Abstract
Onion (Allium cepa L.) is a common vegetable, widely consumed all over the world. Onion contains diverse phytochemicals, including organosulfur compounds, phenolic compounds, polysaccharides, and saponins. The phenolic and sulfur-containing compounds, including onionin A, cysteine sulfoxides, quercetin, and quercetin glucosides, are the major bioactive constituents of onion. Accumulated studies have revealed that onion and its bioactive compounds possess various health functions, such as antioxidant, antimicrobial, anti-inflammatory, anti-obesity, anti-diabetic, anticancer, cardiovascular protective, neuroprotective, hepatorenal protective, respiratory protective, digestive system protective, reproductive protective, and immunomodulatory properties. Herein, the main bioactive compounds in onion are summarized, followed by intensively discussing its major health functions as well as relevant molecular mechanisms. Moreover, the potential safety concerns about onion contamination and the ways to mitigate these issues are also discussed. We hope that this paper can attract broader attention to onion and its bioactive compounds, which are promising ingredients in the development of functional foods and nutraceuticals for preventing and managing certain chronic diseases.
Keywords: phytochemicals, antioxidant, anticancer, anti-obesity, anti-diabetic, safety
Introduction
Onion (Allium cepa L.) is widely cultivated and consumed around the world (1). The common onion varieties with three different colors, including red, yellow, and white, are normally available in the food market. As a food item, onion is usually served as a vegetable ingredient in warm dishes by cooking, like baking, boiling, braising, grilling, frying, roasting, sautéing, or steaming. It can also be eaten raw in salads, made into juice, pickled in vinegar, or used as a spice. As an herbal medicine, onion is recommended to relieve or prevent several common diseases, such as atherosclerosis, asthma, bronchitis, and coughs. The health benefits of onion are mainly attributed to its diverse bioactive constituents, such as organosulfur compounds, phenolic compounds, polysaccharides, and saponins (2, 3). Recently, accumulated studies demonstrated the remarkable health functions of onion and its bioactive compounds, including antioxidant (4), antimicrobial (5), anti-inflammatory (6), anti-obesity (7), anti-diabetic (8), anticancer (9), cardiovascular protective (10), neuroprotective (11), hepatorenal protective (12), respiratory protective (13), digestive system protective (14), reproductive protective (15), and immunomodulatory properties (16). Generally speaking, onion consumption is quite safe for the consumers. However, several potential health concerns should not be ignored, such as pesticide residue (17), heavy metal-enrichment (18, 19), microbial contamination (20, 21), and nitrate accumulation (22).
Although the bioactive compounds and certain bioactivities of onion have been discussed in recent reviews (3, 23, 24), this review can provide an updated and more comprehensive understanding about the diverse health functions and safety concerns of onion. The literature summarized in this review was mainly collected from Web of Science Core Collection, PubMed, and Scopus databases from 2016 to 2021, with a focus on the bioactive compounds and health functions of onion, with special attention paid to the relevant molecular mechanisms (Figure 1). The potential safety concerns of onion and the strategies to mitigate these health risks are also discussed. It is expected to attract more attention to the health benefits of onion and its consumption and application in the prevention and management of chronic diseases.
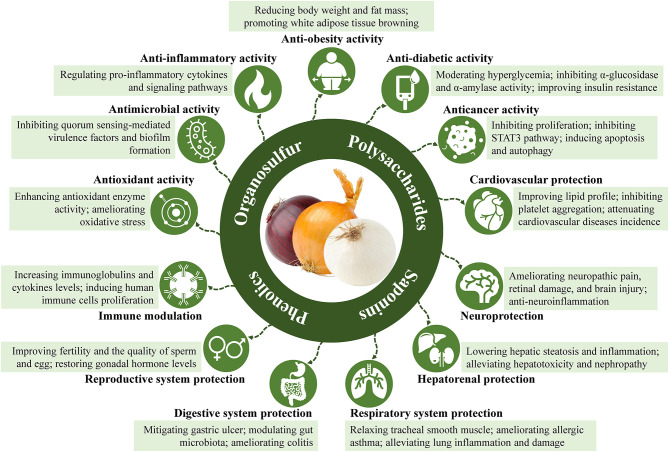
Figure 1.
Bioactive compounds and health functions of onion.
Bioactive Compounds in Onion
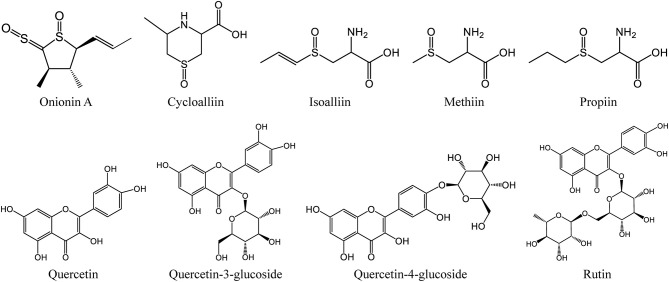
Onion is rich in a variety of phytochemicals with beneficial functional, including organosulfur compounds (25, 26), phenolic compounds (27–29), polysaccharides (30), and saponins (31, 32). The major bioactive compounds of onion are sulfur-containing compounds, such as onionin A and cysteine sulfoxides, as well as the phenolic compounds, such as rutin, quercetin, and quercetin glucosides (Figure 2). It is different for the contents of bioactive compounds among different onion varieties (5). Red onion had the highest contents of anthocyanins and flavonols, followed by the yellow onion, but the white onion contained the lowest amount (33). Besides, the major compounds varied in different layers of onion (34). Quercetin was the major compound in the skin of red onion, while quercetin-4-glucoside was the main compound in its bulb (35).
Figure 2.
The chemical structures of the main organosulfur and phenolic compounds in onion.
Processing can change the bioaccessibility and content of bioactive compounds in onion. The bioaccessibility of total flavonols in onion was not affected by high-pressure processing, but the onion matrix could improve the bioaccessibility of its flavonol (36). It has been found that onion skin quercetin aglycone was more bioavailable than pure quercetin dihydrate in humans (37). The quercetin content was not significantly changed by sautéing (38), but the content and bioaccessibility of phenolic compounds, especially quercetin-derivatives, were found increased by cooking, such as baking, grilling, and frying (39). Besides, the contents of the cysteine sulfoxides, including cycloalliin, isoalliin, methiin, and propiin, were changed differently in onion by heat processing, depending on the cooking methods (40). For instance, their contents were decreased during boiling, but increased during frying, microwaving, and steaming. Furthermore, the flavonoid content was decreased during the processing of black onion, while the contents of isoalliin and fructose were significantly increased (41).
Health Functions of Onion
Many plant-based foods, such as garlic (42), ginger (43), sweet tea (44), dark tea (45), germinated edible seeds and sprouts (46), as well as their bioactive compounds, including resveratrol (47), curcumin (48), rutin (49), quercetin (50), citrus flavonoids (51), and spice essential oils (52) have been demonstrated to possess a variety of health functions. As a traditional and popular food, onion has also been reported with multiple health functions based on in vitro, in vivo, and human studies (Table 1). In the following part, the main health functions and related molecular mechanisms of onion and its bioactive compounds are summarized and discussed in detail.
Table 1.
The health functions and potential mechanisms of onion.
| Product/compounds | Study type | Subjects/cell lines | Dose | Main effects and potential mechanisms | References |
|---|---|---|---|---|---|
| Antioxidant activity | |||||
| Onion methanol extract | In vitro | Rat dopaminergic cell line N27-A | 500 μg/mL | Upregulating antioxidant enzyme (HO-1, NQO1, and catalase) expressions | (6) |
| Onion powder | In vivo | Rats | 10% onion powder in diets | Enhancing the activity of CAT, SOD and GPx enzymes in erythrocytes and liver | (10) |
| Onion extract | In vivo | Ovalbumin-sensitized rats | 35, 70, and 140 mg/kg/d for 21 days | Enhancing the levels of SOD, CAT, and thiol | (53) |
| Onion | In vivo | Potassium bromate-induced oxidative damage in rats | 10, 20, and 30% in diets | Protecting against oxidative damage; reducing MDA levels; restoring the architecture of liver and kidney cells | (54) |
| Phenolic-rich onion extract | In vivo | Broiler chicks | 1, 2, and 3 g/kg diet | Increasing CAT, SOD activity, and GSH level | (55) |
| Pawpaw-onion powder | In vivo | African catfish | 2.5, 5, and 10 g/kg diet | Increasing antioxidant enzyme activity | (56) |
| Onion juice | Clinical trial | Healthy subjects | 100 mL for 8 weeks | Reducing total free radicals and superoxide anions levels; elevating the glutathione content and total antioxidant capacity | (57) |
| Antimicrobial activity | |||||
| Onion liquid and lyophilized extracts | In vitro | Staphylococcus aureus; Escherichia coli | Showing high antibacterial efficiency against Gram-positive bacteria, such as S. aureus | (58) | |
| Lemongrass and onion essential oils | In vitro | Escherichia coli; Salmonella Choleraesuis; Listeria monocytogenes; Staphylococcus aureus | Leafy vegetables treated with the essential oils combination showed higher antibacterial protection and odor acceptability | (59) | |
| Red onion polysaccharide fractions | In vitro | Staphylococcus aureus; Escherichia coli; Bacillus subtilis; Salmonella typhimurium | Showing stronger antibacterial effect against B. subtilis than other bacteria | (30) | |
| Graphene using extract of onion | In vitro | Escherichia Coli; Pseudomonas aeruginosa; Staphylococcus faecalis; Staphylococcus aureus | Showing great antibacterial activity | (60) | |
| Bulb extracts from onion and onion varieties | In vitro | Bacillus cereus; Staphylococcus aureus; Listeria innocua; Escherichia coli; Pseudomonas aeruginosa | Against three Gram-positive species (B. cereus, L. innocua, S. aureus) and P. aeruginosa | (5) | |
| Onion husks non-polar fraction; 7-Keto-(5-6-dihydro)-β-Sitosterol | In vitro | Pseudomonas aeruginosa; Chromobacterium violaceum | Inhibiting Quorum sensing effects; inhibiting swimming motilities | (61) | |
| Silver nanoparticles using extracts of neem, onion and tomato | In vitro | Staphylococcus aureus | Against Gram-positive bacteria Staphylococcus aureus in nutrient agar | (62) | |
| Red onion skin extract | In vitro | Staphylococcus epidermidis; Staphylococcus aureus; Listeria innocua; Enterococcus faecalis | Showing great antibacterial activity | (63) | |
| Onion essential oil | In vitro | Aspergillus, Fusarium, and Penicillium species | Showing fungicidal or inhibitory effects on the growth of fungal species from food | (64) | |
| Onion endophytic bacterium Bacillus endophyticus | In vitro | Magnaporthe oryzae | Showing effective antifungal effect against rice blast pathogen | (65) | |
| Red onion ethanol extract | In vitro | Trichophyton rubrum | Preventing tinea pedis caused by fungal infection | (66) | |
| Anti-inflammatory activity | |||||
| Onion methanol extract | In vitro | Lipopolysaccharide-induced BV-2 microglial cells | 50, 250, and 500 μg/mL | Preventing LPS-stimulated increases of proinflammatory cytokines, TNF-α, IL-6, and IL-1β; decreasing iNOS and COX-2 levels; reducing the release of NO | (6) |
| Red onion skin extract | In vitro | LPS-treated RAW 264.7 cells | Inhibiting IL-6 and IL-1; decreasing the release of NO | (63) | |
| Onion-derived nanoparticles | In vitro | LPS-treated RAW 264 cells | Inhibiting NO production | (67) | |
| Onion bulb extract | In vitro | Isolated bone-marrow derived neutrophils | 0.01, 0.1, 1, 10, and 100 μg/ml | Reversing and preventing colitis in mice via inhibition of proinflammatory signaling molecules and neutrophil activity | (14) |
| In vivo | Dextran sulfate sodium-induced colitis in mice | 100 and 200 mg/kg | |||
| Onion extract | In vivo | Rats | 0.175, 0.35, and 0.7 mg/mL in drinking water | Decreasing in total WBC and PLA2 level; decreasing neutrophil and eosinophil counts; increasing in lymphocytes count | (68) |
| Onion bulb extract | In vivo | Dextran sulfate sodium-induced colitis in mice | 30, 60, 100, and 200 mg/kg | Modulating the expression and the activity of important pro-inflammatory molecules and signaling pathways involved in the inflammatory response | (69) |
| Onion bulb extract | In vivo | Mice | 10, 30, 60, and 100 mg/kg | Alleviating house dust mite-induced perivascular and peribronchial inflammation through EGFR, ERK1/2, and AKT pathway | (13) |
| Onion aqueous extract | In vivo | Carrageenan-induced paw edema in rats | 0.1, 0.5, and 1.5 mg/kg I.P injection | Reducing rat paw edema dose dependently | (70) |
| Anti-obesity activity | |||||
| Onion peel extracts; quercetin and isoquercetin | In vitro | 3T3-L1 cells | Extract 50, 100, and 150 μg/ml; quercetin and isoquercetin 25, 50, and 100 μM | Remodeling white adipocytes to brown-like adipocytes | (27) |
| In vivo | HFD-fed mice | 0.5% in diets for 8 weeks | |||
| Onion peel extract | In vitro | 3T3-L1 cells | 25, 50, 100, 150, 200, 300, 400, and 500 μg/mL | Inhibiting lipid accumulation | (71) |
| In vivo | HFD-fed mice | 36, 90, and 144 mg/kg for 8 weeks | Reducing body weight; lowering fat coefficient and improving serum lipid levels | ||
| Quercetin; red onion extract | In vivo | HFD-fed mice | Diets with 17 mg/kg of quercetin equivalents for 9 weeks | Preventing hypermethylation in the Pgc-1α promoter | (72) |
| Onion oil | In vivo | HFD-fed rats | 46.3 and 92.6 mg/kg/d for 60 days | Reducing body weight gain and tending to decrease adipose tissue weight | (73) |
| Quercetin-rich onion peel extract | Clinical trial | 72 subjects with BMI > 23 kg/m2 | 170 mg capsule contains 50 mg quercetin, 2 capsules/d for 12 weeks | Reducing weight and percentage of body fat; decreasing blood glucose and leptin levels | (7) |
| Quercetin-rich onion powder | Clinical trial | 70 healthy Japanese subjects | 9 g/d for 12 weeks | Lowering alanine aminotransferase; reducing visceral fat area in lower high-density lipoprotein cholesterol subjects | (74) |
| Steamed onion | Clinical trial | 70 overweight subjects | 300 mg capsule contains 37.5% steamed onion; 3 capsules/d for 12 weeks | Reducing percentage of body fat and fat mass with no significant effects on lean body mass | (75) |
| Onion peel extract | Clinical trial | 61 overweight and obese subjects | Capsule contains 50 mg quercetin, 119.2 mg total polyphenol, and 65.0 mg total flavonoid; 2 capsules/d for 12 weeks | Regulating erythrocyte n-6/n-3 ratio and preventing fat accumulation in various body regions | (76) |
| Anti-diabetic activity | |||||
| Onion seed extract | In vivo | Streptozotocin-induced male rats | 200 and 400 mg/kg/d for 28 days | Protecting against adverse effects of diabetes on reproductive system | (77) |
| Fenugreek seeds and onion | In vivo | Streptozotocin-induced diabetes in rats | 10% fenugreek seeds or 3% onion or their combination in diet for 6 weeks | Ameliorating hyperglycemia and its associated metabolic disorders | (78) |
| Fenugreek seeds and onion | In vivo | Streptozotocin-induced diabetes in rats | 10% fenugreek seeds or 3% onion or their combination in diet for 6 weeks | Reducing oxidative stress | (79) |
| Fenugreek seeds and onion | In vivo | Streptozotocin-induced diabetes in rats | 10% fenugreek seeds or 3% onion or their combination in diet for 6 weeks | Alleviating cardiac damage | (80) |
| Fenugreek seeds and onion | In vivo | Streptozotocin-induced diabetes in rats | 10% fenugreek seeds or 3% onion or their combination in diet for 6 weeks | Attenuating diabetic nephropathy | (81) |
| Fenugreek seeds and onion | In vivo | Streptozotocin-induced diabetes in rats | 10% fenugreek seeds or 3% onion or their combination in diet for 6 weeks | Ameliorating eye lens abnormalities | (82) |
