Abstract
Background and aims:
Pouchitis is a common complication of ileal pouch-anal anastomosis (IPAA) in patients with ulcerative colitis (UC) who have had colectomy. Pouchitis has been considered a single entity despite a broad array of clinical and endoscopic patterns. We developed a novel classification system based on the pattern of inflammation observed in pouches and evaluated the contributing factors and prognosis of each phenotype.
Methods:
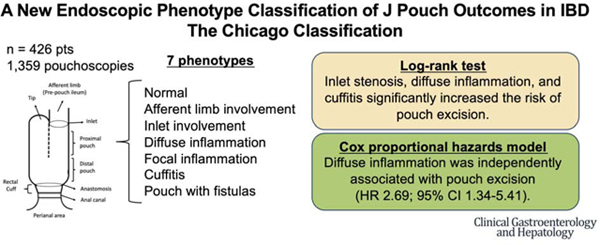
We identified 426 patients (384 with UC) treated with proctocolectomy and IPAA who subsequently underwent pouchoscopies at the University of Chicago between June 1997 and December 2019. We retrospectively reviewed 1,359 pouchoscopies and classified into 7 main pouch phenotypes: (1) normal, (2) afferent limb involvement (AL), (3) inlet involvement, (4) diffuse, (5) focal inflammation of the pouch body, (6) cuffitis, and (7) pouch with fistulas noted after 6 months from ileostomy takedown. Logistic regression analysis was used to assess factors contributing to each phenotype. Pouch survival was estimated by log-rank test and Cox proportional hazards model.
Results:
Significant contributing factors for AL involvement were BMI ≥25 and hand-sewn anastomosis; for inlet involvement was male gender; for diffuse inflammation were extensive colitis and preoperative use of anti-tumor necrosis factor drugs; for cuffitis were stapled anastomosis and preoperative Clostridioides difficile infection. Inlet stenosis, diffuse inflammation, and cuffitis significantly increased the risk of pouch excision. Diffuse inflammation was independently associated with pouch excision (HR 2.69; 95% CI 1.34–5.41; P = 0.005).
Conclusion:
We describe 7 unique IPAA phenotypes with different contributing factors and outcomes, and propose a new classification system for pouch management and future interventional studies.
Keywords: inflammatory bowel disease, ileal pouch-anal anastomosis, pouchitis, endoscopic phenotype, pouch prognosis
Graphical Abstract
Introduction
Surgical intervention is sometimes required in patients with ulcerative colitis (UC) due to medically refractory disease or neoplasia. Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is the most common procedure performed, and a “J” shaped reservoir is the most common pouch configuration. However, pouchitis can develop in up to 70% of patients after IPAA surgery and significantly impairs quality of life.1, 2
The diagnosis of pouchitis has been based on patient symptoms, endoscopic and histologic findings are often reported based on the pouchitis disease activity index (PDAI).3 However, PDAI has limitations. A recent study demonstrated that mucosal erosions or ulcerations are present in nearly a quarter of asymptomatic patients with a pouch and associated with the subsequent development of acute pouchitis.4 Furthermore, the diagnosis of pouchitis is not binary but should account for the heterogenious nature of clinical and endoscopic presentations. A classification of pouchitis based on the endoscopic presentation and severity is needed in order to provide a better clinical approach for these patients.
Pouch failure defined as requiring pouch excision has been reported in up to 10% of patients.5, 6 Previous studies have evaluated the predictors of pouch failure and reported that Crohn’s disease (CD) of the pouch is a significant risk factor of pouch failure. CD of the pouch can develop in about 10% of patients with preoperative diagnosis of UC.7 This phenotype has been characterized by stricturing or inflammation of the afferent limb (AL) or more proximal small intestine and/or fistulizing disease, although additional genetic or pathophysiologic features clarifying that this is similar to the Crohn’s disease of patients without IPAA have not been well described.8 Thus, there is a major unmet need to develop a more predictive classification system for pouch outcomes. The evaluation of endoscopic findings at each anatomical location of the pouch may provide a predictive value of the endoscopic phenotype of the pouch for pouch outcomes.
This study assessed the endoscopic findings and clinical outcomes of IPAA patients in order to develop a novel and clinically meaningful classification system.
Methods
We performed a retrospective cohort study of inflammatory bowel disease (IBD) patients treated by total proctocolectomy with IPAA in J pouch configuration who subsequently underwent pouchoscopies at the University of Chicago between June 1997 and December 2019. This study was approved by the Institutional Review Board of the University of Chicago (IRB # 16–0061, # 15573A). Our standard operating protocol was described in the Supplementary Methods.
Endoscopic Findings
We characterized the findings based on review of images and reports into endoscopic findings of inflammation that include “erythema/edema”, “erosions/friability”, “ulceration”, “stenosis”, “granularity”, and “loss of vascular pattern” based on the previous endoscopic scoring indices including PDAI3, 9 and a surveillance assessing the reliability of items to assess endoscopic disease activity of pouchitis.10 Features of perianal, anal or perineal disease included anal fissures, fistulas, skin tags or hemorrhoids. The authors (SA, LG and DTR) worked together to develop a consensus approach after reviewing the first 60 reports.
