Abstract
Background & aims:
People with a higher genetic risk for obesity are more likely to develop cardiovascular disease (CVD), and healthy plant-based dietary patterns may be associated with decreased risks of obesity and cardiovascular events. We investigated whether adherence to healthy plant-foods-rich dietary patterns might attenuate risks of obesity and related cardiovascular abnormalities for people at genetically higher risk of obesity.
Methods:
This study included 121799 middle-aged adults in UK Biobank who were initially free of metabolic diseases and cancer. We calculated a healthful plant-based diet index (hPDI) based on 17 major food groups as well as a genetic risk score (GRS) for obesity consisting of body mass index (BMI)-associated variants. The incidence of cardiovascular events (myocardial infarction, MI, or stroke) was prospectively followed during a mean (SD) 5.1 (0.9) years.
Results:
We found significant interactions between GRS and hPDI on adiposity (Pinteraction <0.0001); adherence to hPDI was more strongly associated with lower levels of adiposity among participants with higher GRS than those with lower GRS. Further, we found a similar pattern of GRS-hPDI interactions on untreated hypertension (Pinteraction=0.0036). When we tested GRS-hPDI interactions on cardiovascular events, adherence to hPDI was more strongly associated with a decreased risk of MI among people with high GRS (above median) than those with low GRS (Pinteraction=0.006). Among participants with high GRS, high adherence to hPDI (the top tertile of hPDI) was associated with an HR 0.54 (95% CI: 0.39, 0.74) for MI, as compared to low adherence.
Conclusions:
Adherence to healthy plant-based dietary patterns significantly attenuated risks of cardiovascular abnormalities for people at genetically higher risk of obesity. Our results support the precision medicine strategies considering genetics and dietary habits to modify cardiovascular health for people at higher risk of genetically determined obesity.
Keywords: Cardiovascular events, Risk factors, Obesity, Genetic predisposition, Plant-based nutrition
Introduction
Obesity has been related to life-threatening chronic metabolic diseases, including cardiovascular diseases (CVD) [1]. Notably, the obesity epidemic has coincided with major shifts from traditional plant-foods rich dietary patterns to unhealthy dietary habits characterized by higher intakes of animal foods and highly processed foods [2]. When interacting with inherent susceptibility in the genome [3], such shifts may partly contribute to the surge of obesity [4–6]. For example, previous observational studies [7–11] have also shown that genetic predisposition to obesity may promote adiposity when exposed to obesogenic dietary intakes, and also suggested that greater adherence to healthy dietary habits might attenuate the genetic predisposition to obesity and weight gain [7, 10, 12]. Genetic predisposition can be identified in early stage of life [13], and exerts a persistent effect on gaining adiposity throughout adult life [14]. Therefore, investigations of gene-diet interactions may benefit the lifecourse prevention since early life. Mendelian randomization analyses indicate that the genetic predisposition to obesity causally affects risks of its cardiovascular comorbidities (such as hyphenation and coronary heart disease) [15–17]; however, it remains unknown whether healthy plant-foods rich dietary patterns might attenuate the effects of genetic predisposition to obesity on cardiovascular comorbidities.
Epidemiological studies suggest that risks of obesity and its related cardiovascular comorbidities may be modified by greater adherence to healthy plant-based dietary patterns [18–24]. We and others have recently reported that adherence to a healthful plant-based diet index (hPDI) that captured synergistic and graded intakes of healthy and less healthy plant-based foods, as well as animal foods, was significantly associated with the incidence of CVD in the entire study populations [23, 25–27]. To our knowledge, no large-scale cohort study has examined associations of adherence to healthy plant-based dietary patterns and cardiovascular events, considering participants’ genetic predisposition to obesity.
In the present study, we tested interactions between genetic predisposition to obesity and a healthful plant-based diet index (hPDI) for risks of obesity and its cardiovascular comorbidities, such as hypertension and subsequent CVD events in a large number of participants in the UK Biobank. We tested a hypothesis of whether people at genetically higher risk may be more responsive to healthy dietary patterns assessed by hPDI to modify their risks of obesity and related vascular comorbidities (such as hypertension and CVD events).
Materials and Methods
Study population
The UK Biobank is a large population-based, prospective study including over 500,000 individuals aged 40–69 years when recruited in 2006–2010 who underwent a wide range of physical measures (including blood pressure and anthropometric measurements) and provided biological samples. Sociodemographic, lifestyle, and health-related information was collected through a touch screen questionnaire and verbal interview at the recruitment [28]. Participants reported the frequency of consumption of main foods with a touchscreen questionnaire at recruitment; a previous study has reported a moderate to substantial reproducibility of the dietary questions approximately for 4 years after the recruitment [29]. A large sub-sample of participants completed at least one 24-hour dietary assessment (the Oxford WebQ) at the assessment center or online during 2009–2012.
The present analysis included a total of 211009 participants who completed at least one web-based 24-h dietary assessment (the Oxford WebQ) during 2009–2012 and had information on outcome events based on the hospital records and the death registry. We excluded individuals with a history of CVD (myocardial infarction, MI [n=4043] or stroke [n=2492]) at baseline when the 24-hour dietary assessment was performed. Individuals with a history of cancer (n=6641) (either breast cancer, gastrointestinal cancer, urinary tract cancer, or lung cancer) or diabetes (n=8884) were also excluded from the analysis. Since we aimed to examine untreated (i.e., undiagnosed) high blood pressure (BP) as one of the present study outcomes, participants with a self-reported history of doctor-diagnosed high BP or antihypertensive medication use were excluded (n=54546). We further excluded participants with missing data on total energy or food intake, or those with implausible energy intake (e.g., men with <800 or >4200 kcal/day, or women with <600 or >3500 kcal/day). People who were not of white British ethnicity background were also excluded since the selection of genetic variants for BMI was based on results of white European individuals. Subsequently, our analysis included 121799 participants; overall characteristics of participants who were excluded (n=89210) or included (n=121799) in this study are presented in STable 1. The UK Biobank has approval from the North West Multi-Center Research Ethics Committee, which covers the UK; the Community Health Index Advisory Group, which covers Scotland. This study was covered by the general ethical approval for UK Biobank studies from National Research Ethics Service (http://www.ukbiobank.ac.uk/ethics/). All participants had provided written informed consent to participate in the study.
Dietary Assessment and calculation of hPDI
The Oxford WebQ asked about consumption of >200 types of foods and >30 types of drinks during the previous 24 hours using standard categories to indicate the amount consumed. As compared with an interviewer-administrated dietary assessment, the Oxford WebQ captures similar food items and estimates similar nutrient intakes with moderate-to-strong correlations for the majority of nutrients (Spearman’s correlation coefficients ranges 0.5–0.9) [30]. More details on the dietary assessment are addressed in Online-Supplemental Methods.
