Abstract
Background
This study aimed to evaluate the updated disease burden, risk factors, and temporal trends of liver cancer based on age, sex, and country.
Methods
We estimated the incidence of liver cancer and its attribution to hepatitis B virus (HBV) and hepatitis C virus (HCV) in 2018 based on the Global Cancer Observatory and World Health Organization (WHO) Cancer Causes database. We extracted the prevalence of risk factors from the WHO Global Health Observatory to examine the associations by weighted linear regression. The trend analysis used data from the Cancer Incidence in Five Continents and the WHO mortality database from 48 countries. Temporal patterns of incidence and mortality were calculated using average annual percent change (AAPC) by joinpoint regression analysis.
Results
The global incidence of liver cancer was (age-standardized rate [ASR]) 9.3 per 100,000 population in 2018, and there was an evident disparity in the incidence related to HBV (ASR 0.2–41.2) and HCV (ASR 0.4–43.5). A higher HCV/HBV-related incidence ratio was associated with a higher level of alcohol consumption (β 0.49), overweight (β 0.51), obesity (β 0.64), elevated cholesterol (β 0.70), gross domestic product (β 0.20), and Human Development Index (HDI; β 0.45). An increasing trend in incidence was identified in many countries, especially for male individuals, population aged ≥50 years, and countries with a higher HCV/HBV-related liver cancer incidence ratio. Countries with the most drastic increase in male incidence were reported in India (AAPC 7.70), Ireland (AAPC 5.60), Sweden (AAPC 5.72), the UK (AAPC 5.59), and Norway (AAPC 4.87).
Conclusion
We observed an overall increasing trend of liver cancer, especially among male subjects, older individuals, and countries with a higher prevalence of HCV-related liver cancer. More efforts are needed in enhancing lifestyle modifications and accessibility of antiviral treatment for these populations. Future studies should investigate the reasons behind these epidemiological changes.
Keywords: Liver cancer, Epidemiology, Causes, Risk factors, Trend analysis
Introduction
Liver cancer is one of the most common malignancies, with more than 800,000 new cases diagnosed each year globally [1]. It is also a leading cause of cancer mortality, accounting for over 700,000 cancer deaths annually [1]. Liver cancer is more common in sub-Saharan Africa, East Asia, and Southeast Asia than in the Western countries [1]. However, in the USA, the incidence of liver cancer has more than tripled, while its mortality has more than doubled since 1980 [2]. The most common liver cancer is hepatocellular carcinoma, while other less common types include intrahepatic cholangiocarcinoma, angiosarcoma, and hemangiosarcoma [3]. The risk factors for liver cancer include gender, race, chronic viral hepatitis, cirrhosis, inherited metabolic diseases, alcohol drinking, smoking, obesity, type 2 diabetes, and exposure to carcinogenic substances such as aflatoxins [4]. Liver cancer could be prevented by reducing the prevalence of these modifiable risk factors, including hepatitis vaccination and lifestyle changes [5].
It is important to monitor the epidemiological trend of liver cancer using cancer registry data of high quality. Studying the recent trend of incidence and mortality for liver cancer is crucial as it can inform policy formulation for effective public health interventions and clinical practice. Owing to its high disparity in epidemiology across different populations, a comprehensive evaluation of its worldwide temporal patterns of disease burden in different population groups could benefit resource planning and allocation. Evidence has also showed that there is geographical variation in the epidemiology of liver cancer caused by hepatitis B virus (hepatitis B virus) and hepatitis C virus (HCV) [6]. Evaluating the updated disease burden and associated risk factors of liver cancer by different causes in this population infected with HBV and HCV is important as the preventive measures and clinical management would be different for HBV and HCV.
Nevertheless, there is a lack of studies on the most updated epidemiology and risk factors of liver cancer induced by different causes, as well as its trend. Previous literature only investigated certain populations [7, 8, 9], reported relatively old data [10, 11], and did not present the cancer burden by different causes [12]. Although the Global Burden of Disease (GBD) studies [13, 14] evaluated the disease burden of liver disease by specific etiologies, there is a lack of trend analysis for different groups by sex, age, and country using real-world cancer registry data. Also, none of these studies have investigated the difference in risk factors associated with liver cancer related to HBV and HCV at a country level. Therefore, the objectives of this study were to evaluate the (1) updated global epidemiology of liver cancer in 2018, (2) associated lifestyle and metabolic risk factors related to HBV and HCV, and (3) its recent epidemiologic trend by sex, age, and country.
Methods
Data Source
This study adopted methods similar to our previously published studies [11, 15, 16]. In brief, we retrieved the GLOBOCAN database, which contains data of 185 countries to estimate the global and regional incidences and mortality of liver cancer in 2018 [17]. To improve the quality and coverage of estimation of incidence and mortality, several methods were used in GLOBOCAN, including modeling by mortality-to-incidence ratios, predictions, and approximation from neighboring regions. We also estimated the incidence of liver cancer attributable to HBV and HCV in 2018 based on World Health Organization (WHO) Cancer Causes database [18] and previous studies [19, 20]. For the analysis of its lifestyle and metabolic factors, we used the age-standardized prevalence of risk factors for each country from the WHO Global Health Observatory database [21], including smoking, alcohol consumption, physical inactivity, overweight, obesity, diabetes, hypertension, and elevated cholesterol (see online suppl. Table 1; see www.karger.com/doi/10.1159/000515304 for all online suppl. material). We also extracted the gross domestic product (GDP) per capita and Human Development Index (HDI) in 2018 for each country from the World Bank [22] and the United Nations Development Programme [23], respectively. For trend analysis, we extracted incidence and mortality figures of 48 countries from national and global registries for all available calendar years (1980–2017) (online suppl. Table 2). To retrieve the data on incidence, we searched nation-/region-specific cancer registries in Cancer Incidence in Five Continents, volumes I–XI [24]. The Cancer Incidence in Five Continents database contains population-based data of incidence figures from cancer registries by confirming the diagnosis of each cancer case reported in a predetermined time interval. To obtain the most updated figures on incidence and mortality for the USA, we searched the Surveillance, Epidemiology, and End Results, which is a publicly available program covering most cancer registry data in the USA [25]. We also collected the most updated figures on the incidence and mortality for northern European countries, including Denmark, Finland, Sweden, Iceland, Greenland, Norway, and the Faroe Islands from the Nordic Cancer Registries [26]. We used the WHO mortality database for mortality data for other countries/regions out of the USA and northern Europe [27]. Only data with a quality level of medium or above were used to compute mortality figures in the database [28]. All these cancer registries have been regarded as a well-recognized standard reference for trend analysis of cancer burden. We used the International Classification of Diseases and Related Health Problems-10th Revision code C22 to identify “malignant neoplasm of the liver and intrahepatic bile ducts” in the analysis [29]. Age-standardized rates (ASRs) were calculated for all figures based on the Segi-Doll world standard population [30].
Statistical Analysis
All incidence and mortality figures were presented by ASRs. The HCV-/HBV-related liver cancer incidence ratio was calculated by ASRs of liver cancer incidence attributable to HCV divided by that to HBV. The ratio shows the relative burden of liver cancer attributable to HBV and HCV for individual countries without reference to its incidence. We calculated this ratio to examine the incidence of liver cancer attributable to HCV and HBV in association with certain risk factors. We chose these 2 because only these 2 etiologies for liver cancer were described in the database. Countries with a ratio more than 1 had a higher incidence of HCV-related liver cancer than that related to HBV. Countries with a ratio less than 1 had a lower incidence of HCV-related liver cancer than that related to HBV. Countries with a ratio equal to 1 had the same incidence of HCV-related liver cancer as that related to HBV. We also estimated the total attributable fraction (AF) of liver cancer caused by HBV and HCV for each country from the database. The correlations between the lifestyle and metabolic risk factors, GDP per capita, and HDI with the ratio were examined using Pearson's correlation coefficient (r). We also performed the sensitivity analysis by excluding the countries with a total AF of liver cancer caused by HBV and HCV no more than 50 and 60%, respectively. Weighted linear regression by inverse variance was also performed to generate beta coefficient (β) for the associations. The epidemiological trend of incidence and mortality of liver cancer in the recent past 10 years was evaluated for different countries by using joinpoint regression analysis [31]. The results were presented as average annual percent change (AAPC) with its 95% confidence interval (CI) [31]. A logarithmic transformation of the incidence and mortality data was performed, and standard errors were calculated by binomial approximation. Weights equivalent to each segment's length were apportioned for the specified time frame [32]. Countries with “zero” or “missing” values in their figures of the most recent decade were excluded from the regression analysis. A maximum of 3 joinpoints was used as the parameter of analysis. The AAPC was evaluated as an average of annual percent change (APC) using geometric weighting in populations of different age strata, genders, and countries. A p value of <0.05 was considered statistically significant in the analysis.