| Fenugreek seeds and onion | In vivo | Streptozotocin-induced diabetes in rats | 10% fenugreek seeds or 3% onion or their combination in diet for 6 weeks | Countering the deformity and fragility of erythrocytes | (83) |
| Red onion scales extract | In vivo | Streptozotocin-induced diabetes in rats | 150 and 300 mg/kg/d for 4 weeks | Improving fasting blood glucose and advanced glycation end products levels; elevating serum insulin level; down-regulating inflammatory mRNA expression | (84) |
| Heat-processed onion extract | In vivo | Male rats | 500 mg/kg | Showing anti-diabetic effect by suppressing carbohydrate absorption via inhibition of intestinal sucrase, thereby reducing the post-prandial increase of blood glucose | (85) |
| Fenugreek seeds and onion | In vivo | Streptozotocin-induced diabetes in rats | 10% fenugreek seeds or 3% onion or their combination in diet for 6 weeks | Attenuating diabetic nephropathy via suppression of glucose transporters and renin-angiotensin system | (86) |
| Onion peel extract/onion powder | In vivo | Alloxan-induced diabetes in rats | 1 and 3% onion peel extract, 5 and 7% onion powder in bread | Reducing blood glucose and MDA levels; improving antioxidant enzyme activities | (87) |
| Raw red onion | clinical trial | 53 overweight or obese non-diabetic patients with polycystic ovary syndrome | 2 × 40–50 g/d for overweight and 2 × 50–60 g/d for obese patients or 2 × 10–15 g/d for 8 weeks | Improving insulin resistance markers; increasing the chance of menses occurrence | (88) |
| Anticancer activity | |||||
| Onion methanolic extract | In vitro | MDA-MB-231 cells; A1235 cells | 100 μg/mL in a complete medium | Inhibiting tumor cells proliferation | (89) |
| Onion varieties extract | In vitro | Caco-2 cells | 1:10 dilution (10 μL of extract with 90 μL of growth media) | Inhibiting tumor cells proliferation | (90) |
| Onion bulb extract | In vitro | HeLa cells; HCT116 cells; U2OS cells | IC50: 24.79 μg/mL for HeLa; 24.73 μg/mL for HCT116; 36.6 μg/mL for U2OS | Inducing apoptosis in cancer cells | (91) |
| Flavonol glucosides from red onion waste | In vitro | HeLa cells | 5, 10, 20, 50, and 100 μM | QG, QDG, isoquercetin, and spiraeoside showed potent anticancer effect | (92) |
| Spiraeoside from red onion skin | In vitro | HeLa cells | 0.1, 1, 10, 50, and 100 ug/mL | Inhibiting cell growth; promoting apoptosis by activating caspase-3 and caspase-9; inhibiting the expression of cyclin-dependent kinase 2-cyclin-E | (93) |
| Onion extract | In vitro | AsPC-1, MCF-7, HCT116, HEP2, and HepG2 | Encapsulated on nano chitosan | Decreasing IC50 in cancer cell lines; inducing apoptosis by down-regulating BCL-2 level and up-regulating the activity of caspase-3 and caspase-9 | (94) |
| Onionin A from onions | In vitro | LM8 cells | 4 and 20 mg/kg | Inhibiting tumor proliferation by suppressing Stat3 activation; inhibiting subcutaneous tumor development and lung metastasis | (95) |
| In vivo | LM8 murine tumor-implanted model | 20 mg/kg | |||
| Fresh yellow onion | Clinical trial | Breast cancer patients during doxorubicin-based chemotherapy | 30–40 g/d and 100–160 g/d for 8 weeks | Ameliorating hyperglycemia and insulin resistance during doxorubicin-based chemotherapy | (96) |
| Cardiovascular protection | |||||
| Onion methanol fractions and flavonols | In vitro | Collagen-induced platelet aggregation in rat platelet-rich plasma | 0.5, 1, 3, and 5 mg/mL onion methanol fractions; 0.5, 1, and 2 mg/mL quercetin glycosides | Inhibiting platelet aggregation | (97) |
| Onion peel extract | In vivo | High-cholesterol diet-induced male mice | 100 and 200 mg/kg/d for 12 weeks | Lowering liver weight, total cholesterol, LDL cholesterol, triacylglycerol, atherogenic index, and cardiac risk factor; increasing fecal cholesterol levels | (98) |
| Onion bulb powder | In vivo | High-cholesterol diet-induced male rats | 10% onion powder in high-cholesterol diets for 7 weeks | Altering fecal bile acid composition by modulating microbiome | (99) |
| Onion bulb powder | In vivo | High-cholesterol diet-induced male rats | 10% onion powder in high-cholesterol diets for 7 weeks | Modulating hepatic prostaglandins; enhancing ω-3 oxylipins in the liver; modifying sphingolipids in liver and spleen tissue | (100) |
| Onion bulb powder | In vivo | High-cholesterol diet-induced male rats | 10% onion powder in high-cholesterol diets for 7 weeks | Increasing SOD, CAT, and GPx activities; anti-inflammatory response, and cardiovascular risk biomarkers | (10) |
| Onion extract | In vivo | High-cholesterol diet-induced male rats | 0.5, 1.5, and 4.5 g/kg/d for 4 weeks | Alleviating hyperlipidemia with downregulation of HMGCR and upregulation of LDL receptor | (101) |
| Red wine extract of onion | Clinical trial | Healthy hypercholesterolemic volunteers | 250 mL/d (contains 10.5% of alcohol, 1.4 g/L polyphenols, and 170 mg/L total flavonoids) for 10 weeks | Altering cholesterol; improving antioxidation; inhibiting inflammatory marker levels; attenuating cardiovascular disease incidence | (102) |
| Quercetin from onion skin extract powder | Clinical trial | Overweight to obese adults with hypertension | Capsule contains 132 mg onion skin extract powder, eq. 54 mg quercetin | Acute intake of quercetin does not influence post-prandial blood pressure and endothelial function | (103) |
| Quercetin from onion skin extract powder | Clinical trial | Overweight to obese patients with (pre-) hypertension | 3 capsules/d (eq. 162 mg quercetin) for 6 weeks | Quercetin did not affect glucose, insulin, blood biomarkers of liver and renal function, hematology, and serum electrolytes | (104) |
| Neuroprotection | |||||
| Onion methanol extract | In vitro | Rat dopaminergic cell line N27-A | 500 μg/mL | Upregulating antiapoptotic gene (Bcl-2); protecting against MPP+-induced death | (6) |
| Red onion ethanolic extract | In vivo | Streptozotocin-induced rats | 125 and 250 mg/kg/d for 4 weeks | Improving learning and memory impairments in diabetic rats | (105) |
| Onion leave extract | In vivo | Rats with neuropathic pain | 25, 50, and 100 mg/kg | Ameliorating diabetes-induced and chronic constriction injury-induced neuropathic pain | (106) |
| Onion ethanolic extract | In vivo | 6-hydroxydopamine-induced rats | 50, 100, and 200 mg/kg/d | Reducing malondialdehyde levels; ameliorating cognitive dysfunction | (11) |
| Onion water extract | In vivo | Pterygopalatine artery ligated mice | 300 mg/kg | Ameliorating retinal damage by regulating the expression of neurotrophic factors | (107) |
| Onion outer scale extract | In vivo | Mice with cerebral ischemia-reperfusion injury | Ethyl acetate fraction: 85 and 170 mg/kg; aqueous fraction: 115 and 230 mg/kg | Improving the memory and sensorimotor functions in cerebral injury | (108) |
| Hepatorenal protection | |||||
| Onion juice | In vivo | Doxorubicin-induced rats | 1 mL for 14 days | Preventing doxorubicin -induced hepatotoxicity | (109) |
| Red onion peel extract | In vivo | CCl4-induced rat hepatorenal toxicity | 50 and 100 mg/kg | Ameliorating hepatonephro-linked serum and tissue markers dose dependently | (12) |
| Onion bulb powder | In vivo | High-cholesterol diet-induced rats | 10% in diets for 7 weeks | Increasing liver SOD and GPx activity; decreasing liver protein carbonyls | (10) |
| Red onion scales extract | In vivo | Streptozotocin-induced rats | 150 and 300 mg/kg/d for 4 weeks | Ameliorating kidney histopathological alterations | (84) |
| Onion powder | In vivo | High-fat, high sugar diet rats | 7% in diets for 7 weeks | Lowering hepatic steatosis and hepatic TNF-α gene expression | (110) |
| Quercetin-rich onion powder | Clinical trial | Healthy Japanese subjects | 9 g/d for 12 weeks | Improving liver function; lowering alanine aminotransferase level | (74) |
| Respiratory system protection | |||||
| Onion extract | In vitro | Isolated rat tracheal smooth muscle | 2, 4, 8, 16, 32, and 64 mg/ml add to organ bath every 5 min | Relaxing tracheal smooth muscle via calcium channel blockade or β2-adrenergic stimulatory | (111) |
| Onion aqueous-alcoholic extract | In vivo | Asthmatic rats sensitized with ovalbumin | 0.175, 0.35, and 0.7 mg/mL in drinking water | Decreasing tracheal responsiveness, neutrophil and eosinophil counts; increasing lymphocytes count; reducing monocyte count | (68) |
| Onion extract | In vivo | Nicotine-induced lung damage in rats | 50 mg/kg/d | Attenuating the pathological effect of nicotine in the lung | (112) |
| Onion bulb extract | In vivo | House dust mite-challenged male mice | 10, 30, 60, and 100 mg/kg | Mediating anti-inflammatory effects through the inhibition of the EGFR/ERK1/2/AKT-dependent pathway | (13) |
| Digestive system protection | |||||
| Red onion suspension | In vivo | Rats | 200 and 500 mg/kg | Mitigating various experimental triggers of gastric mucosal injury | (113) |
| Onion powder | In vivo | Broiler chicks | 1.5, 2, and 2.5 g/kg in diet | Improving the population of gut microflora and intestinal histomorphology | (114) |
| Onion quercetin monoglycosides | In vivo | High-fat diet fed rats | 0.15% (quercetin:quercetin monoglycosides, 98:2 and 69:31) in diet for 4 weeks | Increasing the enzymatic activity of the intestinal microbiota | (115) |
| Onion quercetin monoglycosides | In vivo | Dextran sulfate sodium-induced colitis in mice | 0.15% (quercetin:quercetin monoglycosides, 98:2 and 69:31) in diet | Reducing dextran sulfate sodium-induced colitis | (116) |
| Onion bulb extract | In vivo | Dextran sulfate sodium-induced colitis in mice | 30, 60, 100, and 200 mg/kg | Reducing colitis severity; regulating expression and activity of pro-inflammatory molecules and signaling pathways | (69) |
| Onion bulb extract | In vitro | Isolated bone-marrow derived neutrophils | 0.01, 0.1, 1, 10, and 100 μg/ml | Reducing the percentage of viable bone-marrow derived neutrophils; increasing spontaneous apoptosis | (14) |
| In vivo | Dextran sulfate sodium-induced colitis in mice | 100 and 200 mg/kg | Reducing colitis severity; regulating colonic expression/activity profile of pro-inflammatory molecules | ||
| Phenolic-rich onion extract | In vivo | Broiler chicken | 1, 2, and 3 g/kg in diet | Improving growth rate by improving amino acid ileal digestibility and intestinal histology | (55) |
| Reproductive system protection | |||||
| Cysteine sulfoxides | In vitro | Testis-derived I-10 cells | 0.3, 1, and 3 mg/mL | Enhancing progesterone production via activation of the protein kinase A pathway | (117) |
| Onion juice | In vivo | Rats | 3 mL/d | Increasing testosterone level | (118) |
| Onion juice | In vivo | Rats | 3 mL/d | Against permethrin-induced testis damages | (119) |
| Onion juice | In vivo | Rats | 3 mL/d | Maintaining reproductive ability and improving sexual activities | (120) |
| Onion juice | In vivo | Rats | 3 mL/d | Restoring permethrin-induced reductions in hormonal of FSH and LH levels, and gene expression of LHCGR and SF1 | (121) |
| Onion juice | In vivo | Rats | 40 mg/kg/d | Improving the sperm quality and fertility after testicular torsion/detorsion | (15) |
| Onion extract | In vivo | Rats | 100 and 1,000 mg/kg | Improving sperm count, motility, and morphology; ameliorating sera testosterone and SOD levels | (122) |
| Onion extract | In vivo | Rats | 500 mg/kg | Protecting against dexamethasone-induced testicular damage in rats | (123) |
| Onion juice | In vivo | Rats | 5 mL/kg for 21 days | Protecting against maternal dexamethasone-induced reproductive toxicity in rat female offspring | (124) |
| Onion juice | In vivo | Rats | Intracavernosal injection of 200 uL | Improving dutasteride-induced erectile dysfunction in rats | (125) |
| Onion extract | In vivo | Brown laying hens | 0.0032% in diet | Improving egg quality and productive performance | (126) |
| Immune modulation | |||||
| Onion bulb extract | In vitro | NK CD16+ immune cells | Inducing the growth of CD16+ natural killer cells | (127) | |
| Onionin A | In vitro | CD4+ and CD8+ cells | 10, 30, 50, and 100 μM | Improving the activity of lymphocytes | (95) |
| In vivo | Tumor-bearing mice | 20 mg/kg/d for 2 weeks | Preventing the immunosuppressive activities of macrophages | ||
| Onion extract | In vivo | Immune-suppressed rats | 500 mg/kg/d for 4 weeks | Increasing the levels of cytokines (TNF and IL-6) and immunoglobulins (IgG and IgM) | (16) |
| Onion extract | In vivo | Ovalbumin-sensitized rats | 35, 70, and 140 mg/kg/d for 21 days | Decreasing the levels of IL-4 and IgE | (53) |
Antioxidant Activity
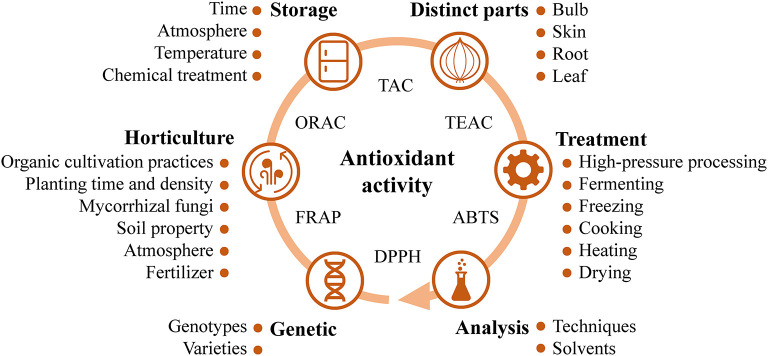
Onion is a good source of natural antioxidants (128). Many studies have been carried out to evaluate the antioxidant activities of onion, and found that onion exhibits strong antioxidant properties by using a series of in vitro assays, including 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 1,1-diphenyl-2-picrilhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), lipid peroxidation, oxygen radical absorbance capacity (ORAC), total antioxidant capacity (TAC), and trolox equivalent antioxidant capacity (TEAC) assays.
Many factors were reported to influence the antioxidant activity of onion, such as the genetic background, horticultural techniques, storage conditions, distinct parts, extraction methods, and processing technologies (Figure 3). Several studies reported that the antioxidant activity varied among different onion cultivars or varieties (5, 129–132), probably related to their genetic background (133). In addition, organic cultivation practices (28, 134), sulfur bentonite-organic-based fertilizers (135), and mycorrhizal fungi (136) were reported to improve the content of bioactive compounds with antioxidant activities in onions. The content of phenolics in the onion bulb and its antioxidant property were increased with the application of mycorrhizal inocula, humic acids, and elevated atmospheric CO2 (137). Planting time and density were also found to influence the antioxidant components of onion seeds (138). Besides, stored atmosphere conditions could affect the quality and bioaccessibility of total phenolics and antioxidant activity of the floral stem of the second-year onion resprout (139). Washing the fresh-cut onions with a combination of nisin and citric acid was reported to increase the total phenolic and flavonoid contents, and antioxidant capacity during storage (140). Sprouting of onion also increased its antioxidant activity, and contents of total phenolics and flavonoids (141, 142). Furthermore, the antioxidant activities in distinct parts of red onion, such as the dry skin and edible portion, were segregated based on the principal component analysis, probably due to the former rich in quercetin while the latter rich in quercetin-4-glucoside (35). The influences of food processing on antioxidant capacities of onions were investigated as well, including drying (143, 144), freezing (145), heating (41, 145), sautéing (38), and high-pressure processing (36). For instance, heating and freezing were found to reduce antioxidant activity of onion (145), while sautéing did not significantly change it (38).
