Abstract
The potential of immunotherapy strategies utilizing BNAbs such as 3BNC117 and 10–1074 to limit viral replication while also facilitating clearance of HIV infected cells has heightened interest in identifying the predominant NK effector subset(s) capable of mediating Antibody Dependent Cellular Cytotoxicity (ADCC). Utilizing Advanced Poly-chromatic Flow Cytometry, we identified that CD57 positive NK cells from ART-suppressed PLWH expressed significantly higher levels of the CD16 FcγR Receptor, 2B4 ADCC co-receptor and HLA-DR activation marker while NKG2C positive NK cells expressed significantly higher levels of the CD2 ADCC co-receptor (p<0.001, n=32). Functionally, CD57 positive NK cells from ART-suppressed PLWH with either high or low NKG2C expansion exhibited significantly enhanced degranulation and IFN-gamma production against heterologous gp120-coated ADCC targets coated with HIV reference plasma compared to CD57 negative NK cells (p=0.002, n=11). CD57 positive NK cells from control donors lacking NKG2C expansion also exhibited significantly more degranulation and IFN-gamma production at every timepoint tested against both heterologous ADCC targets (p=0.019, n=9) and HIV-1 infected autologous CD4+ primary T cells coated with BNAbs. Together, our data supports CD57 positive and NKG2C positive NK cells as the predominant ADCC effector subsets capable of targeting HIV-infected CD4+ cells in the presence of 3BNC117 and 10–1074 immunotherapy.
Keywords: PLWH, NK Cells, ADCC, BNAbs, CD57
Graphical Abstract
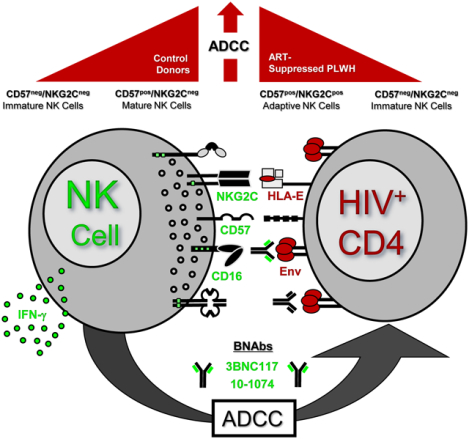
We investigated the NK effector subset(s) that can utilize BNAbs in clearance of HIV-infected targets through ADCC. In control donors, we identified CD57pos mature NK cells exhibited the greatest ADCC poly-functionality against HIV-1 infected targets coated with BNAbs, while NKG2Cpos/CD57pos adaptive NK cells also exhibited strong ADCC capacity in PLWH.
Introduction.
The success of broadly neutralizing antibodies (BNAbs) such as 3BNC117 and 10–1074 in delaying viral rebound during anti-retroviral therapy (ART) interruption (1–3) has highlighted the promise of immuno-therapy approaches utilizing BNAbs in conjunction with Latency Reversing Agents (LRAs) to target the HIV reservoir. In addition to their neutralization capacity, animal models have identified the critical nature of the Fc portion of BNAbs such as 3BNC117 in viral control (4). Together, these results suggest that BNAbs may complement the host antibody response in eliminating infected cells expressing HIV envelope through Antibody-Dependent Cellular Cytotoxicity (ADCC). This is particularly important given the ability of HIV to mutate away from both neutralization and ADCC responses during infection (5–9). Recent evidence from Bruel et al. showing the ability of BNAbs such as 3BNC117 and 10–1074 to trigger robust ADCC by heterologous NK cells against of a wide variety of HIV viral isolates provide further evidence for the advantage of BNAbs in complementing the host humoral ADCC response (10). A critical next step in evaluating the efficacy of BNAb mediated control over HIV-1 in future clinical trials involves identifying and tracking the subset of Natural Killer (NK) cells that serve as effectors during Fc-mediated clearance of HIV.
The ability of NK cells to discriminate between normal and abnormal cells involves complex interactions between inhibitory and activating NK cell receptors (11–14). Under normal conditions, the binding of inhibitory NK cell receptors such as NKG2A, LIR/ILT2 and Killer Immunoglobulin-like Receptors (KIRs) to autologous MHC-class-I (MHC-I) molecules induces negative regulatory signals that switch off NK cells (15–18). During viral infection and tumor transformation, MHC-I downregulation and the displacement of self-peptides leads to a reduction of these inhibitory signals. As a result, NK functional assays using heterologous target cells in vitro (i.e.: targets from different donor than NK cells), may artificially inflate the target cell sensitivity to NK cell lysis due to the potential mismatch in MHC-I ligands and a reduction in NK inhibitory receptor signaling. By contrast, an autologous functional NK assay retains a physiologically relevant KIR/MHC-I interaction and better approximates NK effector cell activity (19–21).
In order to lyse abnormal target cells, NK cells also require the engagement of activating receptors with their ligands on target cells through either the direct or indirect pathway of cytotoxicity. Examples of direct activating NK cell receptors important for natural cytotoxicity include the NKG2D receptor, which recognizes stress-induced ligands, activating KIRs lacking inhibitory motifs, and the Natural Cytotoxicity Receptor Family (NKp46, NKp30, NKp44), which directly recognize viral or cellular antigens (11, 13, 14, 22–25). In contrast, NK lysis through the indirect pathway of killing utilizes the Fc-gamma-RIII (CD16) activating receptor to mediate antigen-dependent NK cytotoxicity (ADCC) in the presence of antibodies specific for viral surface proteins on target cells (16, 26, 27). Additional signals from NK adapter proteins such as 2B4 (23, 28–32) and CD2 (33, 34) may further increase ADCC activity by serving as co-stimulatory signals in synergy with CD16. Cytokines such as IL-2, IL-12, IL-15, IL-21 and Type-I Interferons (IFN-α, β) provided by accessory cells further augment NK lysis of virally infected target cells through both the direct and indirect pathways of cytotoxicity (35–37). While defects in the direct and indirect pathways of NK cytotoxicity have been well documented during viremia in People Living With HIV (PLWH), NK cytotoxic function has been shown to be largely restored following suppression of viremia during prolonged anti-retroviral therapy (ART) (38–43).
