Abstract
Background
The speed with which a pathogen circulates in a sexual network is a function of network connectivity. Cross-sectional connectivity is a function of network features like momentary degree and assortative mixing. Temporal connectivity is driven by partner acquisition rates. The forward-reachable path has been proposed as a summary measure of these two aspects of transmission potential. We use empirical data from San Francisco and Atlanta to estimate the generative parameters of the forward-reachable path and compare results to the HIV/sexually-transmitted infection (STI) epidemics in each city.
Methods
We used temporal exponential random graph models to estimate the generative parameters for each city’s dynamic sexual network from survey data. We then simulated stochastic dynamic networks from the fitted models and calculated the forward-reachable path for each realization, overall and stratified by partnership type and demographics.
Results
The overall mean and median paths were higher in San Francisco than in Atlanta. The overall paths for each city were greater than the sum of the paths in each individual partnership network. In the casual partnership network, the mean path was highest in the youngest age group and lowest in the oldest age group, despite the fact that the youngest group had the lowest mean momentary degree and past-year partner counts.
Conclusions
The forward-reachable path by age group revealed additional utility of the measure beyond the temporal and cross-sectional network connectivity measures. Other non-network factors are still necessary to infer total epidemic potential for any specific pathogen.
Keywords: Sexual networks, Epidemic potential, HIV, Sexually transmitted infections
INTRODUCTION
In HIV and sexually transmitted infection (STI) research, the framework of sexual networks has furthered our understanding of the potential drivers of pathogen prevalence and incidence at the population level.1–3 The empirical network epidemiology literature has mostly focused on key network features that are directly observable in survey data: momentary degree (number of ongoing partnerships at a point in time), assortative mixing (partnership formation between individuals with the same or similar attributes), and cumulative degree (number of partnerships over a given period of time).4 All of these features influence network connectivity and, therefore, epidemic potential. Generally, lower mean momentary degree among individuals means less connectivity in the network.4–7 However, in networks with a low mean degree, the more skewed the distribution of individual degrees, the greater the connectivity within the network.4 Partnership concurrency (momentary degree ≥ 2) also affects epidemic potential by facilitating HIV/STI transmission, specifically during periods of heightened biologic transmissibility (acute HIV infection).2,8,9 Conversely, networks with high levels of assortative mixing and different levels of within-group connectivity may modify transmission by restricting an epidemic within a smaller, high prevalence group.10,11 These cross-sectional network features are only one component of the temporal coevolution of networks and pathogen spread.11–14 In dynamic networks, cross-sectional degree and mixing features interact with the partner acquisition rates, the more familiar “number of partners” accumulated over a period of time.8
The forward-reachable path is one potential informative population-level metric that captures the impact of both cross-sectional and temporal network features on emergent temporal connecitivity.15,16 The forward-reachable path quantifies the maximum number of nodes (persons) each index node is connected to directly (through their own partners) and indirectly (through partners of partners) over time. The forward-reachable path represents an upper threshold for epidemic potential, quantifying the specific contribution of the network to the epidemic dynamics; it does not predict epidemic size, since that also depends on the transmissibility of the pathogen and factors that modify transmissibility, as well as the duration of infection. Comparing the average forward-reachable paths by group (e.g., age or race–ethnicity) can provide insight into differential contributions to network connectivity, without the assumptions of a full-scale infectious disease transmission model.17
The forward-reachable path itself cannot be directly observed from empirical data; however, many of the network features that help to drive variation in the path are observable. These observable features can be used to estimate generative network models for estimating the path.16
There is a small but growing literature on using observable network statistics to infer epidemic potential in sexual networks.14 Research on the forward-reachable path has explored the relative contributions of the cross-sectional and temporal network features on the growth rate of the path, and found that each feature can push temporal network connectivity over an epidemic threshold when the other is too low to do so alone.16 This is consistent with observations that low levels of concurrency (about 5%) could double the path in a population that had very low partner acquisition rates (about 1 every 3 years).2 It is an open empirical question whether the cross-sectional or temporal network features will dominate the generation of the forward-reachable path in any specific population.
Here we investigate the forward-reachable path in the context of men who have sex with men (MSM) sexual networks in San Francisco and Atlanta. There are substantial differences in the epidemiology of HIV and bacterial STIs among MSM between these two cities, reflecting differences in both transmission dynamics and public health responses.18 New HIV diagnoses declined in San Francisco by 13% between 2017 and 2018.19 In Georgia, HIV cases have remained stable over the last decade and Atlanta currently has the second highest rate of new HIV diagnoses among major U.S. cities.18,20 Conversely, the rate of gonorrhea among men in San Francisco was almost double that of men in Atlanta in 2018, with cases increasing faster in San Francisco than in Atlanta.21 Primary and secondary syphilis infection among men has remained mostly stable in both cities, although it was slightly higher in San Francisco. Examining the forward-reachable path in each city-specific network may provide insight into the interaction between network epidemic potential, properties of the pathogens and clinical interventions that impact transmission rates.
Our study investigates the characteristics of the estimated forward-reachable path for these specific local populations, with a model rooted in empirical survey data from those populations. We estimated the path in a multi-step process, using methods recently developed for statistical network estimation and simulation. We described how the path evolves over time, overall and within partnership types, across each city, and examined whether the overall path could be decomposed into the contributions of each partnership type. We also stratified the one-year forward-reachable path estimates by age and race–ethnicity to explore how network reachability differed by demographic sub-groups, and whether these varied consistently with the observable network statistics, and the epidemiologic disparities in pathogen incidence.
METHODS
Study Design.
We used egocentrically sampled sexual network data from ARTnet, a web-based study of MSM conducted between 2017 and 2019, to fit sexual network models for San Francisco and Atlanta MSM.22 Participants for ARTnet were recruited through the American Men’s Internet Survey (AMIS), an annual web-based national survey.23 ARTnet eligibility criteria included male sex at birth, a current male gender identity, lifetime history of sexual activity with another man, and age between 15 and 65. Participants provided information on their sexual partnerships, but the partners were not sampled directly. After deduplication within and across survey waves, ARTnet had a final sample size of 4,904 participants reporting on 16,198 sexual partnerships. The study was approved by the Emory University Institutional Review Board.
We followed a four-step procedure to estimate the forward-reachable path in each city, summarized in Figure 1. The procedure uses empirical data from a sample to make inferences about the complete network of a target population.13 This approach does not directly model an infectious disease process. Instead, it relies on a network model to estimate connectivity within the network via the FRP. Analyses were conducted using the Statnet R packages.24,25 Analysis code may be found at: https://github.com/EpiModel/NetAnalysis-SF-ATL.
Figure 1.
