Abstract
Matrix metalloproteinase (MMP)-2 and MMP-9, also known as gelatinases or type IV collagenases, are recognized as major contributors to the proteolytic degradation of extracellular matrix during tumor invasion. Latent MMP-2 (proMMP-2) is activated by membrane type 1 MMP (MT1-MMP) on the cell surface of tumor cells. We previously reported that cell-bound proMMP-9 is activated by the MT1-MMP/MMP-2 axis in HT1080 cells treated with concanavalin A in the presence of exogenous proMMP-2. However, the regulatory mechanism of proMMP-9 activation remains largely unknown. Transforming growth factor (TGF)-β1 is frequently overexpressed in tumor tissues and is associated with tumor aggressiveness and poor prognosis. In this study, we examined the role of TGF-β1 on MT1-MMP-mediated proMMP-9 activation using human oral squamous cell carcinoma cells. TGF-β1 significantly increased the expression of MMP-9. By adding exogenous proMMP-2, TGF-β1-induced proMMP-9 was activated during collagen gel culture, which was suppressed by the inhibition of TGF-β1 signaling or MT1-MMP activity. This MT1-MMP-mediated proMMP-9 activation was needed to facilitate TGF-β1-induced cell invasion into collagen gel. Thus, TGF-β1 may facilitate MT1-MMP-mediated MMP-9 activation and thereby stimulate invasion of tumor cells in collaboration with MT1-MMP and MMP-2.
Keywords: Oral cancer, MMP, Invasion, ECM, TGF-β1
Abbreviations: ADAM, a disintegrin and metalloproteinase; Con A, concanavalin A; DMEM, Dulbecco's modified Eagle's medium; ECM, extracellular matrix; FBS, fetal bovine serum; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; MT1-MMP, membrane type-1 MMP; OSCC, oral squamous cell carcinoma; PBS, phosphate-buffered saline; TGF, transforming growth factor; TIMP, tissue inhibitor of MMP
1. Introduction
Cell invasion is a complex process that requires controlled degradation of the extracellular matrix (ECM) that is achieved by proteases such as serine proteases and matrix metalloproteinases (MMPs) [1,2]. The MMPs are large family of zinc-dependent proteases that are produced as inactive latent forms and converted into their active forms by removal of N-terminal propeptides [3]. MMP-2 and MMP-9 belong to the gelatinase/type IV collagenase subgroup of the MMP family [4,5]. Although they are highly similar enzymes in many respects, significant differences exist in their substrate specificity and the regulation of gene expression and proenzyme activation. Membrane type 1 MMP (MT1-MMP) was initially identified as an activator of latent MMP-2 (proMMP-2) [6]. MT1-MMP is now known to cleave various types of ECM components, cell adhesion molecules, cytokines, and other proteins [[7], [8], [9]]. The activation of proMMP-2 occurs in a variety of tumor tissues where MT1-MMP is overexpressed. The MT1-MMP/tissue inhibitor of MMP (TIMP)-2 complex serves as a cell surface receptor for proMMP-2, which is then processed to active MMP-2 by adjacent TIMP-2 free MT1-MMP. ProMMP-9 is activated in vitro by various purified proteinases including MMP-2, MMP-3, and serine proteinases [4,5]. However, the mechanism of proMMP-9 activation in vivo remains largely unknown. We previously reported that proMMP-9 binds to a receptor complex containing TIMP-1 and a disintegrin and metalloproteinase 10 (ADAM10) and is activated by the adjacent MT1-MMP/TIMP-2/MMP-2 complex in concanavalin A (Con A)-treated HT1080 fibrosarcoma cells [10].
Transforming growth factor (TGF)-β is frequently overexpressed in tumor tissues and is associated with tumor aggressiveness [11]. It binds to TGF-β receptors 1 and 2 that phosphorylate the canonical downstream transducers, Smad2 and Smad 3. Signaling pathways induced by TGF-β independent of Smad activation include phosphatidylinositol 3-kinase, nuclear factor κB, and mitogen-activated protein kinase (MAPK). Both TGF-β canonical (Smad-dependent) and noncanonical (Smad-independent) signaling cascades occur simultaneously through crosstalk between the core pathway components and combined utilization of Smad/non-Smad transcription factors. TGF-β signaling promotes multiple cellular processes involved in cancer progression, including maintenance of cancer stem cell homeostasis, inhibition of immune response, induction of epithelial-mesenchymal transition, invasion, and metastasis, which are common outcomes of the canonical and the noncanonical pathways [12].