Results
Incidence and Mortality in 2018
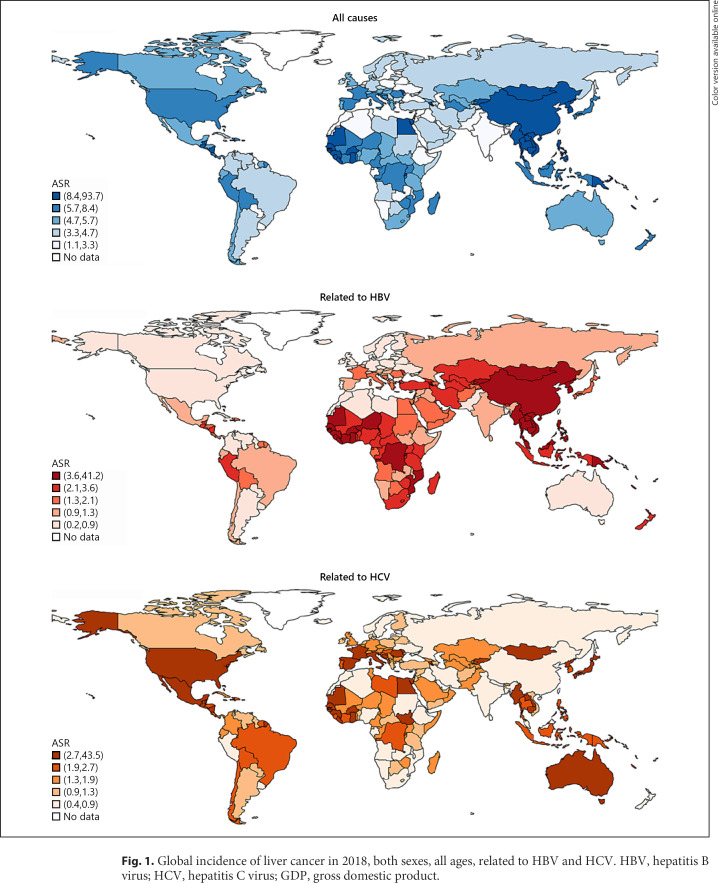
A total of 841,080 (CI 817,635–865,198) new cases were reported in 2018 (Table 1) [17]. The ASR of incidence was 9.3 (CI 9.0–9.6) per 100,000 population and showed 7-fold variation globally (Fig. 1). The highest rates were reported in Eastern Asia (ASR 17.7, CI 17.4–18.0), Micronesia (ASR 15.2, CI 12.4–18.0), and Northern Africa (ASR 14.1, CI 12.8–15.4), while the lowest rates were found in south-central Asia (ASR 2.5, CI 2.3–2.7), Western Asia (ASR 4.0, CI 3.6–4.4), and central and eastern Europe (ASR 4.0, CI 3.9–4.1). Globally, a total of 781,631 (CI 737,605–828,285) related deaths were reported in 2018. The ASR of mortality was 8.5 (CI 8.0–9.0) per 100,000 population and varied 7-fold. The highest mortality rates were found in Eastern Asia (ASR 16.0, CI 15.8–16.2), Northern Africa (ASR 13.9, CI 12.6–15.2), and Southeastern Asia (ASR 13.2, CI 12.3–14.1). The lowest estimated death rates were reported in south-central Asia (ASR 2.3, CI 2.2–2.4), northern Europe (ASR 3.8, CI 3.7–3.9), and central and eastern Europe (ASR 3.9, CI 3.8–4.0).
Table 1.
Incidence and mortality of liver cancer by region
| Incidence |
Mortality |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Both sexes |
Males |
Females |
Both sexes |
Males |
Females |
|||||||
| Region | new cases | ASR | new cases | ASR | new cases | ASR | deaths | ASR | deaths | ASR | deaths | ASR |
| Eastern Africa | 11,550 | 4.8 | 7,011 | 6.2 | 4,539 | 3.6 | 11,251 | 4.7 | 6,799 | 6.2 | 4,452 | 3.5 |
| Middle Africa | 6,010 | 6.5 | 4,137 | 9.4 | 1,873 | 3.9 | 5,853 | 6.5 | 4,056 | 9.5 | 1,797 | 3.9 |
| Northern Africa | 27,935 | 14.1 | 19,912 | 20.8 | 8,023 | 7.8 | 27,505 | 13.9 | 19,570 | 20.4 | 7,935 | 7.7 |
| Southern Africa | 2,710 | 4.9 | 1,692 | 7.4 | 1,018 | 3.2 | 2,597 | 4.7 | 1,614 | 7.1 | 983 | 3.1 |
| Western Africa | 16,574 | 8.3 | 10,778 | 11.1 | 5,796 | 5.7 | 16,356 | 8.2 | 10,747 | 11.1 | 5,609 | 5.6 |
| Caribbean | 2,923 | 5.0 | 1,706 | 6.3 | 1,217 | 3.8 | 2,791 | 4.7 | 1,588 | 5.8 | 1,203 | 3.7 |
| Central America | 11,229 | 6.3 | 5,513 | 6.7 | 5,716 | 6.0 | 10,672 | 5.9 | 5,324 | 6.4 | 5,348 | 5.5 |
| South America | 24,248 | 4.6 | 13,565 | 5.8 | 10,683 | 3.5 | 22,973 | 4.3 | 12,738 | 5.5 | 10,235 | 3.4 |
| North America | 41,851 | 6.6 | 29,900 | 10.1 | 11,951 | 3.4 | 34,339 | 4.8 | 22,889 | 7.1 | 11,450 | 2.8 |
| Eastern Asia | 467,327 | 17.7 | 343,523 | 26.8 | 123,804 | 8.7 | 427,932 | 16.0 | 312,228 | 24.2 | 115,704 | 8.0 |
| Southeastern Asia | 89,010 | 13.3 | 65,407 | 21.0 | 23,603 | 6.6 | 88,429 | 13.2 | 65,238 | 20.9 | 23,191 | 6.5 |
| South-central Asia | 44,010 | 2.5 | 29,027 | 3.4 | 14,983 | 1.7 | 40,812 | 2.3 | 27,060 | 3.2 | 13,752 | 1.5 |
| Western Asia | 9,249 | 4.0 | 5,787 | 5.4 | 3,462 | 2.8 | 9,096 | 4.0 | 5,697 | 5.4 | 3,399 | 2.8 |
| Central and Eastern Europe | 22,784 | 4.0 | 13,737 | 6.2 | 9,047 | 2.5 | 22,745 | 3.9 | 13,581 | 6.1 | 9,164 | 2.4 |
| Western Europe | 23,659 | 5.3 | 17,300 | 8.4 | 6,359 | 2.5 | 22,637 | 4.5 | 15,896 | 7.0 | 6,741 | 2.2 |
| Southern Europe | 25,026 | 6.8 | 17,702 | 10.9 | 7,324 | 3.1 | 21,996 | 5.3 | 14,800 | 8.3 | 7,196 | 2.6 |
| Northern Europe | 10,997 | 4.7 | 7,086 | 6.6 | 3,911 | 2.9 | 9,997 | 3.8 | 6,088 | 5.2 | 3,909 | 2.5 |
| Australia and New Zealand | 2,921 | 5.7 | 2,115 | 8.8 | 806 | 2.7 | 2,709 | 4.7 | 1,881 | 7.0 | 828 | 2.5 |
| Melanesia | 915 | 11.4 | 557 | 14.2 | 358 | 8.9 | 819 | 10.6 | 491 | 13.1 | 328 | 8.3 |
| Polynesia | 67 | 9.2 | 50 | 14.4 | 17 | 4.1 | 54 | 7.4 | 36 | 10.6 | 18 | 4.5 |
| Micronesia | 85 | 15.2 | 69 | 25.6 | 16 | 5.4 | 68 | 12.0 | 54 | 19.8 | 14 | 4.9 |
| World | 841,080 | 9.3 | 596,574 | 13.9 | 244,506 | 4.9 | 781,631 | 8.5 | 548,375 | 12.7 | 233,256 | 4.6 |
ASR, age-standardized rate. Data source: GLOBOCAN 2018 (http://gco.iarc.fr/today).