Data Collection
We reviewed all available reports of pouchoscopies after ileostomy takedown and characterized pouch phenotypes based on the endoscopy report and images. If the endoscopic description was not explicit and the findings were not noted, the endoscopy images were used to report the findings. If the endoscopic description and images were not available, we assigned these data as “not available”. We classified the pouches into 7 main pouch phenotypes based on the anatomic location of abnormalities: (a) normal, (b) AL involvement, (c) inlet (IL) involvement, (d) diffuse inflammation of the pouch body, (e) focal inflammation of the pouch body, (f) cuffitis, and (g) J pouch with fistulas (Figure 1). Further details regarding definition of each endoscopic phenotype, data collection, and statistical analysis were provided in the Supplementary Methods.
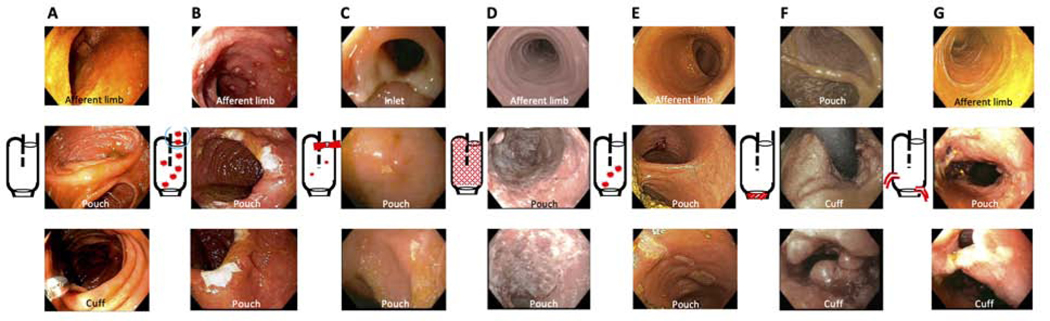
Figure 1.
Endoscopic phenotype of the pouch (The proposed Chicago Classification). (A) normal pouch, (B) afferent limb involvement, (C) inlet involvement, (D) diffuse inflammation of the pouch body, (E) focal inflammation of the pouch body, (F) cuffitis, (G) pouch with fistulas ≥6 months after ileostomy takedown.
Results
Patient background
We identified 426 IBD patients treated by total proctocolectomy and IPAA in J pouch configuration. The demographic characteristics of the patients are summarized in Table 1. Of these patients, 240 were men (56.3%) and 383 Caucasian (89.9%). Included patients had a preoperative diagnosis of UC (384; 90.1%), indeterminate colitis (34; 8.0%), or Crohn’s colitis (7; 1.6%). As for surgical technique, 3-stage IPAA was performed in 224 patients (52.6%) and stapled anastomosis was more frequently performed in 3-stage IPAA (175; 87.5%) compared with 1- or 2-stage IPAA (66; 50.8%) (P <0.001), and hand-sewn anastomosis was more often selected in patients with surgical indication of dysplasia or cancer (29; 78.4%) compared with those with other indications, including medically refractory disease (61; 20.6%) (P <0.001). The majority of patients experienced postoperative complications at stages before ileostomy takedown and it frequently developed within 30 days of each stage (Table S1). Seventeen patients developed fistulas before ileostomy takedown (Table 1) and 12 patients developed fistulas within 6 months after ileostomy takedown.
Table 1.
Demographic characteristics (N = 426)
| Age at diagnosis, n (%) | |
| < 18 years old | 98 (23.0%) |
| ≥ 18 years old | 322 (75.6%) |
| NA | 6 (1.4%) |
|
| |
| Gender, n (%) | |
| Male | 240 (56.3%) |
| Female | 186 (43.7%) |
|
| |
| BMI, n (%) | |
| < 25 | 184 (43.2%) |
| ≥ 25 | 225 (52.8%) |
| NA | 17 (4%) |
|
| |
| Race, n (%) | |
| Caucasian | 383 (89.9%) |
| African American | 16 (3.8%) |
| Asian | 19 (4.5%) |
| American Indian/ Alaska Native | 2 (0.5%) |
| Native Hawaiian or other pacific Islander | 0 (0%) |
| More than one race | 3 (0.7%) |
| Unknown or NA | 3 (0.7%) |
|
| |
| Primary sclerosing cholangitis, n (%) | |
| Yes | 20 (4.7%) |
| No | 406 (95.3%) |
|
| |
| Smoker, n (%) | |
| Current smoker | 14 (3.3%) |
| Past smoker | 109 (25.6%) |
| Non-smoker | 300 (70.4%) |
| NA | 3 (0.7%) |
|
| |
| Family history of IBD, n (%) | |
| Yes | 125 (29.3%) |
| Ulcerative colitis (UC) | 75 (60.0%) |
| Crohn’s disease (CD) | 49 (39.2%) |
| Indeterminate colitis (IC) | 2 (1.6%) |
| Possible inflammatory bowel disease | 11 (8.8%) |
| No | 284 (66.7%) |
| NA | 17 (4.0%) |
|
| |
| Disease duration until surgery, n (%) | |
| < 7 years | 273 (64.1%) |
| ≥ 7 years | 145 (34.0%) |
| NA | 8 (1.9%) |
|
| |
| Diagnosis before colectomy, n (%) | |
| UC | 384 (90.1%) |
| CD | 7 (1.6%) |
| IC | 34 (8.0%) |
| Others | 1 (0.2%) |
|
| |
| Diagnosis after colectomy, n (%) | |
| UC | 279 (65.5%) |
| CD | 133 (31.2%) |
| IC | 10 (2.3%) |
| Others or NA | 4 (0.9%) |
|
| |
| Indication for surgery, n (%) | |
| Medically refractory | 336 (78.9%) |
| Dysplasia/Colorectal Cancer | 47 (11.0%) |
| Fulminant colitis | 55 (12.9%) |
| Toxic megacolon | 9 (2.1%) |
| Others or NA | 36 (8.5%) |
|
| |
| Prior Clostridioides difficile infection, n (%) | |
| Yes | 76 (17.8%) |
| No | 303 (71.1%) |
| NA | 47 (11.0%) |
|
| |
| Disease extent, n (%) | |
| E1: proctitis (proximal extent to the sigmoid colon) | 4 (0.9%) |
| E2: left-sided disease (to the splenic flexure) | 46 (10.8%) |