Elevated MMP-9 level has been shown to positively correlate with invasive and metastatic potential in colon, lung, and oral cancers [4,5]. Transcription of MMP-9 gene is induced by a variety of pro-malignancy factors, such as hypoxia and TGF-β. Tumors with enhanced TGF-β1 signaling show an increase in MMP-9 expression in human oral cancers [[13], [14], [15]]. In this study, we examined whether TGF-β1 is associated with proMMP-9 activation in MT1-MMP expressing cancer cells. Using human oral squamous cell carcinoma (OSCC) cells that express MT1-MMP at high levels, we found that TGF-β1 induced the expression of proMMP-9 and that TGF-β1-induced proMMP-9 was activated by cultivating OSCC cells in collagen gel in the presence of exogenous proMMP-2. This proMMP-9 activation stimulated the invasion of OSCC cells into collagen matrix, which was significantly suppressed by the inhibition of TGF-β1 signaling or MT1-MMP activity. Thus, TGF-β1 facilitates MT1-MMP-mediated proMMP-9 activation, which contributes to promotion of OSCC cell invasions.
2. Materials and methods
2.1. Cell culture and materials
Human embryonic kidney 293 T cells were obtained from ATCC (Manassas, VA, USA). Human OSCC HSC-3 and HSC-4 cells were purchased from the Japan Collection of Research Bioresources Cell Bank (Osaka, Japan). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; D6046, Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich). Acetic acid-extracted type I collagen from pig tendon was purchased from Nitta Gelatin (Cellmatrix Type IA, Osaka, Japan). Human recombinant TGF-β1 (209–16544), TGF-βRI inhibitor SD208 (193–16331), and Con A (033–08773) were obtained from Wako Pure chemical (Osaka, Japan). The synthetic MMP inhibitor (BB94) was a kind gift from the Kotobuki Pharmaceutical (Nagano, Japan). Anti-MT1-MMP (clone 222-2D12), anti-MMP-2 (clone 75-7F7), anti-MMP-9 (clone 56-2A4), and anti-TIMP-1 (clone 67-4H11) antibodies were gifted by Dai-ichi Fine Chemicals (Toyama, Japan). The other immunological reagents used were: anti-phospho-Smad2 (Ser 465/467, 3108), and anti-Smad2 (5339) antibodies (Cell Signaling Technology, Danvers, MA, USA); anti-α-tubulin antibody (T9026, Sigma-Aldrich); anti-ADAM10 antibody (Abcam, ab1997, Cambridge, UK); Hoechst 33,342 and Alexa Fluor-labeled secondary antibodies (Invitrogen, Carlsbad, CA, USA).
2.2. Quantitative RT-PCR
Following treatment of HSC-3 cells with TGF-β1 (10 ng/mL) for 24 h, total RNA was extracted with RNAiso plus (Takara, Ohtsu, Japan) and transcribed to cDNA using SuperScript Vilo cDNA synthesis kit (Invitrogen). Real-time RT-PCR was performed using Faststart SYBR Green master mix kit (Roche) and the cycle threshold (Ct) values were determined by Applied Biosystems ViiA™ 7 Real-Time PCR System. For the standard of MMP-9 copy number, the template was generated in plasmid pCR 2.1 TOPO-TA vector (Life Technologies) including 83 bp amplicon amplified by MMP-9-qPCR primer pair. The standard curve was calculated automatically by plotting the Ct values against each template of known copy number. The averages from at least three independent experiments are shown with standard deviation (SD). Primers used were MMP-9 forward; 5′-GCACGACGTCTTCCAGTACC, MMP-9 reverse; 5′-TCAACTCACTCCGGGAACTC, GAPDH forward; 5′-CGAGATCCCTCCAAAATCAA, GAPDH reverse; 5′-GTCTTCTGGGTGGCAGTGAT.
2.3. Small interfering RNA (siRNA)-mediated protein knockdown
A negative control siRNA was purchased from Qiagen (1027280, Valencia, CA, USA). The siRNA sequences used were as follows: MT1-MMP, CCAGAAGCUGAAGGUAGAA; MMP-9-1, CACAACAUCACCUAUUGGA; MMP-9-2, GGAGUACUCGACCUGUACC; TIMP-1, UCAACCAGACCUUAUA; ADAM10, GAUAUCCAGUCAUGUUAAA. Cells were transfected with 20 nM of siRNA duplexes in Opti-MEM (31,985–070, Invitrogen) using Lipofectamine RNAi MAX (13,778–075, Invitrogen) for 48 h.
2.4. Collagen gel culture
Type I collagen (3 mg/mL) was diluted to 2.1 mg/mL by adding 5 × DMEM containing cells (2 × 105 cells) and 10 × reconstitution buffer (200 mM HEPES, 260 mM NaHCO3, and 50 mM NaOH) on ice, according to the manufacturer's instructions. This cell-collagen mixture was poured into a 48-well tissue culture plate and allowed to polymerize by placing at 37 °C for 30 min. The cells were then stimulated with TGF-β1 in DMEM supplemented with 0.5% FBS. To examine TGF-β1 signaling in collagen gel, the cells (5 × 105 cells) were suspended in 100 μL of collagen solution and the cell-collagen mixture was dropped into a 24-well plate and allowed to polymerize. The cells were cultured in DMEM supplemented with 10% FBS overnight. After serum-starvation for 6 h, the cells were stimulated with TGF-β1 for 24 h.
