Abstract
Background
Combined therapy with tyrosine kinase inhibitors (TKIs) and anti-PD-1 antibodies has shown high tumor response rates for patients with unresectable hepatocellular carcinoma (HCC). However, using this treatment strategy to convert initially unresectable HCC to resectable HCC was not reported.
Methods
Consecutive patients with unresectable HCC who received first-line therapy with combined TKI/anti-PD-1 antibodies were analyzed. Tumor response and resectability were evaluated via imaging every 2 months (±2 weeks) using RECIST v1.1. Resectability criteria were (1) R0 resection could be achieved with sufficient remnant liver volume and function; (2) intrahepatic lesions were evaluated as partial responses or stable disease for at least 2 months; (3) no severe or persistent adverse effects occurred; and (4) hepatectomy was not contraindicated.
Results
Sixty-three consecutive patients were enrolled. Of them, 10 (15.9%) underwent R0 resection in 3.2 months (range: 2.4–8.3 months) after the initiation of combination therapy. At baseline, these 10 patients had a median largest tumor diameter of 9.3 cm, 7 had Barcelona Clinic Liver Cancer stage C (vascular invasion) disease, 2 had stage B, and 1 had stage A. Before surgery, 6 patients were evaluated as a partial response, 3 stable disease, and 1 partial response in the intrahepatic lesion but a new metastatic lesion in the right adrenal gland. Six patients (60%) achieved a pathological complete response. One patient died from immune-related adverse effects 2.4 months after hepatectomy. After a median follow-up of 11.2 months (range: 7.8–15.9 months) for other 9 patients, 8 survived without disease recurrence, and 1 experienced tumor recurrence.
Conclusions
Combination of TKI/anti-PD-1 antibodies is a feasible conversion therapy for patients with unresectable HCC to become resectable. This study represents the largest patient cohort on downstaging role of combinational systemic therapy on TKI and PD-1 antibody for HCC.
Keywords: Hepatocellular carcinoma, Conversion therapy, Anti-angiogenic therapy, Anti-PD-1 antibody
Background
Primary liver cancer is the second leading cancer-related cause of death in China [1]. Although the incidence and mortality of liver cancer are decreasing in China [2, 3], they are increasing in the USA and Europe [4]. Over 90% of primary liver cancers are hepatocellular carcinomas (HCCs), and life expectancy following a diagnosis of HCC is lower than many other cancers [4]. One contributing factor to this poor prognosis is that the majority of patients with HCC are diagnosed at an advanced stage at which they have already missed the opportunity for curative resection [5]. Therefore, systemic therapy is the standard of care for most patients with HCC. In recent years, a number of novel anti-cancer agents have shown effectiveness for the treatment of advanced or unresectable HCC in terms of tumor response and patient survival. However, overall survival (OS) remains poor for patients with HCC receiving systemic therapy [6].
Conversion therapy aims to achieve tumor downstaging and provide patients with initially unresectable or borderline resectable malignancies a chance to receive curative resection. This approach is an established treatment strategy for a number of solid tumors including colorectal cancer, pancreatic carcinoma, gastric cancer, and esophagogastric junction cancer. Conversion therapy for patients with HCC was reported over 2 decades ago [7, 8]. However, until recently, no systemic therapy with potent anti-tumor activity against HCC existed, and few patients were able to achieve downstaging and receive curative surgery following systemic therapy [9]. Other approaches for conversion therapies have mainly included portal vein embolism (PVE), associating liver partition and portal vein ligation for staged hepatectomy [10], and transcatheter arterial chemoembolization [11], which mainly aim to improve feasibility of liver resection.
Although sorafenib has been shown to improve survival for patients with advanced HCC, it is associated with a low tumor response rate (approximately 5%), and therefore, few patients have undergone curative surgery following sorafenib therapy [12, 13, 14]. Since 2017, novel agents, especially combination treatment using anti-angiogenic therapies and anti-programmed cell death protein 1 (PD-1) antibody, have been shown to induce higher tumor response rates than sorafenib (≥30%) [15]. For example, the combination of lenvatinib with anti-PD-1 antibodies pembrolizumab or nivolumab showed objective response rates (ORRs) of 36 and 54.2%, respectively, in early-phase clinical trials [16, 17]. These high tumor response rates have revived the idea of conversion therapy for HCC.
Here, we report 10 patients with initially advanced or unresectable HCC who received anti-angiogenic tyrosine kinase inhibitor (TKI) and anti-PD-1 antibody combination therapy, followed by R0 resection. To our knowledge, this represents the largest reported cohort of patients with unresectable HCC to undergo R0 resection following systemic therapy.
Patients and Methods
Patients
Consecutive patients with unresectable or advanced HCC received first-line systemic therapy with combined TKI/anti-PD-1 antibodies were retrospectively analyzed. HCC was diagnosed based on standard imaging examinations, with or without elevated serum tumor markers, alpha-fetoprotein, and/or protein induced by vitamin K absence-II, based on local and international guidelines [18, 19]. Tumors were considered unresectable either because they were already advanced-stage HCC, intermediate stage, or because of insufficient remnant liver volume after liver resection (<40% for patients with liver cirrhosis; <30% for patients without liver cirrhosis). The study protocol, including treatment regimen and data collection, was complied with the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by Zhongshan Hospital Research Ethics Committee (Approval Number: B2020-177R), and all patients provided written informed consent before receiving combined TKI/anti-PD-1 antibody treatment and before surgery.
