Abstract
In the past two decades, PET/CT has become an essential modality in oncology increasingly used in the management of gastrointestinal (GI)cancers. Most PET/CT tracers used in clinical practice show some degree of GI uptake. This uptake is quite variable and knowledge of common patterns of biodistribution of various radiotracers is helpful in clinical practice. 18F-Fluoro-Deoxy-Glucose (FDG) is the most commonly used radiotracer and has quite a variable uptake within the bowel. 68Ga-Prostate specific membrane antigen (PSMA) shows intense uptake within the proximal small bowel loops. 11C-methyl-L-methionine (MET) shows high accumulation within the bowels, which makes it difficult to assess bowel or pelvic diseases. One must also be aware of technical artifacts causing difficulties in interpretations, such as high attenuation oral contrast material within the bowel lumen or misregistration artifact due to patient movements.
It is imperative to know the common variants and benign diseases that can mimic malignant pathologies. Intense FDG uptake within the esophagus and stomach may be a normal variant or may be associated with benign conditions such as esophagitis, reflux disease, or gastritis. Metformin can cause diffuse intense uptake throughout the bowel loops. Intense physiologic uptake can also be seen within the anal canal. Segmental bowel uptake can be seen in inflammatory bowel disease, radiation, or medication induced enteritis/colitis or infection. Diagnosis of appendicitis or diverticular disease requires CT correlation, as normal appendix or diverticulum can show intense uptake.
Certain malignant pathologies are known to have only low FDG uptake, such as early-stage esophageal adenocarcinoma, mucinous tumors, indolent lymphomas, and multicystic mesotheliomas. Response assessment, particularly in the neoadjuvant setting, can be limited by post-treatment inflammatory changes. Post-operative complications such as abscess or fistula formation can also show intense uptake and may obscure underlying malignant pathology. In the absence of clinical suspicion or rising tumor marker, the role of FDG PET/CT in routine surveillance of patients with GI malignancy is not clear.
Introduction
Gastrointestinal (GI)tract cancers accounted for nearly 213,690 cancer diagnoses and 118,320 cancer-related deaths in 2020.1 Since the introduction of combined PET/CT scanners two decades ago, Positron Emission Tomography combined with Computed Tomography (PET/CT)has become an essential modality in the management of GI cancers, being used for diagnosis, staging, evaluation of treatment response, and assessment of prognosis.2 This is particularly true for esophageal, gastric, and colorectal cancers.
Several radiotracers are used in clinical practice and in the research setting. The specific use of each radiotracer varies based on its metabolic pathway and intracellular stability. Cyclotron produced Fluorine-18 (18F)with a physical half-life of 110 min allows adequate time for labelling as well as transport of radiopharmaceuticals to nearby locations. 18F labeled Fluoro-deoxy-glucose (18F-FDG)remains the most widely used radiotracer in molecular imaging. Carbon-11 (11C) is a short-lived positron emitter with a physical half-life of 20 min that requires an on-site cyclotron. It can be used to label acetate, methionine, or choline for both oncological and non-oncological imaging. Generator-produced Gallium-68 (68Ga)with a physical half-life of 68 min can be used for labeling somatostatin receptor binding compounds such as DOTATATE or DOTATOC (for imaging of neuroendocrine tumors) or small molecules binding to prostate specific membrane agent (PSMA)(for imaging of prostate cancer). Zirconium-89 (89Zr, half-life: 3.3 days), Iodine-124 (124I, half-life: 4.2 days), and Copper-64 (64Cu, half-life: 12.7 h)labeled antibodies, targeting for instance A33 or HER-2/neu, are currently being evaluated in the research setting.
Most of the radiotracers used in the clinical and research setting show GI uptake to some extent (Figure 1). As tracer accumulation within the GI tract is not specific for a single physiological process or pathology, it is imperative to understand physiological distribution of a given tracer within the normal GI tract and potential pitfalls in the interpretation of the imaging study. The purpose of this review is to convey the normal distribution pattern of commonly used tracers, highlight physiological variants, and emphasize pitfalls seen in PET/CT imaging of GI cancers.
Figure 1:
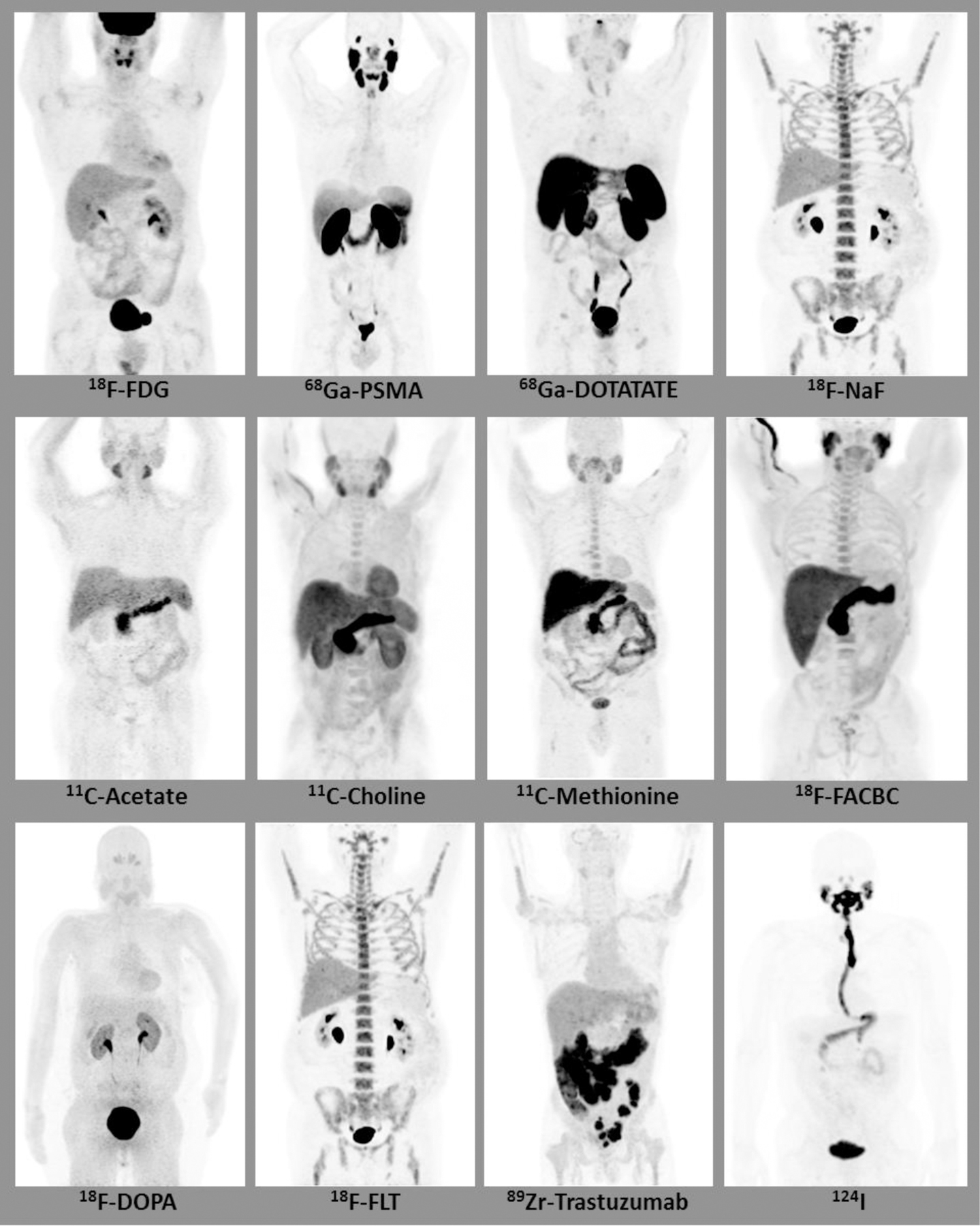
Maximum intensity projection (MIP) images depicting normal gastrointestinal biodistribution of various radiotracers.
Biodistribution of commonly used PET/CT tracers
18F-Fluoro-Deoxy-Glucose (FDG)
18F-FDG is the most commonly used radiotracer in the evaluation of malignancy. One of the key advantages of this radiotracer is its high cellular uptake in many common tumors and its intracellular retention. It acts as a surrogate for glucose and is taken up in tissues with high transmembrane glucose transporter expression and hexokinase activity. 18F-FDG uptake within the GI tract is quite variable and could be mild and diffuse, following the contours of the bowel, or intense and focal, mimicking malignancy. The exact mechanism by which 18F-FDG accumulates in the GI tract is poorly understood. It is postulated that FDG is taken up by the GI muscle and is excreted into the bowel lumen. Other possible causes of GI uptake include swallowed secretions, colonic microbial uptake, mucosal uptake, and uptake by the normal lymphoid tissues, for instance in the terminal ileum.
68Ga-Prostate specific membrane antigen (PSMA)
Prostate specific membrane antigen (PSMA) is structurally similar to glutamate carboxypeptidase II and folate hydrolase I. Although PSMA was first identified in the prostate, it is now known to occur in various tissues.3 Its expression within the stomach is weak to moderate, including weak expression in gastric carcinomas. Small bowel enterocytes are strongly positive to PSMA on immunostaining, whereas intestinal adenocarcinoma shows moderately intense PSMA immunostaining. PSMA expression within both normal colonic mucosa and colonic adenocarcinoma is weak to moderate.4 This pattern of immunostaining is reflected on 68G-PSMA imaging where the proximal small bowel shows intense tracer uptake compared to rest of the GI tract. Uptake is also noted within the celiac, cervical, and sacral ganglia, which can mimic a lymph node metastasis.5,6 Excretion into the saliva may result in esophageal uptake. Hepatobiliary clearance may result in activity within the bowel lumen.7
Somatostatin receptor scintigraphy (SRS)
Somatostatin receptor (SSTR) subtypes 1–5 are widely distributed throughout the body. SSTR-1 is expressed at the highest level in the stomach and jejunum, with low levels seen in the colon. The distribution of SSTR-2 is different from SSTR-1 and is seen in low levels within the jejunum and colon.8 Gallium-68-DOTA-Dphe1, Tyr3-Octreitate (Ga-68 DOTAT AT E) is a somatostatin analog with high affinity for somatostatin receptor-2 (SSTR-2) and is used in neuroendocrine tumor imaging. The GI uptake of the tracer is quite variable. A decreasing proximal to distal gradient with high physiologic uptake is generally seen in the stomach, moderate uptake in the jejunum and ileum, and mild uptake in the colon and rectum.9 This may be explained in part by the diminishing density of somatostatin receptors along the GI tract.10 Salivary excretion of the radiotracer into the GI tract may also contribute to GI activity.
18F-sodiumfluoride (NaF)
18F-N aF is a bone-seeking positron emitting radiotracer that is similar in action to 99mT c-MDP but has better resolution, high bone-to-background ratio, and improved sensitivity.11 It provides image quality and extent of disease evaluation superior to that of 99mT c-MDP. The major route of excretion is via the kidneys. The exact mechanism of uptake within the bowels is not known and is quite varied, ranging from minimal to intense uptake.12
11C-acetate
11C-acetate is a biomarker for lipid synthesis and can be used for the study of myocardial perfusion and oxygen consumption as well as for the imaging of various malignancies such as hepatocellular carcinoma, prostate cancer, renal cancer, and bladder cancer.13,14 The distal esophagus may show prominent tracer uptake, and excreted (usually low-grade)activity can be seen in the small and large bowel.15,16 Metastatic disease to the bowel from HCC, though rare, may be missed on C11-acetate imaging.17
11C/18F-choline
Choline is a precursor of phosphatidylcholine, which is an important element of cell membrane and a marker for lipogenesis,18 and can be labeled with 18F (18F-methylcholine; 18F-FCH and 18F-ethylcholine; 18F-FECH) or 11C (11C-choline). These agents have been investigated for their utility in imaging prostate cancer, hepatocellular carcinoma, and brain tumors such as glioblastoma.19–21 11C-choline has predominant hepatic metabolism that may result in early accumulation within the bowel, compared to 18F-FCH or 18F-FECH, which are primarily excreted via the kidneys.22 In general, colonic activity is lower than small bowel activity, in contrast to 18F-FDG, for which colonic uptake is more prominent than small bowel uptake.23 Intense choline uptake can be seen in inflammation, such as esophagitis, and in colonic adenomas.18,24
Amino acid tracers
Some of the commonly used amino acid tracers include 11C-methyl-L-methionine (MET), Anti-1-amino-3-18F-Fluorocycobutane-1-carboxylic acid (18F-FACBC; commercial name: Ax umin), 18F-Fluoro-L-dihydroxyphenylalanine (F-DOPA), and 18F-Fluoro-ethyl-L-Tyrosine (FET).
Methionine is an essential amino acid that plays an important role in the synthesis of polypeptide chains and formation of S-adenosylmethionine (SAM)required for methylation of DNA, RNA, and protein.25 MET is a biomarker for amino acid transport, which can be used for the imaging of CNS tumors, prostate cancer, and bladder cancer.13,26,27 It shows variable and high accumulation within the bowel, limiting its use in GI and pelvic malignancies.28 The stomach wall uptake rapidly increases during the first 4.5 min and the mean SUV of the stomach wall is usually higher than that of large bowel loops.29 There is some excretion of MET activity from the pancreas into the duodenum, and this has been used to assess pancreatic exocrine function.30 FACBC is a non-natural synthetic L-leucine analog used in the assessment of prostate cancer, breast cancer, and brain tumors.31,32 Mild to moderate esophageal uptake is frequently seen, particularly within the distal esophagus and the gastroesophageal junction, and may indicate inflammation similar to that seen with FDG uptake. Mild to moderate FACBC uptake is also seen within the stomach, small bowel, and colon.33 F-DOPA is a radiolabeled analog of the amino acid L-DOPA and is predominantly used in the imaging of brain tumors and neuroendocrine tumors.34–36 Mild uptake may be seen the esophagus. The bowel may show no or mild diffuse uptake. Less frequently, mild uptake may be seen within the duodenum.34
18F-Fluorothymidine (FLT)
18F-FLT is a biomarker of cell proliferation and thymidine kinase-1 activity. It has been used in non-invasive assessment of cell proliferation activity of several cancers as well as for prediction and monitoring of therapy response.37 Due to the presence of rapidly proliferating mucosal cells within the GI tract, physiological uptake is expected within the intestines and is usually mild and variable. Low background activity results in high tumor-to-background uptake, enabling detection of primary GI neoplasms such as gastric cancers.38
Human epidermal growth factor-2 (HER2)targeted imaging
The antibodies trastuzumab and pertuzumab can be radiolabeled (for instance with 89Zr or 67Cu) and used for HER2 imaging, for instance in breast and gastric cancers. Bowel activity can be seen one to two days after injection, reflecting the primary excretion route of this agent.39–42
Iodine-124 (124I)
124I is a positron emitter with a half-life of 4.2 days; one of the advantages of PET imaging for thyroid cancer is the ability to perform lesional dosimetry.43,44 The isotope can also be used to label various antibodies, peptides, and amino acids.45,46 For lesional dosimetry studies in thyroid cancers, it is administered orally. Normal activity is seen in salivary glands and stomach; lower GI activity may also occur.