| E3: extensive disease (beyond the splenic flexure) | 305 (71.6%) |
| NA | 71 (16.7%) |
|
| |
| Surgical procedure | |
| 3-stage ileal pouch-anal anastomosis (IPAA), n (%) | |
| Yes | 224 (52.6%) |
| No | 159 (37.3%) |
| NA | 43 (10.1%) |
| Anastomosis, n (%) | |
| Staple | 246 (57.7%) |
| Hand-sewn | 91 (21.4%) |
| NA | 89 (20.9%) |
| Postoperative complications, n (%) | |
| Yes | 209 (49.1%) |
| Anastomosis leak | 22 (10.5%) |
| Pelvic sepsis | 6 (2.9%) |
| Abdominal abscess requiring drainage | 49 (23.4%) |
| Fistulas or sinus tracts developed before ileostomy takedown | 17 (8.1%) |
| No | 156 (36.6%) |
| NA | 61 (14.3%) |
|
| |
| Preoperative steroid use, n (%) | |
| Yes | 361 (84.7%) |
| No | 34 (8.0%) |
| NA | 31 (7.3%) |
|
| |
| Preoperative anti-tumor necrosis factor alpha (TNFa) drugs, n (%) | |
| Yes | 188 (44.1%) |
| No | 207 (48.6%) |
| NA | 31 (7.3%) |
|
| |
| Preoperative immunomodulators, n (%) | |
| Yes | 231 (54.2%) |
| No | 164 (38.5%) |
| NA | 31 (7.3%) |
|
| |
| Postoperative metronidazole or ciprofloxacin use, n (%) | |
| Yes | 341 (80.0%) |
| No | 78 (18.3%) |
| NA | 7 (1.6%) |
|
| |
| Postoperative steroid use, n (%) | |
| Yes | 114 (26.8%) |
| No | 305 (71.6%) |
| NA | 7 (1.6%) |
|
| |
| Postoperative anti-TNFα drugs, n (%) | |
| Yes | 123 (28.9%) |
| No | 296 (69.5%) |
| NA | 7 (1.6%) |
|
| |
| Postoperative immunomodulators, n (%) | |
| Yes | 95 (22.3%) |
| No | 324 (76.1%) |
| NA | 7 (1.6%) |
|
| |
| Pouch excision, n (%) | |
| Yes | 48 (11.3%) |
| No | 378 (88.7%) |
BMI, body mass index; IBD, inflammatory bowel disease; CDI, Clostridioides difficile infection;
IPAA, ileal pouch-anal anastomosis; TNF, tumor necrosis factor
We evaluated a total of 1,359 pouchoscopies (mean 3.2 pouchoscopies per patient) with a mean follow-up of 10.9 years. The overall rate of pouch failure was 11.3% (48 patients). We identified 24 patients who had the normal pouch and none of these patients experienced pouch excision during the follow-up (mean 7.8 years). Their surgical indications were dysplasia or cancer in 3 (13.0%) and other indications including medically refractory disease in 21 (87.0%). Four patients were excluded from our study due to missing data.
Frequency and contributing factors of each endoscopic pouch phenotype
We found 131 patients (31.0%) with AL involvement and 178 patients (42.2%) with IL involvement (Table S2). Among 336 patients (79.6%) with pouchitis, diffuse and focal inflammation of the pouch body were detected in 118 patients (35.1%) and 218 patients (64.9%), respectively. We identified 192 patients (45.5%) with cuffitis. The rate of cuffitis in patients who had initial surgery in the 2010s was significantly higher than that in patients who had initial surgery in the 2000s or 1990s. Stapled anastomosis or 3-stage IPAA were often indicated in the 2010s compared with the 2000s or 1990s (Table S3). There were significantly fewer patients in the cuffitis phenotype with the indication for surgery of dysplasia or cancer (33.3%) than all other indications, including medically refractory disease (52.3%) (Table S2). Seventy-eight patients (18.5%) developed pouch fistulas ≥6 months after ileostomy takedown. The data regarding fistula type is described in Table S4. As for preoperative treatment, patients with diffuse inflammation and cuffitis were more likely to be treated with anti-tumor necrosis factor (TNF)α drugs. None of the factors included in this part of our study were significantly associated with pouch excision (Table S2).
Based on the result of univariate analysis (Table S2), logistic regression analysis identified significant factors contributing to each endoscopic pouch phenotype, and included body mass index (BMI) ≥25 and hand-sewn anastomosis for AL involvement (Odds ratio (OR) 1.84, 95% confidence interval (CI) 1.11–3.03 and OR 1.96, 95% CI 1.15–3.33, respectively), male gender for IL involvement (OR 1.58, 95% CI 1.05–2.38), disease extent E3 and preoperative anti-TNFα drugs for diffuse inflammation (OR 2.40, 95% CI 1.02–5.66 and OR 1.79, 95% CI 1.08–2.97, respectively), stapled anastomosis and preoperative Clostridioides difficile infection (CDI) for cuffitis (OR 2.59, 95% CI 1.28–5.22 and OR 2.06, 95% CI 1.09–3.89, respectively), and preoperative diagnosis of CD for pouch fistulas (OR 10.4, 95% CI 1.81–60.0). Meanwhile, E3 was negatively associated with focal inflammation (OR 0.52, 95% CI 0.27–0.98). (Table 2).
