Abstract
Objectives
Non-pharmaceutical interventions against COVID-19 likely have a role in decreasing viral acute respiratory illnesses (ARI). This study aimed to assess the frequency of respiratory syncytial virus (RSV) and influenza ARI in children, before and during the COVID-19 pandemic.
Methods
This study was a prospective, multicenter, population-based ARI surveillance, including children <18 years presenting with fever and/or respiratory symptoms to emergency departments (ED) and inpatient settings in seven United States (US) cities. Respiratory samples were collected and evaluated by molecular testing. Data were analyzed by calendar weeks. Generalized linear mixed-effects models were used to evaluate the association between community mitigation and number of eligible cases, and proportion testing positive for RSV and influenza.
Results
Overall, 45,759 children were eligible; 25,415 were enrolled and tested; 25% were RSV-positive, and 14% were influenza-positive. In 2020, we noted a decrease in eligible and enrolled ARI subjects after community mitigation measures were introduced, with no RSV or influenza detection in 4/5-4/30/2020. Compared to 2016–2019, there was an average of 10.6 fewer eligible ARI cases/week per site and 63.9% and 45.8% lower odds of testing positive for RSV and influenza, respectively, during the 2020 community mitigation period. In all sites except Seattle, the proportions of positive RSV and influenza in the 2020 community mitigation period were lower than predicted.
Conclusion
In March-April 2020, rapid declines in ARI cases and the proportions of RSV and influenza in children were consistently noted across seven US cities, which could be attributable to community mitigation measures against SARS-CoV-2.
Table of Contents Summary
This prospective, multicenter, surveillance study evaluates the frequency of ARI in children before and during the COVID-19 pandemic within seven cities in the United States.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is a highly contagious respiratory virus, primarily transmitted between humans through exposure to respiratory droplets and aerosols from infected individuals.1 Early in the pandemic, national and global efforts have focused on community mitigation strategies to prevent the spread of SARS-CoV-2 throughout the pandemic in the absence of available vaccines and medical interventions. Community mitigation strategies have included hand hygiene, use of face coverings, social distancing, and limiting mass gatherings.2 Moreover, many school closures and stay-at-home orders were widely implemented in the United States (US) by early spring 2020.
Previous studies have demonstrated decreased transmission, infection, and mortality during influenza pandemics when similar community mitigation measures were implemented in a timely, appropriate, and sustainable manner.3 Recent evidence reflects the importance of community mitigation strategies in decreasing COVID-19 cases.4, 5 Several studies during the pandemic have demonstrated a decline in medically attended acute respiratory illnesses (ARI) and decreased activity of seasonal respiratory viruses, including influenza.6–9 Restrictions among available healthcare resources have also altered practice patterns (such as increased use of telemedicine) and clinical and research priorities to slow SARS-CoV-2 spread and alleviate stress on the healthcare system.10, 11 In addition, a decline in child vaccination coverage suggests that patients have been reluctant to procure healthcare during the pandemic.12, 13 These healthcare practices and behavioral changes might result in overall declines in medically attended ARI, especially milder cases that could be managed at home.
However, it is unknown whether such community mitigation strategies have decreased severe cases of ARIs that would require management in emergency department (ED) and inpatient settings. For example, respiratory syncytial virus (RSV) and influenza are among the most common causes of ARI in children, both associated with increased disease severity, and generally peak in winter and early-spring months each year.14–18 Therefore, this study aimed to (1) assess the frequency of ARI in children, including those due to RSV and influenza, in both the ED and inpatient settings, before and during the pandemic within a US multicenter active surveillance network, and (2) whether the proportions of such ARIs testing positive for RSV and influenza during the COVID-19 mitigation period in 2020 were lower than in prior seasons.
Methods
Study Design
Provisional data were obtained from the New Vaccine Surveillance Network (NVSN), a prospective, population-based ARI surveillance platform funded by the Centers for Disease Control and Prevention (CDC).18, 19 Surveillance for ARI in NVSN was reestablished in 2015 and the current seven US pediatric medical centers are located in: Cincinnati, OH; Houston, TX; Kansas City, MO; Nashville, TN; Pittsburgh, PA; Rochester, NY; and Seattle, WA. Subject recruitment occurred ≥4 days/week in the emergency department (ED) and ≥5 days/week in inpatient settings over four respiratory seasons. Analyses were restricted to subjects recruited during December 2016 to April 2017, and October to April each year from 2017 to 2020. The CDC’s and each institution’s Institutional Review Boards approved the study.
Study Population
Children under 18 years were eligible for enrollment if they resided within a site’s surveillance area and visited the ED or were admitted to the hospital within 48 hours of enrollment with ≥1 of the following symptoms: fever, cough, earache, nasal congestion, runny nose, sore throat, post-tussive vomiting, wheezing, shortness of breath/rapid or shallow breathing, apnea, apparent life-threatening event, brief resolved unexplained event, or myalgias; and duration of illness <14 days.18 Children were excluded if they had a known non-respiratory cause for their illness, chemotherapy-associated fever and neutropenia, been transferred from another hospital after an admission of >48 hours, were admitted <5 days after a previous hospitalization, were never discharged home after birth, or had previous enrollment in the study <14 days before current enrollment. Of note, three sites restricted ED enrollment primarily to children under five years during most of the periods of interest (Seattle during December 2016–April 2017, November 2017–April 2018, November 2018–April 2019, and December 2019–March 2020; Pittsburgh during December 2016–April 2018, November 2018–April 2019, and December 2019–March 2020; Kansas City during December 2016–April 2017, November 2017–April 2018, November 2018–April 2019).