Fig. 1.
Global incidence of liver cancer in 2018, both sexes, all ages, related to HBV and HCV. HBV, hepatitis B virus; HCV, hepatitis C virus; GDP, gross domestic product.
Incidence Related to HBV and HCV
Table 2 shows the cause-specific estimated number of new cases, ASRs, and the HCV/HBV-related liver cancer incidence ratios in 2018 for each country. The highest ASRs of HBV-related liver cancer were observed in Mongolia (ASR 41.2, CI 38.8–43.6, ratio 1.055), Vietnam (ASR 16.2, CI 14.9–17.5, ratio 0.079), China (ASR 13.9, CI 13.7–14.1, ratio 0.067), Thailand (ASR 12.8, CI 12.4–13.2, ratio 0.190), Laos (ASR 12.6, CI 7.0–18.2, ratio 0.358), Cambodia (ASR 12.3, CI 6.78–17.8, ratio 0.365), the Gambia (ASR 12.1, CI 10.2–14.0, ratio 0.370), South Korea (ASR 10.9, CI 10.6–11.2, ratio 0.228), Guinea (ASR 10.1, CI 7.9–12. 3, ratio 0.543), and North Korea (ASR 9.3, CI 8.7–9.9, ratio 0.359). The highest ASRs of HCV-related liver cancer were observed in Mongolia (ASR 43.5, CI 41.0–46.0, ratio 1.055), Egypt (ASR 27.0, CI 25.3–28.7, ratio 12.892), Guatemala (ASR 5.6, CI 5.1–6.1, ratio 1.539), Moldova (ASR 5.5, CI 4.7–6.3, ratio 1.855), Guinea (ASR 5.5, CI 4.3–6.7, ratio 0.543), Laos (ASR 4.5, CI 2.5–6.5, ratio 0.358), the Gambia (ASR 4.5, CI 3.8–5.2, ratio 0.370), Cambodia (ASR 4.5, CI 2.5–6.5, ratio 0.365), Japan (ASR 4.5, CI 4.4–4.6, ratio 3.073), and the USA (ASR 4.2, CI 4.1–4.3, ratio 8.746). HBV and HCV were the predominant causes for liver cancer in a majority of the countries. There were 169 (92.9%) and 158 (86.8%) of the 182 countries with a total AF of liver cancer caused by HBV and HCV over 50 and 60%, respectively.
Table 2.
Incidence of liver cancer attributable to HBV and HCV
| Country | HBV |
HCV |
HCV/HBV cancer ratio# | HCV + HBV AF, %* | ||
|---|---|---|---|---|---|---|
| n | ASR | n | ASR | |||
| Mongolia | 986 | 41.2 | 1,040 | 43.5 | 1.055 | 90.4 |
| Egypt | 1,651 | 2.1 | 21,284 | 27.0 | 12.892 | 90.3 |
| Pakistan | 1,170 | 0.8 | 2,440 | 1.7 | 2.086 | 82.4 |
| Myanmar | 2,822 | 5.4 | 1,517 | 2.9 | 0.538 | 81.8 |
| China | 297,401 | 13.9 | 20,036 | 0.9 | 0.067 | 80.8 |
| Tunisia | 69 | 0.5 | 215 | 1.6 | 3.119 | 79.9 |
| Algeria | 120 | 0.3 | 320 | 0.8 | 2.667 | 78.1 |
| Morocco | 91 | 0.2 | 243 | 0.6 | 2.667 | 78.1 |
| Japan | 6,787 | 1.5 | 20,859 | 4.5 | 3.073 | 77.8 |
| South Korea | 10,418 | 10.9 | 2,377 | 2.5 | 0.228 | 77.5 |
| Tajikistan | 106 | 1.8 | 104 | 1.8 | 0.985 | 77.2 |
| Polynesia | 19 | 5.3 | 7 | 2.0 | 0.373 | 77.0 |
| Turkey | 2,316 | 2.4 | 1,043 | 1.1 | 0.450 | 77.0 |
| The Solomon Islands | 23 | 5.8 | 9 | 2.1 | 0.371 | 76.9 |
| Timor-Leste | 21 | 3.0 | 8 | 1.1 | 0.361 | 76.9 |
| North Korea | 3,231 | 9.3 | 1,161 | 3.3 | 0.359 | 76.8 |
| Guam | 18 | 8.5 | 6 | 2.9 | 0.345 | 76.8 |
| Cambodia | 1,431 | 12.3 | 522 | 4.5 | 0.365 | 76.7 |
| Papua New Guinea | 411 | 6.7 | 151 | 2.5 | 0.367 | 76.7 |
| Samoa | 9 | 5.5 | 3 | 2.0 | 0.355 | 76.7 |
| Brunei | 21 | 5.5 | 8 | 2.1 | 0.375 | 76.7 |
| Fiji | 43 | 4.7 | 16 | 1.7 | 0.362 | 76.7 |
| Laos | 584 | 12.6 | 209 | 4.5 | 0.358 | 76.6 |
| New Caledonia | 21 | 5.8 | 8 | 2.1 | 0.365 | 76.6 |
| Mexico | 1,300 | 1.0 | 4,265 | 3.2 | 3.279 | 76.6 |
| Libya | 41 | 0.9 | 105 | 2.3 | 2.563 | 76.6 |
| The Philippines | 5,459 | 6.5 | 1,906 | 2.3 | 0.349 | 76.5 |
| South Sudan | 87 | 1.1 | 216 | 2.8 | 2.477 | 76.5 |
| Somalia | 81 | 1.1 | 113 | 1.5 | 1.391 | 76.5 |
| Vanuatu | 16 | 7.3 | 6 | 2.7 | 0.377 | 76.4 |
| Lebanon | 113 | 1.6 | 60 | 0.8 | 0.531 | 76.1 |
| The Syrian Arab Republic | 162 | 1.3 | 125 | 1.0 | 0.770 | 75.6 |
| Uzbekistan | 642 | 2.5 | 455 | 1.8 | 0.710 | 75.4 |
| Vietnam | 17,658 | 16.2 | 1,393 | 1.3 | 0.079 | 75.2 |
| Sri Lanka | 334 | 1.2 | 243 | 0.9 | 0.729 | 75.2 |
| Georgia | 162 | 2.3 | 123 | 1.7 | 0.759 | 75.1 |
| Senegal | 489 | 5.7 | 318 | 3.7 | 0.651 | 75.1 |
| Kazakhstan | 480 | 2.4 | 362 | 1.8 | 0.755 | 75.1 |