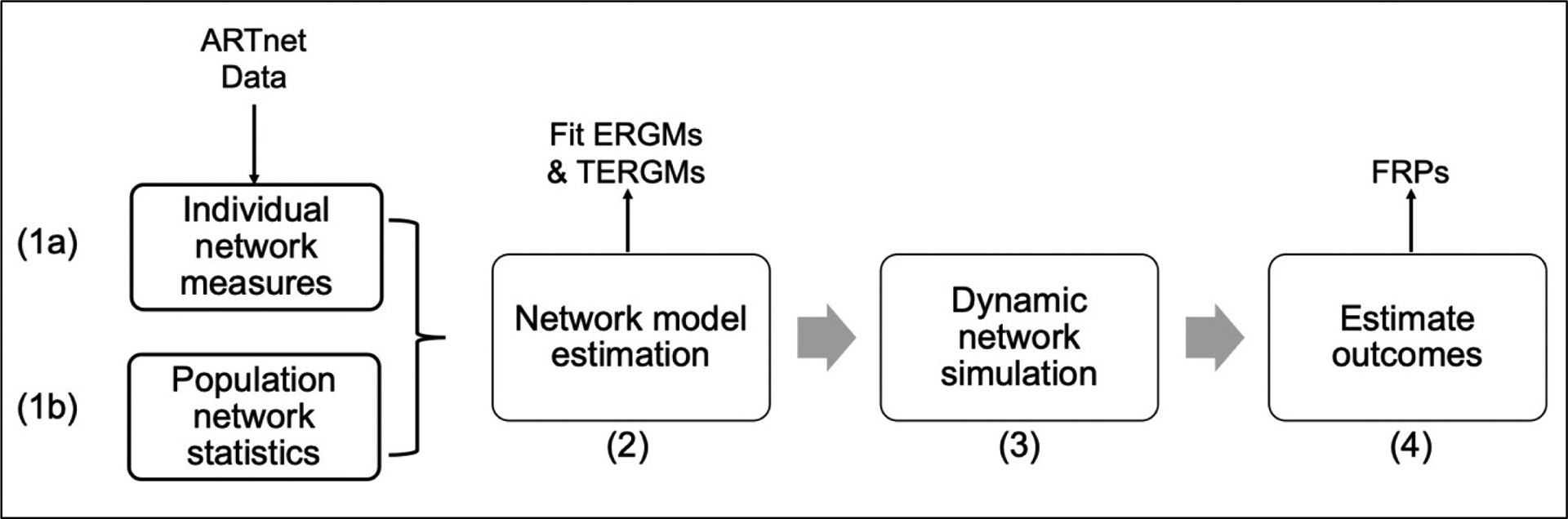
The network analysis process. Step (1a): Estimate individual-level empirical network measures. Step (1b): Combine estimates from 1a with population-level demographic weights to estimate population-level empirical network statistics. Step (2): Fit network models using exponential random graph models (ERGMs) for one-time partnerships and temporal ERGMs for partnerships with duration (main and casual partnerships). Inputs for these models come from step 1b. Step (3): Simulate the complete networks over time for each city. Step (4): Estimate and compare the forward reachable paths (FRPs) for each city.
Step 1a: Individual-Level Empirical Network Measures.
We used the individual-level network measures (Table 1) to derive population-level summary statistics (Step 1b in Figure 1), which we then input as data (technically, the “sufficient statistics”) to estimate the parameters of network models for each city (Step 2 in Figure 1). Descriptive statistics of the participants and their reported partnerships are described elsewhere.22 We categorized partnerships as main (partner considered a boyfriend, significant other, or life partner), casual (an ongoing relationship, but not a main partner), and one-time contacts. Age was categorized into 10-year intervals. Race–ethnicity was dichotomized as Black non-Hispanic/Hispanic versus White non-Hispanic/Other.
Table 1.
Individual-Level Empirical Network Statistics for Sexual Networks of Men Who Have Sex with Men (MSM) in San Francisco and Atlanta
| Main Partnerships | Casual Partnerships | One-Time Partnerships | ||||
|---|---|---|---|---|---|---|
| San Francisco | Atlanta | San Francisco | Atlanta | San Francisco | Atlanta | |
| Momentary Degree, Mean (95% CIa) | ||||||
| Overall | 0.47 (0.36, 0.60) | 0.40 (0.32, 0.49) | 0.88 (0.73, 1.06) | 0.54 (0.45, 0.65) | — | — |
| By Age Group | ||||||
| 15–24 | 0.45 (0.35, 0.59) | 0.37 (0.30, 0.47) | 0.46 (0.38, 0.57) | 0.30 (0.24, 0.36) | — | — |
| 25–34 | 0.57 (0.44, 0.73) | 0.47 (0.38, 0.58) | 0.75 (0.62, 0.90) | 0.48 (0.40, 0.58) | — | — |
| 35–44 | 0.54 (0.42, 0.70) | 0.45 (0.36, 0.56) | 0.96 (0.79, 1.16) | 0.61 (0.51, 0.74) | — | — |
| 45–54 | 0.45 (0.35, 0.58) | 0.37 (0.30, 0.47) | 1.09 (0.91, 1.32) | 0.70 (0.58, 0.85) | — | — |
| 55–64 | 0.34 (0.26, 0.45) | 0.28 (0.22, 0.36) | 1.16 (0.95, 1.40) | 0.74 (0.61, 0.90) | — | — |
| By Race | ||||||
| Black/Hispanic | 0.44 (0.34, 0.58) | 0.37 (0.29, 0.47) | 0.89 (0.73, 1.09) | 0.55 (0.44, 0.67) | — | — |
| White/Other | 0.48 (0.37, 0.62) | 0.40 (0.32, 0.50) | 0.88 (0.73, 1.06) | 0.54 (0.45, 0.65) | — | — |
| Past-Year Cumulative Counts, Mean (95% CI) | ||||||
| Overall | 0.54 (0.42, 0.67) | 0.46 (0.38, 0.56) | 1.2 (1.0, 1.4) | 0.91 (0.78, 1.0) | 10 (9.5, 11) | 4.0 (3.7, 4.2) |
| By Age Group | ||||||
| 15–24 | 0.58 (0.45, 0.74) | 0.49 (0.40, 0.61) | 0.86 (0.73, 1.0) | 0.67 (0.57, 0.78) | 6.1 (5.7, 6.5) | 2.5 (2.3, 2.7) |
| 25–34 | 0.65 (0.51, 0.83) | 0.55 (0.45, 0.67) | 1.1 (0.94, 1.3) | 0.86 (0.74, 0.99) | 9.7 (9.1, 10) | 3.9 (3.7, 4.2) |
| 35–44 | 0.59 (0.46, 0.75) | 0.50 (0.41, 0.61) | 1.3 (1.1, 1.5) | 0.98 (0.84, 1.1) | 12 (11, 10) | 4.7 (4.4, 5.0) |
| 45–54 | 0.48 (0.38, 0.61) | 0.41 (0.33, 0.50) | 1.4 (1.2, 1.6) | 1.1 (0.92, 1.2) | 12 (11, 13) | 4.8 (4.5, 5.2) |
| 55–64 | 0.37 (0.28, 0.47) | 0.31 (0.25, 0.39) | 1.4 (1.2, 1.7) | 1.1 (0.94, 1.3) | 11 (11, 12) | 4.5 (4.2, 4.9) |
| By Race | ||||||
| Black/Hispanic | 0.51 (0.40, 0.66) | 0.44 (0.36, 0.55) | 1.2 (1.0, 1.5) | 0.93 (0.80, 1.1) | 8.9 (8.4, 9.5) | 3.5 (3.3, 3.8) |
| White/Other | 0.54 (0.42, 0.69) | 0.47 (0.38, 0.57) | 1.2 (1.0, 1.4) | 0.90 (0.78, 1.0) | 10 (9.7, 11) | 4.1 (3.8, 4.4) |
| Mean Duration of Partnerships (Weeks)b | ||||||
| Different ages | 216 | 219 | 101 | 105 | — | — |
| Both 15–24 | 70 | 72 | 47 | 48 | — | — |
| Both 25–34 | 247 | 250 | 67 | 70 | — | — |
| Both 35–44 | 521 | 527 | 106 | 110 | — | — |
| Both 45–54 | 664 | 672 | 150 | 155 | — | — |
| Both 55–64 | 1155 | 1168 | 140 | 145 | — | — |
CI indicates confidence interval.