Table 2.
Contributing factors to each endoscopic phenotype of the J pouch (multivariable analysis)
| Phenotype | N | Variables | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|
| Afferent limb involvement | 131 | BMI ≥ 25 | 1.84 (1.11–3.03) | 0.017 |
| Hand-sewn anastomosis | 1.96 (1.15–3.33) | 0.013 | ||
| Inlet involvement | 178 | Male | 1.58 (1.05–2.38) | 0.028 |
| Age at diagnosis ≥ 18 years old | 0.58 (0.33–1.04) | 0.068 | ||
| Age at colectomy ≥ 18 years old | 0.52 (0.22–1.21) | 0.13 | ||
| Diffuse inflammation of the pouch body | 118 | Disease extent E3 | 2.40 (1.02–5.66) | 0.045 |
| Preoperative anti-TNFα drugs | 1.79 (1.08–2.97) | 0.023 | ||
| Family history of IBD | 0.67 (0.38–1.20) | 0.18 | ||
| Age at diagnosis ≥ 18 years old | 0.62 (0.34–1.12) | 0.11 | ||
| Male | 1.55 (0.92–2.61) | 0.10 | ||
| Focal inflammation of the pouch body | 218 | Disease extent E3 | 0.52 (0.27–0.98) | 0.044 |
| Age at diagnosis ≥ 18 years old | 1.40 (0.82–2.39) | 0.22 | ||
| Preoperative immunomodulators | 1.39 (0.89–2.18) | 0.15 | ||
| Cuffitis | 192 | Stapled anastomosis | 2.59 (1.28–5.22) | 0.008 |
| Preoperative CDI | 2.06 (1.09–3.89) | 0.025 | ||
| 3-stage IPAA | 1.50 (0.83–2.70) | 0.18 | ||
| Dysplasia/Cancer (Indication of surgery) | 0.78 (0.31–1.96) | 0.59 | ||
| Preoperative anti-TNFα drugs | 1.42 (0.81–2.47) | 0.22 | ||
| Caucasian | 0.46 (0.19–1.09) | 0.076 | ||
| Male | 1.51 (0.90–2.51) | 0.12 | ||
| Fistulas | 78 | Preoperative diagnosis of Crohn’s disease | 10.4 (1.81–60.0) | 0.009 |
| Family history of IBD | 1.54 (0.90–2.64) | 0.12 | ||
| Preoperative anti-TNFα drugs | 0.63 (0.37–1.07) | 0.085 |
Prognosis of each endoscopic pouch phenotype
The likelihood of developing pouchitis and each phenotype were described in Figure S2. IL involvement, focal inflammation, and cuffitis had the lowest 20-year event-free pouch survival (EFS) (Figure S2). Their 20-year EFSs further declined after 20 years of ileostomy takedown (Figure S2C, S2E, and S2F).
Postoperative treatments were initiated based on clinical symptoms, inflammatory conditions of the pouch, and physician discretion with the patient. Patients with a normal pouch phenotype were less frequently treated with antibiotics, anti-TNFα drugs, immunomodulators, and steroids compared with all other abnormal endoscopic phenotypes. Most phenotypes, except for focal inflammation, cuffitis, and pouch fistulas, were similarly exposed to anti-TNFα drugs (41.0%−45.1%), immunomodulators (35.0%−35.9%), steroids (37.7%−43.0%), and require diverting loop ileostomy (DLI) for symptoms (9.4%−12.1%) (Table S5). However, patients with pouch fistulas were more frequently treated with anti-TNFα drugs (60.3%), immunomodulators (39.7%), and DLI (27.6%) (Table S5). The rate of pouch removal in each phenotype was summarized in Table S2. Adjusted OR (aOR) for pouch excision of diffuse inflammation were statistically significant (aOR 3.32, 95% CI 1.68–6.56). Meanwhile, aOR of focal inflammation was 0.47 (95% CI 0.24–0.94). Pouch excision was statistically significant in patients with pouch fistulas and IL involvement but became insignificant after the adjustment with postoperative treatments (Table S5).