| Bahrain | 13 | 1.5 | 10 | 1.1 | 0.730 | 75.1 |
| Qatar | 18 | 1.8 | 13 | 1.3 | 0.747 | 75.1 |
| Kyrgyzstan | 199 | 4.1 | 143 | 3.0 | 0.720 | 75.0 |
| Cyprus | 32 | 1.4 | 25 | 1.1 | 0.773 | 75.0 |
| Bhutan | 15 | 2.3 | 11 | 1.6 | 0.708 | 75.0 |
| Israel | 144 | 1.1 | 108 | 0.8 | 0.744 | 75.0 |
| Azerbaijan | 169 | 1.6 | 119 | 1.1 | 0.705 | 75.0 |
| Nepal | 120 | 0.5 | 91 | 0.4 | 0.756 | 75.0 |
| Oman | 50 | 1.9 | 37 | 1.4 | 0.738 | 74.9 |
| Bangladesh | 1,342 | 0.9 | 1,001 | 0.7 | 0.746 | 74.9 |
| Gaza Strip and West Bank | 20 | 0.8 | 15 | 0.6 | 0.726 | 74.9 |
| Kuwait | 52 | 2.2 | 39 | 1.7 | 0.752 | 74.8 |
| Maldives | 12 | 3.7 | 8 | 2.5 | 0.694 | 74.7 |
| Armenia | 206 | 4.2 | 147 | 3.0 | 0.715 | 74.6 |
| Afghanistan | 278 | 1.7 | 201 | 1.2 | 0.723 | 74.6 |
| The United Arab Emirates | 43 | 1.8 | 30 | 1.3 | 0.699 | 74.6 |
| Turkmenistan | 122 | 2.6 | 88 | 1.9 | 0.721 | 74.5 |
| Jordan | 82 | 1.3 | 58 | 1.0 | 0.713 | 74.5 |
| Iraq | 234 | 1.2 | 166 | 0.8 | 0.710 | 74.2 |
| Niger | 412 | 3.9 | 160 | 1.5 | 0.388 | 73.7 |
| Yemen | 265 | 1.9 | 190 | 1.3 | 0.715 | 73.4 |
| Thailand | 14,211 | 12.8 | 2,702 | 2.4 | 0.190 | 72.6 |
| Indonesia | 8,588 | 3.5 | 4,802 | 2.0 | 0.559 | 72.5 |
| India | 13,614 | 1.1 | 6,392 | 0.5 | 0.470 | 72.3 |
| Saudi Arabia | 376 | 1.9 | 278 | 1.4 | 0.738 | 72.3 |
| COte d'Ivoire | 516 | 3.9 | 290 | 2.2 | 0.563 | 71.9 |
| Cameroon | 435 | 2.8 | 251 | 1.6 | 0.577 | 71.9 |
| Burundi | 186 | 2.9 | 100 | 1.6 | 0.539 | 71.7 |
| Congo-Kinshasa | 1,669 | 3.7 | 918 | 2.0 | 0.550 | 71.6 |
| Cabo Verde | 21 | 5.0 | 11 | 2.7 | 0.531 | 71.5 |
| Guinea-Bissau | 58 | 5.5 | 32 | 3.0 | 0.551 | 71.5 |
| Mali | 285 | 3.0 | 142 | 1.5 | 0.500 | 71.4 |
| Angola | 269 | 1.7 | 146 | 0.9 | 0.542 | 71.4 |
| Guinea | 731 | 10.1 | 397 | 5.5 | 0.543 | 71.3 |
| Lesotho | 31 | 2.0 | 17 | 1.1 | 0.543 | 71.3 |
| Liberia | 191 | 7.0 | 103 | 3.8 | 0.540 | 71.3 |
| Sierra Leone | 187 | 4.6 | 102 | 2.5 | 0.547 | 71.3 |
| Uganda | 831 | 3.5 | 460 | 1.9 | 0.553 | 71.3 |
| Congo-Brazzaville | 96 | 2.7 | 52 | 1.5 | 0.540 | 71.3 |
| Djibouti | 9 | 1.2 | 5 | 0.7 | 0.550 | 71.3 |
| Namibia | 22 | 1.3 | 12 | 0.7 | 0.547 | 71.3 |
| Ghana | 1,264 | 7.1 | 697 | 3.9 | 0.551 | 71.2 |
| Mauritius | 30 | 1.5 | 15 | 0.8 | 0.518 | 71.2 |
| Equatorial Guinea | 20 | 2.2 | 11 | 1.2 | 0.534 | 71.2 |
| Kenya | 625 | 2.5 | 334 | 1.3 | 0.534 | 71.2 |
| Tanzania | 710 | 2.3 | 384 | 1.2 | 0.541 | 71.2 |
| Eritrea | 43 | 1.5 | 23 | 0.8 | 0.525 | 71.2 |
| Burkina Faso | 600 | 6.4 | 323 | 3.4 | 0.538 | 71.2 |
| Rwanda | 338 | 4.6 | 187 | 2.6 | 0.555 | 71.2 |
| Madagascar | 409 | 2.6 | 230 | 1.5 | 0.563 | 71.1 |
| The Central African Republic | 69 | 2.4 | 37 | 1.3 | 0.539 | 71.1 |
| Zambia | 90 | 1.0 | 48 | 0.5 | 0.536 | 71.1 |
| Mauritania | 146 | 5.1 | 82 | 2.9 | 0.560 | 71.0 |
| Comoros | 14 | 2.7 | 7 | 1.4 | 0.511 | 71.0 |
| Botswana | 29 | 1.8 | 15 | 1.0 | 0.540 | 71.0 |
| Chad | 187 | 2.5 | 101 | 1.4 | 0.540 | 71.0 |
| Benin | 136 | 2.3 | 76 | 1.3 | 0.557 | 71.0 |
| Malawi | 134 | 1.0 | 73 | 0.5 | 0.545 | 70.9 |
| Réunion | 39 | 2.8 | 21 | 1.5 | 0.529 | 70.8 |
| Togo | 146 | 3.2 | 75 | 1.7 | 0.514 | 70.7 |
| Brazil | 2,555 | 1.0 | 6,232 | 2.4 | 2.439 | 70.5 |
| Singapore | 885 | 7.9 | 85 | 0.8 | 0.097 | 70.4 |
| Gabon | 24 | 1.5 | 14 | 0.9 | 0.586 | 69.8 |
| Malaysia | 1,207 | 3.9 | 146 | 0.5 | 0.121 | 69.6 |
| Nigeria | 2,913 | 2.9 | 657 | 0.7 | 0.225 | 69.6 |
| The Gambia | 161 | 12.1 | 60 | 4.5 | 0.370 | 69.3 |
| The USA | 2,694 | 0.5 | 23,566 | 4.2 | 8.746 | 69.2 |
| Australia | 173 | 0.4 | 1,514 | 3.5 | 8.746 | 69.2 |
| Zimbabwe | 267 | 3.2 | 130 | 1.6 | 0.486 | 68.8 |
| Italy | 2,120 | 1.4 | 6,261 | 4.0 | 2.953 | 68.0 |
| South Africa | 1,265 | 2.5 | 424 | 0.9 | 0.335 | 67.7 |
| Ethiopia | 587 | 1.0 | 500 | 0.8 | 0.852 | 67.6 |
| Portugal | 222 | 0.9 | 692 | 2.7 | 3.119 | 65.9 |
| Mozambique | 666 | 3.7 | 121 | 0.7 | 0.182 | 65.6 |
