Abstract
The investigational drug CC34 is a cation peptide with multiple bioactivities. Here, we studied the anti-inflammatory effects of CC34 in lipopolysaccharide (LPS)-treated mouse monocyte-macrophage cells (RAW264.7) and in mice with LPS-induced intestinal inflammation. In vitro, CC34 treatment with less than 50 μg/mL for 24 h did not induce cytotoxicity in RAW264.7 cells. Furthermore, CC34 significantly lowered the levels of select inflammatory cytokines, including TNF-α, IL-1β, and IL-6. Intracellular levels of reactive oxygen species (ROS) were lower in RAW264.7 cells treated with CC34 + LPS than in cells treated with LPS alone. Additionally, CC34 treatment suppressed iNOS and COX-2 mRNA levels in LPS-treated cells. We also observed that CC34 exerted anti-inflammatory activity by suppressing the phosphorylation of IKKβ, IκBα, and NF-κB p65 in vitro. Moreover, CC34 downregulated the release of inflammatory cytokines (TNF-α, IL-1β, and IL-6) in the jejunum tissue and serum of LPS-treated mice. We also found that the myeloperoxidase (MPO) levels were decreased, and the pathological damages were effectively abated in the jejunum tissue of CC34 + LPS-treated mice. In summary, we demonstrated that CC34 exerted anti-inflammatory activities, associated with the neutralization of LPS, inhibition of ROS, inhibition the NF-κB signaling pathway, and down-regulating the secretion of inflammatory cytokines. Thus, CC34 may represent an effective therapeutic strategy for intestinal inflammation.
Keywords: Anti-inflammation, Jejunum, Lipopolysaccharide, Macrophage, ROS
Introduction
Antibiotics, biologics, and immunosuppressive agents are conventional clinical treatment options for inflammatory bowel disease (IBD) (Targan 2010). However, despite their initial clinical efficacy, antibiotics have become less acceptable as clinical therapy due to the widespread emergence of antibiotic resistance and the occurrence of antibiotic residues in animal-derived food. Antimicrobial peptides (AMPs) are considered promising therapeutic candidates with bactericidal activity similar to several antibiotics but without their disadvantages (Brady et al. 2019; Thapa et al. 2020). AMPs are cationic polypeptides with antibacterial, antiviral, anti-inflammatory, and antitumor activities. Interestingly, the immunomodulatory function of AMPs has been the subject of studies aimed at developing new anti-inflammatory drugs (Shim et al. 2015). Some AMPs exert an anti-inflammatory effect by functioning as immunomodulatory agents and repairing the intestinal barrier (Han et al. 2015; Yi et al. 2015). However, the in vivo properties of novel AMPs, especially their biological stability in tissues, have to be thoroughly examined before they can replace antibiotics as new drugs.
Intestinal inflammation is substantially increasing in newly industrialized countries, and the highest hospitalization rates have been reported in developed countries (Limketkai 2019). Furthermore, persistent intestinal inflammation increases the risk of developing intestinal cancers (Chang et al. 2018). Cytokines are the mediators that activate inflammatory cell signaling pathways, their production is stimulated by LPS, which is associated with changes in the microbiome and impaired intestinal epithelial structure and function (Gampierakis et al. 2021; Neurath 2014). AMPs can not only inhibit the activities of intracellular signaling pathways, to attenuate the inflammatory response, but also directly interact with LPS to decrease the inflammatory response (Zhang et al. 2019a, b). LPS, as well as some bacteria and viruses, can stimulate intestinal inflammation by excessively expressing numerous inflammatory factors, which represent measurable inflammation status indicators (Han et al. 2015). Specifically, inflammatory diseases are often characterized by the overexpression of certain inflammatory mediators, including NO, TNF-α, IL-1β, and IL-6 that are upregulated by activated NF-κB (Chen et al. 2019). NF-κB is a nuclear transcription factor, which is closely related to the development of inflammatory diseases. ROS play a crucial role in inflammation-related signal transduction and microenvironmental balance (Baek et al. 2020). It can also activate the NF-κB signaling pathway, which leads to the overexpression of inflammation-associated genes, such as iNOS, COX-2, TNF-α, IL-1β, and IL-6, and aggravates the inflammatory response (Ko et al. 2017; Rod-In et al. 2019). Moreover, inflammatory cell infiltration and apoptosis are associated with concentration variations of MPO, which is a biomarker of oxidative damage that catalyzes the release of potent ROS in colitis (Chami et al. 2018; Liu et al. 2019). Furthermore, testing the integrity of the intestinal mucosa is critical for characterizing the status of intestinal inflammation (Glymenaki et al. 2017; Lorenza et al. 2016).
CC34 is a cationic (+ 5.5) AMP that was constructed from two fragments; its N-terminal part was derived from a cecropin B analog, and the C-terminal part was obtained using a Rana peptide. Sun (2009) already demonstrated that CC34 has a high antibacterial activity and a good biological safety profile, but its potential immunomodulatory effect on intestinal inflammation has not been systematically examined. In this study, we focused on investigating the effect of CC34 on LPS-induced inflammation in RAW264.7 cells and mice.
Materials and methods
Peptide synthesis
CC34 (GWLKKIGKKIERVGQHTRDAILPILSLIGGLLGK) was synthesized by Top-peptide Biotechnology Company (Shanghai, China). The peptide purity of 95% was determined by high-performance liquid chromatography, and the molecular mass (MS) was confirmed to be 3709.51 Da.
Chemicals
LPS was purchased from Sigma (San Diego, CA, USA). The Reactive Oxygen Species Assay Kit and the ELISA kits for TNF-α, IL-1β, and IL-6 were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). TRIzol was obtained from Invitrogen (Carlsbad, CA, USA). Fast Start Universal SYBR Green Master Mix was obtained from Roche Applied Science (Basel, Switzerland). All primary antibodies, including anti-IKKβ, anti-phospho-IKKα/β, anti-IκBα, anti-phospho-IκBα, anti-NF-κB p65, anti-phospho-NF-κB p65, and anti-β-actin antibody, were obtained from Cell Signaling Technology (Danvers, MA, USA). Mouse anti-MPO mAb was purchased from Protein Tech Group (Chicago, USA). Fluorescence conjugated secondary antibody and enhanced chemiluminescence (ECL) kit were purchased from the Institute of Biotechnology Beyotime (Shanghai, China). Polyvinylidene difluoride (PVDF) was bought from Merck Millipore (Billerica, USA). Limulus Amebocyte Lysate Assays Kit was bought from Xiamen Bioendo Technology Company (Xiamen, China). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum, and penicillin–streptomycin solution were bought from Hyclone (Logan, Utah, USA).