Figure 3.
The main influence factors for antioxidant activity of onion.
Onion also exhibits antioxidant activity in cell and animal models. The expression of antioxidant enzymes, including catalase (CAT), NAD(P)H quinone dehydrogenase 1 (NQO1), and hemeoxygenase-1 (HO-1), was upregulated by onion extract in N27-A cells (6). In addition, several studies demonstrated that onion treatment could improve the antioxidant status of animals. Onion was effective for protection against oxidative stress by enhancing the activity of antioxidant enzymes, such as superoxide dismutase (SOD), CAT, and glutathione peroxidase (GPx), in hypercholesterolemic rats (10). The oxidative stress in the liver and kidney was ameliorated by pre-treatment with red onion peel extract in carbon tetrachloride-challenged rats (12). Onion fortified feed ameliorated the liver and kidney oxidative damages in rats administered with potassium bromate (54). Dietary addition of onion extract and combining onion peel powder with pawpaw seed were found to increase antioxidant enzyme activity in broiler chicks (55) and African catfish (56), respectively. Furthermore, a clinical trial revealed that drinking onion juice (100 mL) for 8 weeks could reduce total free radicals and superoxide anions levels, while elevate the glutathione content and total antioxidant capacity in healthy subjects (57).
Overall, onion exhibits strong antioxidant effects, and many factors could affect the antioxidant capability of onion. Although the antioxidant activity of onion has been extensively investigated, the related antioxidant molecular mechanism has been much less explored, which should be further clarified in the future.
Antimicrobial Activity
Onion extracts and their derived bioactive compounds, such as thiosulfinate compounds, phenolic compounds, polysaccharides, and essential oils, have been reported to possess potent antibacterial properties (5, 30, 58, 146), antifungal activities (64, 66), and antiviral effects (147). Different drying methods, such as microwave drying, air drying, and freeze drying, were performed to evaluate the influence of drying processes on its antimicrobial activity, of which freeze-dried onion bulbs showed a stronger antimicrobial property (148).
Onion fiber-based composite materials combining with isolated flavonoids from onion skins were reported to exhibit certain antibacterial activity against Staphylococcus aureus and Escherichia coli (149). Moreover, it was demonstrated that gold nanoparticles (150), silver nanoparticles (151), graphene (60), and polymeric films (63) containing onion extracts exhibited excellent antibacterial properties against both gram-positive and gram-negative bacteria. Quorum sensing is very important for the coordination of bacterial virulence during infection. One result found that onion organic extracts and quercetin had interference on quorum sensing-regulated production of violacein and swarming motility in Pseudomonas aeruginosa and Serratia marcescens, of which quercetin aglycone reduced violacein production while quercetin aglycone and quercetin 3-β-D-glucoside inhibited bacterial motility (152). It is surprising that the biofilm formation, another crucial factor for antimicrobial resistance controlled by quorum sensing system, was not affected by the onion extracts or quercetin (152). In other studies, quorum sensing-mediated virulence factors in pathogens, such as biofilm formation, were found to be inhibited by the β-sitosterol derived compounds from onion husk extract (61) and quercetin 4′-O-β-D glucopyranoside from onion peel extract (153). Besides, onion essential oil was revealed to possess anti-biofilm activity against Listeria monocytogenes as well (154).
Onion essential oil was reported to showed fungicidal or inhibitory effects on the growth of fungal species isolated from food, including Aspergillus, Fusarium, and Penicillium species (64). Biosynthesis of silver nanoparticles using onion endophytic bacterium Bacillus endophyticus showed an effective antifungal effect against rice blast pathogen Magnaporthe oryzae with abnormal mycelia morphology and 88% inhibition rate of mycelium diameter (65). Besides, the ethanol extract of red onion was effective in preventing tinea pedis caused by the fungal infection of Trichophyton rubrum (66).
Therefore, onion has been demonstrated to inhibit the growth of microbes, showing great potential to be used as a natural preservative in the food industry, such as maintaining meat quality during refrigerated storage (155).
Anti-inflammatory Activity
Onion also exhibited anti-inflammatory property, showing protective effects against inflammation-related diseases, such as neuroinflammation (6, 156), allergic inflammation (13), lung inflammation (68), colitis (14, 69), and paw edema (70). The anti-neuroinflammatory activities of onion extract were investigated in lipopolysaccharide (LPS)-induced BV-2 microglial cells. The methanol extract of onion could reduce the nitric oxide (NO) release by down-regulating the mRNA and protein levels of cyclooxygenase-2 (COX2) and inducible NO synthase (iNOS), and attenuate the elevation of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 (6). Onion-derived nanoparticles and flavonoids from onion peels were reported to prevent the LPS-stimulated NO production in RAW264 cells (67) and BV-2 cells (156), respectively. Besides, the extract of onion bulb alleviated house dust mite-induced perivascular and peribronchial inflammation by inhibiting the epidermal growth factor receptor (EGFR)/extracellular signal-regulated kinase (ERK1/2)/protein kinase B (PKB/AKT) signaling pathway (13). Moreover, onion extract significantly reduced lung inflammatory cells, including monocyte, neutrophil, and eosinophil in asthmatic rats (68). Onion bulb extract was reported to both prevent and reverse colitis by regulating some pro-inflammatory signaling pathways, such as mechanistic target of rapamycin (mTOR), mitogen-activated protein kinase family (MAPK), cyclooxygenase-2 (COX-2), and tissue-inhibitors of metalloproteinases (TIMP), as well as several molecules involved in the apoptotic pathway, such as caspase-3, caspase-8, cytochrome c, B-cell lymphoma-extra-large (Bcl-XL), and Bcl-2 in mice (14, 69). In addition, intraperitoneally injection of onion aqueous extract dose dependently reduced Carrageenan-induced paw edema in rats (70). Therefore, onion can be a good food resource with anti-inflammatory activity for the prevention of inflammation-related diseases.
Anti-obesity Activity
Promoting the browning of white adipose tissue is a promising strategy for the prevention of obesity. Quercetin from onion peel has been demonstrated to have browning effects in 3T3-L1 adipocytes and the white adipose tissue of mice (157). Several animal studies and clinical trials have reported that onion is effective in the prevention and management of obesity. Onion peel extract inhibited lipid accumulation in 3T3-L1 cells and reduced body weight in high-fat diet-fed mice via down-regulating the expression of lipogenesis-related genes (71). Red onion extract or quercetin supplementation could ameliorate obesity and insulin resistance in mice fed with a high-fat diet (72). Oral administration of onion oil prevented the body weight gain of rats triggered by high-fat diets (73). Moreover, dietary supplementation of onion peel extract was found to reduce the body weight, body fat mass, and percentage of body fat in overweight and obese Korean subjects (7, 76). Consumption of steamed onion resulted in a positive change of metabolic parameters, lowering the levels of triglycerides and C-peptide, and reduced the percentage of body fat, total body fat, visceral fat, and subcutaneous fat in overweight people (75). In addition, daily intake of onion powder improved the visceral fat area in healthy Japanese subjects with a high-density lipoprotein cholesterol level between 40 and 74 mg/dL (74). Generally, onion and its bioactive compounds have potential application in the management of obesity.
Anti-diabetic Activity
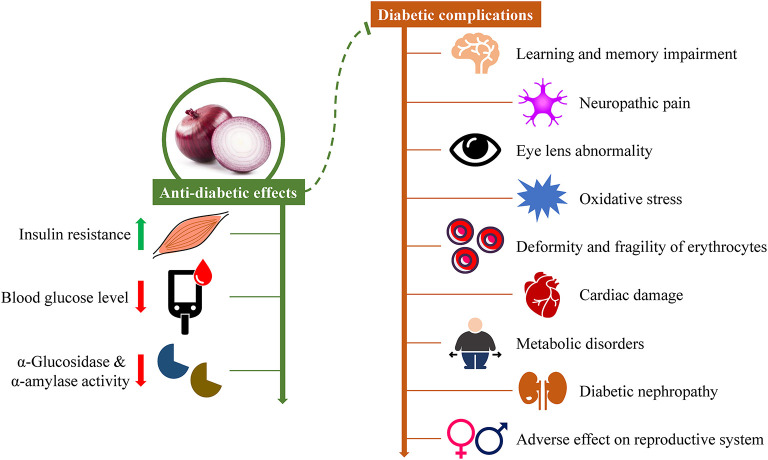
Increasing evidence from in vitro and in vivo studies have demonstrated that onions can not only ameliorate diabetes, but also treat different diabetic complications (Figure 4). Several studies indicate that onion exhibits antidiabetic potential in vitro. The extracts of onion skin or onion solid waste showed a remarkable inhibitory activity toward α-glucosidase and α-amylase, and the enzyme inhibitory activity was in a dose-dependent manner (158, 159). Besides, onion-based green synthesized silver nanoparticles were found to exhibit excellent α-glucosidase and α-amylase inhibitory activities (8). In another study, onion fiber concentrates were revealed to reduce starch digestibility and glucose production rate by suppressing α-amylase activity (160).
Figure 4.
The effects of onion in diabetes and diabetic complications.
Onion also exhibits antidiabetic potential in vivo. The heat-processed onion extract was found to have a high content of Amadori rearrangement compounds, which reduced the post-prandial carbohydrate absorption and blood glucose levels by inhibiting intestinal sucrase activity in rats fed with sucrose or starch meals (85). Moreover, hyperglycemia and its associated metabolic disorders were reported to be ameliorated by dietary supplementation with fenugreek seeds and onion in diabetic rats (78). Hyperglycemia-induced osmotic and oxidative stress is a primary factor in the progression of diabetic complications. It has been reported that intake of fenugreek seeds and onion could also reduce oxidative stress (79), ameliorate eye lens abnormalities (82), alleviate cardiac damage (80), attenuate diabetic nephropathy (81, 86), and counter the deformity and fragility of erythrocytes (83) in STZ-induced diabetic rats. Tamtaji et al. (105) reported that the ethanolic extract of onion had a protective effect on learning and memory deficits in diabetic rats. Furthermore, the anti-diabetic activities of the inedible parts of onion, including skin, seeds, and leaves, have been investigated in STZ-induced diabetic rats as well. Red onion scales extract could improve the levels of fasting blood glucose and advanced glycation end products, enhance serum insulin level, and ameliorate diabetic nephropathy (84). The blood glucose and malondialdehyde (MDA) levels were declined in alloxan-induced diabetic rats fed with wheat bread supplemented with onion powder or onion peel extract (87). Onion seed extract showed a protective activity against the adverse side effects of diabetes in rats (77). The leaf extract of onion was found to ameliorate diabetes-induced neuropathic pain (106).
Onion exhibits antidiabetic potential in humans. In a randomized placebo-controlled clinical trial, daily ingestion of fresh yellow onion in breast cancer patients receiving doxorubicin-based chemotherapy was found to ameliorate the hyperglycemia and insulin resistance (96).
Overall, onion can fight against diabetes by reducing oxidative stress, moderating hyperglycemia, improving insulin resistance, and ameliorating various histopathological changes.
Anticancer Activity
Allium vegetables receive extensive concerns because of their beneficial effects against numerous diseases, especially in treating cancer and alleviating the side effects of current anticancer therapies, which are associated with their bioactive compounds, such as sulfur compounds, flavonoids, and saponins (161, 162). The consumption of Allium vegetables was found to be negatively associated with the risk of diverse cancers, including breast cancer (163), gastric cancer (164), colorectal cancer (165), and upper aerodigestive tract cancers (166).
Many studies have been carried out to evaluate the anticancer activities of the common onion (92, 163, 167–169), other onion varieties, such as Allium cepa L. var. proliferurn Regel and Allium cepa L. Aggregatum group (90, 170, 171), and other Allium species, such as garlic (Allium sativum L.) (163, 172), leek (Allium ampeloprasum L.) (26), chive (Allium schoenoprasum L.), Welsh onion (Allium fistulosum L.) (173), Chinese onion (Allium chinense) (174, 175), and wild edible onions (Allium flavum and Allium carinatum) (176). By in silico approach, it has been found that onion-derived quercetin and diosgenin may take a role in the prevention and treatment of cancer by targeting on axon guidance receptor, neuropilin-1 (167). Onion extract or its major bioactive compounds showed potent anticancer activities, including cytotoxic, antiproliferative, anti-migratory, and apoptosis-inducing activities, in different cancer cells, such as human cervical carcinoma cell line (HeLa), myeloma cancer cell line (P3U1), pancreatic cancer cell line (AsPC-1), larynx cancer cell line (HEP2), colon cancer cell lines (SW620 and HCT116), adenocarcinoma cell line (Caco-2), glioblastoma cell line (A1235), liver cancer cell line (HepG2), and breast cancer cell lines (MDA-MB-231 and MCF-7) (26, 128, 130–133, 139–141]. 8-C-(E-phenylethenyl)quercetin, a novel compound from onion/beef soup, could cause G(2) phase arrest and inhibit the proliferation of colon cancer cells, and induced autophagic cell death, but not apoptotic cell death, by activating extracellular signal-regulated kinase (ERK) (168). Onion A, a sulfur compound from onion, exhibited antitumor effects by inhibiting the activation of suppressing signal transducer and activator of transcription-3 (STAT3) in myeloid lineage cells, and impaired the development of subcutaneous tumor and lung metastasis in tumor-bearing mice (95). Spiraeoside, isolated from red onion skin, exhibited promising anti-cancer effect against HeLa cell, could promote apoptosis by activating the expression of caspase-3 and caspase-9 (94). Recently, it was found that the anticancer activities of onion extract were enhanced by encapsulating on nano chitosan in multiple cancer cell lines (94). Besides, a wild edible onion showed a synergistic anticancer effect with doxorubicin against human hepatoma (HepG2) and lung carcinoma (A549) cells, and could protect from doxorubicin-induced cytotoxicity in human normal fibroblasts (MRC-5) and in vivo zebrafish models (176). Moreover, the intake of fresh onion was reported to reduce fasting blood glucose and improve insulin sensitivity in doxorubicin-treated breast cancer patients (96). Therefore, onion is an excellent anticancer vegetable, but its anticancer effect should be further verified in more clinical trials.
Cardiovascular Protection
Studies have revealed that onion can effectively improve lipid profile and inhibit platelet aggregation, attenuating the incidence of cardiovascular diseases. The methanol extract, quercetin, and quercetin glucosides from onion were found to inhibit collagen-induced platelet aggregation by using rat platelet-rich plasma (97).
Many studies have focused on the hypocholesterolemic effect of onion and its bioactive compounds in rodents fed with high-cholesterol or high-fat diets. Onion could effectively decrease the levels of total cholesterol, triglyceride, and low-density lipoprotein cholesterol in hyperlipidemic animals (98, 101, 177). Li et al. (101) reported that polyphenol-rich onion extract ameliorated hyperlipidemia with upregulation of low-density lipoprotein receptor (LDLR) and downregulation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase (HMGCR) in the liver of Sprague-Dawley rats. Kang et al. (98) found that quercetin-rich onion peel extract resulted in a higher level of fecal cholesterol, and lower values of atherogenic index and cardiac risk factor in high-cholesterol diet-fed mice, with upregulation of LDLR and cholesterol 7-α-monooxygenase (CYP7A1), indicating the cholesterol-lowering effect of onion via fecal excretion. The fecal bile acid content was reported to be modified by the dietary addition of onion in high-cholesterol diet-fed rats (178). Dietary onion significantly increased the activity of antioxidant enzymes and improved the anti-inflammatory response and cardiovascular risk biomarkers in rats fed with a high-cholesterol diet (10). Besides, the high-cholesterol diet-induced shifts in the lipid mediators, such as oxylipin and sphingolipid profiles, were also found to be modified by the dietary supplementation of onion in hypercholesterolemic Wistar rats (100). The antihyperlipidemic potentials of fermented onion and onion volatile oils were investigated as well, and both of them had certain positive effects on hyperlipidemia animals (73, 179). Moreover, the serum triglycerides levels were found to be reduced by consumption of steamed onion in overweight subjects (75). The intake of red wine extract of onion effectively reduced the total cholesterol and low-density lipoprotein cholesterol in healthy hypercholesterolemic subjects, while ameliorated the inflammatory responses and antioxidant defenses as well (102).