Stratified based on whether each partner was in the same age group or different age groups.
Individual-level network measures included momentary degree for main and casual partnership types (overall and stratified by age and race–ethnicity), past-year cumulative partnership counts for all partnership types, partnership durations, and assortative mixing by age and race. We calculated momentary degree as the average of the number of ongoing partnerships on the day of study. We estimated partnership durations (in weeks) for main and casual partnerships from partnership age (the difference between the survey date and the partnership start date) for extant partnerships.22 Assortative mixing by age and race–ethnicity was the proportion of partnerships between individuals in the same demographic category. Additional network measures used in the model (cross-network degree and risk quintile stratification) are provided in the eAppendix, eTable 1.
Step 1b: Population-Level Empirical Network Statistics.
To calculate these statistics we multiplied the individual-level measures from Step 1a by the race–ethnicity distribution for each city and a population size of 10,000 MSM.26 Although this population size is smaller than the estimated MSM population size in each city (approximately 145,972 in San Francisco and 102,642 in Atlanta),27 the FRP measures are standardized so a smaller number is used for computational efficiency.
Step 2: Network Model Estimation.
We fit three exponential random graph models to the population-level network statistics to estimate the generative parameters of the underlying sexual partnership networks: cross-sectional models for one-time partnerships and temporal models for main and casual partnerships. The estimand of an exponential random graph model is the log odds of a partnership between each dyad in the network, conditional on the rest of the network. Temporal exponential random graph models model the likelihood of formation and dissolution of partnerships per unit time.
Step 3: Dynamic Network Simulation.
We then used these three fitted models to simulate complete, multilayer dynamic networks in weekly time steps for 5 years. In the simulation, edges formed and dissolved based on the model parameters. We assumed a closed population, so the node set remained constant. The network was simulated as three interacting layers (one per partnership type) with a shared node set and a unique edge set; dependencies between the layers were captured in “cross-network” terms. The layers were simulated sequentially at each weekly time step to account for any changes in the other edge layers (all time-varying nodal attributes were updated each week). We report on one simulation to demonstrate the variability in the forward-reachable path across individuals.
Step 4: Forward Reachable Path Estimation.
The forward-reachable path is an individual-level count of the temporally ordered chain of edges for each node across a sequence of nodes by a specific time point (see the eAppendix for a conceptual demonstration). Our main outcome measure was the time-varying forward-reachable path, estimated weekly for each node. Descriptive analyses provide estimates of the mean, median, and interquartile range (IQR) for the distribution of this forward-reachable path across nodes. We calculated paths for the complete three-layer partnership network and also the paths in the isolated network layers representing each partnership type. Calculating the forward-reachable path within each partnership type stratum enabled us to evaluate a counterfactual question: what was contribution of each partnership type in isolation compared with the complete three-layer network? This decomposition also demonstrates whether the impact of each network is additive or interactive. For the year one forward-reachable path estimates, we stratified the analysis by race and age group. This analysis allowed us to identify the relative reach of each subgroup.
RESULTS
Empirical Network Statistics.
The individual-level empirical network measures are provided in Table 1 and 2. For all three types of partnerships, momentary degree and cumulative counts were higher in San Francisco than in Atlanta, both overall and within age and race–ethnicity categories. For the persistent partnerships, the mean degree was 0.47 for main and 0.88 for casual in San Francisco and 0.40 for main and 0.54 for casual in Atlanta. The past-year cumulative counts of main partners were only slightly higher than their corresponding momentary degrees, whereas counts for casual partnerships were 40%–70% higher than casual momentary degree. The stratified cumulative counts followed the same patterns as the stratified momentary degree estimates. The one-time partnership counts were over twice as high in San Francisco (10) as in Atlanta (4.0). For both cities, there were small differences in degree and counts by race–ethnicity, but stronger patterns by age group. Among casual partnerships the oldest age group had the highest mean degree (1.2 for San Francisco and 0.74 for Atlanta) and the youngest age group had the lowest mean degree (0.46 for San Francisco and 0.30 for Atlanta).
Table 2.
Assortative Mixing Statistics for Sexual Networks of Men Who Have Sex with Men (MSM) in San Francisco and Atlanta
| Main Partnerships | Casual Partnerships | One-Time Partnerships | ||||
|---|---|---|---|---|---|---|
| San Francisco | Atlanta | San Francisco | Atlanta | San Francisco | Atlanta | |
| Assortative Mixinga | ||||||
| By Age Group | ||||||
| 15–24 | 0.77 | 0.80 | 0.60 | 0.57 | 0.55 | 0.56 |
| 25–34 | 0.67 | 0.70 | 0.48 | 0.44 | 0.43 | 0.45 |
| 35–44 | 0.54 | 0.58 | 0.36 | 0.32 | 0.32 | 0.33 |
| 45–54 | 0.41 | 0.44 | 0.25 | 0.22 | 0.23 | 0.24 |
| 55–64 | 0.29 | 0.32 | 0.17 | 0.15 | 0.16 | 0.16 |
| By Race | ||||||
| Black/Hispanic | 0.47 | 0.55 | 0.52 | 0.51 | 0.53 | 0.54 |
| White/Other | 0.78 | 0.83 | 0.71 | 0.70 | 0.75 | 0.76 |
The assortative mixing statistic is the proportion of partnerships in a demographic group that were between members of the same group (i.e., the diagonals of the mixing matrix).
Assortativity decreased with age: individuals in the youngest age group had the highest probability of a same-age partner and those in the oldest age group had the lowest probability. Within race–ethnicity categories, those in the White/Other group had a higher probability of a same-race partner than those in the Black/Hispanic group.
The relational age of both main and casual partnerships varied by the age group of each partner. For both cities, among partnerships between individuals in the same age group, the mean relational age of the partnerships increased with age of the persons. For main partnerships, the mean relational age of the partnerships in San Francisco ranged from 71 weeks among 15–24-year-olds to 1155 weeks among 55–64-year-olds; in Atlanta, it ranged from 72 weeks to 1168 weeks in those two groups. For casual partnerships, these estimates ranged from 47 weeks to 140 weeks for San Francisco and 48 weeks to 145 weeks for Atlanta.
Forward-Reachable Path.