We followed methods described in the previous publications [23, 31] to create a “healthful” version of PDI (i.e., hPDI) that emphasizes consumption of healthy plant foods, where healthier plant foods received positive scores and less-healthier plant foods and animal foods received negative scores. In the previous studies [23, 31], healthy and less-healthy foods were distinguished based on existing data on associations of foods with cardiovascular outcomes and metabolic abnormalities. Two other PDIs were also created in the previous studies [23, 31], such as an overall PDI that emphasizes consumption of all plant food while reducing animal food intake, and an unhealthful PDI that is calculated by giving positive scores to less-healthy plant foods and negative scores to both healthy plant foods and animal foods. In the present study, we used the hPDI, a diet quality index best reflecting healthy dietary patterns, as a dietary exposure of interest.
In UK Biobank, a total of 17 food groups (whole grains, fruits, vegetables, nuts, legumes and vegetarian protein alternatives, tea and coffee, fruit juices, refined grains, potatoes, sugar-sweetened beverages, sweets and desserts, animal fat, dairy, egg, fish or seafood, meat, and miscellaneous animal-based foods), except for vegetable oils, were available to calculate the hPDI (STable 2). The 17 food groups were ranked into quintiles, and each quintile was assigned a score between 1 and 5. With positive scores, a score of 5 was given for the highest quintile, following on through a score of 1 was given for the lowest quintile. With reverse scores, this pattern of scoring was inverted. The scores of 17 food groups for an individual were summed to calculate hPDI. The hPDI was normally distributed (SFigure 1); higher hPDI reflects higher diet quality of having more healthy plant foods and lesser unhealthy plant-foods and animal foods. Details on intakes of major food groups and nutrients across categories of hPDI are described in STable 3. We confirmed that there was a strong correlation (Pearson correlation coefficient=0.88; P <.0001) between a “hPDI assessed at baseline” and an “averaged hPDI” based on repeated measurements during 2009–2012 (maximum: five times, n of participants=121799) (Online-Supplemental Methods). Therefore, the present study used the earliest data on dietary intake to maximize a follow-up time if participants had completed the dietary assessment more than once.
Calculation of genetic risk score (GRS)
The genetic predisposition to obesity was evaluated as GRS based on 75 common (minor allele frequency of 0.05 (5%) or greater) single nucleotide polymorphisms (SNPs) that showed genome-wide significant associations (P <5×10−8) for BMI in the primary analysis of European-descent individuals [32]. We limited SNPs to those that were associated with BMI in the analysis of European ancestry individuals. Each SNP was re-coded as 0, 1, or 2 according to the number of BMI increasing alleles. We calculated the weighted GRS using the following equation: GRS = (β1 × SNP1 + β2 × SNP2 + … + βn × SNPn) × (n/sum of the β coefficients), where β is the coefficient of each SNP for BMI, and n was 75 (STable 4). The GRS was normally distributed; higher GRS was related to higher BMI with Pearson correlation coefficient of 0.12 (P <0.0001) (SFigure 2). Also, higher GRS was associated with higher risks of obesity and untreated high BP (SFigure 3). We separately investigated several SNPs in the GRS (such as SNPs near FTO, TMEM18, or MC4R) that may be related to dietary macronutrient preference [33–35].
Anthropometric and blood pressure measurements
Anthropometric and BP measurements were collected at UK Biobank assessment at the time of recruitment. Weight and height were measured by trained staff using standard procedures; BMI (kg/m2) was calculated. BP was measured using an automatic digital BP monitor (Omron HEM-7015IT) with an appropriate size cuff; a sphygmomanometer was used when the automatic device could not be employed. Measurements of systolic BP (SBP) and diastolic BP (DBP) were performed twice, and the second set of readings were measured after the participant had rested for about one minute. We calculated averaged values of the two readings of SBP or DBP; participants with only one reading were not included. Mean arterial BP was calculated by the equation: mean arterial BP = ((2 × DBP) + SBP)/3). Untreated high BP was indicated by SBP ≥130 mmHg or DBP ≥80 mmHg based on the ACC/AHA criteria [36]; the present study participants were free of antihypertensive medication use or self-reported history of doctor-diagnosed hypertension.
Ascertainment of CVD
The incidence of CVD was indicated as a composite endpoint of MI or stroke. Follow-up time for incident CVD was calculated from the date of diet questionnaire completed until the time of the event, the time of death, or the end of follow-up (2016) whichever occurred first. The incidence of MI [37] and stroke [38] were based on UK biobank’s algorithms that used inpatient hospital and death registry data linked to the study. The first occurrence of MI was defined as ICD 10 codes: I21.X, I22.X, I23.X, I24.1, I25.2; stroke was defined as total ischemic and hemorrhagic stroke (ICD 10 codes: I60, I61, I63, I64). More detailed information on the definitions of MI and stroke are available elsewhere [37, 38].
Statistical analysis
The primary outcomes were obesity and high BP at the baseline time of recruiting study participants, and the incidence of CVD after baseline. General linear models were performed to estimate β coefficients for differences in BMI or mean arterial BP with adjusting for covariates of age, sex, and the top 5 principal components of ancestry, demographic, lifestyle, and other dietary factors. Details on covariates in adjusted models are described in Online-Supplemental Methods. The logistic regression was performed to calculate the odds ratio (OR) and 95% CIs for untreated high BP. In addition, hypertriglyceridemia and low HDL cholesterol levels have been traditional cardiometabolic comorbidities of obesity to increase CVD risk [1]; therefore, we performed sensitivity analyses using HDL cholesterol and triglycerides as confirmatory outcomes to test whether there were similar interactions between hPDI and GRS on lipids.
The presence of linear or non-linear relationships [39] between hPDI and outcomes were examined by restricted cubic splines with 4 knots; participants with the highest 1% or the lowest 1% of exposure were excluded to minimize the potential impact of outliers. Cox regression model was performed to calculated hazard ratios (HRs) and 95% CIs for CVD incidence. To test interactions between GRS and hPDI for the outcomes, multiplicative interaction was assessed by adding a cross-product term into a model. Considering that we have three disease conditions as the primary outcomes (obesity, high BP, and CVD), we used the significant levels of testing interactions (Pinteraction) at 0.017 (0.05/3). Statistical analyses were performed with the SAS version 9.4 (SAS Institute Inc.) and STATA SE 14.0 (StataCorp).
Results
Individuals with higher scores of hPDI were more likely to be older, females, non-current smokers, multivitamin users, and more likely to have lower intakes of energy and alcohol and higher education history (Table 1). Higher hPDI was related to lower ORs for obesity and untreated high BP among the study participants (SFigure 4).