In addition, dietary fenugreek seeds and onion were reported to protect hyperglycemia-induced cardiac damage by inhibiting the activation of angiotensin-converting enzyme and angiotensin type 1 receptor in the heart of STZ-induced diabetic rats (80). Hypertension is known to be a key risk factor for cardiovascular disorders, however, the intake of quercetin from onion skin appeared to have no beneficial effects on blood pressure and endothelial function in subjects with hypertension (103, 104). More animal studies and clinical trials are needed for better understanding the cardiovascular protective effects and related mechanisms of onions and their bioactive compounds.
Neuroprotection
Several studies have revealed that onion possesses anti-neuroinflammatory activity (6, 156), ameliorates neuropathic pain (106), and exerts neuroprotective effects against Parkinson's disease (11), memory impairment (105), cerebral injury (108), and retinal damage (107).
The anti-neuroinflammatory activity of onion and its bioactive compounds was investigated by using the LPS-stimulated BV2 microglia culture model (6, 156). Onion treatment prevented the LPS-induced increases of NO, TNF-α, IL-6, and IL-1β. The ameliorative effect of onion leave extract on neuropathic pain in rats was demonstrated by using two models, chronic constriction injury model and STZ-induced diabetic model (106). Onion leave extract significantly improved the behavioral and oxidative stress parameters as well as the sciatic nerve histopathological changes in both models. Onion ethanolic extract was reported to reduce malondialdehyde levels, ameliorate cognitive dysfunction, and prevent neuronal injury in 6-hydroxydopamine-induced rat model of Parkinson's disease (11). The neuroprotective effect of onion on learning and memory abilities was assessed in STZ-induced diabetic rats, and it was found that red onion ethanolic extract treatment could improve the learning and memory impairments in diabetic rats with reduced escape latency and traveled distance in Morris water maze test and increased step-through latency in passive avoidance test (105). The neuroprotective effect of onion on cerebral injury was evaluated in a cerebral ischemia/reperfusion mouse model, which was established by bilateral common carotid artery occlusion followed by reperfusion (108). It was revealed that the outer scale extract of onion could improve the memory and sensorimotor functions in cerebral injury mice by reducing cerebral infarct size and oxidative stress. The ischemia/reperfusion-induced retinal injury by pterygopalatine artery ligation in mice was used to investigate the neuroprotective effect of onion on neuronal damage, and it was found that onion water extract may protect from the retinal damage by regulating the expression of neurotrophic factors, such as B-cell lymphoma 2 (BCl-2), glial cell-derived neurotrophic factor (GDNF), glial fibrillary acidic protein (GFAP), and brain-specific homeobox/POU domain protein 3B (Brn3b) (107). Dietary fenugreek seeds and onion showed a protective effect on diabetic-cataract in STZ-induced diabetic rats (82).
Hepatorenal Protection
The intake of functional natural products or constituents has been considered as a complementary way for the management of liver and kidney diseases. Onion is rich in multiple bioactive compounds with hepatorenal protective activities.
The phenolic-rich extract of red onion peels protected against carbon tetrachloride-induced oxidative stress in the liver and kidney tissues of rats (12). Dietary onion ameliorated antioxidant defense in hypercholesterolemic rats with increased activities of SOD, CAT, and GPx in the liver (10). The doxorubicin-mediated hepatotoxicity, parenchymal necrosis, and biliary duct proliferation, were alleviated by pre-treating with onion extract in rats and this hepatoprotective effect was attributed to the antioxidant capabilities of onion extract, which reduced the levels of glutathione and malondialdehyde, while enhanced the levels of SOD and GPx in the liver (109). Diabetic nephropathy, including renal architecture and functional abnormalities as well as podocyte damages, was attenuated by dietary fenugreek seeds and onion in rats by inhibiting the renin-angiotensin system and glucose transporters (86). The STZ-induced diabetic nephropathy in rats was also ameliorated by treating with red onion scales extract, and it was found to be associated with its metabolite fingerprint (84). Besides, several metabolites and related metabolic pathways in the liver were reported to be modulated by the onion supplementation in hypercholesterolemic Wistar rats (178). The liver weight, lipid profile, and lipid mediators were found to be ameliorated by onion in hypercholesterolemic animals (98, 100). Dietary intake of onion was revealed to lower hepatic steatosis, inflammation, and hepatic TNF-α expression in high-fat, high-sugar diet-fed rats (110). Moreover, the levels of alanine aminotransferase (ALT), a liver function marker, were significantly lower in healthy subjects with a daily intake of quercetin-rich onion, indicating that onion may be beneficial for improving liver function in humans (74).
Respiratory System Protection
Onion has been recommended for the treatment of respiratory disorders, such as coughs, asthma, and bronchitis. Several studies have demonstrated that onion could relax tracheal smooth muscle (111), ameliorate allergic asthma (13), reduce lung inflammation (68), and attenuate lung damage (112).
Onion exhibited relatively potent relaxant effects on potassium chloride or methacholine-contracted tracheal smooth muscle in a dose-dependent manner, and the calcium channel blockade and/or β2-adrenergic stimulatory were involved in these effects (111). The onion bulb extract was reported to inhibit house dust mite-induced increase in airway cellular influx and goblet cell hyper/metaplasia, reduce ex vivo eosinophil chemotaxis, and ameliorate peribronchial and perivascular inflammation (13). The preventive and anti-inflammatory activities of onion on tracheal responsiveness and lung inflammation was demonstrated in asthmatic rats, and it was found that drinking water with onion extracts significantly reduced tracheal responsiveness and lung inflammatory cells, including monocytes, neutrophils, and eosinophils (68). Besides, the levels of antioxidant enzymes, SOD and CAT, were increased in bronchoalveolar lavage fluids of the ovalbumin-sensitized rats treated with onion extract, while the levels of oxidant markers, such as malondialdehyde, nitrogen dioxide (NO2), and nitrate (NO3−), were reduced (53). Reduction of lung malondialdehyde and elevation of lung SOD, CAT, and glutathione were also found in nicotine-induced lung damage rats treated with onion extract, which might be the main mechanism for the protective effect on lung damage (112). Moreover, the onion-derived bioactive compound, onionin A, was reported to inhibit lung metastasis in tumor-bearing mice (95).
Since the end of 2019, a new virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has triggered the worldwide pandemic of coronavirus disease 2019 (COVID-19). Up to January 2021, data from the web-based COVID-19 dashboard has shown that more than 100 million people were infected with more than two million deaths (180). The infection of SARS-CoV-2 may result in severe pneumonia, and the common symptoms of COVID-19 include fever, cough, nasal congestion, sore throat, and breathing difficulties (181). Studies have demonstrated that onion and its bioactive compounds could attenuate lung inflammation and protect against diverse respiratory disorders. Therefore, it may be possible that onion and its bioactive compounds have the potential to fight against SARS-CoV-2 and can be consumed in our diets for the prevention of COVID-19, which still needs further investigation.
Digestive System Protection
Onion has been shown to have a protective effect on the digestive system, such as mitigate gastric ulcer (113, 182), modulate gut microbiota (114–116, 183), and ameliorate colitis (14, 69, 116).
It has been reported that raw onion could inhibit histamine-induced gastric acid secretion and mitigate ethanol-stimulated gastric ulcer in rats, whereas boiled onion showed reduced potency (182). Alqasoumi (113) demonstrated that pre-treatment with onion could ameliorate gastric mucosal injury and ulcer index elicited by multiple factors, such as pylorus ligation, hypothermic restrainment, indomethacin, and necrotizing agents. Dietary supplementation of onion powders could modulate gut microbiota with an increased number of lactic acid bacteria in common carp juveniles (183). Onion could also be added into poultry feed as a natural growth promoter, which exhibited a positive effect on gut microbiota and intestinal histomorphology (55, 114). Onion-derived bioactive compounds, such as quercetin and quercetin monoglycosides, were found to enhance the enzymatic activity of gut microbiota in rats (115). Quercetin monoglycosides were reported to modulate the diversity of gut microbiota in colitis mice induced by dextran sodium sulfate (116). Moreover, the severity of colitis in mice was revealed to be reduced by administering with onion bulb extract, no matter it was given before, after, or at the same time of the colitis induction (14, 69). Besides, Allium species, including onion, showed protective effects against upper aerodigestive tract and gastrointestinal cancers (164–166, 168).
Reproductive System Protection
Infertility has been considered a public health problem. Studies have shown that onion exhibited protective effects against adverse effects of chemical toxicity (119–122) and bacterial infection (118) on reproductive system, and could improve fertility and the quality of sperm and egg (15, 126).
Testosterone, a steroid hormone, plays a key role in the development of male sexual characteristics, and its reduction may cause infertility (184). It has been reported that onion extract-contained cysteine sulfoxides could stimulate the production of the testosterone precursor, progesterone, by activating the protein kinase A signaling pathway in a testis-derived cell line (I-10) (117). Bacterial infection is one of the main factors that cause infertility in men. The adverse effects of Escherichia coli infection were reduced in male rats receiving onion juice, while the total antioxidant capacity and testosterone level was increased (118). Besides, onion could restore the permethrin-induced reductions in hormonal levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) as well as the expression of some key genes, such as luteinizing hormone/choriogonadotropin receptor (LHCGR) and steroidogenic factor 1 (SF1) (121). Onion also showed protective effects on testicle parameters and spermatogenesis in rats against the destructive effects of some insecticides or herbicides, such as permethrin, pyrethroid and paraquat (119, 120, 122). Onion seed extract was reported to have a protective activity against the negative effects of diabetes on the reproductive system in STZ-induced diabetic rats (77). Furthermore, the sperm quality and fertility were found to be improved in onion juice-treated adult male Wistar rats after the testicular torsion/detorsion (15). Recent study showed that onion juice could restore the erectile dysfunction induced by dutasteride in rats (125). Onion extract could prevent testicular damage induced by dexamethasone in rats (123) and the reproductive dysfunction in female rat offspring induced by maternal dexamethasone during lactation also found to be ameliorated by onion juice treatment (124). Dietary supplementation of onion extract was revealed to improve the egg quality and productive performance in laying hens (126). Moreover, onionin A showed an inhibitory effect on the progression of ovarian cancer by inhibiting tumor cell proliferation (9). In addition, results from clinical trials indicated that red onion intervention has certain beneficial effects in female subjects with polycystic ovarian syndrome (88, 185).
Immune Modulation
Onion is thought to be beneficial for the immune system. Onion bulb extract showed an in vitro ability to induce the proliferation of human immune cells, particularly the CD16+ natural killer cells (127). Another study demonstrated that onion-derived onionin A could improve the activity of lymphocyte and prevent the immunosuppressive activities of macrophages and myeloid-derived suppressor cell in the tumor microenvironment (95). An in vivo study showed that onion extract had a protective effect against dexamethasone-induced immunosuppressive effects in Wistar rats, such as ameliorating white blood cell counts, enhancing antioxidant activities, and increasing the levels of cytokines (TNF and IL-6) and immunoglobulins (IgG and IgM) (16). The immunomodulatory property of onion was demonstrated in ovalbumin-sensitized rats, and lower levels of IL-4 and IgE were found in sensitized rats treated with onion extract (53). Moreover, onion could be used as a natural immunostimulant added to animal feed to improve the growth performance and reduce the occurrence of diseases (55, 183, 186).
Other Health Functions of Onion
Browning of agro-products can lead to the deterioration of product quality and nutritional value, resulting in a decrease in consumer acceptance. Onion has been demonstrated to possess an inhibitory activity against the enzymatic browning reaction in fresh foods, such as fruit juice, mushrooms, and potato slices (187–190). In addition, intake of onion juice was reported to exhibit a beneficial effect on bone loss and bone mineral density by improving antioxidant capacity (57). Moreover, it has been revealed that fermented onion possessed an anti-photoaging effect against UVB-irradiation, probably by downregulating the expression of tyrosinase in B16F10 melanoma cells and collagenase-1 in UVB-induced HaCaT keratinocyte cells (191).
Potential Safety Concerns of Onion
Onion has been consumed as a vegetable and applied as an herbal medicine for a long history. Normally, onion and its bioactive compounds are quite safe for humans. However, several potential safety risks have raised concerns, for example, the residue of pesticides (17), bioaccumulation of heavy metals (18, 19), and contamination of pathogenic microorganisms (20, 21), which are discussed below.
Pesticide Residue
Pesticides with low toxicity and rapid degradation can be recommended for use in crops. However, the improper use of some pesticides in the onion crop may still cause health risks from onion consumption. Several studies have been carried out to investigate the degradation behavior, residue distribution, and dietary risk of different pesticides, including insecticides (17, 192), fungicides (193–195), and herbicides (196), which were used for pest and disease protection and weed control in onion planting. Overall, the dietary risk of these pesticides through onion could be negligible with the reasonable usage does of pesticides and enough preharvest interval (Table 2).
Table 2.
The residue decline and recommend preharvest interval of commonly used pesticides in onion.
| Pesticides | Applied dosage | Half-life | Preharvest interval | References |
|---|---|---|---|---|
| Thiacloprid | 48 g a.i./ha (1×) | 1.92 days | 9 days | (192) |
| 96 g a.i./ha (2×) | 2 days | |||
| Spinetoram | 0.031 g a.i./ha (1×) | 1.2 days | 1 day | (17) |
| Spinosad | 30 g a.i./ha (1×) | 1.42 days | 0 day | |
| Propiconazole | 120 g a.i./ha (1×) | 6.1–6.2 days | (194) | |
| 180 g a.i./ha (1.5×) | ||||
| Tebuconazole | 215 g a.i./ha (1×) | 1.7 days | 12 days | (195) |
| 430 g a.i./ha (2×) | 2.1 days | |||
| Fluopyram plus Tebuconazole | 75 + 75 g a.i./ha (1×) | 8.8 days | 7 days | (193) |
| 150 + 150 g a.i./ha (2×) | 9.1 days |
a.i./ha, active ingredient per hectare; ×, recommended dosage.
Heavy Metal Enrichment
The enrichment of heavy metals, such as cadmium (Cd), lead (Pb), chromium (Cr), and nickel (Ni), in the farmland growing onion may induce the accumulation of heavy metals in onion and induce food safety issue. Bystricka et al. (197) reported that the content of Cd, Cr, and Pb exceeded the reference limits in the dry matter of onions from contaminated soil in the Slovak Republic, and different heavy metal enrichment capacities were found among the onion varieties. Cd and Pb contents in some Malaysian onions were reported beyond the permissible levels, and the planting site had a greater impact than onion varieties on heavy metal enrichment (19). Gashi et al. (198) demonstrated that the activity of delta-aminolevulinic acid dehydratase could be used as a sensitive biomarker for the risk assessment of bioaccumulation of heavy metals in onion. Yao et al. (199) developed a novel in situ imaging strategy for the fast evaluation of heavy metal enrichment in onion. Besides, Cd uptake in onion and other crops was revealed to be affected by environmental and edaphic factors (200). Indeed, the Cd accumulation in onion was reported to be mitigated by increasing the soil pH (18) or the application of silicon fertilizer to the soil (201).
Microbial Contamination
The use of sanitizer, sodium hypochlorite, combined with elevated CO2/reduced O2 in package atmosphere was found beneficial for the safety and quality of fresh-cut onion, which showed effective inhibitory effects on the growth of Salmonella typhimurium, mesophilic aerobic bacteria, yeasts, and molds during storage (202). Moreover, the pathogenic microorganisms, including Listeria monocytogenes (21), Escherichia coli (203, 204), Salmonella spp. (205), and mold (20) in onion infected by contaminated irrigation water or transferred from contaminated workplace are also considered as potential health risks. The microwave-integrated cold plasma treatment was reported to be a potential technology for non-thermal decontamination of onion powder (206). In addition, improving monitoring of the quality of irrigation water as well as the regular decontamination of the workplace may be the effective ways to reduce the risk of pathogen exposure in onion.
Conclusions
Onion is a widely cultivated and consumed vegetable, and contains various bioactive components. The sulfur-containing compounds, such as onionin A and cysteine sulfoxide, as well as the phenolic compounds, such as quercetin and quercetin glucosides, are the main bioactive constituents in onion and contribute to its multiple health functions, including antioxidant, antimicrobial, anti-inflammatory, and immunomodulatory properties. Moreover, onion can be a promising natural resource to develop functional foods or nutraceuticals for the prevention and management of certain diseases, such as obesity, diabetes, cancers, cardiovascular diseases, neurodegenerative diseases, nephropathy, respiratory disorders, colitis, and infertility.
