Abstract
This paper describes evidence establishing that ultra-low doses of diverse chemical agents at concentrations from 10−18 to 10−24 M (e.g., approaching and/or less than 1 atom or molecule of a substance/cell based on Avogadro’s constant - 6.022 x 1023/mole) are capable of engaging receptor and intracellular signaling systems to elicit reproducible effects in a variety of species, from unicellular organisms to humans. Multiple experimental studies have shown that only one or very few molecules are needed to activate a cell and/or entire organism via cascade(s) of amplification mechanisms and processes. For example, ultra-low dose ligand exposure was able to activate both an individual cell, and ~3,000-25,000 neighboring cells on average, by about 50%. Such activation of cells and whole organisms typically displayed hormetic-biphasic dose responses. These findings indicate that numerous, diverse phylogenetic systems have evolved highly sensitive detection and signaling mechanisms to enhance survival functions, such as defense against infectious agents, responses to diverse types of pheromone communications (e.g., alarm, sexual attraction), and development of several types of cellular protection/resilience processes. This suggests that ultra-low dose effects may be far more common than have been recognized to date. We posit that such findings have important implications for evolutionary theory, ecological and systems biology, and clinical medicine.
Keywords: ultra-low dose, biological amplification, hormesis, pheromone, Avogadro’s constant
Graphical Abstract
1. INTRODUCTION
Phylogentically, cellular and organismal signaling mechanisms have evolved to maximize dose/concentration-response relationships. The effect(s) of concentration ranges of different agents (ligands and environmental factors) are varied, with most receptor-based and enzymatic patterns operating in the 10−6 to 10−9 M or 10−4 to 10−6 M concentration ranges, respectively (i.e., as based on relative dissociation constants). Low doses have generally been considered to be concentrations of a biologically active agent at the site of action that are one or more orders of magnitude lower than the equilibrium dissociation constant [1]. However, there is now evidence of cellular and organismal signaling processes that commonly function at far lower concentrations (viz., in the 10−18 to 10−24 M range). Fortifying and extending these observations are findings of significant biological amplification mechanisms in which a single molecule can activate transcellular signaling via wave-like bystander communication processes affecting up to tens of thousands of neighboring cells. In light of such findings, this paper identifies five areas of ultra-low dose biology (10−18-10−24 M), namely macrophage activation, unicellular receptor signaling, fullerene biology, highly diverse invertebrate pheromone endpoints, and multiple receptor signaling pathways and endpoints in mammalian models. Ultra-low dose effects in these biological systems will be described and addressed in contexts of the quantitative features of both dose response patterns/outcomes, and mechanistic functions.
2. IMMUNE ACTIVATION: Phagocytosis
Macrophages play an important role in innate immunity, acting as initial defense against microbial threats by engaging multi-faceted phagocytosis, cytotoxic and inflammatory molecular mechanisms. Various types and magnitudes of stress may significantly affect (i.e., increase and/or decrease) macrophage phagocytotic capacity. Many of these changes in macrophage responsivity are meditated by changes in levels of circulating and local tissue catecholamines.
Alterations of macrophage activity became the focus of considerable interest based on initial observations indicating that disruption of hibernation in the Wall lizard invariably led to rapid death due to bacterial infection. This suggested that the stress of disrupted hibernation likely elevated catecholamine (CA) levels, thereby suppressing macrophage phagocytic response, leading to increased susceptibility to – and ultimately death from - microbial infection. Experiments with the Wall lizard investigated the effects of several CAs [(i.e., dopamine (DA), norepinephrine (NE) and epinephrine (E)] across a wide range of concentrations (10−15 to 10−5 M) on macrophage phagocytotic function. For each CA ligand, a biphasic concentration gradient was demonstrated, with maximal stimulation of phagocytosis occurring at the lowest concentration tested (10−15 M), with stimulation ranging between 160% to 180%, as compared to the 100% control group. At higher CA concentrations, a progressive decrease in response occurred. Low concentrations of NE- and E-induced stimulation were blocked by pre-administration of ß-adrenergic antagonists. As to be expected, DA acts through DA-(type 1 and 2) receptors, but also via ß-adrenoceptor-dependent mechanisms, as demonstrated by the partial blocking effect(s) of ß-adrenergic antagonists. Putative receptor cross-talk processes (e.g., engaging yoked second-messenger pathways) may be operative in mediating the differential CA-induced macrophage function(s). Supporting this hypothesis are demonstrations that cAMP-induced activation of ß-adrenoreceptor-linked cyclase was essential for macrophage phagocytosis. Thus, blocking the NE/E receptor prevented engagement of adenylase cyclase-mediated cAMP activity.
