Given the many limitations of [18F]-FDG in glioma imaging, such as high background uptake in normal brain tissue and significant uptake in inflammation, the quest for better positron emission tomography (PET) tracers for brain tumor imaging has been constant [1]. Since amino acid transporters (AATs) are indispensable channels for cellular uptake of nutrients and neurotransmitters, radiolabeled amino acids (AAs) have been extensively studied for imaging brain malignancies [2]. In the clinical setting, PET scans with radiolabeled AA (analogues) are often performed following brain tumor diagnosis by either magnetic resonance imaging (MRI) or computed tomography (CT), potentially aiding in personalized treatment planning, monitoring the efficacy of various types of therapy (e.g., radiation therapy, surgery, and/or chemotherapy), and the detection of tumor recurrence [1, 3].
To date, several AA-based tracers have been investigated clinically, such as 11C-methionine ([11C]-MET), 18F-fluorodihydroxyphenylalanine ([18F]-DOPA), 18F-fluoroethyltyrosine ([18F]-FET), nC-α-methyl-L-tryptophan ([11C]-AMT) [2], 4-18F-(2S,4R)-fluoroglutamine ([18F]-FGln) [4, 5], and several others [6, 7]. Among these PET tracers, [11C]-MET has been the most extensively studied, and is highly useful in L-type amino acid transporter 1 imaging [8-10]. However, widespread clinical application of [11C]-MET has been severely hampered by the short half-life of 11C (20.38 min) and low radiochemical yield (usually <5%). By using the more widely available, longer-lived 18F (t1/2: 109.7 min) to label methionine or other AAs (analogues) without interfering with their biological activity, this problem may be solved. Unfortunately, radiolabeling AA (analogues) with 18F without affecting their transport by AATs is very challenging, since even minor modifications to the side chain will significantly alter the biological properties of the resulting PET tracer, which may function differently from the parent AA.
In this issue of European Journal of Nuclear Medicine and Molecular Imaging, Li and Kong et al. reported the first-in-human study of 18F-trifluorobborate-derived tyrosine (denoted as [18F]-FBY, Fig. 1) in six healthy human volunteers and thirteen patients who were suspected with glioma [11]. The significance of this study is that this is the first clinical investigation of a new class of PET tracers (i.e., boramino acid, BAA, Fig. 1), which can potentially have a broad impact in imaging many solid tumor types that exhibit elevated AAT activity. Clinical translation is typically a long process, requiring the concerted efforts of a collaborative team from various disciplines, as well as significant resources and infrastructure. The clinical application of [18F]-FBY has been no different, with the path leading to this important study dating back to a 2005 study conducted by Ting et al.
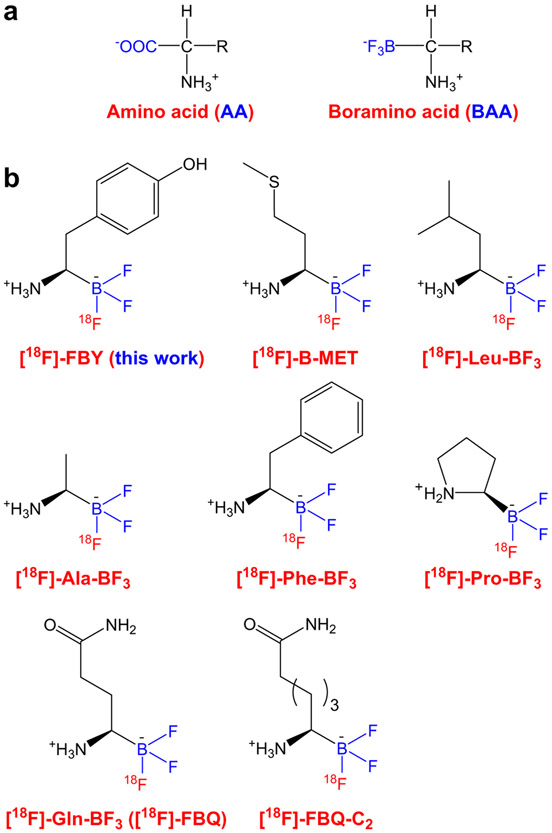
Fig. 1.