2.5. Gelatin zymography
ProMMP-2 supernatant was prepared from 293 T cells transfected with MMP-2 expression plasmids, as described previously [10]. Conditioned medium (CM) was examined by mixing with an equal volume of sample buffer. For detection of cell-associated MMP-2 and MMP-9, cells were washed with phosphate-buffered saline (PBS), and dissolved in sample buffer by sonication. The samples were separated by electrophoresis on SDS-polyacrylamide gel containing gelatin (Difco, 0143–17, sparks, MD, USA) labeled with Alexa Fluor 680 (Molecular Probes). The gels were processed and monitored using an Odyssey infrared imaging system (LI-COR, Lincoln, NE, USA).
2.6. Western blotting
Cells were washed with ice-cold PBS, and lysed in a buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 1% Triton X100, and a protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). To analyze the secreted proteins, StrataClean Resin (Stratagene, 400,714, La Jolla, CA, USA) was added to an appropriate volume of CM and rotated for 2 h at 4 °C. After centrifugation, the supernatant was removed and sample buffer was added to the precipitated resin. The samples were separated by electrophoresis on SDS-polyacrylamide gels and transferred to a nitrocellulose membrane.
2.7. Invading cell trapping (iCT) assay
The iCT assay was performed as described previously [16,17]. Cell strainers with a nylon mesh of 40-μm2 square pores (Funakoshi, FN-HT-AMS-14002, Tokyo, Japan) were placed in an inverted orientation in a culture dish (100-mm diameter). Cell-collagen mixture (8 × 105 cells in 200 μL of volume) was transferred and polymerized on the bottom surface of the nylon mesh of the cell strainer by placing at 37 °C for 30 min. After polymerization, the cell strainer was transferred to a 24-well plate. Then a 200 μL of reconstituted collagen gel solution was poured onto the inner surface of the nylon mesh. After polymerization, the cell strainer was transferred to a 24-well plate filled with 1700 μL DMEM containing 0.5% FBS and proMMP-2. The inner chamber was filled with 400 μL of DMEM containing 0.5% FBS and TGF-β1. Cells in the lower gel were allowed to migrate upward into the upper gel for 3 days. Invaded cells in the upper gel were counted under a light microscope. Alternatively, invaded cells were stained with Hoechst 33,342 and counted using confocal laser scanning microscopy LSM900 (Carl Zeiss, Jena, Germany).
2.8. Data analysis and statistics
Data are expressed as the average ± SD. Differences between two groups were assessed by Student t-test. Statistical significance was considered at P-values < 0.01.
3. Results
3.1. TGF-β1 induces proMMP-9 expression
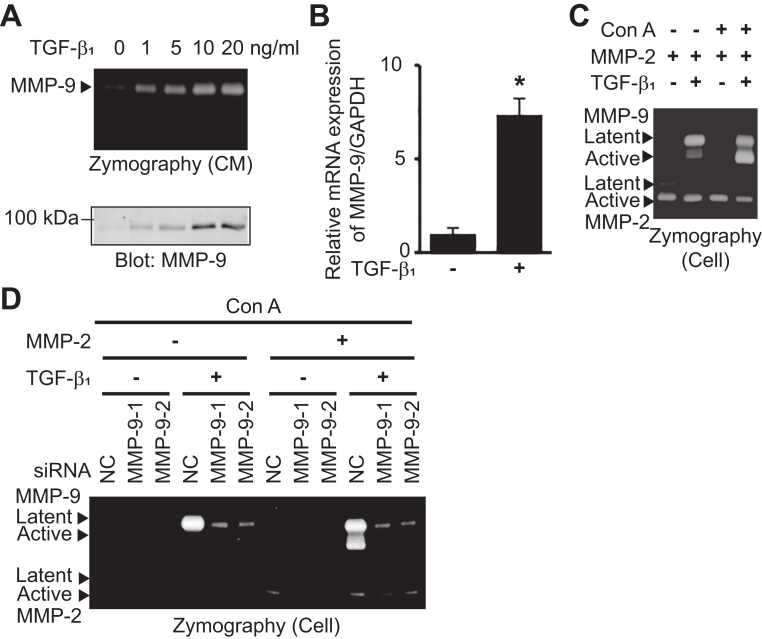
To determine whether TGF-β1 induces the expression of MMP-9 in OSCC cells, HSC-3 cells were treated with various concentrations of TGF-β1 for 24 h (Fig. 1A). Gelatin zymography showed that TGF-β1 induced MMP-9 activity in a dose-dependent manner. Western blotting analysis confirmed that TGF-β1 induced the production and secretion of proMMP-9. Maximal proMMP-9 level was achieved at 10 ng/mL of TGF-β1 in HSC-3 cells. QRT-PCR revealed that TGF-β1 significantly upregulated the expression of MMP-9 mRNA (Fig. 1B). ProMMP-9 bound to the cell surface receptor is activated by the MT1-MMP/MMP-2 axis in Con A-treated human fibrosarcoma HT1080 cells. Addition of exogenous proMMP-2 is necessary for this activation [10]. To examine whether the TGF-β1-induced proMMP-9 was activated by MT1-MMP/MMP-2 axis, HSC-3 cells were treated with TGF-β1, proMMP-2, and Con A. The cell-associated MMP-2 and MMP-9 were examined by gelatin zymography (Fig. 1C). The exogenously added proMMP-2 was converted to its active form as HSC-3 cells expressed MT1-MMP. When the cells were treated with TGF-β1 alone, proMMP-9 was weakly activated. This MMP-9 activation was significantly enhanced following combined treatment with TGF-β1 and Con A. When HSC-3 cells were transfected with siRNA against MMP-9, TGF-β1 treatment failed to induce or activate proMMP-9 even in the presence of Con A and proMMP-2 (Fig. 1D). These results suggest that proMMP-9 induced by TGF-β1 is activated by the MT1-MMP/MMP-2 axis in Con A-treated HSC-3 cells.