Data and Specimen Collection
After obtaining informed consent, the research staff interviewed the child’s parent and/or guardian using a standardized case report form that included demographic information (including age, race and ethnicity). Mid-turbinate nasal swabs and oropharyngeal swabs were collected and combined in viral transport media by study personnel or clinical staff from enrolled children. For intubated patients, tracheal aspirates were accepted as alternatives to oropharyngeal swabs. When nasal, oropharyngeal, or tracheal aspirate specimens were not available, clinically salvaged respiratory specimens were obtained. Specimens were transported to each site laboratory and stored at 2°C to 8°C until processed (within 72 hours). Specimens underwent testing at each site by commercial or institution-specific in-house reverse transcription-polymerase chain reaction assays for RSV and influenza. Diagnostic assay methods varied by site and included Luminex NxTAG Respiratory Pathogen Panel (Cincinnati and Kansas City), BioFire FilmArray Respiratory Panel (Seattle), Applied Biosystems TaqMan Array Microfluidic Card (Rochester), and in-house real-time reverse transcription-polymerase chain reaction assays (Houston, Pittsburgh, and Nashville).18 All sites conducted CDC-sponsored proficiency testing to ensure the validity and consistency of respiratory viral detections at each site.18, 19
COVID-19 Pandemic-related Restrictions and Community Mitigation Dates
Research enrollment activities were paused or limited for several weeks in March at all sites as institutional policies suspended clinical research to conserve supplies and personal protective equipment during the COVID-19 pandemic. Enrollment occurred at a single children’s hospital at six sites and at two children’s hospitals in Rochester; capacity to care for children was not affected in any of these hospitals during the pandemic. Table 1 shows the dates of suspension of enrollment, school-closures, and stay-at-home order implementation by study site, which were provided by principal investigators, as instituted by local orders at each site. The total number of daily eligible and enrolled children in the ED and inpatient settings during the study period were provided by all sites. Community mitigation onset was defined as the earliest of either school closures or stay-at-home orders. The community mitigation period was defined as the first full calendar week following community mitigation initiation (Table 1) until the end of the study period (i.e., April 30, 2020 for all sites).
Table 1.
Dates of Community Mitigation Measures Implementation and Enrollment Suspension Periods, by Study Site.*
| Site | School-closure | Stay-at-home orders | IP suspension of enrollment dates | ED suspension of enrollment dates |
|---|---|---|---|---|
| Nashville, TN | 3/16/2020 | 3/23/2020 | None | None |
| Rochester, NY** | 3/13/2020 | 3/13/2020 | None | None |
| Cincinnati, OH | 3/17/2020 | 3/24/2020 | 3/25/2020 to 3/30/2020 | 3/24/2020 to 3/30/2020 |
| Seattle, WA | 3/17/2020 | 3/23/2020 | None | None |
| Houston, TX | 4/2/2020 | 3/24/2020 | 3/23/2020 to 4/3/2020 | 3/23/2020 to 4/22/2020 |
| Kansas City, MO | 3/16/2020 | 3/24/2020 | 3/18/2020 to 3/29/2020 | 3/18/2020 to 3/28/2020 |
| Pittsburgh, PA | 3/13/2020 | 3/23/2020 | 3/22/2020 to 3/29/2020 | 3/22/2020 to 3/29/2020 |
IP: inpatient; ED: emergency department
Community mitigation onset was defined as the earliest of either school closures or stay-at-home orders. The community mitigation period was defined as the first full calendar week following community mitigation initiation until the end of the study period (i.e., April 30, 2020 for all sites).
Enrollment was ceased from 3/16/2020 to 3/24/2020 in IP and ED settings in one affiliated hospital in the participating network.
Statistical Analysis
Descriptive statistics were summarized as frequency (percentage) for categorical variables or median (interquartile range) for continuous variables. The number of eligible and enrolled children and proportions of RSV and influenza were evaluated by calendar weeks each season. Children who were originally enrolled in the ED but then admitted, we classified their final status as the inpatient setting.
We sought to evaluate whether eligible case numbers and proportions testing positive for RSV and influenza were lower during the COVID-19 pandemic-related community mitigation period as compared to “baseline”. Baseline was defined as the same calendar weeks during 2016–2019. We fit generalized linear mixed-effects models (linear to model the mean number of eligible cases to obtain mean differences, and binomial-logit to model the odds of testing positive for RSV and influenza, separately, among enrolled cases). We included random effects for study site and season and fixed effects for categorical calendar week in each model, further including for each model the two prior weeks of corresponding outcomes (i.e., lagged outcomes) as covariates (flu and RSV cases are understood to be heavily driven by cases occurring in recent weeks).
We also aimed to evaluate whether eligible case numbers and proportions testing positive for RSV and influenza during the COVID-19 pandemic-related community mitigation period were as would have been predicted based on data from previous years. Regression models for eligible cases (linear) and proportions testing positive for RSV and influenza (logistic) were based on data from all sites prior to mitigation. Each model included two weeks of lagged outcomes, a two-way interaction between study site and season alone with each lower-order term, and a restricted cubic spline on calendar week with six knots positioned at equally-spaced quantiles. The fitted regression models were then used to generate predicted means/proportions for the community mitigation period, which were graphically represented in conjunction with pre-mitigation predictions using a locally weighted scatterplot smoother (LOWESS). We used a nominal significance level of 0.05 (two-tailed) for all analyses. All statistical analyses were performed using StataCorp, College Station, TX software (version 15.1), and R version 4.0.2.
Results
Over four respiratory seasons from 2016–2020, 45,759 eligible children with ARI were identified: 27,114 (59%) from the ED, and 18,645 (41%) from the inpatient setting. Overall, 25,415 (56%) were enrolled and had respiratory specimens collected and tested for RSV and/or influenza. The cohort median age was 19 months (interquartile range 7–50), with 56% male and 33% non-Hispanic White. Overall, 25% of the samples were RSV-positive, and 14% were influenza-positive. A total of 12,366 subjects were enrolled from ED and then discharged to home; 18% and 19% were RSV and influenza positive, respectively. A total of 13,049 were enrolled and their final status was the inpatient setting; 32% and 8% were RSV and influenza-positive, respectively.
The number of eligible and enrolled subjects and the proportions of RSV and influenza by calendar week stratified by season are shown in Figure 1. In 2020, each study site had an estimated average of 10.6 (95% CI: [5.92, 15.2]; p < 0.001) fewer eligible ARI cases per calendar week per site during the community mitigation period as compared to the corresponding periods in previous years.
Figure 1.