a Amino acid (AA) and boramino acid (BAA). b Chemical structures of several 18F-labeled BAAs
To overcome the difficulty in 18F-labeling of AAs and other molecules, various chemical approaches were developed by research groups around the globe [12, 13]. In one pioneering study in 2005, Ting et al. reported two new classes of biomolecule precursors (i.e., arylfluoroborates and alkylfluorosilicates) that can enable highly efficient 18F-labeling in one step within an aqueous or mixed solvent media [14]. Subsequently, a series of studies were carried out to optimize the 18F-labeling procedure and yield, and various targeting ligands were studied in small animal models [15-21], which were exquisitely summarized in a review article in 2016 [22]. In brief, various biomolecules can be functionalized with an organotrifluoroborate moiety, which can then be radiolabeled with aqueous 18F− in one step, a fast process (usually <30 min) with a satisfactory non-decay-corrected radiochemical yield of 20–35% [23]. More importantly, the radiolabeling is quite stable with little defluorination (evidenced by low bone uptake in the PET images) when the trifluoroborate is stabilized with a nearby electron-withdrawing group (e.g., −NH3+).
Building upon these findings, a new class of AA mimics, BAAs (Fig. 1a), were developed and readily labeled with 18F for PET imaging, which can serve as general imaging probes for AATs [24]. The structure of a BAA resembles that of the corresponding natural AA, except that −BF3 replaces the carboxylate group. Both in vitro and in vivo, these [18F]-BAAs behaved like AAs and the cellular uptake was mediated by AATs, which was validated by molecular docking and density functional theory structure predictions. In this initial report [24], four 18F-BAAs were synthesized and investigated (i.e., [18F]-Leu-BF3, [18F]-Phe-BF3, [18F]-Ala-BF3, and [18F]-Pro-BF3; Fig. 1b), exhibiting very low background signal in normal tissue (including the brain) and tumor-specific accumulation, suggesting that [18F]-BAAs hold great promise in developing new PET tracers.
Very few AAs have been labeled with 18F due to the lack of proper chemistry, while all BAAs can be readily synthesized and labeled with 18F via 19F-18F exchange reaction. Therefore, all 20 BAAs could potentially be labeled in the same way for PET imaging applications. In one follow-up study, an 18F-labeled alanine derivative ([18F]-Ala-BF3, Fig. 1b) was reported for cancer imaging, which serves to measure alanine-serine-cysteine-threonine transporter type 2 (ASCT2) activity [25]. Good tumor contrast was achieved in xenograft tumor-bearing mice and the tracer showed very low uptake in inflammation, a feature not associated with 18F-FDG. In another report, [18F]-Gln-BF3 (also called [18F]-FBQ, Fig. 1b) was synthesized as a glutamine derivative to explore its potential applications for cancer diagnosis [26]. Even though [18F]-FBQ exhibited fast clearance from normal tissues and tumor uptake was significant, high bone uptake was observed which increased over time, indicating clear defluorination that renders [18F]-FBQ unstable/unsuitable for clinical translation. To overcome the defluorination problem, two methylene groups were added to the side chain of [18F]-FBQ to yield [18F]-FBQ-C2 (Fig. 1b), which was synthesized with good radiochemical yield and purity [27]. [18F]-FBQ-C2 showed excellent stability both in vitro and in vivo with no defluorination observed (< 1 %ID/g uptake of radioactivity in the bone). A competitive inhibition assay revealed that [18F]-FBQ-C2 enters cells via the system ASC and N, similar as glutamine, and can be transported by overexpressed ASCT2 in the tumor cells.
In another report, an 18F-labeled boron-derived methionine analogue, [18F]-B-MET (Fig. 1b), was synthesized and evaluated as a potential substitute of [11C]-MET for glioma PET imaging [28]. The push button synthesis, highly efficient radiolabeling, and good imaging performance in glioma models make this tracer a promising candidate for future clinical translation. However, because there is no trapping mechanism for [18F]-B-MET inside the cell, unlike [18F]-FDG (i.e., tumor uptake of [18F]-FDG usually increases over time), it showed rather rapid clearance from the tumor tissue. Subsequently, a metabolically stable boron-derived tyrosine (denoted as fluoroboronotyrosine, FBY, Fig. 1b) was developed for boron neutron capture therapy (BNCT), and [18F]-FBY was synthesized and employed for PET image guidance, as well as quantitative and non-invasive evaluation of FBY biodistribution in mouse tumor models [29]. When thermal neutron irradiation was applied, B16F10 tumor-bearing mice injected with FBY showed significantly prolonged median survival without exhibiting obvious systemic toxicity. This is an excellent example of true theranostics, given the same chemical entity was used for both imaging and therapy applications (with the only difference being 18F vs 19F for PET and BNCT, respectively). Recently, these investigators also established a bioorthogonal chemical system, in which Phe-BF3 (which can enter cells) desilylates and cleaves a designed linker containing a silyl ether, enabling the controlled release of a drug from an antibody-drug conjugate in tumor-bearing mice [30]. Again, [18F]-Phe-BF3 allowed for non-invasive and quantitative measurement of tumor uptake via serial PET imaging.