Systemic Therapy
When we designed this study, the combination of lenvatinib (a multi-target TKI) with pembrolizumab [20] or the combination of apatinib (a VEGFR2 TKI) with camrelizumab [21] showed high ORRs and acceptable safety profiles for advanced HCC in early-phase clinical trial. Most recently, the combination of lenvatinib and nivolumab also showed similar anti-tumor activities [16]. A number of regimens were used in this study because of local practice and research protocol. The TKIs used in this study were lenvatinib (8 mg/day regardless of patient body weight) or apatinib [22] (250 mg/day). Anti-PD-1 antibodies were intravenously administered as follows: nivolumab 3 mg/kg, or camrelizumab [23] 200 mg, every 2 weeks or pembrolizumab 200 mg, or sintilimab [24] 200 mg, every 3 weeks.
All patients were treated and monitored regularly. Briefly, complete blood count, liver, renal, thyroid, adrenal and cardiac functions, and tumor markers were monitored every 2–3 weeks before each anti-PD-1 antibody treatment cycle. Tumor response and resectability were evaluated via contrast-enhanced magnetic resonance imaging/computed tomography (MRI/CT) and chest CT every 2 months (±2 weeks). Tumor response was assessed according to RECIST v1.1 and modified RECIST criteria [25, 26]. Adverse events were assessed and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
Liver Resection
Preoperative assessment included blood tests and imaging. Patients were classified as unresectable if R0 resection is impossible, or remnant liver volume is below 30% in non-cirrhotic patients or 40% in cirrhotic patients, or tumor stage is Barcelona Clinic Liver Cancer (BCLC) stage B and up-to-seven criteria out [27], or stage C, or tumor recurrence is diagnosed within 12 months after initial resection. Except in the first patient, liver biopsies were performed to assess the severity of liver necroinflammation to exclude latent hepatotoxicity induced by combination therapy. Patients were classified as having resectable HCC if (1) R0 resection could be achieved with sufficient remnant liver volume and function, (2) intrahepatic lesions were evaluated as partial response (PR) or stable disease (SD) for at least 2 months, (3) no severe or persistent adverse effects occurred from systemic therapy, and (4) no contraindications for hepatectomy existed.
Post-hepatectomy liver failure was diagnosed and graded based on an increased international normalized ratio of prothrombin time and hyperbilirubinemia on or after postoperative day 5 as proposed by the International Study Group of Liver Surgery [28]. Postoperative complications were classified using the Clavien-Dindo classification [29]. A standard 7-point baseline sampling protocol were applied for resected tumor specimen [30]. If no viable tumor cells were found, the whole tumor specimen were completely sampled. Pathological complete response (pCR) was defined as no residual viable tumor cells on hematoxylin and eosin staining on slide sections from completely resected primary tumors, tumor thrombosis, and metastatic lesions.
Postoperative Management
Combination therapy was resumed 4–6 weeks post-surgery, after postoperative complications were resolved. Follow-up imaging examinations (contrast-enhanced MRI/CT or abdominal ultrasound) were performed every 2–3 months or when tumor recurrence was suspected based on elevated serum tumor biomarkers. Post-recurrence treatments were administered according to local guidelines [18].
Statistical Analyses
Statistical analyses were performed using PASW Statistics v.18.0 for Windows (IBM Corp., Armonk, NY, USA). OS was defined as the interval between the date that combination therapy was initiated and the date of the patient's death. Recurrence-free survival was defined as the interval between the date of surgery and the date of diagnosis of tumor recurrence or patient death from any cause. Kaplan-Meier analysis was used to determine the survival rates at each time points.
Results
Patients and Treatment
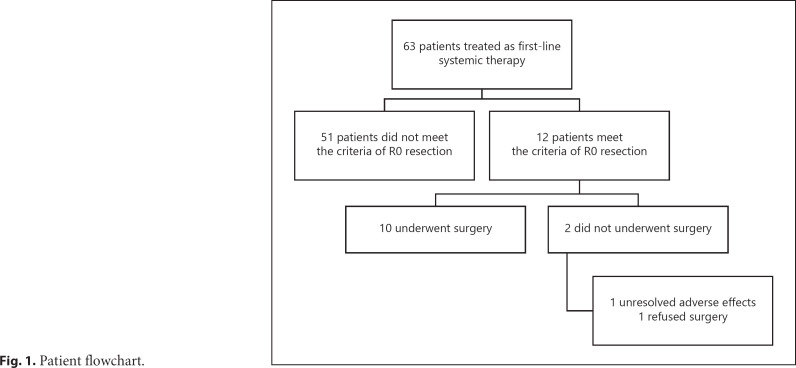
From September 2018 to December 2019, 63 consecutive patients who received TKI plus anti-PD-1 antibody treatment as first-line systemic therapy were analyzed. Of them, 10 patients (15.9%) underwent R0 resection. Other 2 patients were evaluated as resectable after achieving a PR of the original tumor (e.g., met the first and second criterion of resection as mentioned above) but did not undergo liver resection. One experienced unresolved adverse effect from anti-PD-1 antibody treatment (grade II myocarditis), and 1 refused surgery (Fig. 1).
