Abstract
Background
The gut microbiota represents a potential treatment target in heart failure (HF) through microbial metabolites such as trimethylamine N-oxide (TMAO) and systemic inflammation. Treatment with the probiotic yeast Saccharomyces boulardii have been suggested to improve left ventricular ejection fraction (LVEF).
Methods
In a multicentre, prospective randomized open label, blinded end-point trial, we randomized patients with LVEF <40% and New York Heart Association functional class II or III, despite optimal medical therapy, to treatment (1:1:1) with the probiotic yeast Saccharomyces boulardii, the antibiotic rifaximin, or standard of care (SoC) only. The primary endpoint, the baseline-adjusted LVEF at three months, was assessed in an intention-to-treat analysis.
Findings
We enrolled a total of 151 patients. After three months’ treatment, the LVEF did not differ significantly between the SoC arm and the rifaximin arm (mean difference was -1•2 percentage points; 95% CI -3•2 - 0•7; p=0•22) or between the SoC arm and the Saccharomyces boulardii arm (mean difference -0•2 percentage points; 95% CI -2•2 - 1•9; p=0•87). We observed no significant between-group differences in changes in microbiota diversity, TMAO, or C-reactive protein.
Interpretation
Three months’ treatment with Saccharomyces boulardii or rifaximin on top of SoC had no significant effect on LVEF, microbiota diversity, or the measured biomarkers in our population with HF.
Funding
The trial was funded by the Norwegian Association for Public Health, the Blix foundation, Stein Erik Hagen's Foundation for Clinical Heart Research, Ada og Hagbart Waages humanitære og veldedige stiftelse, Alfasigma, and Biocodex.
Keywords: Heart failure, Microbiota, Trimethylamine N-oxide, Probiotics, Antibiotics, Inflammation
Research in context.
Evidence before this study
The gut microbiota is altered in heart failure. A blooming of pathological bacteria and loss of microbial diversity have been described. These dysbiotic changes are believed to influence the heart through gut-specific metabolites and leakage of bacterial products that in turn activate innate immunity. The metabolite trimethylamine N-oxide has been associated with adverse remodelling in heart failure, while several mediators of innate immunity can impact cardiac function.
In a pilot study, three months treatment with the probiotic yeast Saccharomyces boulardii improved left ventricular ejection fraction in patients with heart failure.
Added value of this study
The GutHeart study was designed to examine the effects of microbiota modulation on cardiac function in heart failure. We found that neither microbiota modulation with the antibiotic rifaximin or the probiotic Saccharomyces boulardii affected cardiac function or trimethylamine N-oxide. In fact, our interventions did not significantly change the microbiota diversity. Furthermore, our study participants were well treated and with low symptomatic burden. The degree of dysbiosis at baseline appeared to be low measured by the microbial diversity.
Our study suggests that broad interventions with probiotics and antibiotics might not be sufficient to significantly alter the microbiota in well-treated patients with heart failure.
Implications of all the available evidence
Our study suggests that not all well-treated patients with heart failure have substantial dysbiosis. In these patients, a more precise approach targeting specific bacterial taxa or a gut-related metabolite should be attempted.
Alt-text: Unlabelled box
1. Introduction
Heart failure (HF) with reduced ejection fraction is a progressive disease with high morbidity and mortality. Contemporary treatment of HF centres on blockade of maladaptive neurohormonal activation. Disturbances in metabolic and inflammatory pathways also seem to play an important role in the development and progression of HF, but how to modulate these mechanisms is not clear [1,2].
Over the last two decades, research has suggested that the gut microbiota may play a role in HF [3]. However, the causal pathways behind a proposed gut-heart axis remain elusive. Several small studies have shown that the gut microbiota differs between patients with HF and healthy subjects. Significant differences in bacterial diversity, distribution of the main microbial phyla, specific taxa, and pathogenic microorganisms have been observed [4,5]. At a functional level, the microbiota-related metabolite trimethylamine N-oxide (TMAO) is associated with increased risk of adverse cardiovascular events and susceptibility for the development and severity of HF [6,7]. Furthermore, the microbiota in patients with HF may have a reduced capacity to synthesize beneficial metabolites such as short chained fatty acids [8]. Short chain fatty acids, in particular butyrate, are essential for maintaining the mucosal barrier of the gut [9]. Loss of barrier function might facilitate leakage of bacterial components like lipopolysaccharides (LPS). These compounds may in turn activate the innate immune system through pattern recognition receptors. This mechanism may contribute to the low-grade systemic inflammation observed in HF [10], [11], [12]. Acetate and propionate, two other short chain fatty acids, may affect the renin-angiotensin system through G-protein-coupled olfactory receptors, linking the gut microbiota to activation of neurohormonal pathways in HF [13]. Acetate has also been shown to decrease cardiac hypertrophy, attenuate cardiac fibrosis, and improve cardiac function in experimental studies [14].
In a small pilot trial, Costanza and colleagues randomly assigned 20 patients with HF with reduced ejection fraction to treatment with the probiotic yeast Saccharomyces boulardii (S.boulardii) or placebo [15]. The left ventricular ejection fraction (LVEF) increased more in the active treatment arm. However, the small size of the study and the lack of data on associated changes in the gut microbiota limit our ability to draw conclusions regarding causality.
In the Targeting Gut Microbiota to Treat Heart Failure (GutHeart) trial, we aimed to explore the effect of S.boulardii or the oral non-absorbable antibiotic rifaximin on top of guideline-recommended treatment for HF with reduced ejection fraction. In this proof-of-concept study, we also aimed to assess the effects of the microbiota-directed treatment on the composition and function of bacteria in the gut, on the key microbial metabolite trimethylamine N-oxide (TMAO), and on systemic inflammation.
