Abstract
The knowledge of the essential role of platelets in tissue healing is gradually increasing and as regenerative medicine prompts new solutions, platelet-derived bioproducts have been proposed as a potential tool in this field. In orthopaedics and sports medicine, the use of PRP has been rapidly increasing in popularity as patients seek novel non-surgical approaches to acute and chronic musculoskeletal conditions. The concept of having platelets as a secretory organ other than a mere sponge-like coagulation component opens up new frontiers for the use of the platelet secretome. Platelet lysate is a solution saturated by growth factors, proteins, cytokines, and chemokines involved in crucial healing processes and is administered to treat different diseases such as alopecia, oral mucositis, radicular pain, osteoarthritis, and cartilage and tendon disorders. For this purpose, the abundant presence of growth factors and chemokines stored in platelet granules can be naturally released by different strategies, mostly through lyophilization, thrombin activation or ultrasound baths (ultrasonication). As a result, human platelet lysate can be produced and applied as a pure orthobiologic. This review outlines the current knowledge about human platelet lysate as a powerful adjuvant in the orthobiological use for the treatment of musculoskeletal injuries, without however failing to raise some of its most applicable basic science.
Keywords: Platelet lysate, Regenerative medicine, Orthobiologics, Orthopaedics, Musculoskeletal injuries
1. Introduction
The increasing use of orthobiologics in musculoskeletal injuries, especially cell-based therapies involving mesenchymal stem cells (MSCs), has led to an increasing demand for clarification (or elucidation) of the role of blood components. Platelets play an essential role not only in primary hemostasis but also in wound healing and tissue regeneration.1 Significant knowledge about platelet biology has been gained during the last decades with increased focus on their regenerative properties. Indeed, platelets behave as a natural reservoir with secretion capacity that is now observed through many studies in the literature.2,3 In this context, platelet-rich plasma (PRP) appears to be the most popular platelet-derived product, which represents a biological treatment for various musculoskeletal injuries involving tendons, ligaments, cartilage and bone. PRP is one of the many new developments within the expanding field of regenerative medicine. Although different formulations have been described, it aims to improve the process of tissue repair through local delivery of autologous bioactive agents to influence critical physiological mechanisms such as inflammation, angiogenesis or extracellular matrix (ECM) synthesis.4 Recently, Lana et al. proposed a new classification for PRP in order to standardize PRP procedures.5 Criteria included harvest method, activation, red blood cells, number of spins, image guidance, leukocytes number and light activation in order to propose a consensus for new studies. To be defined as a “working PRP”, the platelet concentration should be around 106 per microliter, since higher concentrations have not shown enhancements tissue healing.6 Platelet-rich plasma (PRP) is also defined as platelets concentrated over the basal number (four-to nine-fold) in a small plasma volume.
By its secretome, platelets have a central role in hemostasis, secreting a cargo of proteins that are found in alpha granules, dense granules, lysosomes and micro-particles. Growth factors include vascular endothelial growth factor, epidermal growth factor, transforming growth factor-B, hepatocyte growth factor, brain-derived neurotropic factor, basic fibroblast growth factor, connective tissue grow factor, angiopoietin-1, SDF-1, thrombospondin and bone morphogenetic proteins (BMP-2, 4, 6),7 chemokine (CXC) ligand 12 (SDF-1), CXCL10, CXCL5, CXCL4 (PF4), CXCL3, CXCL2, CXCL1, and inflammatory cytokines such as interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte-colony-stimulating factor (G-CSF), interleukin 8 (IL-8), IL-7, IL-6, IL-1a, macrophage inflammatory protein 1a (MIP- 1a), and MIP-1b.8 Regenerative functions may also come from microbicidal and antimicrobial factors like complement C3 and C4 precursors, complement factor D and matrix metalloproteinases. Factors released from dense granules include thymosin B2, cathepsin D and E, ADP, ATP, histamine, Ca++, serotonin, epinephrine and a range of chemokines (CCL-2, 3, 5, CXCL-1, 5, 8, 12, 7). The stimulatory and inhibitory effects of these mediators cause attraction and proliferation of macrophages, fibroblasts, satellite muscle cells and progenitors/endothelial cells, resulting in vessel remodeling and tissue repair. Platelets also have a role in modulating innate and adaptive immune responses suggesting a putative influence on inflammatory and tumor diseases.9 Particularly, platelets release microvesicles from the plasma membrane, which contain miRNA and mRNA to signal new protein synthesis in loci or inside other progenitor cells when internalized.10
Platelet lysate (PL) is one of the proper sources of bioactive molecules found in platelet releasate. It is used in cell growth and proliferation and is a good alternative to fetal bovine serum. In recent years, the clinical use of PL has gained more attention since its preparations are acellular, thereby reducing concerns over immunogenicity, and contains high concentrations of growth factors and cytokines. In addition, platelet lysate preparations can be filtered to remove immunoglobulins, cryopreserved and made available for future use. PL is considered to be a novel and multifactorial material containing comparably more growth factors than other blood-derived products and can be stored for longer periods of time in lower temperatures in a freezer, whereas PRP is a temperature sensitive mixture and cannot be preserved below 4 °C (8).11 The acellular nature of PL can potentially surpass the traditional PRP in terms of non-autologous application and patient variability, with some claims that platelet lysate is more growth-factor-concentrated than PRP.12
However, this new concept of using a product that had long been considered a “waste” of blood manipulation comes with some concerns regarding preparations and standardizations, especially when the use is non-autologous. There is often little information in terms of product characterization and definition. Lack of product information includes parameters used for PRP isolation from blood (such as g force, time, leukoreduction) as well as variable implementation of a clotting step.