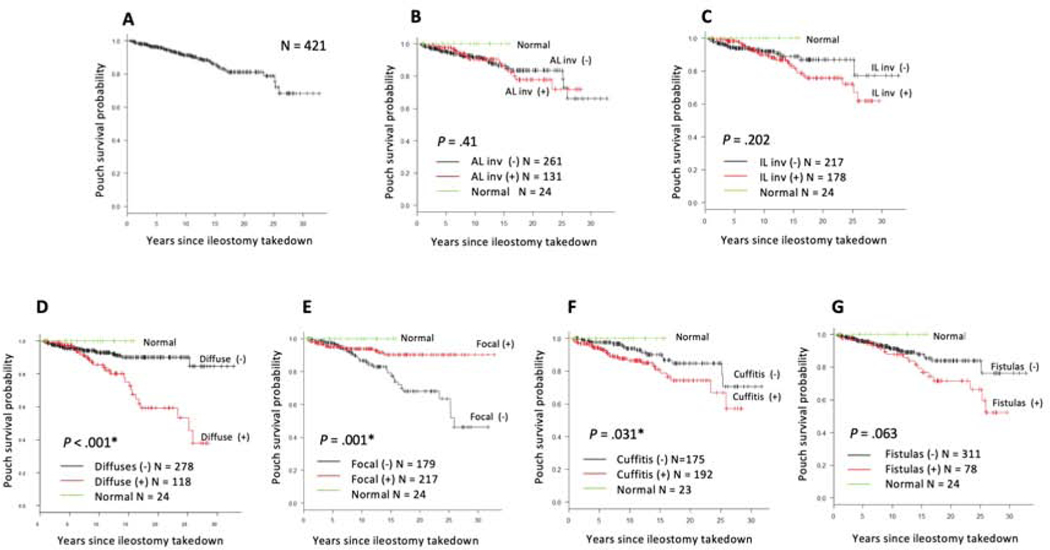
Kaplan-Meier curves for pouch excision demonstrated that the 20-year pouch survival rate for all patients was 81.1% (95% CI 74.4%−86.2%) (Figure 2A). Patients with diffuse inflammation (P < 0.001) and cuffitis (P = 0.031) were at significantly increased risk for pouch excision over time (Figure 2). The likelihood of pouch survival at 20 years after ileostomy takedown was 59.2% (95% CI 43.6%−71.9%) in patients with diffuse inflammation compared with those without diffuse inflammation (90.1%, 95% CI 84.3%−93.8%) (Figure 2D). In contrast, the 20-year pouch survival rate was 90.3% (95% CI 83.6%−94.3%) in patients with focal inflammation compared with those without focal inflammation (68.1%, 95% CI 55.6%−77.7%) (Figure 2E). Among patients without focal inflammation, we identified 118 patients with diffuse inflammation (Figure S3A). We compared the effects of focal and diffuse inflammation on pouch survival and found that the pouch survival rate in diffuse inflammation was significantly lower than that in focal inflammation over time (Figure S3B). Meanwhile, the 20-year pouch survival was 74.2% (95% CI 61.9%−83.1%) in patients with cuffitis compared with those without cuffitis (84.7%, 95% CI 74.4%−91.1%) (Figure 2F).
Figure 2.
Kaplan-Meier curves to evaluate pouch survival for (A) overall population, (B) afferent limb involvement, (C) inlet involvement, (D) diffuse inflammation of the pouch body, (E) focal inflammation of the pouch body, (F) cuffitis, and (G) pouch with fistulas developed ≥6 months after ileostomy takedown.
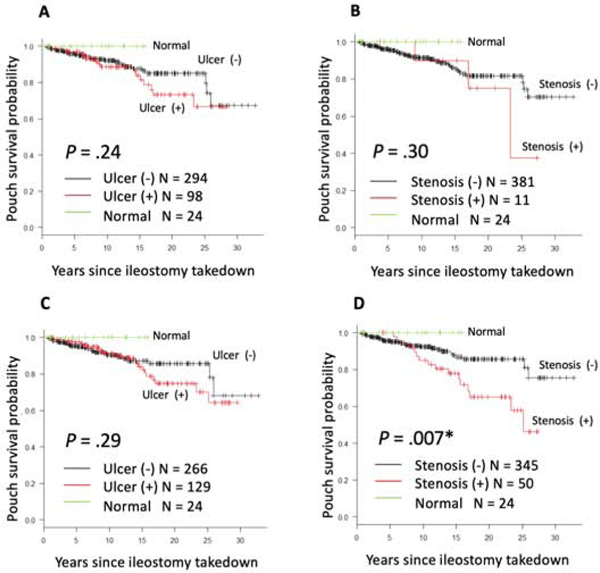
AL and IL involvement were not associated with pouch excision. (Figure 2B, 2C). In the AL involvement, the rate of ulcers and stenosis was 74.8% (98/131) and 8.4% (11/131), respectively. Patients with AL ulcers or stenosis did not show a significant risk of pouch excision compared with those without (Figure 3A, 3B). As for IL involvement, the frequency of ulcers and stenosis was 72.5% (129/178) and 28.1% (50/178), respectively. Although inlet ulcers were not associated with the risk of pouch excision, inlet stenosis significantly increased the risk of pouch excision over time (P = 0.007) (Figure 3C, 3D). The 20-year pouch survival of patients with inlet stenosis was 65.2% (95% CI 47.7%−78.1%) compared with patients without inlet stenosis 85.7% (95% CI 78.8%−90.4%).
Figure 3.
Kaplan-Meier curves to evaluate pouch survival for (A) afferent limb ulcers, (B) afferent limb stenosis, (C) inlet ulcers, and (D) inlet stenosis.
As for pouches with fistulas that occurred ≥6 months after ileostomy takedown, the 20-year pouch survival was 71.5% (95% CI 56.4%−82.2%), which was lower than that of pouch without fistulas (83.9%, 95% CI 76.2%−89.4%), although this was not statistically significant (P = 0.063) (Figure 2G). We also found the 20-year pouch survival of fistulas that developed in the first 6 months after ileostomy takedown was significantly lower than that of fistulas that developed ≥6 months after ileostomy takedown (Figure S4).
