Abstract
Background:
Pre-injury running biomechanics are an ideal comparator for quantifying recovery of running biomechanics following anterior cruciate ligament reconstruction (ACLR), allowing for assessments within both the surgical and non-surgical limbs. However, availability of pre-injury running biomechanics is rare and has only been reported in case studies.
Hypothesis/Purpose:
To determine if running biomechanics return to pre-injury levels within the first year post-ACLR among collegiate athletes. We hypothesized that surgical knee biomechanics would be significantly reduced shortly after ACLR and not return to pre-injury levels by 12 months, and non-surgical limb mechanics would change significantly from pre-injury.
Study Design:
Analysis of routinely collected athletic performance data; Level of Evidence, 3.
Methods:
Thirteen Division I collegiate athletes were identified between 2015 and 2020 (6 female; 20.7±1.3 years old) who had whole body kinematics and ground reaction forces recorded during treadmill running (3.7 ± 0.6 m/s) prior to sustaining an ACL injury. Running analyses were repeated at 4 (4M), 6 (6M), 8 (8M), and 12 (12M) months post-ACLR. Linear mixed effects models were used to assess differences in running biomechanics between post-ACLR time-points and pre-injury within each limb, reported as Tukey-adjusted p-values.
Results:
Compared to pre-injury, the surgical limb displayed significant deficits at all post-operative assessments (p-values <0.01, reported as least square mean difference ± standard error): peak knee flexion angle (4M: 13.2°±1.4, 6M: 9.8°±1.4, 8M: 9.7°±1.4, 12M: 9.0°±1.5); peak knee extensor moment (4M: 1.32±0.13, 6M: 1.04±0.13, 8M: 1.04±0.13, 12M: 0.87±0.15 Nm/kg; 38 to 57% deficit); and rate of knee extensor moment (4M: 22.7±2.4, 6M: 17.9±2.3, 8M: 17.5±2.4, 12M: 16.1±2.6 Nm/kg/s; 33 to 46% deficit). No changes for these variables from pre-injury (p-values > 0.88) were identified in the non-surgical limb.
Conclusion:
Following ACLR, surgical limb knee running biomechanics were not restored to the pre-injury state by 12M, while non-surgical limb mechanics remained unchanged compared to pre-injury.
Clinical Relevance:
Collegiate athletes post-ACLR demonstrate substantial deficits in running mechanics compared to pre-injury that persist beyond the typical return-to-sport timeframe. The non-surgical knee appears to be a valid reference for recovery of the surgical knee mechanics during running, due to the lack of change within the non-surgical limb.
Key Terms: ACL, Running, Gait analysis, Motion Analysis/Kinesiology
Introduction:
Following anterior cruciate ligament reconstruction (ACLR), athletes demonstrate significant alterations in surgical limb running biomechanics compared to both their non-surgical limb and healthy controls.20 These changes include: decreased peak knee flexion angle9,20 and knee flexion excursion,20 decreased peak knee extensor moment,9,20,24 decreased patellofemoral joint force,36 increased initial impact forces,18 and decreased peak vertical ground reaction force.24 Compensatory loading strategies, particularly at the hip,2,18 have also been observed, indicating changes extend beyond the knee joint. Running asymmetries have been observed up to 5 years post-operatively, despite rehabilitation typically ending 6–12 months post-surgery.18 Persistent alterations in movement mechanics and knee joint loading may contribute to the early onset and progression of knee osteoarthritis after ACLR.1,5,36 Understanding the longitudinal changes in running biomechanics during the initial year following ACLR, when athletes typically have access to rehabilitation, is a necessary step toward identifying appropriate strategies aimed at improving running deficits in hopes of mitigating the sequela of joint disease.
Previous studies of running mechanics post-ACLR have used cross-sectional designs where the non-surgical limb or healthy controls are used for comparison.20 The appropriateness of using the non-surgical limb as a comparative reference has come into question due to the potential for bilateral neuromuscular and biomechanical performance deficits to be present after injury and/or surgery.14,22,41 For example, changes in walking mechanics have been observed over time in the non-surgical limb following ACLR.6 As such, the appropriateness of using the non-surgical limb as a reference to evaluate recovery of the surgical limb must be assessed, particularly with regard to running mechanics.
To this end, one must compare longitudinal post-operative data with pre-injury data, allowing for bilateral assessment of within-limb changes over time. Baseline pre-injury data are not commonly available due to the unpredictable nature of ACL injures.17 To date, only a few case studies have been published comparing pre-injury and post-operative running mechanics with limited generalizability.29,30 Preseason assessments of running biomechanics among collegiate athletes at the University of Wisconsin-Madison have established a database of healthy baseline measures on hundreds of athletes. In the event an athlete suffers an ACL injury, the pre-injury baseline provides a unique reference for determining post-operative changes in the surgical and non-surgical limbs.
The purpose of this study, therefore, was to assess the longitudinal changes in running biomechanics throughout the first year post-ACLR compared to the pre-injury state among National Collegiate Athletic Association (NCAA) Division I collegiate athletes. We hypothesized that knee joint kinematics and kinetics and ground reaction forces (GRF) of the surgical limb during running would be significantly reduced shortly after ACLR and not return to the pre-injury state by 12-months. Additionally, we hypothesized that the non-surgical limb kinematics, kinetics, and GRF would change significantly from the pre-injury assessment. Finally, we explored longitudinal changes in hip and ankle joint kinetics (i.e., negative work).
Materials and Methods
Participants
Six years (2015–2020) of routinely collected pre-season performance data and post-ACLR testing available on NCAA Division I athletes in the University of Wisconsin-Madison Badger Athletic Performance database were used for this study (329 unique athletes with baseline running analyses). The record review was approved by the University’s Health Sciences Institutional Review Board. Records were included if the athlete: 1) had an ACL injury and underwent a primary ACLR; 2) had no history of a previous ACL injury on either limb, 3) had a healthy, pre-injury running gait analysis and at least one post-operative running gait analysis; and 4) did not have a lower extremity surgery 12 months prior to the pre-injury running analysis. For athletes that met all of the above criteria, but sustained a second ACL injury or underwent a subsequent lower extremity surgery any time after the initial post-operative running gait analysis, only running gait analyses prior to these injuries were included.
Data Collection and Analysis
The running mechanics collection protocol utilized has been described in detail previously.39 In brief, athletes walked for two minutes to acclimate to the treadmill and motion capture setup. For healthy baseline testing, athletes ran at standardized speeds of 2.68, 2.95, 3.35, 3.80, and 4.47 m/s (10, 9, 8, 7, and 6 min/mile, respectively). Following ACLR, athletes underwent a standardized testing protocol which included running gait analyses performed at 4, 6, 8, and 12 months post-operatively. During post-ACLR testing, speed was increased incrementally, following a similar progression to baseline testing, and athletes were asked to identify when they felt they achieved their maximally comfortable speed; testing was ended following collection of that speed. Fatigue was not directly assessed, but athletes were advised to verbalize if they were feeling fatigued. If fatigue was reported, speed was reduced to a walking pace until the athlete reported readiness to commence running. Fifteen seconds of data were recorded at each running speed after the athlete had acclimated to the speed for at least 30 seconds. Speed was controlled within each athlete by analyzing the maximum speed available that was consistent across all time points (pre-injury and post-ACLR), but speed was allowed to vary between athletes.