Subsequent studies of effects of cAMP-mediated activation of phagocytosis evaluated effects over a 10−18 to 10−2 M concentration range, and demonstrated a biphasic concentration-relationship with ~50% stimulation at the lowest concentration assessed (Figure 1). It was hypothesized that at low concentrations, cAMP stimulated protein synthesis induces enhanced phagocytotic activity. This speculation was supported using the transcription and translation inhibitors actinomycin D and cycloheximide, respectively. Both agents prevented cAMP-induced stimulation of macrophage phagocytosis at low concentrations; however, they failed to attenuate phagocytic induction at higher concentrations. Of note is that cAMP stimulation at 10−18 M occurred with only 120 molecules per 400,000 cells, a ratio of one molecule per ~3,300 cells, thus, revealing a significant amplification effect. The low concentration stimulatory responses were mediated by a genomic pathway, while the higher concentration effects were mediated by a non-genomic pathway [2].
Figure 1.
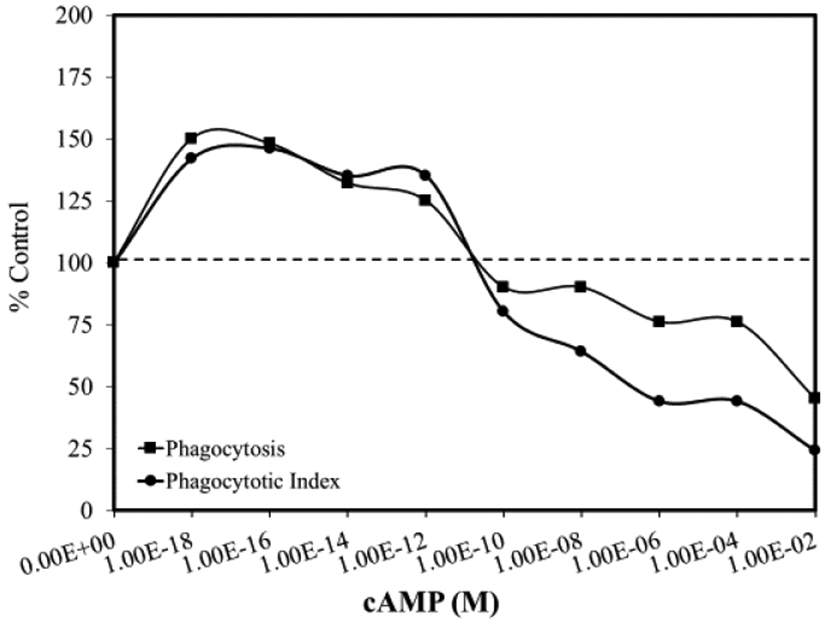
Effects of cAMP on phagocytosis of splenic macrophages (modified from: Roy and Rai [2])
3. UNICELLULAR ACTIVATION: Tetrahymena
During the 1970s, it was first reported that the unicellular organism, Tetrahymena, was capable of binding a number of the endogenous ligands of higher animal species [3, 4]. Further research revealed that Tetrahymena exhibited signal transduction processes similar to mammals [5, 6], and these observations led to a series of studies assessing the sensitivity of Tetrahymena receptors and ligand production. These investigations showed biological effects for multiple endpoints could be produced in Tetrahymena at ligand concentrations of 10−21 M, of serotonin (5-hydroxytryptamine; 5HT) and insulin on tissue histamine content [7-9].