In these limiting scenarios, basic sciences appear to move ahead of clinical studies in humans. To overcome this drawback, an extensive searching of the in vitro and in vivo studies has been outlined in this review. Evidences of practical use of the PL are progressively being made available, albeit in lesser amount in comparison to PRP analogue. For this particular matter, human studies are still lacking and its applicability is here highlighted.
1.1. Platelet lysate and cell proliferation
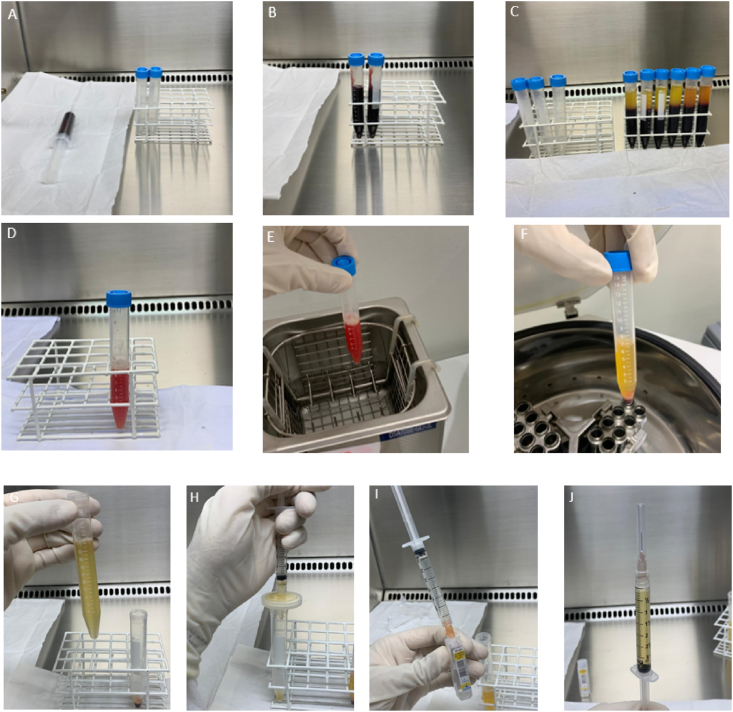
Different methods can be used to produce human platelet lysate (HPL) including platelet apheresis or platelet-rich plasma, through different centrifugation protocols.12 The latter can directly be used to produce platelet concentrate if that is the case. For this purpose, the donor's or patient's whole blood is centrifuged to separate the blood components including platelet-rich plasma, which is then centrifuged to separate platelet-poor plasma from platelet-rich plasma and cell pellet. Ultimately, plasma-, saline-, or phosphate-buffered saline is added to the pellet to reach the desired number of platelets in the platelet concentrate which can then be used to prepare platelet lysate. To do this, ultrasonication or lyophilizations can be used to promote platelet lysis. Then, using a centrifuge, the membranes of the platelet are sedimented and the supernatant containing the molecules released from the platelets is separated and filtered.12 A schematic representation of this process is illustrated by Fig. 1.
Fig. 1.
Preparation of platelet lysate. A) collection of the peripheral blood sample using 3 ml of the ACD anticoagulant; B) adding blood to sterile falcon tubes; C) blood after first centrifugation (300×g for 5 min); D) L-PRP after second centrifugation and resuspended after discarding the PPP; E) L-PRP placed in the ultrasound bath for 30 min under controlled heating; F) Lysate after centrifugation at 800×g for 10 min to separate the debris; G) Separation of the supernatant for filtration; H); Filtering the supernatant using a 0.22 μm filter; I) dilution with saline (1: 4) to optimize protein concentration; J) lysate ready for use.
Human platelet lysate (HPL) can be easily obtained through repeated freeze-thaw cycles, dispensing the need for the addition of substances. Although there is no consensus on the most effective lysis method, optimal concentration of growth factors seems to be achieved with 3–5 freeze/thawing cycles, with possible limited clinical significance beyond this number of cycles.4 Different temperature protocols have been described, most often −80/37 °C.9 By conducting several freeze/thaw cycles the platelet membranes are damaged and growth factors are efficiently released into the plasma. Finally, the platelet fragments are removed by centrifugation to avoid extensive aggregate formation and deplete potential antigens.
