Abstract
Objectives:
State Medicaid programs are the largest single provider of healthcare for pregnant persons with opioid use disorder (OUD). Our objective was to provide comparable, multi-state measures estimating the burden of OUD in pregnancy, medication for opioid use disorder (MOUD) in pregnancy, and related neonatal and child outcomes.
Methods:
Drawing on the Medicaid Outcomes Distributed Research Network (MODRN), we accessed administrative healthcare data for 1.6 million pregnancies and 1.3 million live births in 9 state Medicaid populations from 2014–2017. We analyzed within- and between-state prevalences and time trends in the following outcomes: diagnosis of OUD in pregnancy, initiation and continuity of MOUD in pregnancy, Neonatal Opioid Withdrawal Syndrome (NOWS), and well-child visit utilization among children with NOWS.
Results:
OUD diagnosis increased from 49.6 per 1,000 to 54.1 per 1,000 pregnancies, and the percentage of those with any MOUD in pregnancy increased from 53.4% to 57.9%, during our study time period. State-specific percentages of 180-day continuity of MOUD ranged from 41.2% to 84.5%. The rate of neonates diagnosed with NOWS increased from 32.7 to 37.0 per 1,000 live births. State-specific percentages of children diagnosed with NOWS who had the recommended well-child visits in the first 15 months ranged from 39.3% to 62.5%.
Conclusions:
Medicaid data, which allow for longitudinal surveillance of care across different settings, can be used to monitor OUD and related pregnancy and child health outcomes. Findings highlight the need for public health efforts to improve care for pregnant persons and children affected by OUD.
Keywords: Pregnancy, Opioid Use Disorder, Neonatal Opioid Withdrawal Syndrome, Medication for Opioid Use Disorder, Medicaid
INTRODUCTION
Opioid use disorder (OUD) in pregnancy contributes to adverse pregnancy and child health outcomes.1–3 OUD in pregnancy is a major area of policy focus for state Medicaid programs,4 which collectively cover 43% of all pregnancy care, but cover more than 80% of care for pregnant persons and children affected by OUD.5–7 Therefore, Medicaid has a major role in providing high-quality OUD treatment for pregnant persons, as well as monitoring ongoing care for children born to those with OUD in pregnancy.
Clinical guidelines recommend that persons with OUD in pregnancy initiate or maintain treatment with buprenorphine or methadone, and that children exposed to opioids in utero be monitored for withdrawal symptoms for 7 days and receive ongoing follow-up preventive care in early childhood.2,8 Clinical trials have demonstrated that medication for opioid use disorder (MOUD) in pregnancy reduces the risk of relapse and overdose and improves pregnancy outcomes.9–12 Despite this, observational studies have shown that a significant proportion of persons who are diagnosed with OUD in pregnancy do not receive any MOUD.13,14 Likewise, prior research has suggested low levels of preventive care among children exposed to opioids in utero, relative to children not exposed to opioids in utero.15
A barrier to a more comprehensive understanding of the treatment landscape for pregnant persons and children affected by OUD is a lack of comparable data and measurements across state Medicaid programs. Prior research has used single-state data sources,16 which, although valuable, preclude the ability to identify differences across states. Variability in the structure of Medicaid data across states similarly precludes cross-state comparisons of studies using single-state data. Multi-state data analyses have typically been limited to the use of hospital discharge data,17,18 which are well-suited to studying hospital care but do not provide data on MOUD or any care received before or after the delivery episode.
The present study overcomes limitations of prior single-state Medicaid studies by drawing on the Medicaid Outcomes Distributed Research Network (MODRN) to provide comparable, multi-state measures of the burden of OUD in pregnancy, MOUD treatment, and neonatal and child outcomes. Specifically, we draw on a common data model implemented across 9 states to examine rates of pregnant persons diagnosed with OUD, medication for OUD (MOUD) received in pregnancy, neonatal opioid withdrawal syndrome (NOWS), and well-child visits among children diagnosed with NOWS.
METHODS
Data
The Medicaid Outcomes Distributed Research Network (MODRN) is a multi-state, multi-faceted collaborative project to promote learning about health outcomes in Medicaid programs by drawing on partnerships between universities and state health agencies.19 State Medicaid program data include a wealth of information including all enrollment, inpatient, professional, outpatient, pharmacy, and provider files for services provided in fee-for-service and Medicaid managed care plans; however, each state’s internal data structure is unique. MODRN investigators developed a Medicaid Common Data Model to facilitate centralized development of analytic protocols with results that are comparable between states. The MODRN common data model defines comparable groups within participating state Medicaid populations: pregnant persons, children, disabled adults, non-disabled adults, and newly-eligible expansion adults. It also defines common measure specifications for identifying OUD and related outcomes.
Data for the present study included Medicaid healthcare administrative data for pregnant females (hereafter referred to as pregnant women) and children from 9 states (Kentucky, Maine, Maryland, Michigan, North Carolina, Ohio, Pennsylvania, Virginia, and West Virginia) that participate in MODRN. Data on pregnant and child populations were analyzed separately because not all states had the capability to link maternal-child dyads in Medicaid healthcare data. Study data included 1.6 million pregnancies, 88,927 in which women were diagnosed with OUD; and 1.3 million children, 44,883 of whom were diagnosed with NOWS (see Supplementary Appendix Table A1 for ICD-9 and ICD-10 codes used to identify pregnant women; See Table A2 for state-specific counts of the study population). This study was approved by the University of Pittsburgh Institutional Review Board.
Outcomes
Opioid Use Disorder in Pregnancy
Opioid use disorder (OUD) diagnosis was identified based on ICD-9-CM diagnosis codes prior to Oct 1, 2015 (304.0x, 205.5x) and ICD-10-CM diagnosis codes (F11.xxx) thereafter. Pregnant women in each year who had at least one encounter in an inpatient or outpatient setting with a diagnosis of OUD in any field were defined as having OUD.