2. Methods
2.1. Study design
The GutHeart trial (www.clinicaltrials.govNCT02637167) is a phase II, multicenter, randomized, open label, controlled trial. The trial was conducted at three hospitals in Norway and one hospital in Brazil. Participants were randomly assigned in a 1:1:1 fashion. Both interventional arms were compared to the standard of care (SoC) arm.
The trial complies with the declaration of Helsinki. The Regional Ethics Committees approved the trial (reference No 2015/120/REK sør-øst) and all subjects gave their written informed consent to participate. Independent data monitors oversaw the study [16].
2.2. Participants
We recruited patients from the outpatient clinics at Oslo University Hospital Rikshospitalet (Oslo, Norway), Oslo University Hospital Ullevål (Oslo, Norway), Nordlandssykehuset (Bodø, Norway), and Instituto Nacional de Cardiologia (Rio de Janeiro, Brazil). We enrolled patients with symptomatic HF in New York Heart Association functional class II, III, and LVEF < 40 % at the time of inclusion. The participants had to be on optimal medical treatment for three months prior to inclusion. This includes maximally tolerable doses of angiotensin converting enzyme inhibitors/angiotensin receptor antagonists/angiotensin receptor-neprilysin inhibitors, beta-blockers, and mineralocorticoid receptor antagonists if indicated, according to ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2016 [17].
Key exclusion criteria were: treatment with antibiotics or probiotics during the last three months prior to inclusion, significant comorbidities, treatment with immunosuppressive drugs, concurrent infections, or bowel disease. Patients who had received cardiac resynchronization therapy during the past six months were not included. A complete list of inclusion and exclusion criteria is provided in Supplementary Table 1.
Diet is a known major modulator of the gut microbiome. We therefore encouraged all patients not to change their dietary habits during the study period. All patients were equipped with a list of probiotics and food enriched with probiotics, which they were asked to refrain from during the study period.
2.3. Randomization and masking
The research support unit at Oslo University Hospital generated a balanced, permuted block randomization list with varying block sizes. The randomization was stratified by center. We performed treatment allocation on the online platform Viedoc TM (PCG Solutions, Uppsala, Sweden). The study was open label with blinded endpoint analyses.
2.4. Interventions
Patients eligible for participation were randomized to oral treatment with 550 mg of the non-absorbable antibiotic rifaximin twice a day, two capsules of 250 mg of the probiotic yeast S.boulardii (CNCM I-745) twice a day, or conventional treatment for HF only. All interventions were given on top of SoC. The intervention period lasted three months. Patients were asked not to ingest probiotics and food enriched with probiotics during the study period. Follow-up visits were scheduled at month one and three. We consecutively registered all adverse events. Serious adverse events were defined as an event that resulted in death, was immediately life-threatening, required in-patient hospitalization, or prolongation of existing hospitalization, resulting in persistent or significant disability or incapacity, or an important medical event that may jeopardize the subject or may require medical intervention to prevent one of the outcomes listed above.
An independent safety committee oversaw the safety of the trial and assessed all serious adverse events.
2.5. Study endpoints
The primary endpoint was LVEF after three months of intervention after adjustment for baseline values. The secondary endpoints were baseline-adjusted N-terminal pro-B-type natriuretic peptide (NT-proBNP), high sensitivity C-reactive protein (CRP), TMAO, changes in the composition of the microbiota (Shannon index, amplicon sequence variants (ASVs), and other compositional changes).
2.6. Echocardiography
We used a standardized protocol for image acquisition as recommended by the European Association of Cardiovascular Imaging [18]. Echocardiograms were digitally stored and analysed offline in EchoPac version 202 pc. We de-identified the exams and assigned a random identification number to each individual exam. Image analysis was performed at the core lab facility at Oslo University hospital, Oslo, Norway by personnel blinded to treatment allocation and to whether the exam was performed at baseline or follow-up. Due to data storage problems, which occurred at one study site, approximately 10% of the echocardiograms were analysed at the local study site. We performed sensitivity and validation analysis on these data. Left ventricular volumes were calculated using the modified Simpson's rule[19]. We used apical four chamber views in combination with two-chamber views unless the three-chamber view was superior in terms of endocardial border definition.
2.7. Microbiota analyses
At inclusion, all patients received a stool collection device and careful instructions on how to collect the samples. They collected samples immediately prior to and at the end of the intervention period. The samples were delivered in person or by postal mail. All samples were collected in tubes with a deoxyribonucleic acid (DNA) stabilizing solution (PSP Spin Stool DNA kit, Stratec Molecular GMBH, Berlin, Germany). We registered the time from collection to freezer for all samples.
Stool DNA was extracted using the PSP Spin Stool DNA Plus extraction (Stratec Molecular) kit with a protocol modified by adding a bead-beating step, as described elsewhere [20]. The V3–V4 region of the 16S ribosomal ribonucleic acid (rRNA) gene was amplified and libraries sequenced on the Illumina MiSeq platform (San Diego, California, USA) at the Norwegian Sequencing Centre (Oslo, Norway), as previously described in detail [5].