Fig. 1.
TGF-β1 induces proMMP-9 expression. (A) HSC-3 cells were stimulated with TGF-β1 at indicated concentrations for 24 h. CM were analyzed by gelatin zymography and immunoblotted with anti-MMP-9 antibody. (B) HSC-3 cells were stimulated with TGF-β1 (10 ng/mL) in Opti-MEM for 24 h. QRT-PCR was performed to detect the expression of MMP-9. The error bars are SD of the mean values obtained from three independent experiments. *p < 0.01. (C) HSC-3 cells were treated with proMMP-2 and TGF-β1 (10 ng/mL) for 24 h. After that, Con A (50 ng/mL) was added and incubated for 24 h. Cell-associated MMP-2 and MMP-9 were analyzed by gelatin zymography (Cell). (D) HSC-3 cells were transfected with siRNA against negative control (NC), MMP-9-1, or MMP-9-2 for 48 h. Then, cells were treated with Con A, proMMP-2, and TGF-β1 (10 ng/mL) as described above. Cell-associated MMP-2 and MMP-9 were analyzed by gelatin zymography.
3.2. TGF-β1 stimulates proMMP-9 activation in collagen gel
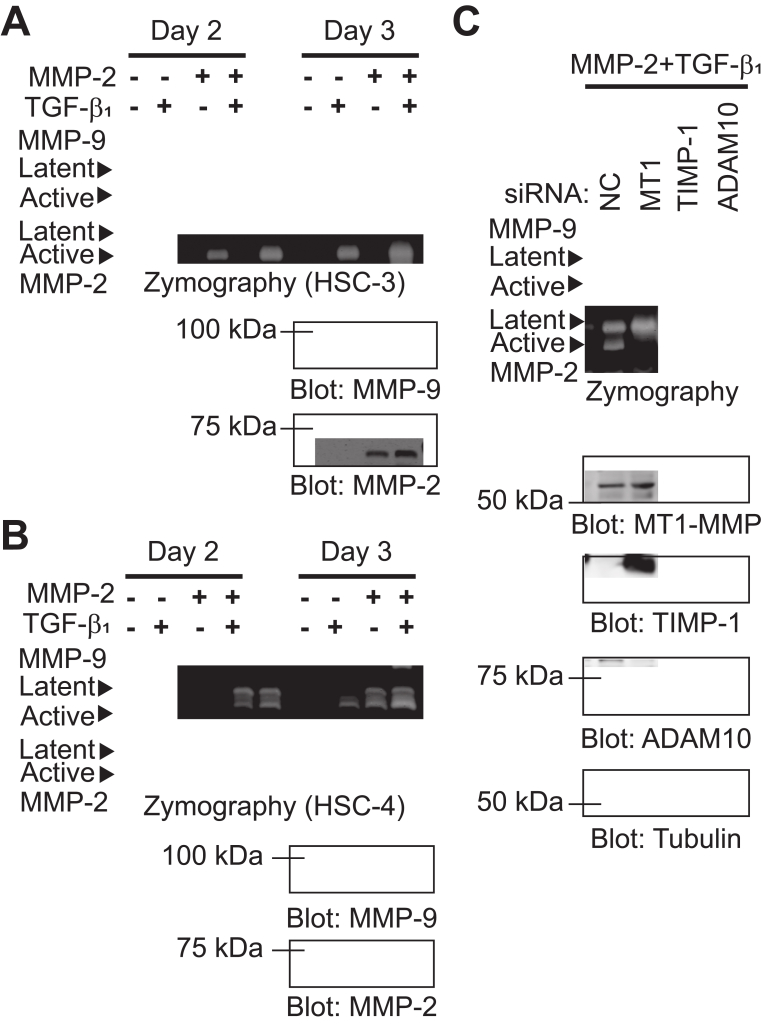
ProMMP-2 activation by MT1-MMP is facilitated by culturing cells in collagen gel [18,19]. When HT1080 cells were cultured in collagen gel, proMMP-9 activation was induced by adding exogenous proMMP-2 [10]. To investigate whether proMMP-9 induced by TGF-β1 is activated in OSCC cells cultured in collagen gel, HSC-3 and HSC-4 cells were embedded in collagen gels and treated with TGF-β1 and/or proMMP-2. TGF-β1 stimulated proMMP-9 expression and proMMP-2 activation in HSC-3 cells, which resulted in the promotion of proMMP-9 activation on day 3 (Fig. 2A). In HSC-4 cells, TGF-β1 stimulated the expression of proMMP-9 and proMMP-2 and effectively induced the activation of proMMP-2 and proMMP-9 on day 3 (Fig. 2B). To assess the role of MT1-MMP, TIMP-1, and ADAM10 in TGF-β1-induced proMMP-9 activation in OSCC cells cultured in collagen gel, siRNAs against MT1-MMP, TIMP-1, or ADAM10 were transfected into HSC-3 cells and proMMP-9 activation was tested (Fig. 2C). MT1-MMP knockdown abrogated proMMP-2 activation and reduced proMMP-9 secretion, resulting in the loss of proMMP-9 activation. Knockdown of TIMP-1 and ADAM10 also disrupted proMMP-9 activation but without affecting proMMP-9 production and proMMP-2 activation. These results demonstrate that TIMP-1 and ADAM10 function as a proMMP-9 receptor in OSCC cells, and suggest that MT1-MMP may participate in TGF-β1-induced proMMP-9 expression and activation in collagen gel.
