Abstract
We investigated whether cerebrovascular disease contributes to neurodegeneration and clinical phenotype in dementia with Lewy bodies (DLB). Regional cortical thickness and subcortical gray matter volumes were estimated from structural magnetic resonance imaging (MRI) in 165 DLB patients. Cortical and subcortical infarcts were recorded and white matter hyperintensities (WMHs) were assessed. Subcortical only infarcts were more frequent (13.3%) than cortical only infarcts (3.1%) or both subcortical and cortical infarcts (2.4%). Infarcts, irrespective of type, were associated with WMHs. A higher WMH volume was associated with thinner orbitofrontal, retrosplenial, and posterior cingulate cortices, smaller thalamus and pallidum, and larger caudate volume. A higher WMH volume was associated with the presence of visual hallucinations and lower global cognitive performance, and tended to be associated with the absence of probable rapid eye movement sleep behavior disorder. Presence of infarcts was associated with the absence of parkinsonism. We conclude that cerebrovascular disease is associated with gray matter neurodegeneration in patients with probable DLB, which may have implications for the multifactorial treatment of probable DLB.
Keywords: Dementia with Lewy bodies, cerebrovascular disease, magnetic resonance imaging, white matter hyperintensities, infarcts, neurodegeneration
1. INTRODUCTION
Although Lewy bodies are the main pathological hallmark of dementia with Lewy bodies (DLB), recent neuropathological and magnetic resonance imaging (MRI) studies have recognized the potential role of cerebrovascular disease in DLB pathogenesis (Donaghy et al., 2020; Jellinger, 2018; Watson and Colloby, 2016). Two common MRI markers of cerebrovascular disease are white matter hyperintensities (WMHs) and infarcts (Wardlaw et al., 2013). Whereas several studies showed that the WMH burden is higher in patients with probable DLB than in healthy controls (Barber et al., 1999; Joki et al., 2018; Kim et al., 2015; Koikkalainen et al., 2016; Sarro et al., 2017), no differences in WMH burden have also been reported (Burton et al., 2006; De Reuck et al., 2016; Oppedal et al., 2012). Further, it is unclear whether the WMH burden in DLB is due to primary vascular pathology or is secondary to neurodegeneration (Barber et al., 2000; Chimowitz et al., 1992; Fazekas et al., 1996). Two studies showed that WMH burden in DLB is associated with increased global brain atrophy and atrophy in the medial temporal lobes (Barber et al., 2000; Joki et al., 2018). Moreover, investigations into the association between WMHs and cognitive functioning have also provided mixed results. No associations have been reported in several studies (Barber et al., 1999; Burton et al., 2006; Fukui et al., 2013; Oppedal et al., 2012), while other reports suggested that the association of WMHs with cognition may depend on the APOE genotype and/or the location of the lesions. For instance, Mirza et al. (2019) reported an association exclusively in APOE ε4 carriers, and Park et al. (2015) showed that increased WMHs in cholinergic white matter pathways is associated with lower cognitive performance. Very few studies investigated the association of WMHs with clinical features of DLB. Sarro et al. (2017) showed no associations with visual hallucinations, parkinsonism, or cognitive fluctuations. However, Barber et al. (1999) reported that although periventricular WMHs were not associated with visual hallucinations, occipital WMHs were associated with the absence of visual hallucinations; while Fukui et al. (2013) showed that periventricular WMHs were associated with the presence of visual hallucinations in DLB. Further, increased WMHs have been associated with a lower frequency of rapid eye movement sleep behavior disorder (RBD) (Sarro et al., 2017). Finally, the WMH burden may increase with age in probable DLB (Barber et al., 1999; Sarro et al., 2017), although some studies did not replicate that finding (Burton et al., 2006; Park et al., 2015), and the WMH burden may be higher in women but not in APOE ε4 carriers (Sarro et al., 2017).
Even less is known about infarcts in DLB. Their frequency seems to be comparable to that in healthy controls (Kim et al., 2015; Koikkalainen et al., 2016; Sarro et al., 2017), and may be associated with older age, but not with APOE genotype, sex, or severity of dementia (Sarro et al., 2017).
Therefore, the current knowledge on how cerebrovascular disease contributes to neurodegeneration and clinical phenotype in DLB is limited and inconclusive. This may be partially explained by the lack of longitudinal studies specifically designed to investigate associations between cerebrovascular disease and neurodegeneration and clinical phenotype in DLB. Further, methodological variability and the use of relatively small samples, has made replication and generalization of findings difficult. In the current multi-center study, we investigated WMHs and cortical and subcortical infarcts in 165 patients with probable DLB, the largest cohort so far in an MRI study of cerebrovascular disease in DLB. We hypothesized that there would be a positive association between WMHs and infarcts, and that a higher burden of cerebrovascular disease would be associated with reduced cortical thickness and volume of subcortical gray matter structures, as MRI markers of neurodegeneration. We also hypothesized that a higher burden of cerebrovascular disease would be associated with a lower frequency of clinical features of DLB and a lower cognitive performance.
2. METHODS
2.1. Participants
We used data from the European DLB consortium (E-DLB) (Oppedal et al., 2019) and the Mayo Clinic DLB cohort (Kantarci et al., 2017), from Rochester, Minnesota, the United States.
The E-DLB consortium archives data from 40 centers across Europe (Kramberger et al., 2017), including patients with probable DLB, Parkinson’s disease with dementia, or AD. For the current study, we included patients with probable DLB from the E-DLB centers that had collected more than 20 DLB patients each and had high-resolution T1-weigthed and fluid attenuation inversion recovery (FLAIR) MRI data (see below). Three E-DLB centers satisfied such criteria, including the Day Hospital of Geriatrics, Memory Resource and Research Centre (CMRR, Strasbourg, France, n = 34), the Motol University Hospital (Prague, Czech Republic, n = 29), and the VU University Medical Center (VUmc, Amsterdam, the Netherlands, n = 34). The diagnostic procedure and clinical examinations are described elsewhere (Kramberger et al., 2017). Briefly, diagnosis was made according to the 2005 International Consensus Criteria for probable DLB (Mckeith et al., 2005), based on detailed history and clinical examinations including physical, neurological, and psychiatric examinations performed by a licensed neurologist. Exclusion criteria were patients with acute delirium, terminal illness, previous major stroke, psychotic or bipolar disorder, craniocerebral trauma, a major neurological illness other than dementia.
The Mayo Clinic DLB cohort is a prospective study of consecutive cases assessed through the Mayo Clinic Alzheimer’s Disease Research Center and the Mayo Clinic Study of Aging. For the current study we included patients assessed between May 2007 and September 2017 who had high-resolution T1-weigthed and FLAIR MRI data (see below). All patients underwent a medical history review, informant interview, neurologic examination, and neuropsychological assessment (Ferman et al., 2018). For the current study, patients with a diagnosis of probable DLB according to the 2005 International Consensus Criteria (Mckeith et al., 2005) were included (n = 68).
All centers recorded whether patients fulfilled criteria for parkinsonism, visual hallucinations (VH), fluctuating cognition, and a clinical history of probable rapid eye movement sleep behavior disorder (RBD). Presence/absence of clinical features was based on the 2005 International Consensus Criteria for probable DLB (Mckeith et al., 2005), to allow harmonized diagnosis across the four centers because many of the patients were assessed prior to 2017 International Consensus Criteria (McKeith et al., 2017). Polysomnogram confirmation was not required for RBD, and the International Classification of Sleep Disorders-II diagnostic criteria B was used for the diagnosis of probable RBD (AASM, 2005). The Mini-Mental State Examination (MMSE) was selected as a measure of global cognition.
An institutional ethics committee at each E-DLB center as well as the Mayo Clinic Institutional Review Board approved the study. Informed consent on participation was obtained from all of the patients or an appropriate surrogate according to the Declaration of Helsinki.
2.2. Magnetic resonance imaging
A high-resolution 3D T1-weigthed magnetization prepared rapid gradient echo (MPRAGE) sequence and a FLAIR sequence were acquired in all four of the centers included in this study. Supplementary Table 1 shows the scanning parameters by center. 3T scanners were used in the Day Hospital of Geriatrics, Memory Resource and Research Centre (CMRR, Strasbourg, France), the Mayo Clinic (Rochester, US), and the VU University Medical Center (VUmc, Amsterdam, the Netherlands). A 1.5T scanner was used in the Motol University Hospital (Prague, Czech Republic).