Paired-end reads were filtered for Illumina Universal Adapters and PhiX, demultiplexed, quality trimmed, and merged using BBDuk 38•86, Cutadapt 2•10, and BBMerge 38•86[21], [22], [23]. Denoising to ASVs, taxonomic classification and filtering of contaminants and rare ASVs were done with QIIME2 version 2020•8 [24]. There were no detectable levels of bacteria in the negative controls, and consequently, no identified contaminants were removed from the dataset before further analyses were performed.
To reduce the effect of heterogeneous sequencing depths, we rarefied all samples to a common level of 7952 reads. We calculated diversity values and tested for differential abundance with this rarefied dataset.
In a subset of patients we estimated the butyrate producing capacity of the microbial communities by the abundance of the Butyrate-acetoacetate CoA transferase gene encoding the rate-limiting step. This represents the capacity of the microbiota to produce butyrate. We used PICRUST2 with default settings on all included samples [25].
2.8. Circulating biomarkers
The patients fasted overnight before we collected blood samples by venipuncture. Samples were collected at baseline and after three months. We separated serum and EDTA-plasma within 1 h by centrifugation at room temperature and at 4°C, respectively, and stored the samples at −80°C until analyses. NT-ProBNP was determined by an electrochemiluminesence immunoassay (ECLIA) (Roche Diagnostics, Mannheim, Germany), plasma TMAO by stable isotope dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described[26], and CRP was analysed by ELISA (DRG Instruments, Marburg/Lahn, Germany). Intra-assay coefficients of variation were 6•9% for CRP, 5% for NT-proBNP, and 4•1% for TMAO.
2.9. Six minute walk test
A six minute walk test was performed at baseline and at 3 months. The patients were asked to bring appropriate clothing and shoes for the test. They rested in a chair for at least 10 minutes before the test started. The patients were encouraged to make his/her best effort during the six minute test. Heart rate and arterial oxygen saturation was recorded at the beginning of the exercise, and again at the end of the exercise after six minutes. We recorded the walking distance and any premature interruption of the test.
2.10. Statistics
We analysed the data according to the intention-to-treat principle. As we were not able to obtain endpoints at 3 months for all patients, we used the full analysis set strategy. The intention-to-treat population is defined as all participants who were randomized regardless of adherence to study drug or follow-up. The per-protocol population is defined as cases with more 80% self-reported investigational drug compliance. We also excluded 15 patients where LVEF was calculated at the local study site and not at the designated core lab in a modified intention-to-treat analysis. We performed sensitivity analyses for both per-protocol and modified intention-to-treat cases.
The trial was powered to detect a 5 percentage points increase in LVEF in either intervention group compared with the SoC arm, with an α of 5 %, and power of 80 %. With a presumed standard deviation of LVEF of 7•5 percentage point, 37 patients would be needed in each group. To compensate for dropouts, we intended to include 50 patients in each group, in total 150 patients.
The primary endpoint was analysed with analysis of covariance (ANCOVA), comparing LVEF after three months between either intervention group and the SoC arm. We adjusted for baseline LVEF. We also used ANCOVA to determine the effect of our interventions on the Shannon Index, ASVs, the abundance of the Butyrate-acetoacetate CoA transferase gene, NT-proBNP, CRP, and TMAO.
Paired sample t-tests or Wilcoxon signed-rank tests were used to explore within-group treatment effects. We log-transformed TMAO, CRP, NT-proBNP, and the abundance of Butyrate-acetoacetate CoA transferase gene because the raw data were skewed.
When we analysed microbiota data at genus level, only significant within-group and between-group differences were reported. Wilcoxon signed-rank tests were used to explore the genus-level within-group treatment effects. P-values were adjusted using the Benjamini-Hochberg False Discovery Rate. Differences between the treatment groups were tested using Mann-Whitney-Wilcoxon on the delta values.
We used IBM SPSS statistics version 25•0 to perform all statistics. Data are presented as means (+/-standard deviation), medians (interquartile range) or number (percent) unless stated otherwise.
2.11. Role of the funding sources
The funding sources had no role in the design of the trial, data collection, analysis, and interpretation, writing of the manuscript, or the decision to publish.
3. Results
3.1. Enrollment and baseline characteristics
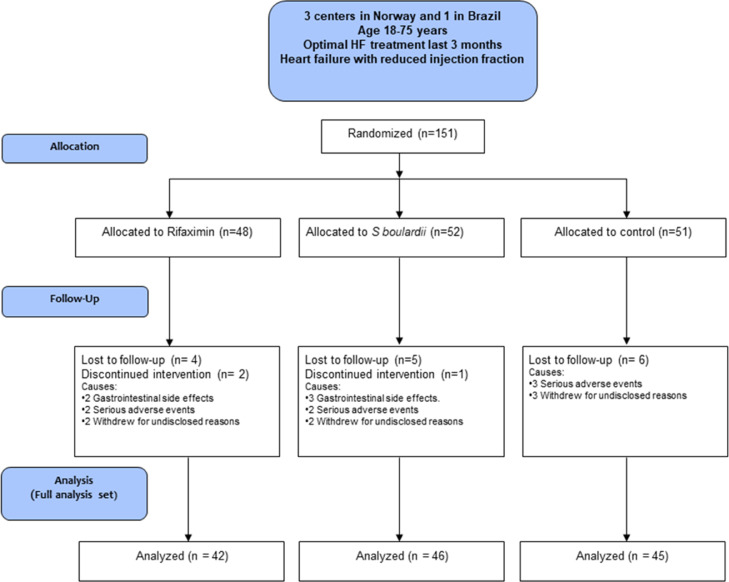
From March 11th, 2016 to May 16th, 2019, we enrolled 151 patients, 124 in Norway, and 27 in Brazil. The last patient completed the three-month follow-up August 30th, 2019. Forty-eight patients were allocated to rifaximin, 52 patients to S.boulardii, and 51 patients were assigned to conventional treatment only. One hundred thirty-two patients were analysed for the primary and secondary endpoints (Fig, 1). The characteristics of the study population are presented in Table 1. Baseline characteristics were balanced between the groups.