We assessed the outcome of J pouches with more than one phenotype with abnormal findings. Among patients with at least one phenotype (N = 306), 110 patients had one phenotype (35.9%), 71 had two phenotypes (23.2%), 67 had three phenotypes (21.9%), and 58 had more than three phenotypes (19.0%). The most common combination of phenotypes was AL involvement and IL involvement (26.8%), followed by cuffitis and IL involvement (23.5%), and diffuse inflammation and IL involvement (22.3%) (Table S6). We found that patients with more than three phenotypes were at significantly increased risk of pouch excision compared with those with three phenotypes or less (P <0.001) (Figure S5). To determine the relationship of phenotypes to time to pouch excision, a Cox proportional hazards model was used. We included AL involvement, inlet stenosis, diffuse inflammation, cuffitis, and pouch fistulas that developed ≥6 months after ileostomy takedown in this analysis and found that diffuse inflammation was independently associated with pouch excision (HR 2.69; 95% CI 1.34–5.41; P = 0.005) (Table 3).
Table 3.
Cox proportional hazards model to determine phenotype associated with pouch excision
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| AL involvement | 0.72 (0.37–1.40) | 0.33 |
| Inlet stenosis | 1.41 (0.70–2.85) | 0.34 |
| Diffuse inflammation | 2.69 (1.34–5.41) | 0.005 |
| Cuffitis | 1.72 (0.87–3.38) | 0.12 |
| Fistulas | 1.53 (0.81–2.91) | 0.19 |
Discussion
The etiology of pouchitis is not known, and while some risk factors for pouchitis have been described,11 it is appreciated that there is significant heterogeneity of pouchitis and limited evaluation to guide therapeutic interventions. In this large study with longitudinal follow-up, we describe unique endoscopic phenotypes and outcomes of patients with the pouch and identified risk factors and predictors of pouch survival. In this proposed “Chicago Classification of Pouchitis,” we found that each phenotype had different contributing factors and corresponding prognosis. Our survival analysis demonstrated that diffuse inflammation, cuffitis, and inlet stenosis were significantly associated with pouch excision. In particular, diffuse inflammation was an independent endoscopic phenotype associated with pouch excision.
Our study is the first to demonstrate that a higher number of distinct anatomic endoscopic findings in the tip of the J, proximal pouch, and distal pouch were associated with a greater likelihood of subsequent pouch excision. We found that one of the pre-operative risk factors of diffuse inflammation was extensive colitis (E3), which has also been previously reported as a risk factors of chronic pouchitis.12, 13 In contrast, E3 was inversely associated with focal inflammation, suggesting that the severity of preoperative disease activity might be correlated with the degree of pouch inflammation. These findings suggest that patients with E3 before surgery might benefit from more intensive follow-up in order to mitigate the risk of pouch loss. Furthermore, we identified that preoperative use of anti-TNFα drugs was associated with a diffuse inflammation. A retrospective case control study at our center found that preoperative exposure to anti-TNFα drugs was an independent risk factor for the development of pouchitis, speculating that anti-TNFα drugs may precondition the pouch to develop pouchitis through alterations in microbiome.14
We found that a stapled anastomosis was significantly associated with cuffitis. Consistent with this finding, the rate of cuffitis was high in the era in which stapled anastomosis or 3-stage IPAA were often indicated. The stapling technique in IPAA normally leaves a 1–2 cm of rectal cuff15 and logically would be associated with a risk of cuffitis if patients had medically refractory proctitis before surgery. Compared to patients with medically refractory disease, our patients with surgical indication of neoplasia were more frequently treated with mucosectomy and hand-sewn anastomosis (no rectal cuff remaining). Therefore, it is expected that patients with indication for neoplasia had a significantly lower rate of cuffitis than those with other indications like medically refractory disease. We may also presume that the preoperative disease severity of patients with neoplasia could be quiescent or milder than that of patients with medically refractory disease, resulting in the lower rate of cuffitis in those with neoplasia. Furthermore, preoperative CDI was also a significant risk factor of cuffitis, which is confirmation of prior work published by our group16 Previous studies demonstrated that preoperative CDI was significantly associated with pouch failure16 and 20.6% of patients with a history of CDI who underwent proctocolectomy with IPAA were found to be positive for Clostridioides difficile toxin in the pouch.17 Therefore, we presumed that if patients were exposed to CDI preoperatively, Clostridioides difficile would continue colonizing in the remnant rectal tissue and may give rise to inflammation and increase the risk of pouch excision. Our analysis suggested that inflammation may develop in the inlet or cuff even after 20 years. While cuffitis may be related to the surgical technique, IL involvement might develop because of the anatomy and a combination of tension and local ischemia in a susceptible IBD patient due to the long duration of the pouch. This hypothesis is indirectly supported by the univariate analysis, which identified that age less than 18 years at diagnosis or colectomy was significantly associated with subcequent IL involvement.
CD of the pouch is the most frequent reason reported by others for pouch failure.7, 8 We avoided such a label of CD of the pouch and approached our analyses based on the endoscopic anatomic phenotypes and the specific outcome of pouch survival. Distinct from other studies, our data showed that AL involvement was not necessarily associated with a risk of pouch removal. Our multivariable analysis identified that BMI ≥25 and hand-sewn anastomosis were significant contributing factors to AL involvement, whereas preoperative diagnosis of CD was not associated with AL involvement. We presumed that this finding might be explained by the alteration of microbiome secondary to obesity18 or by changes in post colectomy pouch colonization due to removal of the diseased rectal cuff that occurs in the hand-sewn technique.15 Meanwhile, patients with pouch fistulas that developed ≥6 months after ileostomy takedown suffered an increasing risk of pouch excision over time. Indeed, a preoperative diagnosis of CD was significantly associated with pouch fistulas, suggesting a common pathophysiology between CD and fistulas in the pouch.
