Abstract
This study aimed to investigate the correlation between monocyte to high-density lipoprotein cholesterol ratio (MHR) and obstructive sleep apnea (OSA) in patients with hypertension. A total of 246 hypertensive patients (67 controls, 65 mild, 51 moderate, and 63 severe OSA) were included. The relationship between MHR and OSA was analyzed. MHR correlated positively with apnea–hypopnea index (AHI), while negatively with mean SpO2 (P < 0.01). MHR was higher in OSA group than the control group (9.2 ± 2.6 vs. 10.8 ± 3.6, P < 0.001). Moreover, MHR in severe OSA group was the highest among all groups (9.2 ± 2.6, 10.2 ± 3.2, 10.4 ± 4.0, and 11.8 ± 3.4 in control, mild, moderate, and severe OSA group, respectively, P < 0.001). Logistic regression analysis demonstrated that MHR was an independent predictor of the presence of OSA (OR = 1.152, P < 0.01) and severe OSA (OR = 1.142, P < 0.01). Area under the curve of MHR was 0.634 (P < 0.05) and 0.660 (P < 0.05) for predicting OSA and severe OSA respectively in the ROC analysis. In conclusion, MHR increased with the severity of OSA. As a practical and cost-effective test, MHR was expected to be an available marker in evaluating OSA risk and severity in hypertensive patients.
Subject terms: Predictive markers, Hypertension, Sleep disorders
Introduction
Obstructive sleep apnea (OSA) is a highly prevalent clinical syndrome affecting more than 10% of the general population1,2. It is characterized by recurrent partial or total obstructions of the upper airway during sleep, leading to intermittent hypoxemia (IH) and sleep fragmentation3. Accumulating studies reveal that OSA is an independent risk factor for hypertension and consequent cardiovascular morbidities. Additionally, treatment of OSA could greatly improve both OSA symptoms and blood pressure (BP) control. Besides, the therapy was more effective in patients with severe OSA4. Therefore, it is of great meaning to detect practical clinical parameters screening the presence and evaluating the severity of OSA in hypertensive patients for early management of OSA, better control of hypertension and further reducing the consequent cardiovascular morbidities.
Increased sympathetic activation, oxidative stress, systemic inflammation, and endothelial dysfunction induced by chronic intermittent hypoxemia (CIH) are taken for the potential mechanism of OSA in leading to the development of hypertension5,6. Recently, the monocyte to high density-lipoprotein (HDL) cholesterol ratio (MHR), a new indicator of inflammation and oxidative stress, has been addressed as a predictor and prognostic marker of cardiovascular diseases7. Several studies have demonstrated the relationship between MHR and OSA in general population. MHR was found increased with OSA severity8, and independently associated with the occurrence of cardiovascular disease in OSA patients as well9,10. However, none of the previous studies have ever investigated the association between MHR and OSA in patients with hypertension. Therefore, the aim of the study was to evaluate the association between MHR and OSA, and to further investigate whether MHR could be used as an independent marker to predict OSA presence and severity in hypertensive patients.
Results
Demographic characteristics
Initially, 318 participants were enrolled, but 72 participants were excluded from the study because 9 participants were lack of monocyte results or HDL results, 6 participants were lack of complete out of center sleep test (OCST) recordings and 57 participants met the exclusion criteria, such as infection, insomnia, congestive heart failure, chronic obstructive pulmonary disease, etc. Finally, a total of 246 patients were included in the study (183 males, aged 56.7 ± 12.7 years, averaged BMI 27.90 ± 4.43 kg/m2), including 179 patients with OSA (OSA group) and 67 patients without OSA (control group).
The baseline characteristics were presented in Tables 1 and 2. The apnea–hypopnea index (AHI), mean oxygen saturation (mean SpO2), lowest pulse oxygen saturation (LSpO2), the percentage of sleep duration with SpO2 < 90% (TS90) and oxygen desaturation index (ODI) were significantly different among all groups (P < 0.05).
Table 1.
Baseline clinical, laboratory, and OCST data of the study population.