Cell culture
RAW264.7 cells were maintained by the Harbin University of Commerce (Harbin, China). Cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin solution at 37 °C in a 5% CO2 incubator.
Cell viability
RAW264.7 cells were seeded in a 96-well plate at a density of 5 × 104 cells/mL in 100 µL DMEM for 24 h. For the viability test, the original medium was discarded, and the cells were treated with 0, 40, 50, 60, 70, 80, 90, and 100 µg/mL of CC34 (100 µL/well) for 24 h. Then, aliquots of 10 µL MTT (5 mg/mL) were added to each well, and incubation was continued for 4 h. After aspirating the original culture solution, aliquots of 150 µL DMSO were added to each well, and the plate was placed on a shaker. The absorbance was measured at 490 nm, and the cell viabilities after treatment were calculated relative to the absorbance value of the control.
Measurement of inflammatory cytokines secreted from RAW264.7 cells
RAW264.7 cells were pretreated with different CC34 concentrations (10, 20, and 40 µg/mL) for 1 h, and stimulated with LPS (50 ng/mL) for 24 h. Cells culture supernatants were collected and analyzed for TNF-α, IL-1β, and IL-6 concentrations measured using commercial ELISA kits according to the manufacturer’s instructions.
Measurement of ROS
To measure intracellular ROS, RAW 264.7 cells were initially seeded in a six-well plate at a density of 5 × 104 cells/mL, and then treated with peptides and LPS for 24 h. After the incubation, the cells were washed twice with phosphate-buffered saline (PBS) and incubated with 10 µM DCFH-DA detection reagent for 40 min at 37 °C in the dark. Before the final analysis, the cells were washed three times with PBS. The intracellular ROS value was measured using a flow cytometer at an excitation wavelength of 500 nm and an emission wavelength of 525 nm.
Real-time PCR
Total RNA was isolated from RAW264.7 cells using TRIzol following the manufacturer’s instructions. Then, cDNA was synthesized by reverse transcription using mRNA as the template and amplified using the following thermal cycle conditions: initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s, 57 °C for 30 s, and 72 °C for 45 s. The following primers were used: iNOS, forward primer, 5′-CTCTAGTGAAGCAAAGCCCAAC-3′, and reverse primer, 5′-CCTCACATACTGTGGACGGG-3′; COX-2, forward primer, 5′-CAGCAAATCCTTGCTGTTCC-3′, and reverse primer, 5′-TGGGCAAAGAATGCAAACATC-3′; β-actin, forward primer, 5′-GTGACGTTGACATCCGTAAAGA-3′, and reverse primer, 5′-GCCGGACTCATCGTACTCC-3′. The gene expression was quantified using the 2−△△CT method.
Animal model
5–6-week-old male Kunming mice (originally derived from Swiss mice) were purchased from the Changchun Yisi Experimental Animal Technology Biotechnology Company (Changchun, China). Mice had free access to food and water, and they were maintained in a designated room at 22 ± 2 °C with a humidity of 60 ± 5% under a daily 12-h light/dark cycle.
The mice were divided into four groups of 10 animals in each, all of which received abdominal injections by body weight (BW) for 7 days. The control injection contained normal saline, the CC34 treatment was 9 mg/kg CC34, the LPS treatment was 7.5 mg/kg LPS, and the CC34 + LPS treatment was an injection of 9 mg/kg CC34 as pretreatment followed by an injection of 7.5 mg/kg LPS. LPS and CC34 were dissolved in normal saline. Acute enteritis was induced in mice using the method described by Zhang et al. (2019a, b). The CC34-treated mice received intraperitoneal injections at 7:00 a.m. for 7 days; the mice of the control and LPS groups were treated with an equal volume of sterile saline. On the 7th day, the LPS- treated and CC34 + LPS-treated mice received the first treatment with intraperitoneally injected LPS, which was followed after 1 h by the second treatment with saline or CC34, according to the treatment group; meanwhile, the control and CC34 treatments were injected with an equal volume of saline. 6 h after the last treatment, the mice were euthanized by cervical dislocation, and blood and tissues were collected.
Assessment of disease activity index (DAI)
The DAI of mice was assessed 6 h after the treatment. BW loss, stool consistency, and the presence or absence of fecal blood were measured and recorded (Loher et al. 2004). The DAI represented the sum of the following scores: BW, 0 points for no weight loss, 1 point for 1%–5% BW loss, 2 points for 5%–10% BW loss, 3 points for 10%–15% BW loss, and 4 points for > 15% BW loss; stool consistency, 0 points for normal granular feces, 2 points for pasty and semisolid feces, and 4 points for liquid feces; fecal blood, 0 points for no blood, 2 points for positive fecal occult blood, and 4 points for gross bleeding.
Measurement of mouse inflammatory cytokines by ELISA
Mouse blood samples were collected and centrifuged for 20 min at 3000 × g at 4 °C. The jejunum tissues and PBS (pH 7.4) were mixed at a weight-to-volume ratio of 1 g to 9 mL and homogenized on ice. The fresh homogenates were centrifuged for 20 min at 3000 × g at 4 °C, and the supernatants were collected used to measure the TNF-α, IL-1β, and IL-6 levels. The inflammatory cytokines in the jejunum tissues and serum were analyzed using commercial ELISA kits, according to the manufacturer’s instructions.
Histopathology and immunohistochemistry
All intestinal tissue samples were fixed overnight using 4% paraformaldehyde. Intestinal tissues were embedded in paraffin and cut into sections of 5 µm thickness. Then, the tissues were stained with hematoxylin and eosin (H&E). Villi and crypts were measured using Image-Pro Plus 6.0.
MPO protein expression levels were assessed using an immunohistochemical method. Samples were dewaxed and rehydrated before using the sodium citrate buffer for antigen retrieval. Unspecific binding sites were blocked with 3% w/v BSA for 30 min at 25 °C, and anti-MPO antibodies (1:800) were added for overnight incubation at 4 °C. Then, the secondary antibody (1:50) was added and the incubation was continued for 50 min at 25 °C. Finally, the samples were dehydrated and analyzed using a confocal microscope. Immunohistochemical data were processed using Image-Pro Plus 6.0.
Western blotting
The protein in the cells and tissue samples was extracted using the whole protein extraction kit. The total protein concentration was measured using the BCA kit. Samples with normalized protein content were separated by SDS–PAGE (using 10% PAA gels) and then transferred to PVDF membranes. The membranes were blocked with 5% skimmed milk for 1 h at 25 °C, and incubated with the primary antibodies overnight at 4 °C, which was followed by incubation with HRP-conjugated secondary antibodies. The blots were detected using an ECL detection kit, and the signal intensity was quantified using a gel imaging system (Bio-Rad, GelDoc XR, USA).