Fig. 1.
CONSORT Flow diagram. S.boulardii = Saccharomyces boulardii.
Table 1.
Baseline characteristics according to randomization.
| Rifaximin N=48 |
S.boulardii N=51 |
SoC N=52 |
||
| Age (years) (SD) | 59(10) | 62(8) | 60(10) | |
| Female gender – n (%) | 13(27) | 10(20) | 13(27) | |
| Body mass index (kg/m2) (SD) | 28(5) | 29(5) | 28(4) | |
| Systolic BP. (mm Hg) (SD) | 118(21) | 119(20) | 123(20) | |
| Diastolic BP (mm Hg) (SD) | 72(11) | 72(11) | 75(11) | |
| Heart rate (beats/minute) (SD) | 66(12) | 66(10) | 69(11) | |
| Atrial fibrillation/flutter – n (%) | 11(23) | 20 (39) | 16(33) | |
| NYHA class II/III – n (%) | 31 (65)/17(35) | 36 (71)/15(29) | 39 (80)/10(20) | |
| Medical history | ||||
| Ischemic etiology – n (%) | 28(58) | 30(59) | 26(53) | |
| History of smoking – n (%) | 21(44) | 13(26) | 21(43) | |
| History of hypertension – n (%) | 17(35) | 19(37) | 29(47) | |
| Diabetes mellitus – n (%) | 14(29) | 15(29) | 12(25) | |
| Implantable cardioverter defibrillator only – n (%) | 12(25) | 12(24) | 18(37) | |
| Cardiac resynchronization therapy – n (%) | 15(31) | 21 (41) | 17(35) | |
| Heart failure medication – n (%) | ||||
| ACE inhibitor/ARB | 47 (98) | 50 (98) | 43 (88) | |
| Sacubitril/Valsartan | 8(17) | 9(18) | 4(8) | |
| Beta blocker | 46 (96) | 51 (100) | 44 (90) | |
| Mineralocorticoid receptor antagonist | 24 (50) | 34 (67) | 37(63) | |
| Diuretics | 35(73) | 29(57) | 28(57) | |
| Laboratory values | ||||
| Hemoglobin (g/dL) (IQR) | 14•5 (13•9 - 15•5) | 14•6 (13•5 - 15•6) | 14•5 (13•5 - 15•3) |
|
| eGFR (IQR) | 72 (58 - 86) | 65 (47 - 83) | 73 (57 - 90) | |
| N-terminal-pro-B-type natriuretic peptide (pg/mL) (IQR) | 1241 (515 - 1707) | 811 (387 - 1618) | 853 (435 - 1893) | |
| C-reactive protein (mg/L) (IQR) | 1•48 (0•74 - 3•44) | 1•79 (0•70 - 3•47) | 1•42 (0•61 - 4•81) |
|
| TMAO (µmol/L) (IQR) | 6•10 (4•07 - 10•54) | 7•00 (4•40 - 12•77) | 6•00 (3•63 - 11•21) | |
| Diversity measures | ||||
| Shannon diversity index (IQR) | 5•52 (4•80 - 5•84) | 5•42 (4•91 - 5•86) | 5•62 (4•95 - 5•89) |
|
| Amplicon sequence variants | 222 (185 - 270) | 219 (175 - 264) | 231 (202 - 269) | |
| Echocardiography | ||||
| Left ventricular ejection fraction (%) (SD) | 28(7) | 30(6) | 31(6) | |
Data are given as number (percent), mean (standard deviation), or median (interquartile range) as appropriate.
SD = Standard deviation, IQR = interquartile range, NYHA class = New York Heart Association functional class, ACE-I = Angiotensin converting enzyme inhibitor, ARB = Angiotensin II receptor blocker, eGFR= estimated Glomerular Filtration Rate, TMAO = Trimethylamine N-oxide, SoC = Standard of care.
Heart failure drug regimens remained stable during the study period. We recorded minor adjustments in either mineralocorticoid receptor antagonists, beta-blockers, or angiotensin converting enzyme inhibitors/angiotensin receptor antagonists/angiotensin receptor-neprilysin inhibitors in eight patients. Six patients either initiated loop diuretics or adjusted their doses of loop diuretic.