| Characteristics | Control (n = 67) | OSA (n = 179) | P value |
|---|---|---|---|
| Clinical parameters | |||
| Age (years) | 56.6 ± 13.5 | 56.7 ± 12.4 | 0.958 |
| Male gender, n (%) | 44 (65.7) | 139 (77.7) | 0.055 |
| Systolic BP (mmHg) | 135.6 ± 19.7 | 136.2 ± 16.0 | 0.824 |
| Diastolic BP (mmHg) | 79.9 ± 13.1 | 82.6 ± 13.2 | 0.163 |
| BMI (kg/m2) | 26.6 ± 3.5 | 28.4 ± 4.6 | 0.002* |
| Obesity, n (%) | 43 (64.2) | 134 (74.9) | 0.068 |
| Alcohol consumption, n (%) | 15 (22.4) | 44 (24.6) | 0.720 |
| Cigarette smoking, n (%) | 29 (43.3) | 79 (44.1) | 0.905 |
| Diabetes mellitus, n (%) | 25 (37.3) | 54 (30.2) | 0.285 |
| Dyslipidemia, n (%) | 53 (79.1) | 150 (83.8) | 0.388 |
| CAD, n (%) | 28 (41.8) | 104 (58.1) | 0.022* |
| Medical therapy | |||
| CCBs, n (%) | 33 (49.3) | 97 (54.2) | 0.490 |
| α- or β-Blockers, n (%) | 29 (43.3) | 97 (54.2) | 0.128 |
| ACEIs/ARBs, n (%) | 36 (53.7) | 114 (63.7) | 0.154 |
| Diuretics, n (%) | 9 (13.4) | 47 (26.3) | 0.033 |
| ≥ 3 classes of anti-hypertensive medications, n (%) | 14 (20.9) | 60 (33.5) | 0.055 |
| Hypertension severity | |||
| Stage-2 hypertension, n (%) | 21 (31.3) | 73 (40.8) | 0.175 |
| Laboratory parameters | |||
| Monocyte count (109/L) | 0.4 (0.3–0.4) | 0.4 (0.3–0.5) | 0.069 |
| Triglyceride (mg/dL) | 132.0 (95.2–180.7) | 152.4 (111.6–219.7) | 0.098 |
| Total cholesterol (mg/dL) | 177.8 ± 41.9 | 173.8 ± 42.3 | 0.507 |
| HDL cholesterol (mg/dL) | 41.7 ± 8.5 | 38.7 ± 8.2 | 0.010* |
| LDL cholesterol (mg/dL) | 99.2 ± 30.9 | 98.3 ± 27.3 | 0.825 |
| MHR | 9.2 ± 2.6 | 10.8 ± 3.6 | < 0.001* |
| OCST parameters | |||
| AHI (events/h) | 2.8 ± 1.4 | 24.8 ± 16.1 | < 0.001* |
| Mean SpO2 (%) | 94 (93–95) | 94 (92–95) | 0.018* |
| LSpO2 (%) | 82 (80–86) | 80 (76–83) | < 0.001* |
| TS90 (%) | 4 (2–38) | 23 (7–66) | 0.002* |
| ODI | 4.0 ± 4.8 | 20.5 ± 15.3 | < 0.001* |
Data are means ± standard deviation, numbers of subjects (%), or medians (range).
OSA obstructive sleep apnea, BP blood pressure, BMI body mass index, CAD coronary artery disease, HDL high-density lipoprotein, LDL low-density lipoprotein, MHR monocyte to high-density lipoprotein cholesterol ratio, OCST out of center sleep testing, AHI apnea–hypopnea index, Mean SpO2 mean oxygen saturation, LSpO2 lowest pulse oxygen saturation, TS90 the percentage of sleep duration with SpO2 < 90%, ODI oxygen desaturation index.
*P < 0.05.
Table 2.
Baseline clinical, laboratory, and OCST data of the study population.
| Characteristics | Control (n = 67) | Mild OSA (n = 65) | Moderate OSA (n = 51) | Severe OSA (n = 63) | P value |
|---|---|---|---|---|---|
| Clinical parameters | |||||
| Age (years) | 56.6 ± 13.5 | 57.6 ± 11.1 | 58.3 ± 10.8 | 54.6 ± 14.6 | 0.407 |
| Male gender, n (%) | 44 (65.7) | 48 (73.8) | 37 (72.5) | 54 (85.7) | 0.071 |
| Systolic BP (mmHg) | 135.6 ± 19.7 | 133.4 ± 15.4 | 138.3 ± 16.8 | 137.5 ± 15.9 | 0.410 |
| Diastolic BP (mmHg) | 79.9 ± 13.1 | 80.7 ± 12.0 | 81.6 ± 12.7 | 85.3 ± 14.5 | 0.104 |
| BMI (kg/m2) | 26.6 ± 3.5 | 27.5 ± 3.8 | 28.0 ± 5.0 | 29.5 ± 4.9*† | 0.002# |
| Obesity, n (%) | 43 (64.2) | 47 (72.3) | 35 (68.6) | 52 (82.5) | 0.122 |