Fig. 2.
TGF-β1 induces proMMP-9 activation in collagen gel. (AB) OSCC cells embedded in collagen gel were treated with proMMP-2 and TGF-β1 (10 ng/mL) in a 48-well culture plate for 2 or 3 days. CM was analyzed by gelatin zymography and immunoblotted with anti-MMP-9 and anti-MMP-2 antibodies. (C) HSC-3 cells were transfected with siRNA against negative control (NC), MT1-MMP (MT1), TIMP-1, or ADAM10 for 48 h. After transfection, the cells were embedded in collagen gel and treated with proMMP-2 and TGF-β1 for 3 days. CM were analyzed by gelatin zymography and cell lysates were immunoblotted with anti-MT1-MMP, anti-TIMP-1, anti-ADAM10, and anti-tubulin antibodies.
3.3. MMP participates in TGF-β1-induced proMMP-9 expression
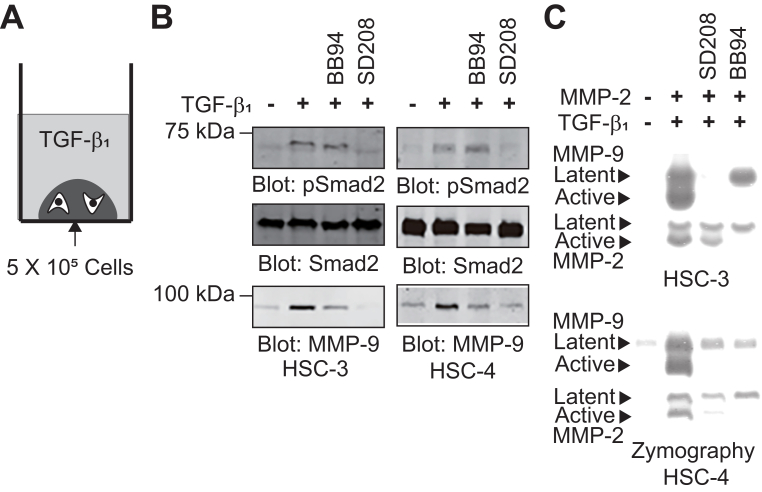
Increasing evidence demonstrates the crosstalk between MMPs, integrins, and type I collagen in TGF-β1 signaling [11,[20], [21], [22]]. SD208, a selective inhibitor of TGF-β type I receptor, has been shown to abrogate TGF-β1-mediated expression of several genes in different cell types [23]. We tested the effect of SD208 and a synthetic broad MMP inhibitor (BB94) on TGF-β1-induced proMMP-9 expression in OSCC cells during collagen gel culture. TGF-β1 induced the sustained phosphorylation of Smad2 and proMMP-9 expression in HSC-3 and HSC-4 cells that was significantly reduced by SD208 treatment (Fig. 3A and B). BB94 treatment slightly decreased the expression of proMMP-9, but did not affect to Smad2 phosphorylation. Next, the effect of SD208 and BB94 on TGF-β1-induced proMMP-9 activation was examined in HSC-3 cells cultured in collagen gel (Fig. 3C). SD208 treatment slightly inhibited proMMP-2 activation but severely suppressed the expression and activation of proMMP-9. BB94 completely blocked the activation both of proMMP-2 and proMMP-9, and reduced TGF-β1-induced proMMP-9 expression. These results suggest that MMP may be involved in TGF-β1-induced proMMP-9 expression and activation in OSCC cells grown in collagen gel.
Fig. 3.