Table 1:
Characteristics of study participants according to quartile (Q) categories of healthful plant-based diet index
| Q1 (n=30550) |
Q2 (n=32123) |
Q3 (n=26255) |
Q4 (n=32871) |
|
|---|---|---|---|---|
| Healthful plant-based diet index | 47 (3) | 54 (1) | 58 (1) | 65 (3) |
| Animal foods, servings/d | 4.9 (2.4) | 3.8 (2.0) | 3.2 (1.8) | 2.6 (1.7) |
| Unhealthful plant-based foods, servings/d | 7.1 (3.1) | 5.2 (2.6) | 4.1 (2.3) | 2.9 (2.0) |
| Healthful plant-based foods, servings/d | 9.2 (4.0) | 11.7 (4.3) | 13.4 (4.4) | 16.5 (5.0) |
| Age, y | 53.8 (8.1) | 55.1 (8.0) | 55.6 (7.8) | 56.0 (7.6) |
| Male sex | 16061 [52.6] | 14338 [44.6] | 10406 [39.6] | 11085 [33.7] |
| Smoking habit | ||||
| Never | 17748 [58.1] | 18879 [58.8] | 15704 [59.8] | 19710 [60] |
| Former | 9689 [31.7] | 10508 [32.7] | 8593 [32.7] | 11314 [34.4] |
| Current | 3060 [10] | 2671 [8.3] | 1904 [7.3] | 1793 [5.5] |
| Missing | 53 [0.2] | 65 [0.2] | 54 [0.2] | 54 [0.2] |
| Income | ||||
| Less than £18,000 | 3540 [11.6] | 3824 [11.9] | 3115 [11.9] | 3901 [11.9] |
| £18,000 to £30,999 | 6097 [20.0] | 6722 [20.9] | 5521 [21] | 6939 [21.1] |
| £31,000 to £51,999 | 8372 [27.4] | 8388 [26.1] | 6801 [25.9] | 8583 [26.1] |
| £52,000 to £100,000 | 7455 [24.4] | 7843 [24.4] | 6367 [24.3] | 7836 [23.8] |
| Greater than £100,000 | 2221 [7.3] | 2280 [7.1] | 1910 [7.3] | 2352 [7.2] |
| Missing | 2865 [9.4] | 3066 [9.5] | 2541 [9.7] | 3260 [9.9] |
| Townsend deprivation index | −1.8 (2.8) | −1.8 (2.7) | −1.9 (2.7) | −1.8 (2.7) |
| College or university degree, yes | 11964 [39.2] | 13616 [42.4] | 11701 [44.6] | 15951 [48.5] |
| Physical activity, MET-hours/wk | 39 (40.6) | 39.1 (38.4) | 40.4 (38.7) | 42.3 (39.8) |
| Hours of TV-watching, hours/d | 2.6 (1.5) | 2.5 (1.5) | 2.4 (1.4) | 2.2 (1.4) |
| Sleep duration, hours/d | 7.2 (1.0) | 7.2 (1.0) | 7.2 (0.9) | 7.2 (0.9) |
| Multivitamin user, yes | 7303 [23.9] | 7961 [24.8] | 6859 [26.1] | 9412 [28.6] |
| Total energy intake, kcal/d | 2293 (623) | 2097 (596) | 1997 (582) | 1923 (565) |
| Saturated fatty acids, %E | 14 (3.7) | 12.9 (3.8) | 12.2 (3.8) | 11.1 (3.6) |
| Alcohol intake, g/d | 18.1 (25.3) | 16.9 (23.5) | 15.6 (22.0) | 13.4 (19.9) |
| Dietary fiber, g/d | 13.6 (5.8) | 15.4 (6.5) | 16.8 (6.8) | 19.8 (7.5) |
| Magnesium, g/d | 324 (97) | 335 (102) | 346 (105) | 375 (115) |
| Potassium, g/d | 3574 (1160) | 3645 (1187) | 3723 (1193) | 3922 (1210) |
| Body mass index (BMI), kg/m2 | 26.9 (4.3) | 26.3 (4.1) | 26.0 (4.1) | 25.5 (3.9) |
| Systolic blood pressure, mmHg | 134 (17) | 134 (17) | 133 (17) | 133 (17) |
| Diastolic blood pressure, mmHg | 81 (10) | 80 (10) | 80 (10) | 79 (9) |
| Mean arterial blood pressure, mmHg | 98 (11) | 98 (11) | 97 (11) | 97 (11) |
| History of dyslipidemia*, yes | 1787 [5.8] | 1897 [5.9] | 1674 [6.4] | 2055 [6.3] |
| Triglycerides, mmol/L | 1.48 (1.05, 2.16) | 1.38 (0.99, 2.00) | 1.34 (0.96, 1.92) | 1.28 (0.93, 1.82) |
| HDL cholesterol, mmol/L | 1.44 (0.37) | 1.50 (0.38) | 1.53 (0.38) | 1.57 (0.39) |
| Genetic risk score of BMI | 70.8 (5.7) | 70.8 (5.7) | 70.9 (5.6) | 71.0 (5.6) |
Data are mean (SD), N [%], or median (25th, 75th).
Self-reported history of dyslipidemia or cholesterol-lowering medication use
GRS × hPDI interactions on obesity and BP
We found significant interactions between GRS and hPDI on obesity in a multivariate-adjusted model controlling for the covariates (Figure 1, panel A); higher adherence to hPDI was more strongly associated with lower levels of BMI (Pinteraction <0.0001) among participants with higher GRS than those with lower GRS. Further, we found a similar pattern of GRS-hPDI interactions on mean arterial BP (Figure 1, panel B); the favorable association of hPDI with mean arterial BP was stronger among participants with higher GRS than among those with lower GRS (Pinteraction=0.02). Figure 2 shows ORs for untreated high BP by hPDI scores according to the genetic risk of obesity. There was a significant GRS-hPDI interaction for untreated (i.e., undiagnosed) high BP (Pinteraction=0.0036); higher hPDI was associated with a lower probability for untreated high BP among people with higher GRS, as compared with those with lower GRS. STable 5 shows differences in BMI or mean arterial BP levels per 10-point increase in hPDI across quartile categories of GRS if we viewed differently. The genetic associations with BMI and mean BP were stronger among participants with lower hPDI than among those with higher hPDI.