2.3. Infarcts and WMHs
MRIs from the four centers contributing to this study were centralized at Mayo Clinic and graded by two neurologists (J.G-R. and Z.N.), both for infarcts and WMHs, according to established standards (Graff-Radford et al., 2020). Infarcts were graded on FLAIR images that were co-registered with T1-weighted MPRAGE images as previously described.
Infarcts were initially identified by trained image analysts and subsequently confirmed by a vascular neurologist (J.G-R.). The intra-rater reliability based on blinded reading of 50 possible infarcts on two separate occasions was excellent (kappa = 0.92) (Graff-Radford et al., 2020). Cortical infarcts were defined as hyperintense lesions (gliosis) on the FLAIR sequence, which were located in the cortical gray matter and extending to the cortical edge with or without involvement of the adjacent white matter. A corresponding T1 hypointensity was required for confirmation. Subcortical infarcts were defined as hyperintense lesions with a dark center on FLAIR images, which were located in the white matter, infratentorial, and central gray-capsular regions. The dark center (tissue loss) was required to be greater than or equal to 3 mm in diameter as measured on the FLAIR or T1 sequences. As previously noted (Graff-Radford et al., 2020), the dark center may not be seen on FLAIR images in infarcts located in the thalamus and caudate, giving fully hyperintense lesions, while having CSF intensity on T1-weighted images. For this reason, infarcts in the thalamus and caudate did not need to meet the 3-mm diameter criteria, central hypointensity, or surrounding rim of hyperintensity thresholds. Further, a subset of cerebellar infarcts with CSF intensity on the FLAIR and T1 sequences may have no hyperintense ring on FLAIR, but the infarcts still needed to meet the 3-mm criteria.
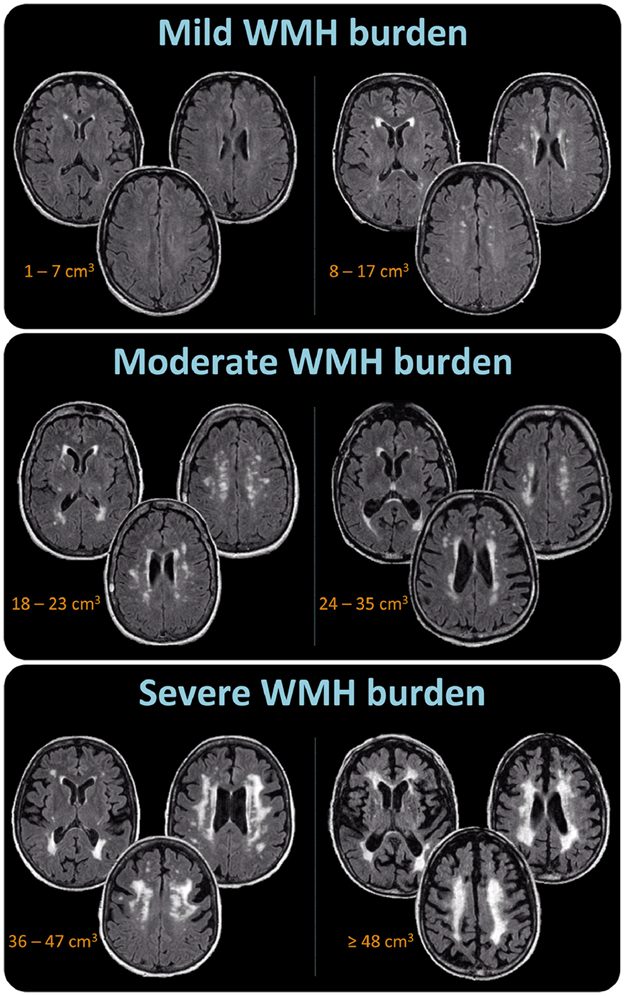
WMH volume was visually assessed by a neurologist (Z.N.), centrally at Mayo Clinic and under the same technical conditions (i.e., monitor, lighting, contrast adjustments, etc.). WMH volume was assessed using a semi-quantitative scale of six volume categories. WMHs were defined as signal abnormalities of variable size in the white matter that present as hyperintensities on FLAIR images and are not associated with infarcts identified on FLAIR images. WMH volume was estimated as in a previous study (Kantarci et al., 2008). Briefly, each subject’s FLAIR image was compared with a bank of ten FLAIR image templates with increasing WMH volume in patients with probable DLB (from 1 to 100 cm3), as determined by a semi-automated image segmentation algorithm (Raz et al., 2013). A continuous scale with a slider bar was used to estimate each subject’s WMH volume by matching to the WMH templates (Wu et al., 2002). The WMH volume estimation algorithm was previously validated against quantification using an automated image segmentation of the WMH volume. The concordance correlation coefficient was 0.88 (95% confidence interval (CI) = 0.83, 0.94) (Kantarci et al., 2008). Intra-rater reliability for WMH assessments in the current study was excellent (weighted kappa = 0.96, 95% CI = 0.92, 0.99). Figure 1 shows representative cases with mild WMH, moderate WMH, and severe WMH burden (similar to Fazekas (Fazekas et al., 1987) scores of 1, 2, and 3, respectively), and their correspondence with the six volume categories of the WMH visual scale. In the current study, the measure used in most of the analyses was the six-level variable, representing WMH volume. When specified, the three WMH burden categories variable was used instead to facilitate clinical interpretability and comparability with similar WMH scales such as the Fazekas scale (Fazekas et al., 1987).
Figure 1. Visual examples of mild, moderate, and severe WMH burden.
The mild WMH burden category includes the two lower volume categories corresponding to WMHs 1 to 7 cm3 large (first volume category), and WMHs 8 to 17 cm3 large (second volume category). The moderate WMH burden category includes the two intermediate volume categories corresponding to WMHs 18 to 23 cm3 large (third volume category), and WMHs 24 to 35 cm3 large (fourth volume category). The severe WMH burden category includes the two higher volume categories corresponding to WMHs 36 to 47 cm3 large (fifth volume category), and WMHs 48 cm3 or larger (sixth volume category). Abbreviations: WMH = white matter hyperintensities.
Assessment of both WMHs and infarcts was blinded to clinical information.
2.4. Cortical thickness and subcortical gray matter volume
Using ANTs (Avants et al., 2008), the Mayo Clinic Adult Lifespan Template (MCALT) (https://www.nitrc.org/projects/mcalt/) atlases were propagated to individuals’ native MPRAGE space and regional estimations of thickness across the cortical mantle and volume across subcortical gray matter structures were calculated. Tissue probabilities were determined for each MPRAGE using the unified segmentation algorithm in SPM12 (Welcome Trust Centre for Neuroimaging, London, UK), with MCALT tissue priors and settings (Schwarz et al., 2017), and cortical thickness was estimated from these probabilities using ANTs DiReCT (Das et al., 2009). Left and right regions of interest (ROIs) were combined, giving a total of 41 bilateral cortical ROIs and 6 subcortical ROIs. The total intracranial volume was calculated from the tissue probabilities and included in statistical models involving subcortical gray matter volumes, to account for between-subject variability in head size.
2.5. Statistical analysis
Demographic and clinical characteristics were reported using means and standard deviations for continuous variables, and counts and percentages for categorical variables. Distributions of infarcts and WMH were described using an exploded pie chart, histogram, and bar chart. Because automated quantification of cortical thickness and subcortical gray matter volumes is influenced by field strength (Guo et al., 2019), we restricted our statistical analyses on cortical thickness and subcortical gray matter volumes to the subsample including 3T scanners (n = 136). In contrast, visual assessment of infarcts and WMHs are less influenced by the variability in scanners and MRI protocols across centers, and were assessed centrally by the same two raters (J.G.R. and Z.N.). Hence, we used the entire cohort (N = 165) when analyzing infarcts and WMHs. We ran linear mixed effects (continuous outcomes) or logistic mixed effects (binary outcomes) models that included the infarcts and WMHs as fixed effect variables and center as a random variable. All models were also adjusted for age and sex. When analyzing the subcortical gray matter volumes as the response variable there was an additional adjustment for total intracranial volume. Heterogeneity across centers was tested by comparing nested models: the above restricted model with MRI measures and centers, and a more general model with MRI measures within centers. The restricted model assumes a common association with the MRI measures across centers, and the more general model allows each center to have different associations with the MRI measures. A significant p-value would indicate heterogeneity in the associations with MRI measures by center, and that a common measure of association is not appropriate. Our results showed that the test for heterogeneity was not significant in any of the models. In addition, we used linear mixed or logistic mixed models to compare women and men, and APOE ε4 non-carriers and carriers (at least one ε4 allele), as well as to test for age associations across demographic and clinical variables. A p-value ≤0.05 (two-tailed) was deemed significant in all these analyses. The False Discovery Rate (FDR) (Genovese CR, Lazar NA, 2002) was applied to deal with multiple testing in the analyses involving the cortical thickness and subcortical gray matter volume ROIs.