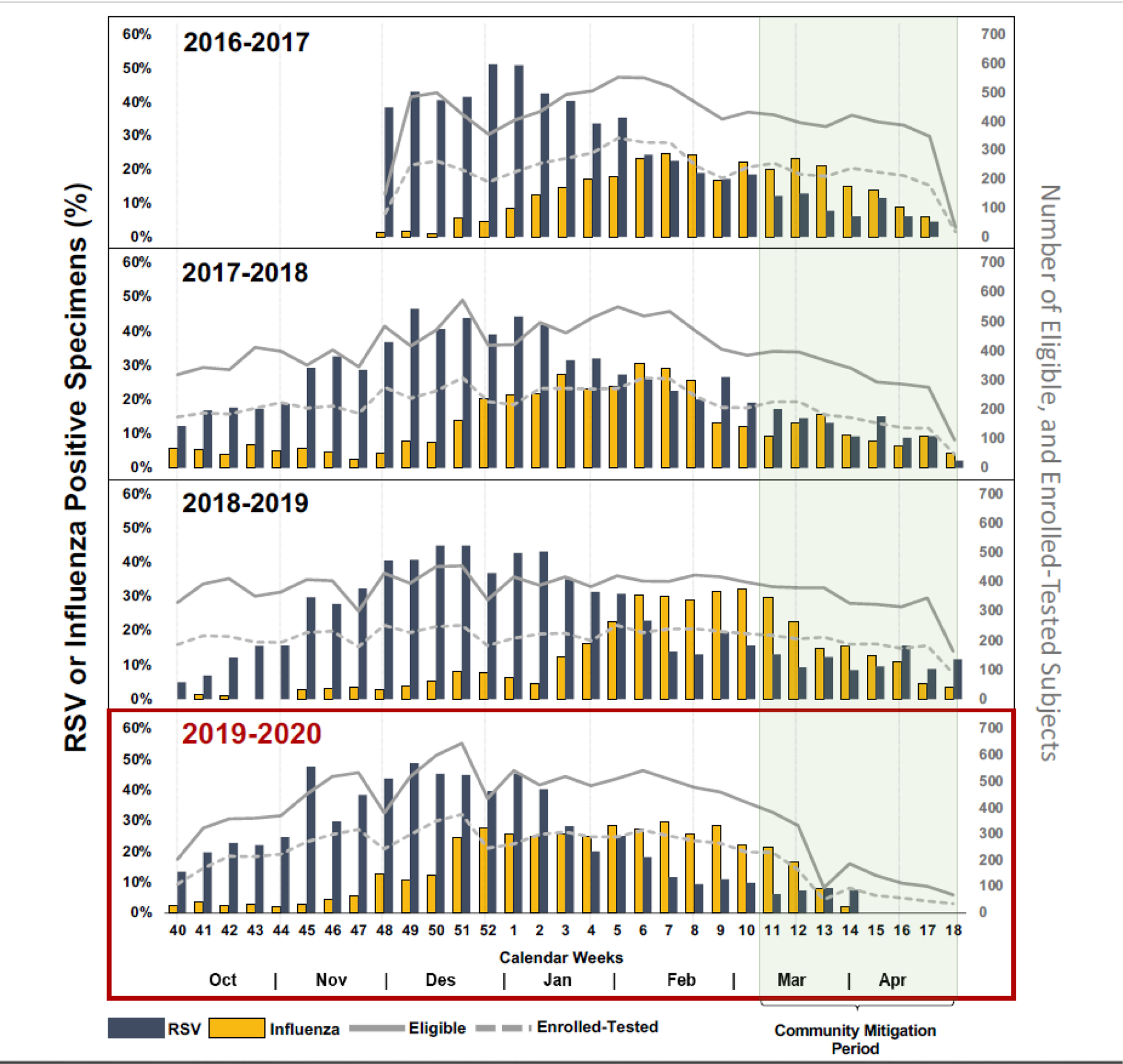
Numbers of Eligible and Enrolled Acute Respiratory Illness Cases, and Proportions of Respiratory Syncytial Virus (RSV) and Influenza Detection by Week, Stratified by Study Season.
The cumulative proportions of weekly RSV and influenza detections by study season are shown in greater detail in Figure 2. Compared to previous seasons, RSV and influenza detections in 2020 stopped after week 15. In 2020, no RSV or influenza detections were observed in weeks 15–18 (4/5–4/30) in either the ED or inpatient settings (Figure 3). Among enrolled cases, the odds of testing positive for RSV was estimated to be 63.9% (95% CI: [23.5%, 82.9%]; p = 0.008) lower in the community mitigation period of 2020 as compared to the corresponding time periods in previous years; for influenza, the odds were 45.8% (95% CI: [7.64%, 68.2%]; p = 0.024) lower.
Figure 2.
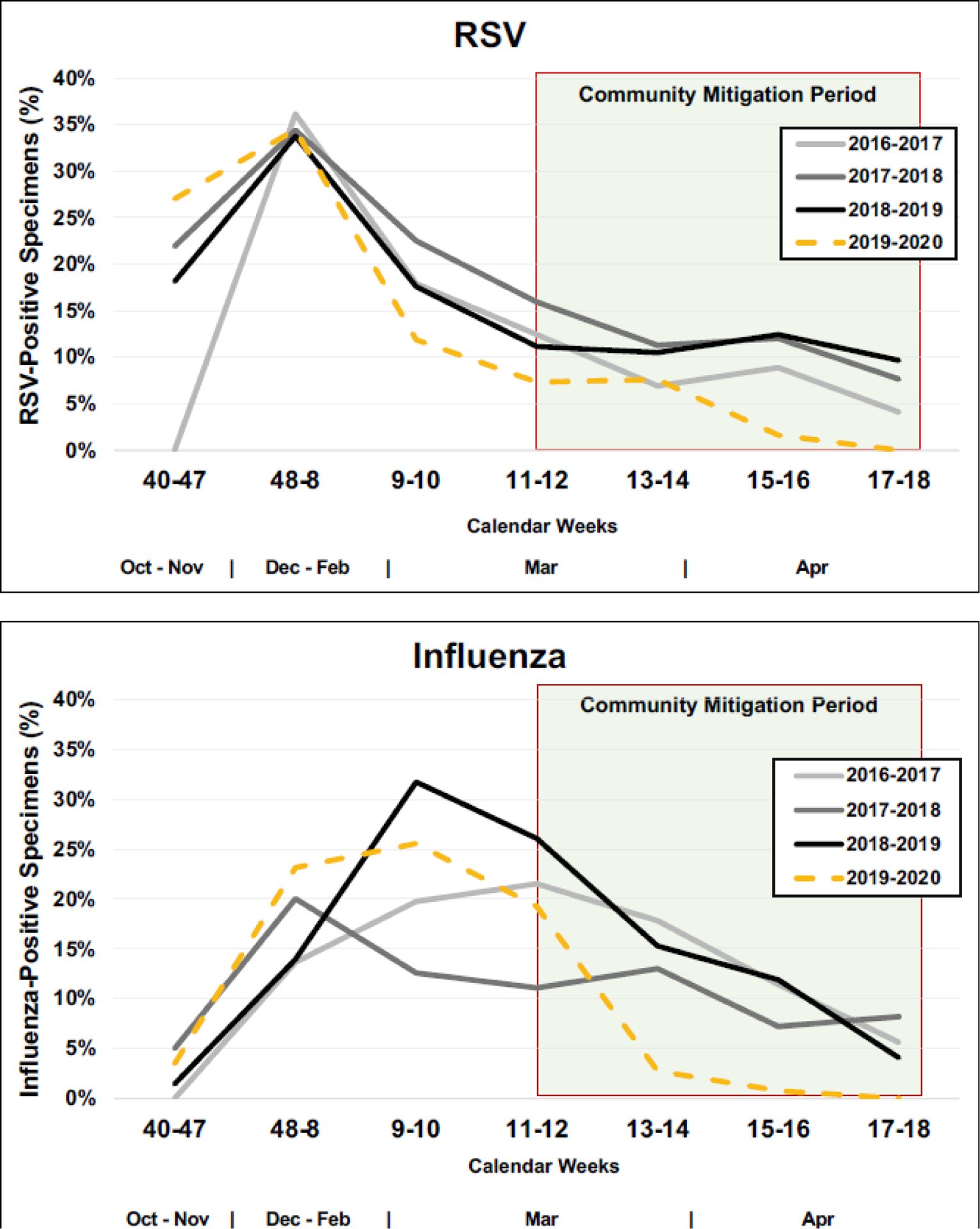
Cumulative Proportions of Weekly Respiratory Syncytial Virus (RSV) and Influenza Detection by Study Season.