Maximum intensity projection (MIP)images that depict the normal biodistribution of the above tracers are shown in Figure 1. Mechanism of action, use, and biodistribution of some of the commonly used radiopharmaceuticals in the clinical and research setting are presented in Table 1.
Table 1:
Mechanism of action, use, and biodistribution of radiopharmaceuticals commonly used in clinical and research settings.
| Radiotracers | Property | Mechanism of Action | Use | GI Biodistribution |
|---|---|---|---|---|
| 18F-Fluoro-Deoxy-Glucose (FDG) | Glucose analog | Glucose analog | Majority of the oncological imaging | Variable, mild or intense, diffuse or segmental, throughout the GI tract |
| 68Ga-Prostate specific membrane antigen (PSMA) | Similar to glutamate carboxypeptidase II and folate hydrolase I | PSMA expressed on cell membrane, internalized on ligand binding | Prostate cancer | Intense uptake within duodenum and jejunum, variable low-grade uptake within the remainder of the bowel loops |
| Somatostatin receptor Scintigraphy (SRS) | Somatostatin receptor analog | Binds to somatostatin receptor (SSTR) subtypes | Neuroendocrine tumor | More intense within the proximal bowel loops compared to the distal bowel |
| 18F-Sodium Fluoride (NaF) | Analog for hydroxyl group in in the bone matrix | Localization in the hydroxyapatite crystal | Osseous metastases | Not common, if present, it is variable, predominantly with the small bowel |
| 11C-Acetate | Substrate for synthesis of acetyl Co-A | Biomarker for cell membrane lipid synthesis | Myocardial perfusion and oxygen consumption various malignancies, such as hepatocellular carcinoma, prostate cancer, renal and bladder cancers | May have prominent esophageal uptake, low-grade within the small and large bowel |
| 11C/18F-Choline | Precursor of phosphatidylcholine | Biomarker for lipogenesis and biosynthesis of cell membrane | Prostate cancer, hepatocellular carcinoma and brain tumors | Variable but generally colonic uptake is less than the small bowel uptake |
| 11C-methyl-L-methionine | Essential amino acid | Protein synthesis | CNS tumor imaging, prostate and bladder cancers | Variable and intense, stomach uptake higher than in large bowel loops |
| FACBC; commercial name: Axumin | Non-natural synthetic Lleucine analog | Amino acid transporter | Prostate cancer, breast cancer and brain tumors | Variable mild to moderate uptake within the GI tract, more prominent with in the esophagus |
| 18F -Fluoro-Ldihydroxyphenylalanine (FDOPA) | L-DOPA analog | Precursor for catecholamines | Brain tumors and neuroendocrine tumors | Mild uptake within the esophagus. Minimal or no uptake within the bowel loops |
| 18F-Fluoro-ethyl-L-Tyrosine (FET ) | Neutral amino acid analog | Amino acid transporter | Brain tumor | Minimal or no uptake |
| 18F-Fluorothymidine (FLT) | Pyrimidine analog | Biomarker for cell proliferation and thymidine kinase −1 activity | Several cancers for prediction and monitoring of therapy response | Variable, usually low-grade |
| Human Epidermal Growth Factor-2 (HER2) targeted imaging | Humanized mAb against HER2 Receptor | Binds to HER2 receptor | Breast and esophagogastric cancer | Seen one to two days after administration and moves distally over time |
| Iodine-124 (124I) | Positron Emitting Iodine | Functions as Iodide | Thyroid | Seen one to three days after ingestion and decreases over the time |
Technical artifacts in imaging
Technical artifacts occur irrespective of the radiotracer used and can lead to inaccurate interpretation. Most relevant for this topic are CT-related and patient-related factors.
CT factors
High-density oral contrast material may cause significant attenuation, leading to overcorrection on attenuation corrected PET images and the appearance of apparent FDG-avid lesions and overestimation of SUV47 (Figure 7C). Metallic implants such as lumbar fixation hardware or intraluminal bowel stents can cause streak artifact and degrade the quality of the CT images. They can also result in overestimation of local tracer uptake. Assessment of the non-attenuation corrected PET images identifies the artifact and clarifies interpretation.48,49
Figure 7:
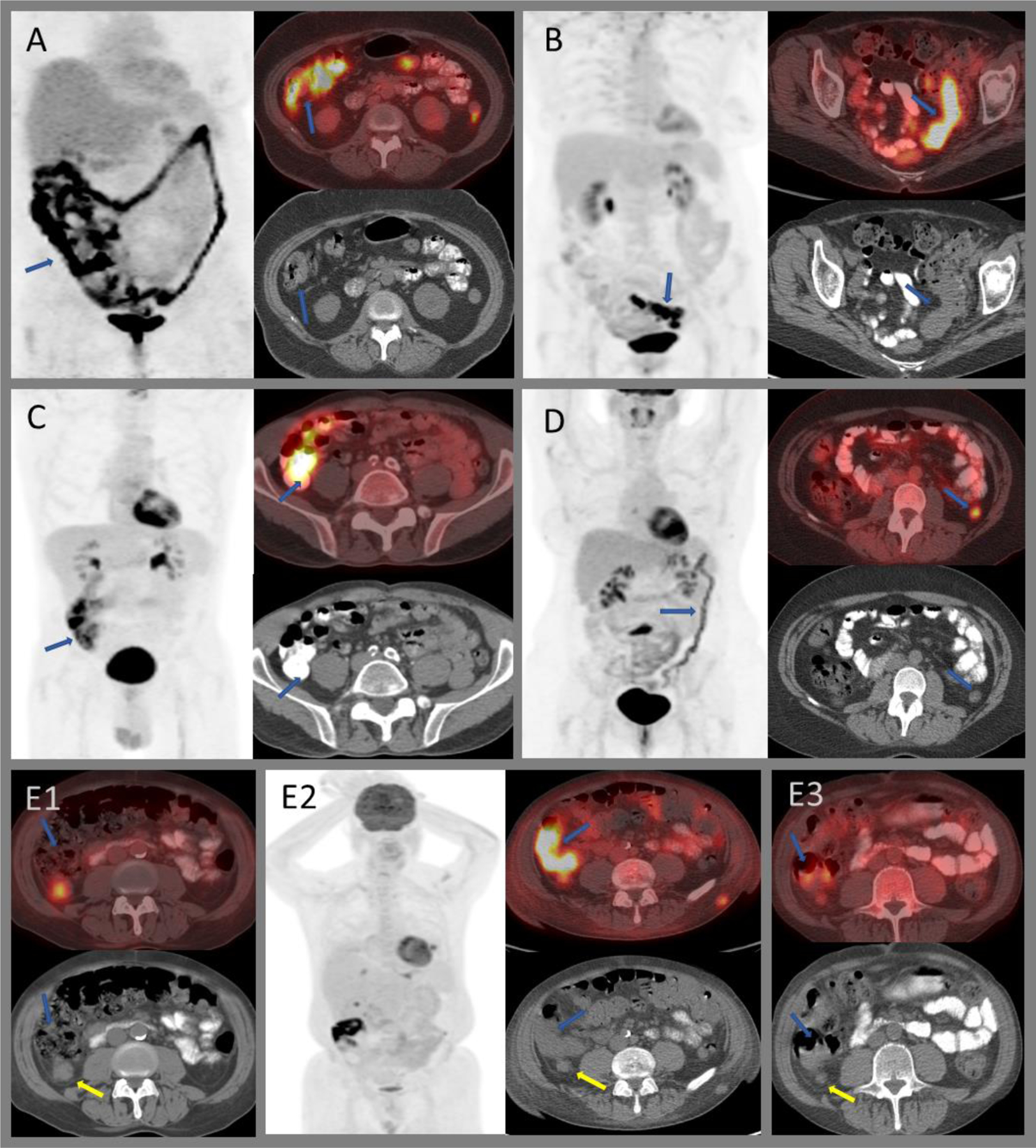
Variable FDG uptake within the colon. A) Intense diffuse uptake throughout the large bowel in patient on metformin. B) Segmental intense uptake within the sigmoid colon with bowel wall thickening in a patient with known ulcerative colitis without active symptoms. C) Intense uptake secondary to attenuation artifact from oral contrast media. D) Moderately intense diffuse uptake within the distal large bowel in a patient with active ulcerative colitis. E) Post-radiation colitis of the hepatic flexure (E2) in a patient who underwent radiation therapy for a moderately intense right posterior peritoneal metastasis (E1; yellow arrows). Post-radiation, the nodule decreased in size; however, intense uptake is seen within the adjacent hepatic flexure (E2; blue arrows). Bowel uptake resolved on subsequent imaging with residual low-grade uptake within the peritoneal nodule (E3).
Patient factors
Patient motion during image acquisition of imaging can lead to PET vs. CT misregistration. PET is usually acquired over a duration of 2 to 3 min at each bed position, whereas CT is acquired over a few seconds. The effect is particularly seen in the peristaltic bowel, which could change its position between the CT and PET acquisition. Respiratory motion can result in misregistration of organ activity in the upper abdomen, possibly giving the appearance of an FDG-avid hepatic metastasis due to misplaced colonic activity on the fused images. Similarly, misregistration within the pelvis can occur due to different filling status of the urinary bladder, shifting the position of bowel loops and the uterus between CT and PET acquisitions. Arms by the side of the patient can cause significant streak and attenuation artifact.
Physiologic and non-physiologic radiotracer uptake that can mimic malignancy and pitfalls in PET/CT imaging of various GI malignancy are presented in Table 2.
Table 2:
Variants and Pitfalls of PET/CT in GI cancers
| Site | Tracer Used | Benign Conditions that can Mimic Malignancy | Malignant Conditions with Unreliable or Low-Grade Uptake |
|---|---|---|---|
| Esophagus | FDG | Esophagitis, leiomyoma | T-staging is unreliable Early-stage adenocarcinoma can be low-grade with low PPV for Stage 1 disease |
| Radiation induced fibrosis or inflammation versus residual disease can have similar appearance | |||
| Stomach | FDG | Physiologic uptake, particularly within the fundus, gastritis, schwannoma, leiomyoma | Distal gastric tumors ca be low-grade compared to proximal tumors Decreased sensitivity for LNs Decreased sensitivity for diffuse-type cancers such as Signet ring cell cancers Decreased sensitivity for some indolent NHL such as gastric MALT |
| Response assessment not possible for non-avid or minimally avid tumors Higher sensitivity for determining treatment failure than to predict response for GIST | |||
| Role is unclear in routine follow up Can have false positive results | |||
| Small Bowel | FDG | Physiologic uptake, IBD, enteritis, | Can be low-grade for MALT lymphoma and neuroendocrine tumors (NET) |
| Ga-DOTATATE | High proliferation index and poorly differentiated NETs and neuroendocrine carcinomas |
||
| Colon and Rectum | FDG | Physiologic uptake, metformin bowel, colonic or ileostomy stoma, polyps, diverticulitis, IBDs, colitis, Inflammatory pseudotumor, sarcoidosis, normal appendix | May not be helpful in staging of localized disease without metastases Mucinous tumors can be low-grade |
| Anastomotic uptake – physiological and inflammation Post-operative changes and complications such as fistula Radiation induced inflammation |
Can have false positive results for response assessment in neoadjuvant setting | ||
| Not recommended for routine follow up; can have false positive results | |||
| Anal Canal | FDG | Physiological, hemorrhoids, anal fistulas | Not for local staging of primary tumor |
| Radiation induced inflammation | Can be false positive if performed soon after chemoradiotherapy | ||
| Role in follow up unclear – uptake within the anal canal on follow up does not necessarily indicate recurrence | |||
| Peritoneum | FDG | Benign conditions as such mesenteric panniculitis, post-operative changes, TB peritonitis Splenules, transposed ovaries, sarcoidosis, portal vein thrombosis, mesh prosthesis, hernia repair plug, |
Decreased sensitivity for small-volume disease, predominantly cystic disease, ascites, multicystic peritoneal mesothelioma, pseudomyxoma peritonei |
| Post hyperthermic intraperitoneal chemotherapy (HIPEC) or operative changes | Response assessment and recurrence – PET/CT may underestimate disease |
Esophagus
Based on location, esophageal tumors can be divided into cervical, upper thoracic, middle thoracic, lower thoracic, and gastroesophageal junctional tumors. Gastroesophageal junctional tumors have epicenters no more than 2 cm off the cardia.50 Squamous cell carcinomas (SCC) and adenocarcinomas (AC) are the two major subtypes of esophageal cancers.
Physiologic FDG uptake within the esophagus could be caused by swallowed saliva or metabolic activity within the esophageal smooth muscle due to peristalsis and can be seen as low-grade linear uptake along the length of the esophagus (Figure 2E).