At present, both in vitro and in vivo evidence suggests that onion powder, juice, and extracts exhibit multiple health functions. Although several bioactive compounds have been found to contribute to these functions, more bioactive compounds from onion or its by-products should be identified, and their health functions, the relevant molecular mechanisms, and whether they have synergistic effects need to be illuminated in the future. Currently, most studies focused on the health functions of the raw onion, it is necessary to investigate whether the cooking processing can impact its health benefits. Moreover, more well-designed clinical trials are still required to verify the health benefits of onion and onion-derived bioactive compounds in humans. Last but not the least, safety issues of onion should always be aware of, not only the contaminations mentioned in this review, but also other potential risks, such as the overdose of bioactive compounds.
Author Contributions
X-XZ, F-JL, and HL drafted the manuscript. H-BL, D-TW, FG, WM, YW, B-HM, and R-YG critically revised the manuscript. R-YG and B-HM conceived the idea and scientific guidance through the process. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was funded by the Central Public-interest Scientific Institution Basal Research Fund of China (No. Y184XK05) and the Local Financial Funds of National Agricultural Science and Technology Center, Chengdu, China (No. NASC2021KR01).
References
- 1.Pareek S, Sagar NA, Sharma S, Kumar V. Onion (Allium cepa Fruit L) and Vegetable Phytochemicals: Chemistry and Human Health. 2nd ed. Hoboken, NJ: Wiley Blackwell. (2017). p. 1145–62. [Google Scholar]
- 2.Marrelli M, Amodeo V, Statti G, Conforti F. Biological properties and bioactive components of Allium cepa L.: focus on potential benefits in the treatment of obesity and related comorbidities. Molecules. (2019) 24:119. 10.3390/molecules24010119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teshika JD, Zakariyyah AM, Zaynab T, Zengin G, Rengasamy KRR, Pandian SK, et al. Traditional and modern uses of onion bulb (Allium cepa L.): a systematic review. Crit Rev Food Sci Nutr. (2019) 59:S39–70. 10.1080/10408398.2018.1499074 [DOI] [PubMed] [Google Scholar]
- 4.Ouyang H, Hou K, Peng WX, Liu ZL, Deng HP. Antioxidant and xanthine oxidase inhibitory activities of total polyphenols from onion. Saudi J Biol Sci. (2018) 25:1509–13. 10.1016/j.sjbs.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loredana L, Giuseppina A, Filomena N, Florinda F, Marisa D, Donatella A. Biochemical, antioxidant properties and antimicrobial activity of different onion varieties in the Mediterranean area. J Food Meas Charact. (2019) 13:1232–41. 10.1007/s11694-019-00038-2 [DOI] [Google Scholar]
- 6.Jakaria M, Azam S, Cho DY, Haque ME, Kim IS, Choi DK. The methanol extract of Allium cepa L. protects inflammatory markers in lps-induced bv-2 microglial cells and upregulates the antiapoptotic gene and antioxidant enzymes in N27-A cells. Antioxidants. (2019) 8:348. 10.3390/antiox8090348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Cha YJ, Lee KH, Yim JE. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: a 12-week, randomized, double-blind, placebo-controlled study. Nutr Res Pract. (2016) 10:175–181. 10.4162/nrp.2016.10.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jini D, Sharmila S. Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Mater Today Proc. (2020) 22:432–8. 10.1016/j.matpr.2019.07.672 [DOI] [Google Scholar]
- 9.Tsuboki J, Fujiwara Y, Horlad H, Shiraishi D, Nohara T, Tayama S, et al. Onionin A inhibits ovarian cancer progression by suppressing cancer cell proliferation and the protumour function of macrophages. Sci Rep. (2016) 6:29588. 10.1038/srep29588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colina-Coca C, Gonzalez-Pena D, de Ancos B, Sanchez-Moreno C. Dietary onion ameliorates antioxidant defence, inflammatory response, and cardiovascular risk biomarkers in hypercholesterolemic Wistar rats. J Funct Foods. (2017) 36:300–9. 10.1016/j.jff.2017.07.014 [DOI] [Google Scholar]
- 11.Salami M, Tamtaji O, Mohammadifar M, Talaei S, Tameh A, Abed A, et al. Neuroprotective effects of onion (Allium cepa) ethanolic extract on animal model of parkinson's disease induced by 6-hydroxydopamine: a behavioral, biochemical, and histological study. Gazi Med J. (2020) 31:25–9. 10.12996/gmj.2020.07 [DOI] [Google Scholar]
- 12.Ahmed AF, Al-Yousef HM, Al-Qahtani JH, Al-Said MS. A hepatonephro-protective phenolic-rich extract from red onion (Allium cepa L.) peels. Pak J Pharm Sci. (2017) 30:1971–9. [PubMed] [Google Scholar]
- 13.El-Hashim AZ, Khajah MA, Orabi KY, Balakrishnan S, Sary HG, Abdelali AA. Onion bulb extract downregulates EGFR/ERK1/2/AKT signaling pathway and synergizes with steroids to inhibit allergic inflammation. Front Pharmacol. (2020) 11:551683. 10.3389/fphar.2020.551683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khajah MA, El-Hashim AZ, Orabi KY, Hawai S, Sary HG. Onion bulb extract can both reverse and prevent colitis in mice via inhibition of pro-inflammatory signaling molecules and neutrophil activity. PloS ONE. (2020) 15:e0233938. 10.1371/journal.pone.0233938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shokoohi M, Madarek EOS, Khaki A, Shoorei H, Khaki AA, Soltani M, et al. Investigating the effects of onion juice on male fertility factors and pregnancy rate after testicular torsion/detorsion by intrauterine insemination method. Int J Women's Health Reprod Sci. (2018) 6:499–505. 10.15296/ijwhr.2018.82 [DOI] [Google Scholar]
- 16.Alkhedaide A, Soliman MM, Ismail TA. Protective effect of onion extract against experimental immunesuppression in Wistar rats: biological and molecular study. Afr J Tradit Complementary Altern Med. (2017) 14:96–103. 10.21010/ajtcam.v14i5.13 [DOI] [Google Scholar]
- 17.Malhat F, Abdallah O. Residue distribution and risk assessment of two macrocyclic lactone insecticides in green onion using micro-liquid-liquid extraction (MLLE) technique coupled with liquid chromatography tandem mass spectrometry. Environ Monit Assess. (2019) 191:584. 10.1007/s10661-019-7752-1 [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh JAE, Yi Z, Gray CW, Munir K, Lehto N, Robinson BH. Cadmium uptake by onions, lettuce and spinach in New Zealand: implications for management to meet regulatory limits. Sci Total Environ. (2019) 668:780–9. 10.1016/j.scitotenv.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 19.Kharisu CS, Koki IB, Ikram R, Low KH. Elemental variations and safety assessment of commercial onions (Allium cepa) by inductively coupled plasma-mass spectrometry and chemometrics. Int Food Res J. (2019) 26:1717–24. [Google Scholar]
- 20.Mayer S, Twaruzek M, Blajet-Kosicka A, Grajewski J. Occupational exposure to mould and microbial metabolites during onion sorting-insights into an overlooked workplace. Environ Monit Assess. (2016) 188:154. 10.1007/s10661-016-5150-5 [DOI] [PubMed] [Google Scholar]
- 21.Scollon AM, Wang HQ, Ryser ET. Transfer of Listeria monocytogenes during mechanical slicing of onions. Food Control. (2016) 65:160–7. 10.1016/j.foodcont.2016.01.021 [DOI] [Google Scholar]
- 22.Haftbaradaran S, Khoshgoftarrnanesh AH, Malakouti MJ. Assessment, mapping, and management of health risk from nitrate accumulation in onion for Iranian population. Ecotoxicol Environ Saf. (2018) 161:777–84. 10.1016/j.ecoenv.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 23.Arshad MS, Sohaib M, Nadeem M, Saeed F, Imran A, Javed A, et al. Status and trends of nutraceuticals from onion and onion by-products: a critical review. Cogent Food Agric. (2017) 3:1280254. 10.1080/23311932.2017.1280254 [DOI] [Google Scholar]
- 24.Galavi A, Hosseinzadeh H, Razavi BM. The effects of Allium cepa L. (onion) and its active constituents on metabolic syndrome: a review. Iran J Basic Med Sci. (2021) 24:3–16. 10.22038/ijbms.2020.46956.10843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno-Rojas JM, Moreno-Ortega A, Ordonez JL, Moreno-Rojas R, Perez-Aparicio J, Pereira-Caro G. Development and validation of UHPLC-HRMS methodology for the determination of flavonoids, amino acids and organosulfur compounds in black onion, a novel derived product from fresh shallot onions (Allium cepa var. aggregatum). LWT-Food Sci Technol. (2018) 97:376–83. 10.1016/j.lwt.2018.07.032 [DOI] [Google Scholar]
- 26.Zamri N, Abd Hamid. H. Comparative study of onion (Allium cepa) and leek (Allium ampeloprasum): identification of organosulphur compounds by UPLC-QTOF/MS and anticancer effect on MCF-7 cells. Plant Foods Hum Nutr. (2019) 74:525–30. 10.1007/s11130-019-00770-6 [DOI] [PubMed] [Google Scholar]
- 27.Lee SG, Parks JS, Kang HW. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J Nutr Biochem. (2017) 42:62–71. 10.1016/j.jnutbio.2016.12.018 [DOI] [PubMed] [Google Scholar]
- 28.Ren FY, Reilly K, Kerry JP, Gaffney M, Hossain M, Rai DK. Higher antioxidant activity, total flavonols, and specific quercetin glucosides in two different onion (Allium cepa L.) varieties grown under organic production: results from a 6-year field study. J Agric Food Chem. (2017) 65:5122–32. 10.1021/acs.jafc.7b01352 [DOI] [PubMed] [Google Scholar]
- 29.Viera VB, Piovesan N, Rodrigues JB, Mello RD, Prestes RC, dos Santos RCV, et al. Extraction of phenolic compounds and evaluation of the antioxidant and antimicrobial capacity of red onion skin (Allium cepa L.). Int Food Res J. (2017) 24:990–9. [Google Scholar]
- 30.Ma YL, Zhu DY, Thakur K, Wang CH, Wang H, Ren YF, et al. Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.). Int J Biol Macromol. (2018) 111:92–101. 10.1016/j.ijbiomac.2017.12.154 [DOI] [PubMed] [Google Scholar]
- 31.Lanzotti V, Romano A, Lanzuise S, Bonanomi G, Scala F. Antifungal saponins from bulbs of white onion, Allium cepa L. Phytochemistry. (2012) 74:133–9. 10.1016/j.phytochem.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 32.Dahlawi SM, Nazir W, Iqbal R, Asghar W, Khalid N. Formulation and characterization of oil-in-water nanoemulsions stabilized by crude saponins isolated from onion skin waste. RSC Adv. (2020) 10:39700–7. 10.1039/D0RA07756A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang SL, Deng P, Xu YC, Lu SW, Wang JJ. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J Integ Agric. (2016) 15:2175–81. 10.1016/S2095-3119(16)61385-0 [DOI] [Google Scholar]
- 34.Beesk N, Perner H, Schwarz D, George E, Kroh LW, Rohn S. Distribution of quercetin-3, 4'-O-diglucoside, quercetin-4'-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chem. (2010) 122:566–71. 10.1016/j.foodchem.2010.03.011 [DOI] [Google Scholar]
- 35.Park MJ, Ryu DH, Cho JY, Ha IJ, Moon JS, Kang YH. Comparison of the antioxidant properties and flavonols in various parts of Korean red onions by multivariate data analysis. Horticulture Environ Biotechnol. (2018) 59:919–27. 10.1007/s13580-018-0091-2 [DOI] [Google Scholar]
- 36.Fernandez-Jalao I, Sanchez-Moreno C, De Ancos B. Influence of food matrix and high-pressure processing on onion flavonols and antioxidant activity during gastrointestinal digestion. J Food Eng. (2017) 213:60–8. 10.1016/j.jfoodeng.2017.02.015 [DOI] [Google Scholar]
- 37.Burak C, Brull V, Langguth P, Zimmermann BF, Stoffel-Wagner B, Sausen U, et al. Higher plasma quercetin levels following oral administration of an onion skin extract compared with pure quercetin dihydrate in humans. Eur J Nutr. (2017) 56:343–53. 10.1007/s00394-015-1084-x [DOI] [PubMed] [Google Scholar]
- 38.Watanabe J, Muro T, Kobori M, Takano-Ishikawa Y. Changes in quercetin content and antioxidant capacity of quercetin-rich onion cultivar 'quer-gold' during cooking. J Jpn Soc Food Sci Technol Nippon Shokuhin Kagaku Kogaku Kaishi. (2018) 65:63–6. 10.3136/nskkk.65.63 [DOI] [Google Scholar]
- 39.Cattivelli A, Conte A, Martini S, Tagliazucchi D. Influence of cooking methods on onion phenolic compounds bioaccessibility. Foods. (2021) 10:1023. 10.3390/foods10051023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Lee S, Shin D, Yoo M. Change in organosulfur compounds in onion (Allium cepa L.) during heat treatment. Food Sci Biotechnol. (2016) 25:115–9. 10.1007/s10068-016-0017-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno-Ortega A, Pereira-Caro G, Ordonez JL, Munoz-Redondo JM, Moreno-Rojas R, Perez-Aparicio J, et al. Changes in the antioxidant activity and metabolite profile of three onion varieties during the elaboration of ‘black onion’. Food Chem. (2020) 311:125958. 10.1016/j.foodchem.2019.125958 [DOI] [PubMed] [Google Scholar]
- 42.Shang A, Cao S-Y, Xu X-Y, Gan R-Y, Tang G-Y, Corke H, et al. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods. (2019) 8:246. 10.3390/foods8070246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao Q-Q, Xu X-Y, Cao S-Y, Corke H, Li H-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. (2019) 8:185. 10.3390/foods8060185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shang A, Liu H-Y, Luo M, Xia Y, Yang X, Wu D-T, et al. Sweet tea (Lithocarpus polystachyus rehd.) as a new natural source of bioactive dihydrochalcones with multiple health benefits. Crit Rev Food Sci Nutr. (in press). 10.1080/10408398.2020.1830363 [DOI] [PubMed] [Google Scholar]
- 45.Lin F-J, Wei X-L, Liu H-Y, Zhang P-Z, Gandhi GR, Hua-Bin L, et al. State-of-the-art review of dark tea: from chemistry to health benefits. Trends Food Sci Technol. (2021) 109:126–38. 10.1016/j.tifs.2021.01.03029495598 [DOI] [Google Scholar]
- 46.Gan R-Y, Lui W-Y, Wu K, Chan C-L, Dai S-H, Sui Z-Q, et al. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: an updated review. Trends Food Sci Technol. (2017) 59:1–14. 10.1016/j.tifs.2016.11.010 [DOI] [Google Scholar]
- 47.Meng X, Zhou J, Zhao CN, Gan RY, Li HB. Health benefits and molecular mechanisms of resveratrol: a narrative review. Foods. (2020) 9:340. 10.3390/foods9030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu XY, Meng X, Li S, Gan RY, Li Y, Li HB. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients. (2018) 10:1553. 10.3390/nu10101553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farha AK, Gan R-Y, Li H-B, Wu D-T, Atanasov AG, Gul K, et al. The anticancer potential of the dietary polyphenol rutin: current status, challenges, and perspectives. Crit Rev Food Sci Nutr. (in press). 10.1080/10408398.2020.1829541 [DOI] [PubMed] [Google Scholar]
- 50.Ulusoy HG, Sanlier N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit Rev Food Sci Nutr. (2020) 60:3290–303. 10.1080/10408398.2019.1683810 [DOI] [PubMed] [Google Scholar]