Classically, progenitor cells have been cultured in fetal bovine serum (FBS) in initial studies upon regenerative cells cultures.13 The risks of xeno-immunization against bovine antigens and transmission of pathogens have prompted a need for human alternatives for cell therapy protocols. In the early 1980s, human platelet lysate (HPL), mostly prepared by repeated freeze/thaw cycles and sonication from fresh blood or outdated platelet concentrates, was found to stimulate the proliferation of specific cell lines (e.g. fibroblasts and endothelial cells).14,15 Since 2005, beginning with the study of Doucet et al.16 describing the preparation and efficient use of human platelet lysate (HPL) for expanding MSC, a series of reports have shown that both allogeneic and autologous HPL are superior to FBS for stimulation of cell proliferation,17 resulting in larger colony-forming unit-fibroblast and higher differentiation capacity.18 A recent and comparative study demonstrated that growth factor (PDGF, VEGF, IGF and TGF-b) concentration in PL surpassed in 148.7 fold the corresponding parameters in FCS, and replacement of FCS by PL increased the number of MSCs by 396,5 %.19,20 These features of HPL have met the idea that platelets have more to do with a “secreting factory” than storage as previously thought.
Similar to the non-consensus situation observed inin vivo use of PRP, there is still no uniform procedure for HPL preparation. Platelet count and centrifugation settings vary in the pertinent literature (REFS). The activation and release methods of platelet content are mainly induced by repeated freeze/thaw cycles (mostly freezing at −20 °C and thawing at 37 °C), direct platelet activation with thrombin or calcium chloride or sonication at different frequencies (ultrasonic bath at frequencies around 20 kHz) (1). The authors favor the use of 2 centrifugation steps (320 g for 5 min then 700 g for 17 min), followed by ultrasonic bath for 30 min and a final centrifugation at a high intensity for 10 min.21, 22, 23
In regard to the sonication procedure to produce PL, it has been described alone or in combination with a freezing–thawing cycle.24 Ultrasounds are soundwaves having a frequency ≥20 kHz. Their effect is based on the transmission of ultrasounds in a liquid where they generate thermal and nonthermal effects. For the latter, ultrasound waves act on the gas dissolved, where the compression of the liquid is followed by its rarefaction.25 As a consequence, the micro bubbles expand with each cycle of the applied ultrasonic energy until they reach an unstable size, and then they collide and/or violently collapse in a process called “cavitation”. The efficiency of the lysis has been estimated after quantification of the platelet-derived growth factor- AB (PDGF-AB). After 30 min of sonication, 74 % of PDGF-AB was released from the platelet granules in the medium.24
After isolation of PRP, platelet concentrates can be stored for up to 5–7 days at 22+-2 °C under agitation or frozen for further processing into HPL.26 Typically, the platelet concentrates are shock-frozen at −30 °C and thawed at 37 °C to fragment platelets and then submitted to an additional centrifugation step. The number of cycles varies from one to five (REFS), however, the optimal condition is still unclear. Another procedure involves platelet degranulation by addition of calcium chloride recombinant thrombin, which raises concern due to the involvement of exogenous substances. Sonication alone or complementary to freezing/thawing cycles seems to be a rapid and cost effective method to release the platelet cargo. Sonication for up to 30 min at a frequency of 20 kHz was found to be efficient in degranulating platelet content.27 Although freezing and thawing have not altered biological activity of some growth factor in vitro, the impact of temperature alteration and long periods of storage of HPL preparations, in terms of nutrient functionality to be used in MSC expansion, has not been fully elucidated.28 In vivo, the use of autologous HPL for BM-MSCs culture was successfully described by Centeno et al.29 and autologous use in this context has the advantage of not exposing MCSs to xenogeneic proteins to be internalized by these cells. When utilized by progenitor cells, platelet debris and microvesicles carry regenerative capacity in expanded cell products, with immunological side effects.
Similarly to PRP, HPL content of key growth factors includes mostly IGF1; PDGF-AA, -BB, -AB; TGF-b1; BDNF; EGF; VEGF; HGF; bFGF. Some reports indicate that TGF-b1, PDGF-AB, IGF1, and BDNF are present in higher quantities than other growth factors.30 Other plasma proteins includes albumin, protease inhibitors such as alpha 1-antitrypsin and alpha 2-macroglobulin, and immunoglobulins G, A and M. Minerals like sodium, potassium, magnesium, iron as well as vitamins and glucose are also present in HPL.