4. FULLERENES
Fulleronols represent specific allotropic forms of carbon, being nanosized polyhydroxylated water-soluble derivatives of fullerenes. Cellular and enzymatic assays have been used to evaluate general toxicity responses under oxidative stress, using solutions of organic or inorganic oxidizers (1,4-benzoquinones or potassium ferricyanide, respectively). An enzymatic bioassay can also assess the oxidative toxicity (OXT) of solutions. The OXT enzymatic assay describes the redox features of toxic agents, whereas the general toxicity (GT) assay integrates the net toxic effects on the system. As well, luminous marine bacteria and bacterial enzymes have been used to assess the anti-oxidant properties of several fulleronols. The GT assay measures the maximum bioluminescence intensity while the OXT employs specific kinetic parameter values for the induction bioluminescence period.
The GT bioassay showed biological effects for selected fulleronols at concentration ranges as low as 1 x 10−20 to 1 x 10−21 M [10]. Veoikov and Yablonskaya [11] reported that hydrated fullerenes enhanced luciferase activity by ~75% at 10−23 M (Figure 2). These findings demonstrated that the hydrated fullerene, C60, displayed an antioxidant effect within highly diluted solutions in bacterial-based assays. Specifically, C60 (10−19 M) exhibited a pronounced stabilizing effect on luciferase that prevented loss of its activity for prolonged periods at room temperature.
Figure 2.
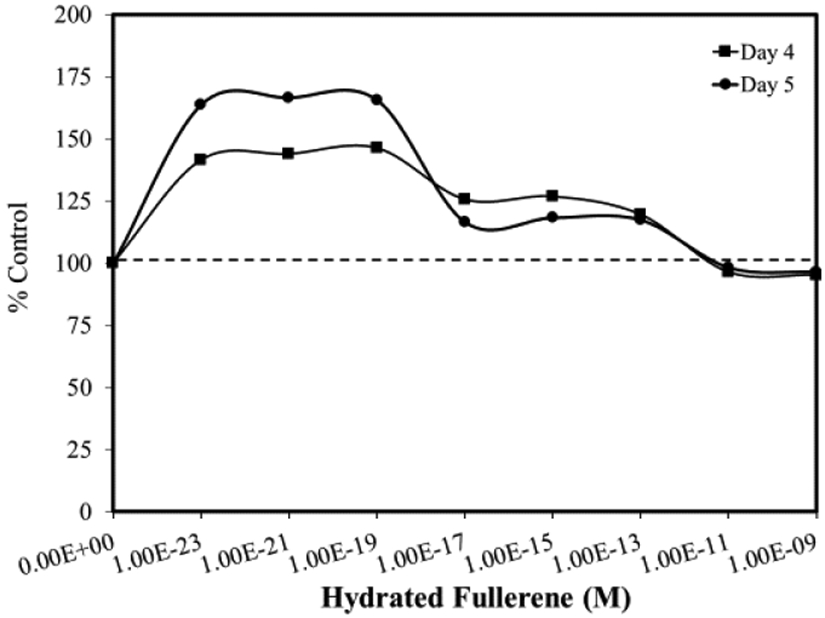
Effects of hydrated fullerene (HyFn C60) on luciferase activity (modified from: Veoikov and Yablonskaya [11])
Important in the aforementioned example is that the dose response features followed a hormetic pattern, with enhanced biological activity induced by low concentrations of agents. An underlying mechanism for these hormetic effects at such highly diluted concentrations was suggested to involve the aqueous envelope surrounding C60 that is negatively charged, and likely to possess electron density capacity. Thus, the agents tested may have direct free radical scavenging activity and effect(s). C60 effects have additionally been reported to induce hormetic responses in cultures of human fetal lung fibroblasts [12, 13].