Figure 3.
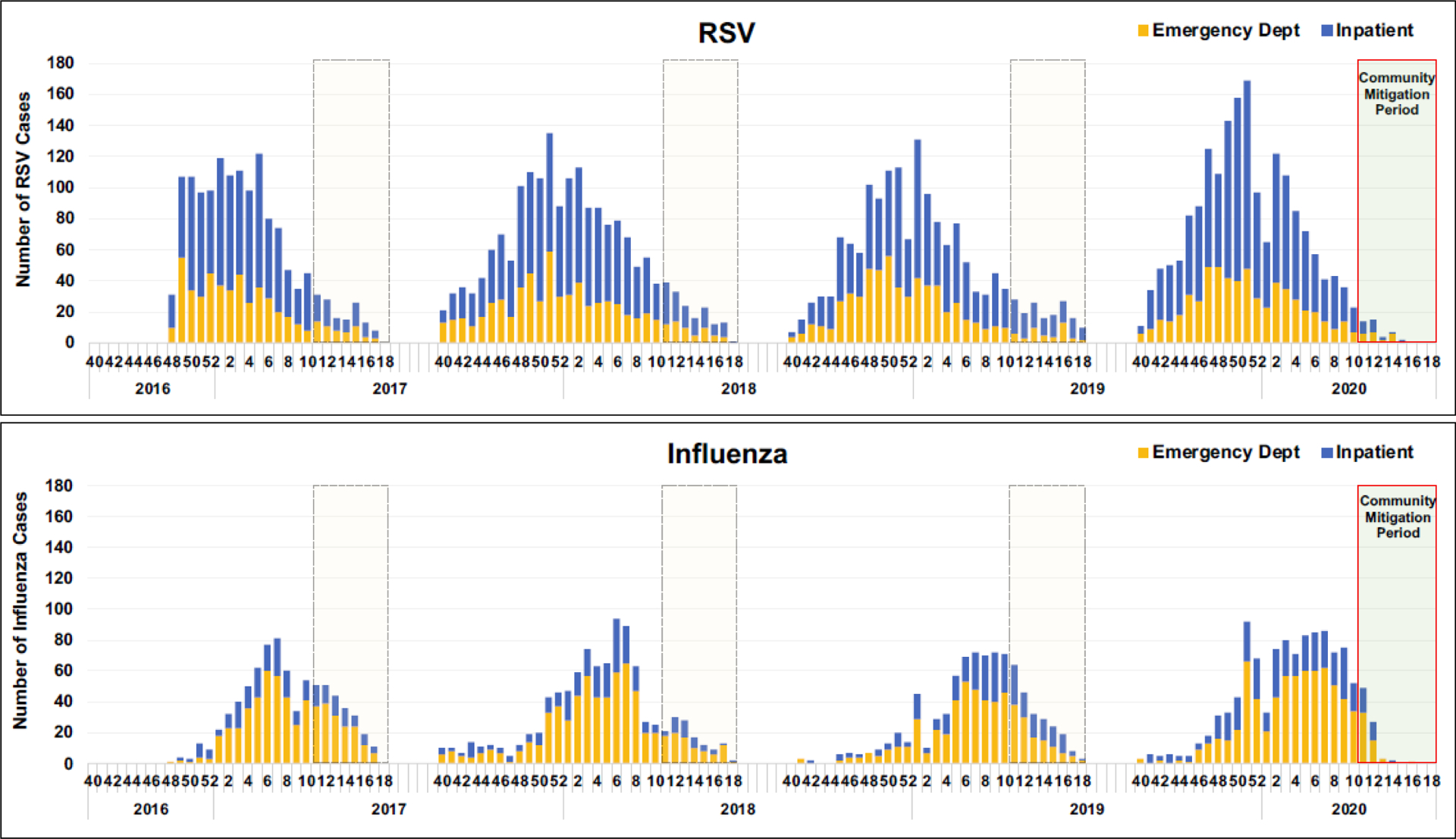
Number of Cases of Respiratory Syncytial Virus (RSV) and Influenza Detection by Week Over the Four-Year Study Period, Stratified by Clinical Setting.
Regression models were developed for all seven sites by study year for total eligible (Figure 4A), proportion positive for RSV (Figure 4B), and proportion positive for influenza (Figure 4C). Based on fitted lines by season, for all sites except Seattle, the number of eligible ARI cases and proportion positive for RSV and influenza among those enrolled in the 2020 community mitigation period was lower than what would have been predicted based on data from site- and time-adjusted trajectories that were present pre-mitigation (Figures 4A, B, C).
Figure 4.