Whole-body kinematics were collected using 42 reflective markers placed on the body segments of each athlete, 23 of which were located on anatomical landmarks.7 Markers were placed by the same researcher [MRSJ] for all data collections on all athletes. A static standing position was also recorded to establish joint centers and for model scaling. Marker kinematics were collected at 200 Hz using an 8-camera passive marker system (Motion Analysis Corporation, Santa Rosa, CA). Ground reaction forces were recorded at 2000 Hz using an instrumented treadmill (Bertec Corporation, Columbus, OH) and synchronized with the kinematic marker data. To identify foot contacts and calculate GRF metrics, the GRFs were low-pass filtered using a bi-directional, 3rd-order Butterworth filter with a cutoff frequency of 50 Hz.39 Gait cycles were identified by foot contacts, with the stance phase of the gait cycle defined from initial contact to toe off, when the vertical GRF rose above and fell below 50 N, respectively.
Biomechanical modeling and analyses have been described in detail.7 Briefly, the body was modeled as a 14-segment articulated linkage and body segments were scaled using the athlete’s height, mass, and segment lengths.15 Joint angles were computed at each time step using a global optimization routine to minimize the weighted sum of squared differences between the measured and the model marker positions.16 In addition, a segment-by-segment inverse dynamics analysis was used to calculate joint moments from the kinematic data and GRFs, both low-pass filtered using a bi-directional, 4th-order Butterworth filter at 12 Hz. Joint powers were computed as the product of the moment and angular velocity for each joint, with mechanical work determined by numerically integrating the respective portions of each joint power curve.
Fifteen strides were analyzed on both limbs from each athlete and included the following biomechanical variables: peak knee flexion during stance; peak knee extensor moment during stance; rate of knee extensor moment during stance; hip, knee, ankle, and total negative work during stance (summation of hip, knee, and ankle negative work); vertical GRF impulse; and braking impulse. All signal and data processing were conducted using MATLAB (Release 2018b, Mathworks, Inc., Natick, MA).
Statistical Analysis
Standard descriptive statistics (means/standard deviations and frequencies/percentages) were used to describe the patient population. Linear mixed effects models were used to assess the influence of time-point and limb, and a potential interaction effect, on each biomechanical variable of interest. Subject and limb were assigned as random effects. For variables in which a significant interaction was detected, Tukey-adjusted p-values were used for pairwise comparisons between pre-injury and post-operative time-points for the surgical and non-surgical limb separately. Speed was controlled within each athlete by using the maximum speed that was consistent across all collections for that athlete; however, due to the small sample size between-athlete variability due to speed was not assessed. Least-square mean differences and associated standard errors are reported. All analyses were conducted using SAS v9.4 (SAS Institutes, Cary, NC) and significance was assessed at α ≤ 0.05.
Results
Records for 13 athletes met eligibility criteria and were included in the final dataset (Figure 1). All athletes underwent ACLR with bone-patellar-tendon-bone autograft. Five athletes underwent ACLR only, 3 underwent ACLR + meniscectomy, and 5 underwent ACLR + meniscus repair. Athletes participated in football (7), basketball (2), soccer (3), or track and field (1). All athletes completed a pre-injury running analysis (14.6 ± 11.9 months prior to surgery). Post-operatively, 9, 10, 9, and 7 athletes completed testing at 4, 6, 8, and 12 months, respectively. One athlete sustained a contralateral ACL injury (10-months post-op) and two others underwent additional lower extremity surgeries (5 and 7 months post-op). The majority of the athletes (9/13 athletes) in our study started running between 3- and 4-months post-surgery. The average speed used for comparisons was 3.65±0.59 m/s (7:20 min/mile) and no athletes reported fatigue during testing. Additional patient demographics appear in Table 1.
Figure 1.
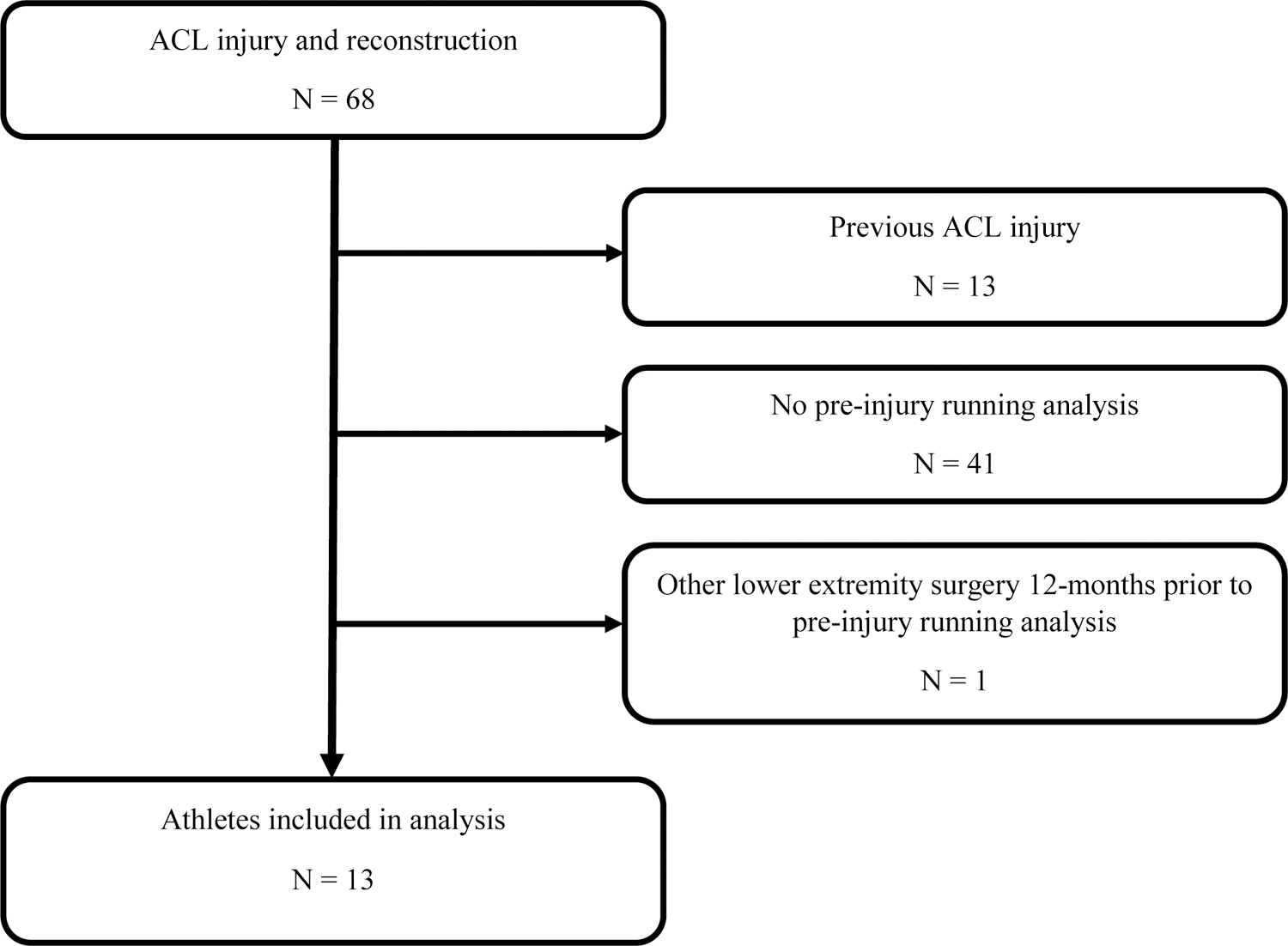
Records extraction process. All athletes included in the analysis had running data from pre-injury and at least one post-operative time point. ACL, anterior cruciate ligament.