Once NK cells mediate cytotoxicity against a target cell, they retain potential to mediate degranulation against additional targets (44) and differentiate into mature NK cells bearing the CD57 maturation marker over time (45, 46). In contrast to T cells where CD57 is expressed upon senescence, the CD57 maturation marker is expressed on a subset of NK cells (typically 40–80% of CD56dim NK cells) that correlates with age and the diversity of the NK repertoire (45, 46). NK cells expressing CD57 have been shown to possess higher NK cytokine production (47) and enhanced ADCC potential (45). In PLWH however, the role of CD57 in marking a specialized NK ADCC effector subset is often confounded by the increased presence of the NKG2C receptor (48, 49). Following acute viremia or viral reactivation by Cytomegalovirus (CMV), a large increase NKG2C positive (NKG2Cpos) NK cells arises in approximately 50% of ART-suppressed PLWH (48, 49). CD57 is co-expressed with NKG2C on these CMV-specific “adaptive” NK cells due to their differentiated status, but CD57 is not directly involved in recognition of virally infected cells (50–52). Rather, NKG2C, due to its interaction with HLA-E presenting peptides from the viral UL40 protein (which mimics the leader peptides from classical MHC Class-1 proteins), represents the NK activating receptor responsible for recognition of CMV infected cells (53–56). A subset of these NKG2Cpos NK cells become deficient in the Fcγ signaling chain over time which allows them to mediate stronger ADCC signaling following utilization of the CD3ζ signaling chain (57–59). NKG2Cpos NK cells from CMV sero-positive individuals also possess increased levels of the CD2 co-receptor for mediating strong ADCC in conjunction with CD16 (33, 34, 60, 61). Together, these phenotypic results help to explain the strong ADCC activity of NKG2Cpos NK cells against CMV infected target cells coated with CMV-specific antibodies (62, 63). However, this observation adds uncertainty as to whether NKG2C or CD57 positive NK subset best tracks with ADCC activity among ART-suppressed PLWH. In this report, we define the phenotypic and functional role of CD57 and NKG2C positive NK cells from ART-suppressed PLWH and control donors to identify the predominant NK effector cell subset that can utilize BNAbs in the clearance of HIV-infected cell targets through ADCC.
Results.
Advanced Poly-chromatic Flow Cytometry Showing ADCC Receptor Expression Among NK Subsets in PLWH.
In order to identify the main NK effector subset capable of facilitating Fc-mediated HIV target clearance in the presence of BNAbs, we measured the phenotypic expression of activation markers and cytotoxicity receptors relevant to ADCC on NK cells expressing CD57 and/or NKG2C from ART-suppressed PLWH utilizing Advanced Poly-chromatic Flow Cytometry (see Supplementary Figure 1A for gating strategy). Among the CD56dim NK subset, we identified a dramatic difference between CD57pos and CD57neg NK cells from ART-suppressed PLWH at the population level by bh-SNE analysis (Figure 1A). In contrast, no clear delineation was observed between NKG2Cpos and NKG2Cneg NK cells from ART-suppressed PLWH (Data Not Shown). One of the most dramatic differences among CD57pos and CD57neg NK cells pertained to NK activation. The geometric mean fluorescence intensity (GMFI) of HLA-DR per cell among CD57pos NK cells was significantly higher (p<0.0001, n=32) compared to undifferentiated NK cells lacking expression of CD57 or NKG2C (Figure 1B). In contrast, NKG2Cpos NK cells did not exhibit higher levels of HLA-DR compared to undifferentiated NK cells, although NK cells expressing both CD57 and NKG2C exhibited the highest level of HLA-DR activation among the NK subsets (Figure 1B).
Figure 1. Advanced Poly-chromatic Flow Cytometry Data Investigating the Expression of Receptors Important for ADCC Activity Among the Different Subsets of NK cells in ART-suppressed PLWH.
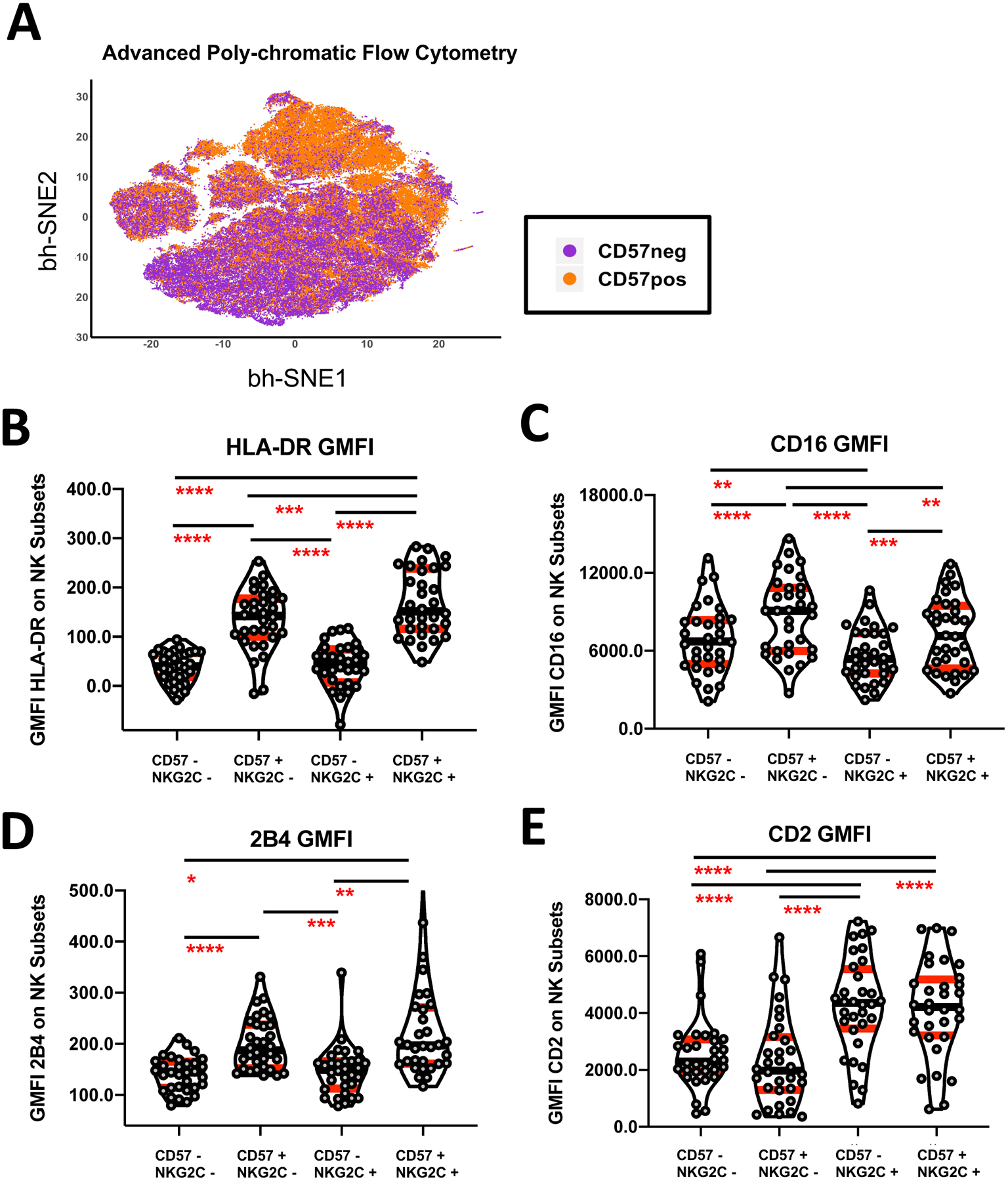
(A) bh-SNE analysis of CD57pos and CD57neg gated NK cells from a cohort of 32 ART-suppressed PLWH stained for Advanced Polychromatic Flow Cytometry. (B-E) Composite data showing the Geometric Mean Fluorescence Intensity (GMFI) of the HLA-DR activation marker (B), the CD16 FcγR receptor (C), the 2B4 ADCC adaptor protein (D), and the CD2 adaptive co-stimulatory marker (E) on CD56dim NK cells from ART-suppressed PLWH after stratification based upon the expression of CD57 and NKG2C (n=32). All ART-suppressed PLWH samples were processed on different days but analyzed for Advanced Poly-chromatic Flow Cytometry together in one experiment to reduce the impact of batch effect. Statistical analyses of four groups was performed using a Repeating-Measure ANOVA with a Geisser-Greenhouse Correction and a Tukey’s Multiple Comparison Test (p value less than 0.05 was considered significant). Error bars in all graphs are displayed as median with interquartile range shown in red.