Fig. 1.
Patient flowchart.
The demographics and baseline characteristics of all 63 patients who received combination therapy are summarized in Table 1. No patients with extrahepatic spread (n = 20) underwent surgery after treatment, whereas 10 out of 43 patients without extrahepatic spread underwent surgery (p = 0.023). All 10 patients who underwent surgery were men, with a median age of 52 years (range: 44–72 years), 7 had BCLC Stage C disease with macrovascular invasion, including Vp3 in 2 patients, Vp4 in 3 patients according to Liver Cancer Study Group of Japan (LCSGJ) classification [31], invasion of the hepatic vein in 1 patient (Vv2 by LCSGJ classification [31]), and invasion of the inferior vena cava/right atrium (Vv3) in 1 patient. Two patients had BCLC stage B HCC, and 1 had BCLC stage A disease with insufficient future remnant liver volume if resected. The median diameter of the largest liver nodule was 9.3 cm (range: 4.2–17.6 cm), and 5 patients had multiple lesions in the liver (up-to-seven criteria out) (Table 2).
Table 1.
Baseline patient demographics and disease characteristics
| Characteristics | Patients who underwent surgery (n = 10) | Patients who did not undergo surgery (n = 53)* | p values |
|---|---|---|---|
| Sex (male/female), n | 10/0 | 46/7 | 0.352 |
| Median age, years (range) | 52 (44–72) | 57 (25–74) | 0.547 |
| ECOG performance status (0/1/2), n | 5/5/0 | 12/33/8 | 0.155 |
| Etiology of HCC (HBV/HCV/non-viral), n | 10/0/0 | 51/0/2 | 1.000 |
| BCLC stage (A/B/C), n | 1/2/7 | 1/13/39 | 0.466 |
| China liver cancer stage (Ib/IIa/IIb/IIIa/IIIb), n | 1/1/1/7/0 | 1/1/12/19/20 | 0.087 |
| Macrovascular invasion (yes/no), n | 7/3 | 27/26 | 0.319 |
| Portal vein tumor thrombus (Vp0/Vp1-2/Vp3/Vp4), n | 5/0/2/3 | 28/1/10/14 | 1.000 |
| Hepatic vein tumor thrombus (Vv0-1/ Vv2/Vv3), n | 8/1/1 | 46/1/6 | 0.538 |
| Extrahepatic disease (yes/no), n | 0/10 | 20/33 | 0.023 |
| Child-Pugh class (A/B), n | 10/0 | 50/3 | 1.000 |
| Baseline AFP, median (range), ng/mL | 1,122 (17.5–60,500) | 860 (1–60,500) | 0.807 |
| Baseline AFP ≥400 ng/mL, n (%) | 7 (70.0) | 32 (60.4) | 0.729 |
| Baseline PIVKA-II, median (range), mAU/mL | 3,927 (111–75,000) | 16,245 (43–75,000) | 0.194 |
| Baseline PIVKA-II ≥1,000 mAU/mL, n (%) | 8 (80.0) | 35 (66.0) | 0.481 |
ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HBV, hepatitis B; HCV, hepatitis C; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II.
Including 2 patients who met the criteria for R0 resection but not did not undergo surgery.
Table 2.
Characteristics of surgical and postoperative features
| Patient No | Intrahepatic tumor size, cm | Number of intrahepatic tumors | BCLC stage | CNLC stage | Vascular invasion | TKI used | Anti-PD-1 antibody used | Tumor response, by RECIST v1.1 | Tumor response, by mRECIST |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.2 | 1 | C | Ilia | Vp4 | Len | Niv | PR | CR |
| 2 | 9.0 | >3 | C | Ilia | Vp4 | Len | Pem | SD | SD |
| 3 | 11.6 | >3 | B | IIb | − | Len | Pem | PR | PR |
| 4 | 9.0 | >3 | C | Ilia | Vp4 | Len | Sin | PR | CR |
| 5 | 4.2 | 2 | B | IIa | − | Len | Pem | SD | SD |
| 6 | 17.6 | >3 | C | IIIa | Vp3 | Apa | Sin | PR | PR |
| 7 | 9.6 | 1 | C | IIIa | Vv2 | Len | Pem | SD | SD |
| 8 | 6.8 | 1 | C | IIIa | Vv3 | Apa | Cam | PR | PR |
| 9 | 15.4 | 1 | C | IIIa | Vp3 | Apa | Sin | PD | PD |
| 10 | 7.3 | 1 | A | Ib | − | Len | Pem | PR | CR |
BCLC, Barcelona Clinic Liver Cancer; CNLC, Chinese Liver Cancer Stage; TKIs, tyrosine kinase inhibitors; Len, lenvatinib; Apa, apatinib; Niv, nivolumab; Pem, pembrolizumab; Sin, sintilimab; Cam, camrelizumab; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
The TKIs and the anti-PD-1 antibodies of the 10 patients received are listed in Table 2. Tumor responses evaluated via pre-hepatectomy imaging were PR (n = 6), SD (n = 3), and progressive disease (n = 1; the intrahepatic tumor was evaluated as a PR; however, a new metastatic lesion was found in the right adrenal gland during systemic therapy).