5. SYNTHETIC TRIPEPTIDES: Mud-crab Pumping Pheromones
There is considerable research demonstrating that mud-crabs produce a pheromone that is released from hatching eggs, which stimulates larval release via a stereotypical pumping process that lyses egg membranes and extrudes the larvae from the mother’s body. The search for the native ligand led to (1) observations that pheromones can be produced by enzymatic degradation of egg membranes via serine proteases; and (2) subsequent testing of synthetic tripeptide pheromone on mud-crab larval pumping function. These studies generally investigated the number of released larvae and/or the percentage of ovigerous crabs that display a pumping response to different concentrations of test agents when placed in 40 mL of seawater. Pheromone mimics produced hormetic-biphasic concentration responses for both endpoints [14]. Of particular interest is that five synthetic tripeptides were examined over concentrations as low as 10−22 M. In these assays, typical hormetic patterns were shown, with stimulatory responses being evoked by ligand concentrations ranging from 10−22 M to 10−16 M. Since the lowest concentration tested (i.e., 10−22 M) still displayed a 40% stimulatory response, a concentration threshold was not established [15] (Figure 3). At the lowest concentration tested (10−22 M), it was calculated that 24 molecules were present in the 40 mL of seawater at the start of testing. Ten mud crabs were consecutively tested in the 40 mL seawater solution prior to using a new test solution. This suggests that at the lowest concentration tested, ultra-low levels (e.g., only one-to-several molecules per crab) of ligand were capable of activating the pumping function response.
Figure 3.
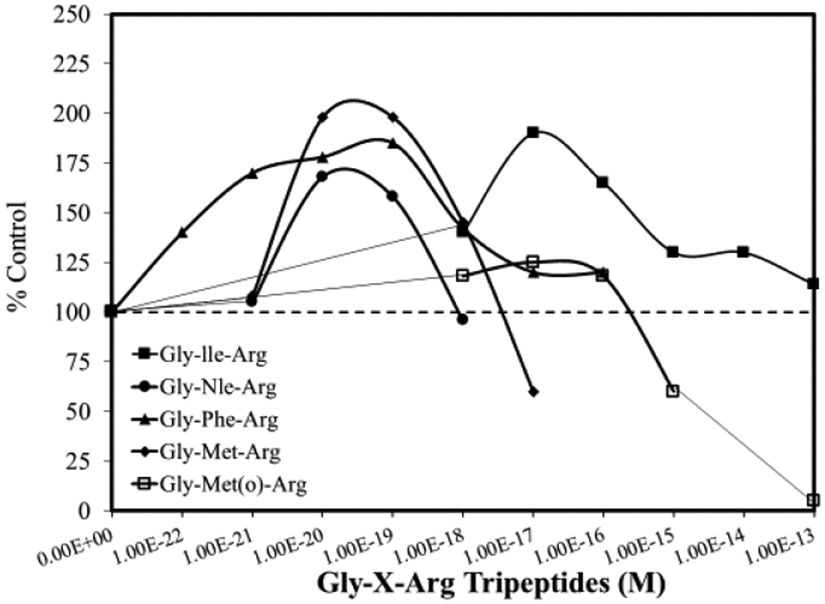
Effects of five gly-x-arg tripeptides on abdominal pumping of egg-bearing mud-crabs (modified from: Rittschoff et al. [14])
6. G PROTEIN-COUPLED RECEPTORS (GPCR): Low-dose Effects of Relaxin and Other Agents
Halls and Cooper [16] reported that the binding of relaxin to its receptor activates assembly of a large signaling complex, and in the process, amplifies relaxin signaling such that far fewer relaxin molecules [i.e., ~attomolar (10−18 M) concentrations] are required to evoke a functional response in key target tissues. Relaxin receptors are a (sub)class of four closely related G protein-coupled receptors (GCPR). GPCR efficiency is increased by the formation of signalosomes which are receptor-linked protein complexes that enhance the preferential activation of downstream targets. Sub-picomolar concentrations of peptide act to engage the complex to couple the relaxin receptor to cAMP to evoke amplified intracellular signaling mechanisms. Halls and Cooper [16] conceptualized the relaxin-GCPR-signalosome process based on observations that circulating relaxin activates organs, such as the heart, in which it is not produced, and circulating concentrations of relaxin were lower than those shown to be effective with other signaling systems.
Civciristov and Halls [17] generalized these observations, noting that such high sensitivity, while fairly common, has been generally overlooked in scientific (and biomedical translational) research, despite over 35 papers showing GCPR responses to ultra-low concentrations of ligands. The biological activities in these reports were typically in the 10-15 to 10−18 M range, with several as low as 10−19 to 10−21 M, and all showing hormetic dose response patterns [18-23].