Human allogeneic platelet lysate was studied on cells involved in the different phases of wound healing (monocytes, endothelial cells, fibroblasts and keratinocytes). PL was able to substitute growth supplements such as FBS and growth factors, with a dose-related effect, with peculiar characteristics at higher PL concentrations evaluated. This evidence gives way to a new field of application of platelet-derived products for efficient tissue regeneration. Stabilized freeze-dried platelets (HPL from outdated platelet concentrates) were also able to induce tubule formation as the freeze-drying process preserved the growth factors which are essential for wound healing, such as PDGF-BB and TGF-B1 in quantities similar to the ones obtained from fresh platelets.31,32 One other possible advantage of PL is that it can be prepared from outdated platelet concentrates (older than 5 days) that are no longer suitable for use in blood transfusion. Platelet lysate (PL) derived from outdated platelets has been shown to have the same impact on MSC cultures and osteogenic differentiation as PL from fresh platelets.33
1.2. Platelet lysate in vitro
In a recent study, human tenocytes were cultured within increasing concentrations of HPL.34 Leukocyte-poor PRP was collected following centrifugations at 200 g and 2300 g for 10 min both to form a platelet pellet. The pellet was resuspended in 1/35 of initial plasma volume, resulting in concentrated PRP (being approximately 20 times more concentrated than original PRP). Solutions were subjected to three freeze/thaw cyles at −80 °C and 37 °C to generate concentrated HPL. Fragmented platelet bodies were pelleted and removed by a last centrifugation at 4500g for 10 min. Final HPLs were diluted in different concentrations in pooled platelet-poor plasma (PPP). Higher concentrations of HPL promoted higher levels of platelet proteins (FGF-b, HGF, PDGF-BB, VEGF and tissue inhibitor of metalloproteinases), and improved tenocyte proliferation and migration in comparison to controls in a dose-dependent manner. These results suggested that HPL at higher concentrations might be more beneficial than lower HPL concentrations for promotion of tendon repair, especially in ageing patients.34
In an in vitro study, equine synoviocytes were stimulated with either interleukin-1B (IL-1B) or lipopolysaccharide (LPS) followed by no treatment or treatment with platelet lysate (PL).35 Chondrocytes were co-cultured with synoviocytes conditioned media for 48 h. Treatment of inflamed (IL-1B or LPS) synoviocytes with PL resulted in increased synoviocytes growth and production of hyaluronic acid and IL-6, which implies a protective effect in the joint. Co-cultured chondrocytes produced increased amounts of collagen type II (COL2A1) and aggrecan gene expression (ACAN) and decreased MMP-13 gene expression, which are consistent with previous studies on the anti-inflammatory effects of PL on cells.36 In a report of biological effects of PL on human articular cartilage, the expanded cells maintained a chondrogenic dedifferentiation potential in vivo. Results showed a significant reduction of the NF-kB activity and the repression of the inflammatory enzyme cyclooxygenase-2, triggering the resolution of the inflammation and beneficial effects in cartilage repair. Moreover, the medium of chondrocytes cultured in the simultaneous presence of PL and IL-1a, showed a significant enhancement of the chemoattractant activity versus untreated chondrocytes.36
In an in vitro comparative analysis of different platelet product preparations to stimulate tendon cell biology, PL products showed higher concentrations of growth factors (TGF-β1, VEGF, PDGF-AB, and bFGF), increased expression of COL1A1 and decreased COX1 expression on tenocytes.37 Also, the stronger effect on HGF (pain antagonist) expression of the PL product in the study suggested better relief from pain and inflammation compared to conventional PRP (Arthrex (PRP-ACP); RegenLab (PRP-BCT)). These findings indicated that pooled and well-characterized PL could have a promising future in tendon tissue regeneration.
In a comparison between PL and FCS in cultures of human adipose-derived stem cells (ASCs) and human articular chondrocytes expansion (HACs), PL strongly enhanced proliferation rates. Glycosaminoglycan (GAG), collagen type II and also collagen type X were mainly present in PL group. Platelet lysate enhanced proliferation, while still retaining the chondrogenic differentiation potential of ASCs and redifferentiation of HACs.38 The chondroprotective features of platelet lysates have also been shown to diminish multiple inflammatory IL-1 beta-mediated effects on human osteoarthritic chondrocytes, including inhibition of NFkB activation, a major pathway involved in the pathogenesis of osteoarthritis.3 Regulator genes of matrix degradation were less expressed (ADAMTS4 and PTGS2) and inhibition of COL2A1 and aggregan (ACAN) by IL-1b was diminished by PL addition to the culture medium. Considering these findings, HPL seems to be a suitable alternative in OA treatment.
In a recent investigation of effects of PL in primary cultures of human umbilical endothelial cells, PL exerted a protective effect against IL-1a activated inflammatory milieu, by inhibiting the NF-KB pathway.39 Endothelial cells represent the first cell population responding to blood extravasation and coagulation, platelet activation and degranulation, and the resulting inflammatory milieu.40 Moreover, PL enhanced proliferation of progenitor endothelial cells, keeping their capability of forming tube-like structures, and activated resting quiescent cells to re-enter cell cycle. These findings suggested a possible in vivo contribution of HPL to new vessel formation after wound by activation of cells resident in vessel walls.
