Cancer patients are commonly considered at higher risk to develop a worse course COVID-19, and to have increased mortality rates due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1,2]. Thus, screening programs for COVID-19 [3] and preventive vaccination programs against SARS-CoV-2 in frail subjects have identified cancer patients among the earliest populations to be included [4,5], with the necessity of high adherence rates [6,7]. However, the immunogenicity of SARS-CoV-2 vaccines in cancer patients is still uncertain, and several questions remain to be addressed for the best efficacy of ongoing vaccination campaigns, and to optimally face the prospective risk posed by emerging, more infectious, SARS-CoV-2 variants [8]. Among the many issues one could list are: (1) the effectiveness of vaccination on seroconversion of cancer patients compared to healthy individuals; (2) the appropriate timing of vaccination in the course of treatment; (3) the interference on the immunization against SARS-CoV-2 of ongoing anticancer therapies, including their possible differential effect(s); and (4) the immunogenicity of diverse SARS-CoV-2 vaccine types. Providing experimental evidence to elucidate these yet unanswered questions is a priority to eventually optimize the protection of cancer patients from SARS-CoV-2 infection.
To gain an initial understanding on the immunogenicity of SARS-CoV-2 messenger RNA (mRNA)-based vaccination in patients with solid malignancies on active therapy for their disease, we prospectively investigated levels of circulating anti-SARS-CoV-2 spike immunoglobulin G (IgG) antibodies (anti-spike IgG) in vaccinated cancer patients compared to healthy subjects. The potential interference of timing and type of anticancer therapies on anti-spike IgG titers at pre-defined timepoints was also evaluated.
Serum samples were collected from consecutive cancer patients (131 subjects) treated on an outpatient basis at our center with immune checkpoint(s) inhibitors (ICI) (70 subjects), chemotherapy (CT) (28 subjects), targeted therapy (TT) (23 subjects), or TT combined with ICI (10 subjects). Main tumor histotypes included skin cancer (57%), thoracic malignancies (22%), and glioblastoma (12%). Investigated subjects had no history of SARS-CoV-2 infection. As per institutional guidelines, patients and healthy subjects tested negative to nasopharyngeal swabs within 48 h prior to hospital admissions and every 10 d, respectively. Patients received a first dose of the mRNA-1273 (Moderna) vaccine (T0), followed by a second dose after 28 d (T1). Vaccine administration was planned and performed at least 7 d apart from the last/next due cycle of therapy. Healthy hospital personnel (42 subjects) received a first dose of BNT162b2 (Pfizer-BioNTech) vaccine (T0), and a second dose 21 d apart (T1). Sera from blood drawings for routine workup of patients were collected at T0 and T1, and after the second vaccination (T2) (median 18 d, range 12–35); sera from healthy controls were collected at T2 (median 14 d, range 13–29).
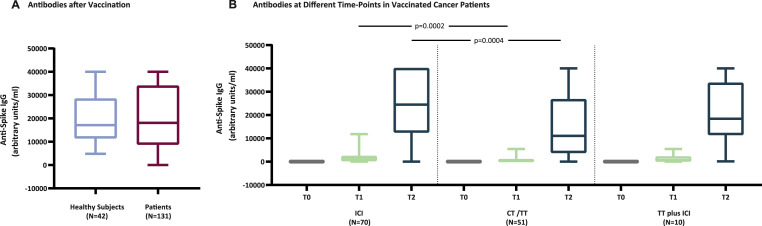
As a whole, cancer patients vaccinated with the mRNA-1273 vaccine while on active therapy for their underlying disease, immunized against SARS-CoV-2 alike healthy individuals. Median values of anti-spike IgG at T2 were 17,132 arbitrary units per milliliter (AU/ml) (95% confidence interval [CI] 14,222–25,353) and 18,064 AU/ml (95% CI 14,270–24,452), for healthy subjects and cancer patients, respectively (Fig. 1 A). Taken together, this positive finding is undoubtedly encouraging from a practical viewpoint; however, its teaching needs to be balanced and dissected against the type of anticancer therapy that patients receive. Indeed, median values of anti-spike IgG for patients treated with ICI, CT or TT (CT/TT), or TT plus ICI were 1020 AU/ml (95% CI 814–1524), 323 AU/ml (95% CI 113–532), and 605 AU/ml (95% CI 94–4762) at T1, and 24,457 (95% CI 18,064–32,652), 11,086 (95% CI 7231–17,168), and 18,447 (95% CI 9115–35,534) at T2, respectively (Fig. 1B). Noteworthy, median values of anti-spike IgG were significantly higher for patients receiving ICI compared to those receiving CT/TT at T1 (P = 0.0002) and T2 (P = 0.0004) (Fig. 1B). Additionally, 112 patients (85%) tested positive for anti-spike IgG at T1, while among the 19 patients (15%) who did not develop anti-spike IgG, 3 (16%), 15 (79%), and 1 (5%) patients were on therapy with ICI, CT, or TT plus ICI, respectively. Finally, 1 and 4 patients treated with ICI or CT remained negative for anti-spike IgG also at T2.