3. RESULTS
Table 1 shows the main demographic and clinical characteristics of the whole cohort (N = 165) and the subsample with available 3T MRI data (n = 136). The 3T MRI subsample involved 82% of the whole cohort and was largely comparable in terms of demographic and clinical characteristics.
Table 1.
Demographic and clinical characteristics
| Whole sample (N = 165) |
3T MRI subsample (n = 136) |
|||
|---|---|---|---|---|
| N | Mean (SD) / count (%) |
N | Mean (SD) / count (%) |
|
| Age, years | 165 | 69.1 (8.6) | 136 | 68.0 (8.3) |
| min - max | 45 - 88 | 45- 88 | ||
| Sex, men | 165 | 119 (72%) | 136 | 105 (77%) |
| Education, years | 165 | 13.6 (3.9) | 136 | 13.3 (4.0) |
| APOE, ε4 carriers | 159 | 69 (43%) | 131 | 59 (45%) |
| MMSE, total score | 164 | 22.9 (5.2) | 135 | 23.1 (5.4) |
| Visual hallucinations, presence | 162 | 89 (55%) | 133 | 75 (56%) |
| Cognitive fluctuations, presence | 157 | 131 (83%) | 128 | 112 (88%) |
| Parkinsonism, presence | 163 | 142 (87%) | 134 | 118 (88%) |
| Probable RBD, presence | 150 | 117 (78%) | 124 | 101 (81%) |
| Center | 165 | 136 | ||
| CMRR, n | 34 (21%) | 34 (25%) | ||
| Mayo Clinic, n | 68 (41%) | 68 (50%) | ||
| Motol University Hospital, n | 29 (17%) | - | ||
| VUmc, n | 34 (21%) | 34 (25%) | ||
Mean (standard deviation, SD) is listed for the continuous variables and count (%) for categorical variables. Abbreviations: 3T MRI = 3 tesla magnetic resonance imaging; APOE = Apolipoprotein E; MMSE = mini-mental state examination; RBD = rapid eye movement sleep behavior disorder; CMRR = Day Hospital of Geriatrics, Memory Resource and Research Centre (Strasbourg, France); VUmc = VU University Medical Center (Amsterdam, the Netherlands).
3.1. Infarcts and WMHs
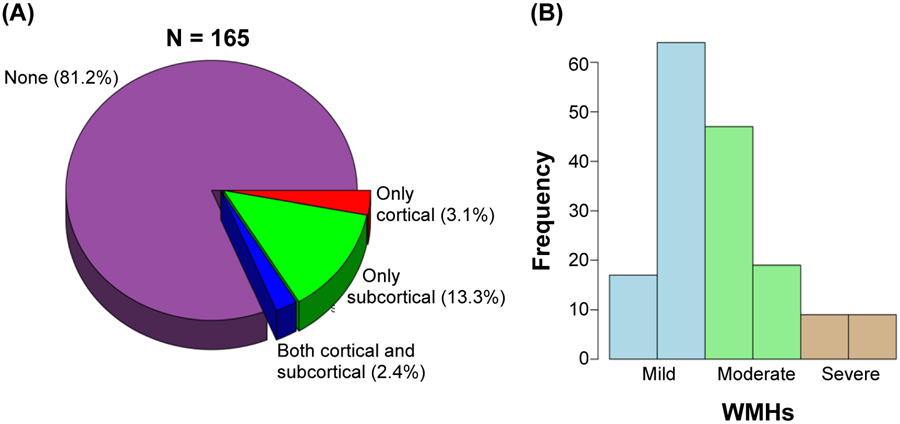
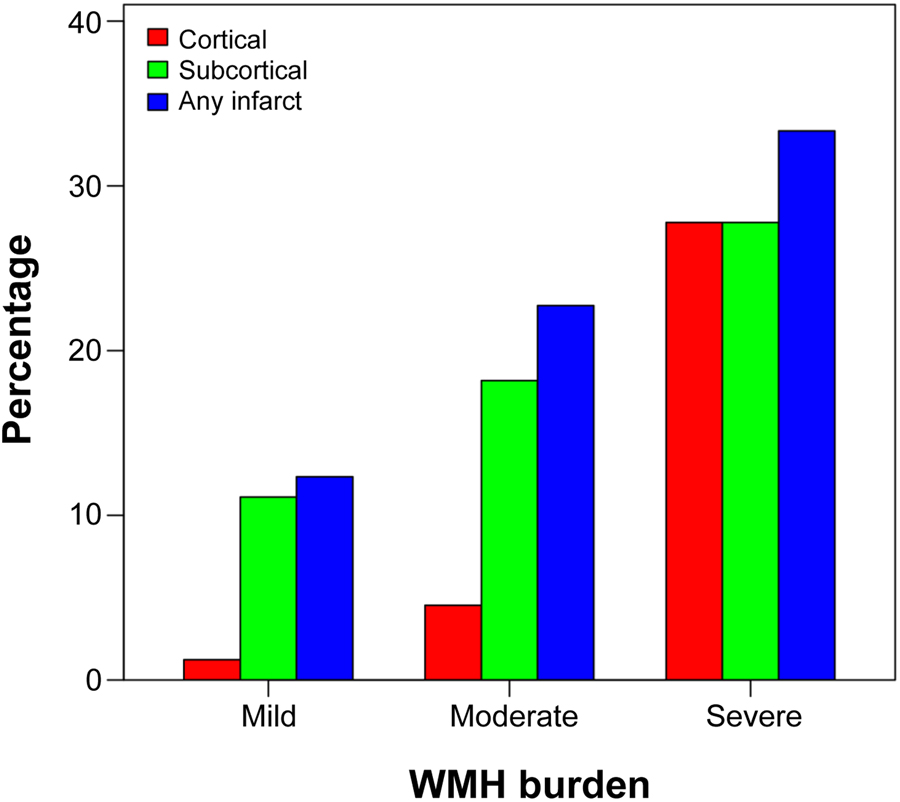
Figure 2A shows that 81.2% of the patients had no infarcts while 18.8% of the patients had cortical or subcortical infarcts. The overall frequency of cortical infarcts was 5.5% and subcortical infarcts was 15.7%. In particular, only subcortical infarcts were present in 13.3% and only cortical infarcts were present in 3.1% of the patients, while cortical and subcortical infarcts were jointly present in 2.4% of the patients. Figure 2B shows that 49.1% of the patients had a mild WMH burden, 40.0% had a moderate WMH burden, and 10.9% had a high WMH burden. There was a significant association between having an infarct and WMH burden (p = 0.015). Figure 3 shows that a higher number of subcortical infarcts was associated with greater WMH burden, while a higher number of cortical infarcts tended to associate with severe WMH burden. We also tested for associations with age, sex, and APOE genotype. Older patients had more infarcts (p = 0.006) and a higher WMH volume (p<0.001), while no significant associations were found for sex. Further, APOE genotype was significantly associated with WMHs (p = 0.044) when entered in a model together with age (p<0.001) and sex (p = 0.77), indicating a higher WMH volume in APOE ε4 non-carriers than in carriers. In contrast, there was no significant association between APOE genotype and the infarcts.
Figure 2. Infarcts and WMHs.
(A) Pie chart displaying percentage of DLB patients with only cortical infarcts (in red), only subcortical infarcts (in green), or both cortical and subcortical infarcts (in blue). (B) Histogram displaying the distribution of the six WMH volume categories (bars) colored by WMH burden (mild, moderate, and severe), as follows: WMHs 1 to 7 cm3 large (first volume category), 8 to 17 cm3 large (second volume category), 18 to 23 cm3 large (third volume category), 24 to 35 cm3 large (fourth volume category), 36 to 47 cm3 large (fifth volume category), and 48 cm3 or larger (sixth volume category) (please see also Figure 1). Abbreviations: WMHs = white matter hyperintensities.