Neutralization of LPS
The neutralization of LPS by the CC34 was assessed using a Limulus Amebocyte Lysate (LAL) Assays Kit. Experiments were according to the manufacturer’s instructions. LPS at a final concentration of 1.0 EU/mL was incubated with multiple concentrations of CC34 (5, 10, 20, 40, 80, and 160 μg/mL) at 37 °C for 15 min, then the mixtures were incubated with LAL assay reagent at 37 °C. After incubation for 6 min the absorbances were measured at 405 nm.
Statistical analysis
The data of all experiments were expressed as the mean ± SD, and data were compared by one-way analysis of variance (ANOVA) using IBM SPSS Statistics 19.0 software. Differences associated with P values of less than 0.05 were considered statistically significant.
Results
Effect of CC34 on RAW264.7 cell viability
The effect of CC34 on RAW264.7 cell viability was assessed using the MTT assay. The cell viability rate was decreased at CC34 concentrations of 50 µg/mL and higher (Fig. 1). The treatment of cells with less than 50 μg/mL CC34 did not induce toxicity.
Fig. 1.
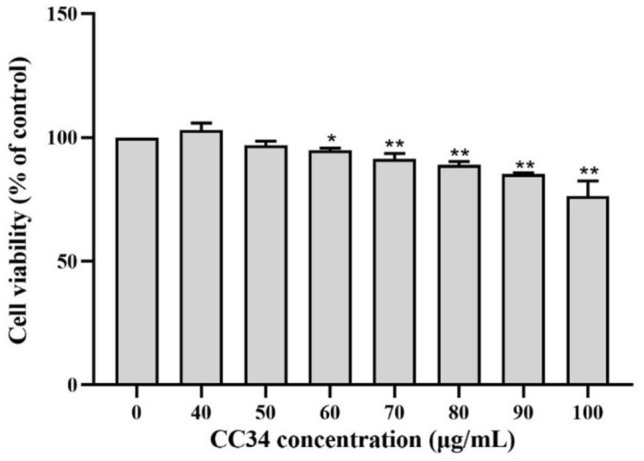
Effect of CC34 on RAW264.7 cell viability. Data are presented as mean ± SD (n = 9). The differences between the groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01
Effect of CC34 on inflammatory cytokine levels in LPS-treated RAW264.7 cells
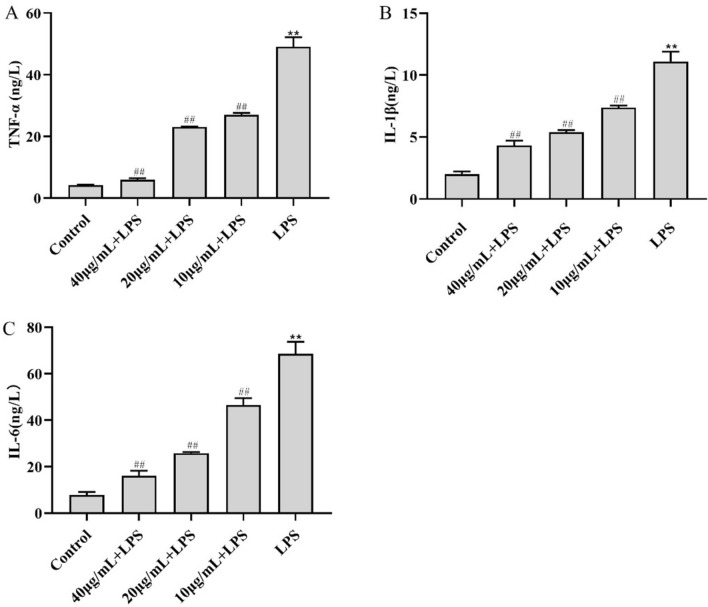
The ELISA-based analysis examined the secreted amounts of select inflammatory cytokines. We found that LPS-treated RAW264.7 cells had significantly higher levels of secreted TNF-α (49.07 ng/L), IL-1β (11.09 ng/L), and IL-6 (68.61 ng/L) than the control cells (P < 0.01). However, the levels of TNF-α, IL-1β, and IL-6 were significantly downregulated in cells treated with 10 μg/mL CC34 + LPS compared with those levels in cells treated with LPS alone (P < 0.01) (Fig. 2).
Fig. 2.
Effects of CC34 on inflammatory cytokines levels in RAW264.7 cells. Concentration of LPS: 0.05 μg/mL. Data are presented as mean ± SD (n = 9). The differences between the groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
CC34 reduced the release of ROS
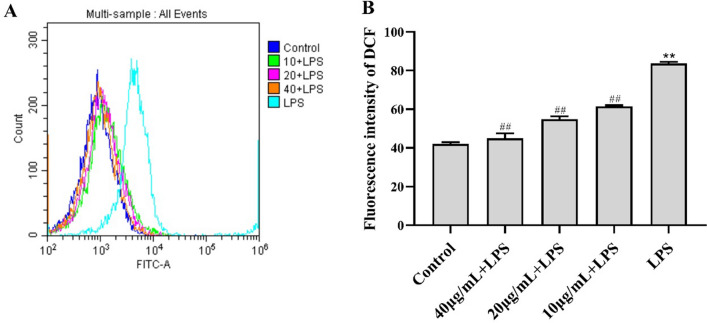
The DCF fluorescence intensity values indicated that the release of ROS was significantly higher in LPS-treated RAW264.7 cells than in control cells (P < 0.01) (Fig. 3). Cells treated with 10 μg/mL CC34 had substantially lower intracellular ROS levels than LPS-treated cells. There was no significant difference between treatment with 40 μg/mL CC34 and the control treatment, and adding CC34 attenuated LPS-stimulated ROS levels significantly in a dose-dependent manner (P < 0.01).
Fig. 3.
Effects of CC34 on ROS in RAW264.7 cells. A Effects of CC34 on fluorescence intensity of ROS. B Quantification of fluorescence intensity of DCF. Concentration of LPS: 0.05 μg/mL. Data are presented as mean ± SD (n = 3). The differences between the groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
Effects of CC34 on mRNA levels of cellular mediators of inflammation
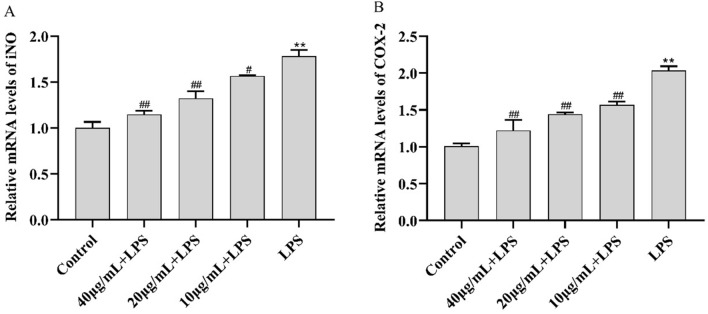
The mRNA levels of iNOS and COX-2 were significantly higher in LPS-treated RAW264.7 cells than in control cells. As shown in Fig. 4, both mRNA levels were significantly lower in CC34 + LPS-treated RAW264.7 cells than in control cells. Moreover, both CC34-associated tendencies were dose-dependent, similar to the effect of CC34 on cellular ROS.