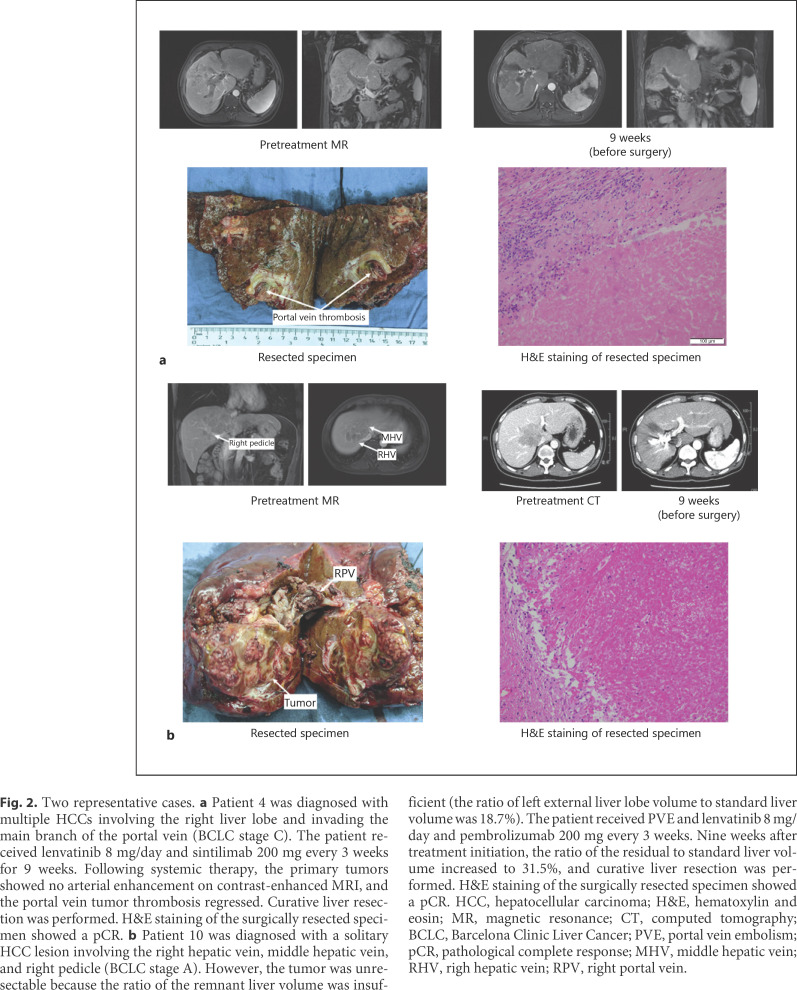
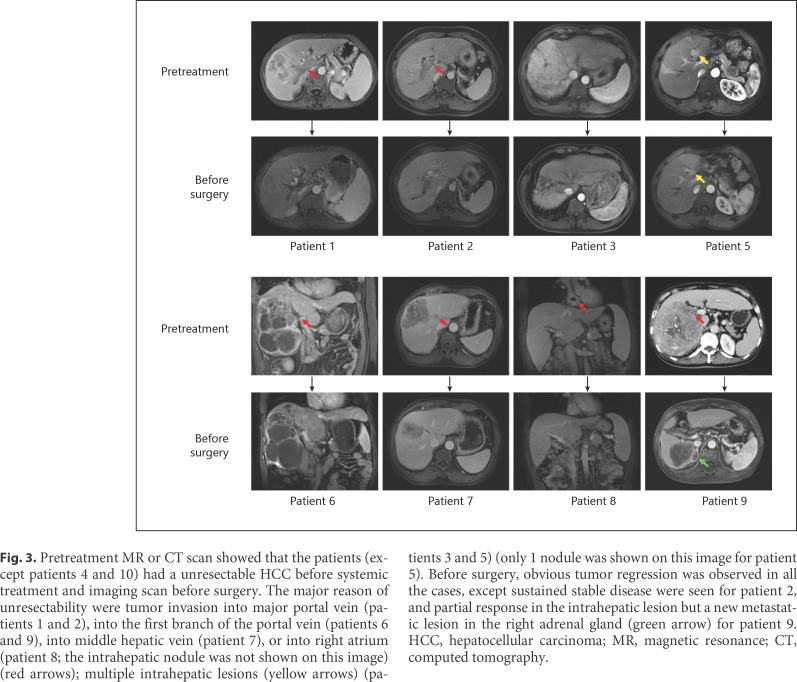
Figure 2 shows 2 representative cases. Patient 4 (Fig. 2a) was diagnosed with BCLC stage C HCC, as tumor invaded into main branch of the portal vein. After the treatment with lenvatinib plus sintilimab for 9 weeks, significant necrosis was found in the portal vein thrombosis by MRI study. No viable tumor cell can be found in the resected liver lesion and tumor thrombus by pathological study. Patient 10 (Fig. 2b) received a PVE to enlarge the future liver volume before initiation of combination therapy (lenvatinib plus pembrolizumab) because the baseline remnant liver volume was 18.7%. Nine weeks after the initiation of combination therapy, the remnant liver volume was increased to 31.5%. The patient then received right trisectionectomy. pCR was also diagnosed by pathological study. MR/CT scans before systemic treatment and before surgery for other 8 patients are shown in Figure 3.