3.2. Primary endpoint
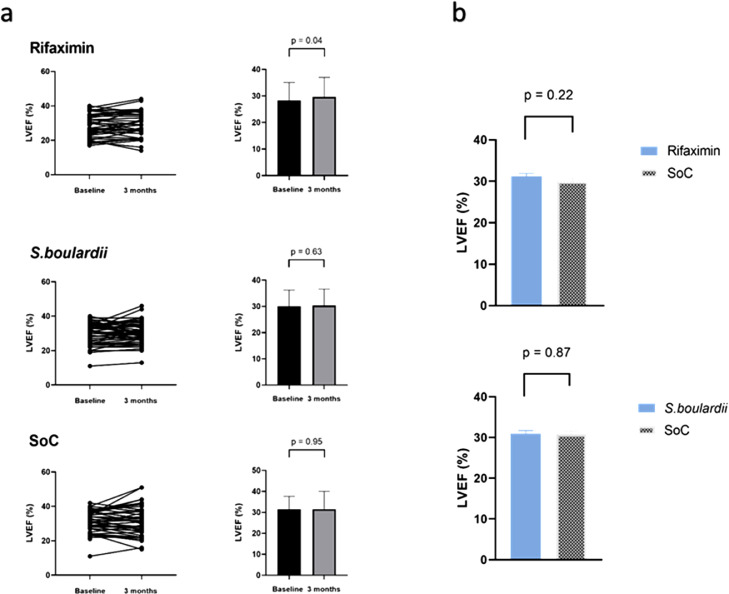
After three months’ intervention, the mean LVEF was 29•6 (7•3) percent in the rifaximin arm, 30•3 (6•3) percent in the S.boulardii arm, and 31•5 (8•6) percent in the SoC arm. The baseline-adjusted difference between the rifaximin and the SoC arm was 1•2 percentage points (95% CI -0•7 - 3•2, P=0•22) (Fig. 2) and 0•2 percentage points (95% CI -1•9 - 2•2, P=0•87) between the S.boulardii arm and the SoC arm (Fig. 2).
Fig. 2.
Baseline-adjusted mean LVEF and within-group changes in LVEF. (Graph a) The graphs shows individual and within-group change in LVEF from baseline to 3 months. (Graph b) The bars indicates baseline-adjusted mean at 3 months. The upper panel shows rifaximin vs control and the lower panel shows S.boulardii vs control. The notation above the bars indicates the p-value for difference between the values. LVEF = Left ventricular ejection fraction. S.boulardii = Saccharomyces boulardii. SoC = Standard of care.
Changes within all treatment arms are shown in Fig. 2. Analyses of modified intention-to-treat and per-protocol cases did not change the results (Table 2).
Table 2.
Sensitivity analysis of adjusted mean difference in left ventricular ejection fraction.
| Analysis | Rifaximin vs SoC | S.boulardii vs SoC | |||||
| ANCOVA (baseline-adjusted mean difference | ANCOVA (baseline-adjusted mean difference | ||||||
| N | Mean (95% CI) | P-value | N | Mean (95% CI) | P-value | ||
| Per- protocol | 83 | 1•1 (-0•93 - 3•14) | 0•28 | 85 | 0•51 (-1•58 - 2•61) | 0•63 | |
| Intention-to-treat | 87 | 1•14 (-0•83 - 3•12) | 0•25 | 91 | 0•17 (-1•85 - 2•18) | 0•87 | |
| Intention-to-treat* | 75 | 0•58 (-1•56 - 2•72) | 0•59 | 84 | 0•07 (-2•0 - 2•13) | 0•95 | |
Per-protocol is defined as cases with more than 80% study drug compliance. Intension-to-treat indicates all participants with full data set (full analysis set). *Indicates intention-to-treat analysis excluding 15 subjects where left ventricular ejection fraction was not calculated at the designated core lab. S.boulardii = Saccharomyces boulardii, SoC = Standard of care.
3.3. Secondary endpoints
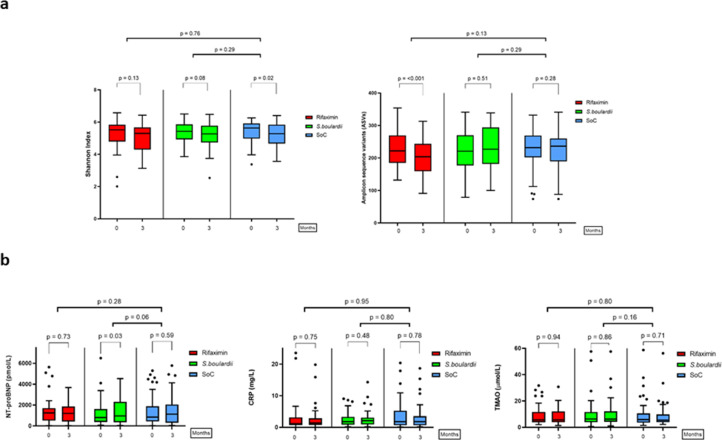
There were no significant differences in the levels of NT-proBNP, CRP, or TMAO after three months for any of the intervention groups versus SoC (Fig. 3). NT-proBNP increased from baseline to three months in the S.boulardii arm, but not in the rifaximin arm or in the SoC arm (Fig. 3). Neither TMAO nor CRP changed significantly from baseline to three months in any group (Fig. 3).
Fig. 3.
Baseline-adjusted microbiota diversity (upper panel a) and circulating biomarkers (lower panel b). The bars within each sector show baseline levels vs levels after three months for all groups. The notation above the thin brackets indicates p-value for within-group change. The notation above the thick brackets indicates the p-value for difference between baseline-adjusted mean after three months for investigational drugs vs control. The p-value above the thin brackets indicate within-group differences. CRP = C-reactive protein. NT-proBNP = N-terminal pro-B-type natriuretic peptide. S.boulardii = Saccharomyces boulardii. SoC = Standard of care. TMAO = Trimethylamine N-oxide.
In total, 135 patients were included in the microbiota analyses. Twenty-one from Brazil and 114 from Norway. We observed no significant differences in global microbiota composition (beta diversity) or bacterial richness (alpha diversity) from baseline to the end of the intervention between either of the intervention groups and SoC (Fig. 3). However, there were False Discovery Rate corrected changes in several bacterial genera in the rifaximin arm. The genus Clostridia_UCG-014, Christensenellaceae_R-7_group, and Clostridiales family XIII were significantly reduced, and Flavonifractor, was significantly increased compared to SoC. In contrast, there were no compositional changes in the S.boulardii arm or in the SoC arm (Supplementary Fig. 1).