| Alcohol consumption, n (%) | 15 (22.4) | 10 (15.4) | 17 (33.3) | 17 (27) | 0.139 |
| Cigarette smoking, n (%) | 29 (43.3) | 26 (40.0) | 21 (41.2) | 32 (50.8) | 0.619 |
| Diabetes mellitus, n (%) | 25 (37.3) | 24 (36.9) | 13 (25.5) | 17 (27.0) | 0.347 |
| Dyslipidemia, n (%) | 53 (79.1) | 55 (84.6) | 44 (86.3) | 51 (81.0) | 0.718 |
| CAD, n (%) | 28 (41.8) | 34 (52.3) | 32 (62.7) | 38 (60.3) | 0.084 |
| Medical therapy | |||||
| CCBs, n (%) | 33 (49.3) | 35 (53.8) | 25 (49.0) | 37 (58.7) | 0.671 |
| α- or β-Blockers, n (%) | 29 (43.3) | 30 (46.2) | 29 (56.9) | 38 (60.3) | 0.165 |
| ACEIs/ARBs, n (%) | 36 (53.7) | 41 (63.1) | 31 (60.8) | 42 (66.7) | 0.483 |
| Diuretics, n (%) | 9 (13.4) | 14 (21.5) | 10 (19.6) | 23 (36.5) | 0.015# |
| ≥ 3 classes of anti-hypertensive medications, n (%) | 14 (20.9) | 19 (29.2) | 13 (25.5) | 28 (44.4) | 0.024# |
| Hypertension severity | |||||
| Stage-2 hypertension, n (%) | 21 (31.3) | 23 (35.4) | 16 (31.4) | 34 (54.0) | 0.027# |
| Laboratory parameters | |||||
| Monocyte count (109/L) | 0.4 (0.3–0.4) | 0.4 (0.3–0.4) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5)* | 0.022# |
| Triglyceride (mg/dL) | 132.0 (95.2–180.7) | 147.1 (106.3–201.1) | 168.3 (120.1–252.5) | 151.5 (110.3–237.9) | 0.262 |
| Total cholesterol (mg/dL) | 177.8 ± 41.9 | 169.3 ± 38.2 | 179.2 ± 41.4 | 174.2 ± 46.9 | 0.566 |
| HDL cholesterol (mg/dL) | 41.7 ± 8.5 | 37.8 ± 7.3* | 41.6 ± 9.3 | 37.2 ± 7.6* | 0.001# |
| LDL cholesterol (mg/dL) | 99.2 ± 30.9 | 95.3 ± 25.0 | 100.2 ± 27.3 | 99.9 ± 29.7 | 0.754 |
| MHR | 9.2 ± 2.6 | 10.2 ± 3.2 | 10.4 ± 4.0 | 11.8 ± 3.4*†§ | < 0.001# |
| OCST parameters | |||||
| AHI (events/h) | 2.8 ± 1.4 | 9.6 ± 4.4* | 22.7 ± 4.6*† | 42.3 ± 12.4*†§ | < 0.001# |
| Mean SpO2 (%) | 94 (93–95) | 94 (93–95) | 94 (93–95) | 93 (92–95)*§ | 0.002# |
| LSpO2 (%) | 82 (80–86) | 81 (78–83) | 80 (76–83) | 77 (68–82)*† | < 0.001# |
| TS90 (%) | 4 (2–38) | 12 (4–30) | 19 (7–44) | 55 (18–133)*†§ | < 0.001# |
| ODI | 4.0 ± 4.8 | 8.6 ± 4.4* | 17.2 ± 7.9*† | 35.4 ± 14.9*†§ | < 0.001# |
Data are means ± standard deviation, numbers of subjects (%), or medians (range).
OSA obstructive sleep apnea, BP blood pressure, BMI body mass index, CAD coronary artery disease, HDL high-density lipoprotein, LDL low-density lipoprotein, MHR monocyte to high-density lipoprotein cholesterol ratio, OCST out of center sleep testing, AHI apnea–hypopnea index, Mean SpO2, mean oxygen saturation, LSpO2 lowest pulse oxygen saturation, TS90 the percentage of sleep duration with SpO2 < 90%, ODI oxygen desaturation index.
*vs. Control, P < 0.05; †vs. mild OSA, P < 0.05; §vs. moderate OSA, P < 0.05; #P < 0.05.
Compared with the control group, body mass index (BMI) value and the prevalence of coronary artery disease (CAD) were higher (P = 0.002 and P = 0.022, respectively) while the level of HDL cholesterol was lower in the OSA group (P < 0.05; Table 1). The mean MHR value was significantly higher in the OSA group (P < 0.001; Fig. 1).
Figure 1.
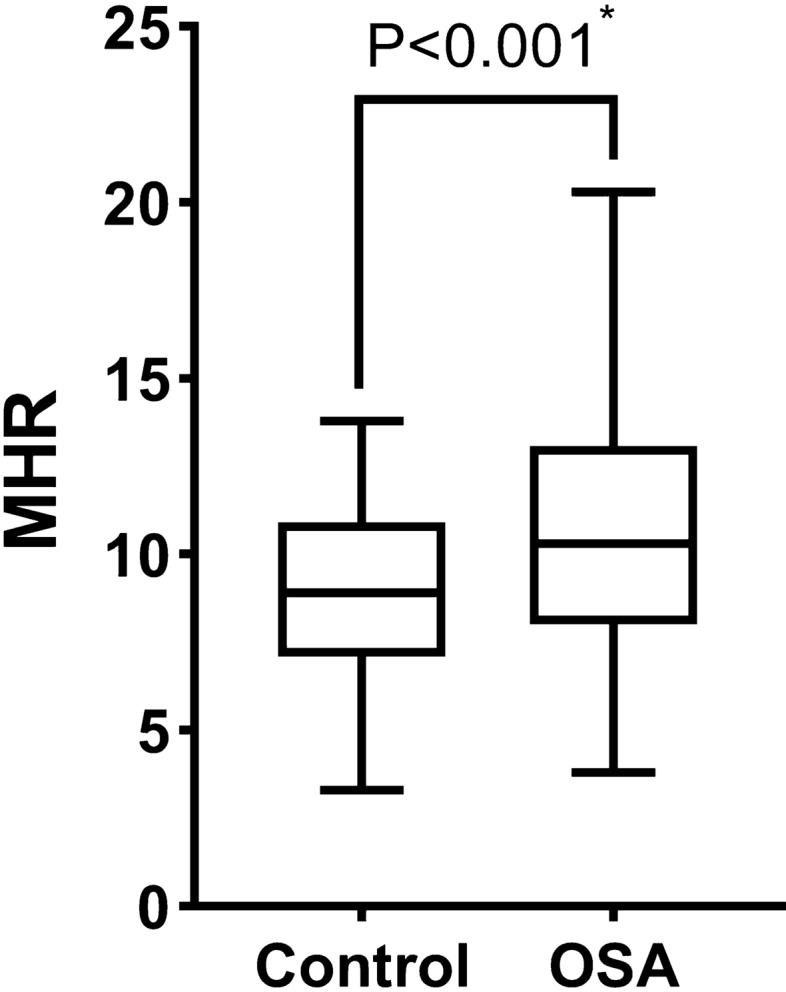
MHR levels in control group and the OSA group. MHR monocyte to high-density lipoprotein cholesterol ratio, OSA obstructive sleep apnea. *P value < 0.05.
