52 (20%) | 47 (18%) | 47 (18%) | |||||||||
| ≥30kg/m2 | 24 ( 9%) | 58 (23%) | 82 (32%) | |||||||||
F2 missing on race, n=36.
F0 missing on education, n=20.
F2 missing on education, n=44.
Table 1 also presents distributions for the serum DDT compounds measured in grandmothers (F0) during their pregnancy. High levels of both DDT isomers (p,p’-DDT and o,p’-DDT) result from the timing of the blood draw. F0 serum was taken when DDT was in active use in the U.S., prior to its ban in 1972 (46).
As shown in Table 1, there was strong representation among African Americans in all three generations that derives from the founding generation which was an archetypal population-based sample of Alameda County, CA with a robust community of African Americans (47). In the ensuing generations, care was taken to ensure continued participation from African Americans by diligently recruiting them to the follow-up 3Gs Study to achieve representation in proportion to the baseline population. Table 1 also demonstrates an upward progression of educational level across F0 and F1 generations. The greater number of women at the highest level of education in the F1 generation compared to the F2 is likely due to the differences in the upper age boundary in the two generations. The F1 are older and have had more opportunity to attain post-graduate credentials.
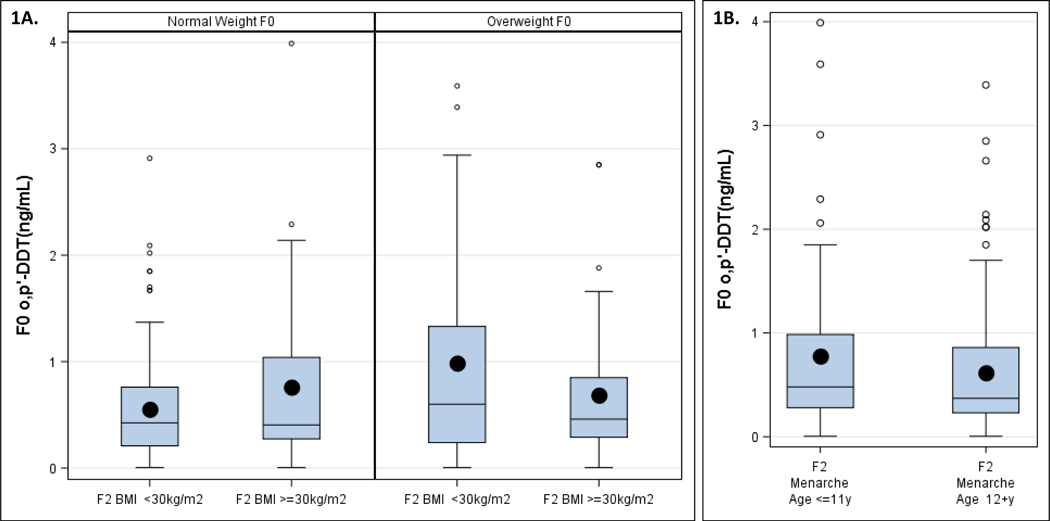
Figure 1, panel A provides the distributions of grandmothers’ (F0) pregnancy serum o,p’-DDT (ng/mL) levels by granddaughter (F2) obesity (BMI ≥30 vs. <30 kg/m2) within F0 weight sub-groups (BMI ≥25 vs. <25 kg/m2). F2 obesity was correlated with higher F0 o,p’-DDT for normal weight F0 but this pattern was opposite for overweight F0. Thus, in further analyses we stratified F2 obesity analyses by F0 BMI (≥25 vs. <25 kg/m2). The rationale to stratify by F0 overweight is provided by the statistically significant p-value for the product term, o,p’-DDT x F0 BMI (Pinteraction (o,p’-DDT x F0 BMI)= 0.0276) in a model adjusted for all DDT congeners.
Figure 1.
Distribution of grandmothers’ (F0) serum o,p’-DDT (ng/mL) levels for: A. Granddaughter (F2) obesity (BMI ≥30 vs. <30 kg/m2) stratified by grandmothers’ (F0) overweight (BMI ≥25 vs. <25kg/m2) and B. Granddaughter (F2) early menarche (age at menarche ≤11 vs >11 years). Overall, F0 serum o,p’-DDT ranged from 0.0004 – 3.99 ng/mL.