We next measured the expression of activating NK receptors and co-receptors relevant to ADCC on the different sub populations of NK cells. While the percentage of NK cells expressing the CD16 FcγR ADCC Receptor did not differ among the different subsets of NK cells we studied (Data Not Shown), we observed that CD57pos NK cells expressed higher GMFI of CD16 (p<0.0001, n=32) when compared to undifferentiated NK cells lacking expression of CD57 or NKG2C (Figure 1C). In contrast, NKG2Cpos NK cells lacking CD57 expressed lower GMFI levels of CD16 (p<0.01, n=32), while NK Cells expressing both CD57 and NKG2C had no difference in expression of CD16 compared to undifferentiated NK cells (Figure 1C). In addition to CD16, we measured the expression of adapter proteins such as 2B4 and CD2 on NK cells that may serve as co-stimulatory signals along with CD16 in driving ADCC. CD57pos NK cells expressed a significantly greater (p<0.0001, n=32) GMFI of the 2B4 ADCC adapter protein compared to CD57neg NK cell subsets irrespective of the presence or absence of NKG2C (Figure 1D). In contrast, NKG2Cpos NK cells expressed a significantly greater (p<0.0001, n=32) GMFI of the CD2 ADCC adapter protein compared to NKG2Cneg NK cell subsets irrespective of the presence or absence of CD57 (Figure 1E). Together, these results indicate that both the CD57pos and NKG2Cpos NK cell subsets exhibit phenotypic hallmarks associated with the potential to mediate enhanced ADCC activity in ART-suppressed PLWH.
CD57pos NK cells Exhibit Heightened ADCC in ART-suppressed PLWH Regardless of NKG2C Expansion.
To assess the ADCC function of the different NK subsets in ART-suppressed PLWH, we incubated PBMC with heterologous gp120-coated CEM NK resistant (CEM NKres) targets in the presence of HIV reference plasma from an Elite Controller individual as we previously described (64) and measured the NK cell poly-functional response of CD3−/CD56+ gated NK cells. Importantly, the expression of CD57 on CD56dim NK subset NK cells was unchanged in the presence or absence of ADCC targets during a 3 hour ADCC assay allowing us to evaluate degranulation frequencies between CD57 positive and negative NK subsets over time (data not shown). We further stratified the individuals in our cohort of ART-suppressed PLWH based upon their NK cell expression of NKG2C into two groups: high NKG2C expansion (median 46%, red dots, n=15) and low NKG2C expansion (median under 3%, gray dots, n=17) (Figure 2A). Interestingly, we observed significantly higher percentage of CD57 on NK cells from ART-suppressed PLWH exhibiting higher levels of NKG2C (p<0.0001) compared to those individuals expressing lower levels of NKG2C (Figure 2B). As shown for a representative ART-suppressed PLWH lacking CMV-driven NKG2C expansion, the gp120-coated CEM NKres targets induced strong CD107a degranulation and IFN-gamma production as compared to the No Target Control or NK cells incubated with uncoated CEM NKres targets in the presence of HIV reference plasma (Figure 2C). In accordance with the phenotypic prediction by Advanced Poly-chromatic Flow Cytometry of strong ADCC activity by CD57pos NK cells, we observed that the CD57pos NK cell subset showed a superior ADCC dual-functional response as evidenced by the absolute frequency of NK cells mediating CD107a degranulation and IFN-gamma production compared to the CD57neg NK cell subset (Figure 2C).
Figure 2. The CD57pos NK subset has heightened ADCC activity in ART-suppressed PLWH when compared to CD57neg NK subset in the presence or absence of NKG2C expansion.

(A) Composite graph displaying the percentage of NKG2C positive NK cells within the CD56dim NK cell repertoire of 32 ART-suppressed PLWH. The cohort of ART-suppressed PLWH was divided into two groups based upon having high (red dots, median 46%, n=15) or low (gray, median less than 3%, n=17) levels of NKG2C expansion. (B) Composite graph displaying the significantly increased percentage of CD57pos NK cells among 32 ART-suppressed PLWH with high NKG2C expansion (red dots) as compared to low NKG2C expansion (gray dots). (C) Analysis of dual-functional NK cell response from a representative ART-suppressed PLWH lacking high NKG2C expansion against heterologous ADCC targets as measured by CD107a Degranulation and IFN-gamma production. PBMC were incubated at a 5:1 effector to target cell ratio for three hours alone (No Target Control) or with uncoated CEM NKres (negative control target) or gp-120 coated CEM NKres cells (ADCC target) in the presence or absence of plasma from an Elite Controller PLWH reference sample. The data is shown in a three-parameter density plot with CD107a degranulation on the X-axis, CD57 on the Y-axis, and IFN-gamma production super-imposed on top as red dots. The frequency of CD56dim/CD3− gated NK cells staining positive for CD107a (black) and IFN-gamma (red) is shown in the right-hand quadrants. (D) Composite graph showing the frequency of CD57 positive and CD57 negative NK cells mediating a dual-functional ADCC response (CD107a degranulation and IFN-gamma production simultaneously) within the CD56dim/CD3− NK cell gate of ART-suppressed PLWH exhibiting high (red dots, n=5) or low (gray dots, n=6) levels of NKG2C expansion. (E) Composite graph showing the percentage of NK cells mediating a dual-functional ADCC response within either the CD57 positive or CD57 negative CD56dim/CD3− NK cell gate of ART-suppressed PLWH exhibiting high (red dots, n=5) or low (gray dots, n=6) levels of NKG2C expansion. All Flow Cytometry samples ART-suppressed PLWH were processed on different days in individual experiments for functional analysis. Statistical analyses of two groups was performed using a paired, non-parametric Wilcoxon Signed-Rank Test with a two-tailed p-value (p value less than 0.05 was considered significant). Error bars in all graphs are displayed as median with interquartile range.