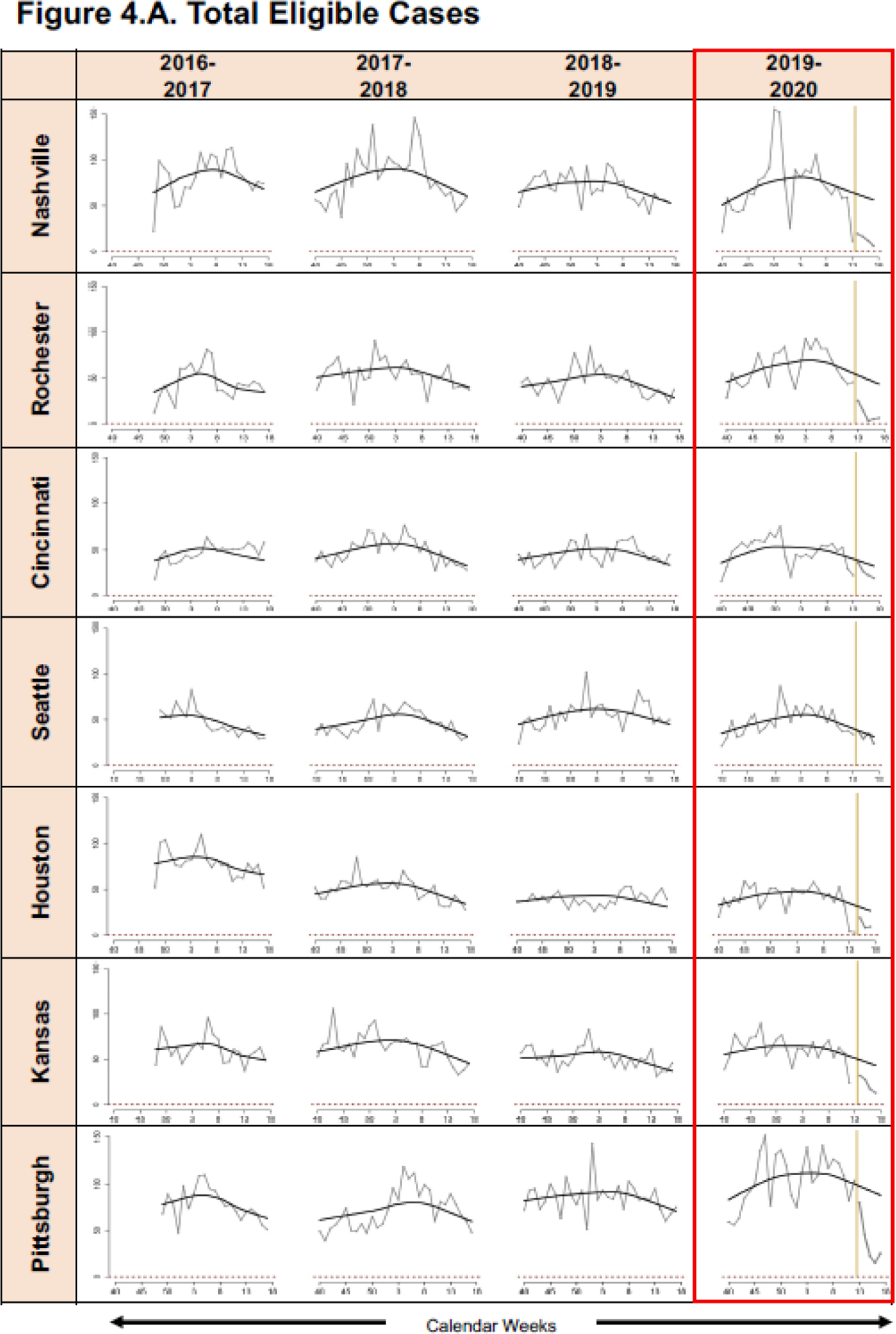
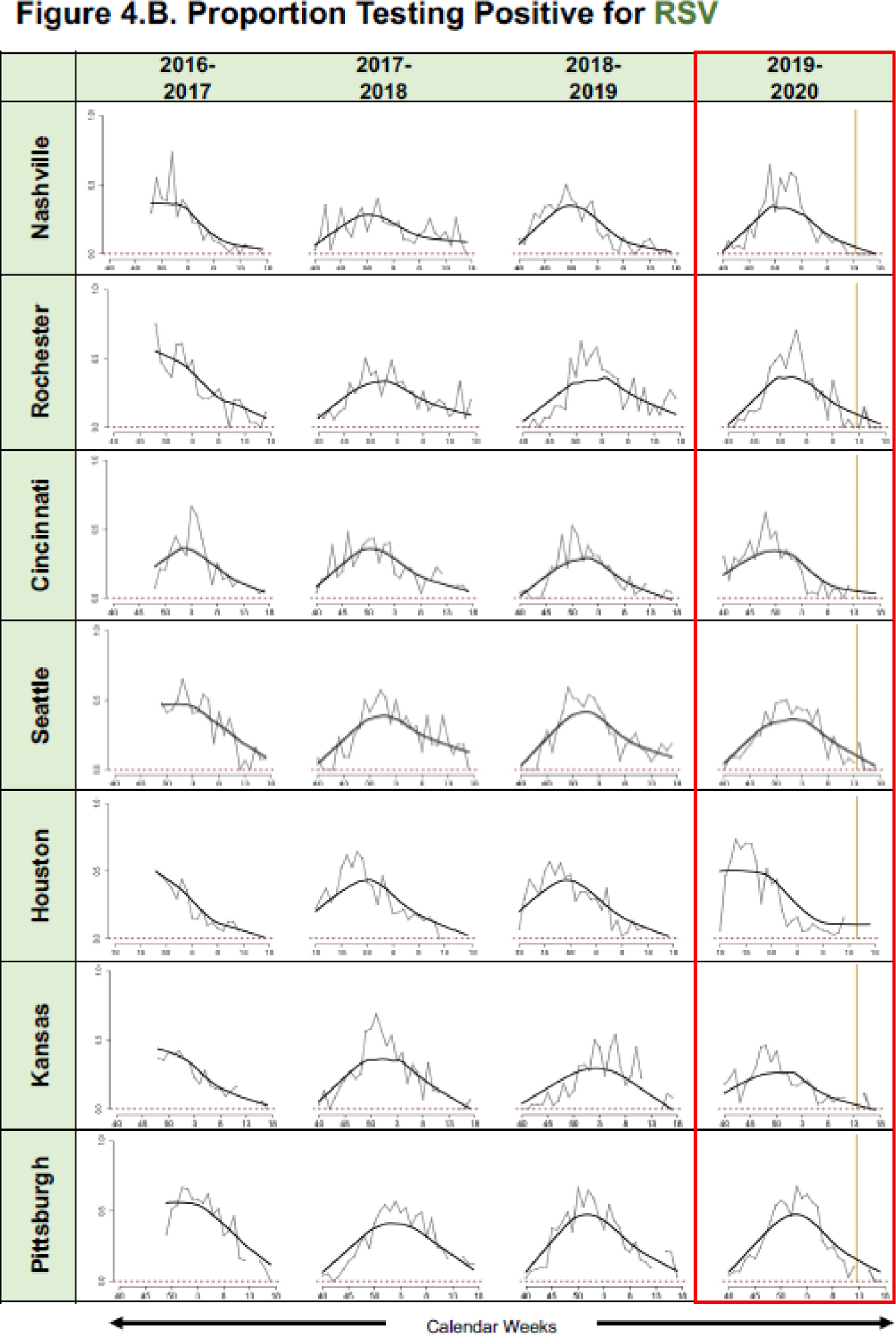
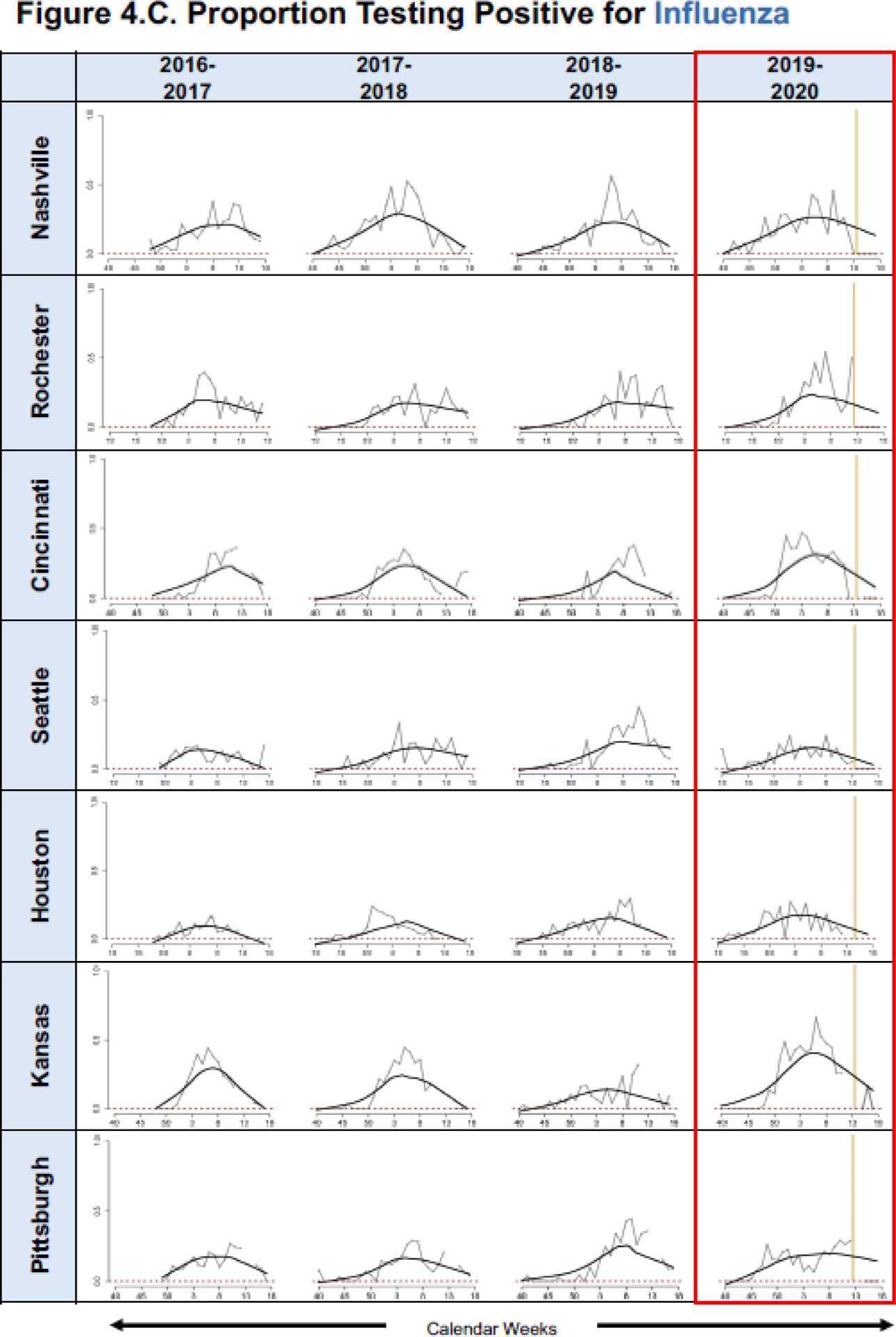
Graphic Representation of Predicted Mean A. Eligible Cases and Proportions of B. Respiratory Syncytial Virus (RSV) and C. Influenza Positive Enrolled Cases for the Community Mitigation Period, in Conjunction with Pre-Mitigation Predictions Using A LOWESS Smoother. (*please see Table 1 for specific halting of enrollments at each specific site).
Discussion
This prospective multicenter population-based ARI surveillance study found a substantial and sustained decline in medically attended ARI visits and the proportion of enrolled children with RSV or influenza infection in seven distinct geographic cities in the United States. These declines occurred promptly after community mitigation measures were implemented at each site in response to the pandemic.
Published studies have documented that these measures effectively decreased the reported burden of COVID-19 cases, with a robust temporal association between the timing of these strategies and the decrease in the trend in the epidemic growth rate of reported COVID-19 cases.4, 5, 20 The majority of community mitigation strategies in each of the seven study sites were implemented around mid-March 2020; following these dates, we noted significantly fewer estimated eligible cases and lower odds of RSV and influenza-positive children compared to the same periods in previous seasons. A reduction in eligible case numbers during community mitigation periods can be explained in part by several other factors, including changes in healthcare-seeking behaviors, and limited access to medical care.10–13 However, it is less plausible that such factors explain the reduction in the proportions of positive detections of RSV and influenza among enrolled subjects, or in the frequency of cases of severe ARIs that are hospitalized. Our study results are consistent with the hypothesis that community mitigation can slow the spread of severe RSV and influenza infections, similar to the experiences with SARS-CoV-2.