Fig. 4.
Effects of CC34 on mRNA levels of iNOS and COX-2. Data are presented as mean ± SD (n = 4). The differences between the groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
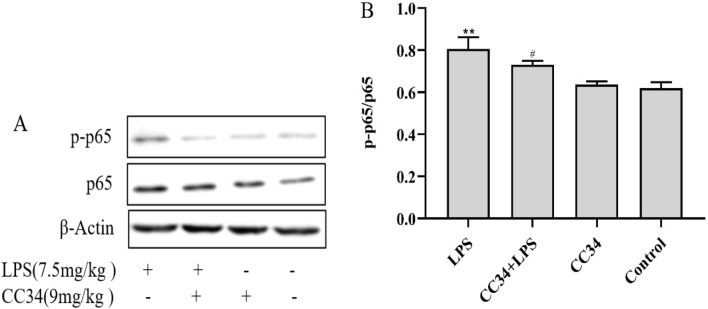
Effects of CC34 on NF-κB inactivation in LPS-treated RAW264.7 cells
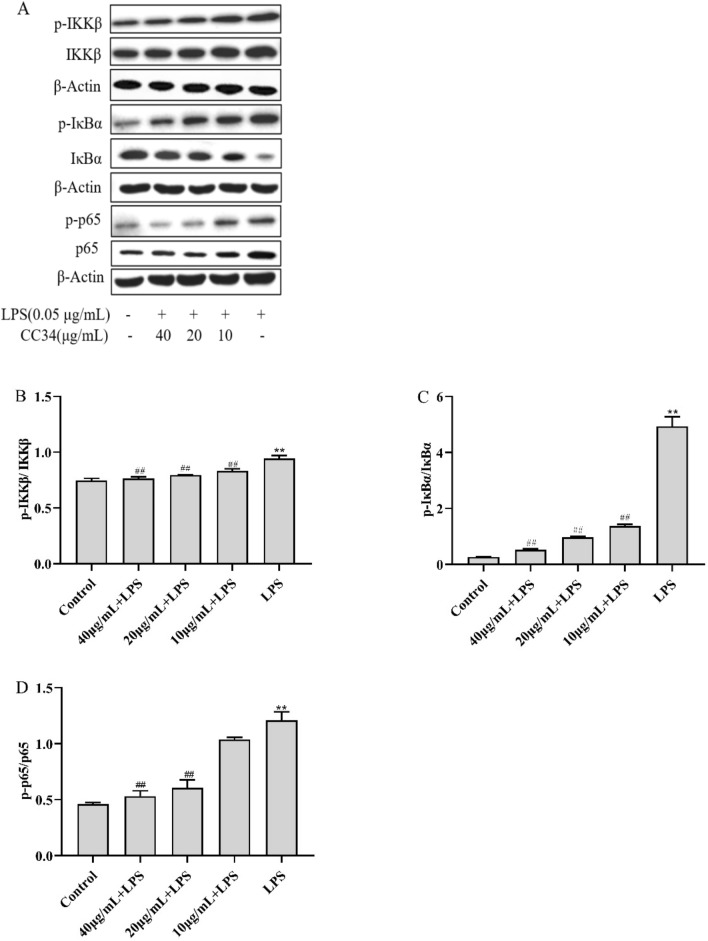
We assessed whether CC34 exerted anti-inflammatory effects via the NF-κB signaling pathway by measuring the phosphorylation levels of NF-κB in vitro (Fig. 5). LPS-treated RAW264.7 cells had significantly higher levels of phospho-IKKβ, phospho-IκBα, and phospho-NF-κB p65 than the control cells (P < 0.01). The levels of phosphorylated IKKβ, IκBα, and NF-κB p65 were significantly lower in RAW264.7 cells treated with 20 μg/mL and 40 μg/mL CC34 than in cells exposed to LPS without CC34 (P < 0.01).
Fig. 5.
Inhibitory effects of CC34 on the NF-κB signaling pathway. A Phosphorylated and total protein levels of NF-κB in RAW264.7 cells were analyzed via western blot. In B–D, the relative protein levels of phospho-IKKβ/IKKβ, phospho-IκBα/IκBα, and phospho-NF-κB/NF-κB in cells are shown. Data are presented as mean ± SD (n = 3). The differences between groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
Effects of CC34 on the DAI score
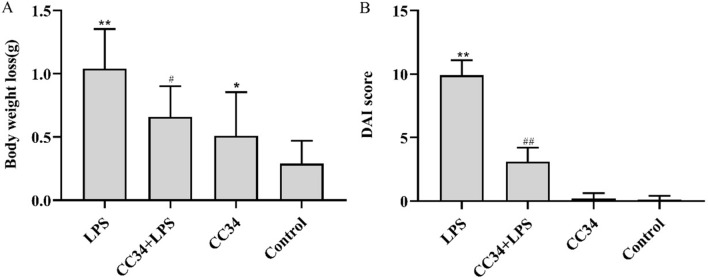
The LPS-induced mouse model of intestinal inflammation was used to further explore the immunomodulatory effects of CC34. After LPS induction, the mice showed a severe disease progression of acute intestinal inflammation. Specifically, the LPS-induced mice displayed a subdued behavior and preferred a curled-up posture, their movements were slow and sluggish, and the fur was moist and not groomed. However, CC34 significantly attenuated these symptoms. As shown in Fig. 6A, the BW loss significantly lowers in CC34 + LPS-treated mice than in LPS-treated mice (P < 0.05). LPS treatment changed the stool consistency and caused mucopurulent bloody stool associated with weight loss. These three clinical signs are used as scoring criteria to derive the DAI. It was consistent with the BW loss (Fig. 6B) that the DAI score was significantly lower in mice treated with CC34 + LPS than in LPS-treated mice (P < 0.01). Furthermore, the DAI score of the mice treated with CC34 alone was close to that of the control group.
Fig. 6.