Figure 1:
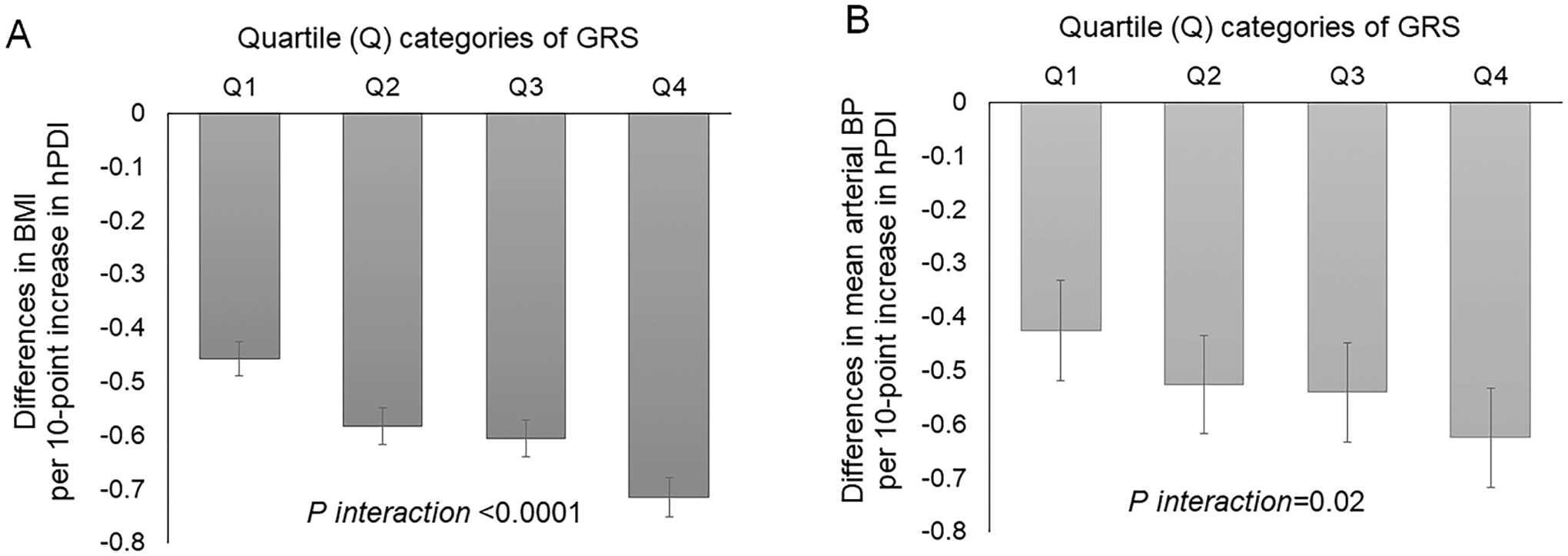
Differences in BMI (panel A) and mean arterial blood pressure (panel B) per 10-point increase in healthful plant-based diet index (hPDI) according to quartile (Q) categories of genetic risk score (GRS) of BMI.
Data are effect sizes (β coefficients [±SE]) after controlling for covariates of age, sex, and the top five principal components of ancestry, college education history, the Townsend deprivation index, smoking habit, total energy intake, multivitamin supplement use, alcohol intake, physical activity, sleep duration, and TV watching hours.
Figure 2:
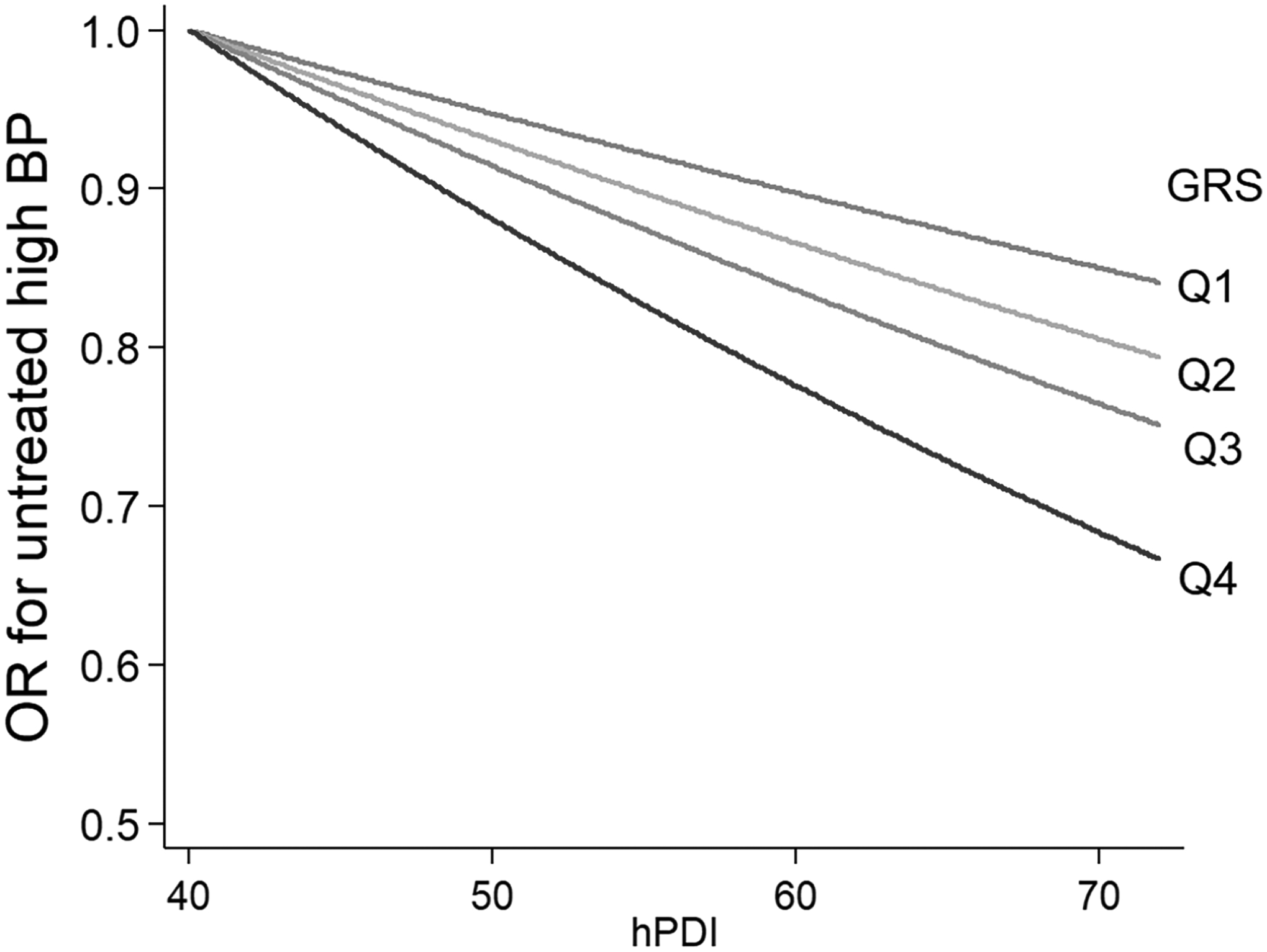
Odds ratio (OR) for untreated high blood pressure by the healthful plant-based diet index (hPDI), according to quartile (Q) categories of the genetic risk score (GRS) of BMI.
Data after adjusted for the same covariates of Figure 1.