Figure 3. Infarcts within mild, moderate, and severe WMH burden.
The association between infarcts and WMH burden. Abbreviations: WMH = white matter hyperintensities.
3.2. Clinical features and cognitive performance
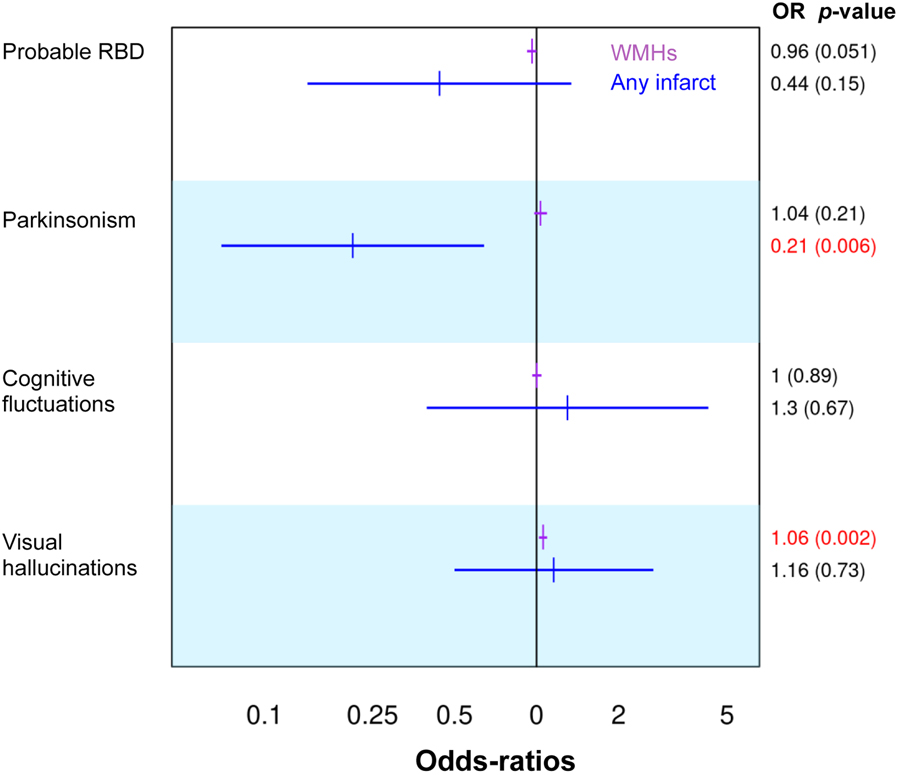
A higher WMH volume was significantly associated with the presence of visual hallucinations (odds ratio = 1.06; 95% confidence interval (CI) = 1.02, 1.09; p = 0.002) and with lower MMSE scores (estimate = −0.09; 95% CI = −0.16, −0.03; p = 0.008). We also observed a trend towards a significant association between a higher WMH volume and the absence of probable RBD (odds ratio = 0.96; 95% CI = 0.92, 1; p = 0.051). In addition, the presence of any infarct was significantly associated with the absence of parkinsonism (odds ratio = 0.21; 95% CI = 0.07, 0.64; p = 0.006) (Figure 4).
Figure 4. The association of infarcts and WMHs with clinical features.
Odds-Ratios and p-values for infarcts and WMHs from a linear mixed model with center as a blocking variable included as a random effect in the models, and age and sex adjustment. Significant p-values (≤0.05) are displayed in red. Abbreviations: RBD = rapid eye movement sleep behavior disorder; WMHs = white matter hyperintensities volume; OR = odds-ratios.
3.3. Cortical thickness and subcortical volumes
For these analyses, we excluded estimations of cortical thickness for ROIs displaying cortical infarcts (exclusively for those patients and ROIs that displayed a cortical infarct).
Supplementary Figure 1 shows that after the FDR adjustment for multiple testing, a higher WMH volume was associated with a thinner cortex in three regions of the orbitofrontal cortex (gyrus rectus and superior and medial orbital regions), and a lower volume of the thalamus and pallidum. Furthermore, a higher WMH volume was associated with a higher volume of the caudate. Figure 5 shows the cluster of cortical regions with the highest estimates (strongest association between higher WMH volume and lower cortical thickness, fully detailed in Supplementary Figure 1). These regions included the middle and inferior orbitofrontal, retrosplenial, and anterior and posterior cingulate cortices, in addition to gyrus rectus and superior and medial orbital regions). No significant associations were observed between infarcts and cortical thickness or subcortical volumes.
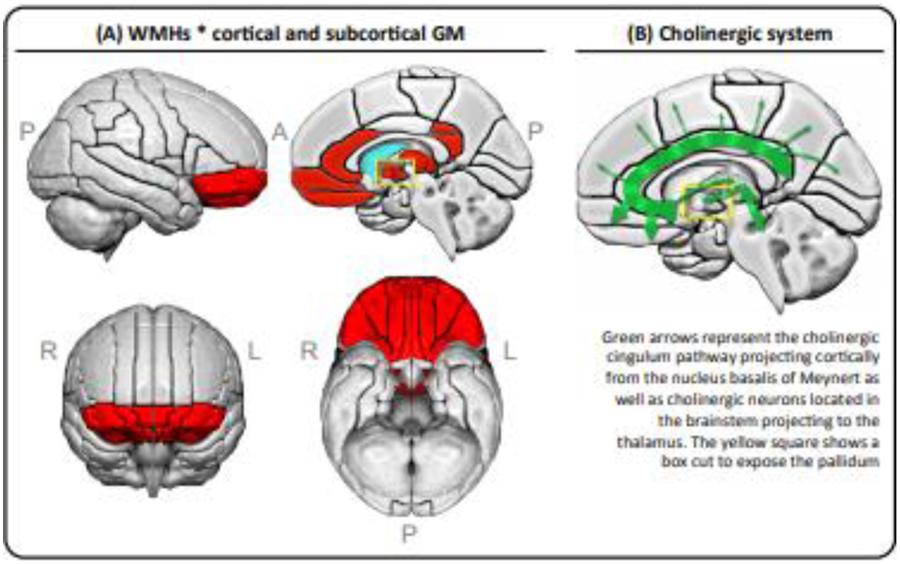
Figure 5. The association of WMHs with cortical thickness and subcortical gray matter volumes.
(A) Red areas represent combined results from two models: the cluster of cortical regions with the highest estimates from a linear mixed effects model with WMHs as a fixed effect variable and center as a random variable, adjusted for age and sex; plus all the significant regions from a linear mixed effects model for subcortical volume ROIs with WMHs as a fixed effect variable and center as a random variable, adjusted for age, sex, and total intracranial volume (please see Supplementary Figure 1 for forest plot results including estimates, confidence intervals, and both uncorrected and FDR-adjusted p-values). (B) A schematic representation of the cholinergic system. Abbreviations: A = anterior; FDR = False Discovery Rate; GM = gray matter; L = left; P = posterior; R = right; WMHs = white matter hyperintensities.
4. DISCUSION
This multi-center study demonstrates that WMHs and infarcts are associated with each other in probable DLB. Moreover, we demonstrated that a higher WMH burden in DLB is associated with increased neurodegeneration, lower cognitive performance, the presence of visual hallucinations, and the absence of RBD. The presence of infarcts was associated with the absence of parkinsonism.