Table 1.
Summary of participant information.
| Participant Information (N = 13)a | |
|---|---|
| Values | |
| Age, y* | 20.7 ± 1.3 |
| Body Mass, kg* | 84.7 ± 18.5 |
| Height, cm* | 179.3 ± 8.9 |
| Females, n (% of total participants) | 6 (46) |
| Running Speed, m/s | 3.65 ± 0.59 |
| Running Analysis From Time of ACLR, mo | |
| Pre-injury | 14.6 ± 11.9 |
| 4-month | 3.9 ± 0.4 |
| 6-month | 6.1 ± 0.3 |
| 8-month | 8.4 ± 0.8 |
| 12-month | 12.2 ± 1.3 |
| IKDC, % | |
| 4-month | 62.6 ± 18.7 |
| 6-month | 75.3 ± 14.4 |
| 8-month | 90.3 ± 7.2 |
| 12-month | 91.0 ± 14.3 |
Values are presented as mean ± SD unless otherwise indicated.
Based on 4-month post-operative assessment. ACLR, anterior cruciate ligament reconstruction IKDC, International Knee Documentation Committee survey.
A significant time*limb interaction was detected for peak knee flexion angle, peak knee extensor moment, rate of knee extensor moment, and knee negative work (all p-values<0.001). No significant changes in the non-surgical limb knee biomechanics compared to pre-injury were identified throughout the first 12 months post operatively (Figure 2, Table 2); however, the surgical knee demonstrated significant reductions in all of these metrics at all time-points compared to pre-injury (all p-values<0.001; Table 2). The largest deficits (indicated by the negative sign) in the surgical knee mechanics compared to pre-injury mechanics were observed at 4 months and did not return to pre-injury levels by 12 months [peak knee flexion angle: 4-months = −13.2°±1.4 (p<0.001), 12-months = −9.0°±1.5 (p<0.001); peak knee extensor moment: 4-months = −1.32±0.13 Nm/kg (p<0.001), 12-months = −0.87±0.15 Nm/kg (p<0.001); rate of knee extensor moment: 4-months = −22.7±2.4 Nm/kg/s (p<0.001), 12-months = −16.1±2.6 Nm/kg/s (p<0.001)]. All time-points are reported in Figure 2 and Table 2. Knee negative work was significantly reduced at all follow-up time-points within the surgical limb (4 months: −0.33±0.04 J/kg, p<0.001; 6 months: −0.27±0.04 J/kg, p<0.001; 8 months: −0.25±0.04 J/kg, p<0.001; and 12 months: −0.22±0.05 J/kg, p=0.002) (Figure 3).
Figure 2.
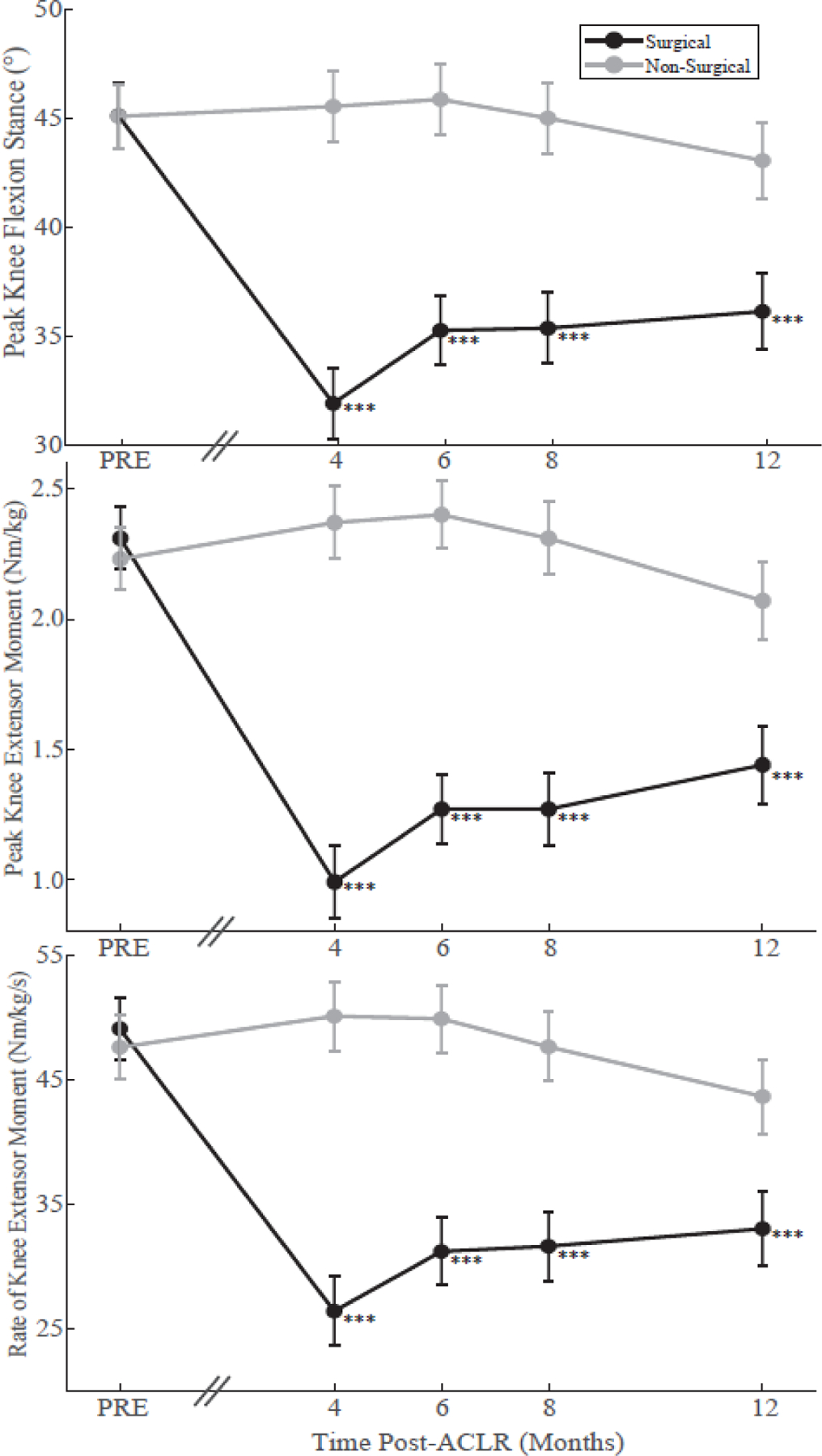
Least-square mean values of peak knee flexion angle during stance (top), peak knee extensor moment during stance (middle), and rate of knee extensor moment during stance (bottom) from pre-injury (PRE) to 12-months post-anterior cruciate ligament reconstruction (ACLR) within the surgical (black) and non-surgical limb (grey). Error bars depict the standard error of the least-square mean. The axis break between pre-injury and 4-months demonstrates that the time interval between pre-injury and 4-months varies between participants. *** Significant within-limb difference from pre-injury p < 0.001.
