Abstract
The extended-spectrum cephalosporin resistant E. coli from food animals transferring to community settings of humans causes a serious threat to public health. Unlike phylogroup B2 E. coli strains, the clinical significance of isolates in phylogroup F is not well revealed. Here, we report on a collection (n = 563) of phylogroup F E. coli isolates recovered from chicken colibacillosis tissues and retail raw chicken meat samples in Eastern China. There was an overlapped distribution of MLST types between chicken colibacillosis-origin and meat-source phylogroup F E. coli, including dominant STs (ST648, ST405, ST457, ST393, ST1158, etc). This study further investigated the presence of extended-spectrum β-lactamase (ESBL/pAmpC) producers in these chicken-source phylogroup F E. coli strains. The prevalence of extended-spectrum cephalosporin resistant strains in phylogroup F E. coli from chicken colibacillosis and raw meat separately accounted for 66.1 and 71.2%. The resistance genotypes and plasmid replicon types of chicken-source phylogroup F E. coli isolates were characterized by multiplex PCR. Our results revealed β-lactamase CTX-M, OXA, CMY and TEM genes were widespread in chicken-source phylogroup F E. coli, and blaCTX-M was the most predominant ESBL gene. Moreover, there was a high prevalence of non-lactamase resistance genes in these β-lactam-resistant isolates. The replicons IncB/O/K/Z, IncI1, IncN, IncFIC, IncQ1, IncX4, IncY, and p0111, associated with antibiotic-resistant large plasmids, were widespread in chicken-source phylogroup F E. coli. There was no obvious difference for the populations, resistance spectrums, and resistance genotypes between phylogroup F E. coli from chicken colibacillosis tissues and retail meats. This detail assessment of the population and resistance genotype showed chicken-source phylogroup F E. coli might hold zoonotic risk and contribute the spread of multidrug-resistant E. coli to humans.
Key words: phylogroup F E. coli, population, resistance spectrum, ESBL genes, chicken retail meats
INTRODUCTION
Bacterial antimicrobial resistance (AMR), as one of the major public-health concern, causes a great impact on humans, animals, and the environment. Recent reports reveal that bacteria isolated from poultry exhibit clinically relevant AMR and harbor extended-spectrum β-lactamase (ESBL) genes, carbapenemase genes, colistin resistance genes, and other plasmid-mediated quinolone genes. Over the last 2 decades, ESBL-producing E. coli isolates have been detected with increasing occurrence in human and animal samples (Boswell et al., 2018; Kawamura et al., 2018; Paitan, 2018). ESBLs induce resistance to extended-spectrum (3rd and 4th generation) cephalosporins (e.g., ceftazidime, cefoperazone, cefixime, and cefpirome) and monobactams (Pitout and Laupland, 2008; Magiorakos et al., 2012; Nicolas-Chanoine et al., 2014). In addition, E. coli strains harbor plasmid-carrying cephalosporinases (pAmpCs) to exhibit a broader spectrum of drug resistance, including the great majority of cephalosporins and cephamycins, and pAmpC property is not repressed by β-lactamase inhibitors, leading to conferring almost all therapeutically accessible β-lactam drugs (Pitout, 2012). In veterinary medicine, the β-lactam drugs are undoubtedly the most important and commonly used antimicrobial category to inhibit bacterial infections. The increasing selective pressure of antibiotics could promote the rapid spread of bacterial resistance genes (Collignon and Voss, 2015). Besides the extensive usage of antimicrobial drugs in animal disease treatment, antibiotics consumption for widely subtherapeutic-dose addition in animal feedstuff is a major reason to accelerate the dissemination of antibiotic-resistant bacteria (Liu et al., 2016; Johnson et al., 2017). The occurrence of ESBL-produced E. coli from poultry source in China is increasing reported since 2006. Due to E. coli as the widespread gram-negative bacteria, the high occurrence of ESBL-produced E. coli in animal and food products caused both livestock industry and public-health challenges (Manges and Johnson, 2012; Pitout, 2012; Manges and Johnson, 2015).
E. coli displays wide-ranging phylogenetic substructure. Six phylogroups (A, B1, B2, C, D, and E) are initially delineated by multilocus enzyme electrophoresis with 35 enzyme loci (Selander et al., 1987). In 2000, an E. coli strain can be assigned to one of particular phylogroups (A, B1, B2, and D) by a triplex PCR method as pronounced by Clermont et al. (2000). The increasing multilocus sequence data and comparative genomics analysis is helpful for understanding of E. coli phylogroup structure, and the Clermont E. coli rapid phylo-typing method is updated to enhance the specificity and detection of new phylogroups (Clermont et al., 2013). E. coli isolates are divided into 8 phylogroups (A, B1, B2, C, D, E, F, and clade I) by the new multiplex PCR method, which is validated to assign over 95% of E. coli strains into a different special phylogroups. When E. coli core-genome phylogenetic tree is rooted on Escherichia fergusonii, the strains assigned to 3 phylogroups (B2, F, and D) are located in the most basal and share closest relationships (Beghain et al., 2018). Phylogroup E then appears, followed by E. coli strains in phylogroups C, B1, and A, act as the most recently separated phylogroup (Beghain et al., 2018). Importantly, a historic evolutionary development of the species is associated to the lifestyle of the strains. The most anciently separated phylogroups (B2, F, and D) contain the majority of extraintestinal pathogenic E. coli (ExPEC) strains (Escobar-Paramo et al., 2004). However, the intestinal pathogenic E. coli (InPEC) isolates, commensal or environmental strains belong to the most newly diverged phylogroups, such as E. coli O157:H7 isolates located in phylogroup E and responsible for the severe intestinal pathologies (Zhu Ge et al., 2014).
Avian pathogenic E. coli (APEC) contaminated chicken products are associated with infection or colonization of humans (Ewers et al., 2014; Manges and Johnson, 2015). It is noteworthy that ST73, ST95, ST131, and ST141 APEC isolates in phylogroup B2 generally exhibit high virulence and zoonotic risk. In our recent research, chicken-origin E. coli within phylogroup F are identified as truly merging APECs and display close relationship with phylogroup B2 APEC strains, holding high virulence and zoonotic potential (Zhuge et al., 2020). Population of phylogroup F APEC isolates is revealed and limited to a few dominant STs (such as ST59, ST354, ST405, and ST648) (Zhuge et al., 2020). Recent reports show the occurrences of ESBL/pAmpC-positive E. coli in broiler flocks are existing in China (Li et al., 2010; Tong et al., 2015; Wu et al., 2018; Song et al., 2020). However, there is few report on the ESBL/pAmpC-producing E. coli isolated from retail raw chicken meats in China. Furthermore, the systematic assessment of the antibiotic resistance potential among chicken-source phylogroup F E. coli strains is described to be substantially lower. In this study, we had characterized the antibiotic resistance of chicken-source E. coli isolates in phylogroup F both phenotypically and genotypically. This was a comparison for the genetic background in antibiotic resistant phylogroup F E. coli recovered from chicken colibacillosis tissues or retail meats in Eastern China.
MATERIALS AND METHODS
Sample Collection and Bacterial Isolation
In our previously described, E. coli isolates were recovered from diseased/dead chicken (diagnosed with typical colibacillosis) in broiler farms among Jiangsu, Zhejiang, Anhui, and Shandong provinces in China, 2012 to 2017 (Zhuge et al., 2020). Phylogroup F E. coli strains were detected by the updated Clermont PCR protocols (Clermont et al., 2013), and a total of 289 Phylogroup F E. coli were recovered from chicken colibacillosis (Zhuge et al., 2020).