We performed several sensitivity analyses. If we examined individual SNPs in or near FTO, TMEM18, and MC4R, there were significant interactions between hPDI and FTO SNP rs1558902 (Pinteraction=0.025), TMEM18 SNP rs13021737 (Pinteraction= 0.002), or MC4R SNP rs6567160 (Pinteraction=0.004) on BMI. Further, if we performed analyses using HDL cholesterol and triglycerides as sensitivity outcomes to examine the GRS-hPDI interactions, we observed similarly significant hPDI-GRS interaction patterns for triglyceride levels (Pinteracion= 0.002) and HDL cholesterol levels (Pinteracion=0.012) (STable 6). The GRS-hPDI interaction on triglycerides was also significant among participants without a history of known dyslipidemia.
GRS × hPDI interactions on CVD
We then performed prospective analyses to examine whether there was significant evidence of gene × diet (i.e., GRS × hPDI) interactions for CVD events. During a mean (SD) 5.1 (0.9) years of follow-up after baseline, there were 1033 incident cases of CVD, including 637 cases of MI. First, we confirmed a significant main effect of hPDI for CVD incidence (multivariate-adjusted HR per 10 points of hPDI: 0.86 [95% CI: 0.78, 0.94]) as well as for MI incidence (HR 0.80 [0.71, 0.91]). As compared to participants with less adherence to the hPDI (in the lowest tertile, T1), those with the highest adherence to hPDI (in the top tertile, T3) had a 20% decreased risk of CVD (HR 0.80 [95% CI: 0.68, 0.94]) and a 26% decreased risk of MI (HR 0.74 [0.60, 0.91]). On the other hand, we observed GRS-hPDI interaction on CVD, indicating that high/low genetic risk of obesity significantly modified the associations of hPDI with CVD incidence (Pinteration=0.03). When we tested GRS-hPDI interactions for MI and stroke separately, we found a significant GRS-hPDI interaction on MI (Pinteraction=0.006), but not on stroke (Pinteration=0.71). Figure 3 shows HRs for CVD (panel A) or MI (panel B) according to high/low GRS categories (based on the median value) and hPDI categories (low, middle, or high adherence according to the tertile categories). Among individuals with high GRS, risks for developing CVD or MI were in particular decreased if participants had the highest adherence to the dietary patterns (i.e., those in the T3 of hPDI) (HR 0.66 [95% CI: 0.52, 0.83] for CVD; HR 0.54 [0.39, 0.74] for MI) as compared to those with less adherence (in the T1 of hPDI). The interaction effect was not appreciably changed (Pinteratios=0.037 for CVD; Pinteration=0.007 for MI) when we additionally controlled for the presence of obesity, high BP, and dyslipidemia (a self-reported history of treated dyslipidemia, triglyceride level of 1.69 mmol/L or greater, or HDL cholesterol of less than 1.03 mmol/L in men or less than 1.29 mmol/L in women) in the model.
Figure 3:
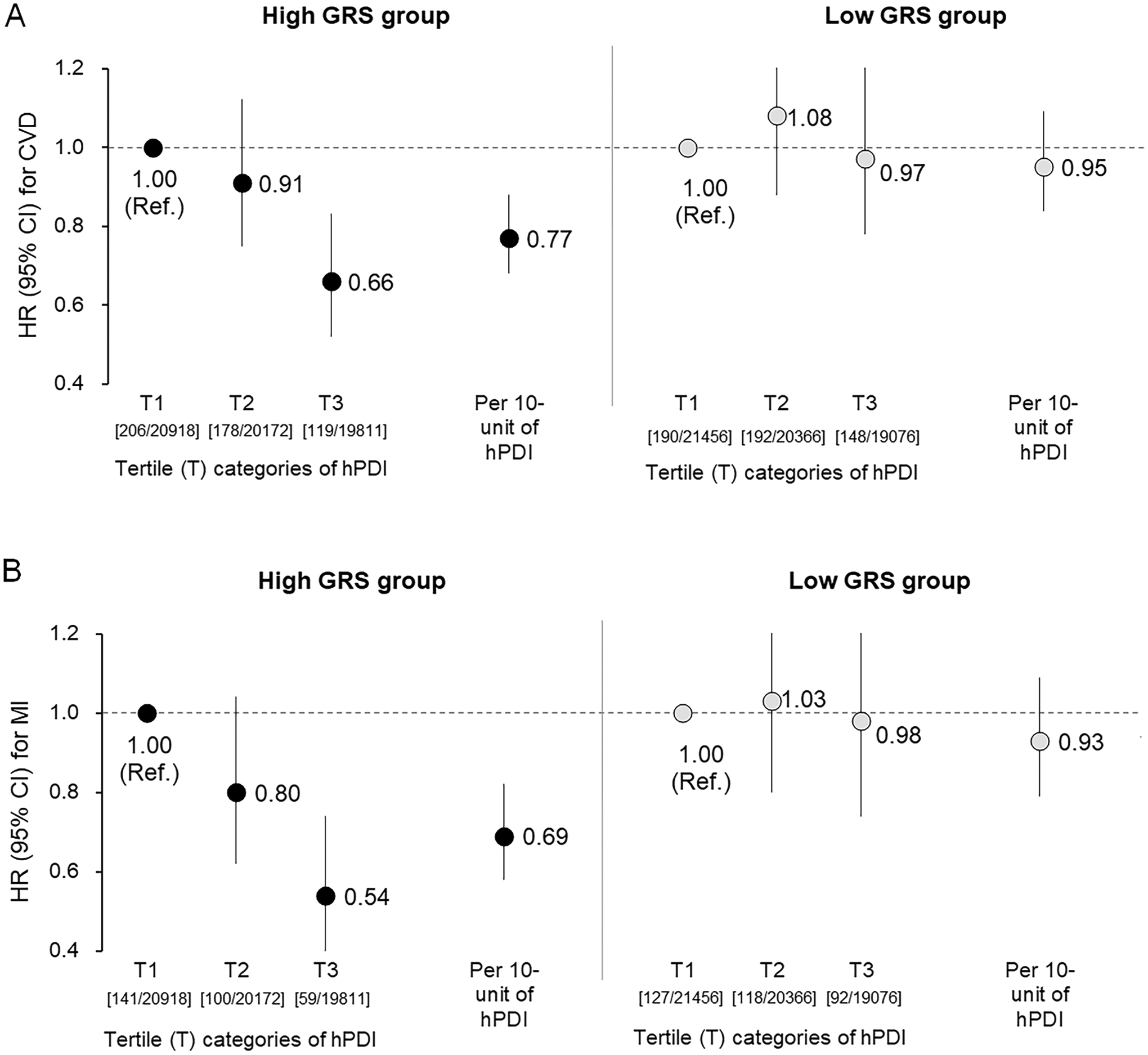
Hazard ratios (HRs) for CVD (A) or MI (B) according to tertile (T) categories or 10-unit increment of healthful plant-based diet index (hPDI) among individuals with high or low GRS of BMI.