Table 2.
Least-square means, standard errors (SE), mean differences from pre-injury, and Tukey-adjusted p-values for surgical and non-surgical limb running biomechanical variables of interest at all time-points. A negative value notes a deficit in the post-operative collection compared to pre-injury.
| Biomechanical Variable | Limb | Pre-Injury | 4-Months | 6-Months | 8-Months | 12-Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean Difference from Pre-Injury (% Change) | p-value | Mean (SE) | Mean Difference from Pre-Injury (% Change) | p-value | Mean (SE) | Mean Difference from Pre-Injury (% Change) | p-value | Mean (SE) | Mean Difference from Pre-Injury (% Change) | p-value | ||
| Vertical Ground Reaction Force Impulse (Ns/kg) | Surgical | 3.58 (0.07) | 3.27 (0.07) | −0.31 (−8.7%) | <0.001 | 3.34 (0.07) | −0.24 (−6.7%) | <0.001 | 3.45 (0.07) | −0.13 (−3.6%) | 0.13 | 3.46 (0.08) | −0.12 (−3.4%) | 0.36 |
| Non-Surgical | 3.54 (0.07) | 3.65 (0.07) | 0.11 (3.1%) | 0.22 | 3.66 (0.07) | 0.12 (3.4%) | 0.13 | 3.69 (0.07) | 0.15 (4.2%) | 0.04 | 3.57 (0.07) | 0.03 (0.8%) | 0.99 | |
| Braking Ground Reaction Force Impulse (Ns/kg) | Surgical | 0.21 (0.007) | 0.17 (0.008) | −0.04 (−19.0%) | <0.001 | 0.18 (0.008) | −0.03 (−14.3%) | <0.001 | 0.19 (0.008) | −0.02 (−9.5%) | 0.01 | 0.20 (0.008) | −0.01 (−4.8%) | 0.87 |
| Non-Surgical | 0.21 (0.007) | 0.24 (0.008) | 0.03 (14.3%) | <0.001 | 0.25 (0.008) | 0.04 (19.0%) | <0.001 | 0.25 (0.008) | 0.04 (19.0%) | <0.001 | 0.24 (0.008) | 0.03 (14.3%) | 0.004 | |
| Peak Knee Flexion Angle During Stance (°) | Surgical | 45.2 (1.5) | 32.0 (1.6) | −13.2 (−29.2%) | <0.001 | 35.3 (1.6) | −9.9 (−21.9%) | <0.001 | 35.4 (1.6) | −9.8 (−21.7%) | <0.001 | 36.2 (1.74) | −9.0 (−19.9%) | <0.001 |
| Non-Surgical | 45.1 (1.5) | 45.6 (1.6) | 0.5 (1.1%) | 0.99 | 45.9 (1.6) | 0.8 (1.8%) | 0.99 | 45.0 (1.6) | −0.1 (−0.2%) | 0.99 | 43.1 (1.7) | −2.0 (−4.4%) | 0.94 | |
| Peak Knee Extensor Moment (Nm/kg) | Surgical | 2.31 (0.12) | 0.99 (0.14) | −1.32 (−57.1%) | <0.001 | 1.27 (0.13) | −1.04 (−45.0%) | <0.001 | 1.27 (0.14) | −1.04 (−45.0%) | <0.001 | 1.44 (0.15) | −0.87 (−37.7%) | <0.001 |
| Non-Surgical | 2.23 (0.12) | 2.37 (0.14) | 0.14 (6.3%) | 0.98 | 2.40 (0.13) | 0.17 (7.6%) | 0.94 | 2.31 (0.14) | 0.08 (3.6%) | 0.99 | 2.07 (0.15) | −0.16 (−7.2%) | 0.98 | |
| Rate of Knee Extensor Moment (Nm/kg/s) | Surgical | 49.10 (2.52) | 26.42 (2.76) | −22.68 (−46.2%) | <0.001 | 31.21 (2.70) | −17.89 (−36.4%) | <0.001 | 31.63 (2.77) | −17.47 (−35.6%) | <0.001 | 33.04 (2.97) | −16.06 (−32.7%) | <0.001 |
| Non-Surgical | 47.61 (2.52) | 50.10 (2.76) | 2.49 (5.2%) | 0.99 | 49.89 (2.70) | 2.28 (4.8%) | 0.99 | 47.64 (2.77) | 0.03 (0.1%) | 0.99 | 43.65 (2.97) | −3.96 (−8.3%) | 0.88 | |
Figure 3.