Building upon the many years of prior work as summarized above, in this issue of European Journal of Nuclear Medicine and Molecular Imaging, Li and Kong et al. reported the first-in-human study of [18F]-FBY (Fig. 1b) [11]. [18F]-FBY exhibited rapid renal clearance and was well tolerated by all healthy volunteers, with no adverse symptoms observed or reported. More importantly, [18F]-FBY uptake had a good correlation with tumor grade, with maximum standardized uptake values (SUVmax) of 0.28 ± 0.14 and 2.84 ± 0.46, tumor-to-normal contralateral activity ratio of 2.30 ± 1.26 and 24.56 ± 6.32 in low-grade (i.e., WHO grade II) and high-grade (i.e., WHO grades III and IV) tumors, respectively. The difference in [18F]-FBY uptake between low-grade and high-grade gliomas was corroborated by immunohistochemical staining for large neutral amino acid transporter type 1 (LAT-1) expression and Ki-67 index. In addition, there was a significant linear correlation between SUVmax from [18F]-FBY PET and the expression level of LAT-1 (r2 = 0.80, P < 0.0001), as well as the Ki-67 labeling index (r2 = 0.79, P < 0.001), traits highly desirable for patient staging, prognosis, and monitoring of therapeutic efficacy. Overall, in addition to potentially providing image guidance for future BNCT of glioma patients, [18F]-FBY is a PET tracer with favorable pharmacokinetic/dosimetry profiles and clean background, as well as no observable bone uptake, features that should make it suitable for tumor imaging virtually anywhere of the human body besides the kidneys and bladder.
For routine clinical use, a PET tracer needs to be synthesized in a simple/fast way with good radiochemical yield and purity [22, 31-33]. These characteristics are reflected by [18F]-FBY, which was synthesized in good non-decay-corrected radiochemical yield (38 ± 14%, n = 13) with excellent radiochemical purity (> 99%). Since the radiolabeling was a simple isotope exchange in aqueous solution, it is straightforward to automate for large-scale synthesis and more broad clinical applications in the future. In terms of radiolabeling stability, there was some concern about defluorination with one of the 18F-labled BAAs (i.e., [18F]-FBQ [26]), with questions arising on whether the radiolabeling method optimized for minimal defluorination in animal studies could translate to clinical application. We are delighted to see that almost no bone uptake was observed during the dynamic PET imaging in healthy volunteers, indicating minimal levels of free fluoride were released from [18F]-FBY, making it suitable for further clinical investigations.
Now that this important first-in-human study has been completed, future investigation/validation of [18F]-FBY in a larger cohort of patients is warranted, not only in brain tumor but also in various other solid tumor types. Direct comparison with [18F]-FDG, [11C]-MET, and/or [18F]-FET in glioma patients will be needed to determine whether [18F]-FBY is superior to the current gold standard. The positive correlation of [18F]-FBY uptake with tumor grade, LAT-1 expression level, and Ki-67 index adds promise. If this correlation is further validated in large cohort studies, [18F]-FBY will have a broad impact in glioma patient diagnosis, staging, prognosis, treatment, and monitoring of treatment efficacy.
In this study with a small number of patients, [18F]-FBY showed good tumor contrast in WHO grade III and IV glioma, whereas the tumor uptake in WHO grade II patients was very low at the background level [11]. During the brain tumor development process, some grade II lesions may become grade III or IV. A combination of [18F]-FBY PET with various other imaging techniques or other PET tracers that can better delineate early-stage, low-grade brain tumors may be needed for the sub-population of low-grade cancer patients, since early and accurate detection of brain tumor is crucial for longer survival and better clinical outcome. Much future work lies ahead, and we look forward to follow-up studies in this exciting area of cancer imaging with 18F-labeld BAAs, as well as future investigations in BNCT, where [18F]-FBY holds the potential to measure local boron concentration via PET imaging to overcome the shortcoming of previous BNCT strategies.