Medication for Opioid Use Disorder (MOUD) in Pregnancy and Continuity of MOUD
The utilization of MOUD (either methadone or buprenorphine) in pregnancy was measured using pharmacy records and procedure codes. Naltrexone is not indicated for use in pregnancy, and was not included. First, we considered women to have any MOUD use if they had at least one pharmacy record with a National Drug Code (NDC) for buprenorphine or buprenorphine/naloxone (full list of NDC codes not shown; available from the authors upon request). Second, we considered women to have any MOUD use if they had a record in any care setting that included a HCPCS code for any of the following OUD medications: oral buprenorphine or buprenorphine/naloxone administration (J0571, J0572, J0573, J0574, J0575) or methadone administration (H0020).
Among women with OUD in pregnancy who initiated MOUD in pregnancy, we calculated the continuity of pharmacotherapy for 90 days or 180 days, the latter being a National Quality Forum measure.20 The continuity of pharmacotherapy measure was calculated among those who had any MOUD in pregnancy and who were continuously enrolled in Medicaid for at least 6 months after the first encounter for MOUD. The continuity of pharmacotherapy measures were calculated based on the number of days’ supply of buprenorphine prescriptions or number of days methadone was dispensed, relative to the length of enrollment (90 days or 180 days). Pregnant women were considered to have continuity of pharmacotherapy if they had continuous treatment with buprenorphine or methadone for either 90 or 180 days, respectively, with gaps in treatment <=7 days. (See Supplementary Appendix Table A3 for more detailed information about outcome measure specifications.)
Neonatal Opioid Withdrawal Syndrome (NOWS)
We constructed 2 measure specifications to examine prevalence of NOWS, including diagnoses that occurred in both inpatient and outpatient settings (See Supplementary Appendix Tables A3 and A4 for detailed measurement specifications). Multiple measures were created to account for the mixed reliability and validity of diagnosis codes for NOWS.21,22 First, we constructed a standard definition of NOWS including diagnosis codes indicating neonatal withdrawal symptoms in the first 7 days of life based on prior studies using hospital discharge data.23 We examined diagnosis rates in the first 7 days of life because clinical guidelines recommend that neonates with known in-utero exposure to opioids be monitored for withdrawal symptoms for 7 days.2 Second, because we were able to follow children over time and observe outpatient care after the birth hospital stay, we also examined diagnosis rates in the first 12 months of life. This second measure allowed us to determine prevalence of NOWS among children who were not screened or diagnosed at birth. For both measures, we followed previous methods to exclude iatrogenic cases,23 i.e., withdrawal symptoms caused by in-hospital administration of opioids. Both measures were calculated as annual rates per 1,000 live births within each state.
Well-child visit utilization
We calculated the count of well-child visit encounters in the first 15 months of life, and the percentage of children with NOWS or opioid exposure who had the recommended number (≥6) of well-child visits (see Appendix Table A3 for detailed measure specifications).24
Data Analysis
We first calculated time trends of pregnancy, neonatal, and child healthcare outcomes pooling numerator and denominator data from all states. Next, we calculated state-specific time trends of the percentage of women who receive any MOUD in pregnancy, the percentage of women with 90- and 180-day continuity of pharmacotherapy in MOUD, the rate of NOWs and/or in-utero illicit substance exposure identified in neonates, and the distribution of well-child visit counts in the first 15 months among children identified with NOWS or opioid exposure. Because data include a census of all Medicaid enrollees in the participating 8 states, and not a sample of enrollees, we do not provide tests of statistical significance for differences between states or over time.
RESULTS
The rate of OUD diagnosis in pregnancy in all 9 state Medicaid programs increased from 49.6 per 1,000 to 54.1 per 1,000 pregnancies from 2014 to 2017, and the percentage of pregnant women receiving any MOUD increased from 53.4% to 57.9% (Table 1). The rate of neonates diagnosed with NOWS increased from 32.7 per 1,000 to 37.0 per 1,000. The percentage of children identified with NOWS who received the recommended number of well-child visits in the first 15 months increased over time, from 47.2% to 51.5%.
Table 1.
Time trends in pregnancy, neonatal, and child outcomes among women and children affected by OUD, 9 state Medicaid programs, 2014–2017
| 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|
| OUD Prevalence and Treatment in Pregnancy a | ||||
| Pregnancies with OUD diagnosis, N | 17,355 | 20,318 | 26,318 | 24,936 |
| OUD diagnosis per 1,000 pregnancies | 49.6 | 55.8 | 61.4 | 54.1 |
| MOUD in pregnancy among women with OUD, N | 9,264 | 11,054 | 14,142 | 14,432 |
| Any MOUD in pregnancy among women with OUD,d % | 53.4 | 54.4 | 53.7 | 57.9 |
| Neonatal Opioid Withdrawal Syndrome (NOWS) b | ||||
| NOWS cases, N | 10.296 | 11,283 | 11,901 | 11,403 |
| NOWS per 1000 live births | 32.7 | 35.1 | 36.8 | 37.0 |
| Well-Child Visits c | ||||
| 0–1 well child visits in first 15 months, % | -- | 6.7 | 6.2 | 5.8 |
| 2–3 well-child visits in first 15 months, % | -- | 15.1 | 14.6 | 14.4 |
| 4–5 well-child visits in first 15 months, % | -- | 31.0 | 30.3 | 28.4 |
| ≥6 well child visits in first 15 months,e % | -- | 47.2 | 48.9 | 51.5 |
N=1,602,978 pregnancies; n=88,927 pregnancies with OUD diagnosis; n=48,892 pregnancies where any treatment with methadone or buprenorphine was observed
N=1,267,961 live births; n=44,883 neonates with NOWS diagnosis in the first 12 months
N=22,913 children with NOWS diagnosis who were continuously enrolled in Medicaid from birth through age 15 months
Medication for opioid use disorder (MOUD) includes any methadone or buprenorphine utilization in pregnancy
Recommended number of well-child visits according to Medicaid Core Quality Metrics
Figure 1 shows state- and year-specific percentages of pregnant women with OUD who received MOUD. Percentages ranged from 37.7% to 66.0%. Among most states, the percentage of pregnant women with OUD who received MOUD increased from 2016 to 2017. The change from 2016 to 2017 ranged from a 4 percentage-point increase (States D and E) to a 14 percentage-point increase (State G).