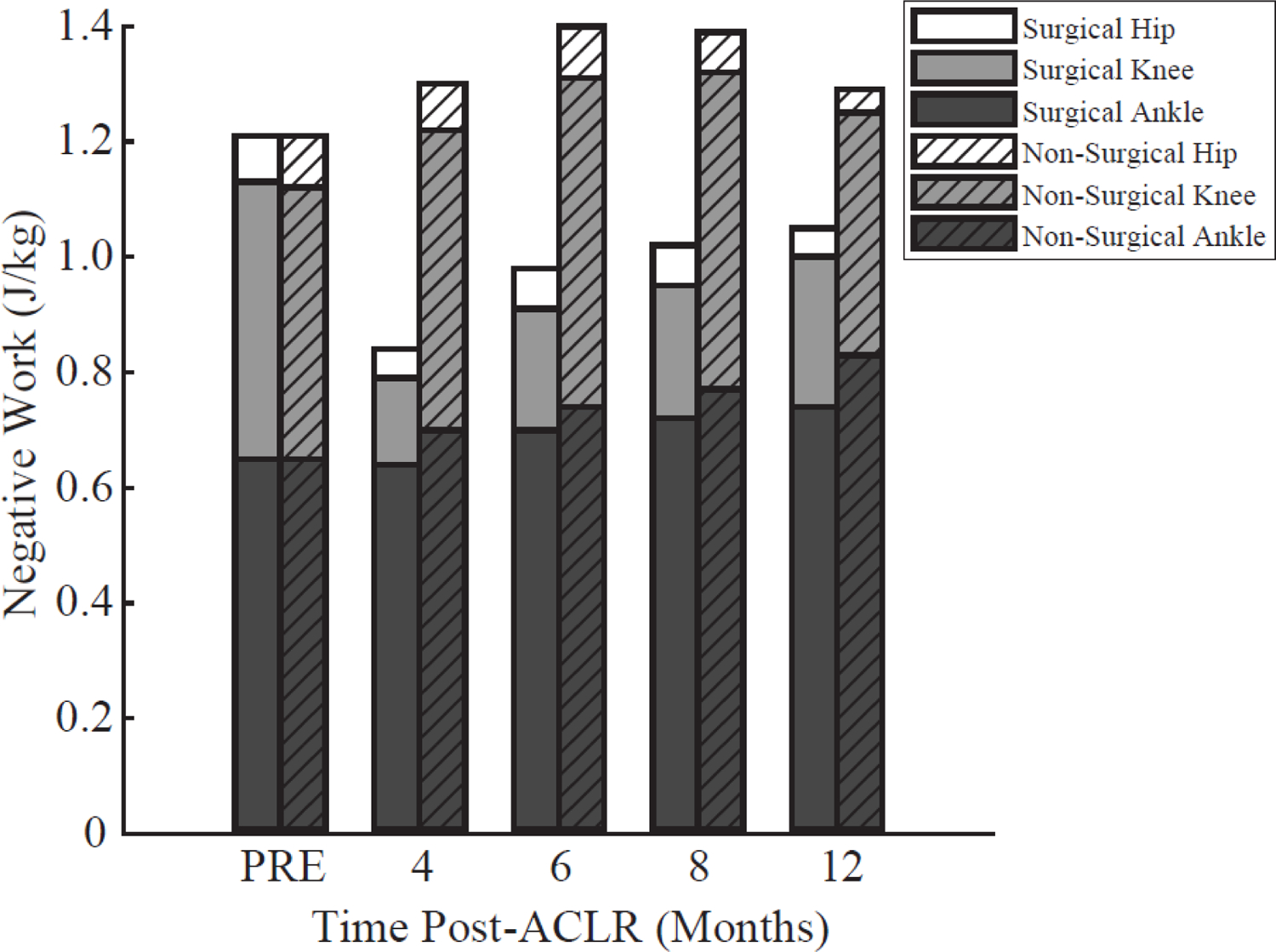
Negative work performed by the hip, knee and ankle of each limb during the stance phase of running at each time point. Knee negative work of the surgical limb was significantly (all p-values <0.001) reduced at all post-anterior cruciate ligament reconstruction (ACLR) time-points relative to pre-injury (PRE). Solid filled bars depict the surgical limb, while diagonal slash filled bars depict the non-surgical limb.
A significant time*limb interaction was detected for total negative work (p-value < 0.001), but not for hip or ankle negative work (p values>0.71). Total negative work was significantly reduced within the surgical limb at 4 (−0.37±0.06 J/kg, p<0.001) and 6 months (−0.23±0.06 J/kg, p=0.01), but not at 8 (−0.18±0.06 J/kg, p=0.12) or 12 months (−0.15±0.07 J/kg, p=0.38) (Figure 3). A time main effect was observed for ankle negative work averaged across both limbs demonstrating an increase at 12 months only (0.13±0.07 J/kg, p=0.01) compared to pre-injury. Within the non-surgical limb, no significant differences in total negative work were detected at any time-point compared to pre-injury (all p-values>0.07) (Figure 3).
A significant time*limb interaction was detected for vertical GRF impulse and braking impulse (p-values<0.001). The surgical limb demonstrated significant reductions in vertical GRF impulse at 4 and 6 months (both p<0.001) and braking impulse at 4, 6, and 8 months (all p-values<0.01). In contrast, the non-surgical limb demonstrated a significant increase in vertical GRF impulse at 8 months (p=0.04) and braking impulse at all time-points (all p-values<0.004; table 2).
Discussion
The purpose of this study was to assess bilateral changes in running biomechanics from pre-injury throughout the first year post-ACLR in NCAA Division I collegiate athletes. Significant alterations were observed, with most metrics not returning to pre-injury values by 12 months post-ACLR on the surgical limb. These data partially supported our primary hypothesis that GRFs and knee joint kinematics and kinetics of the surgical limb during running would not return to pre-injury levels by 12-months following ACLR. This pattern was observed for knee kinematics and kinetics; however, surgical limb vertical GRF and braking impulses were significantly reduced shortly after ACLR, but restored to within pre-injury levels by 6 and 8 months, respectively. Our secondary hypothesis was also partially supported as non-surgical limb GRF impulses were significantly altered, but knee kinematics and kinetics did not change significantly from the pre-injury assessment.
This is the first study, to the authors’ knowledge, to longitudinally quantify changes in running biomechanics throughout the first year post-ACLR compared to pre-injury. Within the surgical limb, significant reductions in peak knee flexion angle, peak knee extensor moment, rate of knee extensor moment, and knee negative work were observed at all time-points compared to the pre-injury values. Previous cross-sectional studies observed significant deficits compared to the non-surgical limb in peak knee flexion angle (pooled mean difference −2.72° [95% confidence interval (CI): −4.45,−0.99]) and stance phase peak knee extensor moment (pooled standardized mean difference −0.62 [95% CI: −0.87,−0.36]) between 3 months and 5 years following ACLR.20 While not directly comparable as we assessed differences from pre-injury, our results demonstrate a greater magnitude of deficit in the surgical limb, with knee flexion deficits during stance ranging from −13.2° to −9.0° and standardized mean difference of peak knee extensor moment during stance ranging from −3.09 to −2.07 throughout the first 12 months post-ACLR (Figure 2). This discrepancy in the magnitude of change within the surgical limb between our study and the systematic review of cross sectional studies may be due, in part, to the majority of studies included in the review reporting data greater than 12 months post-operative. As our study is the first to assess bilateral changes in running mechanics within the first year post-ACLR compared to the pre-injury state, an appropriate comparison dataset does not exist. In a cohort of healthy collegiate athletes, normative between limb asymmetries were 2.2° ± 0.2 for peak knee flexion angle, 9.7±1.0% for peak knee extensor moment, and 15.8±1.5% for knee negative work.38 In the current study, we observed surgical limb deficits at 12-months post-ACLR of 9.0° for peak knee flexion angle, 37.7% for peak knee extensor moment, and 45.8% for knee negative work, which are well outside of these normative ranges. Despite not reporting between-limb asymmetries, comparing our findings to this healthy cohort may provide greater clinical context to the magnitude of deficits observed in our sample. Regardless of the differences in magnitude found in prior studies and the present work, it is evident that surgical limb knee mechanics during running remain significantly altered beyond a time when most athletes return to sport and have completed post-operative rehabilitation. It remains unknown if these altered movement patterns are adopted as a protective mechanism in an attempt to reduce joint stresses or if they may simply be the result of impaired neuromuscular performance.