For E. coli strains recovered from retail meats, 2,361 chicken samples for retail slaughtered fresh chicken, raw chicken meat portions (including livers, necks, skeletons, etc.), and residual tissues in chicken meat packaging were obtained from 138 different supermarkets and food markets in Eastern China (major cities in the Yangtze River Delta) during the period from 2015 to 2019. These samples of chicken retail meats and packaging were transported quickly under cooling environments to our laboratory and stored at 4°C waiting for the next processes within 24 h. Small pieces (2 g) of chicken meat tissues were incubated overnight at 37°C in 10 mL of Luria-Bertani (LB) broth, and plated onto MacConkey agar plates. Then, one bacterial colony per plate of meat sample was isolated and purified in LB broth. Finally, these purified strains were added in peptoneglycerol medium and stocked at −80°C freezer.
Phylogenetic Screening for Phylogroup F E. coli Isolates
Phylogroups of chicken-source E. coli isolates were identified according to the previously described multiplex PCR (Clermont et al., 2013). E. coli isolates were usually distributed in six phylogroups, including A, B1, B2, D, E, and F.
All phylogroup F E. coli isolates were further performed MLST typing, according to 7 housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) (Maiden et al., 1998). Seven pairs of primers were used to amplify these genes, and PCR amplicons were purified and sequenced for both the forward and reverse strands. DNA sequences for each E. coli isolates were matched to EnteroBase, available in E. coli MLST database (http://enterobase.warwick.ac.uk/species/ecoli/allele_st_search). Allele numbers corresponding to seven gene sequences of each E. coli isolate were obtained, and ST type of each phylogroup F isolate was specially designated by combining 7allelic profiles.
Serogrouping for Phylogroup F E. coli Isolates
O-serogroups of phylogroup F E. coli were detected by multiplex PCR using special primer pairs as the previously described (Iguchi, et al., 2015). Then, O-serogroups were confirmed by O antigen diagnostic serum (Tianjin Biochip) (Johnson et al., 2008; Zhuge et al., 2020).
Antimicrobial Susceptibility Testing
Based on the characterization standard for MDR, extensively drug-resistant (XDR), and PDR (pandrug-resistant) bacteria (Magiorakos et al., 2012), the susceptibility testing was performed by 27 antibiotics, classified into 16 antimicrobial types as follows. Aminoglycosides: amikacin (AK), gentamicin (GEN), kanamycin (KAN), and streptomycin (STR). Anti-MRSA cephalosporin: Ceftaroline (CPT). Antipseudomonal penicillins + β-lactamase inhibitor: piperacillin/tazobactam (TZP). Carbapenem: imipenem (IPM). Nonextended spectrum cephalosporins: cefazolin (CZO) and cefuroxime (CXM). Third and fourth generation cephalosporins: cefotaxime (CTX), ceftriaxone (CRO), ceftazidime (CAZ), and cefepime (FEP). Cephamycin: cefoxitin (FOX). Fluoroquinolone: ciprofloxacin (CIP) and levofloxacin (LEV). Folate pathway inhibitor: sulfisoxazole (SMZ) and trimethoprim/sulfamethoxazole (SXT). Glycylcycline: tigecycline (TGC). Monobactam: aztreonam (ATM). Penicillin: ampicillin (AMP). Penicillins + β-lactamase inhibitors: amoxicillin/clavulanic acid (AMC) and ampicillin-sulbactam (SAM). Phenicol: chloramphenicol (CHL). Phosphonic acid: fosfomycin (FOS). Tetracycline: tetracycline (TET). Polymyxins: Colistin (polymyxin E, PE). E. coli strains were cultivated at MH agar plates, and the paper disks containing each antibiotic were attached to these plates. The diameter of the inhibition zone for each agent was measured and recorded. E. coli ATCC25922 acted as the quality control. The antibiotic susceptibility of phylogroup F E. coli strains was determined according to the CLSI standard (CLSI, 2018). These phylogroup F isolates were judged as resistant (R), intermediately resistant (I), or susceptible (S).
Identifying the Types of Lactamase Resistance Genes in E. coli Isolates
The presence of ESBL and plasmid-mediated AmpC genes in phylogroup F E. coli strains were distinguished by multiplex PCR. ESBL genes (CTX-M-1, -2, -8, and -9 groups), lactamase genes (TEM, OXA, and SHV), and pAmpC genes (CMY, FOX, and DHA) were screened using special primer pairs (Table S1) (Dallenne et al., 2010; Poirel et al., 2011; Johnson et al., 2012a; Kawamura et al., 2018). PCR sequencing method was used to detect the specific types of lactamase resistance genes (ESBL, pAmpC, and other lactamase genes). Full-length nucleic acid sequences were used to decide β-lactamase types by BLAST analysis (http://www.ncbi.nlm.nih.gov/) and β-lactamase classification system (http://www.lahey.org/studies/webt.asp).
Identifying the Types of Non-lactamase Resistance Genes and Plasmid Replicons
Non-lactamase antibiotic resistance genes were detected in phylogroup F E. coli isolates by PCR amplification (Dallenne et al., 2010; Poirel et al., 2011; Johnson et al., 2012a; Kawamura et al., 2018), including plasmid-carried fluoroquinolone resistance genes (aac [6’]-Ib-cr, qepA, qnrA, qnrB, and qnrS), sulfonamides resistance genes (sul1, sul2, and sul3), streptomycin resistance genes (aadA, strA, and strB), kanamycin resistance genes (aph [3’]-Ia), tetracycline resistance genes (tetA, tetB, and tetC), fosfomycin resistance genes (fosA and fosA3), colistin resistance genes (mcr-1 to mcr-3), etc (Table S1).
The replicon types of plasmid carried by ESBL/pAmpC-producing E. coli were detected by multiplex PCR method, as previously described (Carattoli et al., 2005; Johnson et al., 2007). PCR-based replicon typing method could target 19 replicon types, such as FIA, HI1, I1, L/M, A/C, K/B, and FIIA (Carattoli et al., 2005; Johnson et al., 2007).
RESULTS
Comparative Analysis for Population Structure Between Phylogroup F E. coli Recovered From Chicken Colibacillosis and Retail Raw Chicken Meats
In our previous study, 289 phylogroup F E. coli strains account for 21.7% of all E. coli recovered from chicken colibacillosis (Zhuge et al., 2020; Table S2). Almost phylogroup F E. coli isolates are recognized as truly APECs and hold ExPEC-associated pathogenic characteristics (Zhuge et al., 2020). In this study, a total of 2178 E. coli strains were recovered from the retail raw chicken meats. Overall, a majority of chicken meat-source E. coli strains were assigned to eight phylogroups: A (37.2%), B1 (21.7%), B2 (8.7%), C (5.4%), D (7.2%), E (1.4%), F (12.6%), and Clade I (2.2%). Remaining (3.6%) E. coli strains were classified as the nonclassified type.
We further assessed the population of chicken meat-source phylogroup F E. coli using MLST analysis. MLST assigned these meat-source phylogroup F strains (n = 274) to 27 unique STs (Table S3). Similar to the previously described, one ST containing more than 8 strains among the chicken-source E. coli was recognized as a dominant ST (Zhuge et al., 2020). These dominant STs harbored 11 STs (ST648, ST405, ST457, ST393, ST362, ST59, ST117, ST135, ST354, ST1158, ST115, and ST501). Our previous results show that the phylogroup F E. coli isolates from avian colibacillosis are assigned to 29 STs, including 13 dominant STs (Zhuge et al., 2020). We identified overlapped distribution of MLST types between chicken colibacillosis-origin and meat-source phylogroup F E. coli. There were 17 common STs among phylogroup F E. coli isolates, including all dominant STs in these chicken meat-source E. coli. Moreover, there was an overlapped distribution of O serotypes in same dominant STs among between chicken colibacillosis-origin and meat-source phylogroup F E. coli. The dominant ST E. coli strains within phylogroup F were summarized in Table 1, which indicated the close association between O serotypes and ST types.