Fig. 2.
Two representative cases. a Patient 4 was diagnosed with multiple HCCs involving the right liver lobe and invading the main branch of the portal vein (BCLC stage C). The patient received lenvatinib 8 mg/day and sintilimab 200 mg every 3 weeks for 9 weeks. Following systemic therapy, the primary tumors showed no arterial enhancement on contrast-enhanced MRI, and the portal vein tumor thrombosis regressed. Curative liver resection was performed. H&E staining of the surgically resected specimen showed a pCR. b Patient 10 was diagnosed with a solitary HCC lesion involving the right hepatic vein, middle hepatic vein, and right pedicle (BCLC stage A). However, the tumor was unresectable because the ratio of the remnant liver volume was insufficient (the ratio of left external liver lobe volume to standard liver volume was 18.7%). The patient received PVE and lenvatinib 8 mg/day and pembrolizumab 200 mg every 3 weeks. Nine weeks after treatment initiation, the ratio of the residual to standard liver volume increased to 31.5%, and curative liver resection was performed. H&E staining of the surgically resected specimen showed a pCR. HCC, hepatocellular carcinoma; H&E, hematoxylin and eosin; MR, magnetic resonance; CT, computed tomography; BCLC, Barcelona Clinic Liver Cancer; PVE, portal vein embolism; pCR, pathological complete response; MHV, middle hepatic vein; RHV, righ hepatic vein; RPV, right portal vein.
Fig. 3.
Pretreatment MR or CT scan showed that the patients (except patients 4 and 10) had a unresectable HCC before systemic treatment and imaging scan before surgery. The major reason of unresectability were tumor invasion into major portal vein (patients 1 and 2), into the first branch of the portal vein (patients 6 and 9), into middle hepatic vein (patient 7), or into right atrium (patient 8; the intrahepatic nodule was not shown on this image) (red arrows); multiple intrahepatic lesions (yellow arrows) (patients 3 and 5) (only 1 nodule was shown on this image for patient 5). Before surgery, obvious tumor regression was observed in all the cases, except sustained stable disease were seen for patient 2, and partial response in the intrahepatic lesion but a new metastatic lesion in the right adrenal gland (green arrow) for patient 9. HCC, hepatocellular carcinoma; MR, magnetic resonance; CT, computed tomography.
Surgery and Perioperative Findings
The median interval between initiation of systemic therapy and surgery was 3.2 months (range: 2.4–8.3 months). For patients with BCLC stage C disease, only those with vascular invasion (7 out of 34 patients) could be converted to resectable disease, whereas none of those with extrahepatic metastasis (n = 20) could be converted (Table 1). All the patients underwent surgery were classified as Child-Pugh A before surgery. As shown in Table 3, 7 patients underwent a major liver resection (≥3 segments), with a mean intraoperative blood loss of 950 ± 746 mL. The median postoperative hospital stay was 14 days (range: 11–68 days). The prevalence of post-hepatectomy liver failure was 50.0%, with grade A failure experienced by 4 patients and grade C by 1 patient. Five patients experienced postoperative complications, including 1 patient with grade I (bile leakage), 3 with grade IIIa (subphrenic collection requiring additional puncture), and 1 with grade V, who died from immune-related adverse effects (patient 1).
Table 3.
Patient characteristics before and after surgery
| Patient No. | Days from systemic therapy to surgery | TKI withdrawal days before surgery | Anti-PD-1 antibody withdrawal days before surgery | Major resection | PHLF* | Postoperative complication* | Postoperative hospital stay, days | pCR |
|---|---|---|---|---|---|---|---|---|
| 1 | 73 | 7 | 19 | Y | y | V | 68 | y |
| 2 | 128 | 7 | 64 | Y | n | 0 | 11 | n |
| 3 | 185 | 9 | 24 | Y | n | IIIa | 14 | y |
| 4 | 73 | 8 | 39 | Y | y | 0 | 22 | y |
| 5 | 127 | 7 | 43 | N | y | 0 | 13 | n |
| 6 | 95 | 8 | 37 | Y | y | I | 26 | y |
| 7 | 99 | 7 | 34 | N | n | 0 | 11 | n |
| 8 | 79 | 7 | 36 | N | n | IIIa | 14 | y |
| 9 | 251 | 8 | 183* | N | y | 0 | 14 | n |
| 10 | 73 | 7 | 31 | Y | n | IIIa | 18 | y |
PHLF, post-hepatectomy liver failure; TKIs, tyrosine kinase inhibitors; pCR, pathological complete response.
Classified according to the International Study Group of Liver Surgery. † Clavien-Dindo criteria. ‡ Patient 9 had a G3 immune-related dermatitis and stopped sintilimab therapy.