Fig. 1.
Anti-spike IgG response induced by mRNA vaccines in healthy subjects and cancer patients. Levels of circulating anti-spike IgG were assessed in cancer patients treated with ICI, CT/TT, or with TT plus ICI at baseline (T0), after the first (T1) and second (T2) dose of the mRNA-1273 (Moderna) vaccine, and in healthy subjects after the second dose (T2) of BNT162b2 (Pfizer-BioNTech) vaccine. Differences between titers of anti-spike IgG in patients and healthy subjects at T2 (A), and among patients treated with different anticancer therapies at T0, T1, and T2 (Panel B) are reported. In each box-and-whisker plot the horizontal line represents the median, the top and bottom of the box the interquartile range, and the whiskers the minimum and maximum values. Circulating levels of anti-spike IgG were determined using the Abbott SARS-CoV-2 IgG II Quant assay (Abbott Laboratories, Chicago, IL), a chemiluminescent microparticle immunoassay used as an aid in evaluating the immune status of individuals with quantitative measurement of IgG antibodies against the spike receptor-binding domain of SARS-CoV-2. This assay was performed on an Abbott Alinity (Abbott Diagnostics) according to the manufacturer’s instructions. A sample was considered positive when the result was >50.0 AU/ml. Values higher than 40,000 AU/ml were not further investigated and reported as 40,000, being the upper limit of the kit detection. All data were represented as median with two-sided 95% confidence intervals estimated using a normal approximation. Statistical significance for differences between cancer patients and healthy controls was carried out using nonparametric two-sided Mann–Whitney test. With regard to the analysis of changes in anti-spike IgG levels, timepoint comparisons according to treatment status were performed using the nonparametric Kruskal–Wallis test followed by Dunn’s multiple comparison test. The P values <0.05 were considered statistically significant. Statistical analyses were carried out by GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA).
As already evident even after the first dose of vaccine, patients on ICI therapy develop significantly higher titers of anti-spike IgG compared to patients treated with CT/TT, unlike when combined with TT (Fig. 1B). This improved efficacy of vaccination observed with ICI therapy, compared to CT/TT, is undoubtedly remarkable and of potential prospective usefulness in the daily practice. Although speculative at present, the broad immune-potentiating activity of ICI may likely contribute to the higher anti-spike IgG response observed in ICI treated patients. Thus, the next essential question to be answered will be whether maintained ICI therapy may contribute to sustain with time the very high titers of circulating anti-SARS-CoV-2 antibodies detected after the two ‘canonical’ doses of the mRNA-1273 vaccine. Studies along this line will be fundamental to fully unveil the efficacy of vaccination of cancer patients undergoing ICI therapy compared to those treated with CT/TT; the long-term immunity to SARS-CoV-2 observed in COVID-19 convalescent healthy subjects supports this need [9].