The forward-reachable path results are shown in Table 3 and Figures 2–4. Overall, the 1-year forward-reachable paths reached more individuals in San Francisco than in Atlanta: on average 80% of individuals were reachable in San Francisco compared to 50% in Atlanta after 1 year (Table 3, Column 1). Main partnership networks, characterized by long partnership durations (low turnover) and low degree, on average connected only a small proportion of the population in both cities (<0.02%) within 1 year. Greater differences between cities emerged in the casual and one-time partnership networks. The average percent of the population reachable after 1 year among casual partnerships was 1.4% in San Francisco and 0.08% in Atlanta. For one-time partnerships, the 1-year average forward-reachable path was over 20% in San Francisco and about 5% in Atlanta. For both cities, the overall path was substantially larger than the sum of the three isolated network paths, indicating that the cross-network connectivity plays a large role in overall population connectivity.
Table 3.
One-Year Forward-Reachable Path by Partnership Type in San Francisco and Atlantaa
| Overall | Main | Casual | One-Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | IQRb | Mean | Median | IQR | Mean | Median | IQR | Mean | Median | IQR | |
| San Francisco | ||||||||||||
| Overall | 8200 | 9121 | 8999, 9121 | 1.7 | 2 | 1, 2 | 145 | 12 | 1, 134 | 2059 | 65 | 1, 4514 |
| By Age Group | ||||||||||||
| 15–24 | 8432 | 9121 | 9028, 9121 | 1.8 | 2 | 1, 2 | 190 | 36 | 2, 196 | 1835 | 1 | 1, 4435 |
| 25–34 | 8229 | 9107 | 8999, 9121 | 1.8 | 2 | 1, 2 | 115 | 5 | 1, 73 | 2010 | 4 | 1, 4504 |
| 35–44 | 8367 | 9121 | 9064, 9121 | 1.7 | 2 | 1, 2 | 146 | 14 | 1, 147 | 2155 | 869 | 1, 4541 |
| 45–54 | 8433 | 9121 | 9064, 9121 | 1.6 | 1 | 1, 2 | 173 | 23 | 2, 173 | 2257 | 2541 | 1, 4576 |
| 55–64 | 7529 | 9088 | 8660, 9121 | 1.4 | 1 | 1, 2 | 101 | 3 | 1, 61 | 2040 | 75 | 1, 4505 |
| By Race | ||||||||||||
| Black/Hispanic | 8239 | 9121 | 9017, 9121 | 1.6 | 2 | 1, 2 | 157 | 18 | 1, 157 | 2093 | 178 | 1, 4507 |
| White/Other | 8190 | 9121 | 8999, 9121 | 1.7 | 2 | 1, 2 | 142 | 11 | 1, 125 | 2051 | 46 | 1, 4519 |
| Atlanta | ||||||||||||
| Overall | 5159 | 7081 | 12, 7448 | 1.5 | 1 | 1, 2 | 8 | 2 | 1, 8 | 505 | 1 | 1, 790 |
| By Age Group | ||||||||||||
| 15–24 | 4889 | 6932 | 5, 7404 | 1.6 | 1 | 1, 2 | 10 | 2 | 1, 11 | 283 | 1 | 1, 15 |
| 25–34 | 5269 | 7080 | 2601, 7448 | 1.6 | 2 | 1, 2 | 7 | 1 | 1, 6 | 533 | 1 | 1, 959 |
| 35–44 | 5580 | 7249 | 5396, 7452 | 1.6 | 1 | 1, 2 | 9 | 2 | 1, 9 | 562 | 1 | 1, 1015 |
| 45–54 | 5452 | 7249 | 4703, 7452 | 1.5 | 1 | 1, 2 | 10 | 2 | 1, 11 | 619 | 1 | 1, 1225 |
| 55–64 | 4599 | 6920 | 1, 7406 | 1.3 | 1 | 1, 2 | 5 | 1 | 1, 4 | 526 | 1 | 1, 901 |
| By Race | ||||||||||||
| Black/Hispanic | 5085 | 7074 | 5, 7448 | 1.5 | 1 | 1, 2 | 8 | 2 | 1, 8 | 492 | 1 | 1, 718 |
| White/Other | 5258 | 7112 | 2229, 7448 | 1.5 | 1 | 1, 2 | 8 | 1 | 1, 8 | 522 | 1 | 1, 864 |
Forward-reachable paths are standardized to a population of 10,000 MSM.
IQR indicates interquartile range.
Figure 2.
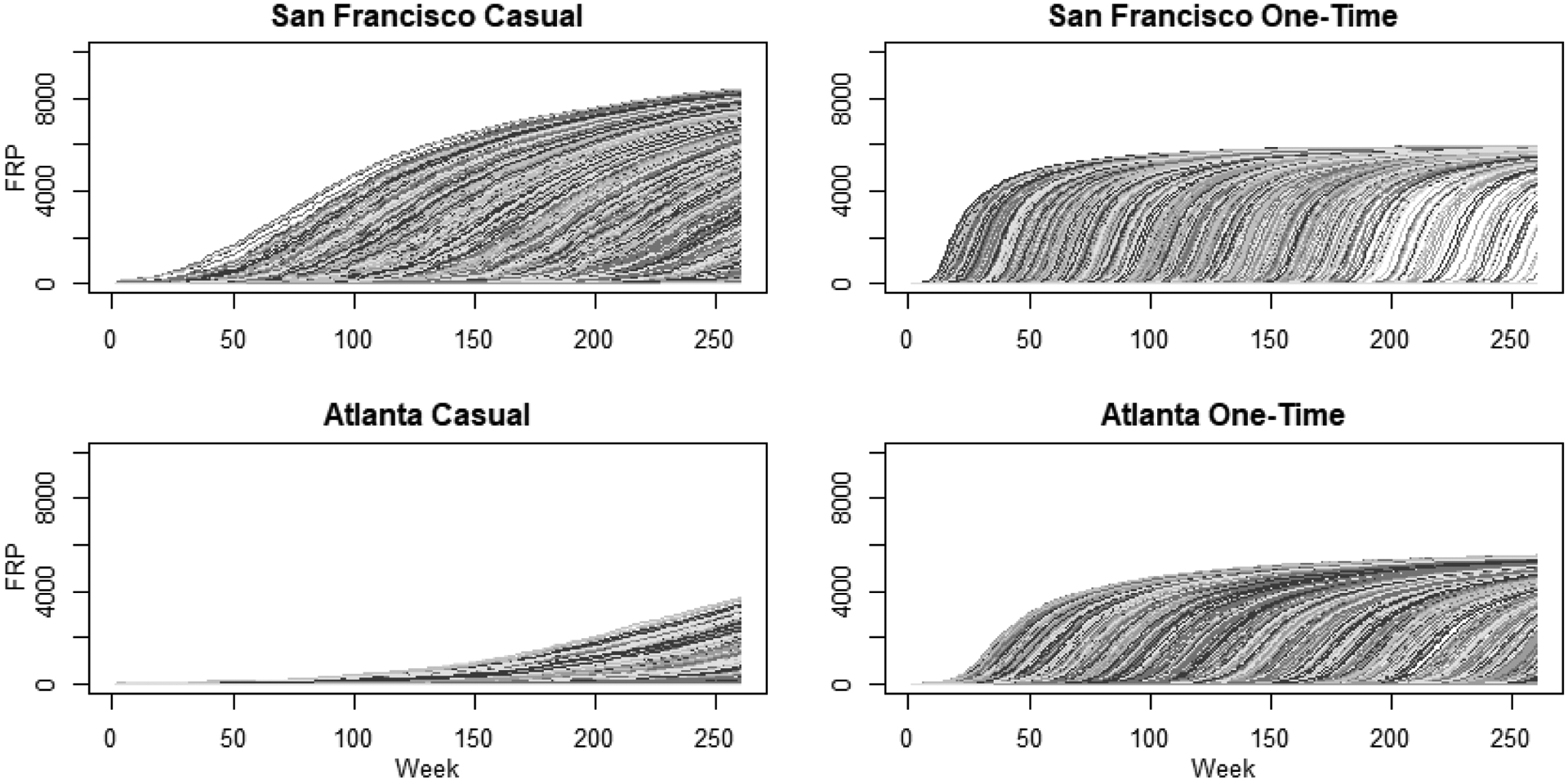
Estimated distribution of forward reachable path (FRP) evolution over a 5-year period for casual and one-time sexual partnership networks of MSM in San Francisco and Atlanta. Each line represents the trajectory of connectivity in the network over time, for a specific node from time 0. Casual partnerships are defined as an ongoing relationship, but not a main partner, and one-time as partnerships are those without duration, occurring only once. The number connected, or reachable within the network, is on the Y-axis and the time step in weeks is the X-axis.