Across the cohort of ART-suppressed PLWH tested, the absolute frequency of CD57pos positive NK cells mediating a dual-functional ADCC response was significantly higher (p=0.0029) than that of the CD57neg NK cells (Figure 2D). This result is not unexpected as CD57pos NK cells make up well over 50% of the CD56dim NK repertoire (median 65% to 80% based upon high or low NKG2C expression, Figure 2B) in ART-suppressed PLWH. Therefore, in order to control for the differences between absolute number of CD57pos NK cells and CD57neg NK cells from each individual, we next measured the percentage of NK cells in the CD57pos and CD57neg subsets that are degranulating and producing IFN-gamma. As shown in Figure 2E, the percentage of ADCC dual-functionality within the CD57pos NK cell subset was also significantly higher (p=0.002) than the percentage of ADCC dual-functionality within the CD57neg NK cell subset.
Importantly, the observed increase in dual-functional ADCC activity by CD57pos NK cells was documented in ART-suppressed PLWH exhibiting either low (gray dots) or high (red dots) NKG2C expansion within the NK repertoire (Figure 2D and E). Together, these results support the notion that CD57pos NK cells possess an increased capacity to mediate ADCC in ART-suppressed PLWH that is not dependent on NKG2C co-expression. However, the ability of pathogens like CMV to drive the expansion of NK cells that express increased NKG2C (and by association increased CD57) further boosts NK mediated ADCC capacity of ART-suppressed PLWH with an expanded CD57pos/NKG2Cpos NK cell repertoire.
CD57pos NK cells Possess Stronger ADCC Against BNAb-coated HIV-1 Infected Autologous CD4+ T Cells.
In addition to assessing the role of CD57pos NK cells in mediating ADCC in ART-suppressed PLWH with varying levels of NKG2C expansion, we also assessed the role of CD57 in control donors who do not exhibit NKG2C expansion (median less than 3% NKG2Cpos). In support of our previous findings in ART-suppressed PLWH, we also observed that the GMFI of the CD16 FcγR Receptor was significantly increased (p<0.001, n=11) on CD57pos NK cells when compared to CD57neg NK cells in control donors lacking NKG2C expansion (Supplementary Figure 1B). Functionally, CD57pos NK cells from control donors exhibited significantly increased CD107a degranulation against heterologous ADCC targets at every timepoint tested (p=0.019 at 1 hour to p=0.002 at 3 hours) indicating a faster and stronger magnitude of an ADCC response compared to CD57neg NK cells (Figure 3A). Likewise, we also observed significantly (p=0.039 at 1 hour to p=0.002 at 3 hours) more rapid and robust IFN-gamma production by CD57pos NK cells against heterologous ADCC targets at every timepoint tested compared to CD57neg NK cells (Figure 3B). Importantly, the enhanced ADCC degranulation and cytokine production by CD57pos NK cells was observed both in terms of the absolute frequency of responding cells (Data not shown) as well as when measuring the percentage of dual-functional NK cells in the CD57pos and CD57neg NK gate (Figure 3A and B).
Figure 3. CD57 Positive NK cells from Control Donors Possess a Stronger Dual-functional Response Against Heterologous ADCC Targets Coated with HIV-plasma and Autologous HIV-1 Infected CD4+ Primary T Cells Coated with HIV-specific BNABs.

(A-B) Composite analysis of the ADCC degranulation and cytokine response of 10 control donors over time. PBMC were incubated at a 5:1 effector to target cell ratio for three hours with gp120-coated CEM NKres cells along with plasma from an Elite Controller PLWH reference sample (ADCC target) and the percentage of CD57pos gated and CD57neg gated CD56dim/CD3− NK cells staining positive for CD107a degranulation (A) and IFN-gamma production (B) was determined every hour by flow cytometry (n=10). Statistical analyses of two groups was performed at each timepoint using a paired, non-parametric Wilcoxon Signed-Rank Test with a two-tailed p-value. Individual samples for ADCC assay were processed on different days in individual experiments for functional analysis. (C) gp120 envelope staining (y-axis) and intra-cellular p24 capsid staining (x-axis) of uninfected or HIV-1 NL4-3 infected CD4+ primary T cells from a representative control donor incubated in the presence or absence of HIV envelope-specific BNABs 10–1074 and 3BNC117 and a fluorescently conjugated secondary antibody specific for the Fc portion of human IgG. The data is representative of 3 independent experiments with one sample per experiment. (D) Analysis of dual-functional NK cell response from a representative control uninfected donor against autologous HIV-1 infected ADCC targets as measured by CD107a Degranulation and IFN-gamma production. PBMC were incubated at a 2:1 effector to target cell ratio for three hours with HIV-1 NL4-3 infected autologous CD4+ primary T cells coated with or without the HIV envelope-specific BNABs 10–1074 and 3BNC117. The data is representative of 3 independent experiments with one sample per experiment and shown in a three-parameter density plot with CD107a degranulation on the X-axis, CD57 on the Y-axis, and IFN-gamma production super-imposed on top as red dots. The frequency of CD56dim/CD3− gated NK cells staining positive for CD107a (black) and IFN-gamma (red) is shown in the upper right-hand quadrant and the gates were determined based upon the background from the No Target control condition.