Figure 1, Panel B provides the distributions of grandmothers’ (F0) pregnancy serum o,p’-DDT (ng/mL) levels for early granddaughter (F2) menarche (age at menarche ≤11 vs. >11 years) and shows that granddaughters with early menarche had higher in ovo exposures to o,p’-DDT.
Table 2 provides modeled associations of grandmothers’ (F0) perinatal serum o,p’-DDT with granddaughter (F2) obesity (BMI ≥30 vs. <30 kg/m2) for various levels of model adjustment, stratified by F0 overweight (BMI ≥25 vs. <25 kg/m2).
Table 2.
Associations1 of grandmothers’ (F0) serum o,p’-DDT with adult F2 Obesity (≥30 v. <30 kg/m2), stratified by grandmothers’ BMI2, n=258 triads
| Estimated o,p’-DDT association by grand-maternal (F0) BMI2 | ||||||
|---|---|---|---|---|---|---|
| Grandmothers’ (F0) BMI <25 (n=182) | Grandmothers’ (F0) BMI ≥25 (n=76) | |||||
| Model Adjustment Level | o,p’-DDT Odds Ratio3 | (95% CI4) | p-value |
o,p’-DDT Odds Ratio3 |
(95% CI4) | p-value |
| DDT congeners | 3.62 | (1.37, 9.56) | 0.0092 | 0.37 | (0.13, 1.02) | 0.0557 |
| + F1 BMI5 Adjusted | 3.43 | (1.47, 8.00) | 0.0044 | 0.36 | (0.11, 1.13) | 0.0809 |
| + F1 BMI5 + Race6 Adjusted | 2.59 | (1.00, 6.73) | 0.0505 | 0.31 | (0.09, 1.01) | 0.0526 |
Associations are estimated from log-linear models adjusted for family clustering and stratified by F0 BMI (categorized as ≤25 kg/m2 vs >25 kg/m2). Unadjusted models include linear terms for p,p’-DDT, p,p’-DDE, o,p’-DDT. Models adjusted for F1 BMI added a linear term for F1 BMI reported at age 30 nearest the age when F1 were pregnant with F2 and nearest the ages when F0 and F2 BMI were captured. Models additionally adjusted for race added a dichotomous variable representing African Americans vs. all other races.
The rationale to stratify by F0 overweight (BMI <25 vs. >25 kg/m2) is based on results from the model estimating F2 obesity that included linear terms for p,p’-DDT, p,p’-DDE, o,p’-DDT and F0 BMI, and the product term between o,p’-DDT (continuous) X F0 BMI (continuous). The p-value for the product term, Pinteraction (o,p’-DDT X F0 BMI)= 0.0276.
Reported o,p’-DDT Odds Ratios are estimated for the median of the 3rd vs. the median of the first tertile. The inter-tertile range for F0 o,p’-DDT at the medians was 0.86 ng/mL.
CI=Confidence Limits
BMI=Body Mass Index, continuous kg/m2.
Race is dichotomized as African American (1) vs. all else (0).
Granddaughter (F2) obesity for normal weight grandmothers (F0).
Risk of obesity in granddaughters was 2 to 3–fold greater when grandmothers’ o,p’-DDT was in tertile 3 compared to tertile 1 (Table 2). A significant >2-fold association of F0 o,p’-DDT with F2 obesity remained after adjustment for F0 African American race and F1 BMI at age 30 (a proxy for F1 BMI during F2 gestation). The adjusted models in Table 2 show that the F0 o,p’-DDT association with F2 obesity persists, even though F0 African American race and F1 BMI at age 30 were positively and significantly correlated with F2 obesity. The F0 o,p’-DDT association with F2 obesity was independent of grandmothers’ p,p’-DDT which showed a significant negative correlation with F2 obesity (Supplementary Table 2A). Supplementary Figure 1 demonstrates that the positive association between grandmothers’ o,p’-DDT and granddaughter BMI is comparable within tertiles of p,p’-DDT, establishing that the positive o.p’-DDT effect is not neutralized by the negative p,p’-DDT effect. This supports the conjecture that the impact of o,p’-DDT exposure per ng/mL on F2 obesity is more potent than that of the p,p’-DDT exposure, consistent with the larger absolute value for the o,p’-DDT coefficient compared to the coefficient for p,p’-DDT (Supplementary Table 2A).