The OSA group was further categorized into the mild (AHI: 5–14.9), moderate (AHI: 15–29.9), and severe (AHI ≥ 30) OSA group. The mean AHI value of OSA group was 24.8/h, and the prevalence of mild (n = 65), moderate (n = 51), and severe (n = 63) OSA were 26.4%, 20.7%, and 25.6%, respectively. As shown in Fig. 2, MHR was found elevated in parallel with the increase of OSA severity. The level of MHR was significantly higher in the severe OSA group than those in the control group (P < 0.001), the mild OSA group (P = 0.006), and the moderate OSA group (P = 0.019). The BMI value was higher in the severe OSA group when compared with the control group (P < 0.001) and the mild OSA group (P = 0.008; Table 2). Monocyte count was higher in the severe OSA group than the control group (P = 0.048; Table 2). Serum HDL cholesterol level in severe OSA group was the lowest among all 4 groups (P = 0.026; Table 2). In addition, the systolic BP and diastolic BP increased with the severity of OSA although there was no statistical significance which might be contributed to the intensive anti-hypertensive medication management in OSA group (Table 2). Patients in the severe OSA group achieved the highest percentage of ≥ 3 classes of anti-hypertensive medications among all 4 groups (Table 2). Taken both the level of BP and the use of anti-hypertensive medications into consideration of hypertension severity, the percentage of stage-2 hypertension was the highest in the severe OSA group (54.0%, P = 0.027, Table 2), indicating that hypertension severity increased with OSA severity.
Figure 2.
MHR levels in control group, the mild OSA group, the moderate group and the severe group. MHR monocyte to high-density lipoprotein cholesterol ratio, OSA obstructive sleep apnea. *P value < 0.05.
The association between MHR and OSA
As shown in Table 3, MHR was positively correlated with AHI (r = 0.244, P < 0.001), ODI (r = 0.250, P < 0.001), while negatively with mean SpO2 (r = − 0.135, P = 0.035).
Table 3.
Correlations between OCST parameters and MHR.
| Variables | MHR | |
|---|---|---|
| r | P value | |
| AHI (events/h) | 0.244* | < 0.001 |
| Mean SpO2 (%) | − 0.135* | 0.035 |
| LSpO2 (%) | − 0.110 | 0.085 |
| TS90 (%) | 0.041 | 0.525 |
| ODI | 0.250* | < 0.001 |
OCST out of center sleep testing, MHR monocyte to high-density lipoprotein cholesterol ratio, AHI apnea–hypopnea index, Mean SpO2 mean oxygen saturation, LSpO2 lowest pulse oxygen saturation, TS90 the percentage of sleep duration with SpO2 < 90%, ODI oxygen desaturation index.
*P value < 0.05.
Potential risk factors related to the presence and the severity of OSA were further investigated in both univariate and multivariate logistic regression analysis. Univariate logistic regression analysis showed that BMI (OR = 1.102, 95% confidence interval [CI]: 1.027–1.184, P = 0.007) and MHR (OR = 1.173, 95% CI 1.067–1.289, P = 0.001; Table 4) were associated with OSA; male sex (OR = 2.512, 95% CI 1.158–5.446, P = 0.020), BMI (OR = 1.118, 95% CI 1.046–1.194, P = 0.001), stage-2 hypertension (OR = 2.403, 95% CI 1.341–4.309, P = 0.003) and MHR (OR = 1.182, 95% CI 1.083–1.289, P < 0.001; Table 5) were associated with severe OSA. Further, multivariate logistic regression analysis identified that BMI and MHR were independently associated with the presence of OSA (BMI: OR = 1.081, 95% CI 1.005–1.161, P = 0.036; MHR: OR = 1.152, 95% CI 1.047–1.268, P = 0.009; Table 4), BMI, stage-2 hypertension and MHR were independently associated with severe OSA (BMI: OR = 1.089, 95% CI 1.016–1.168, P = 0.016; stage-2 hypertension: OR = 2.089, 95% CI 1.125–3.878, P = 0.020; MHR: OR = 1.142, 95% CI 1.041–1.252, P = 0.005; Table 5) after adjusted for other potential risk factors.
Table 4.