We next investigated if the dominant role of by CD57pos NK cells in mediating ADCC against heterologous HIV gp120-coated ADCC targets also extended to detection of autologous HIV-1 infected CD4+ primary T cells coated with HIV-specific BNABs. Utilizing our previously described autologous assay system (20, 64, 65), we documented high levels of infectivity with the HIV-1 CXCR4-tropic NL4-3 isolate as measured by intracellular p24 expression (Figure 3C). We then coated the HIV-1 infected CD4+ primary T cells targets with the HIV envelope-specific BNAbs 10–1074 (V3 loop) and 3BNC117 (CD4 binding site) at 10 μg/ml which has been shown to represent a readily achievable clinical plasma concentration of BNAb (3). Incubation of HIV-1 infected CD4+ primary T cells with 10–1074 and 3BC117 led to strong envelope expression on p24 positive infected cells (Figure 3C), but an extremely low level of staining against uninfected CD4+ primary T cells (Supplementary Figure 1D). Functionally, the combined presence of 10–1074 and 3BNC117 was able to trigger robust ADCC-specific degranulation and IFN-gamma cytokine response by CD56dim gated NK cells against HIV-1 infected CD4+ primary T cells (Figure 3D). In contrast, the recognition of HIV-1 infected CD4+ primary T cells by CD56dim gated NK cells in the absence of BNAbs was minor as documented by low levels of NK cell degranulation and IFN-gamma production (Figure 3D). Consistent with our previous observations utilizing heterologous ADCC targets, the HIV-specific ADCC response triggered by BNAbs against autologous targets was primarily mediated by the CD57pos NK subset as measured by the absolute frequency of cells exhibiting CD107a degranulation and IFN-gamma production (Figure 3D). Likewise, CD57pos NK cells possessed a significantly higher percentage (p=0.0156) of dual-functional NK cells compared to CD57neg NK cells in response to autologous HIV-1 infected CD4+ primary T cells coated with BNABs 3BNC117 and 10–1074 or HIV reference plasma from an Elite Controller when used separately or in combination (Supplementary Figure 1C). Together, these results indicate that similar to our previous observations in ART-suppressed PLWH, CD57pos NK cells from control donors without NKG2C expansion mediate enhanced ADCC activity against HIV-1 infected autologous CD4+ primary T cells coated with BNAbs.
Discussion.
Phenotypic characterization of the NK cells mediating ADCC against HIV-1 infected targets coated with BNAbs will be a critical next step in evaluating the efficacy of BNAb immunotherapy in viral control during future clinical trials. Here, we identified that CD57pos NK cells represent the dominant subset associated with ADCC activity against HIV-1 infected autologous CD4+ primary T cells coated with the HIV-specific BNAbs 3BNC117 and 10–1074 in control donors (Figure 3D and Supplementary Figure 1C). CD57pos NK cells from control donors also exhibited a significantly greater CD107a degranulation and IFN-gamma response against heterologous ADCC targets at every timepoint tested indicating a faster ADCC response compared to CD57neg NK cells (Figure 3A and B). The use of control donors with minimal NKG2C expansion (median less than 3%) provided a controlled system for measuring the role of CD57 maturation in NK-mediated ADCC activity due to the absence of cofounding effects from CMV re-activation typically associated with PLWH. In ART-suppressed PLWH, CD57pos NK cells also exhibited a significantly stronger ADCC-specific degranulation and cytokine response against gp120-coated targets irrespective of high or low NKG2C expression (Figure 2D and E). However, our observation showing that NKG2C is expanded on approximately half of the ART-suppressed PLWH in our cohort, and this NKG2C expansion is associated with significantly higher expression of CD57 on the NK repertoire (Figure 2B), suggests that both CD57 and NKG2C work in concert as dominant phenotypic markers for ADCC activity within the NK repertoire in the context of HIV infection. This assumption is corroborated by our Advanced Poly-chromatic Flow Cytometry data (Figure 1B through E) showing that both CD57pos and NKG2Cpos NK cell subsets exhibit phenotypic hallmarks associated with enhanced ADCC activity in ART-suppressed PLWH. Together, our work highlights the importance of tracking the frequency of both CD57pos and NKG2Cpos NK cells as potential immune determinants of control in clinical studies administering BNAbs into ART-suppressed PLWH with the intent to increase viral control and clearance.
While “adaptive” NK immune responses mediated by NKG2C are triggered by CMV re-activation (53, 66, 67), they may also be able to target HIV/SIV infected cells directly through HLA-E presentation of viral proteins (66, 68). The additional activating receptor signaling provided by NKG2C on adaptive NK cells may allow them to facilitate greater clearance of HIV infected targets through the direct cytotoxicity pathway. Similarly, NKG2Cpos NK cells have also been shown to downregulate the low-strength Fcγ signaling chain which enhances their ADCC capacity following utilization of the high-strength CD3ζ signaling chain (57–59). While we did not measure the expression of the Fcγ signaling chain in our Advanced Poly-chromatic Flow Cytometry panel in this study, our data showing that NKG2Cpos NK cells from ART-suppressed PLWH possess enhanced levels of the ADCC co-receptor CD2 (Figure 1E) suggests that NKG2Cpos NK cells possess a phenotypic signature of enhanced ADCC capacity. Here, we could not directly explore the ADCC function of CD57+/NKG2C+ NK cells from healthy controls as we only identified one out of sixteen heathy control donors from our cohort with CMV-driven NKG2C expansion. However, in ART-suppressed PLWH where NKG2C expansion is much more prevalent, we documented that NK cells expressing various levels of NKG2C expression maintained strong HIV-specific ADCC poly-functionality (Figure 2D and E). Together, these observations may provide a mechanistic explanation for the inverse correlation observed between the NKG2C marker and the levels of HIV viral replication during acute infection prior to ART (69, 70). The observed clinical correlation between NKG2C expression on NK cells and viral load before the administration of ART may reflect the direct effect of the NKG2C activating receptor on NK function. Alternatively, it may reflect an indirect relationship of NKG2C expression on NK function when adaptive NK expansion drives an increase in CD57 maturation, which may in itself be associated with a reduction in viral load. Future studies will need to address the impact of baseline frequencies of CD57pos and NKG2Cpos NK cells on virological measures in PLWH treated with BNABs following ART interruption.
Materials and Methods.
Subject Criteria and Clinical Assessment.
16 Uninfected control donors and 32 ART-Suppressed People Living with HIV (PLWH) were enrolled from the greater Philadelphia metropolitan area according to Informed Consent Principles. All participants were recruited according to IRB guidelines approved by the Institutional review boards of the University of Pennsylvania, Presbyterian Hospital, Philadelphia FIGHT, and The Wistar Institute (IRB Approval Number 2110176). All ART-suppressed PLWH received anti-retroviral therapy with undetectable viral replication (below 50 copies viral RNA/mL) for a period of at least one year prior to recruitment. All ART-suppressed PLWH were recruited with CD4+ T cell counts above 350 cells/ microliter at the time of draw.