Our unique dataset provides novel insights into whether the non-surgical limb is an appropriate comparator for the surgical limb. In short, the knee joint metrics of the non-surgical limb were consistent over time compared to the pre-injury state, which may support the use of the non-surgical limb as a comparator for knee-specific biomechanics throughout the rehabilitation process. This particular finding has important clinical implications as most sports medicine facilities utilize the non-surgical limb as a reference when assessing return-to-sport readiness and generally do not have pre-injury data as a comparator. Further studies with larger sample sizes will be needed to corroborate these findings.
Conversely, substantial alterations in GRFs of the non-surgical limb were detected. Braking impulse within the non-surgical limb was increased by 14.3% to 19.0% at all time-points post-ACLR compared to pre-injury, while the surgical limb demonstrated a 19.0% to 9.5% decrease at 4 and 8 months, respectively. Additionally, the non-surgical limb demonstrated a significant increase (4.2%) in vertical GRF impulse at 8 months, while the surgical limb decreased 8.7% and 6.7% at 4 and 6 months, respectively, compared to pre-injury. As such, utilizing the contralateral limb as a reference of recovery for these particular variables will likely conflate the true deficits when compared to using pre-injury as a reference. Moreover, recovery of running biomechanics post-ACLR should not be based solely on GRF variables, as these returned to pre-injury levels within the surgical limb earlier than knee specific kinematics and kinetics. Finally, the greater GRFs observed on the non-surgical limb may increase that limb’s injury risk to a running-related injury, and warrants further investigation.
We also sought to determine the distribution of negative work performed by the joints of the surgical and non-surgical limbs to assess for compensatory movement strategies, as biomechanical and neuromuscular compensations have been observed during walking, running, squatting, and jumping following ACLR.2,10,19,33,35 The underlying cause of these observed compensations may be due, in part, to persistent joint pain,32 impaired quadriceps performance,10,11,21,35,37 reduced joint proprioception,27 or lack of confidence following ACLR.23,28,40 We found significant reductions in total negative work in the surgical limb at 4 and 6 months (p-values<0.01). The significant reduction in knee negative work at all time-points (all p-values<0.002) and the lack of time*limb interaction for hip and ankle negative work suggests that the knee is the primary contributor to the change in the surgical limb’s total negative work (Figure 3). Within the non-surgical limb, a statistically significant change from the pre-injury state was not detected for total negative work, but a potential increase in total negative work post-operatively was observed (p-values = 0.07 and 0.16 at 6 and 8 months, respectively) (Figure 3).
By 3 to 4 months post-surgery the majority of the athletes in our study initiated running. This is consistent with prior research indicating that most athletes resume running ~3 months following ACLR.26 This time period coincides with when the knee mechanics displayed the greatest deficit from pre-injury. Initiating running with such significant gait abnormalities may lead athletes to adopt compensatory movement patterns that are detrimental to long-term joint health.1,5,36 Abnormal quadriceps function is likely a primary contributor to the substantial running deficits observed at the 4- and 6-month time-points. Indeed, deficits in quadriceps neuromuscular performance and strength are nearly universal early after ACLR13,25 and have been associated with reduced knee flexion angles and extensor moments during hopping21 and walking37, as well as rate of knee extensor moment during running.22 Further, subtle changes in quadriceps force production, neuromuscular coordination, and associated movement kinematics can significantly increase knee cartilage loads during activities including walking and running.4,11,12,34,42 The altered running biomechanics we observed throughout the initial year post-ACLR likely have a significant effect on tibiofemoral3,31 and patellofemoral cartilage loading.3,8,36 However, previous studies in this area are of cross-sectional design, which limits our understanding of how knee cartilage loading changes over time. Understanding longitudinal changes in knee cartilage loading is an important next step in this line of research, as altered knee joint loading has been suggested to contribute to early onset osteoarthritis.1,5,36
This is the first study to our knowledge to assess changes in running biomechanics in elite collegiate athletes. As the athletes in this study were highly trained prior to sustaining an ACL injury, results may not generalize to other age groups or levels of competition. Despite frequent and unrestricted access to sports medicine facilities and resources, substantial biomechanical deficits were observed in this cohort. In a general sports medicine population with more restricted access to post-surgical care, the magnitude of the observed running alterations may be even more pronounced. Although not all athletes underwent testing at every time-point, we used linear mixed effects models to account for missing data within subjects. Additionally, all athletes had bone-patellar tendon-bone grafts, limiting the potential generalizability to other graft types. Due to the small sample size of this study, the observed effects may be inflated. The sample size also limited our ability to investigate the effects of potential confounders such as sex and concomitant surgical procedures (e.g. meniscus repair). However, the results from this study support the need for future healthy baseline screening and development of larger datasets to assess for the potential influence of these confounders.
In conclusion, substantial reductions in knee flexion angle, extensor moment, and negative work during running were evident in the surgical limb throughout the first 12 months post-ACLR, when compared to pre-injury. Additionally, the non-surgical limb appears to be a valid reference of recovery for the surgical knee-specific running mechanics following ACLR, but not for GRF variables.
What is known about the subject:
To date, it remains unknown how running biomechanics post-ACLR change from the pre-injury state. Pre-injury data, although rarely available, provides unique insight into the post-surgical deficits in running biomechanics by providing a within-subjects view of individual gait mechanics. Moreover, using pre-injury data as a reference allows for the assessment of changes within both the surgical and non-surgical limb. Previous literature demonstrates that deficits in running biomechanics exist post-ACLR, particularly peak knee flexion angle and peak knee extensor moment during stance, which have been observed from 4 months to 5 years post-ACLR compared to the non-surgical limb or healthy controls. However, previous literature provides limited understanding of longitudinal changes in running biomechanics, as most studies have been of cross-sectional design. Further, previous studies have used the non-surgical limb as a comparator, despite it being unknown if changes in this limb occur. As a result, the non-surgical limb may provide an inaccurate representation of running biomechanical changes post-ACLR.