Figure 1. Percentage of pregnant women who received any medication for opioid use disorder, 9 state Medicaid programs, 2014–2017.
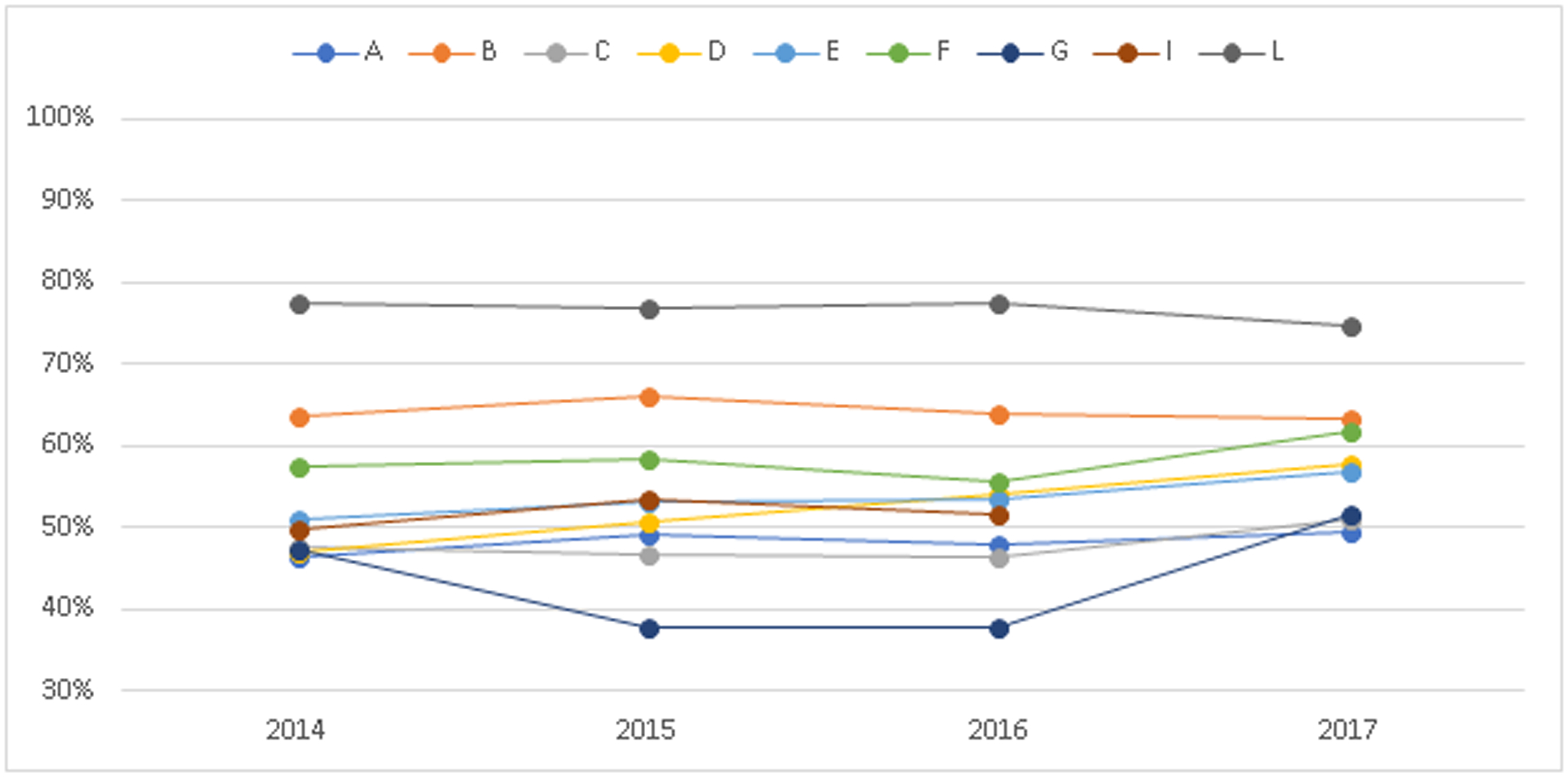
Includes pregnancies in which a diagnosis of OUD was recorded (N=88,927). For all states except A, medication treatment was defined as having any use of buprenorphine or methadone during the pregnancy. For state A, medication treatment was defined as having any use of buprenorphine during the pregnancy.
Figure 2 presents 90- and 180-day continuity of pharmacotherapy among women who initiated MOUD treatment in pregnancy, pooled across all study years (2014–2017). State-specific percentages of 90-day continuity of pharmacotherapy ranged from 69.8% to 93.3%. State-specific percentages of 180-day continuity of pharmacotherapy among women who initiated MOUD treatment in pregnancy ranged from 40.9% to 84.5%. In all states, the percentage of pregnant women with 180-day continuity of MOUD was lower than the percentage of pregnant women with 90-day continuity of MOUD.
Figure 2. Continuity of pharmacotherapy for pregnant women with OUD who initiate medication treatment in 9 state Medicaid program.