Data and 95% CIs after adjusted for the same covariates of Figure 1. Data in bracket are [incident cases/N] across tertile categories of hPDI.
Results of sensitivity analyses
We tested interactions between GRS and individual food groups for MI incidence; each 1 SD unit increase in nuts (Pinteracion=0.01) and vegetables (Pinteracion=0.01) showed the most robust interactions with GRS for MI (Figure 4). Given such interaction effect was observed for GRS of BMI, we tested interactions between phenotype BMI and hPDI. There was significant evidence of interactions between hPDI and BMI levels (Pinteraction= 0.006) or BMI categories (<25, 25 to 29.9, or 30.0 or more; Pinteraction=0.03) for the MI incidence. Additionally, we performed other sensitivity analyses to estimate HRs for CVD only among participants with obesity or high BP at baseline since those individuals would be more affected by the gene-diet interactions (n=82169; 890 incident cases of CVD, including 566 incident cases of MI). We found similar significant evidence of GRS-hPDI interaction patterns on risks of CVD (Pinteration=0.014) and MI (Pinteration=0.0017) in the subpopulation (SFigure 5). Finally, all analyses were repeated after excluding participants (n=22785) who reported that their dietary intake yesterday was not a fairly typical diet; the gene-diet interaction patterns for the outcomes were statistically significant even after excluding these participants (STables 7–9).
Figure 4:
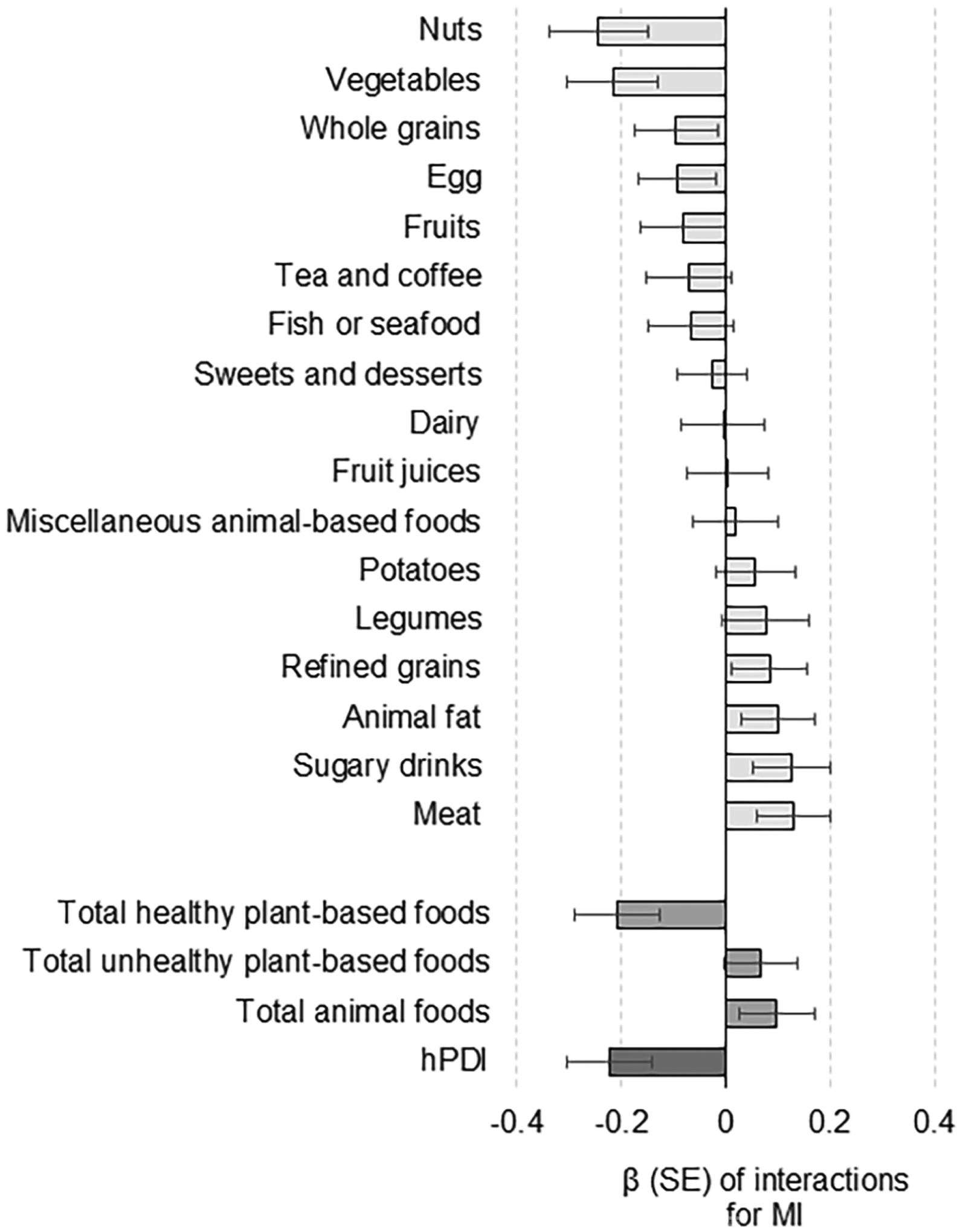
Interaction of genetic risk score (GRS) of obesity with individual food groups for the incidence of MI.
Data and error bars indicate β coefficients and SE for interactions between GRS and dietary components (per 1 SD increment) for the risk of MI after controlling for the covariates.
Discussion
In this study, we newly found significant interactions of adherence to healthy plant-based dietary patterns with genetic susceptibility to obesity regarding risks of CVD events, particularly the risk of MI. Similar patterns of interactions were found on obesity-related cardiometabolic comorbidities. Our results highlight the importance that people at genetically higher risk may be more responsive to benefits of plant-based diets to modify risks of obesity and its cardiometabolic comorbidities.
We showed that the genetic risk of obesity was modified by the adherence to healthy plant-based dietary patterns, and these findings are in line with our previous studies showing that a higher general diet quality (such as assessed by the Alternate Healthy Eating Index (AHEI) and Dietary Approaches to Stop Hypertension (DASH) diet scores) attenuated genetic predisposition to obesity or weight gain in US cohorts [7, 10]. As compared to other diet indices (such as AHEI, DASH, and Mediterranean diet scores), the hPDI is based on more food items to capture synergistic and graded intakes of various food items. Food and nutrient intakes that have been reported to interact with the genetic susceptibility to obesity (such as intakes of sugar-sweetened beverages, coffee, and saturated fat) [8, 11, 40–43] were included as components in the hPDI, which might contribute to the observed strong GRS-hPDI interactions on obesity in this study.