MMP inhibition suppressed TGF-β1-induced proMMP-9 activation. (A) Schematic illustration of TGF-β1 stimulation of the cells grown in collagen gel. (B) HSC-3 (left panel) and HSC-4 (right panel) cells were dropped in collagen gels and cultured in 10% FBS/DMEM overnight. After washing twice with PBS, the cells were serum-starved for 6 h and then treated with TGF-β1 (10 ng/mL) for 24 h. Cell lysates were immunoblotted with anti-phosphoSmad 2 (pSmad2), anti-Smad2, and anti-MMP-9 antibodies. (C) HSC-3 cells were embedded in collagen gel as described in Fig. 2A and treated with proMMP-2 and TGF-β1 (10 ng/mL) plus SD208 (1 μM) or BB94 (1 μM) for 3 days. CM were analyzed by gelatin zymography.
3.4. ProMMP-9 activation stimulates TGF-β1-induced invasion
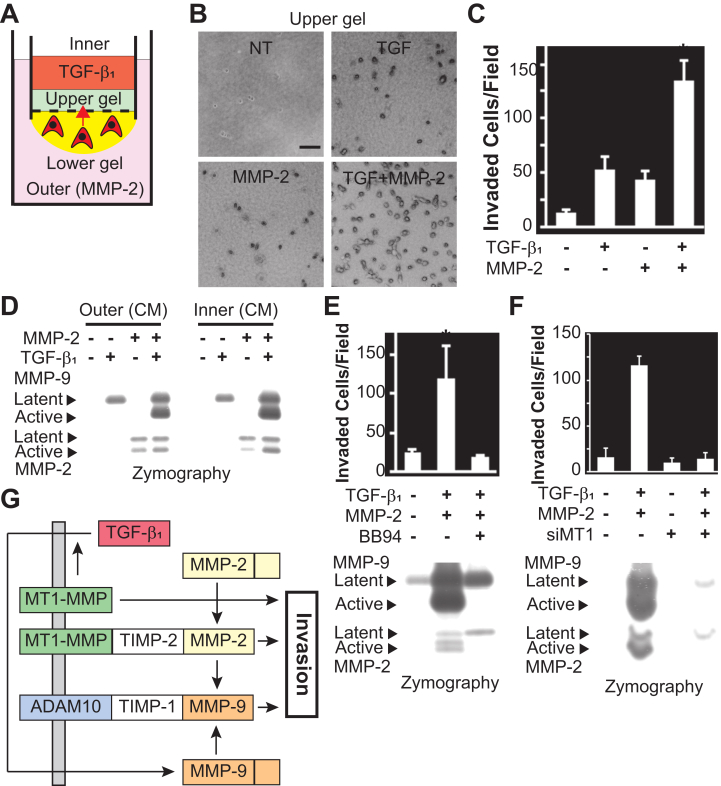
To investigate the role of TGF-β1-induced proMMP-9 activation on cell invasion, we employed a novel invasion assay [16,17] (Fig. 4A). The number of HSC-3 cells invading from the lower gel to the upper gel was increased following treatment with either TGF-β1 or proMMP-2, and was remarkably augmented by their combined treatment (Fig. 4B and C). Activation of proMMP-9 was observed in CM from both inner and outer chambers when the cells were treated with TGF-β1 and proMMP-2 (Fig. 4D). The increase of cell invasion by TGF-β1 and proMMP-2 treatments was inhibited by BB94 treatment or MT1-MMP silencing, which was accompanied with the blockade of activation of proMMP-2 and proMMP-9 (Fig. 4E and F). Similar results were obtained for HSC-4 cells (Fig. S1). The number of invaded cells were significantly increased by combined treatment with TGF-β1 and proMMP-2, which was suppressed by treatment with BB94, SD208, or MT1-MMP knockdown. These results suggest that TGF-β1-induced proMMP-9 is activated by the MT1-MMP/MMP-2 axis, which is needed to promote the invasion of OSCC cells into collagen gel.
Fig. 4.
TGF-β1-induced proMMP-9 activation stimulates invasion of OSCC cells. (A) Schematic view of the iCT assay. (B) Phase contrast images of the HSC-3 cells that invaded into the upper collagen gel. Bars, 200 μm. (C) Quantification of invaded cells in the upper gel. (D) CM obtained from either the outer or inner chamber were subjected to gelatin zymography. (E) The iCT assay was carried out using HSC-3 cells in the presence or absence of BB94 (both chamber; 1 μM). CM from the inner chamber were analyzed by gelatin zymography and immunoblotted with anti-MMP-9 and anti-MMP-2 antibodies. (F) The iCT assay was carried out using MT1-MMP-silenced (siMT1) HSC-3 cells. CM from the inner chamber were analyzed by gelatin zymography. (G) A schematic illustration of TGF-β1-induced cell invasion. Data represent mean number of cells per field ±SD. *p < 0.01. L, latent form; A, active form of MMP-2 or MMP-9.