Civciristov et al. [24] noted that two GPCRs, the B2-adrenergic receptor (ß2AR) and the muscarinic acetylcholine M3 receptor (M3R) were activated at femtomolar (fM) (10−15 M) concentrations of particular ligands. Based on mathematical modeling, it was predicted that 1 fM of ligand can activate approximately 40% of cells in a preparation within a temporal interval of five (5) minutes, as a result of activation by two possible receptor binding events. Receptor activation at low concentrations qualitatively differs from that at higher concentrations, having both spatially and temporally distinct patterns of intercellular signaling, and a distinct response pattern at the cellular level [24]. It was shown that similar forms of highly efficient and amplified signaling would demand an activation rate constant (Kact) that is of sufficient magnitude to affect a response by one or two binding events per cell. Further, it was demonstrated that binding of CHO-K1 cells with 10−18 M concentration of thrombin ligand enhanced the production of cAMP. Civciristov et al. [24] incubated 30,000 cells in 200 μL at a concentration of 10−18 M (~120 molecules per 200 μL), yielding approximately 1 molecule/250 cells. As well, it was shown that CHO-K1 cells were responsive to 10−20 M thrombin concentrations (i.e., an estimated one molecule per 25,000 cells).
7. DISCUSSION
Herein we provide evidence for the induction of reproducible biological responses in multiple biological systems at ligand concentrations from 10−18 M to 10−24 M. These findings elucidate the capacity of a number of biological systems to display extraordinary sensitivity to ultra-low concentrations of ligand. Until quite recently, this capacity of cells (and to some extent organ systems and whole organisms) to possess and engage functional ultra-low level detection and signaling mechanisms that affect a broad spectrum of biological processes has been poorly recognized and acknowledged. Findings such as those presented in this paper reveal these capabilities to occur across phylogenetic species, from unicellular to mammalian organisms. Yet, these extremely sensitive detection, signaling and amplification mechanisms, activities and effects in microbial, plant, and animal domains, and their constitutive and/or adaptive functions are only coming to light and being identified as the foci of basic and translational research.
Civciristov and Halls [17] hypothesized that some ultra-low dose effects may occur via preassembly of signaling proteins within the receptor complex, which occupies only a limited spatial domain of the plasma membrane. Such structures may account for how some ligand-receptor binding effects occur at concentrations that are orders of magnitude below the receptor Kd. It is possible that protein interactions within the complex induce and/or mediate allosteric changes of the ligand binding region of certain (GPC) receptors, creating an open-type configuration that exhibits enhanced affinity for the ligand. Indeed, such changes have been shown to increase binding affinity by greater than 15,000-fold [25]. Civcirostov and Halls [17] further speculated that since ultra-sensitive binding complexes are likely to have restricted domains within the plasma membrane, this area might become receptor enriched, creating a “ligand sink”, increasing affinity, and creating a signaling activity hot spot.
While the number of molecules capable of interacting directly with cells in the 10−18 to 10−24 M range is extremely small, experimental evidence indicates a biological amplification process that is integrated within a “bystander-broadcasting effects” system. For example, a ratio of one cAMP molecule/3,300 macrophages statistically increased the average phagocytic response by 50%. A similar bystander amplification broadcasting effect was reported by Offen [26] in the neuroprotective actions of two vasoactive intestinal peptide (VIP) derived peptides on PC12 cells, in a Parkinson’s disease model system. The concentration for both agents at 10−18 M (600 molecules/one mL) enhanced the survival of 300,000 PC 12 cells by 50% to 60%; an amplification response of ~ 5,000 fold. Sowa et al. [27] demonstrated the capacity of morphine to reduce macrophage phagocytosis at 10−20 M by 8.5%, affecting ~3,500 cells/molecule (NB: a value similar to that reported for phagocytosis induced by cAMP). These examples of apparent activation of several thousand cells by a single molecule represent an important conceptual development in furthering understanding of (the ubiquity and potency of hormesis.
