Abstract
HPV infection drives tumorigenesis in the majority of cervical, oropharyngeal, anal, and vulvar cancers, amongst other cancer types. Genetic and epidemiologic evidence have highlighted the role of immunosuppression in the oncogenesis of HPV-related malignancies. Here we review how HPV modulates the immune microenvironment and subsequent therapeutic implications. We describe the landscape of immunotherapies for these cancers with a focus on findings from early phase studies exploring antigen-specific treatments, and we discuss future directions. Whilst responses across these studies have been modest to date, a deeper understanding of HPV-related tumor biology and immunology may prove instrumental for the development of more efficacious immunotherapeutic approaches
I. INTRODUCTION
Human papillomavirus (HPV) infection with high-risk subtypes is linked to squamous cell carcinomas and adenocarcinomas that arise in several different organs but share many common biological and immunologic properties. Collectively referred to as HPV-associated cancers, these tumors arise in organs with a high incidence of HPV infections and include the more prevalent cervical, anal, and oropharyngeal cancers as well as the less prevalent vaginal, vulvar and penile cancers. In cervical and anal cancers, HPV is the etiology for nearly 90% of malignancies whereas for oropharyngeal, vaginal, vulvar and penile cancers up to 30% of tumors diagnosed in the United States are still driven by carcinogen exposure or other causes (1). Persistent infection with high-risk HPV subtypes results in malignant transformation, canonically by the expression of HPV oncogenes E6 and E7, which inhibit the tumor suppressors p53 and Rb, respectively (2). These oncoproteins additionally inhibit apoptosis, promote genomic instability, inhibit telomere shortening, promote angiogenesis, and facilitate invasion and metastasis, amongst other emerging functions (3-5). While the association between global immune compromise and HPV-related malignancies is well established, our understanding of how HPV creates a state of immune suppression in the microenvironment is still limited. Further complicating this issue is the fact that different subtypes of HPV preferentially infect each tumor sub-site, and there may be tissue-specific immune barriers in each microenvironment. Modulation of the immune system by the most frequently implicated high-risk HPV subtypes, HPV 16 and HPV 18, in pre-invasive lesions, as well as sustained immune evasion and resistance in invasive HPV malignancies, has been the subject of many recent studies. Simultaneously, advances in tumor immunology and immunotherapy in the past decade have reinvigorated interest in immunotherapeutic approaches to treat HPV-related malignancies. HPV-related malignancies theoretically have unique viral antigens that can be targeted by novel immunotherapeutic approaches such as adoptive cell therapies and therapeutic vaccines. Although early clinical studies have shown promise, further work is needed to define the basis of the modulation of the immune system in HPV-related cancers. This review focuses on the interactions of HPV and the immune system, particularly the adaptive immune system, which promote a persistent state of oncogenesis, and it also explores novel therapeutic avenues that target the immune system for the treatment of HPV-related malignancies. We focus particularly on HPV-related cervical and head and neck cancers, for which more robust pre-clinical and clinical data are available; however, we also highlight relevant studies on other HPV-related malignancies.
II. Population-based studies support a role for the host immune system in HPV-related carcinogenesis
1. Epidemiologic studies link global immune compromise to HPV cancer risk
Given the increasing prevalence of immunocompromised patients in the past decades a significant correlation between immune suppression and the risk of cancer development has been demonstrated in population-based studies. Particularly, HPV-associated malignancies have been shown to be enriched in immunocompromised patients and cervical cancer is now an AIDS-defining illness (1). Likewise, reports from multiple global populations have now demonstrated increased incidence of penile, anal, and oropharyngeal cancers in patients with HIV/AIDS, with risks increasing between 3 to 33 fold compared to a baseline population (6-9).
Further, solid organ transplantation is also associated with an increased risk of HPV-associated malignancies. Patients with solid organ (liver, heart, kidney) transplants in the United States showed a clear increase in all infection-related malignancies, in particular oropharyngeal, vulvar, penile and anal cancers (10). This increase in risk in HPV-associated malignancies after solid organ transplant has been corroborated in other populations across the globe (11,12). A, a meta-analysis combining both HIV/AIDS patients and solid organ transplant patients demonstrated a uniform increase in standardized incidence ratios of all HPV-related malignancies that ranged from 2 to 30 depending on the cancer subtype. These data strongly suggest that immune compromise, rather than an intrinsic variable of either HIV/AIDS or solid organ transplantation, underlies an increase in HPV-related cancer risk across these populations (13).
2. Genomic analyses identify mutations in immune function associated with HPV cancer risk
Large scale genetic studies have identified both germline and somatic genetic alterations in genes involved in immune function and HPV oncogenesis. After multiple reports initially suggested an association between cervical cancer and single nucleotide polymorphisms at human leukocyte antigen (HLA) loci, a genome-wide association study (GWAS) of 1000 cervical cancers and 4000 controls in the Danish population identified multiple loci associated with increased cancer risk: a locus adjacent to the MHC class I polypeptide-related sequence A gene (MICA), a locus between HLA-DRB1 and HLA-DQA1, and a locus at HLA-DPB2 (14). The first locus encodes a frameshift mutation in MICA resulting in decreased surface expression. Surface expression of MICA is essential in binding NKG2D and activating natural killer cells and T cells early in the infection (15). The increased risk associated with the second locus, however, lacks a mechanistic explanation at present; hence, further studies are required to establish its causal relationship. The third locus maps close to HLA-DPB2 which encodes the β chain of the peptide antigen receptor HLA-DP, a gene found to be associated with genetic susceptibility to carcinoma in situ (16). Furthermore the HLA class II haplotype DRB1*1301-DQA1*0103-DQB1*0603 was associated with decreased risk of HPV-related cervical malignancies (14). The mechanistic basis for the protection against HPV-induced oncogenesis conferred by these particular HLA class II alleles remains to be elucidated. Another GWAS of 2600 oropharyngeal cancers and 6500 controls also identified HLA loci as primary risk loci. Similarly, the authors noted the observation that DRB1*1301-DQA1*0103-DQB1*0603 was protective and associated with a statistically-significant decreased risk of HPV-positive oropharyngeal cancers, but not HPV-negative oropharyngeal cancers (17).
Analysis of somatic genetic alterations from The Cancer Genome Atlas (TCGA) demonstrated that in cervical cancer, 8% of patients appear to have novel mutations in HLA-A and 6% in HLA-B. Intriguingly, 8% of cervical cancers also demonstrated a predominant gain of function mutation in CD274, the gene coding for PD-L1 (18). In head and neck cancer, TCGA data demonstrated dysregulated immune pathways due to somatic mutations in 7% percent of all HPV-negative tumors and 11% of HPV-positive tumors (19). Taken together, these data describe a genetic profile of both germline and somatic mutations that affect critical immune pathways of antigen presentation and immune checkpoints. While these mutations appear to arise in fewer than 10% of patients, their presence sheds light on possible mechanisms that allow for HPV immune evasion and resistance.
III. Strategies employed by HPV-positive tumors to evade the adaptive immune system
Modulation of effector T cells has gradually become the cornerstone of immunotherapies across the vast majority of cancers, and more recently HPV-related malignancies. As such, understanding the mechanisms governing T cell activation, reactivity, suppression, and exhaustion in HPV-related malignancies is of paramount importance. Multiple reports have described the enrichment of tumor infiltrating lymphocytes (TIL) in HPV-positive tumors. Analysis of TILs from 12 cervical tumors showed that 9/12 had CD4+ T cells and 8/12 patients had CD8+ T cells that were specific to HPV antigens when stimulated with overlapping peptides derived from E6 and E7 ex-vivo, and most patients had polyclonal responses (20). A more comprehensive effort using peptides derived from all HPV genes was performed in HPV16+ head and neck squamous cell carcinoma (HNSCC), where HPV antigen-specific T cell responses were seen in 43 of 66 patient samples and displayed significant heterogeneity. T-cells from patients pre- and post-treatment were expanded and re-stimulated in vitro using peptides spanning all the known early and late genes of HPV. The results showed that both CD4+ and CD8+ T cells were reactive against peptides from all HPV genes with a predominance for E1 and L1. Interestingly, the levels of activated T cells increased with tumor stage and decreased post-curative therapy, suggesting that the prevalence of HPV antigen-primed cytotoxic T cells is dependent on tumor burden, which serves as the source of antigen (21). On the other hand, it is intriguing that these cells are unable to control HPV-infected cells in vivo despite retaining reactivity when stimulated ex-vivo. Taken together, these findings imply that HPV-associated cancers employ mechanisms mediating immune evasion and/or resistance. Indeed, several recent studies have examined HPV immune escape and regulatory pathways within the tumor microenvironment, which we have broadly classified into different categories described below. It is worth noting that many of these mechanisms have been studied using in vitro systems and as such the rate of their occurrence in vivo is variable.
1. Downregulation of MHC molecules to prevent cytotoxic T and NK cell activation
Activation of CD4+ and CD8+ T cells and T cell-mediated tumor killing are dependent on intact antigen presentation by antigen-presenting cells and tumor cells. As a result, tumors frequently harbor mutations in genes affecting the antigen presentation pathway of both major histocompatibility complex (MHC) class I and II molecules (22). As discussed above, GWAS identified mutations in the host genome at HLA loci that may affect predisposition and susceptibility to HPV-related malignancies. In addition, HPV modulates the antigen presentation pathway through its own gene expression program to prevent presentation of viral antigens on MHC class I and class II molecules during infection. The HPV E5 protein causes alkalization of late endosomes, preventing peptide-bound MHC class I and class II molecules from reaching the cell surface (Figure 1). Expression of E5 early in infection allows HPV to “hide” from anti-viral CD4+ and CD8+ T cells, which leads to increased viral persistence, replication, and spread to surrounding cells, contributing to malignant transformation (23).
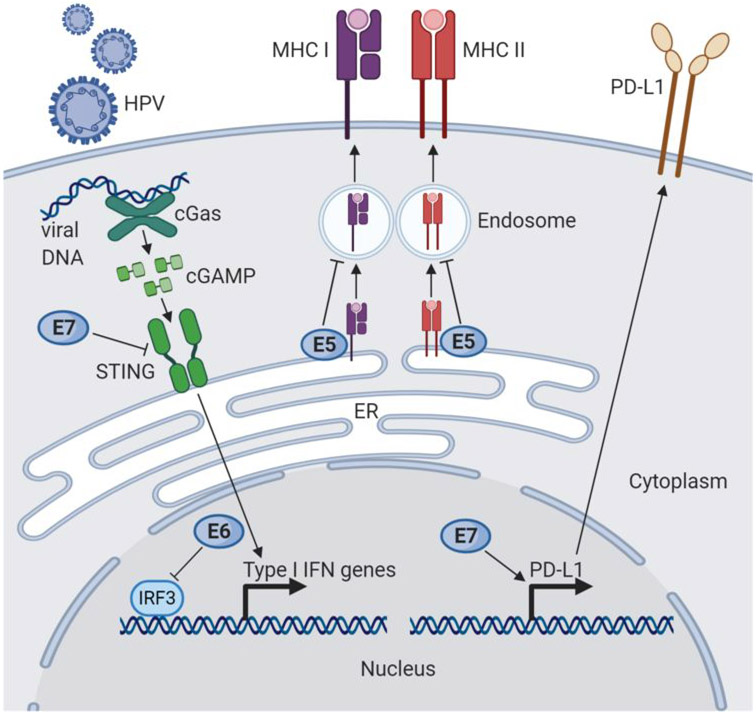
Figure 1: Cell-intrinsic mechanisms of HPV immune modulation.