Patient 1 died 2.4 months post-surgery after experiencing immune-related adverse effects that began from postoperative day 7 in the liver, skin, lung, and pancreas, demonstrated via liver and skin biopsies. Preoperative laboratory examination did not show any sign of liver inflammation. His liver function was classified as Child-Pugh A6 without ascites or hepatic encephalopathy, serum total bilirubin was 13.8 μmol/L, serum albumin was 33 g/L, international normalized ratio was 1.14, serum alanine transaminase was 9 U/L, aspartate aminotransferase was 19 U/L, γ-glutamyl transferase was 71 U/L, serum HBV-DNA was undetectable, the shockwave elastography of liver parenchyma was 21.0 kPa, and the ratio of future liver volume to standard liver volume was 44.6%. The last dosage of anti-PD-1 antibody (nivolumab) was given 19 days before surgery and last dose of TKI (lenvatinib) was given 7 days before surgery. Retrospective analysis of the surgical specimen revealed an active inflammation of the liver parenchyma. Thereafter, all the other patients underwent liver biopsies before surgery to exclude active inflammation in the liver parenchyma. We did not encounter any prominent liver inflammation in the following liver biopsy, and no immune-related adverse events were observed in the subsequent 9 patients following hepatectomy.
Six patients (60%) achieved a pCR in all surgical specimens. For patient 7, a pCR was found in the resected tumor thrombosis in the middle hepatic vein, but viable tumor cells were found in the intrahepatic lesion, indicating a downstaging from BCLC stage C to stage A.
Follow-Up
The cutoff date for the present analysis was November 3, 2020. One patient died from multisystemic immune-related adverse events without tumor recurrence (patient 1). After a median follow-up of 11.2 months (range: 7.8–15.9 months), the other 9 patients remained alive. Tumor recurrence was detected in 1 patient, who received locoregional therapy plus combination therapy with lenvatinib and anti-PD-1 antibodies and remained tumor-free at the last follow-up; the other 8 patients are tumor-free. All surviving patients received combination therapy and regular surveillance. The 12-month survival rate of the 10 patients after the initiation of combination therapy was 90.0% (standard error, 9.5%), and 12-month recurrence-free survival rate after surgery was 80.0% (standard error, 12.6%).
Discussion
The present study showed that conversion therapy allowed successful R0 resection in 15.9% (10/63) of the patients with initially unresectable HCC. The results suggest that combination therapy with an anti-angiogenic TKI and anti-PD-1 antibodies is a feasible conversion therapy for patients with unresectable HCC to achieve a successful resection with potential long-term recurrence-free survival.
Macrovascular invasion is not indicated for resection as recommended by BCLC guideline, while some Asian guidelines or consensus recommends surgical resection to patients with portal vein invasion [18, 32, 33], and R0 resection has been achieved in highly selected patients meeting these criteria. However, the median recurrence-free survival for these patients following upfront surgery is around 6 months, and the median OS is approximately 1 year [34]. One study found that in patients with HCC who received adjuvant apatinib therapy following surgery, the median recurrence-free survival was 7.6 months [35]. So, the outcomes of surgery with or without postoperative adjuvant therapy for patients with vascular invasion are unsatisfactory, especially in the era of agents with high anti-tumor efficacy, by which the median survival of HCC patients in advanced stage has been extended to over 20 months [17, 36, 37]. The treatment sequence (systemic therapy followed by liver resection, or liver resection followed by systemic therapy) needs to be updated, as suggested by the present study.
Although the value of resection in patients with objective response needs more study, the pCR rate of 9.5% (6/63) or 60% (6/10) in the patients who received resection is definitely very encouraging. In comparison, previous reports have shown that nivolumab or nivolumab plus ipilimumab in patients with early HCC yields a pCR of 24% [38]. These finding supports the efficacy of systemic treatment in HCC. However, it may raise a question on why we still need to remove the tumor. We believe that tumor must be completely removed whenever feasible because tiny cluster of viable tumor cells could be left and become a source of relapse, and it is almost impossible to guarantee pCR before resection. Experience from patients with colorectal cancer liver metastasis shows that a pCR is achieved by approximately two-thirds of patients with resected liver metastasis, and if the disappearing liver metastasis leaves unresected, more than half will experience recurrence [39]. Therefore, patients with tumor responses evaluated by imaging are likely to benefit from resection of the residual lesions to achieve a longer term of tumor-free survival.
The present study also demonstrated that major hepatectomy after the combination treatment is safe. The incidence of postoperative complications was similar to the previous reports [40, 41]. One patient died following hepatectomy, which was attributed to severe immune-related adverse effects (liver tissue necrosis) began on postoperative day 7, as demonstrated via liver biopsy. Subsequently, the patient experienced immune-related adverse effects on the skin as indicated by skin biopsies, lungs, pancreas and pituitary gland, and the onset of type 1 diabetes. The patient eventually died from multisystem failure on postoperative day 72. Following this case, pre-hepatectomy liver biopsies became mandatory to exclude active inflammation in the liver parenchyma in all the other patients. Further studies are warranted to fully examine the value of liver biopsies before surgery, particularly following downstaging with immune therapy.