Also, we newly found significant GRS-hPDI interactions on undiagnosed high BP, suggesting that better adherence to hPDI significantly modified obesity-related vascular comorbidities for people at genetically higher risk of obesity. Similar interactions were found for lipid markers. Previous evidence has shown that vegetarian diets and healthier plant-based foods would be beneficial for controlling levels of BP and HDL cholesterol [20, 21, 44] while the associations for triglycerides have been conflicting across studies [21]. Hypertension, hypertriglyceridemia, and low HDL cholesterol levels are major comorbidities of obesity to increase the risk of CVD [1], and genetic variants of obesity may also increase risks of these comorbidities [15, 32, 45]. Nonetheless, our findings on GRS-BMI and hPDI interactions on cardiometabolic markers were based on cross-sectional analyses, and expanded prospective cohort studies in various populations are necessary to clarify whether adherence to healthy dietary patterns is related to greater improvements in the cardiometabolic health among people at a genetically high risk of obesity.
On the other hand, we also assessed the incidence of CVD prospectively and found similar patterns of GRS-hPDI interactions on CVD. Mendelian randomization analyses have shown a causal effect of obesity for the risk of coronary disease [15, 16], and our results highlight that having greater adherence to healthy plant-based dietary patterns could attenuate the CVD risk for people at genetically higher risk of obesity. For example, we observed that the highest adherence to hPDI was associated with a 26% decreased risk of MI in the total study participants; a meta-analysis also reported that vegetarian diet was related to a 25% reduced risk of ischemic heart disease [24]. On the other hand, there was a more (i.e., 46%) decreased risk by having the adherence to hPDI-assessed dietary patterns in people with a higher risk of obesity. Our findings suggest that people carrying higher obesity GRS may befit more to modify the risks of not only obesity but also subsequent CVD by following dietary recommendations of increasing intake of healthy plant foods, while reducing intake of less healthy plant foods and animal foods. These findings are supported by a meta-analysis of diet or lifestyle intervention trials (including the PREDIMED trial, a randomized trial aimed at assessing the effect of the Mediterranean diet for CVD prevention) showing that individuals carrying obesity predisposing allele of FTO gene may lose more weight through diet/lifestyle interventions as compared to non-carriers [46]. As for the biological mechanisms, adherence to plant-based dietary patterns may be related to improved profiles of obesity-related inflammation [47] and insulin sensitivity [48], which may also affect vascular complications of obesity. Other pathways, such as effects of plant bioactives in regulating non-shivering thermogenesis and energy expenditure [49, 50] as well as in stimulation of antioxidant activities [51] might be involved. Some obesity genes included in our GRS are expressed in the hypothalamus [32, 52], which controls energy balance and regulation of food intake [53, 54], and FTO and MC4R gene variants may be related to determining total energy intake and preference of macronutrients [33–35]. In our previous study, interactions with the Alternative Healthy Eating Index on obesity were more significant for central nervous system-related genetic variants [10]. The present study also showed significant interactions between hPDI and several specific genes (FTO, TMEM18, and MC4R) on BMI, suggesting that dietary preference and appetite control may be partly involved in the gene-diet interactions on obesity. We also speculate that people who carry higher-risk alleles of the GRS may be more sensitive to dietary intake and plant-based biological compounds to being overweight or obese, which may, in part, modify the risks of cardiometabolic comorbidities of obesity and CVD. Interestingly, we observed that the interaction effect for MI did not completely become null after controlling for obesity, suggesting that the effect of GRS-hPDI interactions on CVD events may be related to the risk through mechanisms other than gaining adiposity. Also, higher GRS has been associated with risk of obesity since early life and gaining adiposity throughout the life course [13, 14], as well as cardiovascular risk [15, 16]. Our findings suggest that people at higher genetic risk of obesity may gain more benefits of lowering CVD risk by following healthy plant-based diet patterns, regardless of the presence of obesity at baseline. Such finding has important implication regarding the lifecourse prevention of CVD since early life.
Our study has several strengths. Individual food items were extensively assessed to evaluate the adherence to hPDI based on various food groups. The consistent directions of gene-diet interactions on obesity and its related vascular comorbidities strengthen the conclusion. Our study also has several potential limitations. Firstly, it is challenging to quantify individuals’ diet accurately in large cohorts, and dietary assessment might be subject to recall error, as well as limitations in representing habitual, long-term dietary habits. Nonetheless, we acknowledged the hPDI used in our main analysis was correlated the averaged hPDI based on repeated assessments at different time points; we also showed results only among people who reported dietary intake as a normal diet. Secondly, analyses for BMI and BP were performed in a cross-sectional design, although we excluded participants with histories of chronic diseases. Thirdly, there might be a potential bias of the studied population, and the generalizability of our findings needs to be replicated in other independent prospective cohorts.
In conclusion, our study underscores the importance of adherence to healthy plant-based dietary patterns to modify the risks of cardiovascular abnormalities for people at genetically higher risk of obesity. These results would be important in the precision medicine strategy considering genetics and plant-based dietary habits to modify the cardiovascular health for people at higher risk of genetically determined obesity.
Supplementary Material
Acknowledgments:
We appreciate all the participants of the UK biobank for their continued cooperation. We thank the UK Biobank Management team for their assistance.
Funding Sources:
The study is supported by National Institutes of Health (NIH) grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States-Israel Binational Science Foundation Grant 2011036. LQ was a recipient of the American Heart Association Scientist Development Award (0730094N). YH was a recipient of a Grant-in-Aid for Scientific Research and Postdoctoral Fellowship for Research Abroad from the Japan Society for the Promotion of Science. YH is a recipient of the 2019 American Heart Association postdoctoral fellowship award (19POST34380035). The sponsors had no role in the design or conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors declare no conflict of interest associated with this publication.