Effects of CC34 on BW and DAI score. A BW loss in mice. B Effects of CC34 on DAI in mice. CC34-treated mice were intraperitoneally injected with 9 mg/kg of CC34; LPS treatment was an intraperitoneal injection of 7.5 mg/kg. Data are presented as mean ± SD (n = 10). The differences between the groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
Effects of CC34 on inflammatory cytokine in jejunum and serum of LPS-treated mice
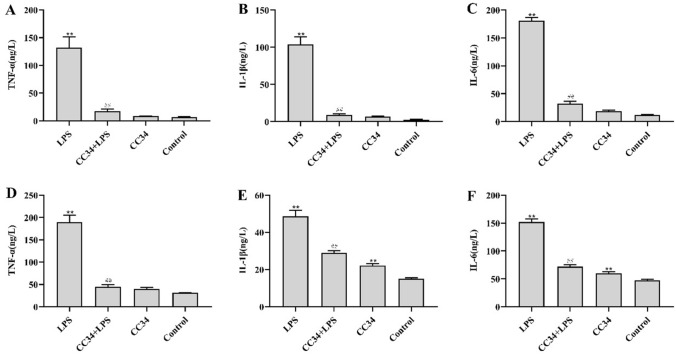
As shown in Fig. 7, LPS-treated mice had substantially higher levels of TNF-α, IL-1β, and IL-6 in the jejunum than the control mice. We also observed that CC34 + LPS-treated mice displayed significantly lower inflammatory cytokine levels in the jejunum and serum than the mice treated with LPS alone. The inflammatory cytokine levels in the jejunum did not significantly differ between mice treated with CC34 alone and the control mice.
Fig. 7.
Effects of CC34 on inflammatory cytokines in LPS-induced jejunum tissue and serum. ELISA for TNF-α (A), IL-1β (B), and IL-6 (C) in jejunum; ELISA for TNF-α (D), Il-1β (E), and Il-6 (F) in serum. CC34-treated mice were intraperitoneally injected with 9 mg/kg of CC34; LPS treatment was an intraperitoneal injection of 7.5 mg/kg. Data are presented as mean ± SD (n = 9). The differences between the groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
Pathological damage of jejunum tissue
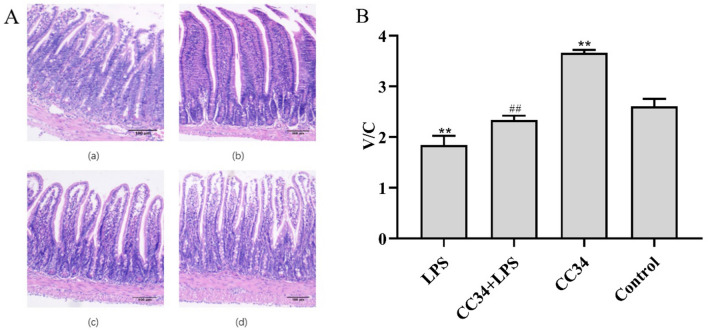
Histological analysis demonstrated that LPS treatment damaged the jejunal villus structure, which appeared to be related to goblet cell loss. The infiltration of neutrophils and lamina propria mononuclear cells in the jejunum tissue was observed, but the appearance of jejunum did not indicate any differences between CC34-treated and control mice (Fig. 8A). Jejunum tissue injuries and the degree of inflammation were attenuated in CC34 + LPS-treated mice compared with those in LPS-treated mice (P < 0.01). Mice with CC34 treatment had a significant increase in both the villus height and the villus height-to-crypt depth (V/C) ratio compared with those in LPS-treated mice (P < 0.01) (Fig. 8B).
Fig. 8.
Effects of CC34 on jejunum tissue morphology. A Pathological evaluation of jejunal tissue was performed using H&E staining. (a) LPS, (b) LPS + CC34, (c) CC34, and (d) Control. Original magnification ×100, scale bar = 100 μm. B Villus height-to-crypt depth (V/C) ratio. CC34-treated mice were intraperitoneally injected with 9 mg/kg of CC34; LPS treatment was an intraperitoneal injection of 7.5 mg/kg. Data are presented as mean ± SD (n = 9). The differences between the groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
CC34 injections administered to mice for seven consecutive days resulted in an increased jejunal V/C ratio compared with that in control mice (P < 0.01).
MPO levels
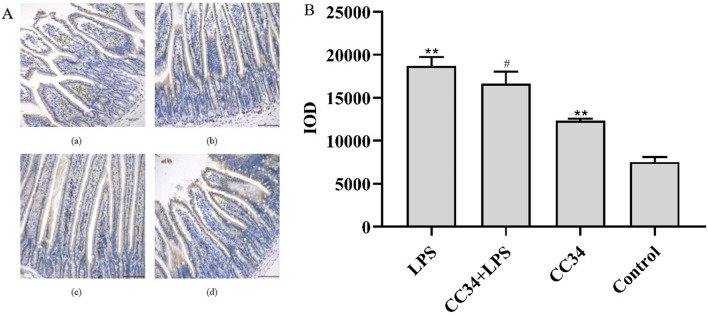
Immunohistochemistry analysis showed that the jejunal MPO level was significantly higher in LPS-treated mice than in control mice (P < 0.01). CC34 + LPS-treated mice had significantly lower MPO levels than mice treated with LPS alone (Fig. 9A). As shown in Fig. 9B, CC34 treatment effectively inhibited the expression of MPO in the jejunum of LPS-stimulated mice.
Fig. 9.
Effect of CC34 on MPO in LPS-treated mice. A Immunohistochemistry analysis of MPO in jejunum tissue. (a) LPS, (b) LPS + CC34, (c) CC34, and (d) Control. Original magnification ×200, scale bar = 100 μm. B IOD was used to accurately determine the total protein expression of MPO. CC34-treated mice were intraperitoneally injected with 9 mg/kg of CC34; LPS treatment was an intraperitoneal injection of 7.5 mg/kg. Data are presented as mean ± SD (n = 3). The differences between the groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
Effect of CC34 on the NF-κB signaling pathway in mice
We also examined the effects of CC34 on the NF-κB signaling pathway in the jejunum of LPS-stimulated mice by western blotting. We found that NF-κB phosphorylation was significantly higher in LPS-treated mice than in control mice (P < 0.01). Remarkably, the phosphorylation of NF-κB proteins in the jejunum was effectively inhibited in mice treated with 9 mg/kg CC34 compared with that in mice treated with LPS alone (P < 0.05) (Fig. 10).
Fig. 10.
Effects of CC34 on phosphorylated NF-κB in mice. A Phosphorylated and total protein levels of NF-κB in the jejunum were analyzed using western blot. B Relative protein expression levels of phospho-NF-κB/NF-κB in mice. Data are presented as mean ± SD (n = 3). The differences between groups were assessed using ANOVA followed by Duncan’s test. Compared to control: *P < 0.05, **P < 0.01. Compared to LPS: #P < 0.05, ##P < 0.01
LPS neutralization activity of CC34
The LAL assay was used to test whether CC34 has an activity to neutralize LPS. As shown in Fig. 11, CC34 caused partial neutralization of endotoxin in a dose-dependent manner. At the concentrations of 5, 10, 20, 40, 80, and 160 μg/mL, CC34 inhibited 6.05, 22.9, 32.0, 52.3, 72.7, and 84.2% of LPS, respectively.