Figure 2:
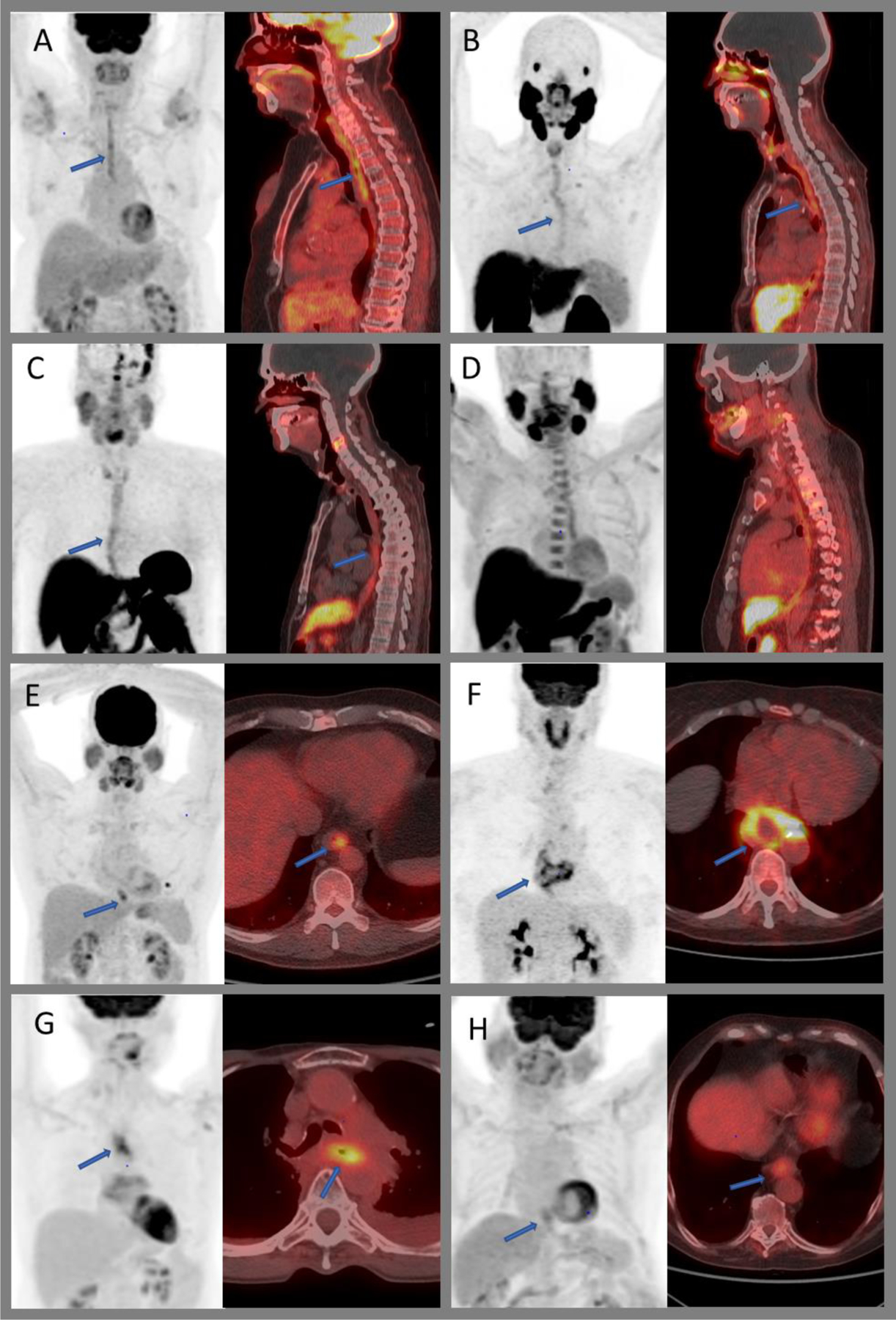
Physiologic and non-physiologic radiotracer uptake within the esophagus. MIP and fused sagittal images demonstrating mild to moderate diffuse uptake of benign esophagitis on 18F-FDG PET/CT (A), 68Ga-PSMA PET/CT (B), 68Ga-DOTATATE PET/CT (C), and 18F-FACBC PET/CT (D). MIP and axial fused images of the chest showing benign focal uptake within the distal esophagus (E), moderately intense peripheral uptake within a benign leiomyoma (F), post-radiation inflammation within the mid esophagus (G), and low-grade uptake within a biopsy proven esophageal adenocarcinoma (H).
Variable moderate to intense, diffuse, or focal FDG uptake can be seen in esophagitis due to various causes such as gastroesophageal reflux disease, recent radiation therapy, medication, or infection (Figure 2A, B, C and D). Benign esophageal leiomyoma (Figure 2F) and glycogen acanthosis are also known to show FDG uptake.51,52 Uptake within the distal esophagus and gastroesophageal junction, similar to FDG uptake with reflux disease, can be seen with 11C-acetate and 18F-FACBC33. 68Ga PSMA has shown to have uptake within achalasia.53
During cancer staging, FDG PET-CT is not sufficiently accurate to assign the T-stage, as it cannot reliably assess esophageal wall definition or the depth of tumor invasion. Lowe et al. reported that 18F-FDG PET accurately staged the T category in 43% of patients, compared to 71% by endoscopic ultrasound.54 18F-FDG PET-CT is of limited value in the assessment of early-stage adenocarcinoma when the endoscopy and biopsy indicate cT is and cT1 disease.55 In a meta-analyses by Shi et al., FDG PET/CT had a sensitivity of 55–62% and a specificity of 76–96% for detection of regional lymph node metastasis.56 Interim FDG PET/CT after induction chemotherapy may differentiate responders from non-responders early in the course of treatment. A decrease in 35% of initial FDG uptake has been shown to predict clinical response in patients with locally advanced adenocarcinoma of the gastroesophageal junction with sensitivity and specificity of 93% and 95%, respectively.57 However, the value of FDG PET/CT after completion of chemoradiotherapy remains unclear. In the post-chemoradiotherapy setting, the esophagus may have a very heterogenous appearance due to radiation induced inflammation and fibrosis (Figure 2G). FDG tends to perform worse in adenocarcinomas than in squamous cell carcinomas57,58 (Figure 2H). In a systematic review, FDG PET/CT showed pooled sensitivity and specificity of 62% and 73%, respectively, for detecting pathologically complete response in primary tumor and regional lymph nodes; the authors noted that this was not sensitive enough to guide treatment decisions.59
FDG PET/CT has proven useful in the detection of recurrent esophageal cancer. In a meta-analysis, the pooled sensitivity and specificity were 96% and 78% for detecting recurrence after primary treatment with curative intent.60 Of note, pre-test probability matters, and the positive predictive value of FDG PET/CT was lower for patients with initial stage I disease compared to those with initial stage II or III disease.61
Stomach
Most gastric cancers are adenocarcinomas, followed in prevalence by lymphoma and gastrointestinal stromal tumors (GIST). Gastric carcinoid and gastric metastases are rare. Some precancerous pathologies such as H. pylori infection, autoimmune gastritis, and atrophic gastritis are known to precede the development of the intestinal type (Lauren’s classification) of gastric adenocarcinoma, which include the tubular (most common), mucinous, and papillary subtypes. Poorly cohesive carcinoma, which includes signet ring cell carcinoma, belongs to the diffuse type (Lauren’s classification) of gastric cancer.62 The stomach is the most common site of primary extranodal non-Hodgkin lymphoma (NHL), accounting for nearly 60–75% of cases, with diffuse large B-cell lymphoma (DLBCL) and mucosa associated lymphoid tissue (MALT)the most prevalent subtypes.63
The mechanism of physiologic gastric FDG uptake is unclear, ranging from intense and localized to the fundus/cardia to mild to moderate and diffuse throughout the stomach (Figure 3A). Koga et al. postulated that FDG uptake may be related to number of parietal cells in each region of the stomach, with higher mean FDG uptake in the upper part of the stomach, where the parietal cells are most numerous.64 The uptake is usually within the wall, but it is not understood whether it is in the smooth muscles of the gastric wall or within the layers of the gastric mucosa. Use of gastric distension using water or carbonated liquids and an antimotility agent such as Buscopan was shown to be an effective tool in discriminating patients with physiological uptake in up to 64% of cases.65 However, in most cancer treatment centers, it is not a routine practice to use this protocol for patients undergoing FDG PET-CT without a known gastric pathology. Physiologic FDG uptake can also be related to the normal lymphoid tissues.
Figure 3:
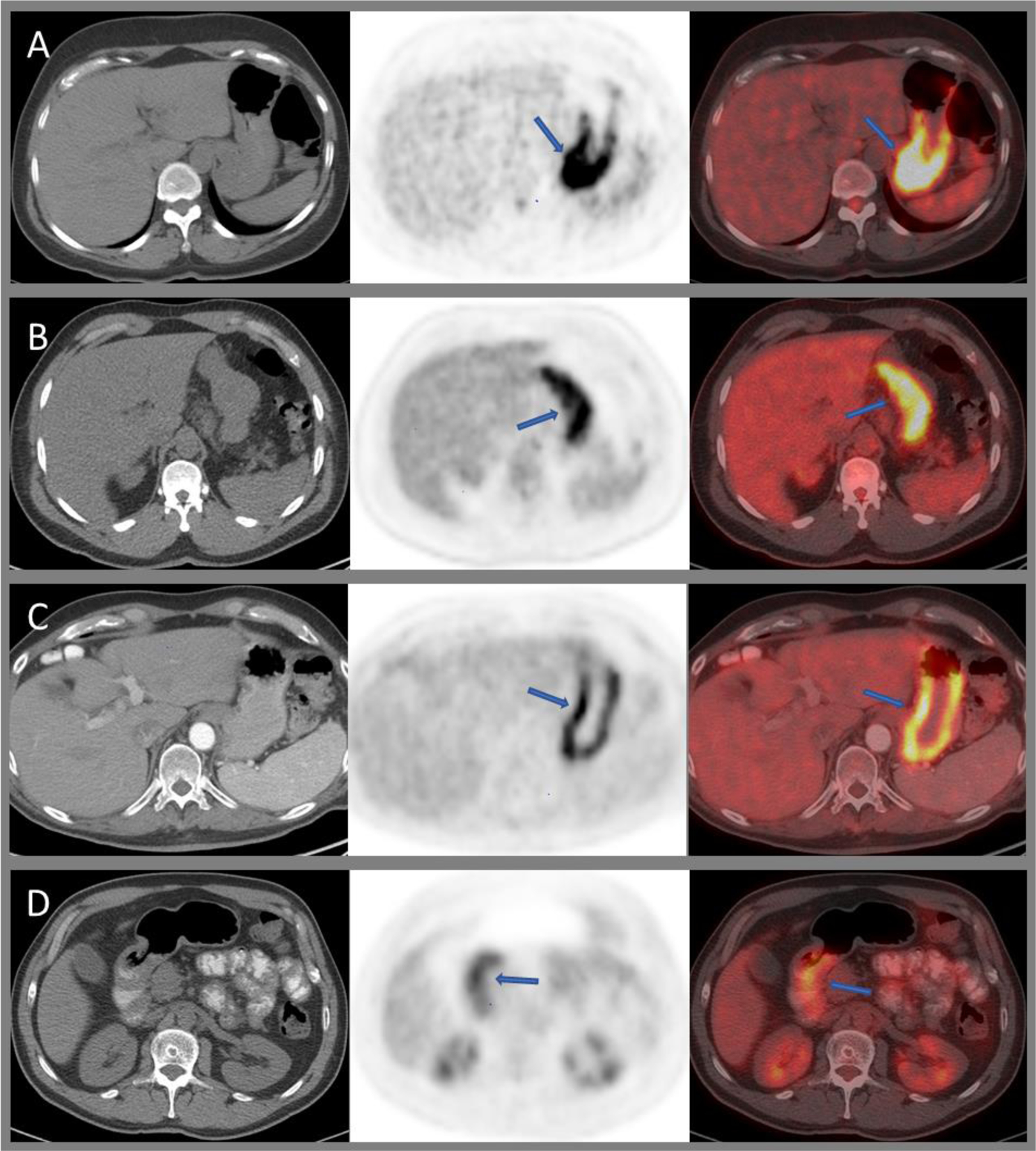
Axial CT, PET, and fused images of 18F-FDG PET/CT demonstrating intense, predominantly proximal, physiologic gastric uptake (A); moderately intense diffuse uptake in H. pylori infection (B) and chronic inactive gastritis (C); mild diffuse uptake within the pylorus post-radiation (D).
Benign pathologies of the stomach include mucosal ulcerations, for example secondary to peptic ulcer disease or H. pylori infection (Figure 3B); benign polyps such as hyperplastic, adenomatous, or inflammatory polyps; and diffuse mucosal abnormalities such as acute gastritis, medication, or radiation induced gastritis and chronic and atrophic gastritis (Figure 3C and D). All of these can cause variable gastric FDG uptake and can mimic primary gastric malignancy. Elevated gastric FDG uptake can be seen in H. pylori infection and chronic atrophic gastritis. Benign pathologies such as xanthogranulomatous gastritis and benign tumors such as gastric schwannoma and leiomyoma can mimic GIST on PET/CT66–68 (Figure 4A and B).
Figure 4:
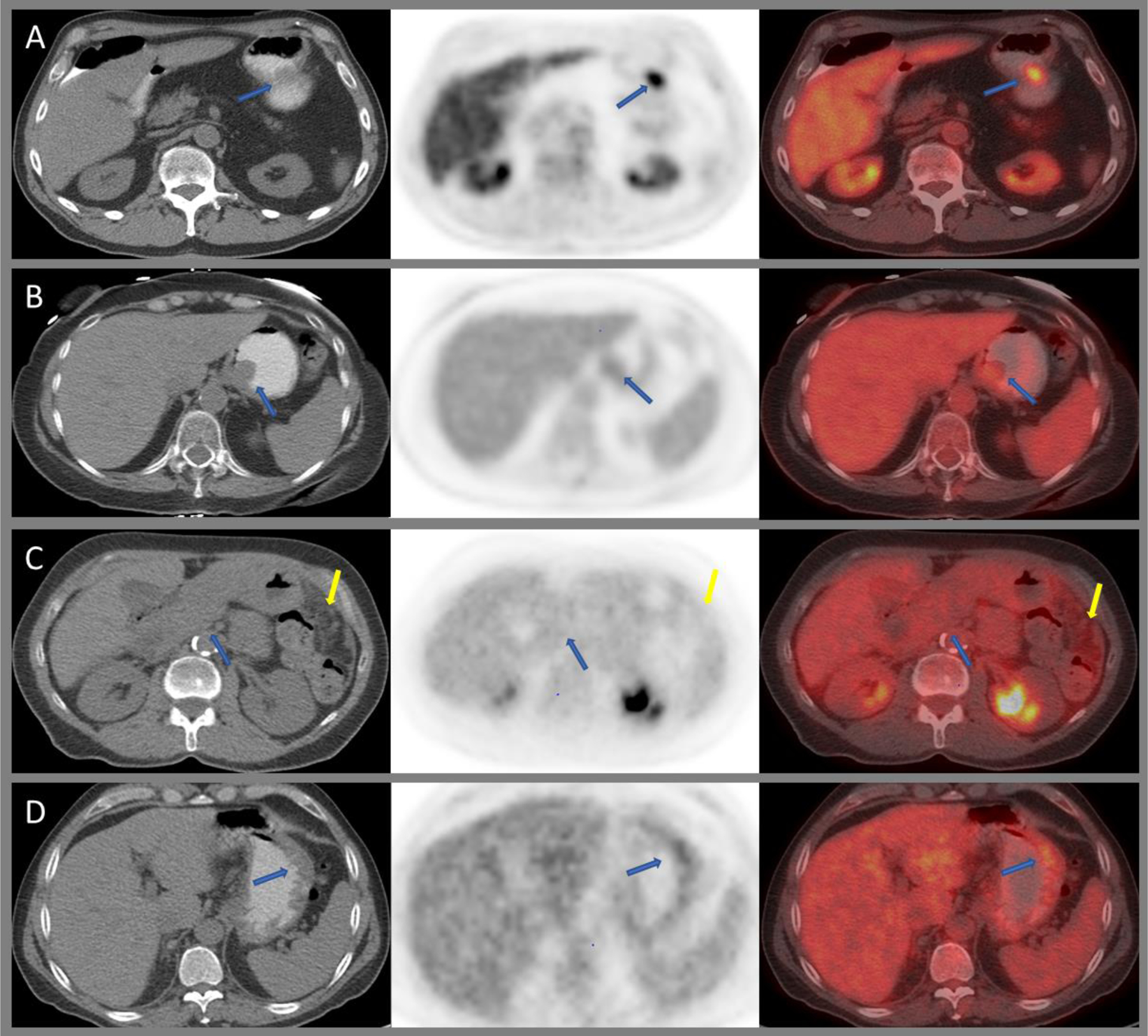
Axial CT, PET, and fused images of 18F-FDG PET/CT demonstrating intensely avid gastric schwannoma along the greater curvature of the stomach (A), mildly avid leiomyoma at the gastroesophageal junction (B), diffuse low-grade uptake within an infiltrating poorly differentiated adenocarcinoma with signet ring cell features (blue arrows) and peritoneal carcinomatosis (yellow arrows), (C) and mild diffuse uptake within a biopsy proven gastric MALT lymphoma (D).