Granddaughter (F2) obesity for overweight/obese grandmothers (BMI ≥25 kg/m2).
Risk of obesity in granddaughters was about 70% lower when grandmothers’ o,p’-DDT was in tertile 3 compared to tertile 1 (Table 2). Adjustment for F0 African American race and F1 BMI at age 30 had little effect on these associations (Table 2). The sample size of overweight/obese grandmothers is small, reflecting the lower incidence of overweight and obesity in the 1960’s compared to current obstetric populations. This smaller sample size likely reduced statistical power in the F0 overweight subset.
Granddaughter (F2) abdominal fat.
All but one of the F2 participants who were obese also had waist circumference ≥80 cm. For this reason, models that predicted F2 obesity, also predicted F2 waist circumference ≥80 cm.
Granddaughter (F2) Age at Menarche.
Unlike F2 obesity, the F0 o,p’-DDT association with F2 age at menarche did not vary significantly according to F0 BMI. Therefore, we present results for F2 age at menarche for all F0 (Table 3). Risk of early menarche (≤ age 11 vs >age 11 years) in F2 was estimated to be about 2 times more likely when F0 o,p’-DDT levels were in tertile 3 compared to tertile 1. Adjustment by F0 African American race and age at menarche, and by F1 age at menarche and BMI at age 30 did not exert a notable effect on estimated F0 o,p’-DDT associations with F2 age at menarche (Table 3). The association of F1 BMI at age 30 with F2 early menarche did not reach statistical significance (p=.0572), but still is unlikely to have occurred by chance. p,p’-DDT was associated with a lower risk of F2 early menarche in the fully adjusted model (Supplementary Table 2B).
Table 3.
Associations of grandmothers’ (F0) serum o,p’-DDT with F2 early menarche1, n=235 triads
| Estimated o,p’-DDT Association | |||
|---|---|---|---|
| Model Level of Adjustment | Odds Ratio2 | (95% CI3) | p-value |
| DDT congeners and F0 Age at Menarche Adjusted | 2.23 | (1.24, 4.01) | 0.0076 |
| + F1 Age at Menarche Adjusted | 2.25 | (1.24, 4.08) | 0.0077 |
| + Race Adjusted | 2.06 | (1.10, 3.87) | 0.0235 |
| + F1 BMI Adjusted | 2.08 | (1.11, 3.90) | 0.0222 |
Early menarche was defined as age ≤11 vs. >11 years based on the 25th percentile for the distribution of F2 age at menarche. Associations are estimated from log-linear models adjusted for family clustering. Models include linear terms for p,p’-DDT, p,p’-DDE, o,p’-DDT and F0 age at menarche (continuous). Subsequent models were adjusted by cumulatively adding: F1 age at menarche (continuous), race dichotomized as African American (1) vs. all other races (0), and F1 BMI (continuous) reported at age 30 nearest the age when F1 were pregnant with F2 and nearest the ages when F0 and F2 BMI were captured.
Reported o,p’-DDT odds ratios are estimated for the median of the 3rd vs. the median of the first tertile. The inter-tertile range for F0 o,p’-DDT at the medians was 0.86 ng/mL.
CI=Confidence Interval.
When o,p’-DDT is represented categorically (Supplementary Tables 3A and 3B), results are consistent with the linear associations estimated from models in Tables 2 – 3 where o,p’-DDT was represented as a continuous variable. As seen in Supplementary Table 2A and Supplementary Figure 1, p,p’-DDT and o,p’-DDT, which are positively correlated, have associations in opposite directions with F2 obesity and also with F2 age at menarche (Supplementary Table 2B). Thus, we include both in all models due to mutual confounding. Removing p,p’-DDE from models (null associations with F2 outcomes, Supplementary Tables 2A and 2B) has no substantial impact on findings, but we include p,p’-DDE in final models as it is the DDT compound measured in many studies and readers often wish to see or rule out its impact on health outcomes. Exclusion of outlier F0 o,p’-DDT values did not materially affect the associations.
DISCUSSION
Results show that grandmothers’ o,p’-DDT was associated with an increase in granddaughter obesity and early age at menarche, presenting a consistent pattern of associations that support the concept that in egg exposure to o,p’-DDT has potential for altering risk of breast cancer across generations. To the best of our knowledge, such a study has never been previously conducted in humans.