Figure 4.
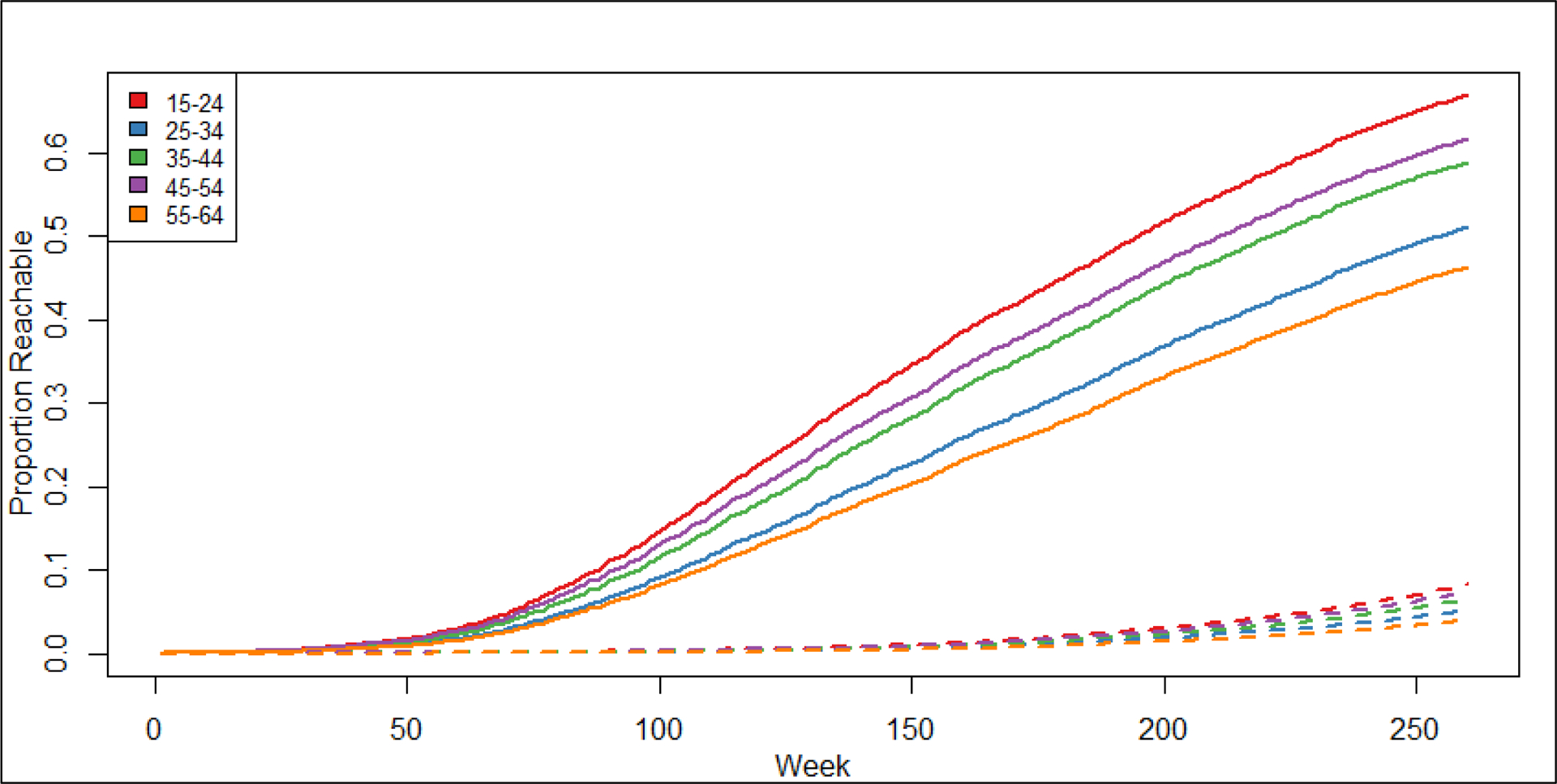
Average proportion of the San Francisco (solid lines) and Atlanta (dashed lines) populations connected within sexual networks of casual partnerships of MSM over a 5-year period by age category. The Y-axis shows the average proportion of the 10,000 MSM connected, or reachable, in the network over time. The X-axis shows the time step in weeks. In San Francisco, reachability ranged from around 40-65% over 5-years, whereas in Atlanta, reachability ranged from around 4–8% over 5-years.
The distribution of individual 5-year forward-reachable path trajectories by partnership type and city are visualized in Figures 2 (casual and one-time partnerships) and 3 (main partnerships). The main partnership forward-reachable paths rose slowly and reached few individuals (less than 30 per 10,000 in both cities). For San Francisco, the casual partnership paths rose more slowly than one-time partnership paths but reached a higher fraction of the population by year 5. In Atlanta, the one-time partnership paths both rose faster and reached more individuals than the casual partnership FRPs. Figures 2 and 3 also shows the profound variation in the individual FRP trajectories, indicating a substantial degree of heterogeneity in reachability across persons in each city. This variability in individual trajectories is also reflected in the 1-year FRPs, as shown in the IQRs in Table 3. For example, in Atlanta the IQR for the overall network was 12 to 7448 per 10,000.
Figure 3.