Table 1.
The association of ST types and O serotypes among chicken-source phylogroup F E. coli isolates.
| Colibacillosis-related isolates |
Meat-related isolates |
|||
|---|---|---|---|---|
| STsa | No. of isolates | O serotypes | No. of isolates | O serotypes |
| ST59 | 21 | O1 | 18 | O1 |
| ST62 | 8 | O7 | 6 | O7 |
| ST115 | 17 | O5, O8, O9, O21, O136 | 9 | O5, O8, O9, O21 |
| ST117 | 22 | O24, O85, O78, O109, O161 | 17 | O24, O78, O109, O111, O161 |
| ST135 | 12 | O2, O50, O83 | 16 | O2, O50, O83 |
| ST354 | 23 | O1, O3, O11, O25, O45, O51 | 14 | O11, O25, O45, O51 |
| ST362 | 9 | O15, O86, O25 | 19 | O7, O15, O86, O25 |
| ST393 | 16 | O11, O15, O25, O86 | 21 | O11, O15, O25, O86, O101 |
| ST405 | 22 | O2, O45, O102 | 34 | O2, O21, O45, O102, |
| ST457 | 47 | O11, O154, O102 | 27 | O11, O154, O102 |
| ST501 | 12 | O17, O44, O77, O86, | 8 | O17, O44, O77, |
| ST648 | 29 | O1, O2, O25, O45, O102 | 36 | O1, O2, O25, O45, O50, O102 |
| ST1158 | 8 | O17, O44, O92, O102 | 11 | O17, O44, O92, O102 |
| Total Percent (%) | 246 (85.1%) |
- | 236 (86.1%) |
- |
The dominant STs, and each ST harbors more than 8 strains.
Antimicrobial Susceptibility of Phylogroup F E. coli From Chicken Colibacillosis
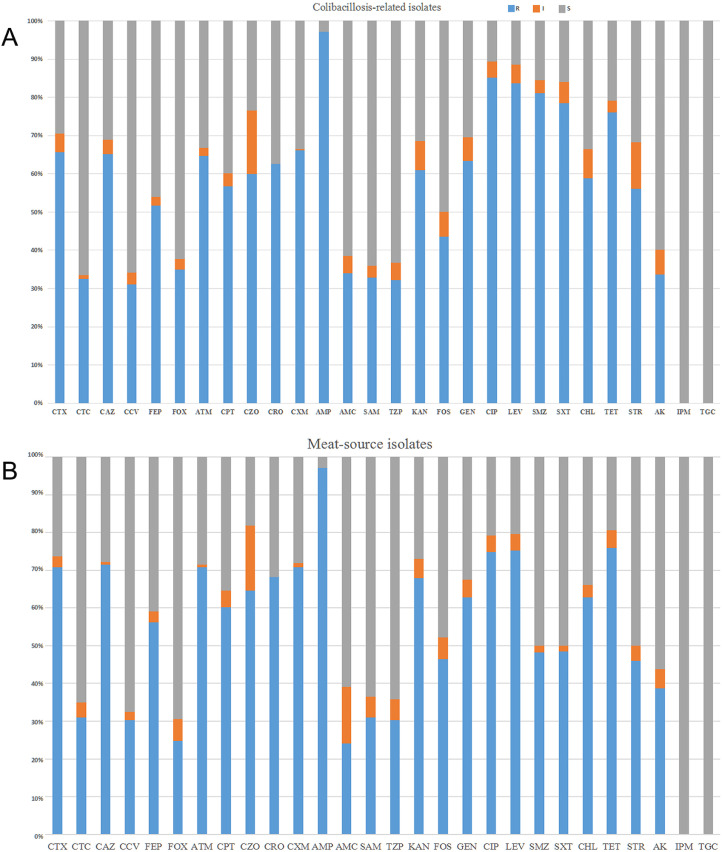
Surveillance of antimicrobial resistance in chicken-source phylogroup F E. coli strains is critical to possibility of controlling avian colibacillosis. For the susceptibility of phylogroup F E. coli from chicken colibacillosis, all strains were tested with 27 antibiotics from 16 categories. More than half of phylogroup F E. coli from chicken colibacillosis presented the resistance to cephalosporin antibiotics, including CXM (66.1%), CTX (65.7%), CAZ (64.4%), ATM (64.7%), FEP (51.6%), and CPT (56.7%) (Figure 1A). About 32% phylogroup F E. coli isolates from chicken colibacillosis were resistant to β-lactamase inhibitors, including CTC, AMC, SAM, CCV, and TZP. Moreover, 34.9% E. coli isolates were resistant to FOX. There were high resistance rates of phylogroup F E. coli isolates from chicken colibacillosis to non-cephalosporin antibiotics. For 289 colibacillosis-related isolates, 97.2% resistant to AMP, 85.1% were resistant to CIP, and 81.0% resistant to SMZ. And around 60% of colibacillosis-related isolates were resistant to other non-cephalosporin antibiotics, such as GEN, KAN, TET, and STR (Figure 1A). Despite this, there was relatively low resistance to FOS (37.0%) and AK (33.6%). Importantly, 27 (9.3%) colibacillosis-related isolates conferred resistance to colistin with MICs ≥4 mg/L. It was worthy highlighting that all phylogroup F isolates were susceptible to IPM and TGC. The antimicrobial susceptibility tests showed all colibacillosis-related phylogroup F E. coli were MDR strains, and 95.5% isolates conferred resistance to more than 5 antimicrobial agents (Table S2). Furthermore, 4 phylogroup F E. coli isolates were resistant to 13 drug categories, apart from colistin, carbapenem, and glycylcycline. According to the definition of MDR, XDR, and PDR microbes (Magiorakos et al., 2012), 3 isolates resistant to 14 categories could be considered as XDR strains (Table S2). Based on the resistance spectrum for cephalosporins and β-lactamase inhibitors, more than 66.1% colibacillosis-related phylogroup F E. coli might produce ESBLs or pAmpCs. The majority of cephalosporin-resistance isolates were located in the dominant ST117, ST354, ST405, ST457, and ST648.
Figure 1.
(A) Antimicrobial susceptibility for phylogroup F E. coli from chicken colibacillosis. The columns showed the percentages of 289 strains that were resistant (blue), intermediate (orange), or sensitive (gray) to 28 common antibiotics. Abbreviations were indicted in materials and methods. (B) Antimicrobial susceptibility for 274 phylogroup F E. coli isolates from chicken retail meats.
Antimicrobial Susceptibility of Phylogroup F E. coli From Retail Meats
The antimicrobial susceptibility tests were performed to evaluate antimicrobial resistance traits of phylogroup F E. coli from retail meats, which is critical to the poultry food safety. Similar to the resistance spectrums of E. coli recovered from chicken colibacillosis, isolates from retail meats held the high resistance rates (≥60%) to cephalosporin antibiotics, including CTX (70.8%), CAZ (71.5%), CRO (68.2%), CPT (60.2%), and others. Moreover, those isolates resistant to β-lactamase inhibitors presented about 24.0 to 30% rates, such as AMC (24.1%) and CTC (31.0%) (Figure 1B). Phylogroup F E. coli from chicken meats held low resistance rates to FOS (46.4%) and AK (38.7%). Moreover, 17 (6.2%) isolates were resistant to colistin. Similar to colibacillosis-related isolates, the antimicrobial susceptibility tests showed all meat-related phylogroup F E. coli were MDR strains, and 98.9% isolates conferred resistance to more than 5 antimicrobial agents. Furthermore, 5 phylogroup F E. coli from retail meats were resistant to 13 drug categories, apart from colistin, carbapenem, and glycylcycline (Table S3). About 71.2% chicken meat-related phylogroup F E. coli might produce ESBLs or pAmpCs, and the majority of cephalosporin-resistance isolates also belonged to the dominant ST117, ST354, ST393, ST405, ST457, and ST648.