Gastric GIST has been detected on PSMA PET,69,70 and 18F-choline uptake has been reported in gastric neuroendocrine tumors.71
The variable, sometimes intense physiological FDG uptake within the stomach wall can lead to difficulty in detection of primary gastric malignancies. T-stage cannot be assessed reliably due to limited spatial resolution. Smyth et al. reported lower FDG avidity in distal gastric tumors than in proximal cancers (59% vs. 81%), as well as lower FDG avidity in diffuse-type gastric cancers than in those of intestinal subtype (44% vs. 97%).72 In terms of nodal staging, FDG PET/CT has a lower sensitivity than EUS (50% vs. 73.3%), and again, its accuracy is lower for diffuse-type than for intestinal/mixed-type tumors (48.6% vs. 58.8%).73 FDG is not reliable for the assessment of signet ring carcinoma subtype (SRCC); these tumors show lower FDG uptake than tumors of other histology and may be missed completely (Figure 4C). Interestingly, high 68Ga PSMA uptake was reported in signet ring cell gastric cancer.74
The reported detection of gastric MALT of FDG PET/CT varies widely, ranging from 50–71%; in general, FDG uptake in gastric MALT is lower than in more aggressive lymphomas75–78 (Figure 4D).
As noted above, FDG PET/CT has been used for response assessment at different time points during and after completion of therapy. Long term results of a prospective study showed sensitivity and specificity of interim FDG PET/CT in predicting responders of 69% and 82%.79 A pilot study showed an improvement in disease-free survival among metabolic non-responders when the neoadjuvant chemotherapy was switched to a non-cross resistant regimen.80
The exact role of FDG PET/CT in detecting recurrent gastric cancer is still unclear. One study evaluated its role for surveillance in asymptomatic gastric cancer patients after curative surgery. In patient-based analysis, sensitivity and specificity were 84% and 88%, with no clear difference when stratified by early vs. advanced gastric cancer or time between surgical resection and PET scan. However, false positive FDG uptake was observed in 11% of patients, resulting in low positive predictive value (PPV) of only 43%.81 In a recent meta-analysis, the pooled sensitivity and specificity of the FDG PET/CT for detecting recurrent gastric cancer were reported to be 81% and 83%.82
Small bowel
Primary tumors of the small bowel such as adenocarcinomas, GIST, lymphoma, and neuroendocrine tumors (NET) are relatively uncommon, accounting for about 5% of all GI malignancies.1 Some tumors have predilection for certain parts of the small bowel; for example, adenocarcinomas are more frequently found in the duodenum, and NET are more commonly found in the distal ileum.83,84
The majority of commonly used radiotracers show physiologic small bowel avidity, either due to hepatobiliary excretion, active secretion into the lumen, or swallowed activity moving along the GI tract. Physiologic FDG uptake in the terminal ileum/caecum can be very intense (Figure 6A). As the small bowel is quite mobile within the peritoneal cavity, misregistration of PET and CT images is not uncommon, which may make it difficult to localize FDG activity.
Figure 6:
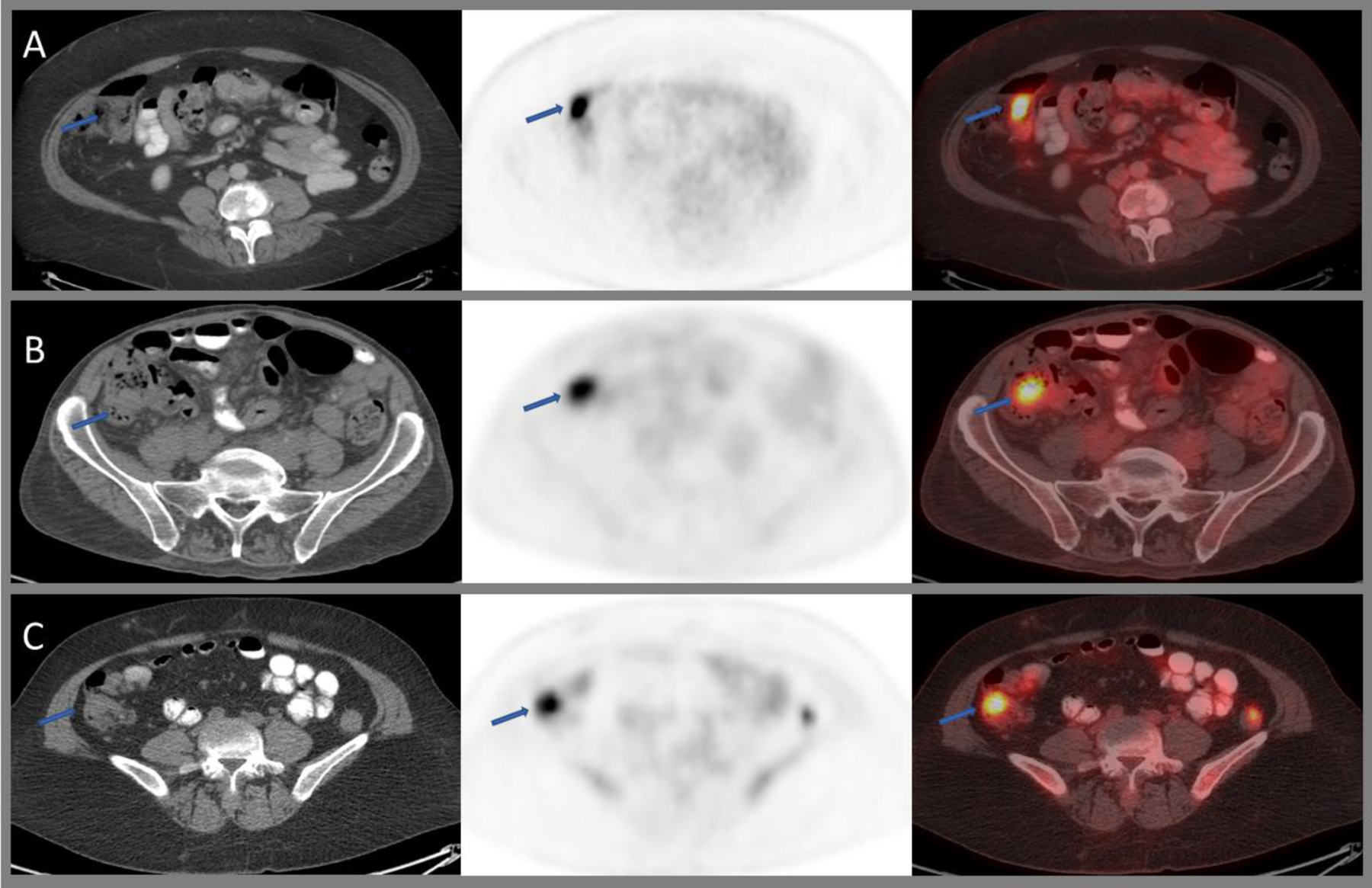
Axial CT, PET, and fused images of 18F-FDG PET/CT through the lower abdomen showing intense physiological uptake within the ileocecal junction (A), benign sessile tubular adenoma (B), and metastatic deposit at the ileocecal junction from patient’s known poorly differentiated gastric adenocarcinoma with signet ring cell features (C).
Of note, PSMA shows high uptake within the duodenum and the proximal part of the jejunum where there is abundance of brush borders (microvilli) showing increased PSMA immunostaining85,86 (Figure 1).
Inflammatory FDG uptake can be seen in inflammatory bowel disease (IBD), radiation or medication induced enteritis, or infection. Usually, this uptake appears linear, along the length of involved segments or portions of the bowel, rather than focal.87 Louis et al. reported a sensitivity of 72.9% in the assessment of IBD when compared with endoscopy. The overall specificity was only 55.3%, increasing to 72.3% when the FDG uptake was associated with CT changes such as bowel wall thickening.88 Intussusception can be associated with increased FDG uptake. In adults, the underlying cause of intussusception is tumor-associated in up to 77% of patients.89
Small bowel adenocarcinomas usually show increased FDG uptake with CT features of a mass (Figure 5C and 6C). However, benign adenomas can present in similar fashion (Figure 5A and 6B). Like the stomach, involvement of the small bowel by high-grade lymphoma, most commonly DLBCL, is associated with intense FDG uptake. However, indolent lymphomas may show diffuse mild uptake.90 Small bowel GIST can be intensely FDG-avid (Figure 5B). In contrast, small bowel NET can be missed easily (FDG sensitivity 36%), in particular when showing a low proliferation index of less than 2%.91
Figure 5:
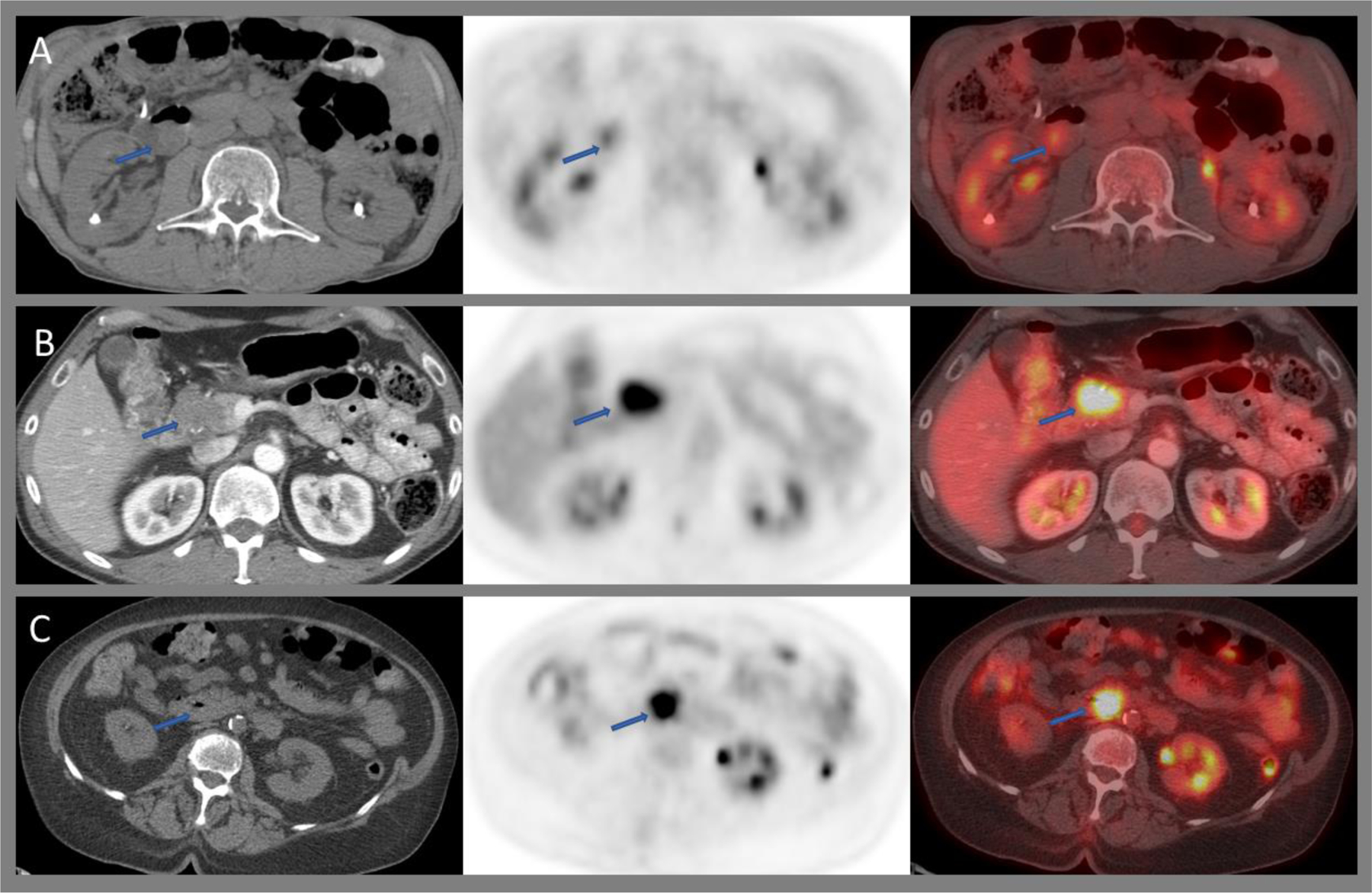
Axial CT, PET, and fused images of 18F-FDG PET/CT demonstrating low-grade uptake within a duodenal tubulovillous adenoma (A), intensely avid gastrointestinal stromal tumor (GIST) of the duodenum(B), and duodenal carcinoma (C).