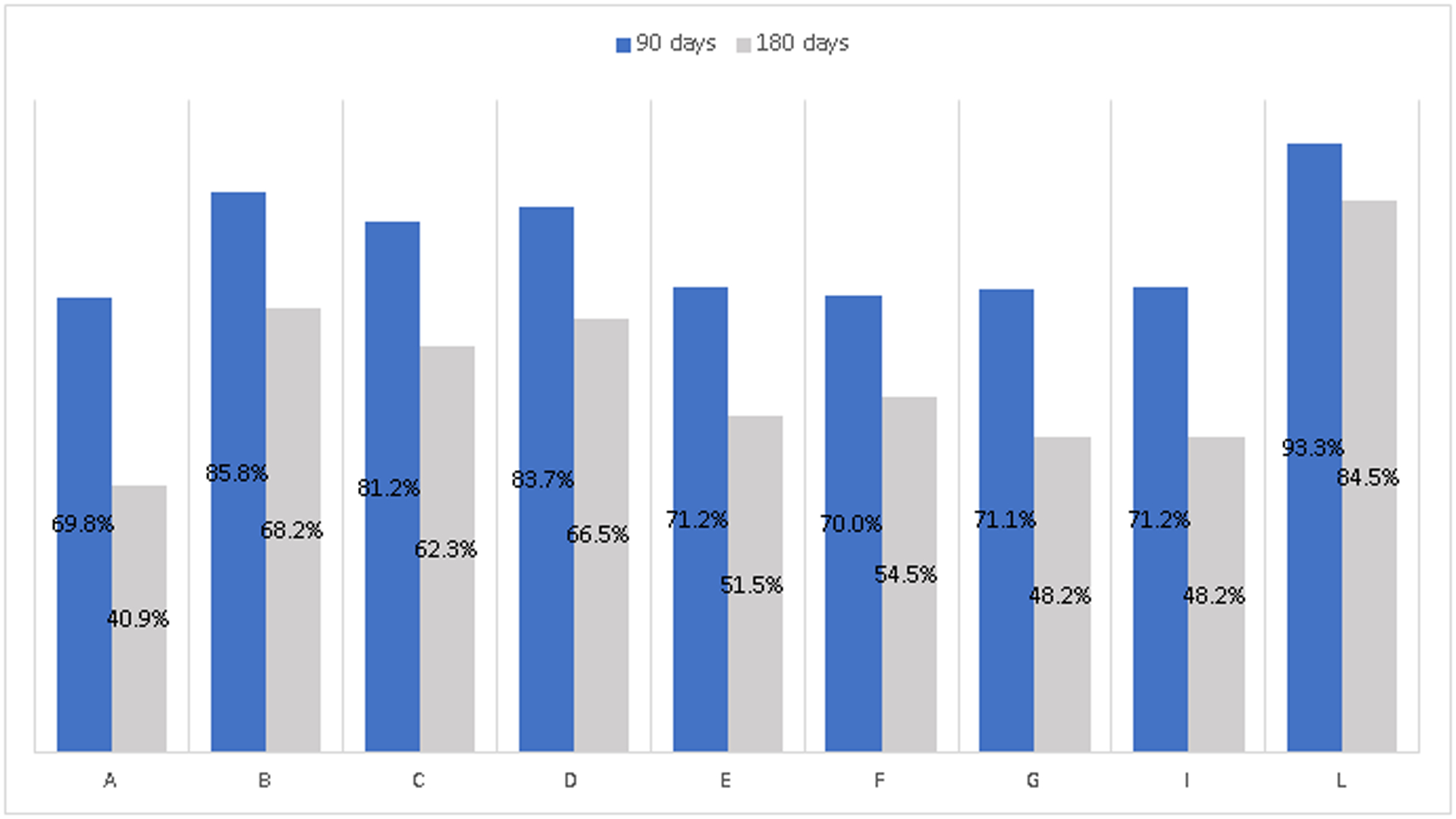
Includes pregnancies in which pharmacotherapy with methadone or buprenorphine for OUD treatment was initiated (N=48,892). For all states except A, M OUD was defined as having any use of buprenorphine or methadone during the pregnancy. For state A, MOUD was defined as having any use of buprenorphine during the pregnancy. Data shown are an average across years 2014–2017.
State- and year-specific time trends of NOWS among children by 7 days and 12 months of age are shown in Figure 3. For each state, 2 time trend lines are shown: the rate of NOWS per 1,000 live births by age 7 days and 12 months of age. In 2017, NOWS rates within the first 7 days ranged from 18 to 79 cases per 1,000 live births across states. Six states had consistent or slightly increasing prevalences over time, while 2 states (D and I) showed larger relative temporal increases of an average of 40% and 27%, respectively, in the rates of NOWS diagnosed in the first 7 days. Allowing a 12-month diagnostic time resulted in higher prevalence estimates in all states; ranging from a 9% to a 24% relative increase in prevalence estimates of NOWS.
Figure 3. Rates of Neonatal Opioid Withdrawal Syndrome (NOWS) diagnosis among children by 7 days and 12 months of age, 9 state Medicaid programs, 2014–2017.