Infection by HPV and expression of HPV proteins E5, E6, and E7 can lead to immune suppression, evasion, and resistance. HPV E7 has been shown to downregulate the cGAS STING pathway, an important innate response pathway to viral DNA that induces the expression of type I IFN genes, by directly inhibiting STING (left). HPV E6 can also dampen type I IFN gene expression by inhibiting the IFN regulatory factor IRF3. HPV E5 inhibits transport of MHC to the cell surface, which may be bound to either viral or tumor antigens, by alkalization of late endosomes. Finally, overexpression of E7 in a preclinical cervical cancer model has been shown to upregulate the PD-L1 immune checkpoint.
Expression of E5 within HPV-infected tumor cells may also prevent the presentation of non-viral tumor-associated antigens on MHC molecules and the activation of anti-tumor T cells (24). Overexpression of E5 in HNSCC cell lines renders them resistant to immune checkpoint blockade, likely through the acquired loss of antigen presentation. Interestingly, this effect can be reversed with rimantadine, an anti-viral medication that inhibits E5 and upregulates MHC class I in vitro (25).
Interestingly, natural killer (NK) cells, which normally recognize cells without surface MHC expression, did not kill HPV-infected cells in the above work. This is consistent with previous reports that HPV dysregulates specific HLA molecules and renders NK cells incapable of clearing virally-infected cells (26) (Figure 2). For example, HPV strongly down-regulates HLA-A, HLA-B, and HLA-C molecules, but upregulates HLA-E, which binds to the CD94-NKG2A inhibitory receptor on NK cells (24,27). However, cytotoxic NK cell activity does not seem to be completely abrogated in patients with advanced HNSCC, as patients with high CD56dim (cytotoxic NK cell phenotype) infiltration have improved survival (28). In addition, HPV-positive oropharyngeal carcinoma is associated with higher CD56+ cell infiltration than HPV-negative head and neck squamous cell carcinomas, and the level of CD56+ infiltration correlates with outcomes (29,30).
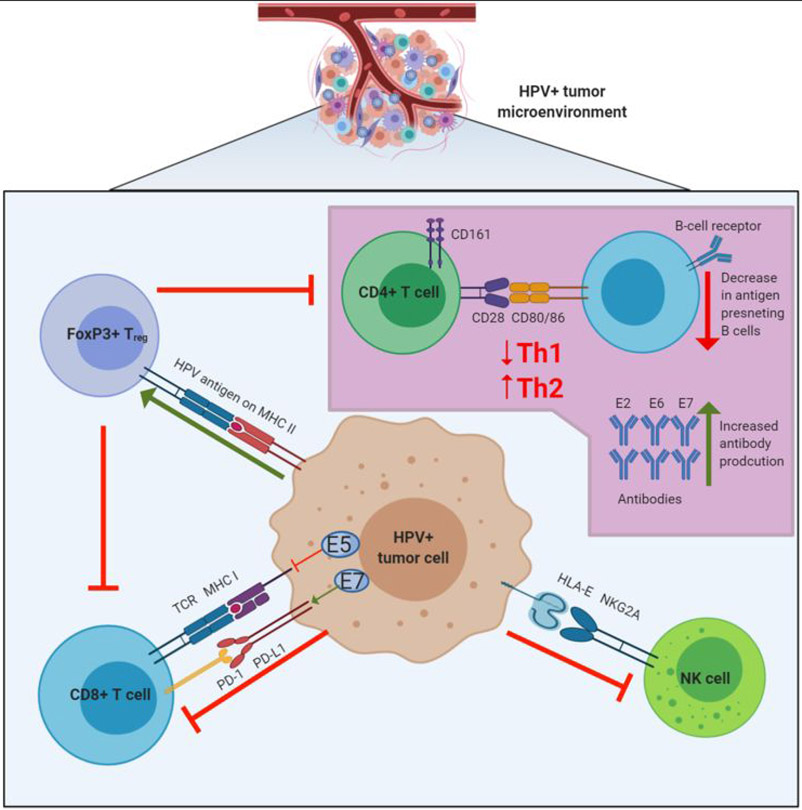
Figure 2: HPV immune modulation of the tumor microenvironment.
HPV infected tumor cells promote an immunosuppressive microenvironment through several mechanisms. Downregulation of antigen presentation on MHC by HPV E5 or mutations in antigen presentation pathway genes leads to decreased recognition by effector T cells. Upregulation of the PD-L1 immune checkpoint on infected tumor cells can lead to inhibition of cytolytic T cell activity. HPV also modulates HLA expression to engage NK cell inhibitory receptors, for example through the interaction of HLA-E molecules with NKG2A. There is also evidence of HPV-antigen specific FOXP3+ T regulatory cells, which function to suppress both CD8+ and CD4+ cells in the tumor microenvironment. CD4+ cells are typically skewed towards a Th2 response due to an upregulation of Th2 cytokines, which stimulates a humoral response. In some HPV-positive malignancies, a high frequency of antibodies specific to E2, E6, and E7 is also detected.
2. Modulation of CD4+ T-cells activation
Population studies have linked the depletion of CD4+ T cells in HIV/AIDS patients to the development of HPV-related malignancies. This finding was recapitulated in mice where CD4+ T cell depletion resulted in the failure of CD8+ T cells to mount an effective immune response against E7, which was required to maintain tumor control (31). Furthermore, in cervical cancer patients, CD4+ T cell response to HPV-derived peptide stimulation was impaired when compared to healthy controls suggesting an important role for CD4+-mediated immune surveillance (32). Data from clinical samples suggest that the phenotype, rather than absolute number of CD4+ T cells is critical for anti-HPV immunity. For example, cervical cancers display a surprising heterogeneity in the absolute number of CD4+ tumor infiltrates, with no correlation between the overall number of CD4+ T cells and survival. A subset of CD4+ T cells, CD4+CD161+ T cells, was positively correlated with survival, and cervical cancers overall had fewer CD4+CD161+ effector T cells than oropharyngeal cancers (33,34). In a similar study in HPV-related oropharyngeal cancers, the absolute number of CD4+ tumor infiltrating lymphocytes also did not appear to correlate with outcomes (35).
Interestingly, cervical cancer patients appear to have a skewed CD4+ TH cell response towards a TH2 response (humoral immunity) rather than TH1 (cell mediated immunity) (Figure 2). This appears to coincide with a decline in peripheral IFNγ levels (36). In cervical tumors, altered levels of cytokines favoring a TH2 response have been observed as well (32), but that has not been well characterized in HPV-positive HNSCC. In concordance with these studies, a TH1 response with CD161+ T cells and CD103+ T cells has been associated with better outcomes in HPV-positive oropharyngeal squamous cell carcinoma (33). Finally, a pro-tumorigenic IL-17-associated TH17 response mediated by stromal fibroblasts through the secretion of CCL20 has been recently described. This response was found to mediate progression of high-grade cervical neoplasia to invasive cancer. Whether this TH17 response persists in the invasive cancer stage and represents a therapeutic target is still unknown (37).
3. The inhibitory function of regulatory T cells
A subset of CD4+ T cells, Foxp3+ regulatory T cells (Treg), can suppress anti-tumor immunity by downregulating induction and proliferation of effector T cells. Tregs play an important role in dampening the host immune response in autoimmune diseases and viral infections. Interestingly, a high number of Tregs has been identified in cervical intraepithelial neoplasms (CIN) and cervical cancers, and the frequency of Tregs correlated with severity of disease, suggesting that Tregs may be associated with interference of anti-HPV immunity (38,39). This is further supported by studies demonstrating that tumor-infiltrating Tregs in cervical cancer patients exhibit specificity for HPV antigens (Figure 2). Interestingly, CD4+ lymphocytes expressing a regulatory phenotype with low proliferative response are found in the primary site, lymph node metastases, and peripheral blood in patients with cervical cancer, suggesting that the induction of immune tolerance is systemic and may promote metastatic spread (40).
A high number of infiltrating Tregs have also been found in HNSCC tumors; however, there are conflicting data whether this is more prevalent in HPV-positive vs HPV-negative HNSCC (41,42). Some data point to the ratio of Treg to CD8+ T cells as a predictor of clinical outcomes irrespective of HPV status in HNSCC (43,44). Moreover, an increased ratio of Tregs to CD3+ cells in the peripheral blood of HNSCC patients has been associated with a poorer outcome, consistent with other cancer types (45).
4. Modulation of the cGas-STING pathway
Activation of the cGas-STING pathway in response to cytosolic DNA is a conserved anti-viral pathway for sensing DNA viruses. Signaling via cGas-STING in response to viral-associated molecular patterns results in the expression of genes encoding type I interferons and pro-inflammatory cytokines. In turn, this leads to upregulation of cell adhesion molecules, co-stimulatory molecules, and MHC class I and class II molecules on tumor and stromal cells within the tumor microenvironment. The immunostimulatory environment results in the recruitment of both innate and adaptive immune effector cells and a highly cytotoxic T cell infiltration to clear the viral infection consistent with a Th1 response (46).
The role of cGas-STING in HPV-related cancers is beginning to be elucidated (Figure 1). In biochemical studies, HPV 18 E7 protein exerts a direct inhibitory action on STING through its LCXCE motif (47). However, there is little sequence homology between the E7 protein of HPV 16 and 18 and unlike HPV-18 E7, HPV-16 E7 modulates STING stability through the NOD-like receptor NLRX1 (48). Depletion of NLRX1 improved type I interferon- dependent T cell infiltration and tumor control in mice (48). More recent data demonstrated that in HPV 16-positive HNSCC cell lines, dampening of the cGas-STING pathway appears to be mediated by the LCXCE domain in HPV 16 E7 (49). Taken together, these data suggest that HPV E7 directly downregulates STING activation and mitigates the type I interferon response in a strain-specific manner, although differences in the mechanism across various tumor types have yet to be elucidated. Finally, HPV 16 E6 has also been demonstrated to inhibit the interferon regulatory transcription factor IRF3, an adaptor of STING, thereby independently decreasing type I IFN production (50).