Furthermore, we analyzed the abundance of the Butyrate-acetoacetate CoA transferase gene at 3 months in the Norwegian study participants. We found no difference in levels at 3 months between groups (SoC vs rifaximin, log mean difference -0•05, 95% CI -0•31 - 0•22, P=0•73 and SoC vs S.boulardii, log mean difference 0•22, 95% CI -0•05 - 0•48, P=0•10).
Finally, we found no significant differences in the six minute walk test performance. The baseline-adjusted difference was 6•8 meters (95% CI -12•5 - 26•1, P =0•48), between the rifaximin and the SoC arm and 7•4 meters (95% CI -10•7 - 25•4, P=0•42), between the S.boulardii and the SoC arm.
3.4. Compliance
Compliance was measured by counting the number of tablets returned at the 1-month and 3-month visits. Forty patients (83%) in the rifaximin arm and 41 patients (80%) in the S.boulardii arm had a per-protocol study drug compliance above 80%. In the rifaximin group, the within-group change in bacterial richness was used as an indirect measure of compliance. There was a significant reduction from baseline to the end of intervention (Fig. 3), as expected based on previous experiences [27]. In the S.boulardii group, visual inspection of an end-point PCR specific for the S.boulardii TY Delta element was performed as a coarse measure of compliance, revealing a visible band in the majority of the S.boulardii group and only in a small number of the control group at 3 months (Supplementary text 1).
3.5. Safety
Safety endpoints are defined in supplementary text 2.
Eight patients stopped the study medication due to side effects, three participants in the rifaximin arm and five in the S.boulardii arm. Abdominal pain, obstipation, diarrhea, and bloating were the main causes of discontinuation. One patient developed an allergic exanthema on the torso while two patients experienced acute dyspnea as reported below. All the symptoms subsided after discontinuation of the study drug.
There were nine serious adverse events, four of which occurred in the rifaximin arm, two in the S.boulardii arm, and three in the SoC arm. In the rifaximin arm, three patients experienced shortness of breath, two of whom were admitted to the hospital for stabilization. The third patient was treated by his primary physician. All patients recovered after diuretic treatment. The third patient died three months after the event. The death was out of hospital and not witnessed. One patient in the rifaximin arm received appropriate treatment from his implantable cardiac defibrillator. Two of the serious adverse events were adjudicated to be “not related to the investigational drug” by the independent safety committee, while the other two were adjudicated to be “probably not related to the investigational drug”.
In the S.boulardii arm, one patient was hospitalized due to pulmonary edema related to a paroxysm of atrial fibrillation. He spontaneously converted to sinus rhythm and improved with diuretic treatment. The patient was later hospitalized due to melena and an International Normalized Ratio (INR) of 6•4 during warfarin treatment. Another patient in the S.boulardii arm was hospitalized with dyspnea. He recovered after intensified diuretic treatment. Both were adjudicated as “probably not related to the investigational drug”.
In the SoC arm, one patient was hospitalized with ventricular tachycardia and syncope. He received an appropriate shock from his implantable cardiac defibrillator and was discharged the following day. One patient was hospitalized with pneumonia and ventricular tachycardia. One patient was hospitalized with dyspnea and concurrent atrial flutter. He responded well to rate control and intensified HF medications. All events in the SoC arm were adjudicated as “not related to the GutHeart trial”.
4. Discussion
In the GutHeart trial, we aimed to investigate if modulation of the gut microbiota could improve cardiac function in patients with HF with reduced ejection fraction. Three months of intervention with the probiotic yeast S.boulardii or the locally acting oral antibiotic rifaximin had no effect on LVEF.
Furthermore, the interventions had no effect on any of the secondary endpoints related to cardiac function, exercise capacity, systemic inflammation, and global microbiota function, or composition. However, in the rifaximin group the composition of the microbiota changed significantly from baseline to the end of the intervention. These changes comprised a reduction of three commensals and an increase in one commensal in the class of Clostridia. We cannot rule out that there were more changes further down the phylogenetic tree or that our interventions impacted the gut metagenome. However, none of these potential effects were transferable into any detectable clinical change or increase in the butyrate producing capacity of the microbiota.
The rationale for the trial was based on the premise that the interventions would improve cardiac function by affecting microbiota composition and to subsequently modulate metabolic pathways and gut-related inflammation. However, no evidence of such effects was found in this trial.
We chose S.boulardii as an investigational drug because a pilot trial comprising 20 Brazilian patients with HF showed that intervention with S.boulardii was associated with a decrease in CRP levels and an increase in LVEF [15]. The other investigational drug was rifaximin, an oral antibiotic with bactericidal activity against a broad array of enteric pathogens. Oral antibiotics have been reported to decrease levels of TMAO in animal models [28]. Both drugs have microbiota-restoring effects in conditions associated with dysbiosis and they have been shown to promote butyrate producers in the gut [29], [30], [31].
One could speculate if the different dietary and genetic background between the Norwegian and Brazilian participants might have affected our results. However, as dysbiosis has been demonstrated in heart failure patients in different European as well as Asian populations, we believed that a potential effect is independent of dietary patterns or genetic background [8,32,33].