These examples provide the lowest doses/concentrations tested to date, without evidence of a threshold, thereby suggesting the possibility that similar effects may occur at even greater cell to molecule ratios (e.g., increases in biological activity of in vitro conditions tested involved 300,000 to 1,000,000 cells/6-120 molecules). Several examples of broadcasting effects were assessed in whole organisms (viz., activation of abdominal pumping in the pregnant mud-crab at 10−22 M of synthetic peptide, in which significant effects were induced by an estimated 2-3 molecules per crab [15]). Similar heightened sensitivity was reported in the green algae, Volvox, which displays both sexual and asexual reproduction. In 1973, Pall [28] reported sexual induction as a function of hormone concentration. Based on studies with Volvox carteri, it was determined that only two molecules of hormone are required to make the gonidium form a sexual rather than an asexual embryo.
We posit that such ultra-sensitive signaling may be of evolutionary significance – and importance - as a common and highly generalized survival strategy. This is strengthened by the work of Civciristov et al. [24] in citing 35 established examples of ultra-sensitive detection/signaling in a variety of biological systems. We speculate that ligand ultra-sensitivity may be important to establishing (cellular and organismal) responsivity to various environmental niches and conditions, whereby ultra-low dose sensitivities may enable hormetic signal amplification in relatively “noisy” environments, thereby creating more resilient phenotype(s) that are more allostatically capable, and adaptable [29].
The effects elicited by very low concentrations of ligand(s) are biologically diverse, and include immune responses, such as macrophage phagocytosis [2]; hormonal functions (e.g., in Tetrahymena [8]); pressor effects in the renal vasculature [18]; pheromonotropic and melanotropic receptor effects in insect species [20]; sex signaling in algae [28]; several enzyme activities [12]; cAMP production [24], and mud crab pheromone pumping factor [15]. The diverse range of endpoints affected by ultra-low concentrations characteristically occurs in the <3,300-25,000 cells/molecule range, indicating that activated cells can exhibit a broadcasting function to engage a large number of other cells in a contained environment of a 1 mL (precluding assessment of the spatial distance or volume in which the activities and effects may occur).
Hormetic patterns of dose response were commonly observed in these investigations of ultra-low dose effects. Thus, the generality of the hormetic dose response occurs across biological models, endpoint(s) measured, inducing agent(s), level of biological organization, and concentration range(s) studied [30]. Many adaptive hormetic responses in various animal models and cell types, and affecting diverse endpoints have recently been shown to be mediated via activation of Nrf2 [31]. How the hormetic-like effects reported with ultra-low doses relates to other, identified hormetic mechanisms remains uncertain, and therefore requires further research – and an openness to scientific self-critique, and paradigmatic revision [32, 33].
Hence, we opine that the findings presented herein afford viable possibilities for new exploratory horizons of biomedical research [34]. These include the role of ultra-low doses in cellular and whole animal biology, as well as investigation(s) of how such low doses affect intercellular biological broadcast communication to induce and/or mediate a host of survival functions [29]. This represents an opportunity for an interdisciplinary approach that can employ emerging technologies (of assessment, and site-specific and ultra-low dose administration of ligands and other trophic factors) to engage hormetic mechanisms to elicit (biologically, clinically, and environmentally) valid and valuable endpoints. Our ongoing work remains committed to such enterprise.
8. ACKNOWLEDGEMENTS
EJC acknowledges long time support from the US Air Force (AFOSR FA9550-19-1-0413) and ExxonMobil Foundation (S18200000000256). JG’s work was supported in part by funding from the Henry Jackson Foundation for Military Medicine; Leadership Initiatives; Brain NeuroBio International; NeuroGen Corporation; Coburg University Distinguished Visiting Professorship in Integrative Health Promotions; and federal funds 2UL1TR001409-06 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards (CTSA) Program, a trademark of the US Department of Health and Human Services, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise.” The U.S. Government is authorized to reproduce and distribute for governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the author and should not be interpreted as necessarily representing policies or endorsement, either expressed or implied. Sponsors had no involvement in study design, collection, analysis, interpretation, writing and decision to and where to submit for publication consideration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
COMPETING INTEREST DECLARATION
The authors declare no competing interests.