PL has been shown to direct chondrogenic differentiation in MSCs cultures using PL supplemented medium in one study.41 It is well known that mesenchymal stem cells are capable of differentiating into chondrocytes in presence of TGF-b. Results showed high concentrations of TGF-b1 in the medium and increased expression of SOX9 (early chondrogenic marker) in PL cultures. In this study, MSCs maintained stemness and potential for differentiation into adipocytes and osteocytes as well. PL seems to be a natural source of TGF-b and could be further extended to in vivo utility by being injected into injured cartilage.
1.3. Platelet lysate in vivo
HPL has been increasingly adopted as an alternative for fetal bovine serum (FBS) for MSC expansion. However, its therapeutic and regenerative potential in vivo is largely unexplored. Recently, periosteum derived cells were able to form significantly higher amount of bone when implanted in small animal models in comparison with FBS. This capacity was linked to activation of WNT and BMP pathways, both osteogenic routes of several progenitor cells.42 Chakar et al. evaluated the effects of PL on rabbit's skull bone regeneration. It was reported that PL-induced vertical bone regeneration and promoted bone formation.43
In a comprehensive animal-cellular model study,44 PL significantly attenuated pain symptoms in rats and exerted chondrocyte-protective and extracellular matrix (ECM)-modifying effect on the arthritic cartilage in a dose-dependent manner in vivo. The in situ expressions of type II collagen and matrix metalloproteinase 13 in the arthritic cartilage was abnormal and was restored by PL. In vitro, PL significantly restored TNF-α-suppressed anabolic gene expression (Col 2 and aggrecan) and TNF-α-increased catabolic gene expression (Col 10, MMP13, Adamts5, and Adamts9) in chondrocytes. The effects were mediated by TNF-α downstream signaling, including inhibition of NF-κB activity. This study provides certain knowledge of anti-OA effect and TNF signaling-related mechanism of PL, proposing it as a promising alternative strategy for OA treatment.
In a wound model study, authors compared the effect of HPL preservation for 9 months using refrigerator (4 °C) or deep freezer (cryopreservation at −80 °C) with lyophilization. Results showed that growth factors contained in lyophilized HPL maintained at 4 °C for up to 9 months are still stable, but less bioactivated than in cryopreservation group (−80 °C). However, the bioactivity of the lyophilized HPL was sufficient for proliferation of fibroblast and the proliferation was superior to that observed with FBS.45 These findings suggested that lyophilization of HPL is a safe and reproducible method for long-term preservation and would be a versatile tool in clinical practice. Tenci et al. found that PL exhibits a positive effect on chronic skin ulcer in rats. Developed ulcers on the dermis and epidermis have been successfully treated with PL when compared with the controls.46
Platelet lysates have also been shown to stimulate angiogenesis, neurogenesis and neuroprotection after stroke. In an in vivo model, rats had their middle cerebral artery occluded and were then injected with PL whereas controls were treated with platelet-poor plasma or FGF2.47 Immunohistochemistry showed increased endogenous neural stem cells in the peri-lesion cortex and augmented angiogenesis in the subventricular zone (which is rich in stem cell niche and blood vessels). Neurological severity scores were significantly improved and infarct volumes were significantly reduced in rats treated with PL, which reveals a new potential of human platelet lysates in reducing behavioral deficits after brain ischaemia.
1.4. Clinical use of human platelet lysate
In a clinical study evaluating the treatment of knee osteoarthrosis with autologous HPL, results of intra-articular injections in 48 patients demonstrated a safe, economical, and easy method to apply growth factors in osteoarthritis.48 Autologous PL (frozen and thawed twice, centrifuged and filtered before application) was administered into the knee joints every 3 weeks for a total of 3 injections. Statistically significant improvements in symptoms, stiffness, pain, daily living and sport scores evaluated at weeks 32 and 52 occurred in comparison to the baseline. In another study by Samara et al. on knee osteoarthritis, magnetic resonance imaging (MRI) results indicated improved thickness of the knee cartilage after PL injection (every 3 weeks for a total of three injections).49 Quantitative MRI demonstrated significant improvement in cartilage thickness for both tibial plate (p = 0.044) and femoral plate (p = 0.028) at twelve months following PL injection.
Since HPL contains a myriad of bioactive growth factors, its usefulness has been tested for the promotion of bone healing. In a report of tibia delayed union with implant breakage, bony union was observed after three weekly injections of autologous PL.50 Detection of growth factors (PDGF-BB, TGF-B1, IGF-1 and EGF) was found to be much higher in concentration in HPL in comparison to the whole blood in the study.