According to the comprehensive findings above, numerous practical questions need to be primarily addressed due to the fragility of cancer patients against SARS-CoV-2 infection, and to the need to ameliorate vaccination campaigns in the months ahead. Among these is whether the results observed with the mRNA-1273 vaccine represent a general feature, possibly shared by other mRNA vaccines and/or by non-mRNA SARS-CoV-2 vaccines. Also, the reciprocal interference between timing of treatment and vaccine administration needs to be further clarified at different timepoints. In fact, while planning to vaccinate cancer patients before therapy initiation may solve this issue, this strategy is not always feasible unless anticancer treatment is delayed; in addition, this approach would clearly exclude fully vaccinated cancer patients who may prospectively require additional doses of vaccine. Indeed, the necessity to re-vaccinate already seroconverted healthy subjects is rapidly emerging, due to the decline with time of anti-spike IgG titers in fully vaccinated individuals [8]. If additional vaccination(s) will be proved necessary also for cancer patients, the decision might eventually be: What patients should we re-boost, and when according to the therapy they receive?
Funding source
None.
Conflict of interest statement
AMDG has served as a consultant and/or advisor to Incyte, Pierre Fabre, Glaxo Smith Kline, Bristol-Myers Squibb, Merck Sharp Dohme, and Sanofi, and has received compensated educational activities from Bristol Myers Squibb, Merck Sharp Dohme, Pierre Fabre, and Sanofi.
MM has served as a consultant and/or advisor to Roche, Bristol-Myers Squibb, Merck Sharp Dohme, Incyte, AstraZeneca, Amgen, Pierre Fabre, Eli Lilly, Glaxo Smith Kline, Sciclone, Sanofi, Alfasigma, and Merck Serono, and owns shares in Epigen Therapeutics, Srl.
GG, CG, MFL, and MGC declare no conflicts of interest.
Acknowledgements
We thank Vincenzo D’Alonzo, M.D., Luana Calabrò, M.D., and Maresa Altomonte, M.D., Center for Immuno-Oncology, Medical Oncology and Immunotherapy, Department of Oncology, University Hospital of Siena, Siena, Italy, for their conceptual and clinical contribution to this work, and Diana Giannarelli, Ph.D., Istituto Nazionale Tumori Regina Elena, Rome, Italy, for supervising the statistical analyses of this study.
References
- 1.Sharafeldin N., Bates B., Song Q., et al. Outcomes of COVID-19 in patients with cancer: report from the National COVID Cohort Collaborative (N3C) J Clin Oncol. 2021;39(20):2232–2246. doi: 10.1200/JCO.21.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Giacomo A.M., Gambale E., Monterisi S., Valente M., Maio M. SARS-COV-2 infection in patients with cancer undergoing checkpoint blockade: clinical course and outcome. Eur J Cancer. 2020;133:1–3. doi: 10.1016/j.ejca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maio M., Hamid O., Larkin J., et al. Immune checkpoint inhibitors for cancer therapy in the COVID-19 era. Clin Cancer Res. 2020;26(16):4201–4205. doi: 10.1158/1078-0432.CCR-20-1657. [DOI] [PubMed] [Google Scholar]
- 4.Desai A., Gainor J.F., Hegde A., et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18(5):313–319. doi: 10.1038/s41571-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corti C., Crimini E., Tarantino P., et al. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Cancer. 2021 May;148:316–327. doi: 10.1016/j.ejca.2021.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Noia V., Renna D., Barberi V., et al. The first report on Covid-19 vaccine refusal by cancer patients in Italy: early data from a single-institute survey. Eur J Canc. 2021;153:260–264. doi: 10.1016/j.ejca.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curigliano Giuseppe, Alexander MM Eggermont. Adherence to COVID-19 vaccines in cancer patients: promote it and make it happen! Eur J Cancer. 2021;153:257–259. doi: 10.1016/j.ejca.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021 Jul 8 doi: 10.1038/s41586-021-03777-9. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Jung J.H., Rha M., Sa M., et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Commun. 2021;12(1):4043. doi: 10.1038/s41467-021-24377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]