Univariate and multivariate logistic regression analysis for the presence of OSA.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.001 (0.979–1.023) | 0.958 | ||
| Male | 1.816 (0.982–3.359) | 0.057 | ||
| BMI | 1.102 (1.027–1.184) | 0.007* | 1.081 (1.005–1.161) | 0.036* |
| Smoking | 1.035 (0.588–1.824) | 0.905 | ||
| Alcohol consumption | 1.130 (0.580–2.203) | 0.72 | ||
| Diabetes mellitus | 0.726 (0.403–1.308) | 0.286 | ||
| Stage-2 hypertension | 1.509 (0.831–2.738) | 0.176 | ||
| MHR | 1.173 (1.067–1.289) | 0.001* | 1.152 (1.047–1.268) | 0.009* |
BMI body mass index, MHR monocyte to high-density lipoprotein cholesterol ratio.
*P value < 0.05.
Table 5.
Univariate and multivariate logistic regression analysis for severe OSA.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 0.982 (0.960–1.005) | 0.122 | ||
| Male | 2.512 (1.158–5.446) | 0.020* | 2.134 (0.949–4.797) | 0.067 |
| BMI | 1.118 (1.046–1.194) | 0.001* | 1.089 (1.016–1.168) | 0.016* |
| Alcohol consumption | 1.241 (0.645–2.387) | 0.518 | ||
| Smoking | 1.453 (0.818–2.582) | 0.202 | ||
| Diabetes mellitus | 0.721 (0.382–1.361) | 0.313 | ||
| Stage-2 hypertension | 2.403 (1.341–4.309) | 0.003* | 2.089 (1.125–3.878) | 0.020* |
| MHR | 1.182 (1.083–1.289) | < 0.001* | 1.142 (1.041–1.252) | 0.005* |
BMI body mass index, MHR monocyte to high-density lipoprotein cholesterol ratio.
*P value < 0.05.
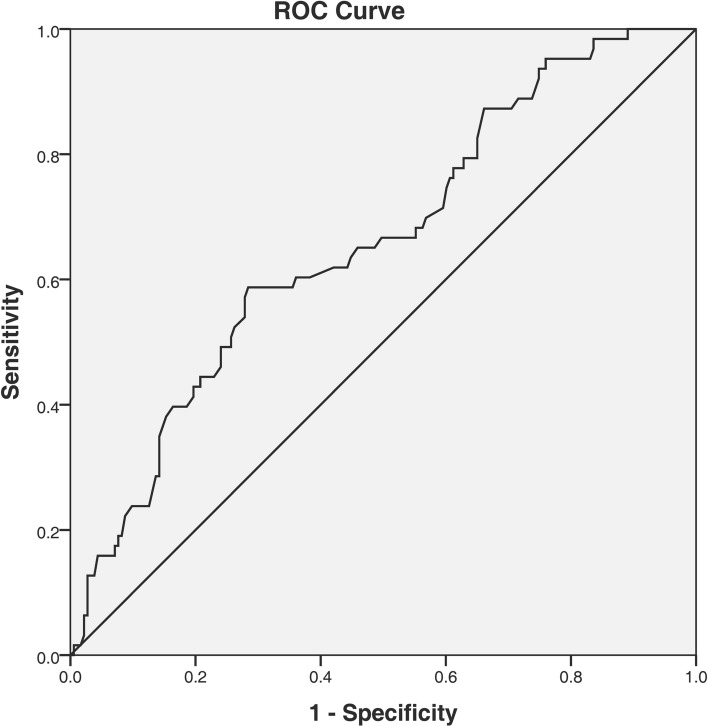
For the prediction of OSA in hypertensive patients, the receiver-operating characteristic (ROC) curve analysis performed the cut-off value of MHR (> 10.3) with the greatest sum of sensitivity (53.1%) and specificity (68.7%), and area under the curve (AUC) of 0.634 (95% CI 0.560–0.708; P = 0.038; Fig. 3). In addition, the optimal MHR index cut-off value used for predicting severe OSA was 11.4 with the greatest sum of sensitivity (58.7%) and specificity (71.6%), and AUC of 0.660 (95% CI 0.583–0.737; P = 0.039; Fig. 4).
Figure 3.
The ROC curve analysis for MHR in predicting the presence of OSA. The cut-off value of MHR was 10.3, with a sensitivity of 53.1% and a specificity of 68.7%, AUC: 0.634 (95% CI 0.560–0.708; P = 0.038). ROC receiver operating characteristic, MHR monocyte to high-density lipoprotein cholesterol ratio, OSA obstructive sleep apnea, AUC area under the curve.
Figure 4.
The ROC curve analysis for MHR in predicting severe OSA. The cut-off value of MHR was 11.4, with a sensitivity of 58.7% and a specificity of 71.6%, AUC: 0.660 (95% CI 0.583–0.737; P = 0.039). ROC receiver operating characteristic, MHR monocyte to high-density lipoprotein cholesterol ratio, OSA obstructive sleep apnea, AUC area under the curve.
Discussion
To our knowledge, this is the first article in the literature that evaluates the relationship between MHR and OSA in hypertensive patients. The results showed that MHR was significantly higher in hypertensive patients with OSA than those without OSA. MHR increased along with the severity of OSA. Moreover, we first demonstrated that MHR acted as an independent predictor of the presence and severity of OSA in hypertensive patients, suggesting that MHR could be used as a reliable parameter for OSA screening and severity evaluation in clinical practice of hypertension management.