The present study builds on a body of evidence from the CHDS that o,p’-DDT exposure during F0 pregnancy or immediately after birth increases risk of breast cancer in F1 daughters (9), and increases the prevalence of breast cancer risk factors among F1 daughters, such as obesity (11) and breast density (10) and altered DNA methylation of breast cancer related genes in midlife (25). The impact of grandmaternal DDT exposure on F2 breast density is unknown but could soon be investigated in this cohort either using new, age-safe techniques for estimating density in young adulthood such as ultrasound (48) or waiting until the F2 achieve the age of mammography screening.
Results in the F1 generation are consistent with another study based in California which found that prenatal o,p’-DDT exposure was associated with childhood adiposity (49). We are not aware of previous human studies that have examined associations between perinatal o,p’-DDT exposure and age at menarche. The two studies which only examined p,p’-DDE or p,p’-DDT prenatally, reported conflicting results, one found no association between prenatal p,p’-DDE or p,p’-DDT and the reported timing of menarche (50) while the other study reported prenatal p,p’-DDE was associated with earlier menarche (51), consistent in principle with our results. We are also unaware of any studies of age at menarche or obesity after grandparent o,p’-DDT exposure. We suspect that perinatal o,p’-DDT is most consistently associated with obesity, menarche, and breast cancer across the CHDS generations because o,p’-DDT is relatively short lived in humans and thus is the best biomarker for perinatal exposure among these related compounds in humans.
Several experimental studies have reported that both p,p’-DDT and o,p’-DDT are toxic to ovarian follicles (24,52–54) (those which exist in the ovaries of an F1 fetus at the time of F0 exposure during pregnancy). Although the multigenerational evidence of rat ovarian disease was only demonstrated for p,p’-DDT in the F1 and F3 generations, and did not examine F2 rats or o,p’-DDT exposure, this body of experimental evidence raises the possibility that o,p’-DDT could directly target the F2 follicles within the F1 fetus at the time that F0 serum o,p’-DDT is measured. We speculate that changes in DNA methylation could unite the relationship of perinatal o,p’-DDT exposure to breast cancer risk. Perinatal o,p’-DDT has been associated with altered blood DNA methylation in adult CHDS F1 daughters at loci associated with breast cancer and /or menarche (25), and obesity (11,55–58). In addition, epigenetic changes were observed in F3 rats with heightened obesity and ovary disease prevalence after ancestral p,p’-DDT exposure (22–24). The F2 daughters are too young to examine whether F0 o,p’-DDT exposure will be associated with increased breast cancer risk. However, if obesity and/or age at menarche are on a causal pathway between o,p’-DDT and breast cancer then the impact of grandmaternal o,p’-DDT exposure across generations cannot be ruled out here.
Increased grandmothers’ BMI appeared to have a protective effect on the association between grandmothers’ o,p’-DDT and granddaughter obesity. Because DDT and DDE isoforms are lipophilic, excess body fat mass dilutes a given quantity of DDT or DDE exposure. However, if these DDT ‘dilution’ effects of BMI were the only or primary explanation for the observed interaction, we would expect grandmothers’ BMI to also modify the DDT effect on age at menarche, which it did not. Thus, we suspect that the interplay between grandmothers’ BMI and o,p’-DDT may be more complex than a simple decrease in dose due to ‘dilution’ and/or ‘excretion’ effects, possibly linked to activation of differential metabolic pathways in high BMI environments.
Weaknesses of this study include a lack of data on diet and exposures to chemicals at other periods of the lives of these three generations and lack of consideration of other unknown exposures that are correlated with DDTs. The F0 samples have been stored since the 1960’s and DDTs were measured about 40 years later. While we cannot rule out some desiccation of the samples, desiccation is likely to apply similarly or at random for the samples in this study. The NIH repository that stores our samples reports when a sample we have requested appears desiccated. This is an infrequent occurrence, and in this case, we do not assay that sample. As this is an observational study, there is uncertainty regarding whether o,p’-DDT is a marker of recent exposure or is itself the primary toxic influence among the DDTs measured. We lack data from other geographic locations and our sample has limited representation of Hispanics and Asians. However, our F0 cohort represents in-migration from East and South geographic areas of the U.S, where DDT was in heavy use (59); and, reflects the racial and ethnic composition of Alameda County California in the 1960’s. We do not know how grandfather exposures may have contributed to our findings. The strengths of this study are its prospective design, direct measurement of multiple DDTs in F0 perinatal serum during active exposure, the ability to account for the contribution of grandmother and mother obesity and age at menarche to observed associations of grandmother DDTs with granddaughter obesity and age at menarche.