We found that the proportion of influenza-positive cases in the ED or inpatient setting in 2020 community mitigation period is lower than what would have been predicted at most site locations, except Seattle. However, there were no cases of influenza detected in Seattle after week 15, consistent with the other sites. Similar non-medical interventions were found effective in prior influenza pandemics, including the 1918–1919 influenza pandemic, when medical interventions were unavailable, and in more recent severe epidemics, when they were used together with medical interventions.3, 21–23 A surveillance study that monitored influenza-like illnesses (ILI) and laboratory-confirmed influenza in New York showed that ILI decreased with an average decrease of 96.9–98.9% in daily laboratory-confirmed influenza case rate/100,000 in different regions in New York during the COVID-19 pandemic.24 Similarly, Sakamoto et al. evaluated the effects of social isolation and adherence to personal hygiene on influenza weekly activity in all age groups in Japan.6 Compared to the same periods in preceding years in their study, influenza activity was significantly lower in January–March 2020, coinciding with mitigation efforts. Young et al. evaluated the 2019–2020 influenza season compared to previous years, and noted steeper declines in new influenza cases following the seasonal influenza peak in China and in Italy, which coincided with a substantial increase in COVID-19 cases in these countries.25 While the mainstay for preventing influenza remains annual vaccination, our findings suggest that in future severe influenza pandemics, similar community mitigation measures might reduce the incidence of severe influenza infection in children.