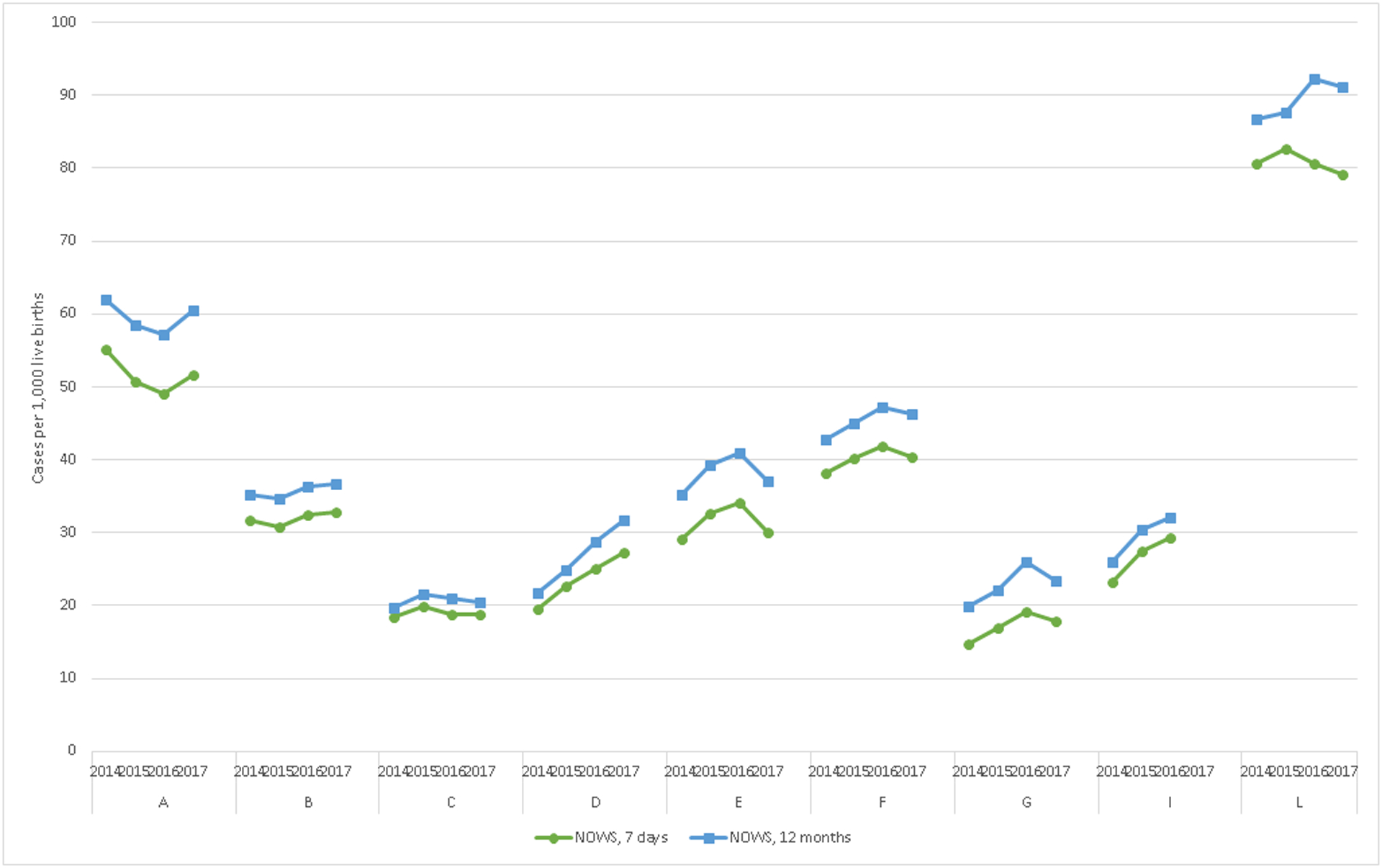
Includes live births (N=1,267,961). NOWS defined as having a diagnosis indicating withdrawal symptoms related to in-utero opioid exposure, exclusive of iatrogenic withdrawal cases.
Among children identified with NOWS within the first 12 months, state-specific percentages of those receiving the recommended ≥6 well-child visits in the first 15 months of life ranged from 39.3% to 62.5% (Figure 4). The percentage of children with 2–5 well-child visits in the first 15 months ranged from 30.3% to 50.9%, and the percentage with 0–1 well-child visits in the first 15 months ranged from approximately 5% to 15%. Increases in the proportion of children receiving the recommended number of visits increased in most states over a relatively short period of time.
Figure 4. Distribution of well-child visits in the first 15 months of live among children who had NOWS diagnosis, 9 state Medicaid programs, 2015–2017.