Advanced Polychromatic Flow Cytometry of NK cell Phenotypes.
PBMC from 32 ART-suppressed PLWH were processed on different days and cryo-preserved for subsequent analysis. Frozen PBMC were thawed together and stained for Advanced Polychromatic Flow Cytometry in one experiment to reduce the impact of batch effect. To assure viability, thawed cells were stained with DAPI Live/Dead for 30 minutes at 4 °C in the dark followed by a wash step (PBS + 2% FBS). Cells were then stained according to the Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019. 49: 1457 −1973. The clone and company of origin for all Fluorochrome-conjugated antibodies are listed in Supplementary Table 1. Data acquisition was performed on a BD FACSymphony A5 cytometer and FlowJo software (version 9.9.4, Tree Star, Ashland, OR) was used for all analyses. For SNE plots, manual gating analysis was used to select live NK cells (CD14−/CD19−/CD3−/CD56+/CD16+) as defined in the gating strategy shown in Supplemental Figure 1A and multiparametric analyses were performed using the Cyt program. Briefly, data were transformed, concatenated and Barnes-Hut t-distributed stochastic neighboring embedding (bh-SNE) and Principal Component Analysis (PCA) were performed. Manual gating was used to overlay color on target populations.
Flow Cytometry for NK Functional Assessments.
All cell surface antibodies and isotype controls used at the recommended dilution of 0.25 μg antibody per million cells. Peripheral Blood Mono-nuclear Cells (PBMC) were stained according to the Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019. 49: 1457 −1973. Briefly, PBMC were incubated with fluorescently antibodies to phenotypic and functional markers for 15 minutes at room temperature in the dark, washed twice and fixed for 5 minutes at 4°C with Cytofix Buffer (BD Biosciences, San Jose, CA). The following surface antibodies (with clones shown in parentheses) and their appropriate isotype controls were obtained from BD Biosciences unless otherwise noted: CD69 BV421 (FN50), CD107a PE (H4A3), CD56 PERCP Cy5.5 (B159), CD57 APC (NK-1), CD16 APC-H7 (3G8), CD3 BV510 (UCHT1), CD4 APC-H7 (RPA-T4). Intra-cellular staining for IFN-gamma BV421 (B27) and p24 FITC (Kc57, Beckman Coulter, Pasadena CA) was carried out in 1X Perm/Wash Buffer (BD Biosciences) as described by the manufacturer. For HIV-1 envelope surface staining of gp120, BNAB coated or uncoated HIV-1 infected CD4+ primary T cells were incubated with a 1:100 dilution of Goat anti-human IgG Fc-specific PE conjugated secondary antibody. A minimum of two hundred thousand events were collected on a BD LSR-II Flow Cytometer and samples were subsequently analyzed with FlowJo software (Tree Star Incorporated, Ashland OR).
NK Poly-functional Assay Against Direct or ADCC Targets.
To study heterologous NK ADCC function in PLWH and control donors, 1×106 PBMC were washed and incubated in the presence or absence of 2×105 target cells at a 5:1 effector/target ratio along with 20 μl anti-CD107a monoclonal antibody and 0.133 μl of Golgi-stop (BD Biosciences) in a 200 μl total volume for three hours. Following the degranulation assay, samples were stained with antibodies to NK cell phenotypic and functional markers as described above. The percentage of CD56+/CD3− gated NK cells staining positive for CD107a degranulation and/or cytokine production was assessed every hour following incubation with target cells and calculated after subtraction of background levels of staining in the absence of target cells (No Target Control). A 5:1 effector to target ratio was utilized for Heterologous ADCC targets because it ensures a saturating amount of targets so that every NK cell has access to a target thereby normalizing for differing NK frequency per PBMC among donors. As heterologous ADCC targets, CEM NK resistant (CEM NKres) tumor targets (NIH AIDS Reagent Resource) were coated with 1 μg gp120 from the HIV-1 IIIB isolate (ProSpec Protein Specialists, East Brunswick, NJ) for 30 minutes, washed, and then incubated with a 1/1000 dilution of heat inactivated plasma from an Elite Controller PLWH reference subject for 15 minutes as previously reported (64). As autologous targets, HIV-1 infected or uninfected autologous CD4+ primary T cells were incubated with 10 μg/ml of HIV-specific BNAbs 10–1074 and 3BNC117 for 15 minutes and utilized at a 2:1 E/T ratio. A 2:1 effector to target ratio was utilized to enhance NK degranulation and cytokine response against HIV-1 infected or uninfected autologous CD4+ primary T cells due to the strong KIR/MHC driven NK inhibitory signal present with autologous targets. All ART-suppressed PLWH samples were processed on different days in individual experiments for functional analysis. The cohort of ART-suppressed PLWH was divided into two groups based upon having high (red dots, median 46%) or low (gray dots, median less than 3%) levels of NKG2C expansion, which represents two standard deviations from the mean of NKG2C expression.
HIV-1 Infection.
CD4+ primary T cells were isolated to 99% purity by positive selection using CD4 magnetic bead isolation kit as described by the manufacturer (Miltenyi Corporation). To trigger activation, CD4+ primary T cells were incubated in the presence of 10 μg/ml PHA-p (Sigma Aldrich Corporation, St. Louis, MO) and 100 IU/ml hIL-2 (PeproTech, Rocky Hill, NJ) for 48 hours. 5×106 activated CD4+ T cells were spinfected at 1800 rpm for two hours with 150 ng of p24 containing supernatant of the CXCR4-tropic HIV-1 isolate IIIB or NL4-3 as previously described (20). HIV-1 infected cells were enriched to greater than 75% p24 positivity utilizing CD4 depletion column (Miltenyi Corporation) to remove uninfected cells as previously described (65). HIV-1 infection was determined four days later by measuring intra-cellular levels of the p24 capsid protein and surface levels of gp120 envelope levels by Flow Cytometry as described above. All viral strains were generated and tittered by the University of Pennsylvania Centers for AIDS Research (CFAR).
Statistical Analysis.
All graphic presentations were performed with Prism 8 software (GraphPad Software, La Jolla, CA) and displayed as median with interquartile range. Statistical analyses of two groups was performed using a paired, non-parametric Wilcoxon Signed-Rank Test with a two-tailed p-value. Statistical analyses of four groups was performed using a Repeating-Measure ANOVA with a Geisser-Greenhouse Correction and a Tukey’s Multiple Comparison Test (alpha = 0.05). No data was purposefully excluded from the analysis and all missing data from any subjects is due to technical issues with the assay. Due to limited sample size, reported p-values are not adjusted for multiple testing.