Clinically, the data for the involvement of cGas-STING pathway are mostly correlative, and a positive association between STING expression and TILs is seen in tissue microarrays from diverse tumor types in the TCGA database. Furthermore, STING expression in tumors, but not the microenvironment, correlates positively with survival outcomes on multivariate analysis (48). One report found higher levels of STING mRNA in HPV-positive HNSCC patient samples as compared to HPV-negative HNSCC, indicating that while HPV oncoproteins may inhibit STING, STING expression in HPV-infected cells is still higher than non-infected cells. Whether or not the STING pathway was intact in these samples despite high levels of mRNA expression was not studied; however, STING agonism appeared to modulate cetuximab-mediated NK cell activation and resulted in tumor regression (51). While this hints at a potential role for STING agonism in augmenting immune directed therapy effects, standalone STING agonism strategies have yet to yield convincing data in early-phase trials. Further pre-clinical and clinical studies are needed to uncover a role for STING agonism in HPV-related malignancies.
5. Regulation of the PD1/PD-L1 immune checkpoint
The PD-1/PD-L1 immune checkpoint is a conserved inhibitory mechanism regulating the immune system in order to prevent auto-immunity. Multiple cancers, including HPV-related tumors, upregulate this checkpoint to facilitate immune tolerance, and inhibitors targeting both PD-1 and PD-L1 have been used successfully in the clinic to overcome this resistance mechanism (Figures 1, 2). Multiple studies have suggested HPV positivity increases PD-L1 on tumors and infiltrating immune cells, however this effect has been hard to disentangle from increased immune infiltrate in these malignancies. An initial analysis of 27 HNSCC tumors suggested that PD-L1 expression was higher in HPV-positive vs HPV-negative tumors (70% vs 29%), although HPV-positive tumors more frequently had TILs present (52). This study also identified PD-L1+ CD68+ tumor associated macrophages (TAMs) frequently in HPV-positive diseases. A subsequent larger study of 214 patients with oropharyngeal cancer showed 85.2% HPV-positive cancers expressed tumor PD-L1 staining compared with only 57.1% of HPV-negative cancers (53). Interestingly, there was no difference in PD-L1 staining of tumor cells at the tumor stromal interface between HPV-positive and HPV-negative tumors. This study also validated that HPV-positive tumors were more likely to have higher intensity of TIL infiltrate than HPV-negative tumors (>25% TIL, 85.2% vs. 51.1%) and TAMs were found to more likely be PD-L1 positive in HPV-positive disease (67.9% vs 49.6%). Of note, an earlier study of 133 patients with oropharyngeal cancer did not identify a difference in tumor PD-L1 expression between HPV-positive and HPV-negative tumors (71% vs 61%), although a much higher cut-off for PD-L1 positivity was used in this study (>20% of tumor cells staining PD-L1+) (54). In another study of 161 patients with HNSCC, 53.7% of HPV-positive cases had tumor surface PD-L1 expression, compared to 28.7% of HPV-negative cases, although this study included cancers from subsites of HNSCC other than the oropharynx (55). In this study, CD8+ TILs expressing PD-1 and PD-L1 were also quantitated and associated with overall survival. In the HPV+ cohort, CD8+ TILs with high expression of PD-L1 were associated with better overall survival than CD8+ TILs with low expression of PD-L1. Interestingly the expression of PD-1 on TILs was not correlated with survival. When PD-1 expression on TILs was analyzed by compartment, however, an association with survival was found for PD-1 expressing TILs in the tumor, as well as at tumor edge but not for stromal TILs expressing PD-1. The discrepancies between these studies that used immunohistochemistry is partially due to the absence of a standardized methodology for reporting PD-L1 expression, the absence of standardized thresholds for positivity, and known differences in staining between antibodies. More recently the combined positive score (CPS), a measure of the number of PD-L1 staining cells divided by the number of viable tumor cells multiplied by 100, has become a useful clinical parameter that predicts response to checkpoint inhibitors clinically (see section IV).
As discussed above, TILs are more abundant in HPV-positive tumors when compared to HPV-negative tumors. Since PD-L1 is a dynamic biomarker that gets upregulated in response to IFNγ secretion by TILs, the increase in PD-L1 expression in HPV-positive tumors may signify a more inflamed tumor microenvironment with recruitment of TILs rather than a direct causative effect of HPV on PD-L1 expression. This is supported by findings from a pooled analysis of the TCGA and MSK-IMPACT cohorts which showed that HPV positivity predicted a better response to immune checkpoint inhibitors in HNSCC, independent of PD-L1 expression level by immunohistochemistry. This effect was positively correlated with increased inflammatory gene expression program and infiltration of CD8+ T cells in the tumor microenvironment of HPV-positive HNSCC. Importantly, an increase in tumor mutational burden and neoantigens was not observed in these tumors compared to HPV-negative HNSCC (56).
HPV positivity is also associated with PD-L1 expression in cervical cancer, where a direct mechanism for HPV oncoproteins in upregulating PD-L1 may exist. In particular, expression of HPV E7, was found to be significantly associated with intra-tumoral surface PD-L1 expression. Overexpression of E7 in an HPV-negative epithelial carcinoma cell line increased PD-L1 expression, and knockdown of E7 in an HPV-positive cervical cancer cell decreased expression of PD-L1 (57). In these experiments, E7-induced PD-L1 expression led to increased CD8+ T cell dysfunction, and vice versa. In mice bearing HPV-positive tumors, administration of vaccines targeting the HPV E6 and E7 proteins leads to induction of tumor-specific T cells, but also results in upregulation of PD-L1 on tumor cells leading to poor tumor control (58). Taken together, these results in pre-clinical models are consistent with clinical data showing that PD-L1 expression in cervical cancer is associated with poor outcomes, likely secondary to cytotoxic T cell inhibition (59).
6. A possible role for the humoral response in regulating cell-mediated cytoxicity
Whilst the importance of the humoral response has been well-established in pre-clinical and clinical HPV preventive vaccine studies, the role of B cells in modulating an effective immune response against established HPV-associated cancers was previously poorly understood. The past decade has seen investigations exploring correlations of B-cells with clinical outcomes, whether they are more frequent in HPV-positive tumors, the type of B cells present in the microenvironment, and whether they are tumor-antigen specific. Early evidence for a role for the humoral response in anti-tumor immunity came from an analysis of 33 patient with HNSCC which identified a strong correlation between B cell infiltration in lymph node metastatic deposits and favorable outcomes (60). A subsequent study analyzing over 800 patients with cervical and HPV-related HNSCC from the TCGA identified the expression of B-cell genes CD19 and IGJ as associated with favorable clinical outcomes (61). Investigators in HNSCC have identified higher level of B-cell infiltrate in HPV-positive vs HPV-negative patients in a cohort of 38 patients samples; however, CD86+/CD21− antigen-presenting B cells were found at a lower proportion in HPV-positive tumors (62). Gene expression analysis of B cells from another small HNSCC patient cohort (N=23 patients) demonstrated a unique B cell-associated signature in HPV-positive cancer compared to HPV-negative tumors, which was further validated with patients from TCGA (63). Finally, single-cell RNA sequencing analysis of 26 patients with HNSCC has revealed an increased proportion of germinal center B cells, likely of tonsillar origin, in HPV-positive tumors, whereas HPV-negative tumors were found to harbor a relatively lower number of B-cells which were less likely to be in germinal center states that in HPV-positive cancers (64). Further work in HNSCC has demonstrated that these B-cells in the tumor microenvironment are enriched in HPV-specific antibody producing cells, and that these B-cells are not solely non-productive passengers as a consequence of inflammation (65). Surprisingly, this work revealed a stronger antibody response to E2 compared to E6 or E7, although this latter effect may be limited to HNSCC, given that E2 is more frequently lost in cervical malignancies. These later investigators also validated the presence of germinal-center state B-cells in the tumor microenvironment of HPV-positive cancers.
Pre-clinical work in murine models of HPV-related HNSCC has begun to evaluate how B-cells influence response to both anti-PD1, radiotherapy and combination treatment. Combination treatment was found to enhance the development of memory B cells, plasma cells, and antigen-specific B cells with B cell receptor sequencing showing increased clonality and somatic hypermutation (61). Taken together, these studies may elucidate a previously underappreciated role for B cells impacting clinical outcomes. The findings also raise multiple interesting questions that warrant further investigation: 1) How does the specific B cell gene signature in HPV positive cancers impact the tumor microenvironment and cell-mediated immunity? 2) Can the antigen-presenting role of B cells differentially activated during treatment mediate an increase in cell-based cytotoxicity? 3) Do antibodies produced by cancer-associated B cells target HPV and other tumor-associated antigens?
IV. Modulation of the immune system for targeting of HPV positive malignancies
Strategies to enhance the immune system’s recognition of HPV-related cancers include inhibition of immune checkpoints and methods to target viral antigens directly. We first review the evolution of immune checkpoint blockade in these cancers, which provides proof-of-concept that the immune system can be activated against this set of malignancies. Next, we review work in progress for antigen-specific immune targeting via two differing approaches: therapeutic vaccination and adoptive cell therapy.
1. Immune Checkpoint Blockade
Immune evasion and resistance in HPV-positive malignancies has led to a natural interest in applying established immunotherapies to these cancers. Immune checkpoint blockade (ICB) is the most rigorously tested strategy to date, with the largest studies in HPV positive cancers occurring in HNSCC. Table 1 summarizes the major clinical trials that have shaped our understanding of how to employ ICB for HPV-positive malignancies. Many of these trials did not restrict participation by HPV status and as such some HPV-negative patients did participate, complicating the interpretation of the results. In recurrent platinum-refractory HNSCC, CHECKMATE-141, a phase III trial of single agent nivolumab versus standard therapy showed a significant improvement in overall survival with nivolumab (HR of 0.70 with a 1-year survival rate of 36% vs. 16%) (66). Similar results in the second line setting in HNSCC were reported in the KEYNOTE-040 trial, a phase III study comparing pembrolizumab with standard of care therapy, with an overall survival benefit in the pembrolizumab arm (HR of 0.80 and a 1-year survival rate of 37% vs. 26%) (67). Both studies included HPV-positive and HPV-negative patients. Exploratory subgroup analyses from CHECKMATE-141 suggested that the effect of anti-PD-1 may be larger in HPV-positive tumors (median OS of 9.1 vs. 4.4 months, compared to 7.5 vs. 5.8 months in HPV-negative tumors); however, an improved benefit in the HPV-positive subgroup was not observed in KEYNOTE-040 trial (66,67). Finally, KEYNOTE-048 was a phase III randomized trial of single agent pembrolizumab versus pembrolizumab and chemotherapy versus cetuximab and chemotherapy in the first line setting for recurrent/metastatic HNSCC. The study demonstrated the superiority of pembrolizumab alone or in combination with chemotherapy compared to standard of care chemotherapy, regardless of HPV status (68). Altogether these results indicate activity of anti-PD-1 agents in HNSCC, although it is unclear whether there is a differential effect on HPV-positive versus HPV-negative tumors. The overall increased sensitivity of both HPV+ and HPV-HNSCC to immune checkpoint blockade as compared to standard of care chemotherapy-based regimens likely represents multiple distinct mechanisms promoting inflammation and immunogenicity. HPV+ HNSCC tumors typically have a low tumor mutational burden; however, viral antigens act as strong immunogens leading to HPV antigen-specific CD8+ T cell infiltration. On the other hand, HPV – HNSCC tumors feature moderate to high tumor mutation burden which likely enriches for neo-antigen-specific CD8+ T cells. This is akin to Merkel cell carcinoma, where both Merkel cell polyomavirus-positive and -negative tumors respond to immune checkpoint blockade (69).