11. REFERENCES
- [1].Gurevich KG, Low doses of biologically active substances: Effects, possible mechanisms, and features, Cell. Biol. Inter 25(5) (2001) 475–484. [DOI] [PubMed] [Google Scholar]
- [2].Roy B, Rai U, Dual mode of catecholamine action on splenic macrophage phagocytosis in wall lizard, Hemidactylus flaviviridis, Gen. Comp. Endocrin 136 (2004)180–191. [DOI] [PubMed] [Google Scholar]
- [3].Csaba G, Lantos T, Effect of hormones on protozoa. Studies on the phagocytotic effect of histamine, 5-hhydroxytrypamine and indoleacetic acid in Tetrahymena pyriformis, Cytobiologie 7 (1973) 361–365. [Google Scholar]
- [4].Csaba G, Lantos T, Effect of insulin on the glucose uptake of Protozoa, Experientia 31 (1975) 1097–1098. [DOI] [PubMed] [Google Scholar]
- [5].Csaba G, Phylogeny an ontogeny of chemical signaling: origin and development of hormone receptors, Int. Rev. Cyto 155 (1994) 1–48. [PubMed] [Google Scholar]
- [6].Csaba G, Hormonal imprinting: its role in the evolution and development of hormones and receptors, Cell Biol. Int 24 (2000) 407–414. [DOI] [PubMed] [Google Scholar]
- [7].Csaba G, Kovacs P, Tolthfalusi L, Pallinger E, Effects of extremely low concentrations of hormones on the insulin binding of Tetrahymena. Cell. Biol. Intern 30:(2006) 957–962. [DOI] [PubMed] [Google Scholar]
- [8].Csaba G, Kovacs P, Pallinger E, How does the unicellular Tetrahymena utlise the hormones that it produces? Paying a visit to the realm of atto-and zeptomolar concentrations, Cell. Tissue Res 327 (2007) 199–203. [DOI] [PubMed] [Google Scholar]
- [9].Csaba G, Laiko E, Pallinger E, Effect of different concentration of serotonin, histamine and insulin on the hormone (serotonin and ACTH) production of Tetrahmena in nutrient-free physiological milieu, Exper. Parasit 129 (2011) 179–182. [DOI] [PubMed] [Google Scholar]
- [10].Sachkova AS, Kovel ES, Churilov GN, Guseynov GA, Bonday AA, et al. (2017). On mechanism of antioxidant effect of fullerenols, Biochem. Biphys. Rep 9 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Veoikov VL, Yablonskaya, O.I. OI, Stabilizing effects of hydrated fullerences C60 in a wide range of concentrations on luciferase, alkaline phosphatase, and peroxidase in vitro, Electromag. Biol. Med 34(2) (2015) 160–166. [DOI] [PubMed] [Google Scholar]
- [12].Ershova ES, Sergeeva VA, Tabakov VJ, Kameneva LA, Porokhovnik LN, et al. 2016. Functionalized fullerene increases NF-kB activity and blocks genotoxic effect of oxidative stress in serum-starving human embryo lung diploid dibroblasts. Oxid. Med. Cell. Long 2016, 9895245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kostyuk SV, Proskurnina EV, Savinova EA, Erschova ES, Kraevaya OA, et al. , Effects of functionalized fullerences on ROS homeostasis determine their cytoprotective or cytotoxic properties. Nanomaterials 10 (2020) 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rittschoff D, Forward RB Jr., Erickson BW, Larval release in brachyuran crustaceans. J. Chem. Ecol 16 (1990) 1359–1370. [DOI] [PubMed] [Google Scholar]
- [15].Pettis RJ, Erickson BW, Forward RB, Ritschoff D, Superpotent synthetic tripeptide mimics of the mud-crab pumping pheromone, Inter. J. Peptide Protein Res 42 (1993) 312–319. [DOI] [PubMed] [Google Scholar]