In a patient tracking study, data from 470 patients treated with autologous HPL epidural injection for lumbar radicular pain syndrome showed significantly lower pain scores and better functional ratings.51 HPL was prepared after PRP acquisition of one centrifugation at 200g for 10 min and then placed at −80 °C for 5–10 min followed by thawing (one cycle). The sample was re-centrifuged to pellet any remaining platelet bodies and finally filtered at the time of injection with a 0.22 μm filter to remove lysed membrane debris. Results from this extensive number of patients, with no reported side effects, brought the idea that HPL may be more advantageous when compared to PRP, as the former does not carry the potential risk of platelet aggregation or vascular occlusion, as ischemic spinal cord lesions have been reported via particulate steroid occlusion of radicular arteries.52 These optimistic results were recently reproduced in patients with lumbar disc herniation through epidural HPL infiltrations with significant disc resorption and clinical improvements.53 The procedure includes 1 ml of PRP injection into posterior structures (spinal ligaments and facet joints) and 3 ml of autologous PL in the epidural space. Authors reported higher PL-derived growth factor concentrations in relation to the mean plasma (BDNF: 69,000 and 4960; EGF: 1324 and 41; IL-1RA: 447 and 72; PDGF-BB: 43,913 and 4123; VEGF: 572 and 140. Pg/ml, respectively).
Tan et al. have evaluated HPL in the treatment of refractory lateral epicondylitis.54 A total of 56 patients received three autologous PL injections (double freeze-thawing method after high-speed centrifugation and final filtration). Significant differences were observed in VAS and Mayo scores with all patients having recovered normal function in the 12-month follow-up and doppler images decreasing to a lower inflammatory activity.
1.5. Future perspectives
As studies suggest, human platelet lysate can be considered as a suitable alternative for other blood products including PRP, platelet gel and human serum, due to the depletion of platelet membranes in PL (and white blood cells in some protocols) as well as the growth factors supply. There is sufficient evidence to recommend that expired platelet products can be used.55 In addition, PL is effective in applications such as regenerative medicine, tissue engineering, cell culture, and cell therapy. However, its preparation needs to be standardized to reach the optimum standard of growth factor profiling.
Due to the possible allogenic application of HPL from pooled samples, HPL presents itself as an advantageous alternative over other blood products. Nevertheless, it may require the implementation of pathogen inactivation procedures (especially virus reduction), removal steps to improve safety of advanced cell therapy products and risk assessments with donation epidemiology. There is also the need for the inclusion of blood bank rules and standardization of ingredients utilized. Altogether, they must still retain the efficacy of HPL.
Finally, the use of a pool of healthy donors could minimize individual variability, offering an advantage in comparison to patients' derivatives, and encouraging application of allogeneic HPL as “off-the-shelf’ in the near future. The development of controlled-release systems to protect growth factors and provide sustained delivery would enhance HPL biological effects for clinical application in musculoskeletal injuries.
2. Conclusion
In this review, we summarized the advantages of human platelet lysate in clinical applications. Some details about its preparation, features and content were introduced as well. Cumulative evidence indicates that HPL has the potential to become one of the standard products for tissue engineering and also for regenerative purposes as a pure orthobiologic. While there is no reported serious side effect in clinical use of HPL, further studies and standardizations are still needed in order to obtain the best version of this enhanced, acellular product.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
We would like to thank Instituto do Osso e da Cartilagem for the support and remarkable contributions towards the production of this manuscript.
Contributor Information
Lucas da Fonseca, Email: ffonsecalu@gmail.com.
Gabriel Silva Santos, Email: gabriel1_silva@hotmail.com.
Stephany Cares Huber, Email: stephany_huber@yahoo.com.br.
Taís Mazzini Setti, Email: medicatais@gmail.com.
Thiago Setti, Email: thiagosetti@hotmail.com.
José Fábio Lana, Email: josefabiolana@gmail.com.
References
- 1.Burnouf T., Strunk D., Koh M.B.C., Schallmoser K. 2016. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. [DOI] [PubMed] [Google Scholar]
- 2.Adelson E., Rheingold J.J., Crosby W.H. The platelet as a sponge: a review. Blood. 1961 doi: 10.1182/blood.v17.6.767.767. [DOI] [PubMed] [Google Scholar]
- 3.Van Buul G.M., Koevoet W.L.M., Kops N. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011 doi: 10.1177/0363546511419278. [DOI] [PubMed] [Google Scholar]
- 4.Strandberg G., Sellberg F., Sommar P. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfus. 2017 doi: 10.1111/trf.13998. [DOI] [PubMed] [Google Scholar]
- 5.Lana J.F.S.D., Purita J., Paulus C. Contributions for classification of platelet rich plasma - proposal of a new classification: MARSPILL. Regen Med. 2017;12:565–574. doi: 10.2217/rme-2017-0042. [DOI] [PubMed] [Google Scholar]