We found that subcortical infarcts were more common than cortical infarcts, in line with previous reports in patients with probable DLB (Kim et al., 2015; Sarro et al., 2017). We also found a positive association between infarcts and WMH burden. The frequency of subcortical infarcts increased with greater WMH burden, while cortical infarcts were observed almost exclusively in the presence of severe WMH burden. Only two MRI studies investigated infarcts and WMHs in DLB (Kim et al., 2015; Sarro et al., 2017). However, none of these previous studies investigated the association between infarcts and WMHs. Our current study may thus help to clarify the association between these two widely used MRI markers of cerebrovascular disease (Wardlaw et al., 2013). This significant association between infarcts and WMHs suggest common pathophysiological mechanisms underlying both infarcts and WMHs in probable DLB. Although the pathogenesis of these lesions is not well understood and could be multifactorial (Gouw et al., 2011), deep WMHs may be primarily related to ischemic damage (Ballard et al., 2000; Barber et al., 1999; Kenny et al., 2004), while cortical infarcts and periventricular WMHs especially in posterior brain areas may be related to neurodegeneration and cerebral amyloid angiopathy (Barber et al., 2000, 1999; De Reuck et al., 2014; Ghebremedhin et al., 2010). Previous research has mostly focused on the association of infarcts or WMHs with vascular factors in probable DLB (Ballard et al., 2000; Barber et al., 2000; Kenny et al., 2004; Sarro et al., 2017). In our current study we sought to fill in the important gap in the DLB literature regarding the association of infarcts and WMHs with markers of neurodegeneration. Although we can not confirm whether the association between WMHs and infarcts in our current study is specific to probable DLB, previous reports showed that the WMH burden is higher in probable DLB patients than in healthy controls (Barber et al., 1999; Joki et al., 2018; Kim et al., 2015; Koikkalainen et al., 2016; Sarro et al., 2017). However, it should be noted that volume of WMHs and frequency of infarcts tend to cluster together as the age increases in non-demented individuals (Graff-Radford et al., 2020). Hence, our current association between WMHs and infarcts may not be entirely exclusive of DLB.
The association between infarcts and neurodegeneration has not been investigated in DLB, and only two MRI studies reported data on the association between WMHs and neurodegeneration (Barber et al., 2000; Joki et al., 2018). Barber et al. (2000) showed that periventricular WMHs were associated with increased global brain atrophy, while no association was found for deep WMHs. Similarly, Joki et al. (2018) showed that periventricular WMHs were associated with increased atrophy in medial temporal lobes, while no association was found for deep WMHs. Our current study extends these previous reports by providing detailed data on the association of infarcts and WMHs with cortical thickness across the whole cortical mantle, as well as with the volume of key subcortical gray matter structures. We found that a higher WMH volume was associated with increased neurodegeneration in the orbitofrontal cortex, thalamus, and pallidum. We also found that the cluster with the highest estimates (i.e., strongest association between higher WMH volume and lower cortical thickness) included the orbitofrontal, retrosplenial, and posterior cingulate cortices.
The orbitofrontal, retrosplenial, and posterior cingulate cortices receive dense cholinergic input from the cholinergic cingulum pathway, which emerges from the anteromedial nucleus basalis of Meynert (Nemy et al., 2020; Selden et al., 1998). The thalamus also receives cholinergic input from cholinergic neurons located in the brainstem and possibly also from the nucleus basalis of Meynert (Mesulam, 2013). The pallidum is closely connected to the thalamus. Hence, our data suggest that these cortical and subcortical regions could be particularly vulnerable to cholinergic disruption due to cerebrovascular disease, an interpretation that is supported by the known interplay between cholinergic and vascular systems (Claassen and Jansen, 2006; Engelhardt et al., 2007; Liu et al., 2017). While it is not possible to determine a cause and effect relationship in this cross-sectional study, future prospective studies with longitudinal MRI may help determine the temporal relationships between cerebrovascular lesions and neurodegeneration.
In addition, we found that a higher WMH volume was associated with a larger volume (less neurodegeneration) of the caudate. The caudate is possibly the subcortical region that receives the least cholinergic input (Mesulam, 2013). Instead, the caudate receives prominent dopaminergic innervation and is heavily targeted by Lewy body pathology affecting the nigrostriatal dopaminergic system (Jellinger, 2018). Early atrophy of the caudate can be detected already in prodromal to moderate DLB patients and has been associated with impairment of attentional processing speed (Botzung et al., 2019). The lack of cholinergic input to the caudate could explain its larger volume in the context of high WMH burden. The dopaminergic system may be more spared in the context of a cholinergic system that is severely targeted by cerebrovascular disease, in mild to moderate DLB patients who are eligible for MRI studies like in our cohort. This interpretation is further supported by a dual finding in our study: (1) the presence of infarcts was associated with the absence of parkinsonism, a symptom that is closely related to dopaminergic deficits; (2) the trend towards a significant association (p = 0.051) between a higher WMH volume and the absence of probable RBD, a feature that is highly specific to Lewy body pathology (McKeith et al., 2017). Other studies also reported an inverse association between Lewy body pathology and cerebrovascular disease (Fukui et al., 2013; Ghebremedhin et al., 2010).
While brain atrophy is not prominent in DLB (Yousaf et al., 2019), a large multi-center study recently identified the signature pattern of atrophy in probable DLB, involving atrophy in the posterior cortex and preservation of the medial temporal lobes (Oppedal & Ferreira et al., 2018). This pattern also involves several subcortical gray matter structures, including the thalamus, caudate, putamen, and pallidum (Watson and Colloby, 2016). An important observation is that this pattern of atrophy overlaps spatially with the hypometabolic pattern characteristic of DLB (McKeith et al., 2017), as well as with the spatial pattern of white matter abnormalities in diffusion MRI (Nedelska et al., 2015b; Watson et al., 2012), the distribution of reduced blood perfusion (Nedelska et al., 2018), and the pattern of tau binding in positron emission tomography (PET) (Kantarci et al., 2017). In a recent study, we further demonstrated that atrophy in the posterior cortex is increased in probable DLB patients with positive amyloid and tau biomarkers (Abdelnour et al., 2020). Further, all the cortical regions reported in our current study spatially overlap with brain areas that show increased longitudinal atrophy in DLB patients with concomitant Alzheimer’s disease pathology (Nedelska et al., 2015a). Hence, our current results together with these previous reports suggest that there seems to be a spatial correspondence between two common pathologies in DLB (i.e., tau neurofibrillary tangles (NFT) and cerebrovascular disease), potentially contributing to the neurodegeneration and cognitive impairment in part through involvement of the cholinergic system. The potential synergistic interaction between Lewy body pathology, tau NFT, and cerebrovascular disease is a topic of utmost interest that cannot be investigated in-vivo at present due to the lack of reliable biomarkers for alpha-synuclein.
Very few studies have investigated the association of infarcts and WMHs with clinical features of DLB. We found that a higher WMH volume was significantly associated with the presence of visual hallucinations, and we already discussed above the trend towards a significant association between a higher WMH volume and the absence of probable RBD. Further, we found that the presence of infarcts was associated with the absence of parkinsonism. Our current study is in agreement with Sarro et al. (2017). Using a different method for WMHs, we also did not find an association between WMH volume and parkinsonism and cognitive fluctuations. However, in contrast to Sarro et al. (2017), we found that an increased WMH volume was associated with visual hallucinations in our larger and more heterogeneous cohort of DLB patients in the current study. Although 24% of the patients from the current study are shared with the sample used in Sarro et al. (2017), only 8% include MRI scans from the same time frame. The positive association between WMHs and visual hallucinations has also been reported by Fukui et al. (2013), but the opposite result was reported when combining DLB patients with other dementias in Barber et al. (1999), i.e., WMHs in occipital areas were associated with the absence of visual hallucinations (Barber et al., 1999). The authors suggested that perhaps an occipital lobe spared of pathology is necessary to develop visual hallucinations, and that the disruption of neural circuits involving visual association areas might prevent hallucinations (Barber et al., 1999). This hypothesis remains to be tested. Previous studies have reported a higher frequency of visual hallucinations in DLB patients with Alzheimer’s disease co-pathology (Lemstra et al., 2017). Hence, visual hallucinations in DLB seem to be more frequent in the context of co-pathologies, whether cerebrovascular disease or Alzheimer’s disease.
Previous studies including small samples did not find any association between WMHs and cognition (Barber et al., 1999; Burton et al., 2006; Fukui et al., 2013; Oppedal et al., 2012). Our relatively large multi-center cohort of probable DLB patients could thus have helped to capture the heterogeneity within probable DLB better and reveal the association between increased WMHs and lower cognitive performance. This interpretation is supported by two studies that further reduced part of that heterogeneity by stratifying DLB patients based on APOE genotype or focused on cholinergic white matter pathways (Mirza et al., 2019; Park et al., 2015). Mirza et al. (2019) reported an association exclusively in APOE ε4 carriers, and Park et al. (2015) showed that when WMHs are located in cholinergic white matter pathways they seem to have an impact on cognitive performance. We did not investigate the location of WMHs but, as discussed above, we observed that they were associated with neurodegeneration of gray matter areas that receive dense cholinergic input.