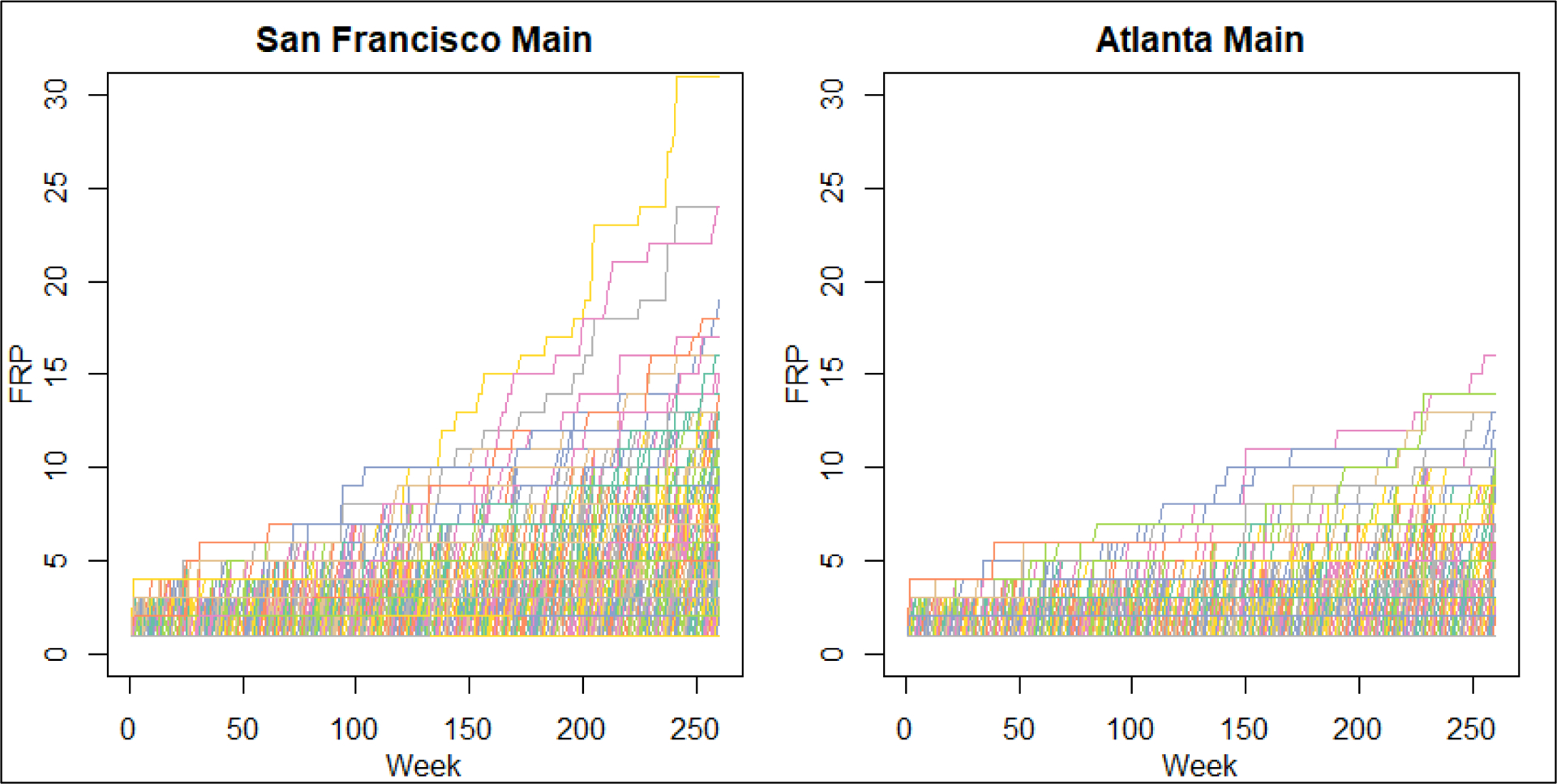
Estimated distribution of forward reachable path (FRP) evolution over a 5-year period for main sexual partnership networks of MSM in San Francisco and Atlanta. Each line represents the trajectory of connectivity in the network over time, for a specific node from time 0. Main partnerships are defined as partners considered a boyfriend, significant other, or life partner. The number connected, or reachable within the network, is on the Y-axis and the time step in weeks is the X-axis.
This variability in forward-reachable path trajectories is strongly associated with age, but not with race–ethnicity. In general, the patterns were consistent across the different path measures. At 1 year, in both cities, the average path among casual partnerships was highest (or tied for highest) in the youngest age group (190 in San Francisco and 10 in Atlanta) and lowest in the oldest age group (101 in San Francisco and 5 in Atlanta) (Table 3). Age variation in the forward-reachable path, particularly in the casual partnership layer, continued to emerge over five years (Figure 4). At year 5, 15–24-year-olds continued to have the highest forward-reachable path, and 55–64-year-olds had the lowest casual network paths in both cities. The ratio of casual paths by these two age groups widened in Atlanta (2.0 to 2.1) but decreased in San Francisco (1.9 to 1.4) as a result of the overall path trajectory in each city (see eAppendix, eTable 2). This age pattern in forward-reachable paths varied inversely with the individual-level empirical network statistics for the casual network (Table 1), where the oldest age group had the highest levels on both momentary degree and cumulative counts of casual partnerships. By contrast, in the one-time network, both the forward-reachable path and the individual-level empirical network statistics varied in the same direction: higher for older age groups.
DISCUSSION
In this study, we examined the forward-reachable path in sexual networks of MSM in San Francisco and Atlanta, two cities with differing HIV and STI epidemic trends. Overall, paths were considerably higher in San Francisco than in Atlanta, suggesting that networks in San Francisco had a greater potential for HIV/STI transmission than networks in Atlanta. In both cities, the overall forward-reachable path was larger than the sum of the paths in the networks for each specific partner type, so these interacting layers combine in a non-additive way. There was profound individual-level variation in the estimated paths that displayed systematic patterns by age for the casual and one-time networks, but little correlation with race–ethnicity. Surprisingly, differences in the casual partnership path by age varied inversely with the differences in individual-level network statistics by age, while differences in the one-time partnership path by age varied consistently with the differences in network statistics by age.
In San Francisco HIV rates have been decreasing but bacterial STI rates (particularly gonorrhea) are increasing faster than in Atlanta. Conversely, in Atlanta HIV rates remain stable and high.19–21 Given these differences, we might expect the underlying sexual networks in these two cities to have unique structural features generating different forward-reachable paths. If the forward-reachable path predicts the relative differences in the observed epidemics well, it might be used as an alternative data-driven approach to estimate epidemic potential in a network in lieu of more complex transmission modeling approaches. The geographic differences in our estimated paths correspond broadly to the bacterial STI diagnosis trends in San Francisco and Atlanta but are less correlated to the geographic differences in HIV diagnoses. We believe this may reflect the impact of clinical interventions, and these comparisons offer a unique window into these dynamics.
Rates of HIV diagnosis have been declining in San Francisco recently, a reflection of their efforts to reduce the HIV epidemic through improved testing, linkage to HIV care, and scaling-up HIV pre-exposure prophylaxis.19 These advances in HIV prevention might mitigate the impact that network factors have on the risk of HIV. If so, then the divergence between the forward-reachable path and the new diagnosis is a metric for assessing the population level efficacy of prevention activities. In this particular comparison, it suggests that San Francisco’s public health HIV prevention and treatment efforts are even more effective than the simple comparison of diagnosis rates suggests: they have achieved greater reductions in HIV diagnoses despite having a higher underlying epidemic potential.