This study compared the proportion of RSV-infected children detected before and after social isolation measures in seven cities in the United States. RSV is a major cause of ARI hospitalizations in young children during the fall and winter months and associated with significant morbidity.16, 17 We found a greater than 70% decrease in RSV-positive ARI in the community mitigation period in 2020 compared to the same period in prior surveillance seasons during which similar enrollment protocols were used. Baker et. al. used laboratory surveillance data from 2020 and estimated that that RSV transmission declined by at least 20% in the United States.26 Further active surveillance is needed during community mitigation periods to understand if RSV circulation in the United States can be affected by these measures.
Several reports indicated that parents were reluctant to take their children to healthcare facilities during this pandemic due to fear of SARS-CoV-2 exposure,12, 13, 27 which could in part contribute to the decline in the number of eligible subjects with ARI. However, we assume that the main effects would be on mild ARI that could be managed outside of the inpatient settings. Although the number of eligible hospitalized ARI subjects decreased in our study during the period of COVID-19 mitigation, healthcare-seeking behavior was less likely to be responsible if we assume presentation of severely ill children would not have been demonstrably altered during this period. Further, local healthcare providers and researchers altered their practices and priorities during the early part of the COVID-19 pandemic, which posed limitations in our enrollment.19 Nonetheless, all sites recorded the number of eligible subjects even when enrollment was not possible due to institution-specific mandates in research curtailment. These data showed a significant decrease in the number of eligible subjects in 2020, as well as in the proportions positive for RSV and influenza. Additionally, a sensitivity analysis only including the three sites with no pauses in enrollments showed significant decreases for eligible and RSV cases, and similar point estimates for influenza cases, albeit not significant, which may be explained by insufficient power given the reduction in sample size. It might be expected, if confounding by limitations of enrollment to only the most ill children was influential, that the proportions of enrolled children with RSV and influenza detection might have increased, not decreased, especially since RSV and influenza are important causes of severe ARI in children.16–18, 28–31
Both RSV and influenza activity show natural fluctuations from season to season such that the decrease in RSV and influenza detections in our study might be partially explained by seasonal variation or even differences in virulence of circulating viral strains.14, 15, 17 The use of four seasons of data from the same study sites using similar enrollment criteria across wide geographic settings makes secular seasonal and virulence trends less likely to explain our findings of abrupt curtailment of RSV and influenza circulation among children in the study sites. We likewise accounted for between-site and between-year variations in prospective surveillance data. Also, we focused on comparing outcomes from the community mitigation period to comparable periods from previous years, and by including lag variables from two weeks leading up to the community mitigation period to reduce the confounding effects of a hiatus in enrollment as the mitigation period began. The major strengths of our study included the use of sensitive, validated molecular virologic testing, analysis of a large sample size from seven geographically diverse cities across the United States, and the use of consistent enrollment procedures over four years of active, prospective, ARI surveillance.
In summary, after COVID-19 community mitigation procedures were implemented, we noted a consistently sharp decline in children with ARI seeking medical care in EDs or being admitted to hospitals at our study institutions across seven US cities early in the pandemic. Furthermore, there were no RSV or influenza cases reported approximately 2–4 weeks after these measures were implemented in weeks 11–13, which contrasted with predictions based on data from prior seasons when mitigation measures were not in effect. This study validates the importance of ongoing active ARI surveillance in children to determine trends in viral positivity before, during, and after interventions, including implementation of community mitigation strategies during public health emergencies and pandemics. Future studies are needed to confirm our findings, especially in the upcoming RSV and influenza respiratory season, as our data suggest that community mitigation measures may have contributed to decreased RSV and influenza disease activity in young children.
What’s Known on This Subject
While decreases in ARI during the COVID-19 community mitigation periods have been described, it is unknown whether such strategies have decreased severe cases of ARIs requiring management in emergency departments and inpatient settings across different cities in the United States.
What This Study Adds
In over 25,000 prospectively enrolled children from seven geographically diverse United States cities, this study showed fewer ARI cases and lower odds of testing positive for RSV and influenza, during the COVID-19 mitigation period compared to 2016–2019.
Acknowledgments
We thank the children and parents who participated in this study.
Funding:
This work was supported by the US Centers for Disease Control and Prevention (cooperative agreement number CDC-RFA-IP16-004) and UL1 TR000445 from NCATS/NIH.
Role of Funder/Sponsor:
The CDC provided funding for subjects enrollment, sample collection and testing, and also participated in the design of the study. NIH grant to support REDCap.
Conflicts of Interest
Jennifer E. Schuster receives support from Merck. John V. Williams is on boards for Quidel and GSK. JenChristopher Harrison’s institution receives support from GlaxoSmithKline, Merck, and Pfizer. Janet Englund is a consultant for Sanofi Pasteur, Meissa Vaccines, and institutional research support from AstraZeneca, GlaxoSmithKline, Pfizer, and Novavax. Natasha Halasa has grant funding from Sanofi, Quidel, and received an honorarium from an educational grant from Genentech. All other authors: No reported conflicts of interest.