CONCLUSIONS
Our findings are consistent with the hypothesis that grandmother (F0) exposures to DDT could have contributed the dramatic increases in obesity in current young adult women including the granddaughter (F2) generation in the CHDS cohort. This conclusion is strengthened by our prior observation that F0 exposures to DDT were also associated with daughters’ mid-life obesity (F1) in this same cohort. Notably, perinatal exposure to DDT is associated with breast cancer risk factors in subsequent generations.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the CHDS families for their steadfast participation in this study of multiple generations. We acknowledge the late Jacob Yerushalmy who had the foresight to design and implement the CHDS; the late Barbara van den Berg, the second Director of the CHDS, who worked indefatigably to preserve the data and serum archive, thus granting the CHDS longevity. We thank Dr. June-Soo Park and his staff at the Environmental Chemistry Laboratory, California Department of Toxic Substances Control, Berkeley, California, USA for their collaboration, expertise and work in measuring the organochlorine levels that supported this research. P. M. Cirillo, N. Y. Krigbaum and B. A. Cohn were funded by the California Breast Cancer Research Grants Program, grants #15ZB-0186 and #22UB-5411, the Breast Cancer Environment Research Program, grants #U01 ES019471, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, contract # HHSN275201100020C. M. M. La Merrill was supported by funding from the National Institute of Environmental Health Sciences (NIEHS), grant #R01 ES024946 and from the USDA National Institute of Food and Agriculture, Hatch project 1002182.
FUNDING SOURCES
This research was supported by the California Breast Cancer Research Grants Program, grants #15ZB-0186 and #22UB-5411, the Breast Cancer Environment Research Program, grants #U01 ES019471 and #R01 ES024946 from the National Institute of Environmental Health Sciences (NIEHS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, contract # HHSN275201100020C, and USDA National Institute of Food and Agriculture, Hatch project 1002182 from the USDA National Institute of Food and Agriculture.
ABBREVIATIONS
- CHDS
Child Health and Development Studies
- DDT
a pesticide widely used worldwide from the 1940’s to the 1970’s and still used in some countries
- DDTs
various chemical compounds related to the commercial insecticide known as DDT
- p,p’-DDT
1,1,1-trichloro2,2-bis(p-chlorophenyl) ethane
- o,p’-DDT
1,1,1-trichloro2-(o-chlorophenyl)- 2-(p-chlorophenyl)ethane
- DDE
1,1-dichloro-2,2-bis (p-chlorophenyl) ethylene
- F0
Founding generation in the Child Health and Development Studies refers to mothers pregnant at entry to the study
- F1
offspring generation in the Child Health and Development Studies refers to daughters of F0 in this study
- F2
grandchildren generation in the Child Health and Development Studies refers to granddaughters of F0 in this study
- BMI
body mass index
Footnotes
CONFLICT OF INTERESTS
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.U. S. Environmental Protection Agency. DDT, a review of scientific and economic aspects of the decision to ban its use as a pesticide. Washington, D.C.: United States Environmental Protection Agency; 1975. July. Report nr EPA-540/1-75-022. [Google Scholar]
- 2.Kutz F, Yobs A, Strassman S, Viar J Jr. Effects of reducing DDT usage on total DDT storage in humans. Pestic Monit J 1977;11:61–3. [PubMed] [Google Scholar]
- 3.Wang M, Park J-S, Petreas M. Temporal Changes in the Levels of Perfluorinated Compounds in California Women Serum over the Past 50 Years. Environmental Science & Technology 2011;45(17):7510–6 doi 10.1021/es2012275. [DOI] [PubMed] [Google Scholar]
- 4.Agency for Toxic Substances and Disease Registry. Toxicological Profile for DDT, DDE, and DDD. U.S. Department of Health and Human Services, Public Health Service.; 2002. [PubMed] [Google Scholar]
- 5.Morgan D, Roan CC. The metabolism of DDT in man. In: Hayes WJ, editor. Essays in Toxicology. Volume 5. New York and London: Academic Press; 1975. p 39–97. [Google Scholar]