Acknowledgments
Funding The authors are grateful for financial support from the National Natural Science Foundation of China (No. 81630049 and 82030052), the University of Wisconsin – Madison, and the National Institutes of Health (P30CA014520).
Footnotes
Studies with human participants or animals This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interesxt The authors declare no competing interests.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view - what is the status quo of positron emission tomography in patients with brain tumors? Neuro-Oncology. 2015;17:1434–44. 10.1093/neuonc/nov118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juhász C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S. Comparison of amino acid positron emission tomographic radio-tracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging. 2014;13. 10.2310/7290.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galldiks N, Langen K-J. Amino acid PET – an imaging option to identify treatment response, posttherapeutic effects, and tumor recurrence? Front Neurol. 2016;7:120. 10.3389/fneur.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. 2015;7:274ra17. 10.1126/scitranslmed.aaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, Zhu H, Liu F, Zhang Y, Yang J, Zhang L, et al. Imaging brain metastasis patients with 18F-(2S,4R)-4-fluoroglutamine. Clin Nucl Med. 2018;43:e392–e9. 10.1097/rlu.0000000000002257. [DOI] [PubMed] [Google Scholar]
- 6.Jung JH, Ahn BC. Current radiopharmaceuticals for positron emission tomography of brain tumors. Brain Tumor Res Treat. 2018;6:47–53. 10.14791/btrt.2018.6.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werner JM, Lohmann P, Fink GR, Langen KJ, Galldiks N. Current landscape and emerging fields of PET imaging in patients with brain tumors. Molecules. 2020;25:1471. 10.3390/molecules25061471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40:615–35. 10.1007/s00259-012-2295-5. [DOI] [PubMed] [Google Scholar]
- 9.Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J. 11C-L-Methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol. 2008;10:1–18. 10.1007/s11307-007-0115-2. [DOI] [PubMed] [Google Scholar]
- 10.Delgado AF, Delgado AF. Discrimination between primary low-grade and high-grade glioma with 11C-methionine PET: a bivariate diagnostic test accuracy meta-analysis. Br J Radiol. 2017;20170426. 10.1259/bjr.20170426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Kong Z, Chen J, Li J, Li N, Yang Z, et al. 18F-Boramino acid PET/CT in healthy volunteers and glioma patients. Eur J Nucl Med Mol Imaging. 2021. 10.1007/s00259-021-05212-7. [DOI] [PubMed] [Google Scholar]
- 12.Preshlock S, Tredwell M, Gouverneur V. (18)F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem Rev. 2016;116:719–66. 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- 13.Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY, Cai W. ImmunoPET: concept, design, and applications. Chem Rev. 2020;120:3787–851. 10.1021/acs.chemrev.9b00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ting R, Adam MJ, Ruth TJ, Perrin DM. Arylfluoroborates and alkylfluorosilicates as potential PET imaging agents: high-yielding aqueous biomolecular 18F-labeling. J Am Chem Soc. 2005;127:13094–5. 10.1021/ja053293a. [DOI] [PubMed] [Google Scholar]
- 15.Ting R, Harwig C, auf dem Keller U, McCormick S, Austin P, Overall CM, et al. Toward [18F]-labeled aryltrifluoroborate radio-tracers: in vivo positron emission tomography imaging of stable aryltrifluoroborate clearance in mice. J Am Chem Soc. 2008;130:12045–55. 10.1021/ja802734t. [DOI] [PubMed] [Google Scholar]