Both infarcts and WMHs increase with age in non-demented individuals (Graff-radford et al., 2020). Since we applied an age correction in our statistical models, our current findings cannot be explained by the effect of aging. Further, our current results do not spatially overlap with the effect of age on cortical thickness and subcortical gray matter volumes commonly seen in healthy individuals (Machado et al., 2018). This suggests that our current results go beyond the association of age with WMHs and neurodegeneration, and are likely specific to DLB. Nonetheless, whether WMHs are due to Lewy-induced cerebrovascular disease or are due to vascular risk factors cannot be answered at present in-vivo, due to the lack of reliable biomarkers for alpha-synuclein. In addition to age, our findings were independent of the effects of sex, APOE genotype, and center.
Two previous studies showed that WMHs and infarcts are associated with older age and female sex, but they do not seem to have an association with APOE genotype in probable DLB (Barber et al., 1999; Sarro et al., 2017). We found that older patients had more infarcts and WMHs, but we did not find any association with sex. Further, APOE genotype was not significantly associated with the infarcts, but it was associated with WMHs after adjusting for age, indicating a higher WMH volume in APOE ε4 non-carriers. This finding has also been reported in Alzheimer’s disease, with APOE ε4 non-carriers (in particular, APOE ε2 carriers) having a higher WMH volume than APOE ε4 carriers (Groot et al., 2018). The suggested mechanism was the reduced integrity of amyloid-affected cerebral vasculature, increasing the risk of cerebral amyloid angiopathy, but amyloid-independent pathways might also be possible (Groot et al., 2018). Importantly, our statistical tests for heterogeneity did not show any significant results. This means that there was no evidence that our results differed across centers, highlighting the generalizability of the reported associations in probable DLB patients across four centers in Europe and the US. Our relatively large multi-center data may thus help to clarify some of the contradictory results from previous studies using smaller samples from a single center.
Our current study has some limitations. A semi-quantitative assessment of WMHs was the preferred method due to the multi-center nature of our study, which included some variability in MRI scanners and scanning parameters (Oppedal et al., 2018). Hence, we were unable to obtain separate estimations of periventricular and deep WMHs, which may differ in their underlying pathophysiology (Barber et al., 1999). Vascular risk factors have previously been associated with increased cerebrovascular disease in DLB (Sarro et al., 2017; Donaghy et al., 2020), so that they might partially explain some of our current findings. However, vascular risk factors were not consistently recorded across the four centers included in this study, which prevented us to consider them in our statistical analysis. Cerebrovascular disease may be one of the factors contributing to neurodegeneration in probable DLB. The spatial concordance between the pattern of tau PET binding and the neurodegeneration identified in our current study claims in favor of future studies investigating the association between tau PET, WMHs, and cortical thickness. Because the data was collected through a consortium on DLB, a matching control group was not available at each site. While this is a limitation of our cohort future studies that compare DLB patients to controls can determine the differences between DLB and normal aging. Finally, the diagnosis of probable DLB was based on clinical grounds, without autopsy confirmation, which has known limitations (Rizzo et al., 2018). However, it is reassuring from previous studies that most of the patients in the E-DLB cohort who had a dopamine transporter SPECT scan available were positive (Oppedal et al., 2018), and that the Mayo patients showed a high rate of Lewy body disease at autopsy (Sarro et al., 2017).
In conclusion, this multi-center study demonstrates an association between WMHs and the neurodegeneration of brain areas that receive dense cholinergic input, and that WMHs and infarcts have an impact on several clinical features and cognitive performance in probable DLB. This suggests a synergistic interaction between cerebrovascular disease and Lewy body pathology, which possibly extends to other highly concomitant pathologies in probable DLB, including amyloid-beta and tau NFT tangles (Ferreira et al., 2020). Hence, prevention and therapy of cerebrovascular disease should be considered for the multifactorial treatment of probable DLB.
Supplementary Material
Highlights.
Subcortical infarcts were more frequent than cortical infarcts in probable DLB
Infarcts were associated with WMH burden in probable DLB
WMH burden was associated with gray matter neurodegeneration in probable DLB
WMH burden was associated with clinical features of DLB and cognitive performance
Our findings may have implications for the multifactorial treatment of probable DLB
5. ACKNOWLEDGEMENTS
The authors particularly thank the patients and their family members for participating in this research. This work was supported by the National Institutes of Health (U01- NS100620, P50-AG016574, U01-AG006786, R37-AG011378, R01-AG041851, R01-AG040042, C06-RR018898 and R01-NS080820), Foundation Dr. Corinne Schuler, the Mangurian Foundation for Lewy Body Research, the Elsie and Marvin Dekelboum Family Foundation, the Little Family Foundation, the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program, the Western Norway Regional Health Authority, the Swedish Foundation for Strategic Research (SSF), the Swedish Research Council (VR), Karolinska Institutet travel grants, Center for Innovative Medicine (CIMED), the Foundation for Geriatric Diseases at Karolinska Institutet, the NIHR biomedical research centre at UCLH, and the Projet Hospitalier de Recherche Clinique (PHRC, IDCRB 2012-A00992-41) and fondation Université de Strasbourg. The sponsors played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
D Ferreira, SA Przybelski, TG Lesnick, AW Lemstra, Z Nedelska, CG Schwarz, H Botha, ML Senjem, JA Fields, DS Knopman, R Savica, NR Graff-Radford, RC Petersen, J Hort, K Oppedal, and E Westman report no disclosures relevant to the manuscript. J Graff-Radford receives research support from NIH. T Ferman receives funding from the Mangurian Foundation for Lewy body research and NIH. VJ Lowe serves as a consultant for AVID Radiopharmaceuticals, Bayer Schering Pharma, Eisai Inc, Philips Molecular Imaging, and Piramal Imaging and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH (NIA, NCI), and the MN Partnership for Biotechnology and Medical Genomics. CR Jack has consulted for Lily, serves on an independent data monitoring board for Roche, and as a speaker for Eisai, but he receives no personal compensation from any commercial entity. He receives research support from NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. F Blanc, has served as national coordinator and principal investigator for clinical trials sponsored by Biogen, Roche, Axovant and Eisai. BF Boeve has served as an investigator for clinical trials sponsored by Biogen and Alector. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program and the Little Family Foundation. D Aarsland has received research support and/or honoraria from AstraZeneca, H. Lundbeck, Novartis Pharmaceuticals and GE Health, and served as paid consultant for H. Lundbeck, Eisai and Evonik. K Kantarci serves on the data safety monitoring board for Takeda Global Research and Development Center, Inc.; receives research support from Avid Radiopharmaceuticals and Eli Lilly, and receives funding from NIH and Alzheimer’s Drug Discovery Foundation.