| Iran | 1,770 | 2.4 | 496 | 0.7 | 0.280 | 64.9 |
| Germany | 1,617 | 0.8 | 3,997 | 1.9 | 2.473 | 63.2 |
| Spain | 1,054 | 1.0 | 3,103 | 3.0 | 2.943 | 62.7 |
| Guatemala | 434 | 3.6 | 668 | 5.6 | 1.539 | 61.7 |
| Haiti | 161 | 2.0 | 247 | 3.0 | 1.531 | 61.5 |
| Guyana | 5 | 0.7 | 7 | 1.0 | 1.531 | 61.5 |
| The Dominican Republic | 174 | 1.6 | 266 | 2.4 | 1.527 | 61.4 |
| Barbados | 3 | 0.6 | 5 | 0.9 | 1.527 | 61.4 |
| Cuba | 208 | 0.9 | 306 | 1.4 | 1.466 | 61.4 |
| Costa Rica | 105 | 1.5 | 157 | 2.3 | 1.492 | 61.3 |
| Jamaica | 27 | 0.7 | 38 | 1.0 | 1.442 | 61.3 |
| Puerto Rico | 87 | 1.3 | 128 | 1.9 | 1.482 | 61.3 |
| Panama | 62 | 1.2 | 91 | 1.8 | 1.472 | 61.3 |
| Paraguay | 45 | 0.7 | 66 | 1.0 | 1.472 | 61.3 |
| Nicaragua | 139 | 2.6 | 207 | 3.8 | 1.488 | 61.2 |
| El Salvador | 126 | 1.6 | 189 | 2.5 | 1.498 | 61.2 |
| Suriname | 8 | 1.4 | 13 | 2.1 | 1.519 | 61.2 |
| Honduras | 99 | 1.4 | 148 | 2.1 | 1.498 | 61.2 |
| Venezuela | 293 | 0.9 | 437 | 1.3 | 1.488 | 61.2 |
| Moldova | 182 | 3.0 | 338 | 5.5 | 1.855 | 61.1 |
| Bolivia | 167 | 1.5 | 247 | 2.2 | 1.484 | 61.1 |
| The Bahamas | 3 | 0.6 | 5 | 0.9 | 1.546 | 61.1 |
| Martinique | 10 | 1.0 | 14 | 1.5 | 1.480 | 61.0 |
| Bulgaria | 119 | 0.8 | 222 | 1.4 | 1.864 | 61.0 |
| Ecuador | 243 | 1.3 | 354 | 1.9 | 1.460 | 61.0 |
| Belarus | 117 | 0.7 | 222 | 1.3 | 1.905 | 61.0 |
| Trinidad and Tobago | 16 | 0.8 | 24 | 1.2 | 1.531 | 61.0 |
| Colombia | 549 | 0.9 | 841 | 1.4 | 1.531 | 61.0 |
| French Guiana | 5 | 2.1 | 8 | 3.1 | 1.466 | 60.9 |
| Belize | 5 | 2.1 | 7 | 3.0 | 1.466 | 60.9 |
| The Netherlands | 212 | 0.5 | 396 | 1.0 | 1.868 | 60.8 |
| Uruguay | 40 | 0.7 | 56 | 0.9 | 1.413 | 60.8 |
| Ukraine | 382 | 0.5 | 704 | 0.9 | 1.841 | 60.8 |
| Croatia | 134 | 1.3 | 254 | 2.5 | 1.890 | 60.7 |
| Malta | 5 | 0.5 | 9 | 0.9 | 1.918 | 60.7 |
| Czechia | 222 | 0.9 | 422 | 1.7 | 1.900 | 60.6 |
| Guadeloupe | 8 | 0.9 | 12 | 1.3 | 1.463 | 60.6 |
| Montenegro | 11 | 0.8 | 21 | 1.5 | 1.872 | 60.6 |
| Luxembourg | 15 | 1.4 | 27 | 2.5 | 1.788 | 60.5 |
| Poland | 550 | 0.7 | 1,004 | 1.3 | 1.827 | 60.5 |
| Albania | 93 | 1.7 | 170 | 3.0 | 1.822 | 60.4 |
| Serbia | 162 | 0.9 | 303 | 1.7 | 1.871 | 60.3 |
| Slovenia | 62 | 1.2 | 114 | 2.3 | 1.853 | 60.2 |
| North Macedonia | 40 | 1.1 | 72 | 1.9 | 1.800 | 60.2 |
| Bosnia and Herzegovina | 116 | 1.6 | 215 | 2.9 | 1.848 | 60.1 |
| Slovakia | 112 | 1.1 | 196 | 1.9 | 1.757 | 60.1 |
| New Zealand | 239 | 2.9 | 51 | 0.6 | 0.213 | 59.9 |
| Hungary | 233 | 1.2 | 418 | 2.1 | 1.799 | 59.9 |
| Switzerland | 203 | 1.0 | 363 | 1.8 | 1.786 | 59.9 |
| Romania | 759 | 1.8 | 1,301 | 3.2 | 1.714 | 59.7 |
| Sudan | 336 | 1.4 | 223 | 0.9 | 0.664 | 59.4 |
| Belgium | 230 | 1.0 | 358 | 1.5 | 1.555 | 58.5 |
| Greece | 609 | 2.1 | 348 | 1.2 | 0.571 | 58.3 |
| Chile | 304 | 1.0 | 614 | 2.1 | 2.021 | 58.0 |
| Argentina | 497 | 0.8 | 820 | 1.3 | 1.651 | 56.2 |
| France | 1,923 | 1.4 | 4,037 | 3.0 | 2.099 | 56.1 |
| Austria | 205 | 1.0 | 392 | 1.9 | 1.913 | 53.3 |
| Peru | 908 | 2.6 | 171 | 0.5 | 0.189 | 46.6 |
| The Russian Federation | 2,887 | 1.1 | 1,852 | 0.7 | 0.642 | 45.8 |
| The UK | 655 | 0.4 | 2,247 | 1.5 | 3.430 | 38.1 |
| Estonia | 7 | 0.2 | 28 | 0.9 | 3.831 | 34.3 |
| Ireland | 25 | 0.3 | 99 | 1.2 | 3.942 | 34.1 |
| Norway | 24 | 0.2 | 89 | 0.9 | 3.761 | 33.8 |
| Finland | 38 | 0.2 | 147 | 1.0 | 3.870 | 33.6 |
| Iceland | 1 | 0.2 | 4 | 0.7 | 3.870 | 33.6 |
| Lithuania | 17 | 0.3 | 64 | 1.0 | 3.662 | 33.1 |
| Denmark | 43 | 0.3 | 158 | 1.3 | 3.648 | 33.0 |
| Latvia | 10 | 0.2 | 40 | 0.8 | 3.853 | 33.0 |
| Canada | 420 | 0.6 | 789 | 1.1 | 1.880 | 31.1 |
| Sweden | 48 | 0.2 | 141 | 0.7 | 2.959 | 19.4 |
HBV, hepatitis B virus; HCV, hepatitis C virus; n, number of cases; ASR, age-standardized rate; AF, attributable fraction.
Ratio of HCV- and HBV-related liver cancer incidence (ASR).
Total attributable faction of liver cancer caused by HBV and HCV.