Table 1:
Summary of resulted/ongoing immune checkpoint blockade trials in HPV-related malignancies
| Trial | Phase | Stage | Comparison | Results |
|---|---|---|---|---|
| Resulted | ||||
| HPV+ OPC* | ||||
| CHECKMATE-141 (66) | 3 | Recurrent platinum-refractory | Single agent Nivolumab vs standard (Methotrexate, Docetaxel or Cetuximab) | 1-year survival rate of 36% for nivolumab vs 16% for standard therapy |
| KEYNOTE-40 (67) | 3 | Recurrent platinum-refractory | Single agent Pembrolizumab vs standard (Methotrexate, Docetaxel or Cetuximab) | 1-year survival rate of 37% for pembrolizumab vs. 26% for standard therapy |
| KEYNOTE-48 (68) | 3 | Recurrent | Single agent Pembrolizumab vs Pembrolizumab + Cisplatin and 5FU vs Cetuximab + Cisplatin and 5FU | Overall survival benefit in Pembrolizumab + chemotherapy vs Cetuximab + chemotherapy in study population (13 months vs 10.7 months). Pembrolizumab alone superior to Cetuximab + chemotherapy in patients with CPS>1 |
| EAGLE (76) | 3 | Recurrent | Single agent Durvalumab vs Durvalumab + Tremelimumab vs standard (Cetuximab, Taxanes, 5FU or Methotrexate) | No statistically significant overall survival benefit. 1-year survival of 37% in Durvalumab monotherapy arm, 30.4% in the combination arm, 30.5% in standard arm |
| GORTEC 2015-01 (PembroRad) (78) | 2 | Locally advanced non-platinum candidates | Cetuximab + RT vs Pembrolizumab + RT | No difference in locoregional control (59% in the Cetuximab-RT arm vs 60% in pembrolizumab-RT). No difference in progression free survival or overall survival. Acute toxicity lower in the pembrolizumab arm (74% vs 92%) |
| Cervical Cancer | ||||
| KEYNOTE-158 (71) | 2 | Recurrent/Metastatic | Single agent Pembrolizumab (2 arms with 2 different doses) | Overall response rate of 12.2%, 83.7% of tumors PD-L1 positive with CPS***>1 |
| CHECKMATE-358 (72) | 1/2 | Recurrent/Metastatic | Non-comparative multi arm single agent nivolumab or in combination with other immunotherapies | Objective response rate of 26.3% for cervical cancers with nivolumab monotherapy and 40% for nivolumab + ipilimumab |
| Anal Cancer | ||||
| NCI9673 (74) | 2 | Recurrent/Metastatic | Single arm phase 2 trial of single agent Nivolumab | Overall response rate of 24% |
| KEYNOTE-28 (75) | 1b | Locally advanced/metastatic | Single arm phase1b trial of single agent Pembrolizumab | Overall response rate of 17% |
| Completed enrollment/Enrolling | ||||
| HPV+ OPC | ||||
| JAVELIN HN100 | 3 | Locally advanced (Stage III/IV) | Avelumab + CRT** vs CRT** alone | Completed accrual but closed early due to interim analysis showing boundary of futility crossed |
| KEYCHAIN | 2 | Locally advanced p16+ | RT + Pembrolizumab vs CRT* | Pending (NCT03383094) |
| HN-005 | 2/3 | Stage I-III p16+ | CRT** vs de-intensified RT + Cisplatin vs de-intensified RT + Nivolumab | Pending (NCT03952585) |
| HN-004 | 2/3 | Locally advanced non-platinum candidates | RT + Durvalumab vs RT + Cetuximab | Pending (NCT03258554) |
| Cervical Cancer | ||||
| CALLA | 3 | Locally advanced | Cisplatin+RT+Durvalumab vs Cisplatin+RT | Pending (NCT03830866) |
| 2 | Locally advanced | Cisplatin+RT+pembrolizumab vs Cisplatin+RT | Pending (NCT02635360) | |
| BEATcc | 3 | Recurrent/Metastatic | Cisplatin + Paclitaxel +Bevacizumab + Atezolizumab vs Cisplatin+ Paclitaxel+ Bevacizumab | Pending (NCT03556839) |
| KEYNOTE-826 | 3 | Recurrent/Metastatic | Cisplatin+ Paclitaxel + Bevacizumab + Pembrolizumab vs Cisplatin + Paclitaxel + Bevacizumab | Completed accrual (NCT03635567) |
| Cemiplimab | 3 | Recurrent/Metastatic | Cemiplimab vs chemotherapy | Completed accrual (NCT03257267) |
| RaPIDS | 2 | Recurrent/Metastatic | AGEN2034 +/−AGEN1884 | Pending (NCT03894215) |
| SKYSCRAPER-04 | 2 | Recurrent/Metastatic | Tiragolumab+Atezolizumab | Pending (NCT04300647) |
OPC: Oropharyngeal cancer
CRT: Chemoradiotherapy
CPS: Combined positive score
The evidence of efficacy for ICB in other HPV-positive malignancies is less well established due to small single arm studies, rather than randomized controlled trials. In cervical cancer, current standard of care chemotherapies for recurrent/metastatic disease carry dismal outcomes (70). KEYNOTE-158 was a basket phase II trial which tested pembrolizumab in a variety of solid tumors. In this trial, 98 patients with cervical cancer were treated with 12 patients achieving an objective response (3 complete responses), of note responses were only seen in PD-L1-positive tumors. The responses were durable and led to the accelerated approval of pembrolizumab by the FDA for advanced PD-L1-positive cervical cancers (71). These results were similar to the phase I/II CHECKMATE-358 trial, a multi-cohort study of nivolumab with or without ipilimumab for virus-associated cancers regardless of PD-L1 expression. In the monotherapy arm of this trial, 19 cervical cancer patients and 5 vulvar/vaginal cancer patients were treated with response rates of 26.3% and 20%, respectively, and median duration of response was not reached for these patients (72). The combination of ipilimumab and nivolumab appeared to increase the response rate, particularly in patients that had not previously received chemotherapy in the advanced disease setting, with a 36% response rate in previously treated and 46% response rate in untreated patients (73).
In anal squamous cell carcinomas, The NCI9673 phase II trial enrolled 37 patients with treatment-refractory metastatic disease to receive nivolumab, with 9 patients responding, 2 of which achieved complete responses (74). Similarly, KEYNOTE-028 enrolled 24 patients with advanced anal squamous cell cancer onto pembrolizumab, and the observed response rate was 17% with a median overall survival of 9.3 months (75). Based on these early single-arm studies, checkpoint inhibitors in the treatment of recurrent/metastatic anal cancer are currently part of the NCCN guidelines.
Lastly, multiple trials have explored the combination of ICB as well as the use of ICB in earlier settings in HNSCC. For example, the EAGLE Trial, a randomized phase III clinical trial in advanced HNSCC, investigated the role of tremelimumab (anti-CTLA4) and durvalumab (anti-PD-L1) therapy. Unfortunately, neither combined ICB therapy nor single agent durvalumab were superior to standard of care, although this may have been due to an unexpected high survival rate in the standard of care arm (76).
In the locally advanced setting, a number of studies are currently evaluating combination of chemoradiation (CRT) with PD-1 or PD-L1 blockade in the upfront setting for locally advanced cervical cancer patients, including CALLA (durvalumab), NCT02635360 (pembrolizumab), and NRG-GY017 (atezolizumab) trials. In locally advanced HNSCC, JAVELIN HN100, a phase III trial of CRT versus CRT plus avelumab (anti-PD-L1) completed accrual but was stopped early when the trial showed that the primary endpoint of progression-free survival was not met. The addition of avelumab did not improve PFS or OS for locoregionally advanced non-metastatic HNSCC with approximately one third of the entire cohort comprised of HPV-positive HNSCC (77). A similar study to JAVELIN 100, KEYNOTE-412 (pemrolizumab) was also closed to accrual, and results have not yet been reported. The PembroRad phase III GORTEC trial was recently reported in ASCO 2020, which showed that pembrolizumab was not superior to cetuximab for cisplatin-ineligible patients with HNSCC (78) Other trials evaluating the concurrent use of immunotherapy with RT in the upfront setting are still actively recruiting and include KEYCHAIN (pembrolizumab for patients with p16+ HNSCC) and HN-005 (nivolumab with de-intensified RT for early stage HPV-positive oropharyngeal carcinoma). Finally, HN-004 is a phase III trial of cetuximab versus durvalumab in locally advanced head and neck cancer in cisplatin-ineligible patients. Further work is needed to elucidate the mechanism explaining the lack of improvement of anti-PD-1/PD-L1 therapy in combination with radiation therapy for HNSCC.
2. Therapeutic vaccination strategies:
Preventive vaccines against HPV utilize capsid proteins from multiple high-risk HPV strains as a means to generate a neutralizing antibody response to prevent subsequent HPV infection. Antibody-generating HPV vaccination has proven to be a successful measure to prevent HPV-related malignancies in healthy individuals by reducing the risk of developing cancer by half (79). On the other hand, since pathogenesis of HPV-related malignancies is closely tied to sustained expression of the viral oncogenic E6 and E7 proteins, the majority of therapeutic HPV vaccines in the setting of pre-invasive or invasive disease have targeted these antigens derived from these proteins. Therapeutic HPV vaccines utilized in clinic have used differing technologies to deliver HPV-related antigens as well as differing adjuvants to stimulate an immune response. Below, we review vaccination strategies for the platforms most frequently tested in clinic, namely, peptide-based, vector-based (bacterial or viral), and DNA-based, and subsequently we discuss lessons learned. For each platform, we first highlight key studies in the pre-invasive arena before discussing results in developed cancers. A summary of vaccination studies in invasive tumors is presented in table 2, 3 and 4 while a presentation of relevant studies in the pre-invasive setting is presented in supplementary table 1..