Fig. 11.
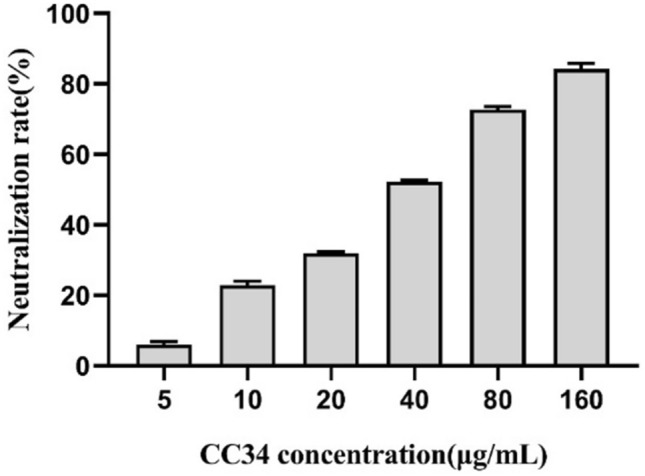
In vitro LPS neutralization by CC34. Data are presented as mean ± SD (n = 3). The differences between groups were assessed using ANOVA followed by Duncan’s test
Discussion
Antimicrobial peptides are studied as a potential treatment option due to the occurrence of antibiotic resistance and residue. AMPs are crucial components of the innate immune system as a defense mechanism in humans and animals (Magrone et al. 2018). However, the undefined effects and mechanisms of AMPs in animal models limit their potential clinical application (Lee et al. 2016). We previously screened many hybrid peptides by testing for antibacterial activity and hemolysis, and we selected the cationic peptide CC34 for further studies because it had a potential antibacterial effect and low toxicity (Sun 2009). Moreover, at concentrations below 50 µg/mL, CC34 displayed a strong anti-inflammatory activity that was not affected by the viability of RAW264.7 cells. Using this dose, the tendency of the cell viability curve of CC34 was similar to that of the hybrid β-hairpin peptides and far higher than that of the PCD-1 (Chen et al. 2015; Liu et al. 2013).
LPS is a major structural and functional component of gram-negative bacteria that can induce intestinal inflammation. Previous studies indicated that the LPS-induced mouse model of intestinal inflammation has the same clinicopathological characteristics as the human IBD (Brandenburg et al. 2016; Zhang et al. 2019a). In our study, we used LPS-induced mice to evaluate the anti-inflammatory activity of CC34. Cationic AMPs have exerted potential for binding to the LPS and inhibit LPS-induced inflammation. Our data showed that the CC34 can neutralize LPS in a dose-dependent manner. Thus, the results indicated that CC34 can attenuate inflammatory response by preventing LPS from binding to LPS-binding protein (LBP), and suppress the regulation of inflammation-related pathways (Mu et al. 2017).
Increased secretion of inflammatory factors, such as TNF-α, IL-6, and IL-1β, is common in intestinal inflammatory diseases, and their excessive accumulation is the cause of severe inflammatory injury in intestinal tissue (Sanchez-Munoz et al. 2008). In the present study, we observed that CC34 treatment diminished the LPS-induced levels of TNF-α, IL-6, and IL-1β in cells, serum, and tissue. Our study suggested that the CC34 attenuated the inflammation by inhibiting the secretion of inflammatory factors. We were also focused on COX-2, a crucial enzyme in the conversion of arachidonic acid to PGE2, and iNOS, which catalyzes the production of NO, because PGE2 and NO play a pivotal role in inflammatory disorders. Importantly, the inhibition of NF-κB activation can downregulate iNOS and COX-2 (Periyasami et al. 2019). Our results showed that treatment with CC34 significantly reduced the levels of iNOS and COX-2.
ROS is a critical upstream signal for the NF-κB signaling pathway, which can directly induce cell apoptosis and the destruction of surrounding tissues in response to various stimuli (Choi et al. 2013; Nakajima and Kitamura 2013). The excessive accumulation of ROS upregulates the expression of NF-κB and its translocation into the nucleus, where it functions as a transcriptional regulator by enhancing the mRNA levels of inflammatory mediators via interaction with NF-κB binding sites (Jeong et al. 2011; Ye et al. 2019). In this study, treatment with the CC34 markedly lowered the levels of ROS in LPS-induced RAW264.7 cells. Reducing the levels of certain inflammatory mediators, including TNF-α, IL-1β, IL-6, COX-2, and iNOS, is achieved by modulating the NF-κB signaling pathway (Xie et al. 2019). Evidence supports that RAW264.7 cells stimulation with LPS enhances intracellular ROS production (Ye et al. 2019). Excessive ROS accumulation causes oxidative damage and affects the nuclear transfer of NF-κB by increasing phosphorylation of the IκBα (Rajakumar et al. 2014). The in vitro treatment with suppressed the NF-κB signaling pathway because the phosphorylation of IKKβ, IκBα, and NF-κB p65 was decreased. Our results suggested that CC34 blocked the ROS production and the NF-κB signaling pathway by regulating inflammatory gene expression.
Furthermore, AMPs can relieve intestinal inflammation in mice by protecting the intestinal mucosal barrier (Nan et al. 2008). The mouse model of LPS-induced intestinal inflammation is associated with weight loss, elevated DAI score, diarrhea, and bleeding, all of which are clinical symptoms of intestinal inflammation in humans (Podolsky 2000; Togre et al. 2018). Excessive inflammatory cytokine production is also a hallmark of inflammatory bowel disease (Kim et al. 2018). Here we found that CC34 treatment also reduced the release of TNF-a, IL-1β, and IL-6 in the serum and jejunum, suggesting that CC34 provided protection in vivo against LPS-induced intestinal inflammation.
Intraperitoneal injection of LPS causes considerable tissue injury and decreases the V/C ratio (Zhang et al. 2019a, b; Zhuang et al. 2019). The histopathology results in our study showed that CC34 attenuated the LPS-induced symptoms of intestinal injury by restoring epithelial integrity and the V/C ratio as indicators for normal growth and health.
The MPO level is another biomarker for the connection between oxidative stress and inflammation in various tissues (Mancini et al. 2017). A previous study indicated that cellular MPO catalyzes the production of ROS, which is released during inflammation, affecting the functions of neutrophils related to cytokine levels (Chami et al. 2018). The degree of inflammation in IBD is associated with the elevated MPO level (Aratani 2018; Hanifeh et al. 2018; Stamp et al. 2012). Interestingly, in our study, we found that CC34 treatment lowered the MPO level in the jejunum of LPS-induced mice with acute jejunal inflammation. Thus, CC34 functioned as a safeguard against LPS-induced acute intestinal inflammation.