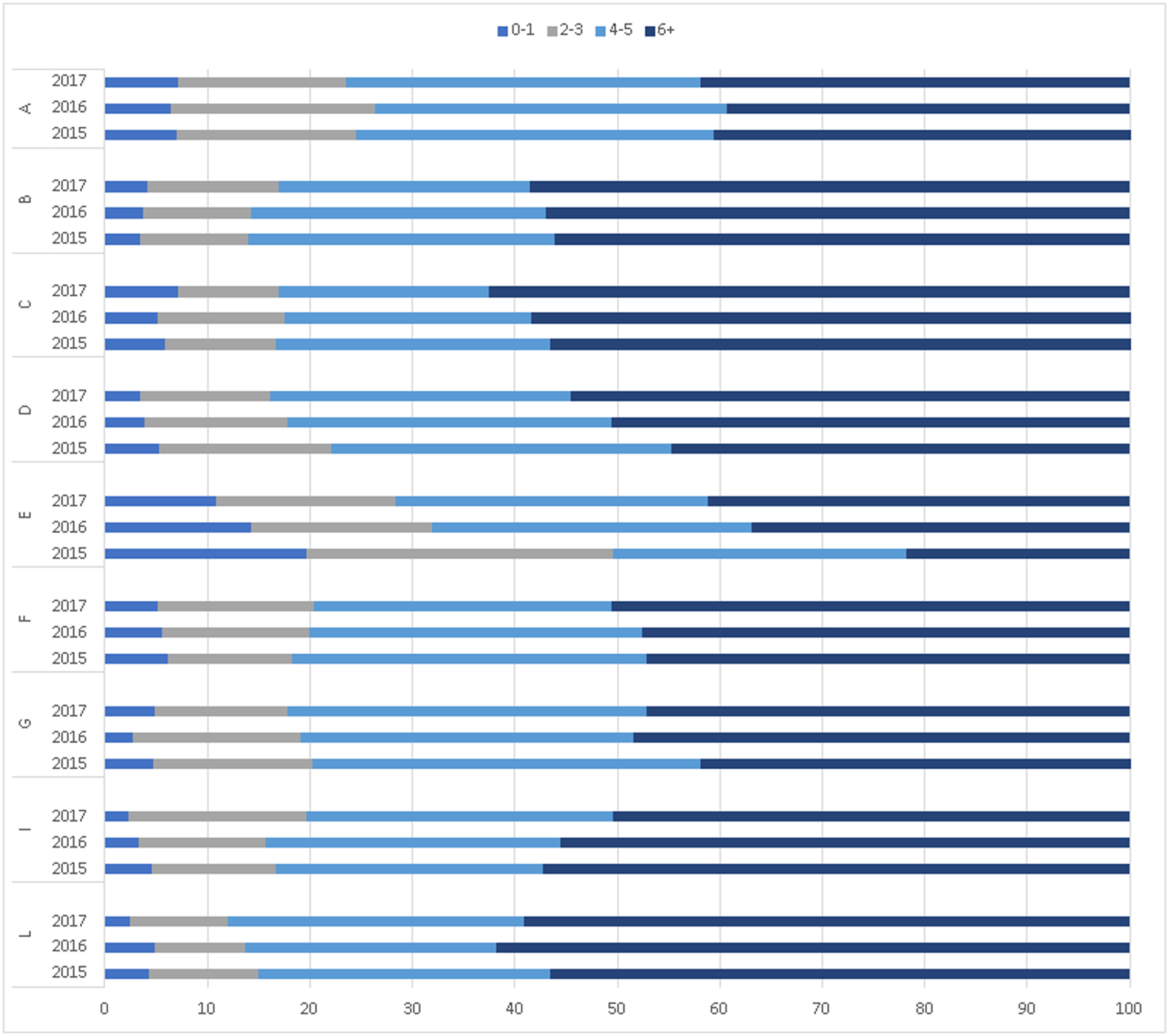
Includes children diagnosed with NOWS by age 12 months and who remained continuously enrolled in Medicaid from birth through age 15 months (N=22,346). Six or more well-child visits in the first 15 months are recommended according to the Medicaid Core Quality Metrics.
DISCUSSION
In this study of 9 state Medicaid populations, the rate of pregnant women diagnosed with OUD increased but MOUD rates among pregnant women with OUD generally remained stable; and rates of identification of NOWS varied across states but well-child visit utilization among these children generally increased over time. These results provide a broad overview of the prevalence of OUD in pregnancy, MOUD treatment, and outcomes among child born to women with OUD in pregnancy in the United States.
Our finding that >5% of pregnant women in Medicaid had an OUD diagnosis is a higher prevalence than has been previously estimated in a different group of states.14 This OUD prevalence is also far higher than reported in national and multi-state surveillance data, which have estimated prevalence of OUD diagnoses rates of 2.5 to 6.5 per 1,000 delivery hospitalizations.17,25–27 There are several explanations for this finding. First, our population includes only Medicaid-enrolled pregnant women. Because Medicaid is a means-tested program for low-income populations, and because of the cyclical relationship between substance use disorders and poverty, Medicaid is the primary insurer for pregnancy care among persons with OUD.28 Second, our data include diagnosis of OUD at any time in pregnancy, not just during the delivery hospital stay. Third, it is likely that the states included in the present study have high rates of OUD prevalence, relative to national estimates, as we include 6 (Maine, Maryland, Kentucky, Ohio, Pennsylvania, West Virginia) of the 10 states with the highest age-adjusted drug overdose mortality rates in the US.29 The increasing diagnosis rate over time could be due to a true increase in OUD prevalence in pregnancy, more comprehensive screening for substance use disorders in pregnancy care, or a combination of both factors.1
Although there is scant evidence on the effects of long-term MOUD on pregnancy outcomes, growing evidence suggests that long-term continuity of MOUD is associated with improved OUD treatment outcomes.30–32 Even with an increase in the proportion of pregnant women diagnosed with OUD, the percent receiving MOUD remained stable from 2014-2-15 or increased in some states from 2016–2017. Among those initiating MOUD in pregnancy, the continuity of pharmacotherapy for 180 days ranged from 41% to 85%. Prior research on the continuity of pharmacotherapy has ranged widely depending on the population under study.33 Recent observational studies among non-pregnant patient populations in the US have found rates of 180-day continuity of MOUD ranging from 42% to 62%.34–36 Although it is encouraging that treatment rates did not decline even as OUD diagnosis rates increased, given that MOUD improves pregnancy and birth outcomes for women with OUD, future work should focus on increasing the proportion of women receiving evidence-based treatment including MOUD.11,37
On average, the prevalence of NOWS diagnoses (at any time in the first 12 months) increased over time, although in 2 states, the prevalence of NOWS slightly declined. These trends, taken together with the increasing prevalence of OUD diagnosis in pregnancy and MOUD utilization in pregnancy, are at odds with a recent study reporting that NOWS cases stopped increasing from 2014–2016.38 Since that study did not stratify by Medicaid insurance status, it is possible that trends in NOWS diagnosis have decreased over time for privately insured neonates and increased for those covered in Medicaid. It is also possible that the states included in this study have different temporal trends in NOWS diagnosis compared to the national hospital discharge sample used for the prior study.