7. REFERENCES
- AASM, 2005. International Classification of Sleep Disorders–2: Diagnostic and Coding Manual. American Academy of Sleep Medicine, Chicago. [Google Scholar]
- Abdelnour C, Ferreira D, Oppedal K, Cavallin L, Bousiges O, Olof L, Hort J, Nedelska Z, Padovani A, Pilotto A, Bonanni L, Kramberger MG, Boada M, Westman E, Pagonabarraga J, Kulisevsky J, Blanc F, Aarsland D The combined effect of amyloid-β and tau biomarkers on brain atrophy in dementia with Lewy bodies. Neuroimage Clin 2020;27:102333. doi: 10.1016/j.nicl.2020.102333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Brien JO, Barber BOB, Scheltens P, Shaw F Mckeith IAN, Anne R Neurocardiovascular Instability, Hypotensive Episodes, and MRI Lesions in Neurodegenerative Dementia. Ann. NY Acad Sci 2000;442–5. doi: 10.1111/j.1749-6632.2000.tb06396.x. [DOI] [PubMed] [Google Scholar]
- Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O’Brien JT MRI volumetric correlates of white matter lesions in dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry 2000;15:911–6. doi: . [DOI] [PubMed] [Google Scholar]
- Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, Perry R, O’Brien J White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J Neurol Neurosurg Psychiatry 1999;67:66–72. doi: 10.1136/jnnp.67.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung A, Philippi N, Noblet V, Sousa PL De, Blanc F Pay attention to the basal ganglia: a volumetric study in early dementia with Lewy bodies. Alzheimers Res Ther 2019; 11:108. doi: 10.1186/s13195-019-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O’Brien JT Progression of white matter hyperintensities in Alzheimer disease, dementia with Lewy bodies, and Parkinson disease dementia: A comparison with normal aging. Am J Geriatr Psychiatry 2006;14:842–9. doi: 10.1097/01.JGP.0000236596.56982.1c. [DOI] [PubMed] [Google Scholar]
- Chimowitz MI, Estes ML, Furlan AJ, Awad IA Further Observations on the Pathology of Subcortical Lesions Identified on Magnetic Resonance Imaging. Arch Neurol 1992;49:747–52. [DOI] [PubMed] [Google Scholar]
- Claassen JAHR, Jansen WMM Cholinergically Mediated Augmentation of Cerebral Perfusion in Alzheimer’s Disease and Related Cognitive Disorders: The Cholinergic – Vascular Hypothesis. J Gerontol A Biol Sci Med Sci 2006;61:267–71. doi: 10.1093/gerona/61.3.267. [DOI] [PubMed] [Google Scholar]
- Das SR, Avants BB, Grossman M, Gee JC Registration based cortical thickness measurement. Neuroimage 2009;45, 867–79. doi: 10.1016/j.neuroimage.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuck J, Auger F, Durieux N, Cordonnier C, Deramecourt V, Pasquier F, Maurage CA, Leys D, Bordet R Topographic distribution of white matter changes and lacunar infarcts in neurodegenerative and vascular dementia syndromes: A post-mortem 7.0-tesla magnetic resonance imaging study. Eur Stroke J 2016;1:122–9. doi: 10.1177/2396987316650780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuck J, Deramecourt V, Auger F, Durieux N, Cordonnier C, Devos D, Defebvre L, Moreau C, Caparros-Lefebvre D, Bordet R, Maurage CA, Pasquier F, Leys D Post-mortem 7.0-tesla magnetic resonance study of cortical microinfarcts in neurodegenerative diseases and vascular dementia with neuropathological correlates. J Neurol Sci 2014;346:85–9. doi: 10.1016/j.jns.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Donaghy PC, Firbank M, Mitra D, Petrides G, Lloyd J, Barnett N, Olsen K, Thomas AJ, O’Brien JT Microbleeds in dementia with Lewy bodies. J Neurol 2020;267:1491–8. doi: 10.1007/S00415-020-09736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt E Moreira DM, Laks J Vascular dementia and the cholinergic pathways. Dement Neuropsychol 2007;1:2–9. doi: 10.1590/S1980-57642008DN10100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA MR Signal Abnormalities at 1.5 T in Alzheimer’s Dementia and Normal Aging. AJR Am J Roentgenol 1987;149:351–6. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kapeller P, Schmidt R, Offenbacher H, Payer F, Fazekas G The relation of cerebral magnetic resonance signal hyperintensities to Alzheimer’s disease. J Neurol Sci 1996;142:121–5. [DOI] [PubMed] [Google Scholar]
- Ferman TJ, Aoki N, Crook JE, Murray ME, Graff-Radford NR, van Gerpen JA, Uitti RJ, Wszolek ZK, Graff-Radford J, Pedraza O, Kantarci K, Boeve BF, Dickson DW The limbic and neocortical contribution of α-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimers Dement. 2018;14:330–9. doi: 10.1016/j.jalz.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D, Przybelski SA, Lesnick TG, Lemstra AW, Londos E, Blanc F, Nedelska Z, Schwarz CG, Graff-Radford J, Senjem ML, Fields JA, Knopman DS, Savica R, Ferman TJ, Graff-Radford NR, Lowe VJ, Jack CR Jr, Petersen RC, Mollenhauer B, Garcia-Ptacek S, Abdelnour C, Hort J, Bonanni L, Oppedal K, Kramberger MG, Boeve BF, Aarsland D, Westman E, Kantarci K Amyloid-β and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology 2020; 10.1212/WNL.0000000000010943. doi: 10.1212/WNL.0000000000010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Oowan Y, Yamazaki T, Kinno R Prevalence and Clinical Implication of Microbleeds in Dementia with Lewy Bodies in Comparison with Microbleeds in Alzheimer’s Disease. Dement Geriatr Cogn Dis Extra 2013;3:148–60. doi: 10.1159/000351423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, N. T Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15:870–8. [DOI] [PubMed] [Google Scholar]
- Ghebremedhin E, Rosenberger A, Rüb U, Vuksic M, Berhe T, Bickeböller H, De Vos RAI, Thal DR, Deller T Inverse relationship between cerebrovascular lesions and severity of lewy body pathology in patients with lewy body diseases. J Neuropathol Exp Neurol 2010;69:442–8. doi: 10.1097/NEN.0b013e3181d88e63. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, Van Der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJG Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011;82:126–35. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- Graff-radford J, Aakre JA, Knopman DS, Schwarz CG, Flemming KD, Rabinstein AA, Gunter JL, Ward CP, Zuk SM, Spychalla AJ, Preboske GM, Petersen RC, Kantarci K, Huston J Prevalence and Heterogeneity of Cerebrovascular Disease Imaging Lesions. Mayo Clin Proc 2020;95:1195–205. doi: 10.1016/j.mayocp.2020.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot C, Sudre CH, Barkhof F, Teunissen CE, van Berckel BNM, Seo SW, Ourselin S, Scheltens P, Cardoso MJ, van der Flier WM, Ossenkoppele R Clinical phenotype, atrophy, and small vessel disease in APOEε2 carriers with Alzheimer disease. Neurology 2018;91:e1851–e9. doi: 10.1212/WNL.0000000000006503. [DOI] [PubMed] [Google Scholar]
- Guo C, Ferreira D, Fink K, Westman E, Granberg T Repeatability and reproducibility of FreeSurfer, FSL-SIENAX and SPM brain volumetric measurements and the effect of lesion filling in multiple sclerosis. Eur Radiol 2019;29:1355–64. doi: 10.1007/s00330-018-5710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm (Vienna) 2018;125:615–50. doi: 10.1007/s00702-017-1821-9. [DOI] [PubMed] [Google Scholar]
- Joki H, Higashiyama Y, Nakae Y, Kugimoto C, Doi H, Kimura K, Kishida H, Ueda N, Nakano T, Takahashi T, Koyano S, Takeuchi H, Tanaka F White matter hyperintensities on MRI in dementia with Lewy bodies, Parkinson’s disease with dementia, and Alzheimer’s disease. J Neurol Sci 2018;385:99–104. doi: 10.1016/j.jns.2017.12.018. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Lowe VJ, Boeve BF, Senjem ML, Tosakulwong N, Lesnick TG, Spychalla AJ, Gunter JL, Fields JA, Graff-Radford J, Ferman TJ, Jones DT, Murray ME, Knopman DS, Jack CR, Petersen RC AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 2017;81:58–67. doi: 10.1002/ana.24825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Petersen RC, Przybelski SA, Weigand SD, Shiung MM, Whitwell JL, Negash S, Ivnik RJ, Boeve BF, Knopman DS, Smith GE, Jack CRJ Hippocampal Volumes, Proton Magnetic Resonance Spectroscopy Metabolites, and Cerebrovascular Disease in Mild Cognitive Impairment Subtypes. Arch Neurol 2008;65:1621–8. doi: 10.1017/CBO9781107415324.