Table 2:
Summary of ongoing/resulted peptide vaccine trials for invasive HPV-associated malignancies
| Vaccine | Vector | Antigen | Disease | Study | Results |
|---|---|---|---|---|---|
| DPX-E7 | No vector | HPV16 E711-19 | HPV+ OPC*, Cervix, anus | Single arm phase I trial in relapsed/metastatic anal, cervical and HPV+ OPC* | Pending (NCT02865135) |
| ISA 101 | No vector | 12 peptides 25-35 amino acids spanning HPV-16 E6 and E7 | HPV+ OPC* | Single arm phase II trial of ISA 101 + Nivolumab in recurrent/relapsed HPV+ OPC* | Pending (NCT02426892) |
| HPV+ OPC* | Single arm Phase II of ISA 101 + Cemiplimab | Pending (NCT04398524) | |||
| HPV+ OPC* | Randomized study of Cemiplimab +/− ISA 101 | Pending (NCT03669718) | |||
| HPV+ OPC* | Single arm Phase II of ISA 101 + Utomilumab | Pending (NCT03258008) | |||
| HPV+ OPC* | IMRT + Pembrolizumab + Cisplatin + ISA 101 | Pending (NCT04369937) | |||
| Cervix | Phase I/II of ISA 101 with carbo/paclitaxel +/− bevacizumab | Pending (NCT02128126) | |||
| Cervix | Phase II Study of Cemiplimab after POD on 1st line therapy | Pending (NCT02128126) | |||
| Hespecta | No vector | HPV16 E6 conjugated to synthetic TLR2 ligand | HPV+ tumors | Phase I study of Hespecta in HPV+ tumors | Pending (NCT02821494) |
| PepCan | No Vector | HPV 16 E6 peptides | HPV+ OPC* | Phase I/II double blinded randomized study of PepCan vs placebo | Pending (NCT03821272) |
| P16_37-63 | No Vector | P16INK4a-37-63 | HPV+ tumors | Pilot study of cisplatin + P16_37-63 | Pending (NCT02526316) |
| HPV+ tumors | Phase I/IIA of P16_37-63 + Montanide ISA-51 VG in advanced HPV+ tumors | Pending (NCT01462838) | |||
| PDS0101 | No Vector | Liposomal HPV16 E6 and E7 peptides | HPV+ HNSCC | Phase II trial of pembrolizumab + PDS0101 in recurrent/metastatic HPV+ HNSCC | Pending (NCT04260126) |
| Cervix | Phase IIA trial of chemoradiation + PDS0101 in stage IB3-IVA cervical cancer | Pending (NCT04580771) | |||
| HPV+ tumors | Phase I/II trial of PDS0101 + Anti-PD-L1/TGF-beta trap (M7824) + IL12 recurrent/metastatic HPV+ tumors | Pending (NCT04287868) |
OPC: Oropharyngeal cancer
Table 3:
Summary of ongoing/resulted viral/bacterial vector vaccine trials for invasive HPV-associated malignancies
| Vaccine | Vector | Antigen | Disease | Study | Results |
|---|---|---|---|---|---|
| ADXS11-001 * | Listeria Monocytogenes | HPV-16 E7 | Anus (90) | Single arm phase II trial of IMRT + Mitomycin + 5-FU + ADXS11-001 | 8/9 (88.9%) patients were progression free at 42 months (NCT01671488) |
| Cervix (91) | Randomized phase II trial of ADXS11-001 +/− Cisplatin | No difference in both arms. Median overall survival of 8 months in both arms (India Trial Registry: CTRI/2010/09/001232) | |||
| Cervix (92) | Single arm phase II study in women with recurrent/metastatic or poor prognosis of ADXS11-001 | Median OS 6.1 months, (1-year OS ~ 38% vs 21% in historical controls). Median PFS 2.8 months (NCT01266460) | |||
| Cervix | Phase III: Randomized study of ADXS11-001 vs. Placebo after CCRT for definitive treatment of cervical cancer | Study closed early, results pending (NCT02853604) | |||
| HPV+ OPC** | Single arm window of opportunity neoadjuvant ADXS11-001 prior to surgery | Pending (NCT02002182) | |||
| TG4001 | Modified vaccinia (MVA) | HPV-16 E6 and E7 + IL2 | HPV+ OPC** | PhaseIb/II single arm trial of TG4001 + Avelumab in recurrent/metastatic setting | Pending (NCT03260023) |
| MG1-E6E7 | Adenovirus vector and Maraba boost | HPV16 E6 and E7 | HPV+ OPC** + Cervix | Dose escalation study with Atezolizumab | Pending (NCT03618953) |
| TheraT | LCMV and Pichinde virus vector | HPV16 E6 and E7 antigens | HPV+ OPC** | Multi-arm dose expansion trial of TheraT in combination with anti-PD-1 therapy | Pending (NCT04180215) |
| PRGN-2009 | Gorilla Adenovirus | Epitopes of HPV 16/18 E6 and E7 | HPV+ tumors | Phase I/II trial of PRGN-2009 +/− Anti-PD-L1/TGF-beta trap (M7824) in recurrent/metastatic HPV+ tumors | Pending (NCT04432597) |
ADXS11-001: Additional small phase I/II studies include: NCT02164461, NCT02002182, NCT02399813, NCT02291055
OPC: Oropharyngeal cancer
Table 4:
Summary of ongoing/resulted DNA/RNA based vaccine trials for invasive HPV-associated malignancies
| Vaccine | Vector | Antigen | Disease | Study | Results |
|---|---|---|---|---|---|
| MEDI-0457 (previously INO-3112) | VGX-3100 plasmid and INO-9012 plasmid | Modified E6 and E7 peptides + IL12 | HPV+ HNSCC | Phase I/II trial of MEDI-0457 with durvalumab in ecurrent/metastatic HPC+ HNSCC | Pending (NCT03162224) |
| HPV+ OPC* | Phase II trial of observation vs durvalumab vs MEDI-0457 + durvalumab in high risk HPV+ OPC | Pending (NCT04001413) | |||
| HPV+ tumors | Phase II trial of MEDI-0457 + durvalumab in recurrent/metastatic HPV+ tumors | Pending (NCT03439085) | |||
| HPV+ HNSCC | Phase I/II of concurrent INO-3112 delivered by electroporation with surgery or chemoradiation | Pending (NCT02163057) | |||
| Cervix | Phase I/II of INO-3112 delivered by electroporation following chemoradiation or in recurrent setting | Pending (NCT02172911) | |||
| GX-188E | Pgx27 plasmid | HPV16 and 18 E6 and E7 + FLT3 | Cervix (97) | Phase II study of pembrolizumab + GX-188E in recurrent cervical cancer | 11/26 patients with response by RECIST, 4 complete at 24 weeks (NCT03444376) |
| VB10.16 | Proprietary | HPV 16 E and E7 | HPV16+ Cervix | Phase II study of atezolizumab + VB10.16 in recurrent HPV16+ cervical cancer | Pending (NCT04405349) |
| HARE-40 | No vector | HPV mRNA + anti-CD40 | HPV+ tumors | Phase I/II study of HARE-40 in upfront OPC in IA arm and recurrent HPV+ tumors in IB arms | Pending (NCT03418480) |
| BNT113 | No vector | HPV16 E6 & E7 | HPV+ OPC* | Randomized study of Pembrolizumab +/− BNT113 in CPS >1 pts | Pending (NCT04534205) |
OPC: Oropharyngeal cancer
Peptide-based vaccines directly deliver short or long peptides encoding HPV-oncoproteins. These have been shown, however, to be weakly immunogenic and need to be delivered with adjuvants to improve potency of both cellular and humoral immunity. As short peptides are HLA-restricted, preclinical work established that HPV16 E712-20 and E786-93, two peptides computationally predicted to bind to HLA A*0201, bind in vitro using T2 assays, and also generate immune responses in mice that clear the HPV virus (80,81). Building on these findings, the first trial to demonstrate efficacy of a therapeutic vaccine in HPV-related disease was a phase Ib trial of these HLA-restricted peptides (i.e E712-20 and E786-93) conducted on 18 patients with high grade vulvar or cervical lesions. Ten patients in this study received HPV 16 E712-20 emulsified with complete Freund’s adjuvant and 8 patients received HPV 16 E786-93 along with an additional lipopeptide consisting of a linker peptide, the T cell helper peptide PADRE-965. Nine of 17 evaluable patients had clinical regression by colposcopy measurements, of which 3 patients had complete regression. Correlative studies identified that 15/18 patients had diminished or eliminated HPV viral DNA by PCR, and 10/18 patients showed increased T cell activation by cytokine release assays. In addition, a limited examination of histologically available tissue showed an increased infiltration of dendritic cells which likely suggests increased antigen presentation (82).
Unlike short peptide vaccines, long peptide vaccines avoid the need for HLA restriction as the longer peptides are processed intracellularly before being presented on MHC molecules. TA-CIN is a synthetic fusion protein of HPV 16 L2, E6 and E7, which has been combined with sequentially delivered imiquimod, a topical TLR7/8 agonist in patients with vulvar intraepithelial neoplasia (VIN). A phase II trial demonstrated robust complete regression of VIN in 12/19 patients receiving TA-CIN and imiquimod at 52 weeks. Correlative analysis identified a marked increase in CD4+ and CD8+ T cell infiltrates in responders while an increase in Treg cells was seen in non-responders. Systemic circulating T cells specific to HPV antigens was detected only in responders (83).Similar results were seen in a phase II trial using overlapping HPV 16 E6 and E7 synthetic long peptides with incomplete Freund’s adjuvant (ISA 101) to treat women with VIN, where 15/19 patients had clinical regression of their lesions, of which 9 had complete regression. These regressions were correlated with a peripheral induction of CD4+ and CD8+ T cells (84).
Given the robust antigen-specific T cell responses of the peptide vaccines in the pre-invasive setting, a strong rationale exists for their addition to ICB in invasive cancers to enhance T cell responses. ISA 101 is being evaluated in a number of single arm studies, in both the localized and metastatic setting (e.g. NCT03669718, NCT04369937, NCT04646005, NCT04398524, NCT02426892) and in a randomized phase II study (NCT03669718). An early readout of a single center phase II trial of ISA 101 with nivolumab in 24 patients with incurable HPV-related cancers (22 of them were oropharyngeal) showed a response rate of 33% by RECIST (25% partial responses and 8% complete responses) with the average response lasting around 10 months (85). Lastly, a short peptide vaccine to HPV 16 E711-19 (DPX-E7), similar to the one used by Muderspach et al. in their pre-invasive study, is now being investigated in an early phase I trial of HLA A*0201 positive patients with oropharyngeal, cervical and anal cancers (NCT02865135).
Vector-based vaccines are genetically engineered live attenuated or inactive, either viral or bacterial vectors modified to express an antigen of interest. Their appeal in vaccine technology stems from the ease of their generation, scalability, as well as their ability to faithfully produce large amounts of antigens in vivo and elicit a strong immune response. The risk of producing the original disease of the vector, especially in live attenuated vectors, does exist. In the pre-invasive setting, a prime-boost vaccination regimen with a DNA vaccine expressive HPV E7 followed by a recombinant vaccinia boost expressing HPV16 and 18 E6 and E7 showed marked T cell expansion and formation of tertiary lymphoid tissue in mucosal lesions in patients with CIN2/3 indicating an immune response (86). TG4001 is another recombinant vaccine composed of a modified vaccinia vector expressing mutated HPV 16 E6 and E7 proteins and the stimulatory T cell cytokine IL-2. Data from a single arm phase II trial of 21 patients with CIN2/3 showed a 48% response rate by colposcopy (87).