- 6.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature 1995;375(15 June):581–5. [DOI] [PubMed] [Google Scholar]
- 7.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect 2007;115(10):1406–14 doi 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn BA. Developmental and environmental origins of breast cancer: DDT as a case study. Reproductive Toxicology 2011;31(3):302–11 doi S0890-6238(10)00315-1 [pii] 10.1016/j.reprotox.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L, et al. DDT Exposure in Utero and Breast Cancer. Journal of Clinical Endocrinology and Metabolism 2015;100(8):2865–72 doi 10.1210/jc.2015-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald JA, Cirillo PM, Tehranifar P, Krigbaum NY, Engman N, Cohn BA, et al. In Utero DDT Exposure and Breast Density in Early Menopause by Maternal History of Breast Cancer. Reprod Toxicol 2019. doi 10.1016/j.reprotox.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Merrill MA, Krigbaum NY, Cirillo PM, Cohn BA. Association between maternal exposure to the pesticide dichlorodiphenyltrichloroethane (DDT) and risk of obesity in middle age. International Journal of Obesity 2020. doi 10.1038/s41366-020-0586-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehr-Green PA. Demographic and seasonal influences on human serum pesticide residue levels. Journal of Toxicology and Environmental Health 1989;27(4):405–21. [DOI] [PubMed] [Google Scholar]
- 13.Cano-Sancho G, Salmon AG, La Merrill MA. Association between Exposure to p,p’-DDT and Its Metabolite p,p’-DDE with Obesity: Integrated Systematic Review and Meta-Analysis. Environ Health Perspect 2017;125(9):096002 doi 10.1289/EHP527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels SI, Chambers JC, Sanchez SS, La Merrill MA, Hubbard AE, Macherone A, et al. Elevated Levels of Organochlorine Pesticides in South Asian Immigrants Are Associated With an Increased Risk of Diabetes. J Endocr Soc 2018;2(8):832–41 doi 10.1210/js.2017-00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Merrill MA, Johnson CL, Smith MT, Kandula NR, Macherone A, Pennell KD, et al. Exposure to Persistent Organic Pollutants (POPs) and Their Relationship to Hepatic Fat and Insulin Insensitivity among Asian Indian Immigrants in the United States. Environ Sci Technol 2019;53(23):13906–18 doi 10.1021/acs.est.9b03373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koepke R, Warner M, Petreas M, Cabria A, Danis R, Hernandez-Avila M, et al. Serum DDT and DDE levels in pregnant women of Chiapas, Mexico. Archives of Environmental Health 2004;59(11):559–65 doi 10.1080/00039890409603434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker DJP. The origins of the developmental origins theory. Journal of Internal Medicine 2007;261(5):412–7. [DOI] [PubMed] [Google Scholar]
- 18.Gluckman PD HM, Bateson P, Beedle AS, Law CM, Bhutta ZA, Anokhin KV, Bougnères P, Chandak GR, Dasgupta P, Smith GD, Ellison PT, Forrester TE, Gilbert SF, Jablonka E, Kaplan H, Prentice AM, Simpson SJ, Uauy R, West-Eberhard MJ. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. The Lancet 2009;373(9675):1654–7. [DOI] [PubMed] [Google Scholar]
- 19.La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mount Sinai Journal of Medicine 2011;78(1):22–48 doi 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heindel JJ, Vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, et al. Parma consensus statement on metabolic disruptors. Environ Health 2015;14(1):54 doi 10.1186/s12940-015-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Merrill M, Karey E, Moshier E, Lindtner C, La Frano MR, Newman JW, et al. Perinatal Exposure of Mice to the Pesticide DDT Impairs Energy Expenditure and Metabolism in Adult Female Offspring . PLoS ONE 2014;9(7):e103337 doi 10.1371/journal.pone.0103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King SE, Nilsson E, Beck D, Skinner MK. Adipocyte epigenetic alterations and potential therapeutic targets in transgenerationally inherited lean and obese phenotypes following ancestral exposures. Adipocyte 2019;8(1):362–78 doi 10.1080/21623945.2019.1693747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King SE, McBirney M, Beck D, Sadler-Riggleman I, Nilsson E, Skinner