Only a small proportion of patients with unresectable HCC are able to achieve downstaging and undergo resection following sorafenib [42] because the ORR associated with sorafenib treatment is <10% [43, 44]. Recent advances in systemic therapies for HCC have made conversion therapy possible [6]. For example, lenvatinib monotherapy is associated with an ORR of 18.8% [45], and results from early-phase clinical trials of combined anti-angiogenic therapies and anti-PD-L1/PD-1 antibodies have shown potent anti-tumor activity. In 2 phase Ib clinical trials, lenvatinib plus pembrolizumab or nivolumab yielded ORRs of 36.0 and 54.3%, respectively, with a PD rate of <10% [16, 17]. In addition, the combination of apatinib and camrelizumab yielded an ORR of 50% in 16 patients with HCC [21]. Most recently, a Phase III trial of the combination of bevacizumab (an anti-VEGFA antibody) and atezolizumab (an anti-PD-L1 antibody) reported an ORR of 27% [36]. Given the high ORRs and low PD rates associated with these combination therapies, patients with borderline resectable tumors may be ideal candidates for conversion therapy. Most patients will respond to combination therapy (tumor shrinkage) and few patients will lose the opportunity of surgery because of tumor progression. Notably, in the present analysis, 1 patient underwent PVE and achieved a significant increase in remnant liver volume while receiving combination therapy, suggesting that combination therapy did not significantly affect liver parenchymal proliferation in this case, and that combination therapy may be further investigated in selected cases.
This study had several limitations that should be discussed. First, the postoperative follow-up period was relatively short, and therefore the long-term outcomes are unknown. Second, all patients had an etiology of HBV infection in this study. The efficacy and safety of the combination therapy in patients with other etiological factors are unknown. Third, the combination of TKIs and anti-PD-1 antibodies used in this study were not unified. All anti-PD-1 antibodies were off-label therapies for HCC and cannot be reimbursed in China; therefore, patients' choice will be an important consideration (mostly the cost and updated information from clinical trials). So far, no evidence showed the effects of these anti-PD-1 antibodies were different.
In summary, we reported the outcomes of 10 patients with initially unresectable HCC who received successful conversion therapy with combined TKI/anti-PD-1 antibodies. The findings show that this conversion therapy strategy is feasible for HCC, and subsequent liver resection is effective and safe given careful preparation and patient assessment. Although the outcomes for patients who underwent resection seems better than for those who did not undergo resection, the exact role of liver resection in patients who achieve downstaging from systemic therapy requires further investigation in a prospective controlled study.
Statement of Ethics
The study protocol was complied with the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by Zhongshan Hospital Research Ethics Committee (Approval Number: B2020-177R). All patients provided written informed consent before receiving combined TKI/anti-PD-1 antibody treatment and before surgery.
Conflict of Interest Statement
H.C.S. has received speaker fees from Hengrui, Bayer, Eisai, and MSD. X.D.Z. has received speaker fees from Eisai and MSD. Dr. Jia Fan is an Editorial Board Member of Liver Cancer.
Funding Sources
This work was supported by the Leading Investigator Program of the Shanghai municipal government (17XD1401100), the National Key Basic Research Program (973 Program; 2015CB554005) from the Ministry of Science and Technology of China, and the National Natural Science Foundation of China (81672326 and 81871928, and 81871929).
Author Contributions
Conception and design: H.-C. Sun, J. Fan, J. Zhou, and Z.-Y. Tang; administrative support: H.-C. Sun, and J. Zhou; provision of study materials or patients: H.-C. Sun, X.-D. Zhu, C. Huang, Y.-H. Shen, Y. Ji, N.-L. Ge, X.-D. Qu, L.-L. Chen, W.-K. Shi, and C.-J. Tan; collection and assembly of data: X.-D. Zhu, C. Huang, Y.-H. Shen, L.-L. Chen, M.-L. Li, and J.-J. Zhu; data analysis and interpretation: H.-C. Sun and X.-D. Zhu; manuscript writing: H.-C. Sun, X.-D. Zhu, C. Huang, and Y.-H. Shen; final approval of manuscript: all authors.
Acknowledgement
We thank the patients and their families.