68Ga-DOTATATE is the standard imaging test for the evaluation of well-differentiated NET,92 with a high sensitivity (>94%) and high specificity (>92%), particularly within the midgut.93 For G3 tumors and those with higher proliferation index (>20%), as well as poorly differentiated neuroendocrine carcinomas, assessment with FDG PET/CT is proposed.94 Focal 68Ga-DOTATATE in the liver secondary to hepatocellular carcinoma can mimic NET metastases.95
Among the other PET radiotracers, PSMA uptake has been reported in duodenal adenocarcinoma96 and in ileal GIST.97
Colon and rectum
Two major distinct precursor pathways are noted for the development of colorectal cancer: the adenoma to carcinoma pathway and the serrated neoplasia pathway.98 Most colon cancers arise from adenomatous polyps (adenoma to carcinoma sequence).99 Adenomas progress through sequential genetic mutations and chromosomal instability, resulting in microsatellite stable tumors. The serrated neoplasia pathway occurs most often due to BRAF and KRASmutation and may result in either microsatellite stable (MSS) or unstable tumors.98 Microsatellite instability (MSI) due to defective DNA repair is also seen in Lynch syndrome. Right-sided colon cancers tend to have more genetic mutation and microsatellite instability, which contributes to the inferior prognosis of advanced stage in right-sided colon cancers compared to left-sided colon cancers.100
Physiological FDG uptake within the cecum, ascending and sigmoid/rectosigmoid colon (usually segmental and of mild to moderate intensity) is frequently greater than in other sections of the large bowel.48,101 Attempts to improve colonic imaging on FDG PET/CT by prior administration of cleansing agents102 may be counterproductive by inducing inflammation with resultant increased uptake.
Metformin is an oral biguanide molecule which accumulates preferentially in the intestinal mucosa, increasing glucose turnover in the splanchnic bed, decreasing hepatic glucose uptake, and reducing fatty acid utilization.103 It causes activation of AMP activated protein kinase, resulting in upregulation of GLUT-1, 2, and 4 transporters.104 The drug may cause significantly higher FDG uptake in the colon (as well as in small bowel)105 (Figure 7A). Discontinuation of metformin 48 h prior to FDG PET/CT is insufficient to suppress this prominent bowel uptake, although uptake is lower than in patients with continued medication.106
Non-physiological benign FDG uptake within the colon can be focal (e.g., due to polyp or diverticulitis), segmental (e.g., in Crohn’s disease, radiation colitis) (Figure 7E1–E3), or diffuse (e.g., in ulcerative or pseudomembranous colitis)107–109 (Figure 7B and 7D).
Focal incidental uptake in the large bowel is seen in approximately 2% of all patients undergoing FDG PET/CT110. In a substantial number of cases, this may be due to malignant or premalignant conditions, and therefore further evaluation with colonoscopy is often recommended24,109,111 (Figure 8C). In a systematic review, the rate of neoplastic, benign, and inflammatory pathologies in incidentally detected focal colorectal FDG uptake were 76%, 19%, and 5%.110 FDG intensity as measured by SUV shows considerable overlap between benign, premalignant, and malignant focal colorectal incidentalomas.111 In asymptomatic adults undergoing cancer screening with same day FDG PET/CT and colonoscopy, Hwang et al. reported a sensitivity and specificity of 6% and 97% for detecting colonic carcinoma and adenoma. The sensitivity increased to 29% in lesions greater than 1 cm.112 Gollub et al. reported that the sensitivity of FDG PET to detect advanced adenoma was 49% and concluded that PET cannot be relied upon for accurate identification of these patients.113
Figure 8:
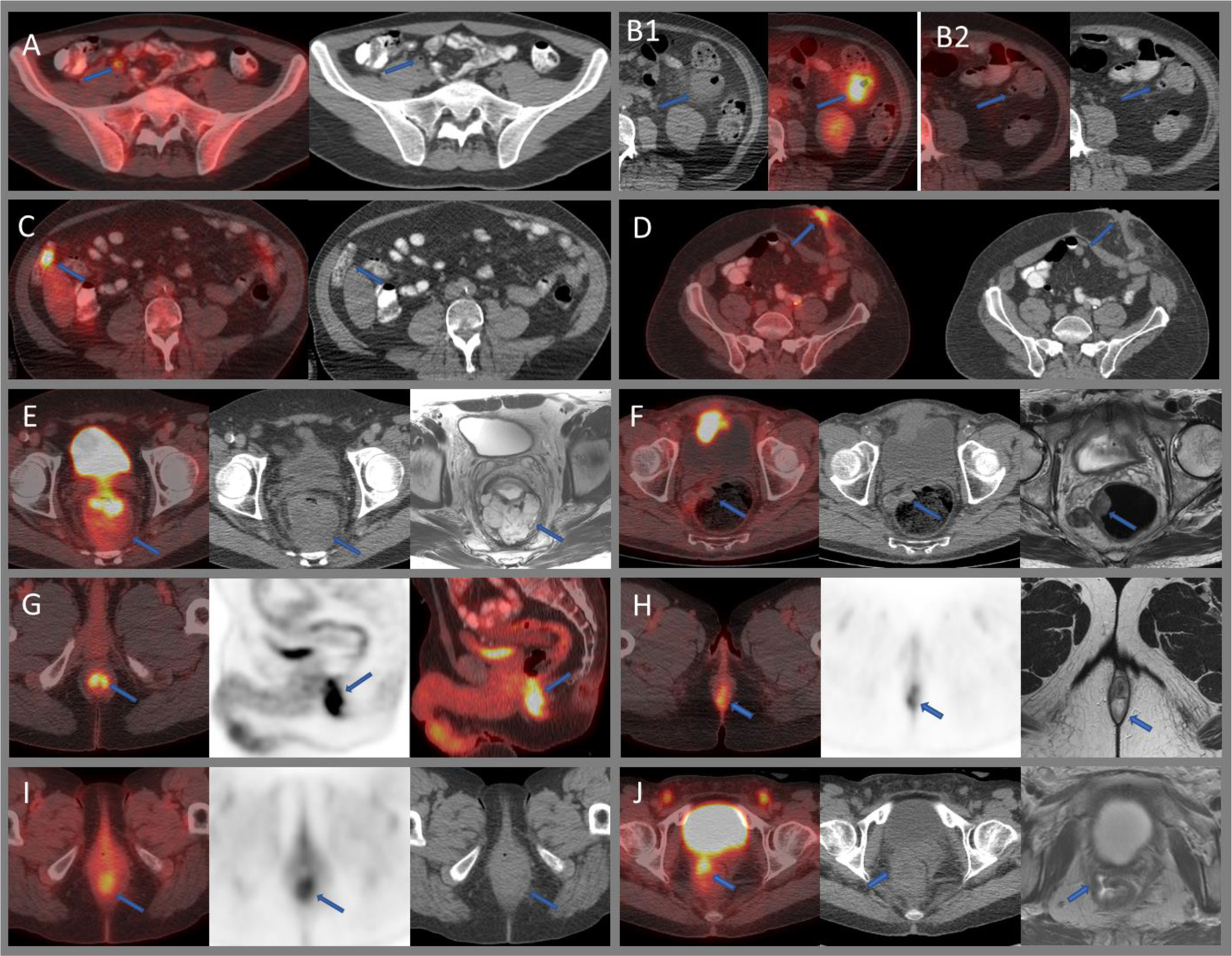
Benign and malignant FDG uptake within the bowel. A) Moderately intense uptake within a gas filled normal appendix. B) Intensely avid diverticulitis within the descending colon (B1), which resolved on follow up imaging two weeks later (B2). C) Intensely avid hepatic flexure tubule-villous adenoma. D) Benign intense uptake within a left anterior abdominal wall stoma. E) Rectal cancer with a mildly avid large mucinous component as seen on axial T2W MRI. F) Mildly avid right lateral wall rectal neuroendocrine tumor. G) Physiologic intense uptake within the anal canal in a patient undergoing 18F-FDG PET/CT for head and neck cancer. H) Moderately avid internal/external hemorrhoids at the anal verge as seen on MRI and also noted on clinical examination. I) Moderately intense radiation proctitis. J) Intense uptake along the rectovaginal fistula as seen on MRI.
Colonic diverticular disease ranges from asymptomatic diverticulosis to symptomatic uncomplicated diverticular disease, uncomplicated diverticulitis, and complicated diverticulitis (including perforation, abscess, bleeding, fistula, peritonitis, or stenosis).114 Focal intense FDG uptake may be seen throughout the spectrum of diverticular disease and should have correlating CT findings for diagnosis115,116 (Figure 8B). Inflammatory pseudotumor or colon sarcoidosis can mimic colonic carcinoma or lymphoma on FDG PET/CT.117,118 Acute appendicitis can be intensely FDG-avid. However, for diagnosing appendicitis on imaging, there should be correlating CT findings, such as thickened appendix, peri-appendiceal inflammatory changes, abscess, or appendicolith, as even normal appendix can sometimes show high FDG avidity119,120 (Figure 8A).
The primary role of FDG PET/CT in the pre-surgical evaluation of newly diagnosed patients with colorectal cancer is to confirm limited (potentially operable) disease and to evaluate patients with unclear or unsatisfactory conventional imaging, including those with allergy to intravenous contrast.121,122 Compared to CT or MRI, FDG PET/CT offers little added value in establishing the local extent of the primary tumor in patients without metastases. Mucinous tumors can show minimal or no FDG uptake, leading to a false negative rate as high as 41%123 (Figure 8E). In a meta-analysis, the pooled sensitivity and specificity of FDG PET for detecting regional lymphadenopathy were 43% and 88%, and 72% and 71% when addressing rectal tumors only.124 Neuroendocrine tumors can show low-grade uptake depending on their proliferation index (Figure 8F).
Neoadjuvant treatment is used in patients with locally advanced rectal cancer and those with potentially resectable metastatic disease.122 No current imaging modality can reliably determine complete pathologic response to select patients for non-surgical management.125 In a meta-analysis, FDG PET/CT had a pooled sensitivity of 52%, which was lower than that of MRI (86%).126 However, FDG PET/CT may help to identify non-responders early and thereby change treatment management. Generally, PET/CT is performed 5–6 weeks after neoadjuvant treatment. Further prolongation of the scan up to 12 weeks after therapy completion does not seem to improve sensitivity or specificity.127
Post-surgically, complications such as intrabdominal fluid collection and fistulas may show intense FDG uptake due to inflammation/infection (Figure 8B). FDG uptake can also be quite variable at a colonic or small bowel anastomosis or at the stoma (Figure 8D). One study showed increased FDG uptake around the anastomosis in 27 of 70 patients, of which 41% had local recurrence; FDG PET/CT was highly sensitive (100%) with suboptimal specificity (73%),128 and SUV measurements did not help in differentiating between recurrence and benign uptake when the post-operative period was less than 12 months. However, another study reported that early post-operative FDG PET/CT detected occult metastases in 6 of 49 patients and changed management in up to 14% of patients in the pN2 subgroup of patients with stage III colorectal cancer.129 Others have reported a false positive rate of 21% in patients with stage III colorectal cancer undergoing early post-operative PET/CT.130
In the absence of clinical suspicion of relapse, there is no significant difference between the diagnostic performance of PET/CT and conventional follow up.131 However, FDG PET/CT is useful in detecting relapse in patients with rising CEA. A meta-analysis including 510 patients reported a pooled sensitivity and specificity of 94% and 77%.132 FDG PET/CT can also provide valuable information in patients with clinical suspicion of relapse, even if the CEA is within normal limits.133 Post-treatment complications such as rectovaginal or rectovesical fistulas show intense FDG uptake and should be differentiated from recurrent disease (Figure 8J). In one study, post-operative surveillance PET/CT of rectal and sigmoid cancer showed FDG uptake to be benign in 13 of 43 hypermetabolic pelvic lesions, including fistulae, anastomotic sinus, perirectal inflammation, and abscess.134
Anal canal
Anal squamous cell carcinoma (ASCC) is the most common histological type, followed by anal adenocarcinoma (AAC).135 There has been an increasing trend in the incidence of ASCC and the risk factors include HPV infection, HIV positive patients, chronically immunosuppressed patients, and organ transplant patients.136
Variable physiological FDG uptake within the anal canal can be due to smooth muscle uptake, lymphatic tissue uptake, or fecal microbes137 (Figure 8G). Very often, intense physiological uptake within the anal canal is caused by increased metabolic activity of the internal and external sphincter 48. Anal FDG uptake is unrelated to sex, age, or blood glucose levels.137
Intense benign FDG uptake has been reported in hemorrhoids,138 either due to inflammation, thrombosis, or vascular proliferation within the hemorrhoids139 (Figure 8H).
FDG PET/CT is less sensitive than MRI in the local staging of primary anal tumors. However, it has a greater sensitivity for detecting metastatic lymph nodes and thereby can alter management.140
The primary mode of treatment for anal cancers is chemoradiotherapy. In a meta-analysis, PET changed the radiotherapy target volume definition in as many as 23% of patients,141 although the impact on clinical outcome remained undefined.
In terms of response assessment, FDG PET/CT had a sensitivity and specificity of 92% and 85% for detecting residual tumor after chemoradiotherapy.142 Test performance may be related to time after completion of therapy: at one month after end of treatment, sensitivity and specificity were 67% and 92%, but were higher at three months after therapy (100% and 97%),143 probably due to persistent inflammation post-radiotherapy (Figure 8I). Patients with complete metabolic response have a better prognosis than do non-responders.144
Post-treatment changes and complications associated with the anal canal can mimic malignancy. Kim et al. reported intense uptake in the presacral anastomotic sinus after low anterior resection mimicking recurrent disease.145 In a study of 299 patients undergoing post-operative surveillance after low anterior resection, Kang et al. reported anastomotic sinuses and fistulas in 2.68% of the cases, with the majority of lesions demonstrating diffuse increased uptake.146 Garg et al. demonstrated intense FDG uptake within a tubercular fistula-in-ano.147
There is limited evidence in the literature to support routine use of FDG PET/CT in the follow up of anal cancer patients in the post-chemoradiotherapy setting. Teagle et al. reported that a negative FDG PET/CT can exclude residual or recurrent disease in patients with anal cancer on follow up post-CRT.148 On the other hand, presence of FDG uptake does not necessarily indicate recurrence. In the study by Teagle et al., 7.1% had false positive uptake and 21.4% had nonspecific uptake, which included low-grade uptake in the anal canal.148
Peritoneum
The peritoneum can be involved by primary malignancies such as peritoneal mesothelioma, primary peritoneal carcinoma, or pseudomyxoma peritonei. Metastatic involvement can occur from adjacent organs such as gastric, ovarian, or colorectal malignancies. Malignant spread within the peritoneum can be nodular, diffuse, military, along the serosal surface of the bowel loops, or solid viscera and ascites.
The peritoneum does not generally show physiologic uptake. However, several benign peritoneal conditions can show FDG uptake, such as mesenteric panniculitis, surgical clip granuloma, post-radiation ascites and hemoperitoneum, fat necrosis, epiploic appendagitis, and abdominal abscesses149 (Figure 9A, B and D). Mesenteric fat stranding associated with inflammatory pathologies can have an appearance similar to peritoneal carcinomatosis.