Based on promising results in pre-invasive lesions, the TG4001 is being further evaluated in a phase Ib/II study with avelumab in recurrent/metastatic oropharyngeal cancers (NCT03260023). A heterologous prime-boost strategy using adenovirus-based prime and Maraba virus-based boost against HPV 16/18 E6 and E7 antigens has reported significant activity in preclinical models, and a clinical trial is currently ongoing (NCT03618953) (88). Finally, an analogous prime-boost vaccine strategy employing two replication-attenuated arenaviruses (lymphocytic choriomeningitis and Pichinde virus) expressing a HPV 16 E6 and E7 fusion protein is being tested in monotherapy and combination therapy regimens, including in combination with anti-PD-1 therapy, in a phase I/II trial of HPV-positive HNSCC (NCT04180215).
One of the most thoroughly studied vector-based vaccines is Axalimogene filolisbac (ADXS11-001), a live attenuated Listeria monocytogenes vaccine that secretes the endogenous Listereolysin O protein fused with the HPV 16 E7 protein. Studies of this vaccine encountered hurdles, including a temporary hold by the FDA following the development of systemic listeriosis in a patient enrolled on a phase I trial for oropharyngeal cancers (89). To date, only phase II results have been reported. These include a 10-patient phase II trial of ADXS11-001 with radiation and mitomycin/5-FU for locally advanced anal cancer. The rationale behind adding the vaccine to chemoradiation was to augment the immune response to the vaccine by combining its administration with radiation. This was the first trial combining therapeutic HPV vaccination with radiation, and it demonstrated the safety of their co-administration. It should be noted, however, that no serologic or T cell analysis was performed on these patients to validate whether or not radiation did actually enhance the HPV-specific immune response (90). A larger phase II trial in India of ADXS11-001 +/− cisplatin for the treatment of recurrent/refractory cervical cancer demonstrated a median survival of about 8 months in both arms. Interestingly, the 12 and 18 months overall survival rates were 1.5-2 times higher than historical rates on GOG trials, however the failure to include a cisplatin-only arm meant that the superiority of ADXS11-001 monotherapy to cisplatin could not be established (91). Since then, the standard of care for recurrent cervical cancer has moved to include doublet platinum-based chemotherapy and bevacizumab when tolerated. A follow-up study of ADXS11-001 in 50 patients with advanced platinum-refractory cervical cancer demonstrated a 12-month overall survival of 38%, which compared favorably to the historical 12-month survival rate of 21% (92). The study did not include correlative analyses; thus, it is unclear whether the clinical benefit observed on the study correlated with an HPV-specific T cell response. The study paved the way for a phase 3 study of ADXS11-011 vs placebo following chemoradiation in cervical cancer patients at high risk of recurrence (AIM2CERV). The study was initially put on hold by the FDA due to an inquiry into manufacturing procedures before being closed in 2019 based on the funding company priorities and before full accrual was reached. A window of opportunity trial of neoadjuvant ADXS11-001 prior to resection of HPV-positive oropharyngeal cancer (NCT02002182) is currently underway.
Finally, DNA vaccination is a strategy which incorporates an antigen-encoding gene into a backbone of a bacterial plasmid. Its main advantage is its ability to activate both innate and adaptive immune responses. Upon its injection, the bacterial plasmid transfects myocytes and triggers the expression of the antigen. The bacterial plasmid contains unmethylated CpG motifs which act as adjuvants and trigger a robust dendritic-cell mediated TLR9-dependent immune response. These dendritic cells subsequently act as antigen presenting cells and activate the adaptive immune response. The variability of the immune response to DNA vaccine (stronger in mice than in humans) is partially attributable to the expression of TLR9 on immune cells (93,94). A recent phase II trial for VGX-3100 is currently accruing (NCT03185013). The construct pNGVL3a-Sig/E7-detox-Hsp70 is a DNA vaccine which produces E7 protein bound to heat shock protein 70. While preclinical models were encouraging, data from a phase I trial of 15 patients with pre-clinical CIN 2/3 showed no effects in regression of CIN as compared to the unvaccinated cohort, and the vaccine failed to elicit a detectable T cell response (95).
In invasive tumors, early phase clinical trials with DNA vaccines are beginning to report outcomes. A phase Ib/II safety data trial of MEDI0457, a DNA based vaccine, in conjunction with ICB showed a robust induction of antigen-specific T cells in p16+ HNSCC in 18/21 patients. The cellular responses persisted out to a year on the study and flow cytometric analysis showed induction of HPV16-specific PD-1+ CD8+ T-cells (96). More recently, an interim analysis of a single arm phase II trial of a DNA vaccine encoding the E6/E7 protein of HPV 16 and 18 in a vector encoding the adjuvant FLT3L (branded GX-188E) with pembrolizumab in recurrent/advanced cervical cancer showed 11/26 patients with response, 4 of which were complete at 24 weeks as assessed by RECIST (97). This provides a rationale for further exploring combination of vaccines and ICB to potentiate T cell specific responses clinically.
In summary, advances in therapeutic vaccination strategies have allowed for the production of highly immunogenic vaccines. The data from studies on pre-invasive lesions and early phase invasive clinical trials show that these vaccines are generally safe. Immunologic correlative assays clearly demonstrate an expansion of antigen-specific adaptive immune cells, particularly when combined with ICB. However, single arm studies remain difficult to fully interpret. Ongoing randomized trials (e.g NCT04534205 and NCT03669718) evaluating the efficacy of different vaccine platforms will be necessary to determine the clinical utility of this approach.
3. Adoptive cell therapy:
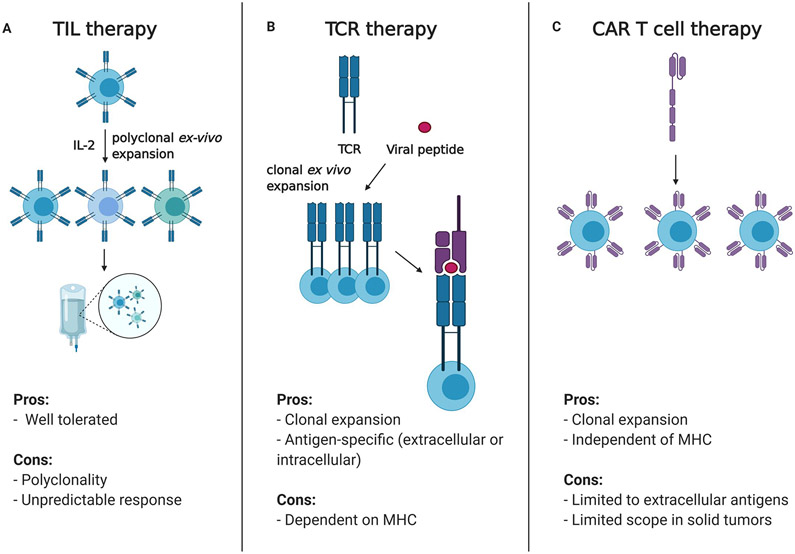
Adoptive cell therapy presents an attractive immunotherapy strategy for HPV-related malignancies since HPV viral antigens are tumor-specific and may be uniformly shared amongst patients with these cancers. Adoptive T cell therapies involve the collection, expansion, selection, and manipulation of T cells from a patient ex vivo, followed by re-infusion into patients. The re-infusion is typically preceded by a myeloablative chemotherapeutic regimen to facilitate engraftment. Three main modalities of adoptive cell therapy include tumor-infiltrating lymphocytes (TILs), genetically modified T cell receptors (TCRs) and T cells with chimeric antigen receptors (CAR T-cells) (Figure 3).
Figure 3: Strategies of adoptive cell therapy in HPV-positive malignancies.
A. Polyclonal TILs are collected from patients, expanded ex-vivo and re-infused into patients. B. An HLA matched, antigen-specific T-cell receptor is identified and cloned and infused into patients for TCR therapy. TCRs in this approach are highly dependent on the patient HLA haplotype C. CAR T cells are dependent on extracellular antigens and circumvent the HLA matching problem seen with TCR based therapies
TIL therapy comprises non-clonal ex vivo expansion of a heterogeneous population of lymphocytes isolated from a surgically resected specimen. Initial trial of infusion of these cells to 9 patients with metastatic cervical cancer showed durable complete responses in 2/9 patients and one partial response in 1/9 patients (98). Interestingly, T cell receptor sequencing from the 2 complete responders revealed T cell reactivity against neo-antigens as well as germline antigens rather than antigens derived from the known HPV oncoproteins (99). A larger phase II trial of adoptive T cell therapy was conducted at the NIH on 28 patients with HPV related cancers, 18 cervical and 11 non-cervical, and response was seen in 28% and 18% of patients, respectively. In this study, it was noted that peripheral blood re-population with HPV-reactive T cells correlated with clinical response (100). C-145-04 is an ongoing phase II trial of TIL therapy open to locally advanced, recurrent or metastatic cervical cancers, and interim analysis presented at ASCO 2019 showed a response rate of 44% in the 27 treated patients with an acceptable safety profile (101).
TCR-engineered T cells are generated by transduction of T cells with a single T cell receptor (TCR) demonstrated to recognize a specific tumor antigen in an HLA-dependent manner. Given the reliance of this technology on TCR-MHC pairing, HLA matching of patients is required for this approach. A phase I/II clinical trial using autologous T cells genetically engineered to express a T cell receptor targeting an HLA-A*02:01-restricted epitope of HPV-16 E6 was recently conducted. Twelve patients were enrolled on the trial and 9 received the highest T cell dose. Of these 9 patients, 2 patients with anal cancer responded (1 complete, 1 partial). Interestingly, responders showed increased E6 TCR T cell presence in resected specimens for several months post-therapy. Genomic analysis of non-responders showed mutations in either antigen recognition pathways (HLA mutations) or interferon response (102). Another phase I study from the NIH demonstrated a slightly better response rate with autologous TCR T cells engineered to target HPV 16-E7 (response in 4/12 patients) (103). The patients on the trial included 5 cervical cancers, 4 HNSCC, 2 anal and 1 vulvar cancers. The updated results from this study show response extended to 6/12 patients by RECIST with a good persistence of the engineered T cells. Genomic analysis in one patient showed mutations in HLA A*0201 to be a late mechanism of resistance to therapy (104). Further trials exploring HPV-specific TCRs are currently being conducted in the United States (NCT02379520) and abroad (NCT03578406).
CAR T cell therapy in HPV-mediated cancers is of interest as it targets surface antigens and thus circumvents the need to target MHC-bound antigens, overcoming defects in the antigen presentation pathway seen in HPV-related malignancies. However, the identification of recurrent and unique cell surface antigens outside of MHC-bound viral antigens in HPV-related malignancies presents a major challenge to this approach. Promisingly, a recent phase I study assessing the safety of CAR-T cells engineered against ErbB in HNSCC showed no dose limiting toxicities with 69% patients demonstrating stable disease (105). In addition, a phase I/II trial using CAR T cells targeting mesothelin, Muc1, PSMA and GD2 in cervical cancer is underway (NCT03356795). These studies will provide data on the effectiveness of CAR therapies in patients with HPV-positive malignancies.