- 6.Milano G., Sánchez M., Jo C.H. Platelet-rich plasma in orthopaedic sports medicine: state of the art. J.ISAKOS.Jt.Disord.Orthop.Sport.Med. 2019 doi: 10.1136/jisakos-2019-000274. [DOI] [Google Scholar]
- 7.Blair P., Flaumenhaft R. Platelet α-granules: basic biology and clinical correlates. Blood Rev. 2009 doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altaie A., Owston H., Jones E. Use of platelet lysate for bone regeneration - are we ready for clinical translation? World J Stem Cell. 2016 doi: 10.4252/wjsc.v8.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semple J.W., Italiano J.E., Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011 doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 10.Edelstein L.C., Mckenzie S.E., Shaw C. MicroRNAs in platelet production and activation. J Thromb Haemostasis. 2013 doi: 10.1111/jth.12214. [DOI] [PubMed] [Google Scholar]
- 11.Rauch C., Feifel E., Amann E.M. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011 doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- 12.Zamani M., Yaghoubi Y., Movassaghpour A. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: are we ready for clinical use? J Cell Physiol. 2019 doi: 10.1002/jcp.28496. [DOI] [PubMed] [Google Scholar]
- 13.Koç O.N., Lazarus H.M. Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant. 2001 doi: 10.1038/sj.bmt.1702791. [DOI] [PubMed] [Google Scholar]
- 14.Hara Y., Steiner M., Baldini M.G. Platelets as a source of growth-dromotina factor(s) for tumor cells. Canc Res. 1980 [PubMed] [Google Scholar]
- 15.King G.L., Buchwald S. Characterization and partial purification of an endothelial cell growth factor from human platelets. J Clin Invest. 1984 doi: 10.1172/JCI111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doucet C., Ernou I., Zhang Y. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005 doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 17.Atashi F., Jaconi M.E.E., Pittet-Cuénod B., Modarressi A. Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng C Methods. 2015 doi: 10.1089/ten.tec.2014.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins H.J., Rozemuller H., Vonk-Griffioen S. Bone-forming capacity of mesenchymal stromal cells when cultured in the presence of human platelet lysate as substitute for fetal bovine serum. Tissue Eng. 2009 doi: 10.1089/ten.tea.2008.0666. [DOI] [PubMed] [Google Scholar]
- 19.Shanskii Y.D., Sergeeva N.S., Sviridova I.K. Human platelet lysate as a promising growth-stimulating additive for culturing of stem cells and other cell types. Bull Exp Biol Med. 2013 doi: 10.1007/s10517-013-2298-7. [DOI] [PubMed] [Google Scholar]
- 20.Cowper M., Frazier T., Wu X. Human platelet lysate as a functional substitute for fetal bovine serum in the culture of human adipose derived stromal/stem cells. Cells. 2019 doi: 10.3390/cells8070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Melo B.A.G., Martins Shimojo A.A., Marcelino Perez A.G. Distribution, recovery and concentration of platelets and leukocytes in L-PRP prepared by centrifugation. Colloids Surf B Biointerfaces. 2018 doi: 10.1016/j.colsurfb.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 22.Perez A.G.M., Lana J.F.S.D., Rodrigues A.A. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISR Newsl.Hematol. 2014 doi: 10.1155/2014/176060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Melo B.A.G., Luzo Â.C.M., Lana J.F.S.D., Santana M.H.A. Centrifugation conditions in the L-PrP preparation affect soluble factors release and mesenchymal stem cell proliferation in fibrin nanofibers. Molecules. 2019 doi: 10.3390/molecules24152729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Astori G., Amati E., Bambi F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther. 2016 doi: 10.1186/s13287-016-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riesz P., Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radic Biol Med. 1992 doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 26.Burnouf T., Goubran H.A., Seghatchian J. Multifaceted regenerative lives of expired platelets in the second decade of the 21st century. Transfus Apher Sci. 2014 doi: 10.1016/j.transci.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Bernardi M., Albiero E., Alghisi A. Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2013 doi: 10.1016/j.jcyt.2013.01.219. [DOI] [PubMed] [Google Scholar]
- 28.Sonker A., Dubey A. Determining the effect of preparation and storage: an effort to streamline platelet components as a source of growth factors for clinical application. Transfus Med Hemotherapy. 2015 doi: 10.1159/000371504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centeno C.J., Busse D., Kisiday J. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells, platelet lysate and dexamethasone. Am J Case Rep. 2008 [PubMed] [Google Scholar]
- 30.Schallmoser K., Bartmann C., Rohde E. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfus. 2007 doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 31.Huber S.C., Junior J.L.R.C., Silva L.Q. Freeze-dried versus fresh platelet-rich plasma in acute wound healing of an animal model. Regen Med. 2019 doi: 10.2217/rme-2018-0119. [DOI] [PubMed] [Google Scholar]
- 32.Sum R., Hager S., Pietramaggiori G. Wound-healing properties of trehalose-stabilized freeze-dried outdated platelets. Transfus. 2007 doi: 10.1111/j.1537-2995.2007.01170.x. [DOI] [PubMed] [Google Scholar]