Figure 9:
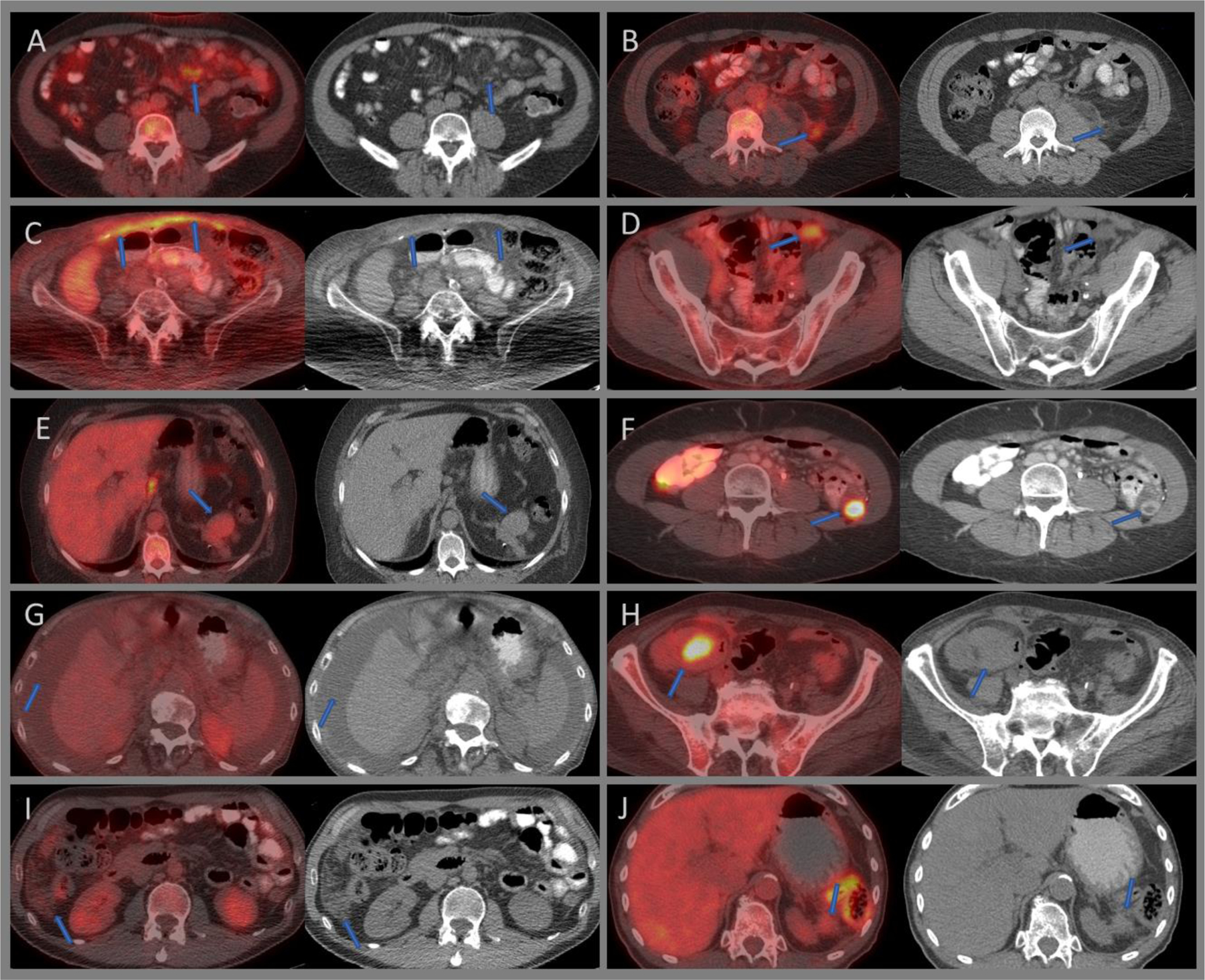
Axial fused and CT images of 18F-FDG PET/CT demonstrating moderately intense mesenteric panniculitis (A), left lower quadrant fat necrosis (B), intense uptake relating to surgical mesh hernia repair (C), moderately intense uptake with a hernia plug (D), mildly avid splenules at the pancreatic tail (E), intensely avid transposed ovary within the left paracolic gutter (F), low-grade malignant ascites from unknown primary (G), intensely avid post-surgical abscess adjacent to the ascending colon (H), minimally avid peritoneal mesothelioma within the right lilac fossa (I), and a minimally avid recurrent peritoneal metastatic deposit at the splenectomy bed from a pancreatic tumor (J).
Post-surgical changes within the abdomen and pelvis may show variable degree of FDG uptake, which can make assessment of peritoneal malignancy difficult. Post-surgical mesh hernia repair may show focal, linear, or non-uniform FDG uptake with no relationship to the time from surgery to post-operative PET/CT or SUVmax150 (Figure 9C).
Splenules (post-surgical or post-trauma) can be FDG-avid and can be mistaken for peritoneal tumors (Figure 9E). They do, however, reliably show uptake of radiolabeled heat-damaged red blood cells.151 Unusual benign conditions such as transposed ovaries, sarcoidosis, abdominal histoplasmosis, or ectopic pregnancy can also show variable FDG uptake and mimic malignancies152–155 (Figure 9F). Portal vein thrombus can present with increased FDG uptake.156
FDG uptake in peritoneal carcinomatosis can be seen as focal intense uptake, irregular heterogenous uptake, or nodular or curvilinear uptake along the surface of the viscera and/or diaphragm.157 Kim et al. reported a pooled sensitivity and specificity of 87% and 92% for PET/CT in detection of peritoneal carcinomatosis.158 Another study reported a false positive rate of 11% on a per patient basis among patients with suspected peritoneal carcinomatosis who underwent FDG PET/CT prior to cytoreductive surgery.159 These may occur due to various causes such as misregistered uptake in normal bowel, benign mesenteric lymphadenopathy, non-malignant inflammatory pathologies such as foreign body reaction around mesh prosthesis, hernia repair plug, infections such as peritoneal abscess, and post-surgical changes (Figure 9D and 9H). In general, FDG PET demonstrates reduced sensitivity for small-volume disease, predominantly cystic or low-grade disease, and ascites160 (Figure 9G and 9J). Multicystic peritoneal mesothelioma (MPM) may show mild or no uptake, whereas epithelioid peritoneal mesothelioma (EPM) is generally FDG-avid (Figure 9I). In patients presenting with ascites as the first symptom, FDG PET/CT may detect the primary tumor in up to 80%161 (Figure 9G). FDG uptake is higher in malignant than in benign ascites, but with considerable overlap.162
Varied GI symptoms have been reported in patients with COVID-19. Most frequently, the GI symptoms include lack of appetite, diarrhea, vomiting, and abdominal pain.163 CT appearances may include thickening of the bowel, pneumatosis, and, rarely, intussusception and ascites;164 variable FDG uptake may be seen in these conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD. In, https://seercancergov/csr/1975_2017/, based on November 2019. SEER data submission, posted to the SEER web site, April 2020 [Google Scholar]
- 2.Beyer T, Townsend DW, Brun T et al. A combined PET/CT scanner for clinical oncology. J Nucl Med 2000; 41: 1369–1379 [PubMed] [Google Scholar]
- 3.Israeli RS, Powell CT, Corr JG et al. Expression of the prostate-specific membrane antigen. Cancer Res 1994; 54: 1807–1811 [PubMed] [Google Scholar]
- 4.Kinoshita Y, Kuratsukuri K, Landas S et al. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J Surg 2006; 30: 628–636 [DOI] [PubMed] [Google Scholar]
- 5.Krohn T, Verburg FA, Pufe T et al. [(68)Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: an important pitfall in clinical practice. Eur J Nucl Med Mol Imaging 2015; 42: 210–214 [DOI] [PubMed] [Google Scholar]
- 6.Rischpler C, Beck TI, Okamoto S et al. (68)Ga-PSMA-HBED-CC Uptake in Cervical, Celiac, and Sacral Ganglia as an Important Pitfall in Prostate Cancer PET Imaging. J Nucl Med 2018; 59: 1406–1411 [DOI] [PubMed] [Google Scholar]
- 7.Minamimoto R, Hancock S, Schneider B et al. Pilot Comparison of ⁶⁸Ga-RM2 PET and ⁶⁸Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. [DOI] [PubMed]
- 8.Yamada Y, Post SR, Wang K et al. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A 1992; 89: 251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moradi F, Jamali M, Barkhodari A et al. Spectrum of 68Ga-DOTA TATE Uptake in Patients With Neuroendocrine Tumors. Clin Nucl Med 2016; 41: e281–287 [DOI] [PubMed] [Google Scholar]
- 10.Gugger M, Waser B Fau-Kappeler A, Kappeler A Fau-Schonbrunn A et al. Cellular detection of sst2A receptors in human gastrointestinal tissue. [DOI] [PMC free article] [PubMed]
- 11.Iagaru A, Mittra E, Dick DW et al. Prospective Evaluation of 99mTc MDP Scintigraphy, 18F NaF PET/CT, and 18F FDG PET/CT for Detection of Skeletal Metastases. Molecular Imaging and Biology 2012; 14: 252–259 [DOI] [PubMed] [Google Scholar]
- 12.Bastawrous S, Bhargava P, Behnia F et al. Newer PET application with an old tracer: role of 18F-NaF skeletal PET/CT in oncologic practice. Radiographics 2014; 34: 1295–1316 [DOI] [PubMed] [Google Scholar]
- 13.Schöder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med 2004; 34: 274–292 [DOI] [PubMed] [Google Scholar]
- 14.Park S, Kim TS, Kang SH et al. 11C-acetate and 18F-fluorodeoxyglucose positron emission tomography/computed tomography dual imaging for the prediction of response and prognosis after transarterial chemoembolization. Medicine (Baltimore) 2018; 97: e12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karanikas G, Beheshti M. ¹¹C-acetate PET/CT imaging: physiologic uptake, variants, and pitfalls. PET Clin 2014; 9: 339–344 [DOI] [PubMed] [Google Scholar]
- 16.Schöder H, Ong SC, Reuter VE et al. Initial results with (11)C-acetate positron emission tomography/computed tomography (PET/CT) in the staging of urinary bladder cancer. Mol Imaging Biol 2012; 14: 245–251 [DOI] [PubMed] [Google Scholar]
- 17.Park JW, Kim JH, Kim SK et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med 2008; 49: 1912–1921 [DOI] [PubMed] [Google Scholar]
- 18.Calabria F, Chiaravalloti A, Cicciò C et al. PET/CT with 18F–choline: Physiological whole bio-distribution in male and female subjects and diagnostic pitfalls on 1000 prostate cancer patients. Nucl Med Biol 2017; 51: 40–54 [DOI] [PubMed] [Google Scholar]
- 19.Michaud L, Touijer KA, Mauguen A et al. (11)C-Choline PET/CT in Recurrent Prostate Cancer: Retrospective Analysis in a Large U.S. Patient Series. [DOI] [PMC free article] [PubMed]
- 20.Giovannini E Fau - Lazzeri P, Lazzeri P Fau - Milano A, Milano A Fau - Gaeta MC et al. Clinical applications of choline PET/CT in brain tumors. [DOI] [PubMed]
- 21.Kirienko M, Sollini M Fau - Lopci E, Lopci E Fau - Versari A et al. Applications of PET imaging with radiolabelled choline (11C/18F-choline). [PubMed]
- 22.Calabria F, Gallo G Fau - Schillaci O, Schillaci O Fau - Cascini GL et al. Bio-Distribution, Imaging Protocols and Diagnostic Accuracy of PET with Tracers of Lipogenesis in Imaging Prostate Cancer: a Comparison between 11C-Choline, 18FFluoroethylcholine and 18F-Methylcholine. [DOI] [PubMed]
- 23.Haroon A, Zanoni L, Celli M et al. Multicenter study evaluating extraprostatic uptake of 11C-choline, 18F-methylcholine, and 18F-ethylcholine in male patients: physiological distribution, statistical differences, imaging pearls, and normal variants. Nucl Med Commun 2015; 36: 1065–1075 [DOI] [PubMed] [Google Scholar]
- 24.Mui M, Akhurst T, Warrier SK et al. Detection of incidental colorectal pathology on positron emission tomography/computed tomography. ANZ J Surg 2018; 88: E122–E126 [DOI] [PubMed] [Google Scholar]
- 25.Kano Y, Sakamoto S, Kasahara T et al. Methionine dependency of cell growth in normal and malignant hematopoietic cells. Cancer Res 1982; 42: 3090–3092 [PubMed] [Google Scholar]
- 26.Okubo S, Zhen HN, Kawai N et al. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neurooncol 2010; 99: 217–225 [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Matsuda H, Kubota K. Imaging Spectrum and Pitfalls of (11)C-Methionine Positron Emission Tomography in a Series of Patients with Intracranial Lesions. Korean J Radiol 2016; 17: 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapela M, Leskinen-Kallio S, Varpula M et al. Metabolic imaging of ovarian tumors with carbon-11-methionine: a PET study. J Nucl Med 1995; 36: 2196–2200 [PubMed] [Google Scholar]
- 29.Isohashi K, Shimosegawa E, Kato H et al. Optimization of [11C]methionine PET study: appropriate scan timing and effect of plasma amino acid concentrations on the SUV. EJNMMI Res 2013; 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takasu A, Shimosegawa T, Shimosegawa E et al. 11C-methionine uptake to the pancreas and its secretion: a positron emission tomography study in humans. Pancreas 1999; 18: 392–398 [DOI] [PubMed] [Google Scholar]
- 31.Ulaner GA, Goldman DA, Gönen M et al. Initial Results of a Prospective Clinical Trial of 18F-Fluciclovine PET/CT in Newly Diagnosed Invasive Ductal and Invasive Lobular Breast Cancers. J Nucl Med 2016; 57: 1350–1356 [DOI] [PubMed] [Google Scholar]
- 32.Michaud L, Beattie BJ, Akhurst T et al. 18 F-Fluciclovine (18 F-FACBC) PET imaging of recurrent brain tumors. Eur J Nucl Med Mol Imaging 2020; 47: 1353–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuster DM, Nanni C, Fanti S et al. Anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med 2014; 55: 1986–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chondrogiannis S, Marzola MC, Rubello D. ¹⁸F-DOPA PET/computed tomography imaging. PET Clin 2014; 9: 307–321 [DOI] [PubMed] [Google Scholar]
- 35.Bozkurt MF, Virgolini I, Balogova S et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur J Nucl Med Mol Imaging 2017; 44: 1588–1601 [DOI] [PubMed] [Google Scholar]
- 36.Humbert O, Bourg V, Mondot L et al. (18)F-DOPA PET/CT in brain tumors: impact on multidisciplinary brain tumor board decisions. Eur J Nucl Med Mol Imaging 2019; 46: 558–568 [DOI] [PubMed] [Google Scholar]
- 37.Salskov A, Tammisetti VS, Grierson J et al. FLT: measuring tumor cell proliferation in vivo with positron emission tomography and 3’-deoxy-3’-[18F]fluorothymidine. Semin Nucl Med 2007; 37: 429–439 [DOI] [PubMed] [Google Scholar]
- 38.Herrmann K, Ott K, Buck AK et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 2007; 48: 1945–1950 [DOI] [PubMed] [Google Scholar]
- 39.Ulaner GA, Hyman DM, Lyashchenko SK et al. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin Nucl Med 2017; 42: 912–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Vega F, Hechtman JF, Castel P et al. EGFR and MET Amplifications Determine Response to HER2 Inhibition in ERBB2-Amplified Esophagogastric Cancer. Cancer Discov 2019; 9: 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulaner GA, Lyashchenko SK, Riedl C et al. First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using (89)Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J Nucl Med 2018; 59: 900–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Donoghue JA, Lewis JS, Pandit-Taskar N et al. Pharmacokinetics, Biodistribution, and Radiation Dosimetry for Pharmacokinetics, Biodistribution, and Radiation Dosimetry for 89 Zr-Trastuzumab in Patients with Esophagogastric Cancer. J Nucl Med 2018; 59: 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eschmann SM, Reischl G, Bilger K et al. Evaluation of dosimetry of radioiodine therapy in benign and malignant thyroid disorders by means of iodine-124 and PET. Eur J Nucl Med Mol Imaging 2002; 29: 760–767 [DOI] [PubMed] [Google Scholar]