Wide Distribution of ESBLs and pAmpCs Genes in Chicken-Source Phylogroup F E. coli Isolates
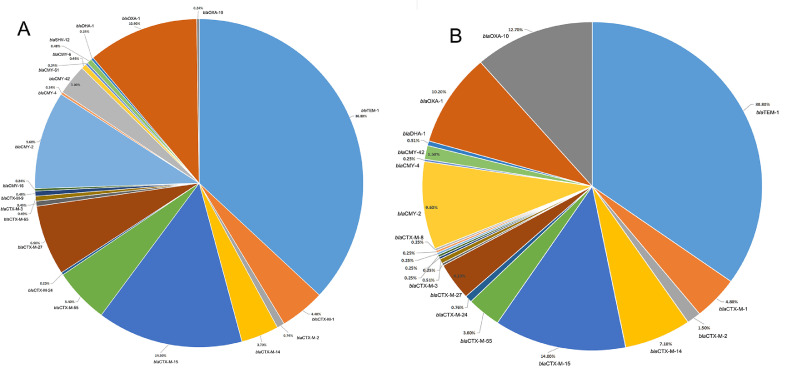
The cephalosporin susceptibility tests suggested that there might be existence of ESBLs and pAmpCs genes among phylogroup F E. coli recovered from chicken colibacillosis tissues and retail raw meats. ESBLs/pAmpCs gene profiles in these chicken-source phylogroup F strains were identified by multiplex PCR. For colibacillosis-related phylogroup F E. coli, about 89.6% isolates harbored β-lactamase TEM, CTX-M groups, OXA, CMY variants, and others (Table S2). The total 18 types of CTX-M, CMY, and OXA genes for 253 copy were detected in colibacillosis-related phylogroup F E. coli isolates (Figure 2A, Table S2). CTX-M genes were detected in 50.2% of colibacillosis-related strains. CTX-M genes presented in these isolates were distributed into 10 types, among which, blaCTX-M-15 (37.7%), blaCTX-M-27 (18.5%), blaCTX-M-55 (13.9%), and blaCTX-M-1 (11.9%) were the dominant blaCTX-M types. Plasmid-encoded pAmpC genes (blaCMY and blaDHA) were presented in 19.4% colibacillosis-related phylogroup F isolates. The blaCMY-2 (69.6%, 39/56) and blaCMY-42 (21.4%, 12/56) were the dominant pAmpC types (Figure 2A, Table S2). A part of colibacillosis-related phylogroup F isolates (15.6%) harbored blaOXA genes, among which, (blaOXA-1 = 44 and blaOXA-10 = 1). Two strains contained the blaSHV-15 gene, and one harbored blaDHA-1 genes. Moreover, β-lactamase TEM genes were detected in 51.6% colibacillosis-related E. coli isolates (Table S2). ESBLs and pAmpC genes were concurrently present in colibacillosis-related E. coli isolates, forming a variety of combinations (such as blaCTX-blaCMY, blaCTX-M-blaCMY-blaOXA, blaTEM-blaCTX-blaCMY, and blaTEM-blaCTX-M-blaOXA).
Figure 2.
(A) The distribution of total CTX-M, OXA, CMY, and TEM genes in phylogroup F E. coli from chicken colibacillosis. (B) The percentages of CTX-M, OXA, CMY, and TEM genes in phylogroup F E. coli recovered from chicken retail meats.
CTX-M, OXA, CMY, and TEM genes were also widespread in phylogroup F E.coli recovered in chicken meats (Figure 2B, Table S3). Similar to colibacillosis-related E. coli, CTX-M genes were detected in 53.6% of chicken meat-related isolates. CTX-M genes in these isolates were categorized into 14 types, among which, blaCTX-M-15 (36.9%), blaCTX-M-14 (17.4%), blaCTX-M-1 (12.8%), blaCTX-M-27 (10.7%), and blaCTX-M-55 (8.1%) were the dominant blaCTX-M types (Figure 2B). Plasmid-encoded pAmpC genes (blaCMY and blaDHA) were presented in 17.2% chicken meat-related isolates. The blaCMY-2 (84.4%, 38/45) were the dominant pAmpC types (Table S3). The blaOXA-1 and blaOXA-10 were presented in 16.4% chicken meat-related phylogroup F isolates. β-lactamase TEM genes were detected in 55.8% chicken meat-related E. coli isolates (Table S3). Co-existence of ESBLs and pAmpCs genes were also widespread detected in meat-related phylogroup F E. coli isolates.
The Presence of Non-lactamase Resistance Genes in Chicken-Source Phylogroup F E. coli Isolates
Besides β-lactamases, many resistance genes located in large plasmids were detected in colibacillosis-related phylogroup F E. coli. The plasmid-mediated strA and strB genes, which conferred streptomycin resistance, were detected together (Moran et al., 2017). The presence of strA/strB (43.6%) could be detected in streptomycin-resistant phylogroup F isolates, and not distributed in streptomycin-susceptible strains. To date, the transferable fosfomycin-resistant genes (fosA, fosA3, fosC2, and fosK) were identified in Enterobacteriaceae, and plasmid-encoded fosA3 mainly conferred the fosfomycin resistance in E. coli (Yao et al., 2016). We detected the fosA and fosA3 in colibacillosis-related phylogroup F E. coli isolates. The presence of fosA (16.6%) and fosA3 (20.4%) were detected in fosfomycin-resistant phylogroup F isolates, and not presented in fosfomycin-susceptible strains (Table S2). The aminoglycoside-resistance genes aph(3′)-Ia, aac(3)-IId and aac(3)-IVa were closely linked with E. coli gentamicin resistance. Our result showed the gentamicin-resistant phylogroup F isolates harbored these genes as the prevalence for 40.5, 29.8, and 20.4%, respectively. The plasmid-encoded catA1, catB, cmlA, and floR genes linked with chloramphenicol resistance could be detected in chloramphenicol-resistant phylogroup F isolates with the prevalence for 21., 15.9, 19.3, and 22.8% (Table S2). The acquisition of sul genes (sul1, sul2, and sul3) mediated E. coli resistance to sulfonamides. The sul1 (42.6%), sul2 (56.4%), and sul3 genes (13.8%) were widespread in sulfonamide-resistant phylogroup F isolates. The plasmid-encoded dfrA gene in clinical E. coli mainly conferred the resistance to sulfisoxazole and trimethoprim-sulfamethoxazole trimethoprim. The 66.7% prevalence of dfrA was detected in colibacillosis-related phylogroup F E. coli. The presence of tet(A), tet(B), and tet(M) for tetracycline resistance were detected in tetracycline-resistant isolates, among which, tet(A) (50.2%), tet(B) (29.8%), and tet(C) (12.1%) widespread in these phylogroup F strains. In addition to the mutations in gyrA and parC genes, plasmid-mediated quinolone resistance genes, including aac(6′)Ib-cr, qnrA, qnrB, qepA, qnrS, oqxA, and oqxB (Gomi et al., 2017; Badi et al., 2018) were closely associated with the resistance to fluoroquinolone. Multiple PCR results indicated that aac(6′)Ib-cr (23.2%), qnrA (9.7%), qnrB (5.9%), qnrS (11.7%), qepA (15.9%), oqxA (12.8%), and oqxB (12.5%) were detected in colibacillosis-related phylogroup F E. coli (Table S2).