- 51.Gandhi GR, Vasconcelos ABS, Wu D-T, Li H-B, Antony PJ, Li H, et al. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: a systematic review of in vitro and in vivo studies. Nutrients. (2020) 12:2907. 10.3390/nu12102907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D, Gan RY, Zhang JR, Farha AK, Li HB, Zhu F, et al. Antivirulence properties and related mechanisms of spice essential oils: a comprehensive review. Compr Rev Food Sci Food Saf. (2020) 19:1018–55. 10.1111/1541-4337.12549 [DOI] [PubMed] [Google Scholar]
- 53.Marefati N, Eftekhar N, Kaveh M, Boskabadi J, Beheshti F, Boskabady MH. The effect of Allium cepa extract on lung oxidant, antioxidant, and immunological biomarkers in ovalbumin-sensitized rats. Med Princ Pract. (2018) 27:122–8. 10.1159/000487885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nwonuma CO, Osemwegie OO, Alejolowo OO, Irokanulo EO, Olaniran AF, Fadugba DO, et al. Antioxidant and the ameliorating effect of Allium cepa (Onion) fortified feed against potassium bromate induced oxidative damage in Wistar rats. Toxicol Rep. (2021) 8:759–66. 10.1016/j.toxrep.2021.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omar AE, Al-Khalaifah HS, Mohamed WAM, Gharib HSA, Osman A, Al-Gabri NA, et al. Effects of phenolic-rich onion (Allium cepa L.) extract on the growth performance, behavior, intestinal histology, amino acid digestibility, antioxidant activity, and the immune status of broiler chickens. Front Vet Sci. (2020) 7:728. 10.3389/fvets.2020.582612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fawole FJ, Adeoye AA, Tiamiyu LO, Samuel FC, Omosuyi OM, Amusa MT. Dietary combination of pawpaw seed and onion peel powder: impact on growth, haematology, biochemical and antioxidant status of Clarias gariepinus. Aquacult Res. (2020) 51:2903–12. 10.1111/are.14629 [DOI] [Google Scholar]
- 57.Law YY, Chiu HF, Lee HH, Shen YC, Venkatakrishnan K, Wang CK. Consumption of onion juice modulates oxidative stress and attenuates the risk of bone disorders in middle-aged and post-menopausal healthy subjects. Food Funct. (2016) 7:902–12. 10.1039/C5FO01251A [DOI] [PubMed] [Google Scholar]
- 58.Orasan O, Oprean R, Saplontai-Pop A, Filip M, Carpa R, Sarosi C, et al. Antimicrobial activity and thiosulfinates profile of a formulation based on Allium cepa L. extract. Open Chem. (2017) 15:175–81. 10.1515/chem-2017-0021 [DOI] [Google Scholar]
- 59.Ortega-Ramirez LA, Silva-Espinoza BA, Vargas-Arispuro I, Gonzalez-Aguilar GA, Cruz-Valenzuela MR, Nazzaro F, et al. Combination of Cymbopogon citratus and Allium cepa essential oils increased antibacterial activity in leafy vegetables. J Sci Food Agric. (2017) 97:2166–73. 10.1002/jsfa.8025 [DOI] [PubMed] [Google Scholar]
- 60.Khanam PN, Hasan A. Biosynthesis and characterization of graphene by using non-toxic reducing agent from Allium cepa extract: anti-bacterial properties. Int J Biol Macromol. (2019) 126:151–8. 10.1016/j.ijbiomac.2018.12.213 [DOI] [PubMed] [Google Scholar]
- 61.Al-Yousef HM, Sheikh IA. Beta-Sitosterol derived compound from onion husks non-polar fraction reduces quorum sensing controlled virulence and biofilm production. Saudi Pharm J. (2019) 27:664–672. 10.1016/j.jsps.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chand K, Abro MI, Aftab U, Shah AH, Lakhan MN, Cao D, et al. Green synthesis characterization and antimicrobial activity against Staphylococcus aureus of silver nanoparticles using extracts of neem, onion and tomato. RSC Adv. (2019) 9:17002–15. 10.1039/C9RA01407A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pagano C, Marinozzi M, Baiocchi C, Beccari T, Calarco P, Ceccarini MR, et al. Bioadhesive polymeric films based on red onion skins extract for wound treatment: an innovative and eco-friendly formulation. Molecules. (2020) 25:318. 10.3390/molecules25020318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kocic-Tanackov S, Dimic G, Mojovic L, Gvozdanovic-Varga J, Djukic-Vukovic A, Tomovic V, et al. Antifungal activity of the onion (Allium cepa L.) essential oil against Aspergillus, Fusarium and Penicillium species isolated from food. J Food Proc Preserv. (2017) 41:e13050. 10.1111/jfpp.13050 [DOI] [Google Scholar]
- 65.Ibrahim E, Luo JY, Ahmed T, Wu WG, Yan CQ, Li B. Biosynthesis of silver nanoparticles using onion endophytic bacterium and its antifungal activity against rice pathogen Magnaporthe oryzae. J Fungi. (2020) 6:294. 10.3390/jof6040294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Genatrika E, Sundhani E, Oktaviana MI. Gel potential of red onion (Allium cepa L.) ethanol extract as antifungal cause tinea pedis. J Pharm Bioallied Sci. (2020) 12:733–6. 10.4103/jpbs.JPBS_256_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamasaki M, Yamasaki Y, Furusho R, Kimura H, Kamei I, Sonoda H, et al. Onion (Allium cepa L.)-derived nanoparticles inhibited lps-induced nitrate production, however, their intracellular incorporation by endocytosis was not involved in this effect on RAW264 cells. Molecules. (2021) 26:2763. 10.3390/molecules26092763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghorani V, Marefati N, Shakeri F, Rezaee R, Boskabady M, Boskabady MH. The effects of Allium cepa extract on tracheal responsiveness, lung inflammatory cells and phospholipase A2 level in asthmatic rats. Iran J Allergy Asthma Immunol. (2018) 17:221–31. [PubMed] [Google Scholar]
- 69.Khajah MA, Orabi KY, Hawai S, Sary HG, El-Hashim AZ. Onion bulb extract reduces colitis severity in mice via modulation of colonic inflammatory pathways and the apoptotic machinery. J Ethnopharmacol. (2019) 241:112008. 10.1016/j.jep.2019.112008 [DOI] [PubMed] [Google Scholar]
- 70.Dimitry MY, Edith DMJ, Armand AB, Nicolas NY. Antioxidant property, anti-inflammatory and analgesic effects of aqueous extracts of two onion bulbs varieties (Allium cepa L.). Clin Phytosci. (2021) 7:49. 10.1186/s40816-021-00284-2 [DOI] [Google Scholar]
- 71.Yu S, Li H, Cui T, Cui M, Piao C, Wang S, et al. Onion (Allium cepa L.) peel extract effects on 3T3-L1 adipocytes and high-fat diet-induced obese mice. Food Biosci. (2021) 41:101019. 10.1016/j.fbio.2021.101019 [DOI] [Google Scholar]
- 72.Devarshi PP, Jones AD, Taylor EM, Stefanska B, Henagan TM. Quercetin and quercetin-rich red onion extract alter Pgc-1 alpha promoter methylation and splice variant expression. PPAR Research. (2017) 2017:3235693. 10.1155/2017/3235693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang C, Li LH, Yang LG, Lu H, Wang SK, Sun GJ. Anti-obesity and hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutr Metab. (2018) 15:43. 10.1186/s12986-018-0275-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishimura M, Muro T, Kobori M, Nishihira J. Effect of daily ingestion of quercetin-rich onion powder for 12 weeks on visceral fat: a randomised, double-blind, placebo-controlled, parallel-group study. Nutrients. (2020) 12:91. 10.3390/nu12010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeong S, Chae J, Lee G, Shin G, Kwon YI, Oh JB, et al. Effect of steamed onion (ONIRO) consumption on body fat and metabolic profiles in overweight subjects: a 12-week randomized, double-blind, placebo-controlled clinical trial. J Am Coll Nutr. (2020) 39:206–15. 10.1080/07315724.2019.1635052 [DOI] [PubMed] [Google Scholar]
- 76.Choi HN, Choue R, Park Y, Yim JE. Onion peel extract increases erythrocyte membrane n-3 fatty acids in overweight and obese Korean subjects. J Med Food. (2020) 23:37–42. 10.1089/jmf.2018.4366 [DOI] [PubMed] [Google Scholar]
- 77.Fallah V, Mahabadi JA, Mahabadi MY, Kashani HH, Nikzad H. Protective effect of Allium cepa (onion) seeds (AC) extract on histopathology of testis in STZ-induced male rats. Int J Morphol. (2017) 35:1517–24. 10.4067/S0717-95022017000401517 [DOI] [Google Scholar]
- 78.Pradeep SR, Srinivasan K. Amelioration of hyperglycemia and associated metabolic abnormalities by a combination of fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) in experimental diabetes. J Basic Clin Physiol Pharmacol. (2017) 28:493–505. 10.1515/jbcpp-2015-0140 [DOI] [PubMed] [Google Scholar]
- 79.Pradeep SR, Srinivasan K. Amelioration of oxidative stress by dietary fenugreek (Trigonella foenum-graecum L.) seeds is potentiated by onion (Allium cepa L.) in streptozotocin-induced diabetic rats. Appl Physiol Nutr Metab. (2017) 42:816–28. 10.1139/apnm-2016-0592 [DOI] [PubMed] [Google Scholar]
- 80.Pradeep SR, Srinivasan K. Alleviation of cardiac damage by dietary fenugreek (Trigonella foenum-graecum) seeds is potentiated by onion (Allium cepa) in experimental diabetic rats via blocking renin-angiotensin system. Cardiovasc Toxicol. (2018) 18:221–31. 10.1007/s12012-017-9431-1 [DOI] [PubMed] [Google Scholar]
- 81.Pradeep SR, Srinivasan K. Alleviation of oxidative stress-mediated nephropathy by dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) in streptozotocin-induced diabetic rats. Food Funct. (2018) 9:134–48. 10.1039/C7FO01044C [DOI] [PubMed] [Google Scholar]
- 82.Pradeep SR, Srinivasan K. Ameliorative influence of dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) on eye lens abnormalities via modulation of crystallin proteins and polyol pathway in experimental diabetes. Curr Eye Res. (2018) 43:1108–18. 10.1080/02713683.2018.1484146 [DOI] [PubMed] [Google Scholar]
- 83.Pradeep SR, Srinivasan K. Haemato-protective influence of dietary fenugreek (Trigonella foenum-graecum L.) seeds is potentiated by onion (Allium cepa L.) in streptozotocin-induced diabetic rats. Biomed Pharmacother. (2018) 98:372–81. 10.1016/j.biopha.2017.12.037 [DOI] [PubMed] [Google Scholar]
- 84.Abouzed TK, Contreras MD, Sadek KM, Shukry M, Abdelhady DH, Gouda WM, et al. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diab Res Clin Pract. (2018) 140:253–64. 10.1016/j.diabres.2018.03.042 [DOI] [PubMed] [Google Scholar]
- 85.Kang YR, Choi HY, Lee JY, Jang SI, Kang H, Oh JB, et al. Calorie restriction effect of heat-processed onion extract (ONI) using in vitro and in vivo animal models. Int J Mol Sci. (2018) 19:874. 10.3390/ijms19030874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pradeep SR, Barman S, Srinivasan K. Attenuation of diabetic nephropathy by dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) via suppression of glucose transporters and renin-angiotensin system. Nutrition. (2019) 67–68:110543. 10.1016/j.nut.2019.06.024 [DOI] [PubMed] [Google Scholar]
- 87.Masood S, Rehman AU, Bashir S, Shazly ME, Imran M, Khalil P, et al. Investigation of the anti-hyperglycemic and antioxidant effects of wheat bread supplemented with onion peel extract and onion powder in diabetic rats. J Diab Metab Disord. (2021) 20:485–95. 10.1007/s40200-021-00770-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ebrahimi-Mamaghani M, Saghafi-Asl M, Niafarc M, Asghari-Jafarabadi M, Mesgari-Abbasi M. Raw red onion intake and insulin resistance markers in overweight or obese patients with polycystic ovary syndrome: a randomized controlled-clinical trial. Prog Nutr. (2018) 20:199–208. 10.23751/pn.v20i1-S.6109 [DOI] [Google Scholar]
- 89.Fredotovic Z, Sprung M, Soldo B, Ljubenkov I, Budic-Leto I, Bilusic T, et al. Chemical composition and biological activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) methanolic extracts. Molecules. (2017) 22:448. 10.3390/molecules22030448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murayyan AI, Manohar CM, Hayward G, Neethirajan S. Antiproliferative activity of Ontario grown onions against colorectal adenocarcinoma cells. Food Res Int. (2017) 96:12–8. 10.1016/j.foodres.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 91.Fredotovic Z, Soldo B, Sprung M, Marijanovic Z, Jerkovic I, Puizina J. Comparison of organosulfur and amino acid composition between triploid onion Allium cornutum Clementi ex Visiani, 1842, and common onion Allium cepa L., and evidences for antiproliferative activity of their extracts. Plants-Basel. (2020) 9:98. 10.3390/plants9010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nile A, Gansukh E, Park GS, Kim DH, Nile SH. Novel insights on the multi-functional properties of flavonol glucosides from red onion (Allium cepa L) solid waste—in vitro and in silico approach. Food Chem. (2021) 335:127650. 10.1016/j.foodchem.2020.127650 [DOI] [PubMed] [Google Scholar]
- 93.Nile A, Nile SH, Cespedes CL, Oh JW. Spiraeoside extracted from red onion skin ameliorates apoptosis and exerts potent antitumor, antioxidant and enzyme inhibitory effects. Food Chem Toxicol. (2021) 154:112327. 10.1016/j.fct.2021.112327 [DOI] [PubMed] [Google Scholar]
- 94.Alzandi AA, Naguib DM, Abas ASM. Onion extract encapsulated on nano chitosan: a promising anticancer agent. J Gastrointest Cancer. (in press). 10.1007/s12029-021-00591-4 [DOI] [PubMed] [Google Scholar]
- 95.Fujiwara Y, Horlad H, Shiraishi D, Tsuboki J, Kudo R, Ikeda T, et al. Onionin A a sulfur-containing compound isolated from onions, impairs tumor development and lung metastasis by inhibiting the protumoral and immunosuppressive functions of myeloid cells. Mol Nutr Food Res. (2016) 60:2467–80. 10.1002/mnfr.201500995 [DOI] [PubMed] [Google Scholar]
- 96.Jafarpour-Sadegh F, Montazeri V, Adili A, Esfehani A, Rashidi MR, Pirouzpanah S. Consumption of fresh yellow onion ameliorates hyperglycemia and insulin resistance in breast cancer patients during doxorubicin-based chemotherapy: a randomized controlled clinical trial. Integr Cancer Ther. (2017) 16:276–89. 10.1177/1534735416656915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ko EY, Nile SH, Jung YS, Keum YS. Antioxidant and antiplatelet potential of different methanol fractions and flavonols extracted from onion (Allium cepa L.). 3 Biotech. (2018) 8:155. 10.1007/s13205-018-1184-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang HJ, Pichiah BT, Abinaya RV, Sohn HS, Cha YS. Hypocholesterolemic effect of quercetin-rich onion peel extract in C57BL/6J mice fed with high cholesterol diet. Food Sci Biotechnol. (2016) 25:855–60. 10.1007/s10068-016-0141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez-Pena D, Gimenez L, de Ancos B, Sanchez-Moreno C. Role of dietary onion in modifying the faecal bile acid content in rats fed a high-cholesterol diet. Food Funct. (2017) 8:2184–92. 10.1039/C7FO00412E [DOI] [PubMed] [Google Scholar]