- 44.Kuker R, Sztejnberg M, Gulec S. I-124 Imaging and Dosimetry. Mol Imaging Radionucl Ther 2017; 26: 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandit-Taskar N, Zanzonico PB, Kramer K et al. Biodistribution and Dosimetry of Intraventricularly Administered (124)I-Omburtamab in Patients with Metastatic Leptomeningeal Tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2019; 60: 1794–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koehler L, Gagnon K, McQuarrie S et al. Iodine-124: a promising positron emitter for organic PET chemistry. Molecules 2010; 15: 2686–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmadian A, Ay MR, Bidgoli JH et al. Correction of oral contrast artifacts in CT-based attenuation correction of PET images using an automated segmentation algorithm. Eur J Nucl Med Mol Imaging 2008; 35: 1812–1823 [DOI] [PubMed] [Google Scholar]
- 48.Cook GJ, Wegner EA, Fogelman I. Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med 2004; 34: 122–133 [DOI] [PubMed] [Google Scholar]
- 49.Beyer T, Antoch G, Müller S et al. Acquisition protocol considerations for combined PET/CT imaging. J Nucl Med 2004; 45 Suppl 1: 25S–35S [PubMed] [Google Scholar]
- 50.D’Journo XB. Clinical implication of the innovations of the 8 th edition of the TNM classification for esophageal and esophago-gastric cancer. J Thorac Dis 2018; 10: S2671–S2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyoshi K, Naito M, Ueno T et al. Abnormal fluorine-18-fluorodeoxyglucose uptake in benign esophageal leiomyoma. Gen Thorac Cardiovasc Surg 2009; 57: 629–632 [DOI] [PubMed] [Google Scholar]
- 52.Kamel EM, Thumshirn M, Truninger K et al. Significance of incidental 18F-FDG accumulations in the gastrointestinal tract in PET/CT: correlation with endoscopic and histopathologic results. J Nucl Med 2004; 45: 1804–1810 [PubMed] [Google Scholar]
- 53.Shakeri S, Farahmandfar F, Gholami G et al. Advanced Case of Achalasia That Appeared on 68Ga-Prostate-Specific Membrane Antigen PET/CT. Clin Nucl Med 2020; 45: 301–302 [DOI] [PubMed] [Google Scholar]
- 54.Lowe VJ, Booya F, Fletcher JG et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005; 7: 422–430 [DOI] [PubMed] [Google Scholar]
- 55.Cuellar SL, Carter BW, Macapinlac HA et al. Clinical staging of patients with early esophageal adenocarcinoma: does FDG-PET/CT have a role? J Thorac Oncol 2014; 9: 1202–1206 [DOI] [PubMed] [Google Scholar]
- 56.Shi W, Wang W, Wang J et al. Meta-analysis of 18FDG PET-CT for nodal staging in patients with esophageal cancer. Surg Oncol 2013; 22: 112–116 [DOI] [PubMed] [Google Scholar]
- 57.Weber WA, Ott K, Becker K et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 2001; 19: 3058–3065 [DOI] [PubMed] [Google Scholar]
- 58.Chhabra A, Ong LT, Kuk D et al. Prognostic significance of PET assessment of metabolic response to therapy in oesophageal squamous cell carcinoma. Br J Cancer 2015; 113: 1658–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Gouw D, Klarenbeek BR, Driessen M et al. Detecting Pathological Complete Response in Esophageal Cancer after Neoadjuvant Therapy Based on Imaging Techniques: A Diagnostic Systematic Review and Meta-Analysis. J Thorac Oncol 2019; 14: 1156–1171 [DOI] [PubMed] [Google Scholar]
- 60.Goense L, van Rossum PS, Reitsma JB et al. Diagnostic Performance of ¹⁸F-FDG PET and PET/CT for the Detection of Recurrent Esophageal Cancer After Treatment with Curative Intent: A Systematic Review and Meta-Analysis. J Nucl Med 2015; 56: 995–1002 [DOI] [PubMed] [Google Scholar]
- 61.Kim SJ, Hyun SH, Moon SH et al. Diagnostic value of surveillance (18)F-fluorodeoxyglucose PET/CT for detecting recurrent esophageal carcinoma after curative treatment. Eur J Nucl Med Mol Imaging 2019; 46: 1850–1858 [DOI] [PubMed] [Google Scholar]
- 62.Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North AM 2013; 42: 261–284 [DOI] [PubMed] [Google Scholar]
- 63.Olszewska-Szopa M, Wróbel T. Gastrointestinal non-Hodgkin lymphomas. Adv Clin Exp Med 2019; 28: 1119–1124 [DOI] [PubMed] [Google Scholar]
- 64.Koga H, Sasaki M, Kuwabara Y et al. An analysis of the physiological FDG uptake pattern in the stomach. Ann Nucl Med 2003; 17: 733–738 [DOI] [PubMed] [Google Scholar]
- 65.Le Roux PY, Duong CP, Cabalag CS et al. Incremental diagnostic utility of gastric distension FDG PET/CT. Eur J Nucl Med Mol Imaging 2016; 43: 644–653 [DOI] [PubMed] [Google Scholar]
- 66.Tsukada T, Nakano T, Miyata T et al. Xanthogranulomatous gastritis mimicking malignant GIST on F-18 FDG PET. Ann Nucl Med 2012; 26: 752–756 [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Li B, Cai L et al. Gastric Schwannoma mimicking malignant gastrointestinal stromal tumor and misdiagnosed by (18)F-FDG PET/CT. Hell J Nucl Med 2015; 18: 74–76 [PubMed] [Google Scholar]
- 68.Hirose Y, Kaida H, Kawahara A et al. ¹⁸F-FDG PET/CT and contrast enhanced CT in differential diagnosis between leiomyoma and gastrointestinal stromal tumor. Hell J Nucl Med 2015; 18: 257–260 [DOI] [PubMed] [Google Scholar]
- 69.Noto B, Weckesser M, Buerke B et al. Gastrointestinal Stromal Tumor Showing Intense Tracer Uptake on PSMA PET/CT. Clin Nucl Med 2017; 42: 200–202 [DOI] [PubMed] [Google Scholar]
- 70.Laurens ST, Witjes F, Janssen M et al. 68Ga-Prostate-Specific Membrane Antigen Uptake in Gastrointestinal Stromal Tumor. Clin Nucl Med 2018; 43: 60–61 [DOI] [PubMed] [Google Scholar]
- 71.Evangelista L, Cassarino G, Capobianco D et al. An Incidental Uptake of 18F-Choline at PET/CT in Gastric Neuroendocrine Tumor. Clin Nucl Med 2020; [DOI] [PubMed]
- 72.Smyth E, Schöder H, Strong VE et al. A prospective evaluation of the utility of 2-deoxy-2-[(18)F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer 2012; 118: 5481–5488 [DOI] [PubMed] [Google Scholar]
- 73.Lehmann K, Eshmuminov D, Bauerfeind P et al. (18)FDG-PET-CT improves specificity of preoperative lymph-node staging in patients with intestinal but not diffuse-type esophagogastric adenocarcinoma. Eur J Surg Oncol 2017; 43: 196–202 [DOI] [PubMed] [Google Scholar]
- 74.Malik D, Kumar R, Mittal BR et al. Ga-labelled PSMA (prostate specific membrane antigen) expression in signet-ring cell gastric carcinoma. Eur J Nucl Med Mol Imaging 2018; 45: 1276–1277 [DOI] [PubMed] [Google Scholar]
- 75.Treglia G, Zucca E, Sadeghi R et al. Detection rate of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with marginal zone lymphoma of MALT type: a meta-analysis. Hematol Oncol 2015; 33: 113–124 [DOI] [PubMed] [Google Scholar]
- 76.Qi S, Huang MY, Yang Y et al. Uptake of [(18)F]fluorodeoxyglucose in initial positron-emission tomography predicts survival in MALT lymphoma. Blood Adv 2018; 2: 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Radan L, Fischer D, Bar-Shalom R et al. FDG avidity and PET/CT patterns in primary gastric lymphoma. Eur J Nucl Med Mol Imaging 2008; 35: 1424–1430 [DOI] [PubMed] [Google Scholar]
- 78.Albano D, Durmo R, Treglia G et al. 18F-FDG PET/CT or PET Role in MALT Lymphoma: An Open Issue not Yet Solved—A Critical Review. Clinical Lymphoma Myeloma and Leukemia 2020; 20: 137–146 [DOI] [PubMed] [Google Scholar]
- 79.Ott K, Herrmann K, Lordick F et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res 2008; 14: 2012–2018 [DOI] [PubMed] [Google Scholar]
- 80.Won E, Shah MA, Schöder H et al. Use of positron emission tomography scan response to guide treatment change for locally advanced gastric cancer: the Memorial Sloan Kettering Cancer Center experience. J Gastrointest Oncol 2016; 7: 506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JW, Lee SM, Son MW et al. Diagnostic performance of FDG PET/CT for surveillance in asymptomatic gastric cancer patients after curative surgical resection. Eur J Nucl Med Mol Imaging 2016; 43: 881–888 [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z, Zheng B, Chen W et al. Accuracy of (18)F-FDG PET/CT and CECT for primary staging and diagnosis of recurrent gastric cancer: A meta-analysis. Exp Ther Med 2021; 21: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benson AB, Venook AP, Al-Hawary MM et al. Small Bowel Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019; 17: 1109–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Modlin IM, Kidd M, Latich I et al. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128: 1717–1751 [DOI] [PubMed] [Google Scholar]
- 85.Demirci E, Sahin OE, Ocak M et al. Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl Med Commun 2016; 37: 1169–1179 [DOI] [PubMed] [Google Scholar]
- 86.Wolf P, Freudenberg N, Bühler P et al. Three conformational antibodies specific for different PSMA epitopes are promising diagnostic and therapeutic tools for prostate cancer. Prostate 2010; 70: 562–569 [DOI] [PubMed] [Google Scholar]
- 87.Cronin CG, Scott J, Kambadakone A et al. Utility of positron emission tomography/CT in the evaluation of small bowel pathology. Br J Radiol 2012; 85: 1211–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Louis E, Ancion G, Colard A et al. Noninvasive assessment of Crohn’s disease intestinal lesions with (18)F-FDG PET/CT. J Nucl Med 2007; 48: 1053–1059 [DOI] [PubMed] [Google Scholar]
- 89.Honjo H, Mike M, Kusanagi H et al. Adult intussusception: a retrospective review. World J Surg 2015; 39: 134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phongkitkarun S, Varavithya V, Kazama T et al. Lymphomatous involvement of gastrointestinal tract: evaluation by positron emission tomography with (18)F-fluorodeoxyglucose. World J Gastroenterol 2005; 11: 7284–7289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Binderup T, Knigge U, Loft A et al. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J Nucl Med 2010; 51: 704–712 [DOI] [PubMed] [Google Scholar]
- 92.Virgolini I, Ambrosini V, Bomanji JB et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging 2010; 37: 2004–2010 [DOI] [PubMed] [Google Scholar]
- 93.Skoura E, Michopoulou S, Mohmaduvesh M et al. The Impact of 68Ga-DOTATATE PET/CT Imaging on Management of Patients with Neuroendocrine Tumors: Experience from a National Referral Center in the United Kingdom. J Nucl Med 2016; 57: 34–40 [DOI] [PubMed] [Google Scholar]
- 94.Bodei L, Ambrosini V, Herrmann K et al. Current Concepts in 68Ga-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. Journal of Nuclear Medicine 2017; 58: 1718. [DOI] [PubMed] [Google Scholar]
- 95.Ulaner GA, Bodei L. Hepatocellular Carcinoma Mimicking Neuroendocrine Tumor Metastasis on 68Ga-DOTATATE PET/CT. Clin Nucl Med 2019; 44: 330–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soeda F, Watabe T, Kato H et al. Duodenal Adenocarcinoma Mimicking Metastasis of Prostate Cancer on 18F-Prostate-Specific Membrane Antigen-1007 PET/CT. Clin Nucl Med 2021; 46: 49–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee DJW, Warner M, Shannon T et al. Incidental finding of ileal gastrointestinal stromal tumour during prostate cancer staging with prostate-specific membrane antigen scan. J Med Imaging Radiat Oncol 2020; [DOI] [PubMed]
- 98.Dekker E, Tanis PJ, Vleugels JLA et al. Colorectal cancer. Lancet 2019; 394: 1467–1480 [DOI] [PubMed] [Google Scholar]
- 99.Cappell MS. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North AM 2008; 37: 1–24, v [DOI] [PubMed] [Google Scholar]
- 100.Lee MS, Menter DG, Kopetz S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J Natl Compr Canc Netw 2017; 15: 411–419 [DOI] [PubMed] [Google Scholar]
- 101.Tatlidil R, Jadvar H, Bading JR et al. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. Radiology 2002; 224: 783–787 [DOI] [PubMed] [Google Scholar]
- 102.Miraldi F, Vesselle H, Faulhaber PF et al. Elimination of artifactual accumulation of FDG in PET imaging of colorectal cancer. Clin Nucl Med 1998; 23: 3–7 [DOI] [PubMed] [Google Scholar]
- 103.Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005; 65: 385–411 [DOI] [PubMed] [Google Scholar]
- 104.Walker J, Jijon HB, Diaz H et al. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J 2005; 385: 485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gontier E, Fourme E, Wartski M et al. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging 2008; 35: 95–99 [DOI] [PubMed] [Google Scholar]