We found that not all patients showed a single phenotype but can develop multiple phenotypes over time. Notably, patients with multiple phenotypes, especially more than three phenotypes with inflammatory conditions, had a significant risk of pouch loss. This finding suggests that the risk of pouch excision can change over time and depend on the number of phenotypes which appear in the endoscopic follow-up. Hence, we believe that continuous pouch monitoring is necessary to stratify the risk of pouch loss based on our classification. Further studies are needed to determine how one phenotype may change over time and to understand whether we can use medical or microbiome-based treatments for such high risk groups to save their pouches.
There are several strengths and limitations to this study. A significant strength is that this study includes a large number of patients with careful and in many cases long-term follow-up at a single tertiary/quaternary center with expertise in medical and surgical approaches to IBD. It is also a strength that we have approached pouchoscopy in a standard manner and re-analyzed pouchoscopy reports in order to develop these phenotypes. However, we acknowledge that it is also a limitation that the study was performed at a single center which might have biased the findings or interpretation. In addition, the retrospective design limited our analysis to those patients who had pouchoscopies, which in many cases were driven by symptomatic, or even antibiotic resistant, patients. Further, although pouchoscopies were frequently performed at our institute based on the standard operating protocol, not all patients underwent a baseline pouchoscopy after their ileostomy takedown. Therefore, the outcomes of each phenotype can be affected by pre- or postoperative variables. However, our analysis did not find any preoperative or demographic factors significantly associated with pouch removal. As for postoperative factors, patients of all phenotypes except for J pouch fistulas and normal pouch were similarly exposed to each of the postoperative treatments. In particular, patients with J pouch fistulas were frequently treated with anti-TNFα drugs and DLI and the OR for pouch excision became insignificant after adjusting for postoperative treatments. This suggests that these treatments might heal fistulas and affect phenotype incidence and pouch outcomes. To validate our findings, prospective and multi-center studies are needed. Furthermore, we used a non-validated definition of pouchitis and did not discriminate between acute and chronic pouchitis. In general, pouchitis is diagnosed based on PDAI, which includes clinical symptoms, endoscopic and histologic findings. However, our study did not assess clinical symptoms and histologic findings. Given that clinical symptoms do not often match endoscopic appearance in practice,4, 19 further assessment of the severity of clinical symptoms according to our classification is warranted. Lastly, we used endoscopic items based on the disease activity indices including PDAI,3, 9 although the impact of each item on pouch outcomes is still unclear. Histological assessment might be helpful to evaluate the influence of each item on pouch outcomes. Further, the possibility of inter- and/or intra-operator biases cannot be excluded as we used endoscopic descriptions by providers to assign endoscopic items.
In conclusion, we describe 7 unique endoscopic phenotypes of IPAA and identified that each phenotype has different contributing factors and outcomes. Our classification suggests that continuous endoscopic assessment of each anatomical location of the J pouch and distribution pattern of inflammation in the pouch body may improve the quality of pouch monitoring and allow for stratification of patients with a high risk of pouch loss. We believe that the Chicago Classification of Pouchitis as proposed in this study may provide a better clinical approach and inform future research and targeted treatments for IBD patients with J pouches.
Supplementary Material
What You Need to Know.
BACKGROUND:
Pouchitis is a common complication of ileal pouch-anal anastomosis (IPAA) after colectomy in ulcerative colitis, but its management is challenging due to heterogeneous inflammatory patterns and inadequate data on long-term outcomes.
FINDINGS:
We classified patients into 7 distinct IPAA endoscopic phenotypes including normal, afferent limb, inlet involvement, diffuse, focal inflammation of the pouch body, cuffitis, and pouch fistulas, and demonstrated differences in contributing factors and pouch outcomes for each phenotype.
IMPLICATIONS FOR PATIENT CARE:
We describe 7 distinct IPAA endoscopic phenotypes and their corresponding contributing factors and outcomes and propose a new classification system for pouch management and future interventional studies.
Acknowledgements
Funding in part provided by NIDDK P30 DK42086, NIDDK RC2 DK122394, and the GI Research Foundation of Chicago
Abbreviations list:
- AL
afferent limb
- BMI
body mass index
- CD
Crohn’s disease
- CDI
Clostridioides difficile infection
- CI
confidence interval
- DLI
diverting loop ileostomy
- EFS
Event-free pouch survival
- HR
hazard ratio
- IBD
inflammatory bowel disease
- IL
inlet
- IPAA
ileal pouch-anal anastomosis
- OR
Odds ratio
- PDAI
pouchitis disease activity index
- PSC
primary sclerosing cholangitis
- TNF
tumor necrosis factor
- UC
ulcerative colitis
Footnotes
DISCLOSURES:
RDC is on the speaker’s bureau from AbbVie and Takeda. He is a consultant/advisor for AbbVie Laboratories, BM/celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, UCB Pharma. He has received clinical trial support/grants from Abbvie, BMS/Celgene, Boehringer Ingelheim, Crohn’s and Colitis Foundation of America, Genentech, Gilead Sciences, Hollister, Medimmune, Mesoblast Ltd., Osiris Therpeutics, Pfizer, Receptos, RedHill Biopharma, Sanofi-Aventis, Schwarz Pharma, Seres Therapeutics, Takeda Pharma, UCB Pharma. His wife is on the board of directors of Aerpio Theraoeutics, Novus Therapeutics, Vital Therapeutics, Inc, and NantKwest.