References
- 1.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394((10204)):1145–58. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387((10015)):251–72. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66((2)):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2019;66((1)):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 5.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35((9)):2155–66. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X-D, Sun H-C. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol. 2019;12((1)):110. doi: 10.1186/s13045-019-0794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang ZY, Uy YQ, Zhou XD, Ma ZC, Lu JZ, Lin ZY, et al. Cytoreduction and sequential resection for surgically verified unresectable hepatocellular carcinoma: evaluation with analysis of 72 patients. World J Surg. 1995;19((6)):784–9. doi: 10.1007/BF00299771. [DOI] [PubMed] [Google Scholar]
- 8.Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma: a strategy to increase resectability. Ann Surg Oncol. 2007;14((12)):3301–9. doi: 10.1245/s10434-007-9549-7. [DOI] [PubMed] [Google Scholar]
- 9.Lau WY. Cure is possible with salvage surgery following downstaging of hepatocellular carcinoma. Chin-Ger J Clin Oncol. 2004;3((3)):130–1. [Google Scholar]
- 10.Wang Z, Peng Y, Hu J, Wang X, Sun H, Sun J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg. 2020;271((3)):534–41. doi: 10.1097/SLA.0000000000002942. [DOI] [PubMed] [Google Scholar]
- 11.Tustumi F, Ernani L, Coelho FF, Bernardo WM, Junior SS, Kruger JAP, et al. Preoperative strategies to improve resectability for hepatocellular carcinoma: a systematic review and meta-analysis. HPB. 2018;20((12)):1109–18. doi: 10.1016/j.hpb.2018.06.1798. [DOI] [PubMed] [Google Scholar]
- 12.Bertacco A, Vitale A, Mescoli C, Cillo U. Sorafenib treatment has the potential to downstage advanced hepatocellular carcinoma before liver resection. Per Med. 2020;17((2)):83–7. doi: 10.2217/pme-2018-0114. [DOI] [PubMed] [Google Scholar]
- 13.Irtan S, Chopin-Laly X, Ronot M, Faivre S, Paradis V, Belghiti J. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int. 2011;31((5)):740–3. doi: 10.1111/j.1478-3231.2010.02441.x. [DOI] [PubMed] [Google Scholar]
- 14.Curtit E, Thiery-Vuillemin A, Nguyen T, Heyd B, Pivot X, Di Martino V, et al. Complete histologic response induced by sorafenib in advanced hepatocellular carcinoma: a case report. J Clin Oncol. 2011;29((12)):e330–2. doi: 10.1200/JCO.2010.32.6785. [DOI] [PubMed] [Google Scholar]
- 15.Zhu XD, Tang ZY, Sun HC. Targeting angiogenesis for liver cancer: past, present, and future. Genes Dis. 2020;7((3)):328–35. doi: 10.1016/j.gendis.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo M, Ikeda M, Motomura K, Okusaka T, Kato N, Dutcus CE, et al. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): study 117. J Clin Oncol. 2020;38((Suppl 4)):513. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38((26)):2960–70. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition) Liver Cancer. 2018;7((3)):235–60. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53((3)):1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC) J Clin Oncol. 2018;36((15_Suppl l)):4076. [Google Scholar]
- 21.Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25((2)):515–23. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Qin S, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as second-line therapy in Chinese patients with advanced hepatocellular carcinoma: a randomized, placebo-controlled, double-blind, phase III study. J Clin Oncol. 2020;38((15_Suppl l)):4507. [Google Scholar]
- 23.Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21((4)):571–80. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Bi X, Sun Y, Yu Y, Zhou J-G, Zeng H, et al. Preliminary results of sintilimab plus different dose of IBI305 (anti-VEGF monoclonal antibody) in patients with advanced hepatocellular carcinoma: a phase Ib study. J Clin Oncol. 2020;38((15_Suppl l)):3079. [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45((2)):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30((1)):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified Bolondi's subclassification (Kinki Criteria) Dig Dis. 2015;33((6)):751–8. doi: 10.1159/000439290. [DOI] [PubMed] [Google Scholar]
- 28.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149((5)):713–24. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240((2)):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22((42)):9279–87. doi: 10.3748/wjg.v22.i42.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29((3)):339–64. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 32.Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 Edition) Liver Cancer. 2020;9((1)):28–40. doi: 10.1159/000503685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3((3–4)):458–68. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter Controlled Study. J Clin Oncol. 2019;37((24)):2141–51. doi: 10.1200/JCO.18.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun H-C, Zhu X-D, Zhou J, Gao Q, Shi Y-H, Ding Z-B, et al. Effect of postoperative apatinib treatment after resection of hepatocellular carcinoma with portal vein invasion: a phase II study. J Clin Oncol. 2020;38((4_Suppl l)):514. [Google Scholar]
- 36.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 37.Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019;37((15_Suppl l)):4012. [Google Scholar]
- 38.Kaseb AO, Duda DG, Tran Cao HS, Abugabal YI, Vence LM, Rashid A, et al. Randomized, open-label, perioperative phase II study evaluating nivolumab alone or nivolumab plus ipilimumab in patients with resectable HCC. J Clin Oncol. 2020;38((4_Suppl l)):486. [Google Scholar]
- 39.Dhir M, Sasson AR. Surgical management of liver metastases from colorectal cancer. J Oncol Pract. 2016;12((1)):33–9. doi: 10.1200/JOP.2015.009407. [DOI] [PubMed] [Google Scholar]
- 40.Chen ZL, Zhang CW, Liang L, Wu H, Zhang WG, Zeng YY, et al. Major hepatectomy in elderly patients with large hepatocellular carcinoma: a multicenter retrospective observational study. Cancer Manag Res. 2020;12:5607–18. doi: 10.2147/CMAR.S258150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai J, Deva S, Lee JS, Lin CC, Yen CJ, Chao Y, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2020;8((1)):e000453. doi: 10.1136/jitc-2019-000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu K, McCaughan GW. How to select the appropriate “neoadjuvant therapy” for hepatocellular carcinoma. Expert Opin Pharmacother. 2018;19((11)):1167–70. doi: 10.1080/14656566.2018.1498843. [DOI] [PubMed] [Google Scholar]
- 43.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 44.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 45.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]