MK. Sperm epimutation biomarkers of obesity and pathologies following DDT induced epigenetic transgenerational inheritance of disease. Environ Epigenet 2019;5(2):dvz008 doi 10.1093/eep/dvz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson E, Klukovich R, Sadler-Riggleman I, Beck D, Xie Y, Yan W, et al. Environmental toxicant induced epigenetic transgenerational inheritance of ovarian pathology and granulosa cell epigenome and transcriptome alterations: ancestral origins of polycystic ovarian syndrome and primary ovarian insufiency. Epigenetics 2018;13(8):875–95 doi 10.1080/15592294.2018.1521223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu HC, Cohn BA, Cirillo PM, Santella RM, Terry MB. DDT exposure during pregnancy and DNA methylation alterations in female offspring in the Child Health and Development Study. Reprod Toxicol 2019. doi 10.1016/j.reprotox.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatric and Perinatal Epidemiology 1988;2(3):265–82. [DOI] [PubMed] [Google Scholar]
- 27.Cohn BA, Terry MB, Plumb M, Cirillo PM. Exposure to polychlorinated biphenyl (PCB) congeners during pregnancy and subsequent risk of breast cancer before age 50. 2012; San Francisco. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohn BA, La Merrill MA, Krigbaum NY, Wang M, Park JS, Petreas M, et al. In utero exposure to poly- and perfluoroalkyl substances (PFASs) and subsequent breast cancer. Reprod Toxicol 2020;92:112–9 doi 10.1016/j.reprotox.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 29.La Merrill MA, Cirillo PM, Krigbaum NY, Cohn BA. The impact of prenatal parental tobacco smoking on risk of diabetes mellitus in middle-aged women. Journal of Developmental Origins of Health and Disease 2015;6(03):242–9 doi doi: 10.1017/S2040174415000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. Canadian Medical Association Journal 2020;192(31):E875–E91 doi 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ardern CI, Janssen I, Ross R, Katzmarzyk PT. Development of health-related waist circumference thresholds within BMI categories. Obesity research 2004;12(7):1094–103. [DOI] [PubMed] [Google Scholar]
- 32.Sholtz RI, McLaughlin KR, Cirillo PM, Petreas M, Park JS, Wolff MS, et al. Assaying organochlorines in archived serum for a large, long-term cohort: implications of combining assay results from multiple laboratories over time. Environ Int 2011;37(4):709–14 doi 10.1016/j.envint.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW. Serial levels of serum organochlorines during pregnancy and postpartum. Archives of Environmental Health 1999;54(2):110–4. [DOI] [PubMed] [Google Scholar]
- 34.Allain C, Poon L, Chan C, Richmond W, Fu P. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470–5. [PubMed] [Google Scholar]
- 35.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. Journal of the American Statistical Association 1979;74: 829–36. [Google Scholar]
- 36.Hu X, Li S, Cirillo P, Krigbaum N, Tran V, Ishikawa T, et al. Metabolome Wide Association Study of serum DDT and DDE in Pregnancy and Early Postpartum. Reprod Toxicol 2020;92:129–37 doi 10.1016/j.reprotox.2019.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohn BA, Cirillo PM, Wolff MS, Schwingl PJ, Cohen RD, Sholtz RI, et al. DDT and DDE exposure in mothers and time to pregnancy in daughters. Lancet 2003;361(9376):2205–6 doi S0140-6736(03)13776-2 [pii] 10.1016/S0140-6736(03)13776-2. [DOI] [PubMed] [Google Scholar]
- 38.Kezios KL, Liu X, Cirillo PM, Cohn BA, Kalantzi OI, Wang Y, et al. Dichlorodiphenyltrichloroethane (DDT), DDT metabolites and pregnancy outcomes. Reprod Toxicol 2013;35:156–64 doi 10.1016/j.reprotox.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohn BA, Cirillo PM, Christianson RE. Prenatal DDT exposure and testicular cancer: a nested case-control study. Arch Environ Occup Health 2010;65(3):127–34 doi 1N8H68P20T708336 [pii] 10.1080/19338241003730887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoaglin DC. John W. Tukey and data analysis. Statistical Science 2003:311–8. [Google Scholar]