MAR has served as a consultant for Pfizer.
SRD has served as a consultant for Pfizer and is on the speaker’s bureau for AbbVie.
JP has received grant support from AbbVie and Takeda. He has served as a consultant for Veraste,. CVS Caremark and is on the advisory board for Takeda, Janssen and Pfizer.
EBC is the founder and chief medical officer of AVnovum Therapeutics.
DTR has received grant support from Takeda; and has served as a consultant for Abbvie, Abgenomics, Allergan Inc., Arena Pharmaceuticals, Bellatrix Pharmaceuticals, Boehringer Ingelheim Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Check-cap, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences S.A., InDex Pharmaceuticals, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Materia Prima, Narrow River Mgmt, Pfizer, Prometheus Laboratories,Reistone, Takeda, and Techlab Inc. He is also co-founder of Cornerstones Health, Inc. and GoDuRn, LLC; on the Board of Trustees of the American College of Gastroenterology.
SA, JEO, VR, LRG, YY, CT, JR, KBS, RDH, KU, BDS, NHH and AS have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg 2013;257:679–685. [DOI] [PubMed] [Google Scholar]
- 2.Lightner AL, Mathis KL, Dozois EJ, et al. Results at Up to 30 Years After Ileal Pouch-Anal Anastomosis for Chronic Ulcerative Colitis. Inflamm Bowel Dis 2017;23:781–790. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Tremaine WJ, Batts KP, et al. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc 1994;69:409–415. [DOI] [PubMed] [Google Scholar]
- 4.Kayal M, Plietz M, Radcliffe M, et al. Endoscopic activity in asymptomatic patients with an ileal pouch is associated with an increased risk of pouchitis. Aliment Pharmacol Ther 2019;50:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manilich E, Remzi FH, Fazio VW, et al. Prognostic modeling of preoperative risk factors of pouch failure. Dis Colon Rectum 2012;55:393–399. [DOI] [PubMed] [Google Scholar]
- 6.Tekkis PP, Lovegrove RE, Tilney HS, et al. Long-term failure and function after restorative proctocolectomy - a multi-centre study of patients from the UK National Ileal Pouch Registry. Colorectal Dis 2010;12:433–441. [DOI] [PubMed] [Google Scholar]
- 7.Barnes EL, Kochar B, Jessup HR, et al. The Incidence and Definition of Crohn’s Disease of the Pouch: A Systematic Review and Meta-analysis. Inflamm Bowel Dis 2019;25:1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lightner AL, Pemberton JH, Loftus EJ Jr, Crohn’s Disease of the Ileoanal Pouch. Inflamm Bowel Dis 2016;22:1502–1508. [DOI] [PubMed] [Google Scholar]
- 9.Shen B, Achkar JP, Connor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum 2003;46:748–753. [DOI] [PubMed] [Google Scholar]
- 10.Samaan MA, Shen B, Mosli MH, et al. Reliability among central readers in the evaluation of endoscopic disease activity in pouchitis. Gastrointest Endosc 2018;88:360–369 e362. [DOI] [PubMed] [Google Scholar]
- 11.Hata K, Ishihara S, Nozawa H, et al. Pouchitis after ileal pouch-anal anastomosis in ulcerative colitis: Diagnosis, management, risk factors, and incidence. Dig Endosc 2017;29:26–34. [DOI] [PubMed] [Google Scholar]
- 12.Achkar JP, Al-Haddad M, Lashner B, et al. Differentiating risk factors for acute and chronic pouchitis. Clin Gastroenterol Hepatol 2005;3:60–66. [DOI] [PubMed] [Google Scholar]
- 13.Hashavia E, Dotan I, Rabau M, et al. Risk factors for chronic pouchitis after ileal pouch-anal anastomosis: a prospective cohort study. Colorectal Dis 2012;14:1365–1371. [DOI] [PubMed] [Google Scholar]
- 14.Bertucci Zoccali M, Hyman NH, Skowron KB, et al. Exposure to Anti-tumor Necrosis Factor Medications Increases the Incidence of Pouchitis After Restorative Proctocolectomy in Patients With Ulcerative Colitis. Dis Colon Rectum 2019;62:1344–1351. [DOI] [PubMed] [Google Scholar]
- 15.Trigui A, Frikha F, Rejab H, et al. Ileal pouch-anal anastomosis: Points of controversy. J Visc Surg 2014;151:281–288. [DOI] [PubMed] [Google Scholar]
- 16.Skowron KB, Lapin B, Rubin M, et al. Clostridium Difficile Infection in Ulcerative Colitis: Can Alteration of the Gut-associated Microbiome Contribute to Pouch Failure? Inflamm Bowel Dis 2016;22:902–911. [DOI] [PubMed] [Google Scholar]
- 17.Sun C, Du P, Wu XR, et al. Preoperative Clostridium difficile infection is not associated with an increased risk for the infection in ileal pouch patients. Dig Dis Sci 2014;59:1262–1268. [DOI] [PubMed] [Google Scholar]
- 18.Bouter KE, van Raalte DH, Groen AK, et al. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017;152:1671–1678. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Bassat O, Tyler AD, Xu W, et al. Ileal pouch symptoms do not correlate with inflammation of the pouch. Clin Gastroenterol Hepatol 2014;12:831–837 e832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.