References
- [1].Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr Rev. 2018;39:79–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005;6:287–98. [DOI] [PubMed] [Google Scholar]
- [4].Hruby A, Manson JE, Qi L, Malik VS, Rimm EB, Sun Q, et al. Determinants and Consequences of Obesity. Am J Public Health. 2016;106:1656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heianza Y, Qi L. Impact of Genes and Environment on Obesity and Cardiovascular Disease. Endocrinology. 2019;160:81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6:223–36. [DOI] [PubMed] [Google Scholar]
- [7].Wang T, Heianza Y, Sun D, Huang T, Ma W, Rimm EB, et al. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ. 2018;360:j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qi Q, Chu AY, Kang JH, Huang J, Rose LM, Jensen MK, et al. Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ. 2014;348:g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ding M, Ellervik C, Huang T, Jensen MK, Curhan GC, Pasquale LR, et al. Diet quality and genetic association with body mass index: results from 3 observational studies. Am J Clin Nutr. 2018;108:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tyrrell J, Wood AR, Ames RM, Yaghootkar H, Beaumont RN, Jones SE, et al. Gene-obesogenic environment interactions in the UK Biobank study. Int J Epidemiol. 2017;46:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang T, Heianza Y, Sun D, Zheng Y, Huang T, Ma W, et al. Improving fruit and vegetable intake attenuates the genetic association with long-term weight gain. Am J Clin Nutr. 2019;110:759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Seyednasrollah F, Makela J, Pitkanen N, Juonala M, Hutri-Kahonen N, Lehtimaki T, et al. Prediction of Adulthood Obesity Using Genetic and Childhood Clinical Risk Factors in the Cardiovascular Risk in Young Finns Study. Circ Cardiovasc Genet. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Song M, Zheng Y, Qi L, Hu FB, Chan AT, Giovannucci EL. Longitudinal Analysis of Genetic Susceptibility and BMI Throughout Adult Life. Diabetes. 2018;67:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lyall DM, Celis-Morales C, Ward J, Iliodromiti S, Anderson JJ, Gill JMR, et al. Association of Body Mass Index With Cardiometabolic Disease in the UK Biobank: A Mendelian Randomization Study. JAMA Cardiol. 2017;2:882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hagg S, Fall T, Ploner A, Magi R, Fischer K, Draisma HH, et al. Adiposity as a cause of cardiovascular disease: a Mendelian randomization study. Int J Epidemiol. 2015;44:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Riaz H, Khan MS, Siddiqi TJ, Usman MS, Shah N, Goyal A, et al. Association Between Obesity and Cardiovascular Outcomes: A Systematic Review and Meta-analysis of Mendelian Randomization Studies. JAMA Netw Open. 2018;1:e183788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Farmer B Nutritional adequacy of plant-based diets for weight management: observations from the NHANES. Am J Clin Nutr. 2014;100 Suppl 1:365s–8s. [DOI] [PubMed] [Google Scholar]
- [19].Turner-McGrievy G, Mandes T, Crimarco A. A plant-based diet for overweight and obesity prevention and treatment. J Geriatr Cardiol. 2017;14:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, et al. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014;174:577–87. [DOI] [PubMed] [Google Scholar]
- [21].Wang F, Zheng J, Yang B, Jiang J, Fu Y, Li D. Effects of Vegetarian Diets on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2015;4:e002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brunner EJ, Mosdol A, Witte DR, Martikainen P, Stafford M, Shipley MJ, et al. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr. 2008;87:1414–21. [DOI] [PubMed] [Google Scholar]
- [23].Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, et al. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol. 2017;70:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57:3640–9. [DOI] [PubMed] [Google Scholar]
- [25].Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-Based Diets Are Associated With a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J Am Heart Assoc. 2019;8:e012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heianza Y, Zhou T, Sun D, Hu FB, Manson JE, Qi L. Genetic susceptibility, plant-based dietary patterns, and risk of cardiovascular disease. Am J Clin Nutr. 2020;112:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q, et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern Med. 2020;180:1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bradbury KE, Young HJ, Guo W, Key TJ. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu B, Young H, Crowe FL, Benson VS, Spencer EA, Key TJ, et al. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011;14:1998–2005. [DOI] [PubMed] [Google Scholar]
- [31].Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, et al. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Qi Q, Kilpelainen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, et al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Hum Mol Genet. 2014;23:6961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qi L, Kraft P, Hunter DJ, Hu FB. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum Mol Genet. 2008;17:3502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Drabsch T, Gatzemeier J, Pfadenhauer L, Hauner H, Holzapfel C. Associations between Single Nucleotide Polymorphisms and Total Energy, Carbohydrate, and Fat Intakes: A Systematic Review. Adv Nutr. 2018;9:425–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484–e594. [DOI] [PubMed] [Google Scholar]
- [37].Definitions of acute myocardial infarction (MI) and main MI pathological types for UK Biobank phase 1 outcomes adjudication. Version 1, January 2017.
- [38].Definitions of stroke and main stroke pathological types for UK Biobank phase 1 outcomes adjudication. Version 1, January 2017.
- [39].Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- [40].Olsen NJ, Angquist L, Larsen SC, Linneberg A, Skaaby T, Husemoen LL, et al. Interactions between genetic variants associated with adiposity traits and soft drinks in relation to longitudinal changes in body weight and waist circumference. Am J Clin Nutr. 2016;104:816–26. [DOI] [PubMed] [Google Scholar]
- [41].Wang T, Huang T, Kang JH, Zheng Y, Jensen MK, Wiggs JL, et al. Habitual coffee consumption and genetic predisposition to obesity: gene-diet interaction analyses in three US prospective studies. BMC Med. 2017;15:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Casas-Agustench P, Arnett DK, Smith CE, Lai CQ, Parnell LD, Borecki IB, et al. Saturated fat intake modulates the association between an obesity genetic risk score and body mass index in two US populations. J Acad Nutr Diet. 2014;114:1954–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Celis-Morales CA, Lyall DM, Gray SR, Steell L, Anderson J, Iliodromiti S, et al. Dietary fat and total energy intake modifies the association of genetic profile risk score on obesity: evidence from 48 170 UK Biobank participants. Int J Obes (Lond). 2017;41:1761–8. [DOI] [PubMed] [Google Scholar]
- [44].Schwingshackl L, Hoffmann G, Iqbal K, Schwedhelm C, Boeing H. Food groups and intermediate disease markers: a systematic review and network meta-analysis of randomized trials. Am J Clin Nutr. 2018;108:576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee MR, Lim YH, Hong YC. Causal association of body mass index with hypertension using a Mendelian randomization design. Medicine (Baltimore). 2018;97:e11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xiang L, Wu H, Pan A, Patel B, Xiang G, Qi L, et al. FTO genotype and weight loss in diet and lifestyle interventions: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103:1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Eichelmann F, Schwingshackl L, Fedirko V, Aleksandrova K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes Rev. 2016;17:1067–79. [DOI] [PubMed] [Google Scholar]
- [48].Kahleova H, Tura A, Hill M, Holubkov R, Barnard ND. A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: A 16-week randomized clinical trial. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kang HW, Lee SG, Otieno D, Ha K. Flavonoids, Potential Bioactive Compounds, and Non-Shivering Thermogenesis. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mele L, Bidault G, Mena P, Crozier A, Brighenti F, Vidal-Puig A, et al. Dietary (Poly)phenols, Brown Adipose Tissue Activation, and Energy Expenditure: A Narrative Review. Adv Nutr. 2017;8:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S–20S. [DOI] [PubMed] [Google Scholar]
- [52].Yeo GS, Heisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nat Neurosci. 2012;15:1343–9. [DOI] [PubMed] [Google Scholar]
- [53].Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–308. [DOI] [PubMed] [Google Scholar]
- [54].Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