- 41.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307(5):491–7 doi 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 42.Herman-Giddens ME. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. International Journal of Andrology 2006;29(1):241–6; discussion 86–90 doi 10.1111/j.1365-2605.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 43.Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Bräuner EV, et al. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr 2020;174(4):e195881 doi 10.1001/jamapediatrics.2019.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr 2012;77(3):137–45 doi 10.1159/000336325. [DOI] [PubMed] [Google Scholar]
- 45.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sørensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics 2008;121 Suppl 3:S172–91 doi 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 46.Kutz FW, Wood PH, Bottimore DP. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Reviews of Environmental Contamination and Toxicology 1991;120:1–82. [DOI] [PubMed] [Google Scholar]
- 47.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 1992;82(5):703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruby L, Sanabria SJ, Obrist AS, Martini K, Forte S, Goksel O, et al. Breast Density Assessment in Young Women with Ultrasound based on Speed of Sound: Influence of the Menstrual Cycle. Medicine (Baltimore) 2019;98(25):e16123 doi 10.1097/md.0000000000016123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warner M, Ye M, Harley K, Kogut K, Bradman A, Eskenazi B. Prenatal DDT exposure and child adiposity at age 12: The CHAMACOS study. Environ Res 2017;159:606–12 doi 10.1016/j.envres.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Namulanda G, Maisonet M, Taylor E, Flanders WD, Olson D, Sjodin A, et al. In utero exposure to organochlorine pesticides and early menarche in the Avon Longitudinal Study of Parents and Children. Environ Int 2016;94:467–72 doi 10.1016/j.envint.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasiliu O, Muttineni J, Karmaus W. In utero exposure to organochlorines and age at menarche. Hum Reprod 2004;19(7):1506–12 doi 10.1093/humrep/deh292. [DOI] [PubMed] [Google Scholar]
- 52.Wojtowicz AK, Gregoraszczuk EL, Ptak A, Falandysz J. Effect of single and repeated in vitro exposure of ovarian follicles to o,p’-DDT and p,p’-DDT and their metabolites. Pol J Pharmacol 2004;56(4):465–72. [PubMed] [Google Scholar]
- 53.Liu KC, Wu RS, Ge W. Luteinizing hormone receptor (lhcgr) as a marker gene for characterizing estrogenic endocrine-disrupting chemicals in zebrafish ovarian follicle cells. Gen Comp Endocrinol 2013;192:89–94 doi 10.1016/j.ygcen.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 54.Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med 2013;11:228 doi 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masson LF, Sharp L, Cotton SC, Little J. Cytochrome P-450 1A1 gene polymorphisms and risk of breast cancer: a HuGE review. Am J Epidemiol 2005;161(10):901–15 doi 10.1093/aje/kwi121. [DOI] [PubMed] [Google Scholar]
- 56.Gorai I, Tanaka K, Inada M, Morinaga H, Uchiyama Y, Kikuchi R, et al. Estrogen-metabolizing gene polymorphisms, but not estrogen receptor-alpha gene polymorphisms, are associated with the onset of menarche in healthy postmenopausal Japanese women. J Clin Endocrinol Metab 2003;88(2):799–803 doi 10.1210/jc.2002-020353. [DOI] [PubMed] [Google Scholar]
- 57.DuBois BN, O’Tierney-Ginn P, Pearson J, Friedman JE, Thornburg K, Cherala G. Maternal obesity alters feto-placental cytochrome P4501A1 activity. Placenta 2012;33(12):1045–51 doi 10.1016/j.placenta.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai PC, Glastonbury CA, Eliot MN, Bollepalli S, Yet I, Castillo-Fernandez JE, et al. Smoking induces coordinated DNA methylation and gene expression changes in adipose tissue with consequences for metabolic health. Clin Epigenetics 2018;10(1):126 doi 10.1186/s13148-018-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.James RA, Hertz-Picciotto I, Willman E, Keller JA, Charles MJ. Determinants of serum polychlorinated biphenyls and organochlorine pesticides measured in women from the child health and development study cohort, 1963–1967. Environmental Health Perspectives 2002;110(7):617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.