Abbreviations:
- ARI
acute respiratory illnesses
- RSV
respiratory syncytial virus
- ED
emergency department
- US
united states
- SARS-CoV-2
severe acute respiratory syndrome
- COVID-19
coronavirus 2 the cause of coronavirus disease 2019
- CDC
centers for disease control and prevention
- ILI
influenza-like illnesses
Footnotes
Publisher's Disclaimer: This is a prepublication version of an article that has undergone peer review and been accepted for publication but is not the final version of record. This paper may be cited using the DOI and date of access. This paper may contain information that has errors in facts, figures, and statements, and will be corrected in the final published version. The journal is providing an early version of this article to expedite access to this information. The American Academy of Pediatrics, the editors, and authors are not responsible for inaccurate information and data described in this version.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. March 26 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebrahim SH, Ahmed QA, Gozzer E, Schlagenhauf P, Memish ZA. Covid-19 and community mitigation strategies in a pandemic. BMJ. March 17 2020;368:m1066. doi: 10.1136/bmj.m1066 [DOI] [PubMed] [Google Scholar]
- 3.Qualls N, Levitt A, Kanade N, et al. Community mitigation guidelines to prevent pandemic influenza—United States, 2017. MMWR Recommendations and Reports. 2017;66(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanu FA, Smith EE, Offutt-Powell T, et al. Declines in SARS-CoV-2 Transmission, Hospitalizations, and Mortality After Implementation of Mitigation Measures-Delaware, March-June 2020. MMWR Morb Mortal Wkly Rep. November 13 2020;69(45):1691–1694. doi: 10.15585/mmwr.mm6945e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong LX, Lin A, He ZB, et al. Mask wearing in pre-symptomatic patients prevents SARS-CoV-2 transmission: An epidemiological analysis. Travel Med Infect Dis. Jul-Aug 2020;36:101803. doi: 10.1016/j.tmaid.2020.101803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto H, Ishikane M, Ueda P. Seasonal Influenza Activity During the SARS-CoV-2 Outbreak in Japan. JAMA. May 19 2020;323(19):1969–1971. doi: 10.1001/jama.2020.6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angoulvant F, Ouldali N, Yang DD, et al. COVID-19 pandemic: Impact caused by school closure and national lockdown on pediatric visits and admissions for viral and non-viral infections, a time series analysis. Clin Infect Dis. June 3 2020;doi: 10.1093/cid/ciaa710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased Influenza Activity During the COVID-19 Pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. September 18 2020;69(37):1305–1309. doi: 10.15585/mmwr.mm6937a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parry MF, Shah AK, Sestovic M, Salter S. Precipitous Fall in Common Respiratory Viral Infections During COVID-19. Open Forum Infectious Diseases. 2020;ofaa511. Published 2020 Oct 23. doi: 10.1093/ofid/ofaa511;doi:10.1093/ofid/ofaa511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keesara S, Jonas A, Schulman K. Covid-19 and Health Care’s Digital Revolution. N Engl J Med. June 4 2020;382(23):e82. doi: 10.1056/NEJMp2005835 [DOI] [PubMed] [Google Scholar]
- 11.Hollander JE, Carr BG. Virtually Perfect? Telemedicine for Covid-19. N Engl J Med. April 30 2020;382(18):1679–1681. doi: 10.1056/NEJMp2003539 [DOI] [PubMed] [Google Scholar]
- 12.Bramer CA, Kimmins LM, Swanson R, et al. Decline in Child Vaccination Coverage During the COVID-19 Pandemic - Michigan Care Improvement Registry, May 2016-May 2020. MMWR Morb Mortal Wkly Rep. May 22 2020;69(20):630–631. doi: 10.15585/mmwr.mm6920e1 [DOI] [PubMed] [Google Scholar]
- 13.Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration - United States, 2020. MMWR Morb Mortal Wkly Rep. May 15 2020;69(19):591–593. doi: 10.15585/mmwr.mm6919e2 [DOI] [PubMed] [Google Scholar]
- 14.Rose EB, Wheatley A, Langley G, Gerber S, Haynes A. Respiratory Syncytial Virus Seasonality - United States, 2014–2017. MMWR Morb Mortal Wkly Rep. January 19 2018;67(2):71–76. doi: 10.15585/mmwr.mm6702a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsitch M, Viboud C. Influenza seasonality: lifting the fog. Proc Natl Acad Sci U S A. March 10 2009;106(10):3645–6. doi: 10.1073/pnas.0900933106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddadin Z, Beveridge S, Fernandez K, et al. Respiratory Syncytial Virus Disease Severity in Young Children. Clin Infect Dis. October 23 2020;doi: 10.1093/cid/ciaa1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus–associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):e341–e348. [DOI] [PubMed] [Google Scholar]
- 18.Campbell AP, Ogokeh C, Lively JY, et al. Vaccine Effectiveness Against Pediatric Influenza Hospitalizations and Emergency Visits. Pediatrics. November 2020;146(5)doi: 10.1542/peds.2020-1368 [DOI] [PubMed] [Google Scholar]
- 19.Rha B, Curns AT, Lively JY, et al. Respiratory Syncytial Virus–Associated Hospitalizations Among Young Children: 2015–2016. Pediatrics. 2020;146(1) [DOI] [PubMed] [Google Scholar]
- 20.Lasry A Timing of Community Mitigation and Changes in Reported COVID-19 and Community Mobility―Four US Metropolitan Areas, February 26–April 1, 2020. MMWR Morbidity and Mortality Weekly Report. 2020;69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey A, Atkins KE, Medlock J, et al. Strategies for containing Ebola in West Africa. Science. November 21 2014;346(6212):991–5. doi: 10.1126/science.1260612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlroth DJ, Glass RJ, Davey VJ, Cannon D, Garber AM, Owens DK. Health outcomes and costs of community mitigation strategies for an influenza pandemic in the United States. Clin Infect Dis. January 15 2010;50(2):165–74. doi: 10.1086/649867 [DOI] [PubMed] [Google Scholar]
- 23.Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA. August 8 2007;298(6):644–54. doi: 10.1001/jama.298.6.644 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg ES, Hall EW, Rosenthal EM, et al. Monitoring COVID-19 through Trends in Influenza-like Illness and Laboratory-confirmed Influenza and COVID-19 - New York State, excluding New York City, January 1 - April 12, 2020. Clin Infect Dis. May 31 2020;doi: 10.1093/cid/ciaa684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young G, Peng X, Rebeza A, et al. Rapid decline of seasonal influenza during the outbreak of COVID-19. ERJ Open Res. July 2020;6(3)doi: 10.1183/23120541.00296-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. December 1 2020;117(48):30547–30553. doi: 10.1073/pnas.2013182117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartnett KP, Kite-Powell A, DeVies J, et al. Impact of the COVID-19 Pandemic on Emergency Department Visits - United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep. June 12 2020;69(23):699-704. doi: 10.15585/mmwr.mm6923e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. February 26 2015;372(9):835–45. doi: 10.1056/NEJMoa1405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zangrillo A, Biondi-Zoccai G, Landoni G, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. February 13 2013;17(1):R30. doi:cc12512 [pii] 10.1186/cc12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flamant C, Hallalel F, Nolent P, Chevalier JY, Renolleau S. Severe respiratory syncytial virus bronchiolitis in children: from short mechanical ventilation to extracorporeal membrane oxygenation. Eur J Pediatr. February 2005;164(2):93–8. doi: 10.1007/s00431-004-1580-0 [DOI] [PubMed] [Google Scholar]
- 31.Khuri-Bulos N, Lawrence L, Piya B, et al. Severe outcomes associated with respiratory viruses in newborns and infants: a prospective viral surveillance study in Jordan. BMJ Open. May 20 2018;8(5):e021898. doi: 10.1136/bmjopen-2018-021898 [DOI] [PMC free article] [PubMed] [Google Scholar]