The degree of dysbiosis is associated with the burden of symptoms in HF [4]. Despite the reduced ejection fraction, most of our patients were in NYHA class II, and the median plasma concentration of NT-proBNP was as low as 964 pg/mL. This corresponds well to a surprisingly high microbiota alpha diversity at baseline compared to that observed in other HF cohorts in which the patients were more symptomatic as evaluated by NYHA-class, had higher levels of NT-proBNP, and used more diuretics [32,34]. Substantial dysbiosis may be a prerequisite for observing eubiotic effects of our interventions.
Several factors, such as diet and commonly used drugs for cardiovascular disease, can influence the composition of the gut microbiota [35,36]. These factors could have confounded our results. However, drugs for HF were evenly distributed across groups (Table 1), and the patients were asked not to change their dietary habits during the intervention period.
Safety concerns have been raised regarding treatments targeting the gut microbiota in HF [37,38]. Importantly, we observed no significant difference in the number or severity of serious adverse events between the intervention groups and the SoC group. Treatment side effects were mild and reversible.
Our trial has several limitations. The main shortcoming is the open label design. Matching placebo products were difficult to obtain due to costs and the distinct smell of yeast of S.boulardii capsules. However, analyses of the predefined endpoints (LVEF, the microbiota composition and function, and circulating biomarkers) were performed in a blinded fashion.
Fecal microbiota transplantation (FMT) represents an interesting interventional concept in microbiota modulation, and would be very interesting as a treatment arm in this trial. However, due to conceivable challenges in study conduction, we opted not to use this form of intervention.
The study was powered to detect a 5 percentage points increase in LVEF; thus, we cannot rule out that a more subtle treatment effect could have been detected in a larger trial. However, the lack of treatment effects both on the diversity of the microbiota composition and on cardiac function strongly suggests that treatment with S.boulardii or rifaximin did not confer a benefit over SoC in our patients with HF.
A major limitation to this trial is that we only have crude measures of study drug compliance. Nevertheless, at group level we observed changes in the microbiota in the rifaximin arm, and we detected S.boulardii DNA in feces in the S.boulardii arm. Combined with a self-reported compliance of about 80%, this suggests that the overall adherence was good..
In conclusion, three months’ intervention with S.boulardii or rifaximin had no clinically significant effect on LVEF, microbiota diversity and function, circulating levels of TMAO, or systemic inflammation in HF with reduced ejection fraction. The treatment was well tolerated.
Our study suggests that optimally treated patients with HF with reduced ejection fraction does not necessarily have a large degree of dysbiosis. Consequently, microbiota modulation using broad-spectrum antibiotics such as rifaximin or unspecific probiotics such as S.boulardii may not be feasible in such patients.
When devising future strategies for targeting the gut-heart axis in HF, one should tailor treatment according to the degree of dysbiosis or directly target specific taxa or metabolites of importance.
Contributors
AA contributed to writing the original draft, visualisation, data curation, formal analysis, investigation, visualization, review, and editing. CM contributed to conceptualisation, project administration, writing original draft, data curation, funding acquisition, investigation, visualization, review, and editing. AF contributed to data curation, investigation, review, and editing. JRH contributed to conceptualisation, data curation, formal analysis, funding acquisition, methodology, review, and editing. SDM, KTL, and AH contributed to investigation, data curation, review, and editing. SH, PA and AL contributed to conceptualization, review, and editing. BH, IG, AS, SS, RKB, and SÅ contributed to data curation, review, and editing. SHH and AG contributed to data curation, formal analysis, methodology, review, and editing. KH contributed to data curation, visualisation, formal analysis, methodology, review, and editing. IS contributed to conceptualisation, data curation, formal analysis, funding acquisition, methodology, review, and editing. LG, MT, and KB contributed to conceptualisation, project administration, data curation, formal analysis, funding acquisition, methodology, review, and editing.
Funding
The trial was funded by grants from the Norwegian Association for Public Health (Nasjonalforeningen for Folkehelsen); Blix foundation for the promotion of medical research, Norway; Stein Erik Hagen's Foundation for Clinical Heart Research, Norway; Ada og Hagbart Waages humanitære og veldedige stiftelse, Norway. Alfasigma and Biocodex kindly provided the investigational medicinal products and Biocodex also provided an independent research grant.
Data sharing statement
The data underlying this article will be shared in accordance with local registration and ethical approval on reasonable request to the corresponding author. There are no publicly available datasets or code.
Declaration of Competing Interest
One co-author discloses a financial relationship with two companies with products or with other financial interests within the field of microbiota. All other authors declare that they have no competing interest, no financial, or other relationships with companies or organizations that might have an interest in the manuscript.
Acknowledgments
We would like to thank members of the Data Safety and Monitoring Board, retired Professors Harald Arnesen and John Kjekshus. We would also like to thank Linn Fosshaug for the assistance with patient recruitment. We would also like to extend our gratitude to all study nurses and laboratory staff at the four study centers for their assistance in the GutHeart trial. At last, we would like to thank all the patients in the GutHeart trial for their participation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103511.