There were differences in the prevalence of NOWS according to the timeframe for diagnosis. A substantial number of NOWS diagnoses occurred after the first 7 days of life, a pattern that occurred uniformly across 9 states with different prevelances of NOWS diagnoses. Typically, withdrawal symptoms in neonates occur 2–6 days after birth, and neonates with known in-utero opioid exposure should be monitored for symptoms for 7 days.39 This study is the first to investigate the timing of NOWS diagnosis in large administrative data. Although Medicaid healthcare data do not permit us to study the reasons for identification of NOWS after the first 7 days of life, the results indicate that 10% to 20% of cases are missed during the birth hospitalization and are identified in follow-up care. This result should be interpreted in the context of the difficulty of accurately identifying NOWS cases in administrative data, due to the nature of NOWS. NOWS is a collection of symptoms caused primarily, but not exclusively, by in-utero opioid exposure, and healthcare systems employ a variety of screening tools and care management models related to NOWS.40 Ultimately, findings point to the importance of considering multiple measure specifications for public health surveillance and intervention activities related to NOWS and in-utero opioid exposure. This is also an important consideration for follow-up care for children, as parental OUD is a chronic condition that can persist in children’s lives well beyond birth.9,41
In 2017, slightly more than half of all children with NOWS or opioid exposure had the recommended number of well-child visits in the first 15 months of life, which is somewhat lower than the percentage among all-Medicaid enrolled children. Among Medicaid-enrolled children in the US, a median of 62% achieve the recommended number of well-child visits in the first 15 months.24 In a 3-year time period, this percentage increased substantially, whereas the percentage of children with 0 or 1 well-child visit declined. Well-child visits are an important component of follow-up care for children affected by OUD, as they provide opportunities for preventive care and developmental screenings.2
Limitations
This study has limitations. First, we relied on administrative healthcare data from state Medicaid programs, meaning that we cannot observe OUD diagnoses or treatment that individuals may receive that is not paid for by Medicaid. If pregnant women with OUD are receiving buprenorphine or methadone treatment separately from Medicaid benefits, for example, our results would underestimate treatment rates. Second, rates of NOWS are based on diagnoses and are not biochemically validated, meaning that rates may be an underestimate. However, there is not a gold standard test for substance exposure in neonates,42 and given that neonates are not universally tested for substances, administrative data are commonly used. Third, we were not able to link data on maternal-child dyads, so we are unable to assess the extent to which maternal MOUD is associated with outcomes among children. Linking maternal-child dyad records is a priority for future studies, however.
Conclusion
Drug overdose deaths continue to increase exponentially and persist as the leading cause of death among persons of reproductive age in the US.43 In the present study of 9 state Medicaid populations, about 5% of pregnant women were diagnosed with OUD in pregnancy, and of those, slightly more than half received any MOUD in pregnancy. Nearly 4% of neonates were diagnosed with NOWS, and of those, approximately half received recommended well-child visits in the first 15 months. Findings demonstrate the value of multi-state Medicaid data, which allow for longitudinal surveillance of care across different settings, to monitor substance use disorders, and related treatment and health outcomes, for pregnant persons and children affected by OUD.
Supplementary Material
Acknowledgement:
We gratefully acknowledge support from the National Institute on Drug Abuse (NIDA) under award number R01DA048029 and the State of Maine, Department of Health and Human Services, agreement number CA-MC-20-100. The funders had no role in the study design, data analysis, or decision to publish the manuscript.
Footnotes
Conflicts of Interest: None.
REFERENCES
- 1.Krans EE, Cochran G, Bogen DL. Caring for Opioid-dependent Pregnant Women: Prenatal and Postpartum Care Considerations. Clinical obstetrics and gynecology. 2015;58(2):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. . HHS Publication No. (SMA) 18–5054. Rockville, MD: 2018. [Google Scholar]
- 3.ACOG Committee on Health Care for Underserved Women and American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstetrics and gynecology. 2012;119(5):1070–1076. [DOI] [PubMed] [Google Scholar]
- 4.Saunders JB, Jarlenski MP, Levy R, Kozhimannil KB. Federal and State Policy Efforts to Address Maternal Opioid Misuse: Gaps and Challenges. Womens Health Issues. 2018;28(2):130–136. [DOI] [PubMed] [Google Scholar]
- 5.Winkelman TNA, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and Costs of Neonatal Abstinence Syndrome Among Infants With Medicaid: 2004–2014. Pediatrics. 2018;141(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2016. Natl Vital Stat Rep. 2018;67(1):1–55. [PubMed] [Google Scholar]
- 7.Admon LK, Bart G, Kozhimannil KB, Richardson CR, Dalton VK, Winkelman TNA. Amphetamine- and Opioid-Affected Births: Incidence, Outcomes, and Costs, United States, 2004–2015. American journal of public health. 2018:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Journal of addiction medicine. 2015;9(5):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaltenbach K, O’Grady KE, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: Early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaman SL, Isaacs K, Leopold A, et al. Treating Women Who Are Pregnant and Parenting for Opioid Use Disorder and the Concurrent Care of Their Infants and Children: Literature Review to Support National Guidance. Journal of addiction medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. The New England journal of medicine. 2010;363(24):2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction. 2012;107Suppl 1:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krans EE, Kim JY, James AE 3rd, Kelley D, Jarlenski MP. Medication-Assisted Treatment Use Among Pregnant Women With Opioid Use Disorder. Obstetrics and gynecology. 2019;133(5):943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemans-Cope L, Lynch V, Howell E, et al. Pregnant women with opioid use disorder and their infants in three state Medicaid programs in 2013–2016. Drug Alcohol Depend. 2019;195:156–163. [DOI] [PubMed] [Google Scholar]
- 15.Jarlenski MP, Krans EE, Kim JY, et al. Five-Year Outcomes Among Medicaid-Enrolled Children With In Utero Opioid Exposure. Health affairs. 2020;39(2):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiff DM, Nielsen T, Terplan M, et al. Fatal and Nonfatal Overdose Among Pregnant and Postpartum Women in Massachusetts. Obstetrics and gynecology. 2018;132(2):466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid Use Disorder Documented at Delivery Hospitalization - United States, 1999–2014. MMWR Morb Mortal Wkly Rep. 2018;67(31):845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villapiano NL, Winkelman TN, Kozhimannil KB, Davis MM, Patrick SW. Rural and Urban Differences in Neonatal Abstinence Syndrome and Maternal Opioid Use, 2004 to 2013. JAMA pediatrics. 2017;171(2):194–196. [DOI] [PubMed] [Google Scholar]
- 19.Adams L, Kennedy S, Allen L, et al. Innovative Solutions for State Medicaid Programs to Leverage Their Data, Build Their Analytic Capacity, and Create Evidence-Based Policy. EGEMS (Wash DC). 2019;7(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Center for Medicare and Medicaid Services. Quality ID #468 (NQF 3175): Continuity of Pharmacotherapy for Opioid Use Disorder (OUD). https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-Measures/2019_Measure_468_MIPSCQM.pdf. Published 2019. AccessedSept 14, 2019.