What this study adds to existing knowledge:
Obtaining pre-injury running biomechanics through the screening of hundreds of healthy collegiate athletes during the pre-season allows for the assessment of the restoration of running biomechanics in athletes that go on to sustain an injury. Using this novel dataset, pre-injury running biomechanics were available for 13 athletes that later went on to sustain an ACL injury. These pre-injury running biomechanics were used as a reference to determine if athletes’ running biomechanics recovered within the first year post-ACLR (assessed at 4, 6, 8, and 12 months post-ACLR). Unique to using pre-injury data as a reference, we were able to determine the changes not only in surgical limb, but also in the non-surgical limb, which is currently unknown. The results of this study highlight that knee kinematic and kinetic deficits persist at 12 months in collegiate athletes compared to the pre-injury state. Moreover, the non-surgical limb knee biomechanics remained unaltered from the pre-injury state throughout the first-year post-operative, validating the use of the non-surgical limb as a reference of recovery for knee specific metrics when pre-injury data is unavailable. As subjects of previous studies assessing running biomechanics post-ACLR have typically been recreational athletes or from the general population, this study provides insight into changes in running mechanics in elite division I collegiate athletes.
References
- 1.Andriacchi TP, Mündermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol 2006;18(5):514–518. doi: 10.1097/01.bor.0000240365.16842.4e [DOI] [PubMed] [Google Scholar]
- 2.Boggess G, Morgan K, Johnson D, Ireland ML, Reinbolt JA, Noehren B. Neuromuscular compensatory strategies at the trunk and lower limb are not resolved following an ACL reconstruction. Gait Posture 2018;60:81–87. doi: 10.1016/j.gaitpost.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 3.Bowersock CD, Willy RW, DeVita P, Willson JD. Reduced step length reduces knee joint contact forces during running following anterior cruciate ligament reconstruction but does not alter inter-limb asymmetry. Clin Biomech 2017;43:79–85. doi: 10.1016/j.clinbiomech.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 4.Brandon SCE, Thelen DG, Smith CR, Novacheck TF, Schwartz MH, Lenhart RL. The coupled effects of crouch gait and patella alta on tibiofemoral and patellofemoral cartilage loading in children. Gait Posture 2018;60:181–187. doi: 10.1016/j.gaitpost.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhari AMW, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc 2008;40(2):215–222. doi: 10.1249/mss.0b013e31815cbb0e [DOI] [PubMed] [Google Scholar]
- 6.Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi TP. Longitudinal changes in knee gait mechanics between 2 and 8 years after anterior cruciate ligament reconstruction. J Orthop Res 2018;36(5):1478–1486. doi: 10.1002/jor.23770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiderscheit BC, Chumanov ES, Michalski MP, Wille CM, Ryan MB. Effects of step rate manipulation on joint mechanics during running. Med Sci Sports Exerc 2011;43(2):296–302. doi: 10.1249/MSS.0b013e3181ebedf4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrington L, Alarifi S, Jones R. Patellofemoral Joint Loads during Running at the Time of Return to Sport in Elite Athletes with ACL Reconstruction. Am J Sports Med 2017;45(12):2812–2816. doi: 10.1177/0363546517716632 [DOI] [PubMed] [Google Scholar]
- 9.Kaur M, Ribeiro DC, Theis J-C, Webster KE, Sole G. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sport Med 2016;46(12):1869–1895. doi: 10.1007/s40279-016-0510-4 [DOI] [PubMed] [Google Scholar]
- 10.Kline PW, Morgan KD, Johnson DL, Ireland ML, Noehren B. Impaired Quadriceps Rate of Torque Development and Knee Mechanics after Anterior Cruciate Ligament Reconstruction with Patellar Tendon Autograft. Am J Sports Med 2015;43(10):2553–2558. doi: 10.1177/0363546515595834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenhart RL, Smith CR, Vignos MF, Kaiser J, Heiderscheit BC, Thelen DG. Influence of step rate and quadriceps load distribution on patellofemoral cartilage contact pressures during running. J Biomech 2015;48(11):2871–2878. doi: 10.1016/j.jbiomech.2015.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenhart RL, Thelen DG, Wille CM, Chumanov ES, Heiderscheit BC. Increasing running step rate reduces patellofemoral joint forces. Med Sci Sports Exerc 2014;46(3):557–564. doi: 10.1249/MSS.0b013e3182a78c3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: A 6-month longitudinal investigation. Scand J Med Sci Sport 2015;25(6):828–839. doi: 10.1111/sms.12435 [DOI] [PubMed] [Google Scholar]
- 14.Lepley AS, Grooms DR, Burland JP, Davi SM, Kinsella-Shaw JM, Lepley LK. Quadriceps muscle function following anterior cruciate ligament reconstruction: systemic differences in neural and morphological characteristics. Exp Brain Res 2019;237:1267–1278. doi: 10.1007/s00221-019-05499-x [DOI] [PubMed] [Google Scholar]
- 15.De Leva P Adjustments to zatsiorsky-seluyanov’s segment inertia parameters. J Biomech 1996;29(9):1223–1230 doi: 10.1016/0021-9290(95)00178-6 [DOI] [PubMed] [Google Scholar]
- 16.Lu TW, O’Connor JJ. Bone position estimation from skin marker co-ordinates using global optimisation with joint constraints. J Biomech 1999;32(2):129–134. doi: 10.1016/S0021-9290(98)00158-4 [DOI] [PubMed] [Google Scholar]
- 17.Montalvo AM, Schneider DK, Yut L, et al. “What’s my risk of sustaining an ACL injury while playing sports?” A systematic review with meta-analysis. Br J Sports Med 2019;53(16):1003–1012. doi: 10.1136/bjsports-2016-096274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noehren B, Wilson H, Miller C, Lattermann C. Long-term gait deviations in anterior cruciate ligament-reconstructed females. Med Sci Sports Exerc 2013;45(7):1340–1347. doi: 10.1249/MSS.0b013e318285c6b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Malley E, Richter C, King E, et al. Countermovement jump and isokinetic dynamometry as measures of rehabilitation status after anterior cruciate ligament reconstruction. J Athl Train 2018;53(7):687–695. doi: 10.4085/1062-6050-480-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pairot-de-Fontenay B, Willy RW, Elias ARC, Mizner RL, Dubé MO, Roy JS. Running Biomechanics in Individuals with Anterior Cruciate Ligament Reconstruction: A Systematic Review. Sport Med 2019;49(9):1411–1424. doi: 10.1007/s40279-019-01120-x [DOI] [PubMed] [Google Scholar]