Pretreatment of mice with CC34 substantially inhibited the phosphorylation-dependent activation of NF-κB. Our data suggested that CC34 exerted anti-inflammatory effects in LPS-treated mice by downregulation of the NF-κB signaling pathway. This result was consistent with our in vitro study.
In summary, our results demonstrated that CC34 effectively improved the clinical manifestations of intestinal inflammation in mice. Overall, our data indicated that CC34 is a potential candidate for a new therapeutic strategy to treat inflammation-related diseases.
Acknowledgements
This study was supported by National Natural Science Foundation (32072759).
Author contributions
Conceptualization: LD, NJ, AZ; data curation: LD, HY; formal analysis: LD, NJ; funding acquisition: NJ, AZ; writing—original draft: LD, NJ; writing—review and editing: LD, NJ, ZW.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Contributor Information
Ning Jiang, Email: byndjn@163.com.
Aizhong Zhang, Email: aizhzhang@sina.com.
References
- Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Baek S, Park T, Kang M, Park D. Anti-inflammatory activity and ROS regulation effect of sinapaldehyde in LPS-stimulated RAW264.7 macrophages. Molecules. 2020 doi: 10.3390/molecules25184089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady D, Grapputo A, Romoli O, Sandrelli F. Insect cecropins, antimicrobial peptides with potential therapeutic applications. Int J Mol Sci. 2019 doi: 10.3390/ijms20235862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg K, Heinbockel L, Correa W, Lohner K. Peptides with dual mode of action: killing bacteria and preventing endotoxin-induced sepsis. Biochem Biophys Acta. 2016;1858:971–979. doi: 10.1016/j.bbamem.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Chami B, Martin NJJ, Dennis JM, Witting PK. Myeloperoxidase in the inflamed colon: a novel target for treating inflammatory bowel disease. Arch Biochem. 2018;645:61–71. doi: 10.1016/j.abb.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Chang M, Chang L, Chang H, Chang F. Intestinal and extraintestinal cancers associated with inflammatory bowel disease. Clin Colorectal Cancer. 2018;17:e29–37. doi: 10.1016/j.clcc.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Chen W, Huang S, Liao C, Sung C, Chen J, Wen Z. The use of the antimicrobial peptide piscidin (PCD)-1 as a novel anti-nociceptive agent. Biomaterials. 2015;53:1–11. doi: 10.1016/j.biomaterials.2015.02.069. [DOI] [PubMed] [Google Scholar]
- Chen M, Qin Y, Ma H, Zheng X, Li D. Downregulating NF-κB signaling pathway with triterpenoids for attenuating inflammation: in vitro and vivo studies. Food Funct. 2019;10(8):5080–5090. doi: 10.1039/C9FO00561G. [DOI] [PubMed] [Google Scholar]
- Choi I, Choi E, Jin J, Park H, Choi J, Kim S. Kaempferol inhibits P. intermedia lipopolysaccharide-induced production of nitric oxide through translational regulation in murine macrophages: critical role of heme oxygenase-1-mediated ROS reduction. J Periodontol. 2013;84(4):545–555. doi: 10.1902/jop.2012.120180. [DOI] [PubMed] [Google Scholar]
- Gampierakis I, Koutmani Y, Semitekolou M, Morianos I, Charalampopoulos I, Xanthou G, Gravanis A, Karalis K. Hippocampal neural stem cells and microglia response to experimental inflammatory bowel disease (IBD) Mol Psychiatry. 2021;26:1248–1263. doi: 10.1038/s41380-020-0651-6. [DOI] [PubMed] [Google Scholar]
- Glymenaki M, Singh G, Brass A, Warhurst G, McBain A, Else K, Cruickshank S. Compositional changes in the gut mucus microbiota precede the onset of colitis-induced inflammation. Inflamm Bowel Dis. 2017;23:912–922. doi: 10.1097/MIB.0000000000001118. [DOI] [PubMed] [Google Scholar]
- Han F, Zhang H, Xia X, Xiong H, Song D, Zong X, Wang Y. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J Immunol. 2015;194:1882–1893. doi: 10.4049/jimmunol.1402300. [DOI] [PubMed] [Google Scholar]
- Hanifeh M, Sankari S, Rajamäki M, Syrjä P, Kilpinen S, Suchodolski J, Heilmann R, Guadiano P, Lidbury J, Steiner J, Spillmann T. S100A12 concentrations and myeloperoxidase activities are increased in the intestinal mucosa of dogs with chronic enteropathies. BMC Vet Res. 2018;14(1):125. doi: 10.1186/s12917-018-1441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Ryu D, Suk D, Lee D. Anti-inflammatory effects of ethanol extract from Orostachys japonicus on modulation of signal pathways in LPS-stimulated RAW264.7 cells. BMB Rep. 2011;44(6):399–404. doi: 10.5483/BMBRep.2011.44.6.399. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lim H, Jang H, Lee S, Jung K, Lee S, Lee S, Rho M. Portulaca oleracea extracts and their active compounds ameliorate inflammatory bowel diseases in vitro and in vivo by modulating TNF-α, IL-6 and IL-1β signalling. Food Res Int. 2018;106:335–343. doi: 10.1016/j.foodres.2017.12.058. [DOI] [PubMed] [Google Scholar]
- Ko E, Cho S, Kwon S, Eom C, Jeong M, Lee W, Kim S, Heo S, Ahn G, Lee K. The roles of NF-κB and ROS in regulation of pro-inflammatory mediators of inflammation induction in LPS-stimulated zebrafish embryos. Fish Shellfish Immunol. 2017;68:525–529. doi: 10.1016/j.fsi.2017.07.041. [DOI] [PubMed] [Google Scholar]
- Lee T, Hall K, Aguilar M. Antimicrobial peptide structure and mechanism of action: a focus on the role of membrane structure. Curr Top Med Chem. 2016;16:25–39. doi: 10.2174/1568026615666150703121700. [DOI] [PubMed] [Google Scholar]
- Limketkai B. IBD hospitalisation trends provide insight for future study. Lancet Gastroenterol Hepatol. 2019;4(4):259–260. doi: 10.1016/S2468-1253(19)30037-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xia X, Xu L, Wang Y. Design of hybrid β-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials. 2013;34(1):237–250. doi: 10.1016/j.biomaterials.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Liu C, Tang X, Zhang W, Li G, Chen Y, Guo A, Hu C. 6-Bromoindirubin-3′-oxime suppresses LPS-induced inflammation via inhibition of the TLR4/NF-κB and TLR4/MAPK signaling pathways. Inflammation. 2019;42(6):2192–2204. doi: 10.1007/s10753-019-01083-1. [DOI] [PubMed] [Google Scholar]