- 100.Gonzalez-Pena D, Checa A, de Ancos B, Wheelock CE, Sanchez-Moreno C. New insights into the effects of onion consumption on lipid mediators using a diet-induced model of hypercholesterolemia. Redox Biol. (2017) 11:205–12. 10.1016/j.redox.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li WY, Yang CY, Mei X, Huang R, Zhang SP, Tang YY, et al. Effect of the polyphenol-rich extract from Allium cepa on hyperlipidemic sprague-dawley rats. J Food Biochem. (2021) 45:e13565. 10.1111/jfbc.13565 [DOI] [PubMed] [Google Scholar]
- 102.Chiu HF, Shen YC, Huang TY, Venkatakrishnan K, Wang CK. Cardioprotective efficacy of red wine extract of onion in healthy hypercholesterolemic subjects. Phytother Res. (2016) 30:380–5. 10.1002/ptr.5537 [DOI] [PubMed] [Google Scholar]
- 103.Brull V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Muller C, et al. Acute intake of quercetin from onion skin extract does not influence postprandial blood pressure and endothelial function in overweight-to-obese adults with hypertension: a randomized, double-blind, placebo-controlled, crossover trial. Eur J Nutr. (2017) 56:1347–57. 10.1007/s00394-016-1185-1 [DOI] [PubMed] [Google Scholar]
- 104.Brull V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Muller C, et al. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-) hypertension: a randomized double-blinded, placebo-controlled crossover trial. Eur J Nutr. (2017) 56:2265–75. 10.1007/s00394-016-1267-0 [DOI] [PubMed] [Google Scholar]
- 105.Tamtaji OR, Hosseinzadeh H, Talaei SA, Behnam M, Firoozeh SMT, Taghizadeh M, et al. Protective effects of red onion (Allium cepa) ethanolic extract on learning and memory impairments in animal models of diabetes. Galen Med J. (2017) 6:249–57. 10.22086/gmj.v6i3.909 [DOI] [Google Scholar]
- 106.Khan D, Mohammed M, Upaganlawar A, Upasani CD, Une HD. Ameliorative potential of Allium cepa Lam. leaves on diabetes induced and chronic constriction injury induced neuropathic pain in experimental rats. Indian J Pharm Educ Res. (2020) 54:143–9. 10.5530/ijper.54.1.17 [DOI] [Google Scholar]
- 107.Kumar S, Modgil S, Bammidi S, Minhas G, Shri R, Kaushik S, et al. Allium cepa exerts neuroprotective effect on retinal ganglion cells of pterygopalatine artery (PPA) ligated mice. J Ayurveda Integr Med. (2020) 11:489–94. 10.1016/j.jaim.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh V, Shri R, Krishan P, Singh IP, Shah P. Isolation and characterization of components responsible for neuroprotective effects of Allium cepa outer scale extract against ischemia reperfusion induced cerebral injury in mice. J Food Sci. (2020) 85:4009–17. 10.1111/1750-3841.15474 [DOI] [PubMed] [Google Scholar]
- 109.Mete R, Oran M, Topcu B, Oznur M, Seber ES, Gedikbasi A, et al. Protective effects of onion (Allium cepa) extract against doxorubicin-induced hepatotoxicity in rats. Toxicol Indust Health. (2016) 32:551–7. 10.1177/0748233713504807 [DOI] [PubMed] [Google Scholar]
- 110.Emamat H, Foroughi F, Eini-Zinab H, Hekmatdoost A. The effects of onion consumption on prevention of nonalcoholic fatty liver disease. Indian J Clin Biochem. (2018) 33:75–80. 10.1007/s12291-017-0636-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Memarzia A, Amin F, Saadat S, Jalali M, Ghasemi Z, Boskabady MH. The contribution of beta-2 adrenergic, muscarinic and histamine (H-1) receptors, calcium and potassium channels and cyclooxygenase pathway in the relaxant effect of Allium cepa L. on the tracheal smooth muscle. J Ethnopharmacol. (2019) 241:112012. 10.1016/j.jep.2019.112012 [DOI] [PubMed] [Google Scholar]
- 112.Zaki SM. Evaluation of antioxidant and anti-lipid peroxidation potentials of Nigella sativa and onion extract on nicotine-induced lung damage. Folia Morphol. (2019) 78:554–63. 10.5603/FM.a2018.0117 [DOI] [PubMed] [Google Scholar]
- 113.Alqasoumi S. The use of onion (Allium cepa L.) treatment can mitigate gastric mucosal injury in rats. Issues Biol Sci Pharm Res. (2015) 3:107–114. 10.15739/ibspr.020 [DOI] [Google Scholar]
- 114.Rahman S, Khan S, Chand N, Sadique U, Khan RU. In vivo effects of Allium cepa L. on the selected gut microflora and intestinal histomorphology in broiler. Acta Histochem. (2017) 119:446–50. 10.1016/j.acthis.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 115.Grzelak-Blaszczyk K, Milala J, Kosmala M, Kolodziejczyk K, Sojka M, Czarnecki A, et al. Onion quercetin monoglycosides alter microbial activity and increase antioxidant capacity. J Nutr Biochem. (2018) 56:81–8. 10.1016/j.jnutbio.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 116.Hong Z, Piao M. Effect of quercetin monoglycosides on oxidative stress and gut microbiota diversity in mice with dextran sodium sulphate-induced colitis. BioMed Res Int. (2018) 2018:8343052. 10.1155/2018/8343052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakayama Y, Ho H-J, Yamagishi M, Ikemoto H, Komai M, et al. Cysteine sulfoxides enhance steroid hormone production via activation of the protein kinase a pathway in testis-derived I-10 tumor cells. Molecules. (2020) 25:4694. 10.3390/molecules25204694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shahverdi S, Khaki A, Jafari B. Investigating the antioxidant effect of Allium cepa after exposure to escherichia coli on biochemical factors, the blood antioxidants, and testis tissue in rats. Crescent J Med Biol Sci. (2017) 4:32–8. [Google Scholar]
- 119.Khaki A, Khaki AA, Rajabzadeh A. The effects of Permethrin and antioxidant properties of Allium cepa (onion) on testicles parameters of male rats. Toxin Rev. (2017) 36:1–6. 10.1080/15569543.2016.1235582 [DOI] [Google Scholar]
- 120.Khaki A, Rajabzadeh A, Khaki AA. Side effects of pyrethroid and supporting role of onion in the male rat's spermatogenesis. Chin Med J. (2017) 130:3015–6. 10.4103/0366-6999.220297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajabzadeh A, Khaki AA, Khaki A. Antioxidant properties of Allium cepa (onion) against permethrin-induced toxicity on LHCGR and SF1 genes in male rats. Crescent J Med Biol Sci. (2018) 5:327–31. [Google Scholar]
- 122.Ofoego UC, Eze ED, Nweke EO, Obiesie IJ, Mbagwu IS, Christian OE. Allium cepa (onion) extract enhances and protects testicular function and architecture against paraquat induced oxidative damage. Int J Life Sci Pharm Res. (2021) 11:194–203. 10.22376/ijpbs/lpr.2021.11.1.L194-203 [DOI] [Google Scholar]
- 123.Nassan MA, Soliman MM, Aldhahrani A, El-Saway HB, Swelum AA. Ameliorative impacts of Allium cepa Linnaeus aqueous extract against testicular damage induced by dexamethasone. Andrologia. (2021) 53:e13955. 10.1111/and.13955 [DOI] [PubMed] [Google Scholar]
- 124.Jeje SO, Akpan EE, Kunle-Alabi OT, Akindele OO, Raji Y. Protective role of Allium cepa Linn (Onion) juice on maternal dexamethasone induced alterations in the reproductive functions of the female offspring of Wistar rats. Curr Res Physiol. (2021) 4:145–54. 10.1016/j.crphys.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yilmaz-Oral D, Onder A, Kaya-Sezginer E, Oztekin CV, Zor M, Gur S. Restorative effects of red onion (Allium cepa L.) juice on erectile function after-treatment with 5α-reductase inhibitor in rats. Int J Impot Res. (in press). 10.1038/s41443-021-00421-y [DOI] [PubMed] [Google Scholar]
- 126.Damaziak K, Riedel J, Gozdowski D, Niemiec J, Siennicka A, Rog D. Productive performance and egg quality of laying hens fed diets supplemented with garlic and onion extracts. J Appl Poul Res. (2017) 26:337–49. 10.3382/japr/pfx001 [DOI] [Google Scholar]
- 127.Lisanti A, Formica V, Ianni F, Albertini B, Marinozzi M, Sardella R, et al. Antioxidant activity of phenolic extracts from different cultivars of Italian onion (Allium cepa) and relative human immune cell proliferative induction. Pharm Biol. (2016) 54:799–806. 10.3109/13880209.2015.1080733 [DOI] [PubMed] [Google Scholar]
- 128.Sidhu JS, Ali M, Al-Rashdan A, Ahmed N. Onion (Allium cepa L.) is potentially a good source of important antioxidants. J Food Sci Technol Mysore. (2019) 56:1811–9. 10.1007/s13197-019-03625-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manohar CM, Xue J, Murayyan A, Neethirajan S, Shi J. Antioxidant activity of polyphenols from Ontario grown onion varieties using pressurized low polarity water technology. J Funct Foods. (2017) 31:52–62. 10.1016/j.jff.2017.01.037 [DOI] [Google Scholar]
- 130.Islam S, Khar A, Singh S, Tomar BS. Variability, heritability and trait association studies for bulb and antioxidant traits in onion (Allium cepa) varieties. Indian J Agric Sci. (2019) 89:450–7. [Google Scholar]
- 131.Yang SJ, Paudel P, Shrestha S, Seong SH, Jung HA, Choi JS. In vitro protein tyrosine phosphatase 1B inhibition and antioxidant property of different onion peel cultivars: a comparative study. Food Sci Nutr. (2019) 7:205–15. 10.1002/fsn3.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sagar NA, Pareek S, Gonzalez-Aguilar GA. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: a comparative study of fifteen Indian cultivars. J Food Sci Technol Mysore. (2020) 57:2423–32. 10.1007/s13197-020-04277-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mitrova K, Hrbek V, Svoboda P, Hajslova J, Ovesna J. Antioxidant activity, S-alk(en)yl-L-cysteine sulfoxide and polyphenol content in onion (Allium cepa L.) cultivars are associated with their genetic background. Czech J Food Sci. (2016) 34:127–32. 10.17221/268/2015-CJFS [DOI] [Google Scholar]
- 134.Ren FY, Reilly K, Gaffney M, Kerry JP, Hossain M, Rai DK. Evaluation of polyphenolic content and antioxidant activity in two onion varieties grown under organic and conventional production systems. J Sci Food Agric. (2017) 97:2982–90. 10.1002/jsfa.8138 [DOI] [PubMed] [Google Scholar]
- 135.Muscolo A, Papalia T, Settineri G, Mallamaci C, Panuccio MR. Sulfur bentonite-organic-based fertilizers as tool for improving bio-compounds with antioxidant activities in red onion. J Sci Food Agric. (2020) 100:785–93. 10.1002/jsfa.10086 [DOI] [PubMed] [Google Scholar]
- 136.Mollavali M, Bolandnazar SA, Schwarz D, Rohn S, Riehle P, Nahandi FZ. Flavonol glucoside and antioxidant enzyme biosynthesis affected by mycorrhizal fungi in various cultivars of onion (Allium cepa L.). J Agric Food Chem. (2016) 64:71–7. 10.1021/acs.jafc.5b04791 [DOI] [PubMed] [Google Scholar]
- 137.Bettoni MM, Mogor AF, Pauletti V, Goicoechea N. The interaction between mycorrhizal inoculation, humic acids supply and elevated atmospheric CO2 increases energetic and antioxidant properties and sweetness of yellow onion. Hortic Environ Biotechnol. (2017) 58:432–40. 10.1007/s13580-017-0122-4 [DOI] [Google Scholar]
- 138.Amalfitano C, Golubkina NA, Del Vacchio L, Russo G, Cannoniero M, Somma S, et al. Yield, antioxidant components, oil content, and composition of onion seeds are influenced by planting time and density. Plants Basel. (2019) 8:293. 10.3390/plants8080293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zudaire L, Vinas I, Abadias M, Simo J, Echeverria G, Plaza L, et al. Quality and bioaccessibility of total phenols and antioxidant activity of calcots (Allium cepa L.) stored under controlled atmosphere conditions. Postharvest Biol Technol. (2017) 129:118–28. 10.1016/j.postharvbio.2017.03.013 [DOI] [Google Scholar]
- 140.Chen C, Hu WZ, Zhang RD, Jiang AL, Zou Y. Levels of phenolic compounds, antioxidant capacity, and microbial counts of fresh-cut onions after treatment with a combination of nisin and citric acid. Hortic Environ Biotechnol. (2016) 57:266–73. 10.1007/s13580-016-0032-x [DOI] [Google Scholar]
- 141.Majid I, Dhatt AS, Sharma S, Nayik GA, Nanda V. Effect of sprouting on physicochemical, antioxidant and flavonoid profile of onion varieties. Int J Food Sci Technol. (2016) 51:317–24. 10.1111/ijfs.12963 [DOI] [Google Scholar]
- 142.Majid I, Nanda V. Total phenolic content, antioxidant activity, and anthocyanin profile of sprouted onion powder. Acta Alimentaria. (2018) 47:52–60. 10.1556/066.2017.0006 [DOI] [Google Scholar]
- 143.Wang YY, Duan X, Ren GY, Liu YH. Comparative study on the flavonoids extraction rate and antioxidant activity of onions treated by three different drying methods. Drying Technol. (2019) 37:245–52. 10.1080/07373937.2018.1482907 [DOI] [Google Scholar]
- 144.Salamatullah AM, Uslu N, Özcan MM, Alkaltham MS, Hayat K. The effect of oven drying on bioactive compounds, antioxidant activity, and phenolic compounds of white and red-skinned onion slices. J Food Proc Preserv. (2021) 45:e15173. 10.1111/jfpp.15173 [DOI] [Google Scholar]
- 145.Cubukcu HC, Kilicaslan NSD, Durak I. Different effects of heating and freezing treatments on the antioxidant properties of broccoli, cauliflower, garlic and onion. An experimental in vitro study. São Paulo Med J. (2019) 137:407–13. 10.1590/1516-3180.2019.004406082019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Al-Yousef HM, Alhowiriny TA, Alam P, Siddiqui NA, Ahmed AF, Al-Qahtani JH, et al. Simultaneous quantification of two phenolic biomarkers by a validated high-performance thin-layer chromatographic method in antimicrobial antioxidant active ethyl acetate fraction of Allium cepa L. (peel). JPC J Planar Chromatogr Modern TLC. (2017) 30:510–5. 10.1556/1006.2017.30.6.8 [DOI] [Google Scholar]
- 147.Ahmadi S, Rajabi Z, Vasfi-Marandi M. Evaluation of the antiviral effects of aqueous extracts of red and yellow onions (Allium cepa) against avian influenza virus subtype H9N2. Iran J Vet Sci Technol. (2018) 10:23–7. [Google Scholar]
- 148.Farag MA, Ali SE, Hodaya RH, El-Seedi HR, Sultani HN, Laub A, et al. Phytochemical profiles and antimicrobial activities of Allium cepa red cv. and A. sativum subjected to different drying methods: a comparative MS-based metabolomics. Molecules. (2017) 22:761. 10.3390/molecules22050761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheng XN, Song RY, Qian YF, Wei J. Preparation of onion-based composite and study on its antimicrobial activity. Adv Eng Mater Appl Mechan. (2016) 2015:529–34. 10.1201/b19268-93 [DOI] [Google Scholar]
- 150.Patra JK, Kwon Y, Baek KH. Green biosynthesis of gold nanoparticles by onion peel extract: synthesis, characterization and biological activities. Adv Powder Technol. (2016) 27:2204–13. 10.1016/j.apt.2016.08.005 [DOI] [Google Scholar]
- 151.Sharma P, Pant S, Rai S, Yadav RB, Sharma S, Dave V. Green synthesis and characterization of silver nanoparticles by Allium cepa L. to produce silver nano-coated fabric and their antimicrobial evaluation. Appl Organomet Chem. (2018) 32:e4146. 10.1002/aoc.4146 [DOI] [Google Scholar]