Supplementary Material
Acknowledgments.
The following reagent was obtained through the NIH HIV Reagent Program, Division of AIDS, NIAID, NIH: CEM.NKR Cells, ARP-458, contributed by Dr. Peter Cresswell. Funding for this work was provided by the NIH Martin Delaney BEAT-HIV (UM1 AI126620), DA048728, DA040554, DA049666, AI136756, AI120828, Kean Family Professorship and the Roberts I. Jacobs Fund of the Philadelphia Foundation.
Footnotes
Data Availability Statement.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest.
The authors have no commercial or financial conflict of interest.
Ethics Approval Statement.
All participants were recruited according to IRB guidelines approved by the Institutional review boards of the University of Pennsylvania, Presbyterian Hospital, Philadelphia FIGHT, and The Wistar Institute (IRB Approval Number 2110176). All authors agree with this submission and this article is not currently submitted elsewhere.
Patient Consent Statement.
16 Uninfected control donors and 32 ART-Suppressed People Living with HIV (PLWH) were enrolled from the greater Philadelphia metropolitan area according to Informed Consent Principles.
References.
- 1.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23(2):185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, and Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isitman G, Stratov I, and Kent SJ. Antibody-Dependent Cellular Cytotoxicity and NK Cell-Driven Immune Escape in HIV Infection: Implications for HIV Vaccine Development. Adv Virol. 2012;2012:637208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, et al. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A. 2011;108(18):7505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahalanabis M, Jayaraman P, Miura T, Pereyra F, Chester EM, Richardson B, et al. Continuous viral escape and selection by autologous neutralizing antibodies in drug-naive human immunodeficiency virus controllers. J Virol. 2009;83(2):662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–12. [DOI] [PubMed] [Google Scholar]
- 9.Arendrup M, Nielsen C, Hansen JE, Pedersen C, Mathiesen L, and Nielsen JO. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J Acquir Immune Defic Syndr (1988). 1992;5(3):303–7. [PubMed] [Google Scholar]
- 10.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7:10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blery M, Olcese L, and Vivier E. Early signaling via inhibitory and activating NK receptors. Hum Immunol. 2000;61(1):51–64. [DOI] [PubMed] [Google Scholar]
- 12.Cooper MA, Fehniger TA, and Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. [DOI] [PubMed] [Google Scholar]
- 13.Middleton D, Curran M, and Maxwell L. Natural killer cells and their receptors. Transpl Immunol. 2002;10(2–3):147–64. [DOI] [PubMed] [Google Scholar]
- 14.Tomasello E, Blery M, Vely F, and Vivier E. Signaling pathways engaged by NK cell receptors: double concerto for activating receptors, inhibitory receptors and NK cells. Semin Immunol. 2000;12(2):139–47. [DOI] [PubMed] [Google Scholar]
- 15.Biassoni R, Ugolotti E, and De Maria A. NK cell receptors and their interactions with MHC. Curr Pharm Des. 2009;15(28):3301–10. [DOI] [PubMed] [Google Scholar]
- 16.Long EO, Burshtyn DN, Clark WP, Peruzzi M, Rajagopalan S, Rojo S, et al. Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev. 1997;155:135–44. [DOI] [PubMed] [Google Scholar]
- 17.Vivier E, and Romagne F. Good news, bad news for missing-self recognition by NK cells: autoimmune control but viral evasion. Immunity. 2007;26(5):549–51. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama WM, and Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–54. [DOI] [PubMed] [Google Scholar]
- 19.Bonaparte MI, and Barker E. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. Aids. 2003;17(4):487–94. [DOI] [PubMed] [Google Scholar]
- 20.Tomescu C, Chehimi J, Maino VC, and Montaner LJ. NK Cell Lysis of HIV-1-Infected Autologous CD4 Primary T Cells: Requirement for IFN-Mediated NK Activation by Plasmacytoid Dendritic Cells. J Immunol. 2007;179(4):2097–104. [DOI] [PubMed] [Google Scholar]
- 21.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, et al. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 2008;4(7):e1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottino C, Biassoni R, Millo R, Moretta L, and Moretta A. The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Hum Immunol. 2000;61(1):1–6. [DOI] [PubMed] [Google Scholar]
- 23.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. [DOI] [PubMed] [Google Scholar]
- 24.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–60. [DOI] [PubMed] [Google Scholar]
- 25.Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31(4):1076–86. [DOI] [PubMed] [Google Scholar]
- 26.Raghavan M, and Bjorkman PJ. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996;12:181–220. [DOI] [PubMed] [Google Scholar]
- 27.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. [DOI] [PubMed] [Google Scholar]
- 28.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, et al. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–14. [DOI] [PubMed] [Google Scholar]
- 29.Bryceson YT, March ME, Ljunggren HG, and Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorsch M, Urlaub D, Bonnemann V, Brode P, Sandusky M, and Watzl C. Quantitative analysis of human NK cell reactivity using latex beads coated with defined amounts of antibodies. Eur J Immunol. 2020;50(5):656–65. [DOI] [PubMed] [Google Scholar]
- 31.Makaryan SZ, and Finley SD. Enhancing network activation in natural killer cells: predictions from in silico modeling. Integr Biol (Camb). 2020;12(5):109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sivori S, Parolini S, Falco M, Marcenaro E, Biassoni R, Bottino C, et al. 2B4 functions as a co-receptor in human NK cell activation. Eur J Immunol. 2000;30(3):787–93. [DOI] [PubMed] [Google Scholar]
- 33.Tang JJ, Sung AP, Guglielmo MJ, Navarrete-Galvan L, Redelman D, Smith-Gagen J, et al. Natural Killer (NK) Cell Expression of CD2 as a Predictor of Serial Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC). Antibodies (Basel). 2020;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voltarelli JC, Gjerset G, and Anasetti C. Adhesion of CD16+ K cells to antibody-coated targets is mediated by CD2 and CD18 receptors. Immunology. 1993;79(3):509–11. [PMC free article] [PubMed] [Google Scholar]
- 35.Biron CA, Nguyen KB, Pien GC, Cousens LP, and Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. [DOI] [PubMed] [Google Scholar]
- 36.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71(2):173–83. [PubMed] [Google Scholar]
- 37.Varchetta S, Oliviero B, Mavilio D, and Mondelli MU. Different combinations of cytokines and activating receptor stimuli are required for human natural killer cell functional diversity. Cytokine. 2013;62(1):58–63. [DOI] [PubMed] [Google Scholar]