For phylogroup F E. coli recovered in chicken meats, the presence of strA (46.4%) and strB (46.7%) could be detected in streptomycin-resistant meat-related isolates. fosA (12.0%) and fosA3 (20.4%) were presented in fosfomycin-resistant meat-related isolates. The gentamicin-resistance genes aph(3′)-Ia, aac(3)-IId and aac(3)-IVa were detected for 58.4, 37.2, and 20.1%, respectively. The catA1 (25.9%), catB (19.7%), cmlA (14.9%), and floR (22.3%) could be detected in chloramphenicol-resistant isolates (Table S3). The sul1 (44.9%), sul2 (56.6%) genes, and sul3 (6.2%) were widespread in sulfonamide-resistant phylogroup F isolates. The 63.9% prevalence of dfrA was detected in chicken meat-related phylogroup F E. coli. The tet(A) (62.4%), tet(B) (65.3%), and tet(C) (28.8%) were also present in these phylogroup F strains. The aac(6′)Ib-cr (27.0%), qnrA (12.4%), qnrB(8.8%), qnrS (17.9%), qepA (21.9%), oqxA (10.6%), and oqxB (10.6%) were detected in meat-related phylogroup F E. coli (Table S3).
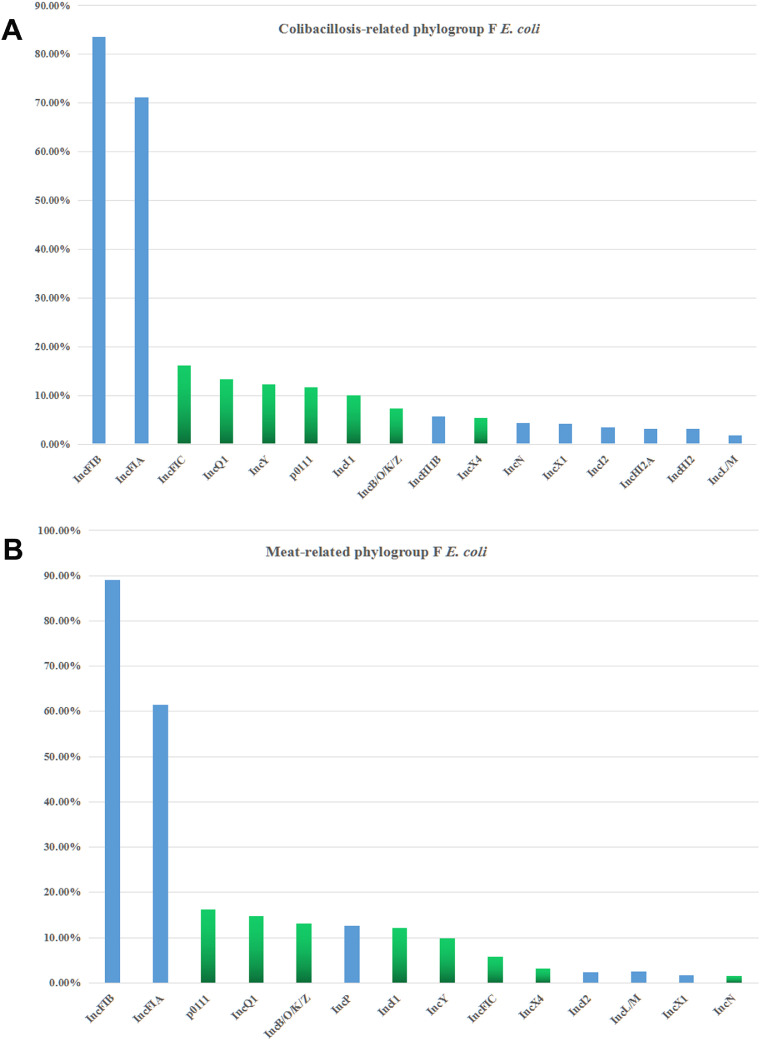
Plasmid Replicon Types in Chicken-Source Phylogroup F E. coli Isolates
Owing to plasmid-encoded resistance genes widespread in chicken-source phylogroup F E. coli isolates, the total 19 replicon types (IncFIA, IncFIB, IncFIC, IncB/O/K/Z, IncP, IncQ1, IncHI2, IncHI2A, IncFII, IncI1, IncI2, IncHI1B, p0111, IncA/C, IncL/M, IncN, IncX1, IncX4, and IncY) were detected in each phylogroup F strain. For colibacillosis-related phylogroup F E. coli, IncFIA, IncFIB, IncFIC, IncFII, IncB/O/K/Z, IncI1, IncN, IncQ1, IncX4, IncY, and p0111 were the most commonly presented in these isolates (Figure 3A). Similar to colibacillosis-related isolates, plasmid replicon types (IncB/O/K/Z, IncFIA, IncFIB, IncFIC, IncFII, IncI1, IncI2, IncL/M, IncQ1, IncX4, IncY, and p0111) were identified in meat-related phylogroup F E. coli (Figure 3B). Apart from IncFIA, IncFIB and IncFII replicons, the widespread replicons IncB/O/K/Z, IncI1, IncN, IncFIC, IncQ1, IncX4, IncY, and p0111 were obviously associated with antibiotic-resistant large plasmids (Johnson et al., 2012b; Musicha et al., 2017; Kawamura et al., 2018). And IncB/O/K/Z, IncFIC, IncL/M, IncN, and IncY replicons exhibited the significant relations with resistance genotypes for ESBLs and pAmpCs genes (Johnson et al., 2012b; Musicha et al., 2017).
Figure 3.
(A) Distribution of plasmid replicon types among the phylogroup F E. coli isolates from chicken colibacillosis. (B) Distribution of replicon types among the phylogroup F E. coli strains from chicken retail meats.
DISCUSSION
Human ExPECs cause a series of extraintestinal disease syndromes, such as urinary tract infections (UTI), bloodstream infections, neonatal meningitis, and wound infections. ExPECs are categorized as several subpathotypes, including uropathogenic E. coli (UPEC), sepsis-associated E. coli (SEPEC), and neonatal meningitis E. coli (NMEC) (Guo, et al., 2015; Mitchell, et al., 2015; Kallonen, et al., 2017). Moreover, the growing body of epidemiological evidences indicate that ExPEC isolates are widespread in nonhuman sources, including poultry, livestock, companion animals, and retail meat products (Bergeron et al., 2012; Manges and Johnson, 2012; Liu et al., 2018). APEC is a typical nonhuman ExPEC subpathotypes. A population assessment of human and avian E. coli strains from extraintestinal infections indicates that the isolates reassigned to phylogroup F hold a higher content of ExPEC-related virulence genes and pathogenicity islands, compared to that in the remaining new D and E groups (Logue et al., 2017). ExPEC strains within phylogroup F, are also highly prevalent in companion animals, swine, horses, cattle, and wild birds (Ewers et al., 2014; Abraham et al., 2015; Blyton et al., 2015; Guo, et al., 2015). Moreover, human ExPEC strains in phylogroup F exhibit antibiotic resistance potential and harbor a series of resistance genes (Abraham et al., 2015; Vangchhia et al., 2016; Logue et al., 2017).
Previous research show that majority of ExPEC isolates were assigned to phylogroups B2 and D, determined by triple PCR method (Clermont et al., 2000; Johnson et al., 2003; Rodriguez-Siek et al., 2005; Chapman et al., 2006; Johnson et al., 2008). In recent epidemiology, the revised Clermont multiplex PCR are performed to reclassify the phylogroups of human or nonhuman ExPECs strains. The isolates, originally classified into phylogroup D by old triple PCR, are reassigned to phylogroups D, F, and a minor group E (Logue et al., 2017). In our previous study, phylogroup F chicken-source E. coli isolates have been revealed as true APECs and hold virulence (Zhuge et al., 2020). Wu et al. (2018) reports the high prevalence of ESBL genes in chicken-source E. coli among the different poultry industries in China (). The findings reveal blaCTX-M as the predominant ESBL gene in ESBL-producing isolates (Wu et al., 2018). However, there is few report on the systematic assessment of the antibiotic resistance potential among chicken-source phylogroup F E. coli strains in China.