Previous data showed that aging, male sex, BMI, smoking and alcohol consumption were strongly associated with OSA11,12. In our study of the hypertensive patients, there were no differences regarding aging, male sex, smoking and alcohol consumption between patients with and without OSA. However, BMI was found increasing with the severity of OSA, and was addressed as independent predictor of OSA, which was in agreement with results in the general population.
OSA has been described as a low-grade chronic inflammatory disease due to CIH, which could exacerbate the progression of hypertension5. Monocytes are essential immune cells that play a key role in the process of inflammation and oxidative stress. It has been proved that hypoxia could increase monocyte counts and the pro-inflammatory effects of monocytes. Alvarez-Martins et al. found that CIH increased monocyte counts via affecting hematopoiesis and the bone marrow microenvironment in a rat model of OSA13. Tamaki et al. found that the invasive ability of monocytes was significantly higher in patients with OSA compared with control subjects14. Additionally, our study in the hypertensive patients showed that monocyte counts were significantly higher in the severe OSA group9,10, indicating the obvious systemic inflammation of severe OSA.
Contrary to the inflammatory property of monocytes, accumulating evidence suggests the anti-inflammatory and antioxidative effects of HDL cholesterol via suppressing cytokines expression and inhibiting monocytes activation and extravagation15,16. Data from the European Sleep Apnea Database showed that HDL cholesterol was significantly reduced in the highest AHI quartile17. Our study of the hypertensive patients further found that the level of HDL cholesterol was the lowest in the severe OSA group, which might be attributed to the aggravated systemic inflammation induced by CIH.
In regard to the inflammatory property of monocytes and the anti-inflammatory property of HDL cholesterol, MHR was proposed as a new marker of systemic inflammation. A number of studies have implicated that MHR was independently associated with the occurrence and prognosis of several cardiovascular diseases. In patients with ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (pPCI), MHR was reported as an independent predictor of stent thrombosis18, no reflow19, contrast-induced nephropathy20, and in-hospital mortality21. In the field of hypertension, MHR was also in accordance with asymptomatic organ damage and non-dipper hypertension22,23. Additionally, MHR was found independently predicted the late recurrence of paroxysmal AF after radiofrequency ablation, with the same predictive value as left atrial diameter24.
Limited studies have explored the relationship of MHR with OSA. Atan et al. found a dose–response correlation between MHR and the severity of OSA8. Both Li et al. and Inonu et al. reported the strong association of MHR with the occurrence of cardiovascular disease in OSA patients9,10. Those findings suggested that MHR was associated with OSA in general population. However, whether MHR is an independent indicator of the presence and the severity of OSA has not been demonstrated. Besides, none of the previous studies have ever investigated the association between MHR and OSA in patients with hypertension. Our study in hypertensive patients found that MHR levels correlated positively with AHI and ODI while negatively with mean SpO2. MHR values in OSA group was significantly higher than the control group. Moreover, MHR values in severe OSA group were the highest among all 4 groups. These findings were consistent with the results in general population8–10. By further logistic regression analysis, MHR was found independently associated with the presence of OSA and severe OSA in hypertensive patients in spite of sex, BMI and hypertension severity, indicating that elevated MHR value might be a predictor for the development and progression of OSA in hypertensive patients. Although the predictive significance of the optimal cut-off value of MHR for OSA was not strong due to the relatively small size of the study, the predictive power of MHR was moderately improved in the prediction for severe OSA, which might be attributed to the increased systemic inflammation in severe OSA patients.
Several limitations of our study should be mentioned. First, given the retrospective cross-sectional nature of this single center study, clinical factors not contained in this study might influence the results. Second, MHR changes before and after continuous positive airway pressure (CPAP) treatment were not investigated. Third, the sample size of this study was relatively small and OCST was used in OSA diagnosis and severity evaluation. Although OCST is widely used and reported comparable to full polysomnography (PSG) both in the diagnosis and severity evaluation of OSA in appropriate clinical settings, the absence of electroencephalogram may reduce the sensitivity of OCST which may result in the underestimation of sleep disordered breathing severity. The result conducting by OCST should be further validated in full PSG. Thus, further multi-center, prospective interventional clinical trials with CPAP treatment on larger populations are needed in the future.
In conclusion, our study found that in hypertensive patients, MHR increased with the severity of OSA, and independently associated with the presence and severity of OSA regardless of sex, BMI and hypertension severity. As OSA is an independent risk factor for hypertension and management of OSA could better control blood pressure and reduce consequent cardiovascular morbidities, MHR, a practical and cost-effective test, might be used as an available marker to evaluate OSA risk and severity in clinical management of hypertension.
Methods
Study population
We retrospectively analyzed consecutive patients who were diagnosed with hypertension and recorded the OCST based on the clinical suspicion of OSA at the cardiovascular department of Peking University Shougang Hospital from July 2016 to September 2019. The diagnosis of hypertension was made based on systolic/diastolic blood BP ≥ 140/90 mmHg, anti-hypertensive medication use, or a previous hypertension diagnosis. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by the local ethics committee of Peking University Shougang Hospital. Informed consent was obtained from all participants.