Risk Factors Associated with HCV/HBV-Related Liver Cancer Incidence Ratio
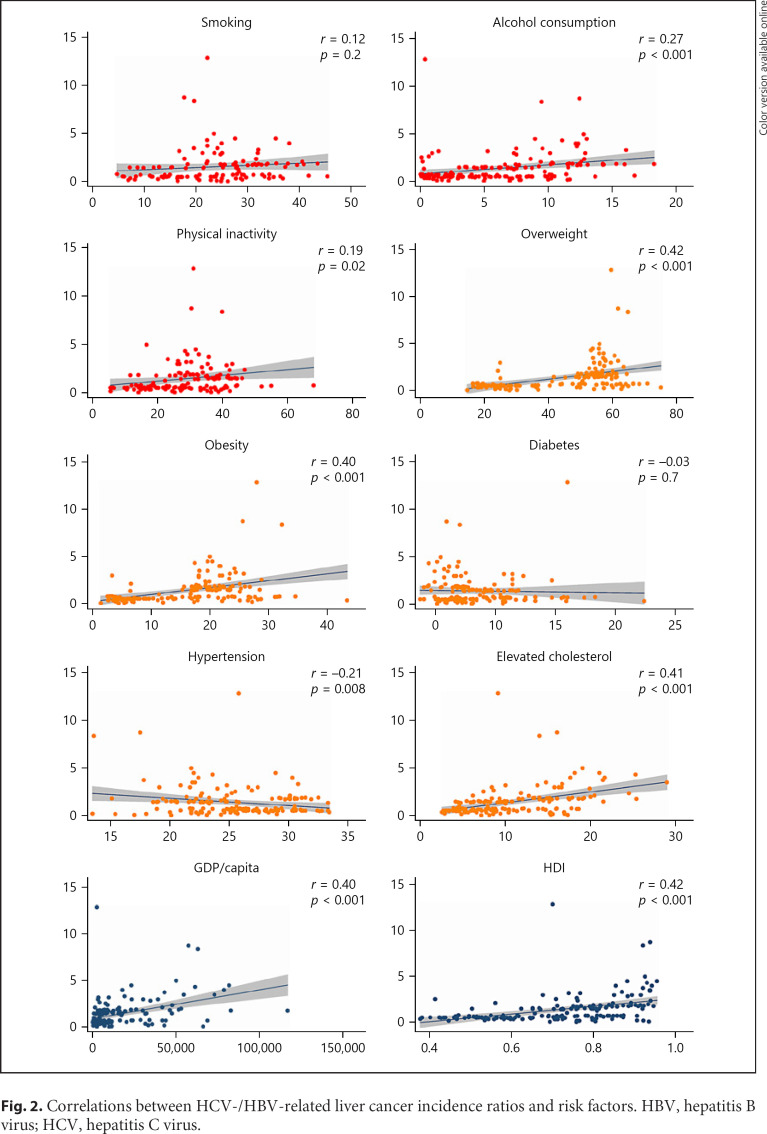
Among the lifestyle risk factors investigated, a higher incidence ratio of HCV/HBV-related liver cancer was associated with a higher prevalence of alcohol consumption (r 0.27, p < 0.001) and physical inactivity (r 0.19, p = 0.02), but not with smoking (p = 0.2) (Fig. 2). For the metabolic risk factors, a higher ratio was associated with a higher prevalence of overweight (r 0.42, p < 0.001), obesity (r 0.40, p < 0.001), and elevated cholesterol (r 0.41, p < 0.001), and a lower prevalence of hypertension (r −0.21, p = 0.008), but not with diabetes (p = 0.7). The higher ratio was also associated with a higher GDP per capita (r 0.40, p < 0.001) and HDI (r 0.42, p < 0.001) for different countries. The correlations remained unchanged when excluding the countries with a total AF of liver cancer caused by HBV and HCV no more than 50% or 60% in the sensitivity analysis (online suppl. Table 3). After conducting the weighted linear regression, the associations remained significant for alcohol consumption (β 0.49, CI 0.03–0.96), overweight (β 0.51, CI 0.39–0.64), obesity (β 0.64, CI 0.38–0.90), elevated cholesterol (β 0.70, CI 0.51–0.90), GDP per capita (β 0.20, CI 0.14–0.26), and HDI (β 0.45, CI 0.33–0.57).
Fig. 2.
Correlations between HCV-/HBV-related liver cancer incidence ratios and risk factors. HBV, hepatitis B virus; HCV, hepatitis C virus.
Temporal Trends of Liver Cancer
The incidence and mortality trends of each country between 1980 and 2017 are shown in online suppl. Figure 1, and the results from the joinpoint regression analysis are plotted in online suppl. Figure 2.
Incidence Trend
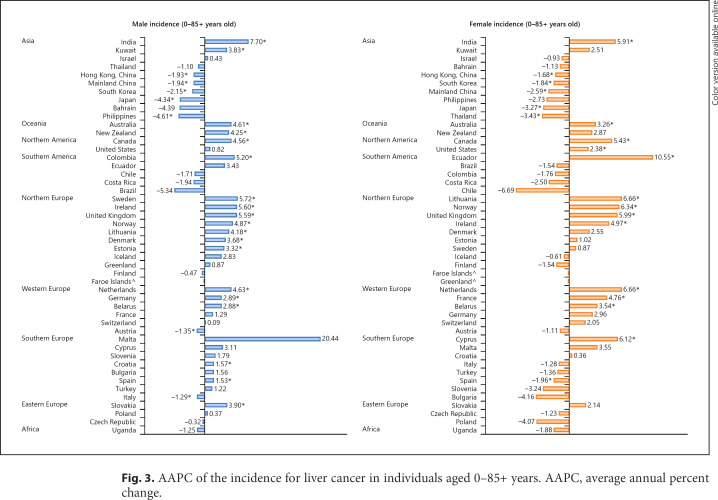
Considering male individuals, 18 countries had an increase in incidence and 23 countries reported stable trends (Fig. 3; online suppl. Table 4). Countries with the most drastic increase were reported in India (AAPC 7.70, CI 4.66–10.82), Ireland (AAPC 5.60, CI 2.53–8.76), Sweden (AAPC 5.72, CI 3.30–8.20), the UK (AAPC 5.59, CI 4.97–6.22), and Norway (AAPC 4.87, CI 2.16–7.66). In contrast, 7 regions, 5 of which were from Asia, showed a decreasing trend. The Philippines (AAPC −4.61, CI 6.91 to −2.24), Japan (AAPC −4.34, CI −4.96 to −3.72), and South Korea (AAPC −2.15, CI −3.11 to −1.18) showed the most significant decrease. Considering female cases, 14 countries had an increase in the incidence and 28 countries reported stable trends. Countries with the most drastic increase included Ecuador (AAPC 10.55, CI 3.92–17.60), Lithuania (AAPC 6.66, CI 4.42–8.95), the Netherlands (AAPC 6.66, CI 3.92–9.47), Norway (AAPC 6.34, CI 4.18–8.56), and Cyprus (AAPC 6.12, CI 0.04–12.56). In contrast, a total of 6 regions showed a decreasing trend. Thailand (AAPC −3.43, CI −6.23 to −0.54), Japan (AAPC −3.27, CI −3.98 to −2.55), and China (AAPC −2.59, CI −3.74 to −1.41) showed the most significant decrease.
Fig. 3.
AAPC of the incidence for liver cancer in individuals aged 0–85+ years. AAPC, average annual percent change.
Mortality Trend
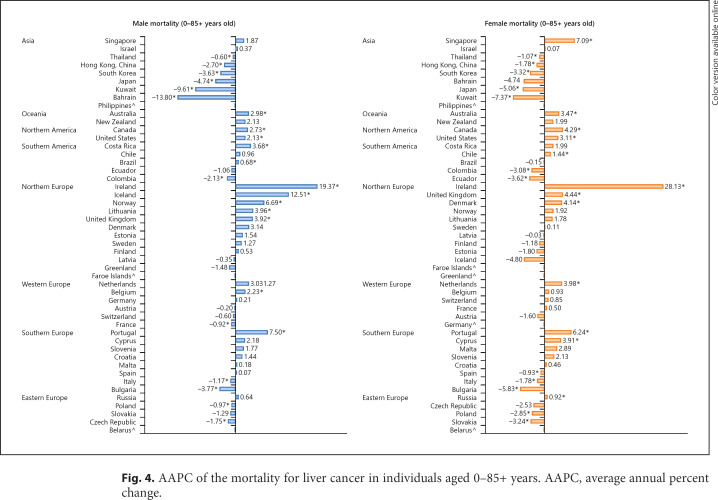
Considering male patients, 13 countries had an increase in mortality and 23 countries reported stable trends (Fig. 4; online suppl. Table 4). Countries with the most drastic increase were reported in Ireland (AAPC 19.37, CI 11.04–28.32), Iceland (AAPC 12.51, CI 0.11–26.45), Portugal (AAPC 7.50, CI 2.55–12.68), and Norway (AAPC 6.69, CI 4.24–9.19). In contrast, 12 regions showed a decreasing trend. Bahrain (AAPC −13.80, CI −20.92 to −6.05), Kuwait (AAPC −9.61, CI 14.41 to −4.54), Japan (AAPC −4.74, CI −5.27 to −4.20), and Bulgaria (AAPC −3.77, CI −5.00 to −2.53) showed the most significant decrease. Considering female patients, 12 countries had an increase in mortality and 25 countries reported stable trends. Countries with the most drastic increase were reported in Ireland (AAPC 28.13, CI 17.52–39.71), Singapore (AAPC 7.09, CI 3.21–11.12), Portugal (AAPC 6.24, CI 1.81–10.87), and the UK (AAPC 4.44, CI 3.72–5.16). In contrast, 11 regions showed a decreasing trend. Kuwait (AAPC −7.37, CI −13.37 to −0.96), Bulgaria (AAPC −5.83, CI −7.59 to −4.03), Japan (AAPC −5.06, CI −5.61 to −4.50), and Ecuador (AAPC −3.62, CI −4.88 to −2.35) showed the most significant decrease.