Conclusion and Future Directions
Epidemiologic and genetic evidence strongly support the role of immunosuppression and immune resistance during oncogenesis of HPV-related malignancies. Further, multiple viral oncogenes promote immune evasion to facilitate viral persistence, some of which are now being explored for pharmaceutical intervention. ICB therapies have shown activity in these cancers in multiple settings and disease sites. The unique shared viral antigens of these malignancies have facilitated the development of both adoptive cell therapies and vaccination strategies based on these shared neo-antigens. Unfortunately, response rates to these interventions have been modest to date, and in some cases, resistance has developed secondary to genetic alterations in antigen processing machinery. However, this shared set of antigens has facilitated rapid important advances in tumor immunology, including the recent identification of antigen-specific B cells in the tumor microenvironment. Furthermore, because of distinct clinical behavior of HPV-related malignancies based on the location of the primary tumor, these solid malignancies present an opportunity to understand more accurately the mechanisms of immune homeostasis in different anatomic sites. Further studies, however, are warranted. Future work will need to identify the best way to harness improvements in antigen-specific immune response to improve oncologic outcomes in patients.
Supplementary Material
Statement of Significance:
HPV modulates the microenvironment to create a pro-tumorigenic state of immune suppression and evasion. Our understanding of these mechanisms has led to the development of immunomodulatory treatments that have shown early clinical promise in patients with HPV-related malignancies. This review summarizes our current understanding of the interactions of HPV and its microenvironment and provides insight into the progress and challenges of developing immunotherapies for HPV-related malignancies.
Acknowledgments:
This work was funded in part by the James and Judith K. Dimon foundation and through NIH /NCI Cancer Center Support Grants P30 CA008748. Figures were created with biorender.com
Footnotes
Conflicts of interests:
No relevant conflicts of interest to the present publication. Authors receive research funding from Bristol Myers Squibb, Pfizer, Repare therapeutics as well as honoraria from Illumina and repare therapeutics
References:
- 1.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015;107(6):djv086 doi 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer 2018;18(4):240–54 doi 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal A, Kundu R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front Microbiol 2019; 10:3116 doi 10.3389/fmicb.2019.03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin-Drubin ME, Munger K. The human papillomavirus E7 oncoprotein. Virology 2009;384(2):335–44 doi 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer 2010;10(8):550–60 doi 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 6.Frisch M, Biggar RJ, Engels EA, Goedert JJ, Group AI-CMRS. Association of cancer with AIDS-related immunosuppression in adults. JAMA 2001;285(13):1736–45 doi 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 7.Grulich AE, Li Y, McDonald A, Correll PK, Law MG, Kaldor JM. Rates of non-AIDS-defining cancers in people with HIV infection before and after AIDS diagnosis. AIDS 2002;16(8):1155–61 doi 10.1097/00002030-200205240-00009. [DOI] [PubMed] [Google Scholar]
- 8.Dal Maso L, Franceschi S, Polesel J, Braga C, Piselli P, Crocetti E, et al. Risk of cancer in persons with AIDS in Italy, 1985-1998. Br J Cancer 2003;89(1):94–100 doi 10.1038/sj.bjc.6601017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005;97(6):425–32 doi 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Pfeiffer RM, Fraumeni JF Jr., Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306(17):1891–901 doi 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birkeland SA, Lokkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet 2000;355(9218):1886–7 doi 10.1016/s0140-6736(00)02298-4. [DOI] [PubMed] [Google Scholar]
- 12.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA 2006;296(23):2823–31 doi 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 13.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370(9581):59–67 doi 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Juko-Pecirep I, Hammer J, Ivansson E, Enroth S, Gustavsson I, et al. Genome-wide association study of susceptibility loci for cervical cancer. J Natl Cancer Inst 2013;105(9):624–33 doi 10.1093/jnci/djt051. [DOI] [PubMed] [Google Scholar]
- 15.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999;285(5428):727–9 doi 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 16.Ivansson EL, Juko-Pecirep I, Erlich HA, Gyllensten UB. Pathway-based analysis of genetic susceptibility to cervical cancer in situ: HLA-DPB1 affects risk in Swedish women. Genes Immun 2011;12(8):605–14 doi 10.1038/gene.2011.40. [DOI] [PubMed] [Google Scholar]
- 17.Lesseur C, Diergaarde B, Olshan AF, Wunsch-Filho V, Ness AR, Liu G, et al. Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer. Nat Genet 2016;48(12):1544–50 doi 10.1038/ng.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research N, Albert Einstein College of M, Analytical Biological S, Barretos Cancer H, Baylor College of M, Beckman Research Institute of City of H, et al. Integrated genomic and molecular characterization of cervical cancer. Nature 2017;543(7645):378–84 doi 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517(7536):576–82 doi 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vos van Steenwijk PJ, Heusinkveld M, Ramwadhdoebe TH, Lowik MJ, van der Hulst JM, Goedemans R, et al. An unexpectedly large polyclonal repertoire of HPV-specific T cells is poised for action in patients with cervical cancer. Cancer Res 2010;70(7):2707–17 doi 10.1158/0008-5472.CAN-09-4299. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt KH, Neller MA, Srihari S, Crooks P, Lekieffre L, Aftab BT, et al. Profiling HPV-16-specific T cell responses reveals broad antigen reactivities in oropharyngeal cancer patients. J Exp Med 2020;217(10) doi 10.1084/jem.20200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves E, James E. Antigen processing and immune regulation in the response to tumours. Immunology 2017;150(1):16–24 doi 10.1111/imm.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmat N, Baghi HB. Human papillomavirus E5 protein, the undercover culprit of tumorigenesis. Infect Agent Cancer 2018;13:31 doi 10.1186/s13027-018-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashrafi GH, Haghshenas MR, Marchetti B, O'Brien PM, Campo MS. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer 2005;113(2):276–83 doi 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- 25.Miyauchi S, Sanders PD, Guram K, Kim SS, Paolini F, Venuti A, et al. HPV16 E5 Mediates Resistance to PD-L1 Blockade and Can Be Targeted with Rimantadine in Head and Neck Cancer. Cancer Res 2020;80(4):732–46 doi 10.1158/0008-5472.CAN-19-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinbach A, Riemer AB. Immune evasion mechanisms of human papillomavirus: An update. Int J Cancer 2018;142(2):224–9 doi 10.1002/ijc.31027. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson R, Ramanakumar AV, Richardson H, Tellier PP, Coutlee F, Franco EL, et al. Human leukocyte antigen (HLA)-E and HLA-G polymorphisms in human papillomavirus infection susceptibility and persistence. Hum Immunol 2011;72(4):337–41 doi 10.1016/j.humimm.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Wagner S, Wittekindt C, Reuschenbach M, Hennig B, Thevarajah M, Wurdemann N, et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer 2016;138(9):2263–73 doi 10.1002/ijc.29962. [DOI] [PubMed] [Google Scholar]
- 29.Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016;1(17):e89829 doi 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, Chen XM, Huang HR, Zhao FP, Wang F, Liu X, et al. Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck 2018;40(6):1245–53 doi 10.1002/hed.25104. [DOI] [PubMed] [Google Scholar]
- 31.Lin CT, Chang TC, Shaw SW, Cheng PJ, Huang CT, Chao A, et al. Maintenance of CD8 effector T cells by CD4 helper T cells eradicates growing tumors and promotes long-term tumor immunity. Vaccine 2006;24(37-39):6199–207 doi 10.1016/j.vaccine.2006.05.108. [DOI] [PubMed] [Google Scholar]
- 32.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res 2004;64(15):5449–55 doi 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 33.Welters MJP, Ma W, Santegoets S, Goedemans R, Ehsan I, Jordanova ES, et al. Intratumoral HPV16-Specific T Cells Constitute a Type I-Oriented Tumor Microenvironment to Improve Survival in HPV16-Driven Oropharyngeal Cancer. Clin Cancer Res 2018;24(3):634–47 doi 10.1158/1078-0432.CCR-17-2140. [DOI] [PubMed] [Google Scholar]
- 34.Santegoets SJ, van Ham VJ, Ehsan I, Charoentong P, Duurland CL, van Unen V, et al. The Anatomical Location Shapes the Immune Infiltrate in Tumors of Same Etiology and Affects Survival. Clin Cancer Res 2019;25(1):240–52 doi 10.1158/1078-0432.CCR-18-1749. [DOI] [PubMed] [Google Scholar]
- 35.Oguejiofor K, Hall J, Slater C, Betts G, Hall G, Slevin N, et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer 2015;113(6):886–93 doi 10.1038/bjc.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bais AG, Beckmann I, Lindemans J, Ewing PC, Meijer CJ, Snijders PJ, et al. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol 2005;58(10):1096–100 doi 10.1136/jcp.2004.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walch-Ruckheim B, Mavrova R, Henning M, Vicinus B, Kim YJ, Bohle RM, et al. Stromal Fibroblasts Induce CCL20 through IL6/C/EBPbeta to Support the Recruitment of Th17 Cells during Cervical Cancer Progression. Cancer Res 2015;75(24):5248–59 doi 10.1158/0008-5472.CAN-15-0732. [DOI] [PubMed] [Google Scholar]
- 38.Adurthi S, Krishna S, Mukherjee G, Bafna UD, Devi U, Jayshree RS. Regulatory T cells in a spectrum of HPV-induced cervical lesions: cervicitis, cervical intraepithelial neoplasia and squamous cell carcinoma. Am J Reprod Immunol 2008;60(1):55–65 doi 10.1111/j.1600-0897.2008.00590.x. [DOI] [PubMed] [Google Scholar]
- 39.Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG, et al. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res 2015;3(1):48–58 doi 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- 40.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A 2007;104(29):12087–92 doi 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heusinkveld M, Goedemans R, Briet RJ, Gelderblom H, Nortier JW, Gorter A, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer 2012;131(2):E74–85 doi 10.1002/ijc.26497. [DOI] [PubMed] [Google Scholar]