- Loher F, Bauer C, Landauer N, Schmall K, Siegmund B, Lehr H, Dauer M, Schoenharting M, Endres S, Eigler A. The interleukin-1 beta-converting enzyme inhibitor pralnacasan reduces dextran sulfate sodium-induced murine colitis and T helper 1 T-cell activation. J Pharmacol Exp Ther. 2004;308(2):583–590. doi: 10.1124/jpet.103.057059. [DOI] [PubMed] [Google Scholar]
- Lorenza P, Federica DC, Pamela V, Cicala M, Cucchiara S, Dallapiccola B. Gut microbiota dysbiosis as risk and premorbid factors of IBD and IBS along the childhood-adulthood transition. Inflamm Bowel Dis. 2016;22(2):487–504. doi: 10.1097/MIB.0000000000000602. [DOI] [PubMed] [Google Scholar]
- Magrone T, Russo M, Jirillo E. Antimicrobial peptides in human disease: therapeutic approaches. Second of two parts. Curr Pharm Des. 2018;24(10):1148–1156. doi: 10.2174/1381612824666180327155230. [DOI] [PubMed] [Google Scholar]
- Mancini S, Mariani F, Sena P, Benincasa M, Roncucci L. Myeloperoxidase expression in human colonic mucosa is related to systemic oxidative balance in healthy subjects. Redox Rep. 2017;22:399–407. doi: 10.1080/13510002.2016.1277049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L, Zhou L, Yang J, Tang J, Liu T, Wu J, Yang HL. The first identified cathelicidin from tree frogs possesses anti-inflammatory and partial LPS neutralization activities. Amino Acids. 2017;49:1571–1585. doi: 10.1007/s00726-017-2449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Kitamura M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med. 2013;65:162–174. doi: 10.1016/j.freeradbiomed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Nan YH, Jeon YJ, Park IS, Shin SY. Antimicrobial peptide P18 inhibits inflammatory responses by LPS but not by IFN-γ-stimulated macrophages. Biotechnol Lett. 2008;30(7):1183–1187. doi: 10.1007/s10529-008-9682-9. [DOI] [PubMed] [Google Scholar]
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Periyasami G, Antonisamy P, Perumal K, Stalin A, Rahaman M, Alothman A. Acompetent synthesis and efficient anti-inflammatory responses of isatinimino acridinedione moiety via suppression of in vivo NF-κB, COX-2 and iNOS signaling. Bioorg Chem. 2019;90:103047. doi: 10.1016/j.bioorg.2019.103047. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Pride and prejudice: inflammatory bowel disease models and drug development. Curr Opin Gastroenterol. 2000;16(4):295–296. doi: 10.1097/00001574-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Rajakumar D, Senguttuvan S, Alexander M, Oommen A. Involvement of oxidative stress, nuclear factor kappa B and the ubiquitin proteasomal pathway in dysferlinopathy. Life Sci. 2014;108(1):54–61. doi: 10.1016/j.lfs.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Rod-In W, Monmai C, Lee SM, Jung SK, Park WJ. Anti-inflammatory effects of lipids extracted from Arctoscopus japonicus eggs on LPS-stimulated RAW264.7 cells. Mar Drugs. 2019;17(10):580. doi: 10.3390/md17100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14(27):4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim DW, Heo KH, Kim YK, Sim EJ, Kang TB, Choi JW, Sim DW, Cheong SH, Lee SH, Bang JK. Anti-inflammatory action of an antimicrobial model peptide that suppresses the TRIF-dependent signaling pathway via inhibition of toll-like receptor 4 endocytosis in lipopolysaccharide-stimulated macrophages. PLoS ONE. 2015;10:e0126871. doi: 10.1371/journal.pone.0126871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp L, Khalilova I, Tarr J, Senthilmohan R, Turner R, Haigh R, Winyard P, Kettle A. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology (oxford) 2012;51:1796–1803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- Sun Y. Design of hybrid antibacterial peptides Cec Md-Che Rc and construction of its gene cloning vector. J Heilongjiang Bayi Agric Univ. 2009;21:47–50. [Google Scholar]
- Targan SR. Current limitations of IBD treatment: where do we go from here? Ann N Y Acad Sci. 2010;1072(1):1–8. doi: 10.1196/annals.1326.032. [DOI] [PubMed] [Google Scholar]
- Thapa RK, Diep DB, Tønnesen HH. Topical antimicrobial peptide formulations for wound healing: current developments and future prospects. Acta Biomater. 2020;103:52–67. doi: 10.1016/j.actbio.2019.12.025. [DOI] [PubMed] [Google Scholar]
- Togre N, Bhoj P, Goswami K, Tarnekar A, Patil M, Shende M. Human filarial proteins attenuate chronic colitis in an experimental mouse model. Parasite Immunol. 2018;40:e12511. doi: 10.1111/pim.12511. [DOI] [PubMed] [Google Scholar]
- Xie Z, Wang Y, Huang J, Qian N, Shen G, Chen L. Anti-inflammatory activity of polysaccharides from Phellinus linteus by regulating the NF-κB translocation in LPS-stimulated RAW264.7 macrophages. Int J Biol Macromol. 2019;129:61–67. doi: 10.1016/j.ijbiomac.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Ye S, Zheng Q, Zhou Y, Bai B, Yang D, Zhao Z. Chlojaponilactone B attenuates lipopolysaccharide-induced inflammatory responses by suppressing TLR4-mediated ROS generation and NF-κB signaling pathway. Molecules. 2019;24(20):3731. doi: 10.3390/molecules24203731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Yu C, Zhang H, Song D, Jiang D, Du H, Wang Y. Cathelicidin-BF suppresses intestinal inflammation by inhibiting the nuclear factor-κB signaling pathway and enhancing the phagocytosis of immune cells via STAT-1 in weanling piglets. Int Immunopharmacol. 2015;28:61–69. doi: 10.1016/j.intimp.2015.05.034. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wei X, Zhang R, Petitte J, Si D, Li Z, Cheng J, Du M. Design and development of a novel peptide for treating intestinal inflammation. Front Immunol. 2019;10:1841. doi: 10.3389/fimmu.2019.01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wei X, Zhang R, Si D, Zhang M. A novel peptide ameliorates LPS-induced intestinal inflammation and mucosal barrier damage via its antioxidant and antiendotoxin effects. Int J Mol Sci. 2019;20(16):3974. doi: 10.3390/ijms20163974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Zhong J, Zhou Q, Zhong Y, Liu P, Liu Z. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int Immunopharmacol. 2019;71:321–327. doi: 10.1016/j.intimp.2019.03.030. [DOI] [PubMed] [Google Scholar]