- 21.Lind JN, Ailes EC, Alter CC, et al. Leveraging Existing Birth Defects Surveillance Infrastructure to Build Neonatal Abstinence Syndrome Surveillance Systems - Illinois, New Mexico, and Vermont, 2015–2016. MMWR Morb Mortal Wkly Rep. 2019;68(7):177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yam P, Lok L, Eastwood J, et al. Validation of hospital discharge coding for neonatal abstinence syndrome. Acta Paediatr. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of Neonatal Abstinence Syndrome - 28 States, 1999–2013. MMWR Morb Mortal Wkly Rep. 2016;65(31):799–802. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare and Medicaid Services. Well-Child Visits in the First 15 Months of Life. https://www.medicaid.gov/state-overviews/scorecard/well-child-visits-first-15-months-of-life/index.html. Published 2020. AccessedMarch 28, 2020.
- 25.Jarlenski M, Krans EE, Chen Q, et al. Substance use disorders and risk of severe maternal morbidity in the United States. Drug Alcohol Depend. 2020;216:108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salemi JL, Raza SA, Modak S, Fields-Gilmore JAR, Mejia de Grubb MC, Zoorob RJ. The association between use of opiates, cocaine, and amphetamines during pregnancy and maternal postpartum readmission in the United States: A retrospective analysis of the Nationwide Readmissions Database. Drug Alcohol Depend. 2020;210:107963. [DOI] [PubMed] [Google Scholar]
- 27.Salihu HM, Mogos MF, Salinas-Miranda AA, Salemi JL, Whiteman VE. National trends in maternal use of opioid drugs among pregnancy-related hospitalizations in the United States, 1998 to 2009. Am J Perinatol. 2015;32(3):289–298. [DOI] [PubMed] [Google Scholar]
- 28.Jarlenski MP, Paul NC, Krans EE. Polysubstance Use Among Pregnant Women With Opioid Use Disorder in the United States, 2007–2016. Obstetrics and gynecology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Drug Overdose Death Data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Published 2016. AccessedMay 5, 2018.
- 30.Samples H, Williams AR, Crystal S, Olfson M. Impact Of Long-Term Buprenorphine Treatment On Adverse Health Care Outcomes In Medicaid. Health affairs. 2020;39(5):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo-Ciganic WH, Gellad WF, Gordon AJ, et al. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction. 2016;111(5):892–902. [DOI] [PubMed] [Google Scholar]
- 32.Lo-Ciganic WH, Donohue JM, Kim JY, et al. Adherence trajectories of buprenorphine therapy among pregnant women in a large state Medicaid program in the United States. Pharmacoepidemiol Drug Saf. 2019;28(1):80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis. 2016;35(1):22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coviello DM, Cornish JW, Lynch KG, et al. A multisite pilot study of extended-release injectable naltrexone treatment for previously opioid-dependent parolees and probationers. Subst Abus. 2012;33(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gryczynski J, Mitchell SG, Jaffe JH, O’Grady KE, Olsen YK, Schwartz RP. Leaving buprenorphine treatment: patients’ reasons for cessation of care. J Subst Abuse Treat. 2014;46(3):356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1–2):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terplan M, Laird HJ, Hand DJ, et al. Opioid Detoxification During Pregnancy: A Systematic Review. Obstetrics and gynecology. 2018;131(5):803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leech AA, Cooper WO, McNeer E, Scott TA, Patrick SW. Neonatal Abstinence Syndrome In The United States, 2004–16. Health affairs. 2020;39(5):764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko JY, Wolicki S, Barfield WD, et al. CDC Grand Rounds: Public Health Strategies to Prevent Neonatal Abstinence Syndrome. MMWR Morb Mortal Wkly Rep. 2017;66(9):242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiff DM, Grossman MR. Beyond the Finnegan scoring system: Novel assessment and diagnostic techniques for the opioid-exposed infant. Semin Fetal Neonatal Med. 2019;24(2):115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones HE, Kaltenbach K, Benjamin T, Wachman EM, O’Grady KE. Prenatal Opioid Exposure, Neonatal Abstinence Syndrome/Neonatal Opioid Withdrawal Syndrome, and Later Child Development Research: Shortcomings and Solutions. Journal of addiction medicine. 2018. [DOI] [PubMed] [Google Scholar]
- 42.Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131(3):e1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heron M. Deaths: Leading Causes for 2017. Natl Vital Stat Rep. 2019;68(6):1–77. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