Patients younger than 18 years of age and with secondary hypertension other than OSAS, comorbid sleep disorders (e.g., central sleep apnea, restless leg syndrome, narcolepsy, insomnia, circadian rhythm disorders, etc.), neural-muscular disease, previous treatment for OSA (e.g., CPAP, surgery, and oral device, etc.), hypoxemic lung disease (e.g., chronic obstructive pulmonary disease, interstitial lung disease, asthma, etc.), hematologic disease, congestive heart failure, liver or kidney disease, malignancy, pregnancy, infection, autoimmune disease, and anti-inflammatory medication use were excluded as previous studies described9,10,25. In total, 246 subjects were included.
Demographic characteristics, systolic BP, diastolic BP, medication use, history of diabetes, dyslipidemia, CAD, smoking and alcohol consumption were retrospectively reviewed. BMI was calculated as the patient’s weight (kg)/height2 (m2). Hypertension severity was divided into 2 stages according to BP levels or medication use (stage-1: BP < 160/100 mmHg or BP under control with 1 or 2 antihypertensive drugs; stage-2: BP ≥ 160/100 mmHg or BP under control with ≥ 3 antihypertensive drugs)26,27.
Laboratory measurements
Blood samples were obtained from the patients in the morning after 12 h of fasting. Monocyte was determined by flow cytometry with a Sysmex XN-2800 automated hematology analyzer (Japan). Lipid profiles including total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol were analyzed with AU5811 automatic biochemical analyzer (Beckman Coulter, USA). MHR was calculated as the monocyte count (103/µL)/HDL cholesterol (mg/dL).
OCST evaluation
All participants performed OCST (Apnea Link Air, ResMed Germany Inc, Germany), which included the following: electrocardiography, pulse oxygen saturation, oral and nasal airflow, nasal air pressure, thoracic-abdominal respiratory movement, snoring microphone, and body position. The application of OCST in OSA screening and severity evaluation has been validated against full polysomnography28,29. Patients with comorbid conditions (including comorbid sleep disorders, neural-muscular disease, hypoxemic lung disease, congestive heart failure, etc.) that were not recommended to receive OCST instead of full PSG were excluded in this study25. OCST was programmed to record automatically, starting from 30 min after the patients went to bed. Recordings that last for less than 300 min were excluded. Polysomnography data were scored manually by trained personnel. According to American Association of Sleep Medicine (AASM) criteria, apnea was defined as a decrease to 0–20% of oronasal air flow for longer than 10 s; hypopnea was defined as a decrease of oronasal air flow by 50% for longer than 10 s, or a decrease of both oronasal air flow by at least 30% and oxygen saturation by 4% for longer than 10 s. The apnea and hypopnea counts per hour were recorded as the apnea–hypopnea index (AHI). The oxygen desaturation index (ODI) was defined as the number of oxygen level drops 3% from baseline per hour. Diagnosis of OSA was made solely when the AHI in the recorded study was ≥ 5 events per hour, irrespective of daytime OSA symptoms, which allowed objective evaluation of the disease severity30. According to the AHI, patients were categorized into the control group (AHI < 5) and the OSA group (AHI ≥ 5). Then the OSA group was further categorized into the mild (AHI: 5–14.9), moderate (AHI: 15–29.9), and severe (AHI ≥ 30) OSA group. The percentage of sleep duration with SpO2 < 90% (TS90), lowest pulse oxygen saturation (LSpO2), mean oxygen saturation (mean SpO2), and ODI were also included.
Statistics
The results were expressed as mean ± SD, median (interquartile range), or number (percentage). Continuous variables were investigated for normal distribution with histograms, probability plots and Kolmogorov–Smirnov test. Differences between groups was assessed by Student unpaired t-test and one-way analysis of variance for normally distributed data; and Mann–Whitney U test and Kruskall–Wallis H test for non-normally distributed data, respectively. Comparison of categorical variables was analyzed by chi square test. Correlations were assessed by Pearson’s rank correlation. The effect of various variables on OSA risk and OSA severity was analyzed with univariate and multivariate logistic regression analysis. Receiver-operating characteristic (ROC) curve with Youden index was used to estimate the predictive validity and determine the optimal MHR cut-off value. P value < 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS version 22.0 (IBM SPSS Statistics for Windows, USA).
Acknowledgements
This work was supported by a grant from the youth scientific research fund of Peking University Shougang Hospital (SGYYQ202004).
Author contributions
M.S. conceived the study, designed the experiments, performed the data analysis, and drafted the manuscript. M.S., Q.T., X.S. and E.Z. participated in data collection. M.S., C.L., H.L. and Y.M. participated in manuscript revision. T.Q. supervised the study. All authors participated in the interpretation of the findings and approved the final version of the manuscript.