- 33.Jonsdottir-Buch S.M., Lieder R., Sigurjonsson O.E. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PloS One. 2013 doi: 10.1371/journal.pone.0068984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger D.R., Centeno C.J., Steinmetz N.J. Platelet lysates from aged donors promote human tenocyte proliferation and migration in a concentration-dependent manner. Bone Jt Res. 2019 doi: 10.1302/2046-3758.81.BJR-2018-0164.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbertie J.M., Long J.M., Schubert A.G. Pooled platelet-rich plasma lysate therapy increases synoviocyte proliferation and hyaluronic acid production while protecting chondrocytes from synoviocyte-derived inflammatory mediators. Front.Vet. Sci. 2018 doi: 10.3389/fvets.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira R.C., Scaranari M., Benelli R. Dual effect of platelet lysate on human articular cartilage: a maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng. 2013 doi: 10.1089/ten.tea.2012.0225. [DOI] [PubMed] [Google Scholar]
- 37.Klatte-Schulz F., Schmidt T., Uckert M. Comparative analysis of different platelet lysates and platelet rich preparations to stimulate tendon cell biology: an in vitro study. Int J Mol Sci. 2018 doi: 10.3390/ijms19010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hildner F., Eder M.J., Hofer K. Human platelet lysate successfully promotes proliferation and subsequent chondrogenic differentiation of adipose-derived stem cells: a comparison with articular chondrocytes. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.1649. [DOI] [PubMed] [Google Scholar]
- 39.Romaldini, Ulivi, Nardini Platelet lysate inhibits NF-κB activation and induces proliferation and an alert state in quiescent human umbilical vein endothelial cells retaining their differentiation capability. Cells. 2019 doi: 10.3390/cells8040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barsotti M.C., Losi P., Briganti E. Effect of platelet lysate on human cells involved in different phases of wound healing. PloS One. 2013 doi: 10.1371/journal.pone.0084753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassan G., Bahjat M., Kasem I. Platelet lysate induces chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells. Cell Mol Biol Lett. 2018 doi: 10.1186/s11658-018-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta P., Hall G.N., Geris L. Human platelet lysate improves bone forming potential of human progenitor cells expanded in microcarrier-based dynamic culture. Stem Cells Transl Med. 2019 doi: 10.1002/sctm.18-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakar C., Naaman N., Soffer E. Bone formation with deproteinized bovine bone mineral or biphasic calcium phosphate in the presence of autologous platelet lysate: comparative investigation in rabbit. Int J Biomater. 2014 doi: 10.1155/2014/367265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan L., Zhou L., Xie D. Chondroprotective effects of platelet lysate towards monoiodoacetate-induced arthritis by suppression of TNF-α-induced activation of NF-κB pathway in chondrocytes. Aging. 2019 doi: 10.18632/aging.101952. Albany NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Notodihardjo S.C., Morimoto N., Kakudo N. Comparison of the efficacy of cryopreserved human platelet lysate and refrigerated lyophilized human platelet lysate for wound healing. Regen.Ther. 2019 doi: 10.1016/j.reth.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenci M., Rossi S., Bonferoni M.C. Particulate systems based on pectin/chitosan association for the delivery of manuka honey components and platelet lysate in chronic skin ulcers. Int J Pharm. 2016 doi: 10.1016/j.ijpharm.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 47.Hayon Y., Dashevsky O., Shai E. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb Haemostasis. 2013 doi: 10.1160/TH12-11-0875. [DOI] [PubMed] [Google Scholar]
- 48.Al-Ajlouni J., Awidi A., Samara O. Safety and efficacy of autologous intra-articular platelet lysates in early and intermediate knee osteoarthrosis in humans: a prospective open-label study. Clin J Sport Med. 2015 doi: 10.1097/JSM.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 49.Samara O., Al-ajlouni J., Najar M.A.- 2017. Intra-articular autologous platelet lysates produce positive mri structural changes in early and intermediate knee intra-articular autologous platelet lysates produce positive mri structural changes in early and inter- mediate knee osteoarthrosis. [Google Scholar]
- 50.Jiang H jiang, Tan X xiang, Ju H yang. Autologous platelet lysates local injections for treatment of tibia non-union with breakage of the nickelclad: a case report. SpringerPlus. 2016 doi: 10.1186/s40064-016-3683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centeno C., Markle J., Dodson E. The use of lumbar epidural injection of platelet lysate for treatment of radicular pain. J Exp Orthop. 2017 doi: 10.1186/s40634-017-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pountos I., Panteli M., Walters G. Drugs R D; 2016. Safety of Epidural Corticosteroid Injections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rawson B. Platelet-rich plasma and epidural platelet lysate: novel treatment for lumbar disk herniation. J Am Osteopath Assoc. 2020 doi: 10.7556/jaoa.2020.032. [DOI] [PubMed] [Google Scholar]
- 54.Tan X., xiang Ju, yang H., Yan W. Autologous platelet lysate local injections for the treatment of refractory lateral epicondylitis. J Orthop Surg Res. 2016 doi: 10.1186/s13018-016-0349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henschler R., Gabriel C., Schallmoser K. Human platelet lysate current standards and future developments. Transfus. 2019 doi: 10.1111/trf.15174. [DOI] [PubMed] [Google Scholar]