- 106.Schreuder N, Klarenbeek H, Vendel BN et al. Discontinuation of metformin to prevent metformin-induced high colonic FDG uptake: is 48 h sufficient? Annals of nuclear medicine 2020; 34: 833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Groshar D, Bernstine H, Stern D et al. PET/CT enterography in Crohn disease: correlation of disease activity on CT enterography with 18F-FDG uptake. J Nucl Med 2010; 51: 1009–1014 [DOI] [PubMed] [Google Scholar]
- 108.Hannah A, Scott AM, Akhurst T et al. Abnormal colonic accumulation of fluorine-18-FDG in pseudomembranous colitis. J Nucl Med 1996; 37: 1683–1685 [PubMed] [Google Scholar]
- 109.Pandit-Taskar N, Schöder H, Gonen M et al. Clinical significance of unexplained abnormal focal FDG uptake in the abdomen during whole-body PET. AJR AM J Roentgenol 2004; 183: 1143–1147 [DOI] [PubMed] [Google Scholar]
- 110.Kousgaard SJ, Thorlacius-Ussing O. Incidental colorectal FDG uptake on PET/CT scan and lesions observed during subsequent colonoscopy: a systematic review. Tech Coloproctol 2017; 21: 521–529 [DOI] [PubMed] [Google Scholar]
- 111.Treglia G, Bertagna F, Sadeghi R et al. Prevalence and risk of malignancy of focal incidental uptake detected by fluorine-18-fluorodeoxyglucose positron emission tomography in the parotid gland: a meta-analysis. Eur Arch Otorhinolaryngol 2015; 272: 3617–3626 [DOI] [PubMed] [Google Scholar]
- 112.Hwang JP, Woo SK, Yoon SY et al. The potential usefulness of (18)F-FDG PET/CT for detecting colorectal carcinoma and adenoma in asymptomatic adults. Ann Nucl Med 2015; 29: 157–163 [DOI] [PubMed] [Google Scholar]
- 113.Gollub MJ, Grewal RK, Panu N et al. Diagnostic accuracy of ¹⁸F-FDG PET/CT for detection of advanced colorectal adenoma. Clin Radiol 2014; 69: 611–618 [DOI] [PubMed] [Google Scholar]
- 114.You H, Sweeny A, Cooper ML et al. The management of diverticulitis: a review of the guidelines. Med J Aust 2019; 211: 421–427 [DOI] [PubMed] [Google Scholar]
- 115.Rainis T, Kaidar-Person O, Keren D et al. Correlation between incidental FDG PET/CT colorectal observations and endoscopic and histopathological results. Oncol Lett 2014; 7: 479–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nishiyama N, Mori H, Kobara H et al. Difficulty in differentiating two cases of sigmoid stenosis by diverticulitis from cancer. World J Gastroenterol 2012; 18: 3623–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xue Q, Miao W. Inflammatory Pseudotumor of Intestine Mimicking Lymphoma on 18F-FDG PET/CT. Clin Nucl Med 2020; 45: 383–384 [DOI] [PubMed] [Google Scholar]
- 118.Mei R, Prediletto I, Nava S et al. Colon Sarcoidosis Mimicking Cancer at 18F-FDG PET/CT. Clin Nucl Med 2020; 45: 387–388 [DOI] [PubMed] [Google Scholar]
- 119.Silman C, Matsumoto S, Ono A et al. 18F-FDG uptake in the normal appendix in adults: PET/CT evaluation. Ann Nucl Med 2019; 33: 265–268 [DOI] [PubMed] [Google Scholar]
- 120.Ahn GS, Barsky AR, Galperin-Aizenberg M et al. 18F-Fluciclovine Uptake in Acute Appendicitis. Clin Nucl Med 2020; 45: e453–e454 [DOI] [PubMed] [Google Scholar]
- 121.Aranda E, Aparicio J, Alonso V et al. SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer 2015. Clin Transl Oncol 2015; 17: 972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodríguez-Fraile M, Cózar-Santiago MP, Sabaté-Llobera A et al. FDG PET/CT in colorectal cancer. Rev Esp Med Nucl Imagen Mol 2020; 39: 57–66 [DOI] [PubMed] [Google Scholar]
- 123.Berger KL, Nicholson SA, Dehdashti F et al. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR AM J Roentgenol 2000; 174: 1005–1008 [DOI] [PubMed] [Google Scholar]
- 124.Lu YY, Chen JH, Ding HJ et al. A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18F-FDG PET or PET/CT. Nucl Med Commun 2012; 33: 1127–1133 [DOI] [PubMed] [Google Scholar]
- 125.Van Cutsem E, Cervantes A, Adam R et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422 [DOI] [PubMed] [Google Scholar]
- 126.van Kessel CS, Buckens CF, van den Bosch MA et al. Preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy: a meta-analysis. Ann Surg Oncol 2012; 19: 2805–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maffione AM, Marzola MC, Capirci C et al. Value of (18)F-FDG PET for Predicting Response to Neoadjuvant Therapy in Rectal Cancer: Systematic Review and Meta-Analysis. AJR AM J Roentgenol 2015; 204: 1261–1268 [DOI] [PubMed] [Google Scholar]
- 128.Smeets P, Ham H, Ceelen W et al. Differentiation between peri-anastomotic inflammatory changes and local recurrence following neoadjuvant radiochemotherapy surgery for colorectal cancer using visual and semiquantitative analysis of PET-CT data. Q J Nucl Med Mol Imaging 2010; 54: 327–332 [PubMed] [Google Scholar]
- 129.Fehr M, Müller J, Knitel M et al. Early Postoperative FDG-PET-CT Imaging Results in a Relevant Upstaging in the pN2 Subgroup of Stage III Colorectal Cancer Patients. Clin Colorectal Cancer 2017; 16: 343–348 [DOI] [PubMed] [Google Scholar]
- 130.Wasserberg N, Purim O, Bard V et al. Early postoperative 18F-FDG PET/CT in high-risk stage III colorectal cancer. Clin Nucl Med 2015; 40: e222–227 [DOI] [PubMed] [Google Scholar]
- 131.Monteil J, Le Brun-Ly V, Cachin F et al. Comparison of 18FDG-PET/CT and conventional follow-up methods in colorectal cancer: A randomised prospective study. Digestive and Liver Disease 2021; 53: 231–237 [DOI] [PubMed] [Google Scholar]
- 132.Lu YY, Chen JH, Chien CR et al. Use of FDG-PET or PET/CT to detect recurrent colorectal cancer in patients with elevated CEA: a systematic review and meta-analysis. Int J Colorectal Dis 2013; 28: 1039–1047 [DOI] [PubMed] [Google Scholar]
- 133.Sanli Y, Kuyumcu S, Ozkan ZG et al. The utility of FDG-PET/CT as an effective tool for detecting recurrent colorectal cancer regardless of serum CEA levels. Ann Nucl Med 2012; 26: 551–558 [DOI] [PubMed] [Google Scholar]
- 134.Kang YH, Han E, Park G. Clinical Etiology of Hypermetabolic Pelvic Lesions in Postoperative Positron Emission Tomography/Computed Tomography for Patients With Rectal and Sigmoid Cancer. Ann Coloproctol 2018; 34: 78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Islami F, Ferlay J, Lortet-Tieulent J et al. International trends in anal cancer incidence rates. Int J Epidemiol 2017; 46: 924–938 [DOI] [PubMed] [Google Scholar]
- 136.Faber MT, Frederiksen K, Palefsky JM et al. Risk of Anal Cancer Following Benign Anal Disease and Anal Cancer Precursor Lesions: A Danish Nationwide Cohort Study. Cancer Epidemiology Biomarkers & Prevention 2020; 29: 185. [DOI] [PubMed] [Google Scholar]
- 137.Sena Y, Matsumoto S, Silman C et al. Physiological 18F-FDG uptake in the normal adult anal canal: evaluation by PET/CT. Annals of Nuclear Medicine 2020; 34: 538–544 [DOI] [PubMed] [Google Scholar]
- 138.Basu S, Nair N. Avid 18F-FDG uptake in rectal hemorrhoid in a patient with metastatic medullary carcinoma of thyroid. Indian J Gastroenterol 2006; 25: 257. [PubMed] [Google Scholar]
- 139.Tsai S-C, Jeng L-B, Yeh J-J et al. Findings of 2-fluoro-2-deoxy-d-glucose positron emission tomography in hemorrhoids. Abdominal Imaging 2011; 36: 548–551 [DOI] [PubMed] [Google Scholar]
- 140.Agarwal A, Marcus C, Xiao J et al. FDG PET/CT in the management of colorectal and anal cancers. AJR AM J Roentgenol 2014; 203: 1109–1119 [DOI] [PubMed] [Google Scholar]
- 141.Albertsson P, Alverbratt C, Liljegren A et al. Positron emission tomography and computed tomographic (PET/CT) imaging for radiation therapy planning in anal cancer: A systematic review and meta-analysis. Critical Reviews in Oncology/Hematology 2018; 126: 6–12 [DOI] [PubMed] [Google Scholar]
- 142.Houard C, Pinaquy JB, Mesguich C et al. Role of (18)F-FDG PET/CT in Posttreatment Evaluation of Anal Carcinoma. J Nucl Med 2017; 58: 1414–1420 [DOI] [PubMed] [Google Scholar]
- 143.Mahmud A, Poon R, Jonker D. PET imaging in anal canal cancer: a systematic review and meta-analysis. The British journal of radiology 2017; 90: 20170370–20170370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwarz JK, Siegel BA, Dehdashti F et al. Tumor response and survival predicted by post-therapy FDG-PET/CT in anal cancer. Int J Radiat Oncol Biol Phys 2008; 71: 180–186 [DOI] [PubMed] [Google Scholar]
- 145.Kim C, Kim DY, Hong IK. Presacral Anastomotic Sinus After Low Anterior Resection Mimicking Recurrent Rectal Cancer. Clin Nucl Med 2020; 45: e171–e173 [DOI] [PubMed] [Google Scholar]
- 146.Kang YH, Park G. Characteristics of 18F-fluorodeoxyglucose uptake in anastomotic sinus and fistula following rectal cancer surgery. Clinical Imaging 2017; 41: 33–36 [DOI] [PubMed] [Google Scholar]
- 147.Garg G, Tripathi M, D’Souza M et al. Demonstration of a tubercular fistula-in-ano on F-18 FDG PET/CT. Clin Nucl Med 2010; 35: 300–302 [DOI] [PubMed] [Google Scholar]
- 148.Teagle AR, Gilbert DC, Jones JR et al. Negative 18F-FDG-PET-CT may exclude residual or recurrent disease in anal cancer. Nucl Med Commun 2016; 37: 1038–1045 [DOI] [PubMed] [Google Scholar]
- 149.Metser U, Miller E, Lerman H et al. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: characterization and incidence. AJR AM J Roentgenol 2007; 189: 1203–1210 [DOI] [PubMed] [Google Scholar]
- 150.Davidson T, Klang E, Goshen E et al. Postoperative changes after surgical mesh hernia repair: a pitfall in interpretation of 18F-FDG PET-CT. Hernia 2017; 21: 629–636 [DOI] [PubMed] [Google Scholar]
- 151.Kok J, Lin M, Lin P et al. Splenosis presenting as multiple intra-abdominal masses mimicking malignancy. ANZ J Surg 2008; 78: 406–407 [DOI] [PubMed] [Google Scholar]
- 152.Davidson T, Komisar O, Korach J et al. Physiologic uptake of 18F-FDG in transposed ovaries may mimic metastasis on 18F-FDG PET/CT imaging. Nucl Med Commun 2018; 39: 171–178 [DOI] [PubMed] [Google Scholar]
- 153.Inoue K, Goto R, Shimomura H et al. FDG-PET/CT of sarcoidosis and sarcoid reactions following antineoplastic treatment. Springerplus 2013; 2: 113–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mohan M, Fogel B, Eluvathingal T et al. Gastrointestinal histoplasmosis in a patient after autologous stem cell transplant for multiple myeloma. Transpl Infect Dis 2016; 18: 939–941 [DOI] [PubMed] [Google Scholar]
- 155.Hao J, Cheng Z, Hu N et al. Imaging of Hepatic Ectopic Pregnancy by 18F-FDG PET/CT. Clin Nucl Med 2016; 41: 697–698 [DOI] [PubMed] [Google Scholar]
- 156.Cowell GW, Thomson LK, Digby M et al. Acute portal vein thrombosis: an important mimic of malignancy on FDG PET/CT. BMJ Case Rep 2011; 2011: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suzuki A, Kawano T, Takahashi N et al. Value of 18F-FDG PET in the detection of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging 2004; 31: 1413–1420 [DOI] [PubMed] [Google Scholar]
- 158.Kim S-J, Lee S-W. Diagnostic accuracy of (18)F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis. The British journal of radiology 2018; 91: 20170519–20170519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Audollent R, Eveno C, Dohan A et al. Pitfalls and mimickers on (18)F-FDG-PET/CT in peritoneal carcinomatosis from colorectal cancer: An analysis from 37 patients. J Visc Surg 2015; 152: 285–291 [DOI] [PubMed] [Google Scholar]
- 160.Anthony MP, Khong PL, Zhang J. Spectrum of (18)F-FDG PET/CT appearances in peritoneal disease. AJR AM J Roentgenol 2009; 193: W523–529 [DOI] [PubMed] [Google Scholar]
- 161.Liu J, Liu G, Liu X et al. Clinical Value of Positron Emission Tomography-Computed Tomography Combined with Ultrasound in Detection of Primary Tumors in Patients with Malignant Ascites. Cancer Biother Radiopharm 2019; 34: 203–207 [DOI] [PubMed] [Google Scholar]
- 162.Zhang M, Jiang X, Zhang M et al. The Role of 18F-FDG PET/CT in the evaluation of Ascites of Undetermined Origin. J Nucl Med 2009; 50: 506–512 [DOI] [PubMed] [Google Scholar]
- 163.Pan L, Mu M, Yang P et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. AM J Gastroenterol 2020; 115: 766–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lui K, Wilson MP, Low G. Abdominal imaging findings in patients with SARS-CoV-2 infection: a scoping review. Abdom Radiol (NY) 2020; 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]