Fig. 4.
AAPC of the mortality for liver cancer in individuals aged 0–85+ years. AAPC, average annual percent change.
Incidence Trend among Younger versus Older Individuals
The incidence of liver cancer increased in 21 countries among individuals aged ≥50 years, and 8 countries reported decreasing trends (online suppl. Fig. 3; online suppl. Table 4). The most marked increase was observed in India (male: AAPC 10.40, CI 7.11 to 6.41; female: AAPC 6.24, CI 1.80–10.87), Ecuador (female: AAPC 11.37, CI 4.66–18.51), and Norway (male: AAPC 7.43, CI 4.79–10.15; female: AAPC 6.99, CI 6.24–7.74). As for individuals aged <50 years, the incidence of liver cancer increased in 4 countries and deceased in 9 countries (online suppl. Fig. 4; online suppl. Table 4). The increase was observed in France (female: AAPC 5.23, CI 0.19–10.52), Spain (male: AAPC 4.83, CI 1.33–8.46), Belarus (male: AAPC 4.77, CI 3.03–6.53), and the UK (male: AAPC 3.13, CI 0.60–5.73).
Discussion
Summary of Major Findings
This study presents the most updated data on the global disease burden of liver cancer by causes and associated risk factors, as well as its epidemiological trends by age, gender, and country. There are several major findings. First, the highest burden tended to predominate in Eastern Asia, and there was an evident epidemiologic disparity in its incidence caused by HBV and HCV in 2018. Second, a higher incidence ratio of HCV/HBV-related liver cancer was associated with a higher level of alcohol consumption, overweight, obesity, elevated cholesterol, GDP, and HDI. Third, many countries reported an increasing trend in liver cancer for the past 10 years, especially among male individuals, those aged ≥50 years, and countries with a higher HCV/HBV-related liver cancer incidence ratio.
Disparities in Epidemiology by Causes
There was a substantial variation in the incidence and mortality of liver cancer in different countries in 2018. We found that the highest incidence and mortality tended to predominate in Eastern Asia, Micronesia, Northern Africa, and Southeastern Asia, while the lowest was found in south-central Asia, Western Asia, central Europe, and eastern Europe. These findings are consistent with those reported from previous studies [10, 11]. In addition to ethnic and racial differences, this variation might be attributed to the different distribution of risk factors for liver cancer across different populations. Chronic HBV and HCV remain important risk factors for liver cancer [33]. Globally, more than 250 million individuals were infected with HBV in 2015, with a significant geographic variation in prevalence [34]. Countries with a prevalence of HBV infection over 8% were mostly in Asia and Africa, and they contributed to approximately 70% of all infected patients. On the contrary, the prevalence of HBV in Western countries was as low as less than 2% [35]. Globally, more than 70 million individuals were infected with HCV in 2015, with a major geographical difference [36]. The prevalence of HCV was high in central Asia and the Mediterranean (>3.5%), while its prevalence was less than 1.5% in North America [37]. Even though the prevalence of HBV and HCV was low in the developed countries, liver cancer caused by HCV was more prevalent in high-income countries like North America and western Europe [38].
Association with Risk Factors, GDP, and HDI
There are some clinical and public health significance for examining the association between the HCV-/HBV-related liver cancer incidence ratio and the prevalence of preventable lifestyle and metabolic risk factors. HBV and HCV are largely predominant risk factors compared with other risk factors in most countries, and there was an evident geographical variation in the epidemiology of liver cancer caused by HBV and HCV. This indicator was devised for the purpose of looking at their relative association with different risk factors, which may help set tailored strategies on liver cancer prevention for individual countries. The current correlation analysis found some lifestyle and metabolic risk factors associated with a higher prevalence of liver cancer incidence attributable to HCV and a lower prevalence of that attributable to HBV at a country-level analysis. These factors included alcohol consumption, overweight, obesity, and elevated cholesterol. The results are generally consistent with the studies at an individual level. There is evidence for a much stronger synergistic effect between alcohol consumption and HCV infection in the development of liver cancer than HBV infection according to a case-control study of 464 patients with liver cancer [39]. A cohort study of 23,820 participants with a follow-up period of 14 years found that obesity was independently associated with a 4-fold risk of liver cancer among patients infected with HCV, but not among patients infected with HBV [40]. Similar associations were found among individuals with diabetes [40] and metabolic syndrome [41]. However, we did not find diabetes as a risk factor for higher HCV-related liver cancer, and this is probably due to the presence of unknown potential confounders or the difference between ecological correlation and individual correlations. A notable finding of the study results is that the ratio was also associated with GDP and HDI, which are 2 important indexes measuring the level of socioeconomic development of different countries.
Increasing Burden in the Past Decade
We observed an overall increasing trend of its incidence and mortality for the past 10 years, especially among male subjects, older individuals, and countries with a higher prevalence of HCV-related liver cancer. The reasons behind the increasing trend of the incidence and mortality of liver cancer remain unclear. As the increase was mostly observed in countries with a higher prevalence of HCV-related liver cancer, the increasing prevalence of alcohol consumption and obesity may have contributed to this epidemiologic transition. For the recent past decade, the global alcohol consumption per capita has increased from 5.5 to 6.4 L (16.4%) among adults [42]. Based on a recent WHO report on the global burden of obesity in 2016, its prevalence has nearly tripled in the past 4 decades [43]. A meta-analysis of more than 14 million participants found that the worldwide prevalence of central obesity has doubled from 1985 (16%) to 2014 (34%) [44]. In addition to HCV-related liver cancer, the increase in burden of liver cancer may also be attributable to the recent increasing trend of nonalcoholic fatty liver disease (NAFLD) [45, 46]. NAFLD can lead to liver cirrhosis and cancer, contributing to liver-related mortality [47]. Evidence showed that there was also a strong association between the risk of NAFLD and obesity, as well as other metabolic diseases [48]. All these factors may be associated with an increasing trend of liver cancer among countries with high GDP and HDI. Considering the increasing prevalence of obesity and metabolic syndrome caused by overnutrition, sedentary lifestyles, and urbanization, the global burden of HCV- and NAFLD-related liver cancer is estimated to increase further in the future.
Strengths and Limitations
This study is an updated analysis of the global burden of liver cancer by causes and its associated risk factors, as well as its recent epidemiological trend by age, gender, and country. The figures were obtained from real-world cancer registries of high quality with a total of more than one million cancer cases. Nevertheless, the study has several limitations. First, there could be underreporting of the cancer figures in lower income countries when compared with higher income countries. In contrast, the figures could also have been overestimated as the figures for incidence and mortality were mainly from the cancer registries of major cities for some countries. Second, direct comparison between some countries could be difficult since cancer registries and causes of death registries might differ by countries and over time. Third, risk factor association with other etiologies could not be assessed from this database, and only the analysis on HCV-/HBV-related liver cancer could be performed. In addition, the increase in incidence in some countries may be attributable to the improvement in diagnosis.
Implications
The reinforcement of country-specific preventive strategies, including a robust implementation of hepatitis vaccination programs and promotion of healthy lifestyle interventions, is important to reduce the burden of liver cancer. Screening programs for high-risk populations can also be organized in primary care settings to detect liver cancer and its related diseases, including HBV, HCV, cirrhosis, and NAFLD [49]. In 2015, the United Nations announced one important goal of Sustainable Development is to eliminate viral hepatitis by 2030 [50]. For HBV-related liver cancer, the decrease in cancer rates will certainly depend on highly effective vaccination campaigns and antiviral treatment. For HCV-related liver cancer, the decline in cancer rates will derive more from an antiviral approach as HCV vaccine is not currently available [51]. However, even though effective antiviral treatment options are available for HBV and HCV, they have not been sufficiently implemented globally, especially in developing countries. The success of the elimination plan will largely depend on the accessibility of antiviral treatment, the availability of treatment and monitoring guidelines, and the capacity to offer screening and treat the high-risk populations. Other strategies to combat liver cancer including community-based health promotion education program (such as those on prevention of needle exchange among intravenous drug users) and environmental modifications (such as initiatives on the promotion of storage technique to avoid aflatoxin contamination) can also be useful for high-risk populations. For patients already diagnosed with liver cancer, it is important to channel efforts and resources in improving available medical and surgical interventions (e.g., surgery, ablation, embolization therapy, radiation therapy, targeted drug therapy, immunotherapy, and chemotherapy) and provide multidisciplinary care to reduce its related morbidity and mortality. Future studies should investigate the plausible reasons behind these epidemiological changes, which may offer further insights into developing an evidence-based globally sustainable, targeted, and individualized public health model in fighting liver cancer at its core.