Data availability
The dataset generated and analyzed in this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, et al. Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–249. [PMC free article] [PubMed] [Google Scholar]
- 3.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol. Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavie P, Hoffstein V. Sleep apnea syndrome: A possible contributing factor to resistant. Sleep. 2001;24:721–725. doi: 10.1093/sleep/24.6.721. [DOI] [PubMed] [Google Scholar]
- 5.Jehan S, et al. Obstructive sleep apnea, hypertension, resistant hypertension and cardiovascular disease. Sleep Med. Disord. 2020;4:67–76. [PMC free article] [PubMed] [Google Scholar]
- 6.May AM, Mehra R. Obstructive sleep apnea: Role of intermittent hypoxia and inflammation. Semin. Respir. Crit. Care Med. 2014;35:531–544. doi: 10.1055/s-0034-1390023. [DOI] [PubMed] [Google Scholar]
- 7.Ganjali S, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell Physiol. 2018;233:9237–9246. doi: 10.1002/jcp.27028. [DOI] [PubMed] [Google Scholar]
- 8.Atan D, Kundi FCS, Özcan KM, Dere H. A new predictor for obstructive sleep apnea syndrome: Monocyte to HDL ratio. Indian J. Otolaryngol. Head Neck Surg. 2017;69:142–146. doi: 10.1007/s12070-016-0980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.InonuKoseoglu H, Pazarli AC, Kanbay A, Demir O. Monocyte count/HDL cholesterol ratio and cardiovascular disease in patients with obstructive sleep apnea syndrome: A multicenter study. Clin. Appl. Thromb. Hemost. 2018;24:139–144. doi: 10.1177/1076029616677803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, et al. Relationship between monocyte to HDL cholesterol ratio and concomitant cardiovascular disease in Chinese Han patients with obstructive sleep apnea. Cardiovasc. Diagn. Ther. 2019;9:362–370. doi: 10.21037/cdt.2019.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drager LF, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am. J. Cardiol. 2010;105:1135–1139. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Kang HH, Kim SW, Lee SH. Association between triglyceride glucose index and obstructive sleep apnea risk in Korean adults: A cross-sectional cohort study. Lipids Health Dis.. 2020;19:182. doi: 10.1186/s12944-020-01358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez-Martins I, et al. The impact of chronic intermittent hypoxia on hematopoiesis and the bone marrow microenvironment. Pflugers Arch. 2016;468:919–932. doi: 10.1007/s00424-016-1797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamaki S, et al. Nocturnal hypoxic stress activates invasive ability of monocytes in patients with obstructive sleep apnoea syndrome. Respirology. 2009;14:689–694. doi: 10.1111/j.1440-1843.2009.01540.x. [DOI] [PubMed] [Google Scholar]
- 15.Barter PJ, et al. Antiinflammatory properties of HDL. Circ. Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 16.Murphy AJ, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 17.Gunduz C, et al. Obstructive sleep apnoea independently predicts lipid levels: Data from the European Sleep Apnea Database. Respirology. 2018;23:1180–1189. doi: 10.1111/resp.13372. [DOI] [PubMed] [Google Scholar]
- 18.Cetin EH, et al. Monocyte/HDL-cholesterol ratio predicts the definite stent thrombosis after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Biomark. Med. 2015;9:967–977. doi: 10.2217/bmm.15.74. [DOI] [PubMed] [Google Scholar]
- 19.Balta S, et al. The relation between monocyte to HDL ratio and no-reflow phenomenon in the patients with acute ST-segment elevation myocardial infarction. Am. J. Emerg. Med. 2016;34:1542–1547. doi: 10.1016/j.ajem.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Sag S, et al. Association of monocyte to HDL cholesterol level with contrast induced nephropathy in STEMI patients treated with primary PCI. Clin. Chem. Lab. Med. 2017;55:132–138. doi: 10.1515/cclm-2016-0005. [DOI] [PubMed] [Google Scholar]
- 21.Karataş MB, et al. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention. Am. J. Emerg. Med. 2016;34:240–244. doi: 10.1016/j.ajem.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 22.Selcuk M, Yildirim E, Saylik F. Comparison of monocyte with high density lipoprotein cholesterol ratio in dipper and nondipper hypertensive patients. Biomark. Med. 2019;13:1289–1296. doi: 10.2217/bmm-2019-0062. [DOI] [PubMed] [Google Scholar]
- 23.Aydin E, Ates I, Fettah Arikan M, Yilmaz N, Dede F. The ratio of monocyte frequency to HDL cholesterol level as a predictor of asymptomatic organ damage in patients with primary hypertension. Hypertens. Res. 2017;40:758–764. doi: 10.1038/hr.2017.36. [DOI] [PubMed] [Google Scholar]
- 24.Chen SA, et al. The preablation monocyte/ high density lipoprotein ratio predicts the late recurrence of paroxysmal atrial fibrillation after radiofrequency ablation. BMC Cardiovasc. Disord. 2020;20:401. doi: 10.1186/s12872-020-01670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markun LC, Sampat A. Clinician-focused overview and developments in polysomnography. Curr. Sleep Med. Rep. 2020 doi: 10.1007/s40675-020-00197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muela HC, et al. Hypertension severity is associated with impaired cognitive performance. J. Am. Heart Assoc. 2017 doi: 10.1161/jaha.116.004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muela HCS, et al. Impact of hypertension severity on arterial stiffness, cerebral vasoreactivity, and cognitive performance. Dement. Neuropsychol. 2017;11:389–397. doi: 10.1590/1980-57642016dn11-040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos-Silva R, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629–636. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chernyshev OY, et al. A pilot study: Portable out-of-center sleep testing as an early sleep apnea screening tool in acute ischemic stroke. Nat. Sci. Sleep. 2015;7:127–138. doi: 10.2147/nss.S85780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Report of an American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667. doi: 10.1093/sleep/22.5.667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and analyzed in this study are available from the corresponding author upon reasonable request.