- 38.Azzoni L, Papasavvas E, Chehimi J, Kostman JR, Mounzer K, Ondercin J, et al. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168(11):5764–70. [DOI] [PubMed] [Google Scholar]
- 39.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur J Immunol. 2003;33(9):2410–8. [DOI] [PubMed] [Google Scholar]
- 40.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100(25):15011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alter G, and Altfeld M. NK cell function in HIV-1 infection. Curr Mol Med. 2006;6(6):621–9. [DOI] [PubMed] [Google Scholar]
- 42.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114(18):3822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia M, Li D, He X, Zhao Y, Peng H, Ma P, et al. Impaired natural killer cell-induced antibody-dependent cell-mediated cytotoxicity is associated with human immunodeficiency virus-1 disease progression. Clin Exp Immunol. 2013;171(1):107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomescu C, Chehimi J, Maino VC, and Montaner LJ. Retention of viability, cytotoxicity, and response to IL-2, IL-15, or IFN-{alpha} by human NK cells after CD107a degranulation. J Leukoc Biol. 2009;85:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116(19):3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheiter M, Lau U, van Ham M, Bulitta B, Grobe L, Garritsen H, et al. Proteome analysis of distinct developmental stages of human natural killer (NK) cells. Mol Cell Proteomics. 2013;12(5):1099–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med. 2015;7(297):297ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babic M, Krmpotic A, and Jonjic S. All is fair in virus-host interactions: NK cells and cytomegalovirus. Trends Mol Med. 2011;17(11):677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geary CD, and Sun JC. Memory responses of natural killer cells. Semin Immunol. 2017;31:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nielsen CM, White MJ, Goodier MR, and Riley EM. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front Immunol. 2013;4:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunemund V, Jungalwala FB, Fischer G, Chou DK, Keilhauer G, and Schachner M. The L2/HNK-1 carbohydrate of neural cell adhesion molecules is involved in cell interactions. J Cell Biol. 1988;106(1):213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cebo C, Durier V, Lagant P, Maes E, Florea D, Lefebvre T, et al. Function and molecular modeling of the interaction between human interleukin 6 and its HNK-1 oligosaccharide ligands. J Biol Chem. 2002;277(14):12246–52. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108(36):14725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendricks DW, Balfour HH Jr., Dunmire SK, Schmeling DO, Hogquist KA, and Lanier LL. Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J Immunol. 2014;192(10):4492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Botet M, Angulo A, and Guma M. Natural killer cell receptors for major histocompatibility complex class I and related molecules in cytomegalovirus infection. Tissue Antigens. 2004;63(3):195–203. [DOI] [PubMed] [Google Scholar]
- 56.Revilleza MJ, Wang R, Mans J, Hong M, Natarajan K, and Margulies DH. How the virus outsmarts the host: function and structure of cytomegalovirus MHC-I-like molecules in the evasion of natural killer cell surveillance. J Biomed Biotechnol. 2011;2011:724607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muccio L, Falco M, Bertaina A, Locatelli F, Frassoni F, Sivori S, et al. Late Development of FcepsilonRgamma(neg) Adaptive Natural Killer Cells Upon Human Cytomegalovirus Reactivation in Umbilical Cord Blood Transplantation Recipients. Front Immunol. 2018;9:1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peppa D, Pedroza-Pacheco I, Pellegrino P, Williams I, Maini MK, and Borrow P. Adaptive Reconfiguration of Natural Killer Cells in HIV-1 Infection. Front Immunol. 2018;9:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, et al. Identification of human NK cells that are deficient for signaling adaptor FcRgamma and specialized for antibody-dependent immune functions. Int Immunol. 2012;24(12):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Comeau EM, Holder KA, Fudge NJ, and Grant MD. Cytomegalovirus-Driven Adaption of Natural Killer Cells in NKG2C(null) Human Immunodeficiency Virus-Infected Individuals. Viruses. 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rolle A, Halenius A, Ewen EM, Cerwenka A, Hengel H, and Momburg F. CD2-CD58 interactions are pivotal for the activation and function of adaptive natural killer cells in human cytomegalovirus infection. Eur J Immunol. 2016;46(10):2420–5. [DOI] [PubMed] [Google Scholar]
- 62.Costa-Garcia M, Vera A, Moraru M, Vilches C, Lopez-Botet M, and Muntasell A. Antibody-mediated response of NKG2Cbright NK cells against human cytomegalovirus. J Immunol. 2015;194(6):2715–24. [DOI] [PubMed] [Google Scholar]
- 63.Michelo CM, van Cranenbroek B, Touw P, Claas FHJ, van der Meer A, and Joosten I. Human Cytomegalovirus Infection Increases Both Antibody- and Non-Antibody-Dependent Cellular Reactivity by Natural Killer Cells. Transplant Direct. 2017;3(12):e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomescu C, Tebas P, and Montaner LJ. IFN-alpha augments natural killer-mediated antibody-dependent cellular cytotoxicity of HIV-1-infected autologous CD4+ T cells regardless of major histocompatibility complex class 1 downregulation. AIDS. 2017;31(5):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomescu C, Mavilio D, and Montaner LJ. Lysis of HIV-1-infected autologous CD4+ primary T cells by interferon-alpha-activated NK cells requires NKp46 and NKG2D. AIDS. 2015;29(14):1767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hammer Q, and Romagnani C. About Training and Memory: NK-Cell Adaptation to Viral Infections. Adv Immunol. 2017;133:171–207. [DOI] [PubMed] [Google Scholar]
- 67.Muntasell A, Vilches C, Angulo A, and Lopez-Botet M. Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: a different perspective of the host-pathogen interaction. Eur J Immunol. 2013;43(5):1133–41. [DOI] [PubMed] [Google Scholar]
- 68.Ram DR, Manickam C, Hueber B, Itell HL, Permar SR, Varner V, et al. Tracking KLRC2 (NKG2C)+ memory-like NK cells in SIV+ and rhCMV+ rhesus macaques. PLoS Pathog. 2018;14(5):e1007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gondois-Rey F, Cheret A, Granjeaud S, Mallet F, Bidaut G, Lecuroux C, et al. NKG2C+ memory-like NK cells contribute to the control of HIV viremia during primary infection: Optiprim-ANRS 147. Clin Transl Immunology. 2017;6(7):e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma M, Wang Z, Chen X, Tao A, He L, Fu S, et al. NKG2C(+)NKG2A(−) Natural Killer Cells are Associated with a Lower Viral Set Point and may Predict Disease Progression in Individuals with Primary HIV Infection. Front Immunol. 2017;8:1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