- 16.auf dem Keller U, Bellac CL, Li Y, Lou Y, Lange PF, Ting R, et al. Novel matrix metalloproteinase inhibitor [18F]marimastat-aryltrifluoroborate as a probe for in vivo positron emission tomography imaging in cancer. Cancer Res. 2010;70:7562–9. 10.1158/0008-5472.Can-10-1584. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Li Y, Lozada J, Wong MQ, Greene J, Lin KS, et al. Kit-like 18F-labeling of RGD-19F-arytrifluroborate in high yield and at extraordinarily high specific activity with preliminary in vivo tumor imaging. Nucl Med Biol. 2013;40:841–9. 10.1016/j.nucmedbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Pourghiasian M, Bénard F, Pan J, Lin KS, Perrin DM. Preclinical evaluation of a high-affinity 18F-trifluoroborate octreotate derivative for somatostatin receptor imaging. J Nucl Med. 2014;55:1499–505. 10.2967/jnumed.114.137836. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Amouroux G, Zhang Z, Pan J, Hundal-Jabal N, Colpo N, et al. (18)F-Trifluoroborate derivatives of [des-arg(10)]kallidin for imaging bradykinin b1 receptor expression with positron emission tomography. Mol Pharm. 2015;12:974–82. 10.1021/acs.molpharmaceut.5b00003. [DOI] [PubMed] [Google Scholar]
- 20.Pourghiasian M, Liu Z, Pan J, Zhang Z, Colpo N, Lin KS, et al. (18)F-AmBF3-MJ9: a novel radiofluorinated bombesin derivative for prostate cancer imaging. Bioorg Med Chem. 2015;23:1500–6. 10.1016/j.bmc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Pourghiasian M, Radtke MA, Lau J, Pan J, Dias GM, et al. An organotrifluoroborate for broadly applicable one-step 18F-labeling. Angew Chem Int Ed Eng. 2014;53:11876–80. 10.1002/anie.201406258. [DOI] [PubMed] [Google Scholar]
- 22.Perrin DM. [(18)F]-Organotrifluoroborates as radioprosthetic groups for PET imaging: from design principles to preclinical applications. Acc Chem Res. 2016;49:1333–43. 10.1021/acs.accounts.5b00398. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Lin KS, Bénard F, Pourghiasian M, Kiesewetter DO, Perrin DM, et al. One-step (18)F labeling of biomolecules using organotrifluoroborates. Nat Protoc. 2015;10:1423–32. 10.1038/nprot.2015.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Chen H, Chen K, Shao Y, Kiesewetter DO, Niu G, et al. Boramino acid as a marker for amino acid transporters. Sci Adv. 2015;1:e1500694. 10.1126/sciadv.1500694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Han Y, Li J, Qin M, Fu Q, Wang C, et al. (18)F-Alanine derivative serves as an ASCT2 marker for cancer imaging. Mol Pharm. 2018;15:947–54. 10.1021/acs.molpharmaceut.7b00884. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Liu H, Duan D, Zhou Z, Liu Z. Preclinical study of an (18)F-labeled glutamine derivative for cancer imaging. Nucl Med Biol. 2018;64–65:34–40. 10.1016/j.nucmedbio.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Li C, Hong H, Liu H, Wang C, Xu M, et al. Side chain optimization remarkably enhances the in vivo stability of (18)F-labeled glutamine for tumor imaging. Mol Pharm. 2019;16:5035–41. 10.1021/acs.molpharmaceut.9b00891. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Liu Z, Zhang H, Li Z, Munasinghe JP, Niu G, et al. Preclinical evaluation of an 18F-trifluoroborate methionine derivative for glioma imaging. Eur J Nucl Med Mol Imaging. 2018;45:585–92. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Shi Y, Zhang Z, Liu H, Lang L, Liu T, et al. A metabolically stable boron-derived tyrosine serves as a theranostic agent for positron emission tomography guided boron neutron capture therapy. Bioconjug Chem. 2019;30:2870–8. 10.1021/acs.bioconjchem.9b00578. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421–6. 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 31.Lau J, Rousseau E, Kwon D, Lin KS, Bénard F, Chen X. Insight into the development of PET radiopharmaceuticals for oncology. Cancers (Basel). 2020;12. 10.3390/cancers12051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandão SCS, Ramos JOX, de Arruda GFA, Godoi E, Carreira L, Lopes RW, et al. Mapping COVID-19 functional sequelae: the perspective of nuclear medicine. Am J Nucl Med Mol Imaging. 2020;10:319–33. [PMC free article] [PubMed] [Google Scholar]
- 33.Bois F, Noirot C, Dietemann S, Mainta IC, Zilli T, Garibotto V, et al. [(68)Ga]Ga-PSMA-11 in prostate cancer: a comprehensive review. Am J Nucl Med Mol Imaging. 2020;10:349–74. [PMC free article] [PubMed] [Google Scholar]