Statement of Ethics
This study was approved by the Survey and Behavioural Research Ethics Committee, Chinese University of Hong Kong (No. SBRE-20-332). We declare the research complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Patient consent was not applicable as the study only used information freely available in the public domain and does not contain any personal or medical information about an identifiable living individual.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding.
Author Contributions
M.C.S.W. and J.H. participated in the conception of the research ideas, study design, interpretation of the findings, writing of the first draft of the manuscript, and provided intellectual input to the translational aspects of the study. J.H., V.L., C.H.N., and C.C. retrieved information from the relevant databases and performed statistical analysis. H.K.P., V.T.C., L.Z., P.C., S.W., X.Q.L., S.L.A.T., W.X., and Z.J.Z. did critical revisions of the manuscripts and provided expert opinions on implications of the study findings.
Supplementary Material
Supplementary data
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Key statistics about liver cancer. 2020. https://www.cancer.org/cancer/liver-cancer/about/what-is-key-statistics.html Accessed 2020 May 27.
- 3.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011 Nov;20((11)):2362–8. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 4.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019 Oct;16((10)):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010 Aug;19((8)):1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Biological Agents IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100B. 2012. https://publications.iarc.fr/119 Assessed 2020 May 25.
- 7.Endeshaw M, Hallowell BD, Razzaghi H, Senkomago V, McKenna MT, Saraiya M. Trends in liver cancer mortality in the United States: dual burden among foreign- and US-born persons. Cancer. 2019 Mar 1;125((5)):726–34. doi: 10.1002/cncr.31869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019 Feb;17((3)):551–e1. doi: 10.1016/j.cgh.2018.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiels MS, O'Brien TR. Recent decline in hepatocellular carcinoma rates in the United States. Gastroenterology. 2020 Apr;158((5)):1503–e2. doi: 10.1053/j.gastro.2019.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer. 2016 Oct 1;139((7)):1534–45. doi: 10.1002/ijc.30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong MC, Jiang JY, Goggins WB, Liang M, Fang Y, Fung FD, et al. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017 Mar 31;7:45846. doi: 10.1038/srep45846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int J Cancer. 2020 Jul 15;147((2)):317–30. doi: 10.1002/ijc.32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017 Dec 1;3((12)):1683–91. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Jiang Y, Yuan H, Fang Q, Cai N, Suo C, et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019 Apr;70((4)):674–83. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Wong MC, Goggins WB, Wang HH, Fung FD, Leung C, Wong SY, et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016 Nov;70((5)):862–74. doi: 10.1016/j.eururo.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 16.Wong MC, Huang J, Lok V, Wang J, Fung F, Ding H, et al. Differences in incidence and mortality trends of colorectal cancer, worldwide, based on sex, age, and anatomic location. Clin Gastroenterol Hepatol. 2020 Feb 20; doi: 10.1016/j.cgh.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 17.International Agency for Research on Cancer WHO Data visualization tools for exploring the global cancer burden in 2018. 2018. https://gco.iarc.fr/today/home Accessed 2020 May 10.
- 18.World Health Organization Cancer causes. 2020. https://gco.iarc.fr/ Assessed 2020 May 25.
- 19.Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018 Jun 15;142((12)):2471–7. doi: 10.1002/ijc.31280. [DOI] [PubMed] [Google Scholar]
- 20.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020 Feb;8((2)):e180–e90. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Global health observatory data repository. 2016. https://apps.who.int/gho/data/node.main Accessed 2020 May 10.
- 22.The World Bank GDP per capita 1960–2019 (current US$) 2020. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD Assessed 25 May 2020.
- 23.United Nations Development Programme Human development data (1990–2018) 2020. http://hdr.undp.org/en/data Assessed 2020 May 25.
- 24.Bray FCM, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J. Cancer incidence in five continents. Vol. XI (electronic version) Lyon: International Agency for Research on Cancer; 2017. https://ci5.iarc.fr Accessed 2020 May 10. [Google Scholar]
- 25.U.S. Department of Health and Human Services NIoH Surveillance, Epidemiology, and End Results (SEER) Program. 2020. https://seer.cancer.gov/about/ Accessed 10 May 2020.
- 26.Danckert BFJ, Engholm G, Hansen HL, Johannesen TB, Khan S, Køtlum JE, Ólafsdóttir E, Schmidt LKH, Virtanen A, Storm HH. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.2 (26.03.2019) 2019. https://www-dep.iarc.fr/NORDCAN/english/frame.asp Accessed 2020 May 10.
- 27.World Health Organization WHO mortality database. 2020. https://www.who.int/healthinfo/mortality_data/en/ Accessed 2020 May 10.
- 28.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005 Mar;83((3)):171–7. [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization International statistical classification of diseases and related health problems 10th revision. 2016. https://icd.who.int/browse10/2016/en Accessed 10 May 2020.
- 30.Segi M, Fujisaku S, Kurihara M. Geographical observation on cancer mortality by selected sites on the basis of standardised death rate. Gan. 1957 Jun;48((2)):219–25. [PubMed] [Google Scholar]
- 31.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000 Feb 15;19((3)):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009 Dec 20;28((29)):3670–82. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015 Oct;62((4)):1190–200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization Global hepatitis report 2017. 2017. http://apps.who.int/iris/bitstream/10665/255017/1/WHO-HIV-2017.06-eng.pdf Accessed 2020 May 27.
- 35.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012 Mar 9;30((12)):2212–9. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization Global hepatitis report 2017. 2017. http://apps.who.int/iris/bitstream/10665/255017/1/WHO-HIV-2017.06-eng.pdf Accessed 2020 May 27.
- 37.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016 Sep 14;22((34)):7824–40. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polaris Observatory HCVC Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017 Mar;2((3)):161–76. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 39.Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002 Feb 15;155((4)):323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 40.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008 Jul;135((1)):111–21. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 41.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012 May;142((6)):1264–e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization Global status report on alcohol and health 2018. 2018. https://apps.who.int/iris/bitstream/handle/10665/274603/9789241565639-eng.pdf Accessed 2020 Oct 10.
- 43.World Health Organization Obesity and overweight. 2019. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight Accessed 10 May 2020.
- 44.Wong MC, Huang J, Wang J. Global, regional and time-trend prevalence of central obesity: a systematic review and meta-analysis of 13.2 million subjects. Eur J Epidemiol. 2020;35:673–83. doi: 10.1007/s10654-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019 Jan;16((1)):57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 46.Wong VW, Hui AY, Tsang SW, Chan JL, Tse AM, Chan KF, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006 Sept;4((9)):1154–61. doi: 10.1016/j.cgh.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008 Oct;49((4)):608–12. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010 Jul;59((7)):969–74. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 49.Wong MCS, Huang J. The growing burden of liver cirrhosis: implications for preventive measures. Hepatol Int. 2018 May;12((3)):201–3. doi: 10.1007/s12072-018-9865-y. [DOI] [PubMed] [Google Scholar]
- 50.United Nations Transforming our world: the 2030 agenda for sustainable development. 2015. https://sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf Accessed 2020 Oct 27.
- 51.Beste LA, Green P, Berry K, Belperio P, Ioannou GN. Hepatitis C-related hepatocellular carcinoma incidence in the veterans health administration after introduction of direct-acting antivirals. JAMA. 2020;324((10)):1003–5. doi: 10.1001/jama.2020.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data