In this study, a total of 563 phylogroup F E. coli strains were recovered from chicken colibacillosis tissues and retail raw chicken meat samples in Eastern China. the antimicrobial susceptibilities of these chicken-source isolates were measured by disk diffusion method referring to the CLSI criteria, and about 68.6% prevalence of ESBL/pAmpC-producing isolates in chicken-source phylogroup F E. coli. In order to identify their molecular characteristics for antibiotic resistance, resistance genotypes and plasmid replicon types of chicken-source phylogroup F E. coli isolates were detected by multiplex PCR. Identification of resistance genotypes and replicon profiling indicated that a majority of chicken-source phylogroup F E. coli isolates harbored plasmid-carrying resistance genes to withstand multiple antimicrobial agents. Our results showed co-existence of ESBLs and pAmpCs genes were widespread detected in chicken-source phylogroup F E. coli isolates. To date, CTX-M type with an increasing occurrence worldwide becomes the dominant beta-lactamases type in Enterobacteriaceae family (Pitout, 2012; Poirel et al., 2018). Our study showed the blaCTX-M-15 was the most common CTX-M type among phylogroup F E. coli recovered from chicken colibacillosis and retail raw meats samples. Besides the cephalosporins, AmpC-type beta-lactamases hold resistance to ESBLs inhibitors and cephamycin (such as cefotetan and cefoxitin) (Poirel et al., 2018). Although plasmid-mediated AmpC including a series of types, CMY-2 type is the most commonly AmpC-type beta-lactamase encountered among chicken-source phylogroup F E. coli isolates.
Previous epidemiological research indicates that ST95 E. coli isolates presented low frequency of antimicrobial resistance and even pan-susceptibility to antimicrobial agents (Stephens et al., 2017). The ST131 E. coli is acknowledged as worldwide high-risk multidrug-resistant clone (Mathers et al., 2015). For improved understanding of the spreading dynamics of ESBL-producing APEC from chicken meats to humans, the ESBL/pAmpC genes and non-lactamase resistance elements and genetic lineages of E. coli from chicken meats were analyzed. There was an overlapped distribution of MLST types between chicken colibacillosis-origin and meat-source phylogroup F E. coli, including dominant STs (ST648, ST405, ST457, ST393, ST1158, etc). Our results showed these dominant STs for chicken-source phylogroup F E. coli isolates were recognized as multidrug-resistant high-risk clones. Moreover, there was similar resistance spectrums and resistance gene contents for phylogroup F E. coli isolates from chicken colibacillosis and retail meats in Eastern China. The latest report by Clermont et al. (2019) shows a new phylogroup G, located intermediately between the phylogroups B2 and F. E. coli isolates in phylogroup G contains 5 sequence types (ST117, ST174, ST454, ST657, and ST738). There are some STs appearing in this study. We found many phylogroup F chicken-source E. coli strains belonged to ST117, ST657, and ST738, based on the identification criteria of phylogroup F in 2013. These strains originally belonging to phylogroup F are generally pathogenic and broadly resistant. This result indicated that this phylogroup G for avian-source E. coli was a zoonotic high-risk group, separated from the phygroup F.
These resistance genes are often positioned at transferable large plasmids of APEC isolates (Poirel et al., 2018; Zhuge et al., 2019). The occurrence of multidrug-resistant APECs not only cause difficulties to the avoidance and prevention of APEC infection, but also brings some challenges in the resistance spread of mobile plasmids to other pathogens and commensals. Therefore, as a long-term strategy, it is critical to discover alternative methods to control colibacillosis in poultry industry. Increased consumption of antimicrobial drugs in food-producing animals to enhance production efficiency have contributed to the emergence and spread of multidrug-resistant APEC/ExPEC, which might promote global increase of ExPEC population diversity in human resistant E. coli infections (Manges et al., 2007; Liu et al., 2016; Wang et al., 2016; Wang et al., 2017; Mellata et al., 2018; Song et al., 2020). However, there is a missing of direct evidence to disclose the causal association between food-original APEC/ExPEC and human extraintestinal infections, because, when establishing habitation in human gut, ExPEC can persist innocuously as commensal microbes in the intestinal tract for months, even to years until environments approving an extraintestinal infection (Manges and Johnson, 2012; Mellata et al., 2018). The improved surveillance of APEC dissemination among poultry reservoirs and chicken-derived food products, and the zoonotic risk of APEC transmission to human is strictly linked with public health implications.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (grant no. 31702252 and 31872479). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Data availability statement: All data generated or analyzed during this study are included in this published article.
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Characterization of Antimicrobial Resistance in Chicken-source Phylogroup F Escherichia coli: Similar Populations and Resistance Spectrums Between E. coli Recovered from Chicken Colibacillosis Tissues and Retail Raw Meats in Eastern China”
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2021.101370.
Appendix. Supplementary materials
Table S1. The detail description of PCR primers for E. coli resistance determinants.
Table S2. The detail STs, resistance spectrums, and the resistance genes prevalence among phylogroup F E. coli isolates from chicken colibacillosis.
Table S3. The detail STs, resistance spectrums, and the resistance genes prevalence among phylogroup F E. coli isolates from chicken retail meats.