- 42.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer 2013;109(10):2629–35 doi 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, et al. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One 2012;7(6):e38711 doi 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109(5):744–52 doi 10.1016/j.tripleo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Ihara F, Sakurai D, Takami M, Kamata T, Kunii N, Yamasaki K, et al. Regulatory T cells induce CD4(−) NKT cell anergy and suppress NKT cell cytotoxic function. Cancer Immunol Immunother 2019;68(12):1935–47 doi 10.1007/s00262-019-02417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol 2015;15(7):405–14 doi 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 47.Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 2015;350(6260):568–71 doi 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 48.Luo X, Donnelly CR, Gong W, Heath BR, Hao Y, Donnelly LA, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest 2020;130(4):1635–52 doi 10.1172/JCI129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaikh MH, Bortnik V, McMillan NA, Idris A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb Pathog 2019;132:162–5 doi 10.1016/j.micpath.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev 1998;12(13):2061–72 doi 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu S, Concha-Benavente F, Shayan G, Srivastava RM, Gibson SP, Wang L, et al. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV(+) status in head and neck cancer. Oral Oncol 2018;78:186–93 doi 10.1016/j.oraloncology.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013;73(6):1733–41 doi 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong AM, Ferguson P, Dodds T, Jones D, Li M, Yang J, et al. Significant association of PD-L1 expression with human papillomavirus positivity and its prognostic impact in oropharyngeal cancer. Oral Oncol 2019;92:33–9 doi 10.1016/j.oraloncology.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Kim HS, Lee JY, Lim SH, Park K, Sun JM, Ko YH, et al. Association Between PD-L1 and HPV Status and the Prognostic Value of PD-L1 in Oropharyngeal Squamous Cell Carcinoma. Cancer Res Treat 2016;48(2):527–36 doi 10.4143/crt.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balermpas P, Rodel F, Krause M, Linge A, Lohaus F, Baumann M, et al. The PD-1/PD-L1 axis and human papilloma virus in patients with head and neck cancer after adjuvant chemoradiotherapy: A multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int J Cancer 2017;141(3):594–603 doi 10.1002/ijc.30770. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Sun H, Zeng Q, Guo XJ, Wang H, Liu HH, et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep 2019;9(1):13404 doi 10.1038/s41598-019-49771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C, Lu J, Tian H, Du W, Zhao L, Feng J, et al. Increased expression of PDL1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol Med Rep 2017;15(3):1063–70 doi 10.3892/mmr.2017.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rice AE, Latchman YE, Balint JP, Lee JH, Gabitzsch ES, Jones FR. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer Gene Ther 2015;22(9):454–62 doi 10.1038/cgt.2015.40. [DOI] [PubMed] [Google Scholar]
- 59.Gu X, Dong M, Liu Z, Mi Y, Yang J, Zhang Z, et al. Elevated PD-L1 expression predicts poor survival outcomes in patients with cervical cancer. Cancer Cell Int 2019;19:146 doi 10.1186/s12935-019-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer 2009;9:292 doi 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SS, Shen S, Miyauchi S, Sanders PD, Franiak-Pietryga I, Mell L, et al. B Cells Improve Overall Survival in HPV-Associated Squamous Cell Carcinomas and Are Activated by Radiation and PD-1 Blockade. Clin Cancer Res 2020;26(13):3345–59 doi 10.1158/1078-0432.CCR-19-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lechner A, Schlosser HA, Thelen M, Wennhold K, Rothschild SI, Gilles R, et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology 2019;8(3):1535293 doi 10.1080/2162402X.2018.1535293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood O, Woo J, Seumois G, Savelyeva N, McCann KJ, Singh D, et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 2016;7(35):56781–97 doi 10.18632/oncotarget.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cillo AR, Kurten CHL, Tabib T, Qi Z, Onkar S, Wang T, et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity 2020;52(1):183–99 e9 doi 10.1016/j.immuni.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wieland A, Patel MR, Cardenas MA, Eberhardt CS, Hudson WH, Obeng RC, et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2020. doi 10.1038/s41586-020-2931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375(19):1856–67 doi 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393(10167):156–67 doi 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 68.Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394(10212):1915–28 doi 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 69.Schadendorf D, Nghiem P, Bhatia S, Hauschild A, Saiag P, Mahnke L, et al. Immune evasion mechanisms and immune checkpoint inhibition in advanced merkel cell carcinoma. Oncoimmunology 2017;6(10):e1338237 doi 10.1080/2162402X.2017.1338237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol 2010;116(1):44–9 doi 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2019;37(17):1470–8 doi 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 72.Naumann RW, Hollebecque A, Meyer T, Devlin MJ, Oaknin A, Kerger J, et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. J Clin Oncol 2019;37(31):2825–34 doi 10.1200/JCO.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naumann RW. Efficacy and safety of nivolumab (Nivo) + Ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: Results from CheckMate 358. Annals of Oncology. Volume 302019. [Google Scholar]
- 74.Morris VK, Salem ME, Nimeiri H, Iqbal S, Singh P, Ciombor K, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(4):446–53 doi 10.1016/S1470-2045(17)30104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ott PA, Piha-Paul SA, Munster P, Pishvaian MJ, van Brummelen EMJ, Cohen RB, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol 2017;28(5):1036–41 doi 10.1093/annonc/mdx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 2020;31(7):942–50 doi 10.1016/j.annonc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourrhis J, Harrington K, Chand PMH, Jin JC, Razaq MA, Teixeira MM, Lovey J, Chamois J, Rueda A, Hu C, Dunn LA, Dvorkin MV, Debeukelaer S, Pavlov D, Thurm H, Cohen E velumab in combination with standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, phase 3 trial. Lancet Oncology 2020;Accepted. [DOI] [PubMed] [Google Scholar]
- 78.Bourhis J, Sire C, Tao Y, Martin L, Alfonsi M, Prevost JB, Rives M, Lafond C, Tourani JM, Biau J, Geoffrois L, Coutte A, Liem X,, Vauleon E, Drouet F, Pechery A, Guigay J, Wanneveich M, Auperin A, Sun X Pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced head and neck squamous cell carcinoma (LA-HNSCC): Results of the GORTEC 2015-01 “PembroRad” randomized trial. Ann Oncol 2020;31:1168. [DOI] [PubMed] [Google Scholar]
- 79.Lei J, Ploner A, Elfstrom KM, Wang J, Roth A, Fang F, et al. HPV Vaccination and the Risk of Invasive Cervical Cancer. N Engl J Med 2020;383(14):1340–8 doi 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 80.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol 1993;23(9):2242–9 doi 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 81.Feltkamp MC, Vreugdenhil GR, Vierboom MP, Ras E, van der Burg SH, ter Schegget J, et al. Cytotoxic T lymphocytes raised against a subdominant epitope offered as a synthetic peptide eradicate human papillomavirus type 16-induced tumors. Eur J Immunol 1995;25(9):2638–42 doi 10.1002/eji.1830250935. [DOI] [PubMed] [Google Scholar]
- 82.Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, Small LA, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res 2000;6(9):3406–16. [PubMed] [Google Scholar]
- 83.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer 2010;102(7):1129–36 doi 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009;361(19):1838–47 doi 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 85.Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019;5(1):67–73 doi 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med 2014;6(221):221ra13 doi 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brun JL, Dalstein V, Leveque J, Mathevet P, Raulic P, Baldauf JJ, et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am J Obstet Gynecol 2011;204(2):169 e1–8 doi 10.1016/j.ajog.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Atherton MJ, Stephenson KB, Pol J, Wang F, Lefebvre C, Stojdl DF, et al. Customized Viral Immunotherapy for HPV-Associated Cancer. Cancer Immunol Res 2017;5(10):847–59 doi 10.1158/2326-6066.CIR-17-0102. [DOI] [PubMed] [Google Scholar]
- 89.Sacco JJ, Evans M, Harrington KJ, Man S, Powell N, Shaw RJ, et al. Systemic listeriosis following vaccination with the attenuated Listeria monocytogenes therapeutic vaccine, ADXS11-001. Human Vaccines & Immunotherapeutics 2016;12(4):1085–6 doi 10.1080/21645515.2015.1121338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Safran H, Leonard KL, Perez K, Vrees M, Klipfel A, Schechter S, et al. Tolerability of ADXS11-001 Lm-LLO Listeria-Based Immunotherapy With Mitomycin, Fluorouracil, and Radiation for Anal Cancer. Int J Radiat Oncol Biol Phys 2018;100(5):1175–8 doi 10.1016/j.ijrobp.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 91.Basu P, Mehta A, Jain M, Gupta S, Nagarkar RV, John S, et al. A Randomized Phase 2 Study of ADXS11-001 Listeria monocytogenes-Listeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer. Int J Gynecol Cancer 2018;28(4):764–72 doi 10.1097/IGC.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huh WK, Brady WE, Fracasso PM, Dizon DS, Powell MA, Monk BJ, et al. Phase II study of axalimogene filolisbac (ADXS-HPV) for platinum-refractory cervical carcinoma: An NRG oncology/gynecologic oncology group study. Gynecol Oncol 2020;158(3):562–9 doi 10.1016/j.ygyno.2020.06.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tudor D, Dubuquoy C, Gaboriau V, Lefevre F, Charley B, Riffault S. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine 2005;23(10):1258–64 doi 10.1016/j.vaccine.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 94.Rottembourg D, Filippi CM, Bresson D, Ehrhardt K, Estes EA, Oldham JE, et al. Essential role for TLR9 in prime but not prime-boost plasmid DNA vaccination to activate dendritic cells and protect from lethal viral infection. J Immunol 2010;184(12):7100–7 doi 10.4049/jimmunol.0803935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res 2009;15(1):361–7 doi 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aggarwal C, Cohen RB, Morrow MP, Kraynyak KA, Sylvester AJ, Knoblock DM, et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin Cancer Res 2019;25(1):110–24 doi 10.1158/1078-0432.CCR-18-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Youn JW, Hur SY, Woo JW, Kim YM, Lim MC, Park SY, et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. Lancet Oncol 2020;21(12):1653–60 doi 10.1016/S1470-2045(20)30486-1. [DOI] [PubMed] [Google Scholar]
- 98.Stevanovic S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 2015;33(14):1543–50 doi 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stevanovic S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 2017;356(6334):200–5 doi 10.1126/science.aak9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stevanovic S, Helman SR, Wunderlich JR, Langhan MM, Doran SL, Kwong MLM, et al. A Phase II Study of Tumor-infiltrating Lymphocyte Therapy for Human Papillomavirus-associated Epithelial Cancers. Clin Cancer Res 2019;25(5):1486–93 doi 10.1158/1078-0432.CCR-18-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jazaeri AA, Zsiros E, Amaria RN, Artz AS, Edwards RP, Wenham RM, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. Journal of Clinical Oncology 2019;37(15_suppl):2538- doi 10.1200/JCO.2019.37.15_suppl.2538. [DOI] [Google Scholar]
- 102.Doran SL, Stevanović S, Adhikary S, Gartner JJ, Jia L, Kwong MLM, et al. T-Cell Receptor Gene Therapy for Human Papillomavirus–Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. Journal of Clinical Oncology 2019;37(30):2759–68 doi 10.1200/jco.18.02424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Norberg S, Nagarsheth N, Sinkoe A, Adhikary S, Meyer T, Lack J, et al. Safety and clinical activity of gene-engineered T-cell therapy targeting HPV-16 E7 for epithelial cancers. Journal of Clinical Oncology 2020;38(15_suppl):101- doi 10.1200/JCO.2020.38.15_suppl.101. [DOI] [Google Scholar]
- 104.Nagarsheth NB, Norberg SM, Sinkoe AL, Adhikary S, Meyer TJ, Lack JB, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med 2021. doi 10.1038/s41591-020-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Papa S, Adami A, Metoudi M, Achkova D, Schalkwyk Mv, Pereira AP, et al. A phase I trial of T4 CAR T-cell immunotherapy in head and neck squamous cancer (HNSCC). Journal of Clinical Oncology 2018;36(15_suppl):3046- doi 10.1200/JCO.2018.36.15_suppl.3046. [DOI] [Google Scholar]
- 106.Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015;386(10008):2078–88 doi 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.