4. Discussion
The expression of MMP-9 is regulated by several cytokines, growth factors and oncogenes [4,5]. TGF-β1, a potent regulator of MMP-9, is the most studied and best described tumor-derived factor. Many studies have reported that TGF-β1 induces the expression, production and secretion of MMP-9 in diverse cell types [[11], [12], [13], [14], [15]]. TGF-β1-mediated upregulation of MMP-9 is associated with invasion and metastasis of various cancer cells. The molecular mechanism of MMP-9 regulation by TGF-β1 is complex and controversial, as it involves TGF-β canonical and noncanonical pathways. ProMMP-9 expression and Smad2 phosphorylation was induced by TGF-β1 in human OSCC cells and was abolished by SD208 treatment (Fig. 1, Fig. 2, Fig. 3). In contrast, treatment with BB94 suppressed TGF-β1-induced MMP-9 expression without affecting Smad2 phosphorylation. In addition, knockdown of MT1-MMP using siRNA also attenuated TGF-β1-induced MMP-9 expression (Fig. 2). MT1-MMP regulates MAPK activation and its knockdown attenuates TGF-β1-mediated MMP-9 expression by reducing MAPK activation in human keratinocyte cells [19,24,25]. MT1-MMP may involve in TGF-β1-induced MMP-9 upregulation in collagen gel. Interestingly, pancreatic cancer cells grown in collagen matrix induce TGF-β1 expression and signaling to promote MT1-MMP expression [20,21]. Although TGF-β1 could not apparently induce MT1-MMP expression in this study (data not shown), the crosstalk between type I collagen, TGF-β1, and MT1-MMP may be important for the transmission of the signal to promote MMP-9 expression.
Consistent with the previous report [10], Con A treatment facilitated the activation of proMMP-9 induced by TGF-β1 in the presence of proMMP-2 (Fig. 1). We also found that proMMP-9, which was induced by TGF-β1, was activated by addition of exogenous proMMP-2 to human OSCC cells cultured in collagen gel. This proMMP-9 activation was inhibited by the treatment with either SD208 or BB94 (Fig. 2, Fig. 3), indicating the importance of TGF-β receptor activation and MMP activity in proMMP-9 activation. MT1-MMP silencing by siRNA blocked proMMP-2 activation and thereby TGF-β1-induced proMMP-9 activation. Moreover, either TIMP-1 or ADAM10 knockdown also suppressed TGF-β1-induced proMMP-9 activation without affecting proMMP-9 expression and proMMP-2 activation. These results indicate that TGF-β1-induced proMMP-9 was activated by MT1-MMP/MMP-2 axis in the collagen gel and that TIMP-1 and ADAM10 may in part serve as proMMP-9 receptors in human OSCC cells. Other molecules such as CD44 and CD63 are reported to bind to TIMP-1 on the cell surface [[26], [27], [28]]. Integrins α3β1, α5β1, αvβ3, and αvβ5 are also known to associate with proMMP-9 [4,5]. Therefore, we cannot rule out the possibility that these molecules may also function as proMMP-9 receptors.
Although MMP-2 and MMP-9 are secreted proteases, at least a fraction of their proteolytic activity is found on the cell surface where they are tethered by cell surface receptors to provide controlled ECM degradation and activation of a variety of latent growth factors. Thus, controlling the proteolytic activity at the cell surface generates focalized pericellular proteolysis, which greatly facilitates cell invasion. This is supported by our results showing that the combined treatment of OSCC cells with TGF-β1 and proMMP-2 stimulated the invasion of OSCC cells into collagen gel, accompanied by the activation of both proMMP-2 and proMMP-9 on the cell surface. Indeed, this enhanced invasion was significantly suppressed by inhibition of TGF-β1 signaling or MT1-MMP activity (Figs. 4 and S1). These data indicate that TGF-β1-induced proMMP-9 is activated by MT1-MMP/MMP-2 axis and promotes the invasion of human OSCC cells into collagen gel, which should be tightly regulated by the balance between MT1-MMP and TIMP-2 [29]. ProMMP-2 is often abundantly expressed by tumor stromal cells and serves as a mediator of tumor-stromal interaction following activation by tumor-specific MT1-MMP, which may activate proMMP-9 (Fig. 4G). Participation of MMP-9 in the coordinated action of MT1-MMP and MMP-2 may facilitate the focalized pericellular proteolysis for tumor invasion and metastasis. In conclusion, proMMP-9 induced by TGF-β1 is bound to the cell surface and then activated by the MT1-MMP/MMP-2 axis. This activated MMP-9 may contribute to pericellular proteolysis for the invasion of OSCC cells into the collagen matrix in collaboration with MT1-MMP and MMP-2.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported partly by the Extramural Collaborative Research Grant of Cancer Research Institute, Kanazawa University and by Grant-in-Aid for Scientific Research C (21K07118) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bbrep.2021.101072.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P., Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Canc. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 3.Cauwe B., Van den Steen P.E., Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 4.Mook O.R., Frederiks W.M., Van Noorden C.J. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Björklund M., Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim. Biophys. Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Sato H., Takino T., Okada Y. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 7.Sato H., Takino T., Miyamori H. Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Canc. Sci. 2005;96:212–217. doi: 10.1111/j.1349-7006.2005.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato H., Takino T. Coordinate action of membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2 enhances pericellular proteolysis and invasion. Canc. Sci. 2010;101:843–847. doi: 10.1111/j.1349-7006.2010.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh Y., Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J. Cell. Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 10.Li Z., Takino T., Endo Y. Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis. Canc. Sci. 2017;108:347–353. doi: 10.1111/cas.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison C.D., Parvani J.G., Schiemann W.P. The relevance of the TGF-β paradox to EMT-MET programs. Canc. Lett. 2013;341:30–40. doi: 10.1016/j.canlet.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadi A., Najafi M., Farhood B. Transforming growth factor-β signaling: tumorigenesis and targeting for cancer therapy. J. Cell. Physiol. 2019;234:12173–12187. doi: 10.1002/jcp.27955. [DOI] [PubMed] [Google Scholar]
- 13.Patel B.P., Shah S.V., Shukla S.N. Clinical significance of MMP-2 and MMP-9 in patients with oral cancer. Head Neck. 2007;29:564–572. doi: 10.1002/hed.20561. [DOI] [PubMed] [Google Scholar]
- 14.Sun L., Diamond M.E., Ottaviano A.J. Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol. Canc. Res. 2008;6:10–20. doi: 10.1158/1541-7786.MCR-07-0208. [DOI] [PubMed] [Google Scholar]
- 15.Joseph M.J., Dangi-Garimella S., Schields M.A. Slug is a downstream mediator of transforming growth factor-beta1-idnuced matrix metalloproteinase-9 expression and invasion of oral cancer cells. J. Cell. Biochem. 2009;108:726–736. doi: 10.1002/jcb.22309. [DOI] [PubMed] [Google Scholar]
- 16.Takino T., Sato H., Seiki M M. Simple and cost-effective assay for isolating invasive living cells. Biotechniques. 2018;65:137–142. doi: 10.2144/btn-2018-0036. [DOI] [PubMed] [Google Scholar]
- 17.Takino T., Suzuki T., Seiki M. Isolation of highly migratory and invasive cells in three-dimensional gels. Curr. Protoc. Cell Biol. 2020;86:e103. doi: 10.1002/cpcb.103. [DOI] [PubMed] [Google Scholar]
- 18.Azzam H.S., Thompson E.W. Collagen-induced activation of the M(r) 72,000 type IV collagenase in normal and malignant human fibroblastoid cells. Canc. Res. 1992;52:4540–4544. [PubMed] [Google Scholar]
- 19.Takino T., Miyamori H., Watanabe Y. Membrane type 1 matrix metalloproteinase regulates collagen-dependent mitogen-activated protein/extracellular signal-related kinase activation and cell migration. Canc. Res. 2004;64:1044–1049. doi: 10.1158/0008-5472.can-03-1843. [DOI] [PubMed] [Google Scholar]
- 20.Shields M.A., Dangi-Garimella S., Redig A. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem. J. 2012;441:541–552. doi: 10.1042/BJ20111240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottaviano A.J., Sun L., Ananthanarayanan V. Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-β1. Canc. Res. 2006;66:7032–7040. doi: 10.1158/0008-5472.CAN-05-4421. [DOI] [PubMed] [Google Scholar]
- 22.Margadant C., Sonnenberg A. Integrin-TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournier P.G.J., Juárez P., Jiang G. The TGF-β signaling regulator PMEPA1 suppresses prostate cancer metastasis to bone. Canc. Cell. 2015;27:809–821. doi: 10.1016/j.ccell.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seomun Y., Kim J.T., Joo C.K. MMP-14 mediated MMP-9 expression is involved in TGF-beta1-induced keratinocyte migration. J. Cell. Biochem. 2008;104:934–941. doi: 10.1002/jcb.21675. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T., Suganuma N., Sato S. Membrane type 1 matrix metalloproteinase regulates anaplastic thyroid carcinoma cell growth and invasion into the collagen matrix. Biochem. Biophys. Res. Commun. 2020;529:1195–1200. doi: 10.1016/j.bbrc.2020.06.043. [DOI] [PubMed] [Google Scholar]
- 26.Chellaiah M.A., Ma T. Membrane localization of membrane type 1 matrix metalloproteinase by CD44 regulates the activation of pro-matrix metalloproteinase 9 in osteoclasts. BioMed Res. Int. 2013;2013:302392. doi: 10.1155/2013/302392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert E., Bridoux L., Devy J. TIMP1 binding to proMMP-9/CD44 complex localized at the cell surface promotes erythroid cell survival. Int. J. Biochem. Cell Biol. 2009;41:1102–1115. doi: 10.1016/j.biocel.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Redondo-Muñoz J., Ugarte-Berzal E., Terol M.J. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia B cell survival through its hemopexin domain. Canc. Cell. 2010;17:160–172. doi: 10.1016/j.ccr.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 29.Cepeda M.A., Pelling J.J., Evered C.L. Less is more: low expression of MT1-MMP is optimal to promote migration and tumourigenesis of breast cancer cells. Mol. Canc. 2016;15:65. doi: 10.1186/s12943-016-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.