- 152.Quecan BXV, Santos JTC, Rivera MLC, Hassimotto NMA, Almeida FA, Pinto UM. Effect of quercetin rich onion extracts on bacterial quorum sensing. Front Microbiol. (2019) 10:867. 10.3389/fmicb.2019.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Al-Yousef HM, Ahmed AF, Al-Shabib NA, Laeeq S, Khan RA, Rehman MT, et al. Onion peel ethylacetate fraction and its derived constituent quercetin 4'-O-beta-D glucopyranoside attenuates quorum sensing regulated virulence and biofilm formation. Front Microbiol. (2017) 8:1675. 10.3389/fmicb.2017.01675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Somrani M, Ingles MC, Debbabi H, Abidi F, Palop A. Garlic, onion, and cinnamon essential oil anti-biofilms' effect against Listeria monocytogenes. Foods. (2020) 9:567. 10.3390/foods9050567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ucak I, Khalily R, Abuibaid AKM, Ogunkalu OA. Maintaining the quality of rainbow trout (Oncorhynchus mykiss) fillets by treatment of red onion peel extract during refrigerated storage. Prog Nutr. (2018) 20:672–8. 10.23751/pn.v20i4.7690 [DOI] [Google Scholar]
- 156.Li QL, Wang YH, Mai YX, Li HY, Wang Z, Xu JW, et al. Health benefits of the flavonoids from onion: constituents and their pronounced antioxidant and anti-neuroinflammatory capacities. J Agric Food Chem. (2020) 68:799–807. 10.1021/acs.jafc.9b07418 [DOI] [PubMed] [Google Scholar]
- 157.Michalak-Majewska M, Solowiej B, Slawinska A. Antioxidant activity, technological and rheological properties of baked rolls containing dried onions (Allium cepa L.). J Food Proc Preserv. (2017) 41:e12914. 10.1111/jfpp.12914 [DOI] [Google Scholar]
- 158.Nile A, Nile SH, Kim DH, Keum YS, Seok PG, Sharma K. Valorization of onion solid waste and their flavonols for assessment of cytotoxicity, enzyme inhibitory and antioxidant activities. Food Chem Toxicol. (2018) 119:281–9. 10.1016/j.fct.2018.02.056 [DOI] [PubMed] [Google Scholar]
- 159.da Silva MGR, Skrt M, Komes D, Ulrih NP, Pogacnik L. Enhanced yield of bioactivities from onion (Allium cepa L.) skin and their antioxidant and anti-alpha-amylase activities. Int J Mol Sci. (2020) 21:2909. 10.3390/ijms21082909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Benitez V, Molla E, Martin-Cabrejas MA, Aguilera Y, Esteban RM. Physicochemical properties and in vitro antidiabetic potential of fibre concentrates from onion by-products. J Funct Foods. (2017) 36:34–42. 10.1016/j.jff.2017.06.045 [DOI] [Google Scholar]
- 161.Nohara T, Fujiwara Y. Antitumor sulfur compounds from Allium species (onion, welsh onion, and garlic). Nat Prod Commun. (2017) 12:1235–6. 10.1177/1934578X1701200821 [DOI] [Google Scholar]
- 162.Asemani Y, Zamani N, Bayat M, Amirghofran Z. Allium vegetables for possible future of cancer treatment. Phytother Res. (2019) 33:3019–39. 10.1002/ptr.6490 [DOI] [PubMed] [Google Scholar]
- 163.Desai G, Schelske-Santos M, Nazario CM, Rosario-Rosado RV, Mansilla-Rivera I, Ramirez-Marrero F, et al. Onion and garlic intake and breast cancer, a case-control study in Puerto Rico. Nutr Cancer Int J. (2020) 72:791–800. 10.1080/01635581.2019.1651349 [DOI] [PubMed] [Google Scholar]
- 164.Turati F, Pelucchi C, Guercio V, Vecchia CL, Galeone C. Allium vegetable intake and gastric cancer: a case-control study and meta-analysis. Mol Nutr Food Res. (2015) 59:171–9. 10.1002/mnfr.201400496 [DOI] [PubMed] [Google Scholar]
- 165.Wu X, Shi J, Fang WX, Guo XY, Zhang LY, Liu YP, et al. Allium vegetables are associated with reduced risk of colorectal cancer: a hospital-based matched case-control study in China. Asia Pacific J Clin Oncol. (2019) 15:e132–41. 10.1111/ajco.13133 [DOI] [PubMed] [Google Scholar]
- 166.Guercio V, Turati F, La Vecchia C, Galeone C, Tavani A. Allium vegetables and upper aerodigestive tract cancers: a meta-analysis of observational studies. Mol Nutr Food Res. (2016) 60:212–22. 10.1002/mnfr.201500587 [DOI] [PubMed] [Google Scholar]
- 167.Yasmin T, Ali MT, Haque S, Hossain M. Interaction of quercetin of onion with axon guidance protein receptor, NRP-1 plays important role in cancer treatment: an in silico approach. Interdiscip Sci Comput Life Sci. (2017) 9:184–91. 10.1007/s12539-015-0137-4 [DOI] [PubMed] [Google Scholar]
- 168.Zhao YL, Fan DM, Zheng ZP, Li ETS, Chen F, Cheng KW, et al. 8-C-(E-phenylethenyl)quercetin from onion/beef soup induces autophagic cell death in colon cancer cells through ERK activation. Mol Nutr Food Res. (2017) 61:1600437. 10.1002/mnfr.201600437 [DOI] [PubMed] [Google Scholar]
- 169.Ravanbakhshian R, Behbahani M. Evaluation of anticancer activity of lacto- and natural fermented onion cultivars. Iran J Sci Technol Trans Sci. (2018) 42:1735–42. 10.1007/s40995-017-0348-0 [DOI] [Google Scholar]
- 170.Abdelrahman M, Mahmoud H, El-Sayed M, Tanaka S, Tran LS. Isolation and characterization of Cepa2, a natural alliospiroside A. from shallot (Allium cepa L. Aggregatum group) with anticancer activity. Plant Physiol Biochem. (2017) 116:167–73. 10.1016/j.plaphy.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 171.Zhou YY, Li C, Feng B, Chen B, Jin LH, Shen YH. UPLC-ESI-MS/MS based identification and antioxidant, antibacterial, cytotoxic activities of aqueous extracts from storey onion (Allium cepa L. var. proliferurn Regel). Food Res Int. (2020) 130:108969. 10.1016/j.foodres.2019.108969 [DOI] [PubMed] [Google Scholar]
- 172.Petrovic V, Nepal A, Olaisen C, Bachke S, Hira J, Søgaard CK, et al. Anti-cancer potential of homemade fresh garlic extract is related to increased endoplasmic reticulum stress. Nutrients. (2018) 10:450. 10.3390/nu10040450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zolfaghari B, Yazdiniapour Z, Sadeghi M, Akbari M, Troiano R, Lanzotti V. Cinnamic acid derivatives from Welsh onion (Allium fistulosum) and their antibacterial and cytotoxic activities. Phytochem Anal. (2021) 32:84–90. 10.1002/pca.2924 [DOI] [PubMed] [Google Scholar]
- 174.Wang YH, Li C, Xiang LM, Huang WJ, He XJ. Spirostanol saponins from Chinese onion (Allium chinense) exert pronounced antiinflammatory and anti-proliferative activities. J Funct Foods. (2016) 25:208–19. 10.1016/j.jff.2016.06.005 [DOI] [Google Scholar]
- 175.Wang YH, Yi XM, Xiang LM, Huang YY, Wang Z, He XJ. Furostanol saponins from Chinese onion induce G2/M cell-cycle arrest an apoptosis through mitochondria-mediate pathway in HepG2 cells. Steroids. (2019) 148:11–8. 10.1016/j.steroids.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 176.Aleksandar P, Dragana MC, Nebojsa J, Biljana N, Natasa S, Branka V, et al. Wild edible onions—Allium flavum and Allium carinatum—successfully prevent adverse effects of chemotherapeutic drug doxorubicin. Biomed Pharmacother. (2019) 109:2482–91. 10.1016/j.biopha.2018.11.106 [DOI] [PubMed] [Google Scholar]
- 177.Grzelak-Blaszczyk K, Milala J, Kolodziejczyk K, Sojka M, Czarnecki A, Kosmala M, et al. Protocatechuic acid and quercetin glucosides in onions attenuate changes induced by high fat diet in rats. Food Funct. (2020) 11:3585–97. 10.1039/C9FO02633A [DOI] [PubMed] [Google Scholar]
- 178.Gonzalez-Pena D, Dudzik D, Garcia A, de Ancos B, Barbas C, Sanchez-Moreno C. Metabolomic fingerprinting in the comprehensive study of liver changes associated with onion supplementation in hypercholesterolemic Wistar rats. Int J Mol Sci. (2017) 18:267. 10.3390/ijms18020267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang WS, Kim JC, Lee JY, Kim CH, Hwang CW. Antihyperlipidemic and antioxidative potentials of onion (Allium cepa L.) extract fermented with a novel Lactobacillus casei HD-010. Evid Based Compl Altern Med. (2019) 2019:3269047. 10.1155/2019/3269047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. (2020) 20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. (2020) 80:401–6. 10.1016/j.jinf.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Amir N, Al Dhaheri A, Al Jaberi N, Al Marzouqi F, Bastaki SM. Comparative effect of garlic (Allium sativum), onion (Allium cepa), and black seed (Nigella sativa) on gastric acid secretion and gastric ulcer. Res Rep Med Chem. (2011) 1:3–9. 10.2147/RRMC.S23032 [DOI] [Google Scholar]
- 183.Mousavi E, Mohammadiazarm H, Mousavi SM, Ghatrami ER. Effects of inulin, savory and onion powders in diet of juveniles carp Cyprinus carpio (Linnaeus 1758) on gut micro flora, immune response and blood biochemical parameters. Turk J Fish Aquat Sci. (2016) 16:831–8. 10.4194/1303-2712-v16_4_09 [DOI] [Google Scholar]
- 184.Kruljac M, Finnbogadottir H, Bobjer J, Giraldi A, Fugl-Meyer K, Giwercman A. Symptoms of sexual dysfunction among men from infertile couples: prevalence and association with testosterone deficiency. Andrology. (2020) 8:160–5. 10.1111/andr.12678 [DOI] [PubMed] [Google Scholar]
- 185.Saghafi-Asl M, Ebrahimi-Mameghani M. The effects of raw red onion consumption on serum levels of adiponectin, leptin, and hs-CRP in overweight/obese females with polycystic ovarian syndrome: a randomized controlled-clinical trial. Iran Red Crescent Med J. (2017) 19:e58674. 10.5812/ircmj.58674 [DOI] [Google Scholar]
- 186.Younes AM, Gaafar AY, Abu-Bryka AEDZ, Awad ES, Abo-Aziza F, Abd El-Aziz, et al. Effects of onion (Allium cepa) in diets of Oreochromis niloticus: growth improvement, antioxidant, anti-inflammatory and disease resistance perspectives. Aquacult Res. (2021) 52:2324–34. 10.1111/are.15084 [DOI] [Google Scholar]
- 187.Lee B, Seo JD, Rhee J-K, Kim CY. Heated apple juice supplemented with onion has greatly improved nutritional quality and browning index. Food Chem. (2016) 201:315–9. 10.1016/j.foodchem.2016.01.092 [DOI] [PubMed] [Google Scholar]
- 188.Yuniarti T, Yuliana ND, Budijanto S. Inhibition of enzymatic browning by onion (Allium cepa L.): investigation on inhibitory mechanism and identification of active compounds. Curr Res Nutr Food Sci. (2018) 6:770–80. 10.12944/CRNFSJ.6.3.19 [DOI] [Google Scholar]
- 189.Bernaś E, Jaworska G. Onion juice and extracts for the inhibition of enzymatic browning mechanisms in frozen A. bisporus mushrooms. J Sci Food Agricult. (2021) 101:4099–107. 10.1002/jsfa.11045 [DOI] [PubMed] [Google Scholar]
- 190.Tinello F, Mihaylova D, Lante A. Valorization of onion extracts as anti-browning agents. Food Sci Appl Biotechnol. (2020) 3:16–21. 10.30721/fsab2020.v3.i1.87 [DOI] [Google Scholar]
- 191.Jeong EJ, Jegal J, Jung Y-S, Chung KW, Chung HY, et al. Fermented onions extract inhibits tyrosinase and collagenase-1 activities as a potential new anti-photoaging agent. Nat Product Commun. (2017) 12:1934578X1701200711. 10.1177/1934578X1701200711 [DOI] [Google Scholar]
- 192.Abdallah OI, Malhat FM. Thiacloprid residues in green onion (Allium cepa) using micro liquid-liquid extraction and liquid chromatography-tandem mass spectrometry. Agricult Res. (2020) 9:340–8. 10.1007/s40003-019-00440-8 [DOI] [Google Scholar]
- 193.Patel BV, Chawla S, Gor H, Upadhyay P, Parmar KD, Patel AR, et al. Residue decline and risk assessment of fluopyram plus tebuconazole (400SC) in/on onion (Allium cepa). Environ Sci Pollut Res. (2016) 23:20871–81. 10.1007/s11356-016-7331-8 [DOI] [PubMed] [Google Scholar]
- 194.Bai A, Chen A, Chen W, Luo X, Liu S, Zhang M, et al. Study on degradation behaviour, residue distribution, and dietary risk assessment of propiconazole in celery and onion under field application. J Sci Food Agricult. (2021) 101:1998–2005. 10.1002/jsfa.10817 [DOI] [PubMed] [Google Scholar]
- 195.Dubey JK, Patyal SK, Katna S, Shandil D, Devi N, Singh G, et al. Persistence and dissipation kinetics of tebuconazole in apple, tomato, chilli and onion crops of Himachal Pradesh, India. Environ Sci Pollut Res. (2020) 27:11290–302. 10.1007/s11356-020-07724-5 [DOI] [PubMed] [Google Scholar]
- 196.Herrmann CM, Goll MA, Phillippo CJ, Zandstra BH. Postemergence weed control in onion with bentazon, flumioxazin, and oxyfluorfen. Weed Technol. (2017) 31:279–90. 10.1017/wet.2016.16 [DOI] [Google Scholar]
- 197.Bystricka J, Arvay J, Musilova J, Vollmannova A, Toth T, Lenkova M. The investigation of sensitivity of different types of onion to heavy metal intake from contaminated soil. Int J Environ Res. (2016) 10:427–40. 10.22059/IJER.2016.58762 [DOI] [Google Scholar]
- 198.Gashi B, Osmani M, Aliu S, Zogaj M, Kastrati F. Risk assessment of heavy metal toxicity by sensitive biomarker delta-aminolevulinic acid dehydratase (ALA-D) for onion plants cultivated in polluted areas in Kosovo. J Environ Sci Health B Pest Food Contam Agricult Wastes. (2020) 55:462–9. 10.1080/03601234.2020.1721229 [DOI] [PubMed] [Google Scholar]
- 199.Yao Z, Lai ZQ, Chen CC, Xiao ST, Yang PH. Full-color emissive carbon-dots targeting cell walls of onion for in situ imaging of heavy metal pollution. Analyst. (2019) 144:3685–90. 10.1039/C9AN00418A [DOI] [PubMed] [Google Scholar]
- 200.Yi ZC, Lehto NJ, Robinson BH, Cavanagh JAE. Environmental and edaphic factors affecting soil cadmium uptake by spinach, potatoes, onion and wheat. Sci Total Environ. (2020) 713:136694. 10.1016/j.scitotenv.2020.136694 [DOI] [PubMed] [Google Scholar]
- 201.Gray CW, Wise BE. Mitigating cadmium accumulation in spinach and onions by the application of silicon fertilizer to soil. Soil Sediment Contam. (2020) 29:532–44. 10.1080/15320383.2020.1747980 [DOI] [Google Scholar]
- 202.Page N, Gonzalez-Buesa J, Ryser ET, Harte J, Almenar E. Interactions between sanitizers and packaging gas compositions and their effects on the safety and quality of fresh-cut onions (Allium cepa L.). Int J Food Microbiol. (2016) 218:105–13. 10.1016/j.ijfoodmicro.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 203.Shock CC, Reitz SR, Roncarati RA, Kreeft H, Shock BM, Klauzer JC. Drip vs. furrow irrigation in the delivery of Escherichia coli to onions. Appl Eng Agricult. (2016) 32:235–44. 10.13031/aea.32.11163 [DOI] [Google Scholar]
- 204.Wright D, Feibert E, Reitz S, Shock C, Waite-Cusic J. Field evidence supporting conventional onion curing practices as a strategy to mitigate Escherichia coli contamination from irrigation water. J Food Prot. (2018) 81:369–76. 10.4315/0362-028X.JFP-17-231 [DOI] [PubMed] [Google Scholar]
- 205.Emch AW, Waite-Cusic JG. Conventional curing practices reduce generic Escherichia coli and Salmonella spp. on dry bulb onions produced with contaminated irrigation water. Food Microbiol. (2016) 53:41–7. 10.1016/j.fm.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 206.Kim JE, Oh YJ, Won MY, Lee KS, Min SC. Microbial decontamination of onion powder using microwave-powered cold plasma treatments. Food Microbiol. (2017) 62:112–23. 10.1016/j.fm.2016.10.006 [DOI] [PubMed] [Google Scholar]