In contrast to HIV, rates of bacterial STIs are rising in San Francisco, and rising faster than rates in Atlanta.21 This is consistent with the higher forward-reachable paths for San Francisco that we estimated based on the empirical network statistics. It is tempting to speculate that this reflects the fact that the prevention services for STIs in San Francisco have not been improved as dramatically as that for HIV, but the reality is likely more complicated. Higher STI case rates in San Francisco may reflect increased screening for STIs among PrEP users.28 However, recent modeling has suggested that PrEP STI screening could decrease STI incidence through this improved case detection.29
For both cities, casual and one-time partnerships contributed the most to the overall size of the forward-reachable paths. This is not surprising: for both partnership types, the turnover rate was faster and the cumulative number of partnerships higher than with main partnerships, resulting in greater temporal connectivity. But as the divergence between the forward-reachable paths and the HIV trends makes clear, reachability is only a measure of potential. Transmission also depends on non-network factors: the properties of a pathogen (e.g., per-act transmission probabilities), the behavior within partnerships (e.g., condom use and frequency of sex), and engagement with clinical care .30–33 In general, the closer the transmission probability is to 100 percent, the better the path will approximate the realized transmission path. Therefore, the forward-reachable path might be less useful for predicting epidemic size for infections like HIV than for higher transmission probability STIs like gonorrhea.34 But the forward-reachable path remains an accurate measure of epidemic potential. Shorter partnerships with high rates of acquisition provide one mechanism of connectivity, longer partnerships with low acquisition rates but overlapping intervals provide another. The relative contribution of each to HIV and STI acquisition and transmission is an empirical question, and here the forward-reachable path may help to quantify this unobserved population characteristic.2
This is the first study to our knowledge to estimate the forward-reachable path using empirically based network models. The findings suggest that estimates of the path can provide unique insights into epidemic potential above and beyond individual-level network statistics like momentary degree and cumulative partnership counts. For example, in the casual partnership networks, the oldest group had the highest momentary degree and cumulative number of partners. Both would suggest that their path should be higher. Instead, the oldest age group had the lowest mean path, and the youngest age group had the highest mean path at all points in time (Figure 4; eTable 2). The reason may be in part due to partnership duration: older MSM may have more ongoing casual partnerships than younger MSM, but their partnerships lasted much longer (Table 1). In the one-time partnerships, the age differences in the network statistics (cumulative rate over time) and the paths were as expected: higher rates in the network statistics for older men translated into larger paths.
Interestingly, the forward-reachable path also provides additional information above the cumulative partner counts, which theoretically reflect this temporal network feature of partnership turnover. This is likely because the empirical survey captured only the past year of partnership history, whereas the mean durations even for the youngest age pairings are approaching one year. The forward-reachable path therefore allows for longer-horizon insights into network connectivity, while still exploiting feasible egocentric network study designs that minimize participant response burden and measurement error.22
Finally, it is worth highlighting the substantial individual-level variability in the forward-reachable path. This suggests that the short-term potential for an epidemic outbreaks or new clusters is dependent upon which nodes seed the transmission process. Observed epidemic indicators, too, may reflect this underlying variation in reachability, with large stochastic variations that should not be overinterpreted. These large variations in individual reachability were more strongly correlated to age than to race–ethnicity. This may reflect the group-level behavioral heterogeneity of nodes as well as mixing between those groups. Because the assortative mixing between demographic groups was less than 100%, the forward-reachable path of one group may be influenced by the average connectivity in another group. Prior literature has demonstrated the potential of assortative mixing to sustain, but not necessarily generate, group-level disparities in disease risk and prevalence over time.1,2
Limitations.
There are several limitations to our analysis. ARTnet relied on participant-reported information, therefore, there is the potential for error in the reporting of the behavioral data we used to parameterize our network models. For instance, there might be biases in the reported number, length, and type of partnerships that favor fewer, longer term partnerships over many, shorter term ones. This could potentially result in an underestimate of the mean degrees and one-time partnership rates or an overestimate of the partnership durations in Table 1. Lower mean degrees or rates coupled with longer partnerships durations would slow the speed with which individuals connect in the network resulting in lower 5-year forward-reachable paths. Mean degree of persistent (main or casual) partnerships might also be underestimated because data on those partnerships were restricted to a participant’s five most recent partnerships in the past year. Only 37 respondents reported 5 or more persistent (main/casual) partners so the resulting underestimate for the forward-reachable paths would small. Further, we modeled the total number of one-time partners in the past year, not constrained to this past-five limit; this accounted for the skewed right tail in that partnership distribution. For example, the rate of one-time partnerships in San Francisco in Table 1 was about 10 one-time partners per year. Another potential limitation is that we recruited a convenience sample of MSM, which might limit the generalizability of our results. In particular, online studies may not well represent racial/ethnic minority MSM.35 This could be one reason for the similar forward-reachable path estimates between Black and White MSM. To minimize the biases associated with this, we weighted our estimates in Table 1 by the race–ethnicity distribution in each city.26
Conclusions.
The forward-reachable path provides a useful approach to estimate connectivity in a dynamic network. The structure of a network corresponds to differences in the path, as was demonstrated in sexual networks of MSM from San Francisco and Atlanta. For some populations, the temporal network structure may provide different conclusions about the epidemic potential in the population than the cross-sectional network structure. Future research should assess how well the forward-reachable path can predict the epidemic potential projected by a full transmission model to determine whether it is a viable alternative approach to inferring epidemic potential.
Supplementary Material
SOURCE OF FUNDING
This work was supported by grants R21 MH112449 and R01 AI138783 from the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST
None declared
SUPPLEMENTAL DIGITAL CONTENT
Supplemental Digital Content 1. Appendix that includes (1) a demonstration of how the forward reachable path is calculated and (2) two tables on additional network statistics (eTable 1) and the estimated forward reachable path in casual networks at four time points (eTable 2). pdf
DATA AND COMPUTING CODE ACCESS
The data are publicly available at: https://github.com/EpiModel/ARTnetData. The code for this study can be found at: https://github.com/EpiModel/NetAnalysis-SF-ATL.