REFERENCES
- Abraham S., Jordan D., Wong H.S., Johnson J.R., Toleman M.A., Wakeham D.L., Gordon D.M., Turnidge J.D., Mollinger J.L., Gibson J.S., Trott D.J. First detection of extended-spectrum cephalosporin- and fluoroquinolone-resistant Escherichia coli in Australian food-producing animals. J. Glob. Antimicrob. Resist. 2015;3:273–277. doi: 10.1016/j.jgar.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Badi S., Cremonesi P., Abbassi M.S., Ibrahim C., Snoussi M., Bignoli G., Luini M., Castiglioni B., Hassen A. Antibiotic resistance phenotypes and virulence-associated genes in Escherichia coli isolated from animals and animal food products in Tunisia. FEMS Microbiol. Lett. 2018;365 doi: 10.1093/femsle/fny088. [DOI] [PubMed] [Google Scholar]
- Beghain J., Bridier-Nahmias A., Le Nagard H., Denamur E., Clermont O. ClermonTyping: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb. Genomics. 2018;4 doi: 10.1099/mgen.0.000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron C.R., Prussing C., Boerlin P., Daignault D., Dutil L., Smith Reid- Chicken as reservoir for extraintestinal pathogenic Escherichia coli in humans. Can. Emerg. Infect. Dis. 2012;18:415–421. doi: 10.3201/eid1803.111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyton M.D., Pi H., Vangchhia B., Abraham S., Trott D.J., Johnson J.R., Gordon D.M. Genetic structure and antimicrobial resistance of Escherichia coli and cryptic clades in birds with diverse human associations. Appl. Environ. Microbiol. 2015;81:5123–5133. doi: 10.1128/AEM.00861-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell T., Mahida N., Montgomery R., Clarke M. Enhanced surveillance of Escherichia coli healthcare-associated bloodstream infections - how many are preventable? J. Hosp. Infect. 2018;100:65–66. doi: 10.1016/j.jhin.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Carattoli A., Bertini A., Villa L., Falbo V., Hopkins K.L., Threlfall E.J. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods. 2005;63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Chapman T.A., Wu X.Y., Barchia I., Bettelheim K.A., Driesen S., Trott D., Wilson M., Chin J.J. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 2006;72:4782–4795. doi: 10.1128/AEM.02885-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont O., Bonacorsi S., Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont O., Christenson J.K., Denamur E., Gordon D.M. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013;5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- Clermont O., Dixit O.V.A., Vangchhia B., Condamine B., Dion S., Bridier-Nahmias A., Denamur E., Gordon D. Characterization and rapid identification of phylogroup G in Escherichia coli, a lineage with high virulence and antibiotic resistance potential. Environ. Microbiol. 2019;21:3107–3117. doi: 10.1111/1462-2920.14713. [DOI] [PubMed] [Google Scholar]
- CLSI . 28th ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2018. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100. [Google Scholar]
- Collignon P., Voss A. China, what antibiotics and what volumes are used in food production animals? Antimicrob. Resist. Infect. Control. 2015;4:16. doi: 10.1186/s13756-015-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallenne C., Da Costa A., Decre D., Favier C., Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010;65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- Escobar-Paramo P., Clermont O., Blanc-Potard A.B., Bui H., Le Bouguenec C., Denamur E. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol. Biol. Evol. 2004;21:1085–1094. doi: 10.1093/molbev/msh118. [DOI] [PubMed] [Google Scholar]
- Ewers C., Bethe A., Stamm I., Grobbel M., Kopp P.A., Guerra B., Stubbe M., Doi Y., Zong Z., Kola A., Schaufler K., Semmler T., Fruth A., Wieler L.H., Guenther S. CTX-M-15-D-ST648 Escherichia coli from companion animals and horses: another pandemic clone combining multiresistance and extraintestinal virulence? J. Antimicrob. Chemother. 2014;69:1224–1230. doi: 10.1093/jac/dkt516. [DOI] [PubMed] [Google Scholar]
- Gomi R., Matsuda T., Matsumura Y., Yamamoto M., Tanaka M., Ichiyama S., Yoneda M. Whole-genome analysis of antimicrobial-resistant and extraintestinal pathogenic Escherichia coli in river water. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.02703-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Wakeham D., Brouwers H.J., Cobbold R.N., Abraham S., Mollinger J.L., Johnson J.R., Chapman T.A., Gordon D.M., Barrs V.R., Trott D.J. Human-associated fluoroquinolone-resistant Escherichia coli clonal lineages, including ST354, isolated from canine feces and extraintestinal infections in Australia. Microbes Infect. 2015;17:266–274. doi: 10.1016/j.micinf.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Iguchi A., Iyoda S., Seto K., Morita-Ishihara T., Scheutz F., Ohnishi M., Japa P.E.C.W.G. Escherichia coli O-Genotyping PCR: a comprehensive and practical platform for molecular O serogrouping. J. Clin. Microbiol. 2015;53:2427–2432. doi: 10.1128/JCM.00321-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.R., Murray A.C., Gajewski A., Sullivan M., Snippes P., Kuskowski M.A., Smith K.E. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob. Agents Chemother. 2003;47:2161–2168. doi: 10.1128/AAC.47.7.2161-2168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.R., Porter S.B., Johnston B., Thuras P., Clock S., Crupain M., Rangan U. Extraintestinal pathogenic and antimicrobial-resistant Escherichia coli, including sequence type 131 (ST131), from retail chicken breasts in the United States in 2013. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.02956-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.R., Urban C., Weissman S.J., Jorgensen J.H., Lewis J.S., 2nd, Hansen G., Edelstein P.H., Robicsek A., Cleary T., Adachi J., Paterson D., Quinn J., Hanson N.D., Johnston B.D., Clabots C., Kuskowski M.A. Molecular epidemiological analysis of Escherichia coli sequence type ST131 (O25:H4) and blaCTX-M-15 among extended-spectrum-beta-lactamase-producing E. coli from the United States, 2000 to 2009. Antimicrob. Agents Chemother. 2012;56:2364–2370. doi: 10.1128/AAC.05824-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.J., Logue C.M., Johnson J.R., Kuskowski M.A., Sherwood J.S., Barnes H.J., DebRoy C., Wannemuehler Y.M., Obata-Yasuoka M., Spanjaard L., Nolan L.K. Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia coli from humans and poultry. Foodborne Pathog. Dis. 2012;9:37–46. doi: 10.1089/fpd.2011.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.J., Wannemuehler Y., Johnson S.J., Stell A.L., Doetkott C., Johnson J.R., Kim K.S., Spanjaard L., Nolan L.K. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl. Environ. Microbiol. 2008;74:7043–7050. doi: 10.1128/AEM.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.J., Wannemuehler Y.M., Johnson S.J., Logue C.M., White D.G., Doetkott C., Nolan L.K. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl. Environ. Microbiol. 2007;73:1976–1983. doi: 10.1128/AEM.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallonen T., Brodrick H.J., Harris S.R., Corander J., Brown N.M., Martin V., Peacock S.J., Parkhill J. Systematic longitudinal survey of invasive Escherichia coli in England demonstrates a stable population structure only transiently disturbed by the emergence of ST131. Genome Res. 2017;27:1437–1449. doi: 10.1101/gr.216606.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K., Hayashi K., Matsuo N., Kitaoka K., Kimura K., Wachino J.I., Kondo T., Iinuma Y., Murakami N., Fujimoto S., Arakawa Y. Prevalence of CTX-M-type extended-spectrum beta-lactamase-producing Escherichia coli B2-O25-ST131 H30R among residents in nonacute care facilities in Japan. Microb. Drug Resist. 2018;24:1513–1520. doi: 10.1089/mdr.2018.0068. [DOI] [PubMed] [Google Scholar]
- Li L., Jiang Z.G., Xia L.N., Shen J.Z., Dai L., Wang Y., Huang S.Y., Wu C.M. Characterization of antimicrobial resistance and molecular determinants of beta-lactamase in Escherichia coli isolated from chickens in China during 1970-2007. Vet. Microbio. 2010;144:505–510. doi: 10.1016/j.vetmic.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Liu C., Diao Y., Wang D., Chen H., Tang Y. Duck viral infection escalated the incidence of avian pathogenic Escherichia coli in China. Transbound Emerg. Dis. 2018;66:929–938. doi: 10.1111/tbed.13107. [DOI] [PubMed] [Google Scholar]
- Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., Yu L.F., Gu D., Ren H., Chen X., Lv L., He D., Zhou H., Liang Z., Liu J.H., Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Logue C.M., Wannemuehler Y., Nicholson B.A., Doetkott C., Barbieri N.L., Nolan L.K. Comparative analysis of phylogenetic assignment of human and avian ExPEC and fecal commensal Escherichia coli using the (previous and revised) clermont phylogenetic typing methods and its impact on Avian Pathogenic Escherichia coli (APEC) classification. Front. Microbiol. 2017;8:283. doi: 10.3389/fmicb.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., Paterson D.L., Rice L.B., Stelling J., Struelens M.J., Vatopoulos A., Weber J.T., Monnet D.L. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Maiden M.C., Bygraves J.A., Feil E., Morelli G., Russell J.E., Urwin R., Zhang Q., Zhou J., Zurth K., Caugant D.A., Feavers I.M., Achtman M., Spratt B.G. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manges A.R., Johnson J.R. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin. Infect. Dis. 2012;55:712–719. doi: 10.1093/cid/cis502. [DOI] [PubMed] [Google Scholar]
- Manges A.R., Johnson J.R. Reservoirs of extraintestinal pathogenic Escherichia coli. Microbiol. Spectr. 2015;3(5) doi: 10.1128/microbiolspec.UTI-0006-2012. [DOI] [PubMed] [Google Scholar]
- Manges A.R., Smith S.P., Lau B.J., Nuval C.J., Eisenberg J.N., Dietrich P.S., Riley L.W. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: a case-control study. Foodborne Pathog. Dis. 2007;4:419–431. doi: 10.1089/fpd.2007.0026. [DOI] [PubMed] [Google Scholar]
- Mathers A.J., Peirano G., Pitout J.D. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015;28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M., Johnson J.R., Curtiss R., 3rd Escherichia coli isolates from commercial chicken meat and eggs cause sepsis, meningitis and urinary tract infection in rodent models of human infections. Zoonoses Public Health. 2018;65:103–113. doi: 10.1111/zph.12376. [DOI] [PubMed] [Google Scholar]
- Mitchell N.M., Johnson J.R., Johnston B., Curtiss R., 3rd, Mellata M. Zoonotic potential of Escherichia coli isolates from retail chicken meat products and eggs. Appl. Environ. Microbiol. 2015;81:1177–1187. doi: 10.1128/AEM.03524-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R.A., Anantham S., Holt K.E., Hall R.M. Prediction of antibiotic resistance from antibiotic resistance genes detected in antibiotic-resistant commensal Escherichia coli using PCR or WGS. J. Antimicrob. Chemother. 2017;72:700–704. doi: 10.1093/jac/dkw511. [DOI] [PubMed] [Google Scholar]
- Musicha P., Feasey N.A., Cain A.K., Kallonen T., Chaguza C., Peno C., Khonga M., Thompson S., Gray K.J., Mather A.E., Heyderman R.S., Everett D.B., Thomson N.R., Msefula C.L. Genomic landscape of extended-spectrum beta-lactamase resistance in Escherichia coli from an urban African setting. J. Antimicrob. Chemother. 2017;72:1602–1609. doi: 10.1093/jac/dkx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas-Chanoine M.H., Bertrand X., Madec J.Y. Escherichia coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 2014;27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitan Y. Current trends in antimicrobial resistance of Escherichia coli. Curr. Top. Microbiol. Immunol. 2018;416:181–211. doi: 10.1007/82_2018_110. [DOI] [PubMed] [Google Scholar]
- Pitout J.D. Extraintestinal pathogenic Escherichia coli: an update on antimicrobial resistance, laboratory diagnosis and treatment. Expert Rev. Anti Infect. Ther. 2012;10:1165–1176. doi: 10.1586/eri.12.110. [DOI] [PubMed] [Google Scholar]
- Pitout J.D.D., Laupland K.B. Extended-spectrum beta-lactamase-producing enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- Poirel L., Madec J.Y., Lupo A., Schink A.K., Kieffer N., Nordmann P., Schwarz S. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 2018;6(4) doi: 10.1128/microbiolspec.ARBA-0026-2017. [DOI] [PubMed] [Google Scholar]
- Poirel L., Walsh T.R., Cuvillier V., Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011;70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Siek K.E., Giddings C.W., Doetkott C., Johnson T.J., Fakhr M.K., Nolan L.K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology. 2005;151:2097–2110. doi: 10.1099/mic.0.27499-0. [DOI] [PubMed] [Google Scholar]
- Selander R.K., Caugant D.A., Whittam T.S. Genetic structure and variation in natural populations of Escherichia coli. In: Neidhardt F.C., editor. Escherichia Coli and Salmonella Typhimurium Cellular and Molecular Biology. American Society for Microbiology; Washington, DC: 1987. pp. 1625–1648. (ed) [Google Scholar]
- Song Y.Y., Yu L.P., Zhang Y., Dai Y., Wang P., Feng C.L., Liu M.D., Sun S.H., Xie Z.J., Wang F.K. Prevalence and characteristics of multidrug-resistant mcr-1-positive Escherichia coli isolates from broiler chickens in Tai'an. China. Poult. Sci. 2020;99:1117–1123. doi: 10.1016/j.psj.2019.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens C.M., Adams-Sapper S., Sekhon M., Johnson J.R., Riley L.W. Genomic analysis of factors associated with low prevalence of antibiotic resistance in extraintestinal pathogenic Escherichia coli sequence type 95 strains. mSphere. 2017;2 doi: 10.1128/mSphere.00390-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong P.P., Sun Y., Ji X., Du X.L., Guo X.J., Liu J., Zhu L.W., Zhou B., Zhou W., Liu G., Feng S.Z. Characterization of antimicrobial resistance and extended-spectrum beta-Lactamase Genes in Escherichia coli isolated from chickens. Foodborne Pathog. Dis. 2015;12:345–352. doi: 10.1089/fpd.2014.1857. [DOI] [PubMed] [Google Scholar]
- Vangchhia B., Abraham S., Bell J.M., Collignon P., Gibson J.S., Ingram P.R., Johnson J.R., Kennedy K., Trott D.J., Turnidge J.D., Gordon D.M. Phylogenetic diversity, antimicrobial susceptibility and virulence characteristics of phylogroup F Escherichia coli in Australia. Microbiology. 2016;162:1904–1912. doi: 10.1099/mic.0.000367. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yi L., Wang Y.X., Wang Y.G., Cai Y., Zhao W.P., Ding C. Isolation, phylogenetic group, drug resistance, biofilm formation, and adherence genes of Escherichia coli from poultry in central China. Poul. Sci. 2016;95:2895–2901. doi: 10.3382/ps/pew252. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang R., Li J., Wu Z., Yin W., Schwarz S., Tyrrell J.M., Zheng Y., Wang S., Shen Z., Liu Z., Liu J., Lei L., Li M., Zhang Q., Wu C., Wu Y., Walsh T.R., Shen J. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2017;2:16260. doi: 10.1038/nmicrobiol.2016.260. [DOI] [PubMed] [Google Scholar]
- Wu C.M., Wang Y.C., Shi X.M., Wang S., Ren H.W., Shen Z.Q., Wang Y., Lin J.C., Wang S.L. Rapid rise of the ESBL and mcr-1 genes in Escherichia coli of chicken origin in China, 2008-2014. Emerg. Microbes Infec. 2018;7:30. doi: 10.1038/s41426-018-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Wu D., Lei L., Shen Z., Wang Y., Liao K. The detection of fosfomycin resistance genes in Enterobacteriaceae from pets and their owners. Vet. Microbiol. 2016;193:67–71. doi: 10.1016/j.vetmic.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Zhu Ge X., Jiang J., Pan Z., Hu L., Wang S., Wang H., Leung F.C., Dai J., Fan H. Comparative genomic analysis shows that avian pathogenic Escherichia coli isolate IMT5155 (O2:K1:H5; ST complex 95, ST140) shares close relationship with ST95 APEC O1:K1 and human ExPEC O18:K1 strains. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuge X., Ji Y., Tang F., Sun Y., Jiang M., Hu W., Wu Y., Xue F., Ren J., Zhu W., Dai J. Population structure and antimicrobial resistance traits of avian-origin mcr-1-positive Escherichia coli in Eastern China, 2015 to 2017. Transbound Emerg. Dis. 2019;66:1920–1929. doi: 10.1111/tbed.13222. [DOI] [PubMed] [Google Scholar]
- Zhuge X.K., Zhou Z., Jiang M., Wang Z.X., Sun Y., Tang F., Xue F., Ren J.L., Dai J.J. Chicken-source Escherichia coli within phylogroup F shares virulence genotypes and is closely related to extraintestinal pathogenic E. coli causing human infections. Transbound Emerg. Dis. 2020;68:880–895. doi: 10.1111/tbed.13755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The detail description of PCR primers for E. coli resistance determinants.
Table S2. The detail STs, resistance spectrums, and the resistance genes prevalence among phylogroup F E. coli isolates from chicken colibacillosis.
Table S3. The detail STs, resistance spectrums, and the resistance genes prevalence among phylogroup F E. coli isolates from chicken retail meats.