- [16].Halls ML, Cooper, D.M.F. DMF, Sub-picomolar relaxin signaling by a pre-assembled RXFP1, AKAP79, AC2, β-arresting 2, PDE4D3 complex, EMBO 29(16) (2010) 2772–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Civciristov S, Halls ML, Signalling in response to sub-picomolar concentrations of active compounds: Pushing the boundaries of GPCR sensitivity, Brit. J. Pharmacol 176 (2019) 2382–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dharmani M, Mustafa MR, Achike FI, Sim MK, Effect of des-aspartate-angiotensin I on the actions of angiotensin II in the isolated renal and mesenteric vasculature of hypertensive and STZ-induced diabetic rats, Reg. Peptides 129 (2005) 213–219. [DOI] [PubMed] [Google Scholar]
- [19]., Hariton-Shalev A, Shalev M, Adir N, Belausov E, Altstein M, Structural and functional differences between pheromonotropic and melanotropic PK/PBAN rectors, Biochim. Biophys. Acta 1830 (2013) 5036–5048. [DOI] [PubMed] [Google Scholar]
- [20].Shalev AH, Altstein M, Pheromonotropic and melanotropic PK/PBAN receptors: Differential ligand-receptor interactions, Peptides 63 (2015) 81–89. [DOI] [PubMed] [Google Scholar]
- [21].Crain SM, Shen K-F, Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment, Proc. Natl. Acad. Sci 92 (1995) 10540–10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu B, Qin L, Yang S-N, Wilson BC, Liu Y, Hong J-S, Femtomolar concentrations of dynorpins protect rat mesencephalic dopaminergic neurons against inflammatory damage, J. Pharm. Exp. Ther 298 (2001) 1133–1141. [PubMed] [Google Scholar]
- [23].Williamson SA, Knight RA, Lightman SL, Hobbs JR, Effects of beta endorphin on specific immune responses in man, Immunology 65 (1988) 47–51. [PMC free article] [PubMed] [Google Scholar]
- [24].Civciristov S, Ellisdon AM, Suderman R, Pon CK, Evans BA, et al. , 2018. Preassembled GPCR signaling complexes mediate distinct cellular responses to ultralow ligand concentrations. Sci. Signal 11, eaan1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Staus DP, Strachan RT, Manglik A, Pani B, Kahsai AW, et al. , Allosteric nanobodies reveal the dynamic range and diverse mechanisms of G-protein-coupled receptor activation, Nature 535 (2016) 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Offen D, Sherki Y, Melamed E, Fidkin M, Brenneman DE, et al. , Vasocative intestinal peptide (VIP) prevents neurotoxicity in neuronal cultures: Relevance to neuroprotection in Parkinson’s disease, Brain Res. 854 (2000) 257–262. [DOI] [PubMed] [Google Scholar]
- [27].Sowa G, Gekker G, Lipovsky MM, Hu S, Chao CC, et al. Inhibition of swine microglial cell phagocytosis of Cryptococcus neoformans by femtomolar concentrations of morphine, Biochem. Pharmacol 53 (1997) 823–828. [DOI] [PubMed] [Google Scholar]
- [28].Pall ML, Sexual induction in Volvox-carteria-quantitative study, J. Cell. Biol 59 (1973) 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Calabrese EJ, Calabrese V, Giordano J, Role of hormesis in functional performance and protection of neural systems, Brain Circ. 3 (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Calabrese EJ, Hormesis: Why it is important to toxicology and toxicologists, Environ. Toxicol. Chem 27 (2008) 1451–1474. [DOI] [PubMed] [Google Scholar]
- [31].Calabrese EJ, Kozumbo WJ, 2021. The hormetic dose-response mechanism: Nrf2 Activation, Pharm. Res 167, 105526. [DOI] [PubMed] [Google Scholar]
- [32].Giordano J, The mechanistic paradox: The relationship of science, technology, ethics and policy, Synesis: A J. Sci. Techn. Ethics Policy 1 (2010) G1–4. [Google Scholar]
- [33].Ives JA, Giordano J, Unusual claims, normative process: On the use and stringency of scientific method, Forsch. Komplement 14 (2007) 138–139. [DOI] [PubMed] [Google Scholar]
- [34].Giordano J, Ives JA, Jonas WB, Hormetic responses in neural systems: Consideration, contexts, and caveats, Crit. Rev. Toxicol 38 (2008) 1–5. [DOI] [PubMed] [Google Scholar]