REFERENCES
- 1.Goodreau SM, Rosenberg ES, Jenness SM, et al. Sources of racial disparities in HIV prevalence in men who have sex with men in Atlanta, GA, USA: a modelling study. The Lancet HIV. 2017;4(7):e311–e320. doi: 10.1016/S2352-3018(17)30067-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S. Concurrent Partnerships and HIV Prevalence Disparities by Race: Linking Science and Public Health Practice. Am J Public Health. 2009;99(6):1023–1031. doi: 10.2105/AJPH.2008.147835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janulis P, Phillips G, Birkett M, Mustanski B. Sexual Networks of Racially Diverse Young MSM Differ in Racial Homophily But Not Concurrency: JAIDS Journal of Acquired Immune Deficiency Syndromes. 2018;77(5):459–466. doi: 10.1097/QAI.0000000000001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moody J, Adams J, Morris M. Epidemic potential by sexual activity distributions. Net Sci. 2017;5(4):461–475. doi: 10.1017/nws.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton DT, Handcock MS, Morris M. Degree Distributions in Sexual Networks: A Framework for Evaluating Evidence: Sexually Transmitted Diseases. 2008;35(1):30–40. doi: 10.1097/OLQ.0b013e3181453a84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handcock MS, Jones JH. Likelihood-based inference for stochastic models of sexual network formation. Theoretical Population Biology. 2004;65(4):413–422. doi: 10.1016/j.tpb.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 7.Jones JH, Handcock MS. An assessment of preferential attachment as a mechanism for human sexual network formation. Proc R Soc Lond B. 2003;270(1520):1123–1128. doi: 10.1098/rspb.2003.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris M, Epstein H, Wawer M. Timing Is Everything: International Variations in Historical Sexual Partnership Concurrency and HIV Prevalence. Jones JH, ed. PLoS ONE. 2010;5(11):e14092. doi: 10.1371/journal.pone.0014092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton JW, Hallett TB, Garnett GP. Concurrent Sexual Partnerships and Primary HIV Infection: A Critical Interaction. AIDS Behav. 2011;15(4):687–692. doi: 10.1007/s10461-010-9787-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. The Lancet. 2012;380(9839):367–377. doi: 10.1016/S0140-6736(12)60821-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry M, Raymond HF, McFarland W. Same race and older partner selection may explain higher HIV prevalence among black men who have sex with men. AIDS. 2007;21(17):2349–2350. doi: 10.1097/QAD.0b013e3282f12f41 [DOI] [PubMed] [Google Scholar]
- 12.Liljeros F, Edling CR, Amaral LAN. Sexual networks: implications for the transmission of sexually transmitted infections. Microbes and Infection. 2003;5(2):189–196. doi: 10.1016/S1286-4579(02)00058-8 [DOI] [PubMed] [Google Scholar]
- 13.Krivitsky PN, Morris M. Inference for social network models from egocentrically sampled data, with application to understanding persistent racial disparities in HIV prevalence in the US. Ann Appl Stat. 2017;11(1):427–455. doi: 10.1214/16-AOAS1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretzschmar M Sexual Network Structure and Sexually Transmitted Disease Prevention: A Modeling Perspective. Sexually Transmitted Diseases. 2000;27(10):627–635. [DOI] [PubMed] [Google Scholar]
- 15.Bender-deMoll S, Morris M, Moody J. Tsna: Tools for Temporal Social Network Analysis.; 2020. Accessed July 1, 2020. https://CRAN.R-project.org/package=tsna
- 16.Armbruster B, Wang L, Morris M. Forward reachable sets: Analytically derived properties of connected components for dynamic networks. Network Science. 2017;5(3):328–354. doi: 10.1017/nws.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray EJ, Robins JM, Seage GR, Freedberg KA, Hernán MA. A Comparison of Agent-Based Models and the Parametric G-Formula for Causal Inference. American Journal of Epidemiology. 2017;186(2):131–142. doi: 10.1093/aje/kwx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. HIV Surveillance Report, 2018. (Updated). Published May 2020. Accessed January 27, 2019. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- 19.San Francisco Department of Public Health. HIV Epidemiology Annual Report 2018. Published September 2019. Accessed January 23, 2020. https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/HIV-Epidemiology-Annual-Report-2018.pdf
- 20.Georgia Department of Public Health, HIV/AIDS Epidemiology Section. HIV Surveillance Summary Georgia, 2017. Published February 2019. Accessed November 1, 2019. https://dph.georgia.gov/georgias-hivaids-epidemiology-section/georgia-hiv-surveillance-data
- 21.Centers for Disease Control and Prevention. 2018 Sexually Transmitted Disease Surveillance. Published January 22, 2020. Accessed July 1, 2020. https://www.cdc.gov/std/stats18/default.htm
- 22.Weiss KM, Goodreau SM, Morris M, et al. Egocentric sexual networks of men who have sex with men in the United States: Results from the ARTnet study. Epidemics. 2020;30:100386. doi: 10.1016/j.epidem.2020.100386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zlotorzynska M, Sullivan P, Sanchez T. The Annual American Men’s Internet Survey of Behaviors of Men Who Have Sex With Men in the United States: 2016 Key Indicators Report. JMIR Public Health and Surveillance. 5(1):e11313. doi: 10.2196/11313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handcock MS, Hunter DR, Butts CT, Goodreau SM, Morris M. statnet : Software Tools for the Representation, Visualization, Analysis and Simulation of Network Data. J Stat Soft. 2008;24(1). doi: 10.18637/jss.v024.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenness SM, Goodreau SM, Morris M. EpiModel: An R Package for Mathematical Modeling of Infectious Disease over Networks. J Stat Soft. 2018;84(8). doi: 10.18637/jss.v084.i08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Census Bureau. U.S. Census Bureau QuickFacts: United States Accessed July 1, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 27.Grey JA, Bernstein KT, Sullivan PS, et al. Estimating the Population Sizes of Men Who Have Sex With Men in US States and Counties Using Data From the American Community Survey. JMIR Public Health Surveill. 2016;2(1):e14. doi: 10.2196/publichealth.5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandra C, Weiss KM, Kelley CF, Marcus JL, Jenness SM. Gaps in Sexually Transmitted Infection Screening among Men who Have Sex with Men in PrEP Care in the United States. Clin Infect Dis. Published online July 23, 2020. doi: 10.1093/cid/ciaa1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenness SM, Weiss KM, Goodreau SM, et al. Incidence of Gonorrhea and Chlamydia Following Human Immunodeficiency Virus Preexposure Prophylaxis Among Men Who Have Sex With Men: A Modeling Study. Clinical Infectious Diseases. 2017;65(5):712–718. doi: 10.1093/cid/cix439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marmor M, Sheppard HW, Donnell D, Bozeman S. Homozygous and Heterozygous CCR5–32 Genotypes Are Associated With Resistance to HIV Infection. Journal of Aquired Immune Deficiency Syndrome. 2001;27(5):472–481. [DOI] [PubMed] [Google Scholar]
- 31.Amirkhanian YA. Social Networks, Sexual Networks and HIV Risk in Men Who Have Sex with Men. Curr HIV/AIDS Rep. 2014;11(1):81–92. doi: 10.1007/s11904-013-0194-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darrow WW, Potterat JJ, Rothenberg RB, Woodhouse DE, Muth SQ, Klovdahl AS. Using Knowledge of Social Networks to Prevent Human Immunodeficiency Virus Infections: The Colorado Springs Study. Sociological Focus. 1999;32(2):143–158. doi: 10.1080/00380237.1999.10571132 [DOI] [Google Scholar]
- 33.Kojima N, Klausner JD. Disparities in the Testing, Treating, Suppressing, and Preventing HIV Infection Among Underserved Populations. AIDS Research and Human Retroviruses. 2019;35(9):786–787. doi: 10.1089/aid.2019.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28(10):1509–1519. doi: 10.1097/QAD.0000000000000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan PS, Khosropour CM, Luisi N, et al. Bias in Online Recruitment and Retention of Racial and Ethnic Minority Men Who Have Sex With Men. J Med Internet Res. 2011;13(2):e38. doi: 10.2196/jmir.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