Appendix. Supplementary materials
References
- 1.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113(6) doi: 10.1161/CIRCRESAHA.113.300376. 709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know? Circ Res. 2016;119(1) doi: 10.1161/CIRCRESAHA.116.308030. 159–76. [DOI] [PubMed] [Google Scholar]
- 3.Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16(3) doi: 10.1038/s41569-018-0108-7. 137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4(3) doi: 10.1016/j.jchf.2015.10.009. 220–7. [DOI] [PubMed] [Google Scholar]
- 5.Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol. 2018;71(10) doi: 10.1016/j.jacc.2017.12.057. 1184–6. [DOI] [PubMed] [Google Scholar]
- 6.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38(11) doi: 10.1093/eurheartj/ehw582. 814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017;38(39) doi: 10.1093/eurheartj/ehx342. 2948–56. [DOI] [PubMed] [Google Scholar]
- 8.Troseid M, Andersen GO, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine. 2020;52 doi: 10.1016/j.ebiom.2020.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9) doi: 10.3945/jn.109.104638. 1619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantz S, Falcao-Pires I, Balligand J-L, Bauersachs J, Brutsaert D, Ciccarelli M. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur J Heart Fail. 2018;20(3) doi: 10.1002/ejhf.1138. 445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(16) doi: 10.1016/j.jacc.2007.07.016. 1561–9. [DOI] [PubMed] [Google Scholar]
- 12.Niebauer J, Volk H-D, Kemp M, Dominguez M, Schumann RR, Rauchhaus M. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353(9167) doi: 10.1016/S0140-6736(98)09286-1. 1838–42. [DOI] [PubMed] [Google Scholar]
- 13.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110(11) doi: 10.1073/pnas.1215927110. 4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10) doi: 10.1161/CIRCULATIONAHA.116.024545. 964–77. [DOI] [PubMed] [Google Scholar]
- 15.Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015;179 doi: 10.1016/j.ijcard.2014.11.034. 348–50. [DOI] [PubMed] [Google Scholar]
- 16.Mayerhofer CCK, Awoyemi AO, Moscavitch SD, Lappegard KT, Hov JR, Aukrust P. Design of the GutHeart-targeting gut microbiota to treat heart failure-trial: a Phase II, randomized clinical trial. ESC Heart Fail. 2018;5(5) doi: 10.1002/ehf2.12332. 977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27) doi: 10.1093/eurheartj/ehw128. 20162129–200. [DOI] [PubMed] [Google Scholar]
- 18.Galderisi M, Henein MY, D'Hooge J, Sicari R, Badano LP, Zamorano JL. Recommendations of the European Association of Echocardiography: how to use echo-Doppler in clinical trials: different modalities for different purposes. Eur J Echocardiogr. 2011;12(5) doi: 10.1093/ejechocard/jer051. 339–53. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3) doi: 10.1093/ehjci/jev014. 233–70. [DOI] [PubMed] [Google Scholar]
- 20.Costea PI, Zeller G, Sunagawa S, Pelletier E, Alberti A, Levenez F. Towards standards for human fecal sample processing in metagenomic studies. Nat Biotechnol. 2017;35(11) doi: 10.1038/nbt.3960. 1069–76. [DOI] [PubMed] [Google Scholar]
- 21.<number>[21]</number>BBtools. BBDuk version 38.25 [Available from: https://sourceforge.net/projects/bbmap/.
- 22.Martin M. No 1: Next Generation Sequencing Data Analysis; 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal; Vol 17. [Google Scholar]
- 23.Bushnell B, Rood J, Singer E. BBMerge – accurate paired shotgun read merging via overlap. PLOS ONE. 2017;12(10) doi: 10.1371/journal.pone.0185056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology. 2019;37(8) doi: 10.1038/s41587-019-0209-9. 852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas GM, Maffei VJ, Zaneveld J, Yurgel SN, Brown JR, Taylor CM. PICRUSt2: an improved and extensible approach for metagenome inference. bioRxiv. 2019 [Google Scholar]
- 26.Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277(6) doi: 10.1111/joim.12328. 717–26. [DOI] [PubMed] [Google Scholar]
- 27.Jørgensen SF, Macpherson ME, Bjørnetrø T, Holm K, Kummen M, Rashidi A. Rifaximin alters gut microbiota profile, but does not affect systemic inflammation - a randomized controlled trial in common variable immunodeficiency. Sci Rep. 2019;9(1):167. doi: 10.1038/s41598-018-35367-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341) doi: 10.1038/nature09922. 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponziani FR, Zocco MA, D’Aversa F, Pompili M, Gasbarrini A. Eubiotic properties of rifaximin: disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol. 2017;23(25) doi: 10.3748/wjg.v23.i25.4491. 4491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16(18) doi: 10.3748/wjg.v16.i18.2202. 2202–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.More MI, Swidsinski A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis - a review. Clin Exp Gastroenterol. 2015;8 doi: 10.2147/CEG.S85574. 237–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luedde M, Winkler T, Heinsen FA, Ruhlemann MC, Spehlmann ME, Bajrovic A. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017;4(3) doi: 10.1002/ehf2.12155. 282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui X, Ye L, Li J, Jin L, Wang W, Li S. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8(1):635. doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui L, Zhao T, Hu H, Zhang W, Hua X. Association study of gut flora in coronary heart disease through high-throughput sequencing. Biomed Res Int. 2017;2017 doi: 10.1155/2017/3796359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayerhofer CCK, Kummen M, Holm K, Broch K, Awoyemi A, Vestad B. Low fibre intake is associated with gut microbiota alterations in chronic heart failure. ESC Heart Fail. 2020 doi: 10.1002/ehf2.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11(1):362. doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Z, Wang J, Chen Y, Cong X, Li N, Ding R. Potential risk associated with direct modulation of the gut flora in patients with heart failure. ESC Heart Fail. 2019;6(3) doi: 10.1002/ehf2.12403. 555–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayerhofer CCK, Awoyemi A, Hov JR, Troseid M, Broch K. Reply: potential risk associated with direct modulation of the gut flora in patients with heart failure. ESC Heart Fail. 2019;6(3) doi: 10.1002/ehf2.12437. 557–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.