- 21.Palmieri-Smith RM, Lepley LK. Quadriceps Strength Asymmetry After Anterior Cruciate Ligament Reconstruction Alters Knee Joint Biomechanics and Functional Performance at Time of Return to Activity. Am J Sports Med 2015;43(7):1662–1669. doi: 10.1177/0363546515578252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pamukoff DN, Montgomery MM, Choe KH, Moffit TJ, Garcia SA, Vakula MN. Bilateral alterations in running mechanics and quadriceps function following unilateral anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2018;48(12):960–967. doi: 10.2519/jospt.2018.8170 [DOI] [PubMed] [Google Scholar]
- 23.Paterno MV, Flynn K, Thomas S, Schmitt LC. Self-Reported Fear Predicts Functional Performance and Second ACL Injury After ACL Reconstruction and Return to Sport: A Pilot Study. Sports Health 2018;10(3):228–233. doi: 10.1177/1941738117745806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perraton LG, Hall M, Clark RA, et al. Poor knee function after ACL reconstruction is associated with attenuated landing force and knee flexion moment during running. Knee Surgery, Sport Traumatol Arthrosc 2018;26:391–398. doi: 10.1007/s00167-017-4810-5 [DOI] [PubMed] [Google Scholar]
- 25.Pua Y-H, Mentiplay BF, Clark RA, Ho J-Y. Associations Among Quadriceps Strength and Rate-of-Torque Development 6 Weeks Post Anterior Cruciate Ligament Reconstruction and Future Hop and Vertical Jump Performance: A Prospective Cohort Study. J Orthop Sport Phys Ther 2017;47(11):845–852. doi: 10.2519/jospt.2017.7133 [DOI] [PubMed] [Google Scholar]
- 26.Rambaud AJM, Ardern CL, Thoreux P, Regnaux JP, Edouard P. Criteria for return to running after anterior cruciate ligament reconstruction: A scoping review. Br J Sports Med 2018;52(22):1437–1444. doi: 10.1136/bjsports-2017-098602 [DOI] [PubMed] [Google Scholar]
- 27.Relph N, Herrington L, Tyson S. The effects of ACL injury on knee proprioception: A meta-analysis. Physiother (United Kingdom) 2014;100(3):187–195. doi: 10.1016/j.physio.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 28.Roe C, Jacobs C, Kline P, et al. Correlations of Single-Leg Performance Tests to Patient-Reported Outcomes After Primary Anterior Cruciate Ligament Reconstruction. Clin J Sport Med 2020;Epub(February 6): doi: 10.1097/JSM.0000000000000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samaan MA, Ringleb SI, Bawab SY, Greska EK, Weinhandl JT. Altered lower extremity joint mechanics occur during the star excursion balance test and single leg hop after ACL-reconstruction in a collegiate athlete. Comput Methods Biomech Biomed Engin 2018;21(4):344–358. doi: 10.1080/10255842.2018.1452203 [DOI] [PubMed] [Google Scholar]
- 30.Samaan MA, Ringleb SI, Bawab SY, Greska EK, Weinhandl JT. Anterior cruciate ligament (ACL) loading in a collegiate athlete during sidestep cutting after ACL reconstruction: A case study. Knee 2016;23(4):744–752. doi: 10.1016/j.knee.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 31.Saxby DJ, Bryant AL, Modenese L, et al. Tibiofemoral contact forces in the anterior cruciate ligament-reconstructed knee. Med Sci Sports Exerc 2016;48(11):2195–2206. doi: 10.1249/MSS.0000000000001021 [DOI] [PubMed] [Google Scholar]
- 32.Seeley MK, Park J, King D, Ty Hopkins J. A novel experimental knee-pain model affects perceived pain and movement biomechanics. J Athl Train 2013;48(3):337–345. doi: 10.4085/1062-6050-48.2.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigward SM, Chan M-SM, Lin PE, Almansouri SY, Pratt KA. Compensatory Strategies That Reduce Knee Extensor Demand During a Bilateral Squat Change From 3 to 5 Months Following Anterior Cruciate Ligament Reconstruction. J Orthop Sport Phys Ther 2018;48(9):713–718. doi: 10.2519/jospt.2018.7977 [DOI] [PubMed] [Google Scholar]
- 34.Smith CR, Brandon SCE, Thelen DG. Can altered neuromuscular coordination restore soft tissue loading patterns in anterior cruciate ligament and menisci deficient knees during walking? J Biomech 2019;82:124–133. doi: 10.1016/j.jbiomech.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 35.Spencer A, Davis K, Jacobs C, Johnson D, Ireland ML, Noehren B. Decreased quadriceps force steadiness following anterior cruciate ligament reconstruction is associated with altered running kinematics. Clin Biomech 2020;72:58–62. doi: 10.1016/j.clinbiomech.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 36.Sritharan P, Schache AG, Culvenor AG, Perraton LG, Bryant AL, Crossley KM. Between-Limb Differences in Patellofemoral Joint Forces During Running at 12 to 24 Months After Unilateral Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2020;48(7):1711–1719. doi: 10.1177/0363546520914628 [DOI] [PubMed] [Google Scholar]
- 37.Di Stasi SL, Logerstedt D, Gardinier ES, Snyder-Mackler L. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am J Sports Med 2013;41(6):1310–1318. doi: 10.1177/0363546513482718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stiffler-Joachim MR, Lukes DH, Kliethermes SA, Heiderscheit BC. Lower Extremity Kinematic and Kinetic Asymmetries during Running. Med Sci Sport Exerc 2020;Epub(November 3): doi: 10.1249/MSS.0000000000002558 [DOI] [PubMed] [Google Scholar]
- 39.Stiffler-Joachim MR, Wille CM, Kliethermes SA, Johnston W, Heiderscheit BC. Foot Angle and Loading Rate during Running Demonstrate a Nonlinear Relationship. Med Sci Sports Exerc 2019;51(10):2067–2072. doi: 10.1249/MSS.0000000000002023 [DOI] [PubMed] [Google Scholar]
- 40.Trigsted SM, Cook DB, Pickett KA, Cadmus-Bertram L, Dunn WR, Bell DR. Greater fear of reinjury is related to stiffened jump-landing biomechanics and muscle activation in women after ACL reconstruction. Knee Surgery, Sport Traumatol Arthrosc 2018;26(12):3682–3689. doi: 10.1007/s00167-018-4950-2 [DOI] [PubMed] [Google Scholar]
- 41.Wellsandt E, Failla MJ, Snyder-Mackler L. Limb Symmetry Indexes Can Overestimate Knee Function After Anterior Cruciate Ligament Injury. J Orthop Sport Phys Ther 2017;47(5):334–338. doi: 10.2519/jospt.2017.7285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willson JD, Sharpee R, Meardon SA, Kernozek TW. Effects of step length on patellofemoral joint stress in female runners with and without patellofemoral pain. Clin Biomech 2014;29(3):243–247. doi: 10.1016/j.clinbiomech.2013.12.016 [DOI] [PubMed] [Google Scholar]