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny RA, Shaw FE, O’Brien JT, Scheltens PH, Kalaria R, Ballard C Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry 2004;75:966–71. doi: 10.1136/jnnp.2003.023812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Chung SJ, Oh YS, Yoon JH, Sunwoo MK, Hong JY, Kim JS, Lee PH Cerebral microbleeds in patients with dementia with lewy bodies and Parkinson disease dementia. Am J Neuroradiol 2015;36:1642–7. doi: 10.3174/ajnr.A4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koikkalainen J, Rhodius-Meester H, Tolonen A, Barkhof F, Tijms B, Lemstra AW, Tong T, Guerrero R, Schuh A, Ledig C, Rueckert D, Soininen H, Remes AM, Waldemar G, Hasselbalch S, Mecocci P, Van Der Flier W, Lötjönen J Differential diagnosis of neurodegenerative diseases using structural MRI data. Neuroimage Clin 2016;11:435–49. doi: 10.1016/j.nicl.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramberger MG, Auestad B, Garcia-Ptacek S, Abdelnour C, Olmo JG, Walker Z, Lemstra AW, Londos E, Blanc F, Bonanni L, McKeith I, Winblad B, de Jong FJ, Nobili F, Stefanova E, Petrova M, Falup-Pecurariu C, Rektorova I, Bostantjopoulou S, Biundo R, Weintraub D, Aarsland D. Long-Term Cognitive Decline in Dementia with Lewy Bodies in a Large Multicenter, International Cohort. J Alzheimers Dis 2017;57:787–95. doi: 10.3233/JAD-161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemstra AW, Beer MH De, Teunissen CE, Schreuder C, Scheltens P, Van Der Flier WM, Sikkes SAM Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2017;88:113–8. doi: 10.1136/jnnp-2016-313775. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhu Z, Teipel SJ, Yang J, Xing Y, Tang Y, Jia J White matter damage in the cholinergic system contributes to cognitive impairment in subcortical vascular cognitive impairment, no dementia. Front Aging Neurosci 2017;9:47. doi: 10.3389/fnagi.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Barroso J, Molina Y, Nieto A, Díaz-Flores L, Westman E, Ferreira D Proposal for a hierarchical, multidimensional, and multivariate approach to investigate cognitive aging. Neurobiol Aging 2018;71:179–88. doi: 10.1016/j.neurobiolaging.2018.07.017. [DOI] [PubMed] [Google Scholar]
- McKeith I, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O'Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K Diagnosis and management of dementia with Lewy bodies: Fourth report of the DLB Consortium. Neurology 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Diagnosis and management of dementia with Lewy bodies Third report of the DLB consortium. Neurology 2005;65:1863–972. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Mesulam M Cholinergic Circuitry of the Human Nucleus Basalis and Its Fate in Alzheimer’s Disease. J Comp Neurol 2013;521:4124–44. 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza SS, Saeed U, Knight J, Ramirez J, Stuss DT, Keith J, Nestor SM, Yu D, Swardfager W, Rogaeva E, St George Hyslop P, Black SE, Masellis M APOE ε4, white matter hyperintensities, and cognition in Alzheimer and Lewy body dementia. Neurology 2019;93, e1807–e19. doi: 10.1212/WNL.0000000000008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M, Shiung M, Weigand S Mayo MRI visual grading scale: validation studies, in: Abstracts of the Annual Meeting of the Society for Neuroscience, San Diego, CA. 2007. [Google Scholar]
- Nedelska Z, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Murray ME, Gunter JL, Senjem ML, Vemuri P, Smith GE, Geda YE, Graff-radford J, Knopman DS, Petersen RC, Parisi JE, Dickson DW, Jack CR, Kantarci K Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging 2015a; 36, 452–61. doi: 10.1016/j.neurobiolaging.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelska Z, Schwarz CG, Boeve BF, Lowe VJ, Reid RI, Przybelski SA, Lesnick TG, Gunter JL, Senjem ML, Ferman TJ, Smith GE, Geda YE, Knopman DS, Petersen RC, Jack CR, Kantarci K White matter integrity in dementia with Lewy bodies: A voxel-based analysis of diffusion tensor imaging. Neurobiol Aging 2015b;36:2010–7. doi: 10.1016/j.neurobiolaging.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelska Z, Senjem ML, Przybelski SA, Lesnick TG, Lowe VJ, Boeve BF, Arani A, Vemuri P, Gra J, Ferman TJ, Jones DT, Savica R, Knopman DS, Petersen RC, Jack R, Kantarci K Regional cortical perfusion on arterial spin labeling MRI in dementia with Lewy bodies: Associations with clinical severity, glucose metabolism and tau. Neuroimage Clin 2018;19:939–47. doi: 10.1016/j.nicl.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemy M, Cedres N, Grothe MJ, Muehlboeck J-S, Lindberg O, Nedelska Z, Stepankova O, Vyslouzilova L, Eriksdotter M, Barroso J, Teipel S, Westman E, Ferreira D Cholinergic white matter pathways make a stronger contribution to attention and memory in normal aging than cerebrovascular health and nucleus basalis of Meynert. Neuroimage 2020;211:116607. doi: 10.1016/j.neuroimage.2020.116607. [DOI] [PubMed] [Google Scholar]
- Oppedal K, Aarsland D, Firbank MJ, Sonnesyn H, Tysnes OB, O'Brien JT, Beyer MK White Matter Hyperintensities in Mild Lewy Body Dementia. Dement Geriatr Cogn Dis Extra 2012;2:481–95. doi: 10.1159/000343480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppedal K, Borda MG, Ferreira D, Westman E, Aarsland D European DLB consortium: diagnostic and prognostic biomarkers in dementia with Lewy bodies, a multicenter international initiative. Neurodegener Dis Manag 2019;9:247–50. doi: 10.2217/nmt-2019-0016. [DOI] [PubMed] [Google Scholar]
- Oppedal K, Ferreira D, Cavallin L, Lemstra A, ten Kate M, Padovani A, Rektorova I, Bonanni L, Wahlund L-O, Engedal K, Nobili F, Kramberger M, Taylor J-P, Hort J, Snædal J, Blanc F, Walker Z, Antonini A, Westman E, Aarsland D A signature pattern of cortical atrophy in dementia with Lewy bodies: a study on 333 patients from the European DLB Consortium. Alzheimers Dement 2019;15:400–9. doi: 10.1016/j.jalz.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Park HE, Park IS, Oh YS, Yang DW, Lee KS, Choi HS, Aim KJ, Kim JS Subcortical whiter matter hyperintensities within the cholinergic pathways of patients with dementia and parkinsonism. J Neurol Sci 2015;353:44–8. doi: 10.1016/j.jns.2015.03.046. [DOI] [PubMed] [Google Scholar]
- Raman MR, Preboske GM, Przybelski SA, Gunter JL, Senjem ML, Vemuri P, Murphy MC, Murray ME, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Dickson DW, Jack CR, Kantarci K Antemortem MRI findings associated with microinfarcts at autopsy. Neurology 2014;82:1951–8. doi: 10.1212/WNL.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, Senjem ML, Gunter JL, Vemuri P, Jack CR Jr, Miller VM, Kantarci K Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology 2013;80:911–8. doi: 10.1212/WNL.0b013e3182840c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G, Arcuti S, Copetti M, Alessandria M, Savica R, Fontana A, Liguori R, Logroscino G Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2018;89:358–66. doi: 10.1136/jnnp-2017-316844. [DOI] [PubMed] [Google Scholar]
- Sarro L, Tosakulwong N, Schwarz CG, Graff-Radford J, Przybelski SA, Lesnick TG, Zuk SM, Reid RI, Raman MR, Boeve BF, Ferman TJ, Knopman DS, Comi G, Filippi M, Murray ME, Parisi JE, Dickson DW, Petersen RC, Jack CR, Kantarci K An investigation of cerebrovascular lesions in dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Dement 2017;13:257–66. doi: 10.1016/j.jalz.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Gunter J, Ward C, Vemuri P, Senjem ML, Wiste H The mayo clinic adult life span template: better quantification across the life span. Alzheimers Dement 2017;13, P93–P4. [Google Scholar]
- Selden NR, Gitelman DR, Salamon-murayama N, Parrish TB, Mesulam M Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998;121:2249–57. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Wardlaw J, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge Rv, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12, 822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R, Blamire AM, Colloby SJ, Wood JS, Barber R, Brien JTO Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology 2012;79:906–14. doi: 10.1212/WNL.0b013e318266fc51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R, Colloby SJ Imaging in Dementia with Lewy Bodies: An Overview. J Geriatr Psychiatry Neurol 2016;29:254–60. doi: 10.1177/0891988716654984. [DOI] [PubMed] [Google Scholar]
- Wu C, Mungas D, Petkov CI, Eberling JL, Zrelak PA, Buonocore MH, Brunberg JA, Haan MN, Jagust WJ Brain structure and cognition in a community sample of elderly Latinos. Neurology 2002;59:383–91. doi: 10.1212/wnl.59.3.383. [DOI] [PubMed] [Google Scholar]
- Yousaf T, Dervenoulas G, Valkimadi PE, Politis M Neuroimaging in Lewy body dementia. J Neurol 2019;266:1–26. doi: 10.1007/s00415-018-8892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.