Abstract
This systematic review aims to evaluate the evidence on the efficacy of mouth rinses on SARS-CoV-2 from in vitro studies. Five electronic databases were searched up to February 2021; no language or time restrictions were used. Two independent reviewers conducted both selection and data extraction processes. The toxicological data reliability assessment tool was used to evaluate the risk of bias. Starting from 239 articles, retrieved by the electronic search, only eight studies were included in our systematic review. Povidone Iodine (PVP-I) was effective in killing SARS-CoV-2, demonstrated higher virucidal activity than other commonly used active ingredients. Conflicting results were found about the effectiveness of Chlorhexidine (CHX) while hydrogen peroxide (H2O2) proved less effective than PVP-I. Other active ingredients, such as quaternary ammonium compounds and Ethanol (particularly when combined with essential oils), have also shown promising results in reducing viral load, with results comparable to PVP-I.
Keywords: Mouth rinse, SARS-CoV-2, Povidone-iodine, Chlorhexidine, Hydrogen peroxide
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a Betacoronavirus linked to the pneumonia outbreak firstly discovered in Wuhan, Hubei province, China, in late 2019 which triggered a pandemic and health crisis. It is mainly transmitted human to human through droplets (small particles >5 μm produced while coughing, sneezing or even speaking), contact with an infected subject or direct or indirect contact with contaminated surfaces [1,2]. Its aerosol transmission has been studied, too, and it occurs primarily in health care facilities through aerosol-generating procedures (AGPs) [2]. AGPs include extubation, intubation, tracheotomy, positive pressure ventilation (CPAP), bronchoscopy and cardiopulmonary resuscitation [3]. High risk is also associated with dental procedures, such as the use of ultrasonic devices, 3-in-1 air-water syringes and high-speed handpieces [4].
According to Xu et al., the primary host cell receptor for SARS-CoV-2 is the angiotensin converting enzyme II (ACE2), which binds with the viral spike protein; epithelial cells of the oral mucosa have a high expression of ACE2 [5]. The presence of a high viral load has been confirmed in the saliva of symptomatic patients with coronavirus disease (COVID-19) with a higher viral load in the first week after the onset of symptom, which then starts to decrease with time [6,7]. Moreover, even in the oropharynx of asymptomatic patients, high viral load has been identified, which emphasises the prominent role of the oral cavity in the transmission of the virus [8].
Therefore, dental professionals are at high risk of SARS-CoV-2 infection because of their proximity to patients and AGP [9]. Since the onset of the pandemic, the scientific community has been focusing on preventing transmission of SARS-CoV-2 infection by using personal protective equipment, limiting the practice of AGPs, and using the rubber dam. With the emerging evidence on the virucidal efficacy of mouth rinse molecules, pre-procedural mouth rinsing is being advocated by several regulatory organisations throughout the world, including the American Center for Disease Control and Prevention, to reduce SARS-CoV-2 transmission in a dental setting [4]. There are many different active ingredients or molecules already effectively used against bacteria or viruses and currently being studied for their potential virucidal effect on SARS-CoV-2. Several studies in vitro and a few in vivo have been conducted on the efficacy of various mouthwashes to reduce viral load, and thus the spread of the infection, before performing any intraoral procedures.
There have been several attempts to conduct systematic reviews on this topic. For instance, the Cochrane collaboration has commissioned three systematic reviews, but none of these could find any robust studies exploring the effectiveness of mouth rinses or nasal sprays on COVID-19 viral load [[10], [11], [12]]. There are several in vivo studies still ongoing. Our systematic review aims to evaluate the current literature on the efficacy of mouthwashes with different active ingredients on SARS-CoV-2 in vitro.
Methods
This review conforms to the preferred reporting items for systematic reviews (PRISMA) reporting guidelines [13].
The PICO question of this review was what is the virucidal activity (outcome) of different mouth rinse molecules (intervention and comparison) on the SARS-COV-2 virus (participants)? Any invitro study evaluating the efficacy of any mouth rinse on SARS-COV2 viral load or any other related outcome were considered for inclusion. All clinical investigations were excluded. Besides, reviews, opinion pieces, perspectives, conference papers, book chapter, abstracts were excluded. We also reviewed the cross-references of the included articles. Any study evaluating the effectiveness of a mouth rinse molecule or new formulation in the form of a mouth rinse on SARS-COV-2 virus from any source was considered for inclusion.
Five databases were searched on 9th February 2021 from inception, which included Medline via Pubmed, Cinahl, Embase, Cochrane and Web of science. The search strategy involved two strings of keywords combined by the Boolean operator ‘AND’. Search strategy used in Pubmed was, “Mouth wash OR mouthwash OR mouth rinse OR mouth rinse OR oral rinse OR rinse” AND “Betacoronavirus OR SARS CoV 2 OR SARSCoV2 OR SARS-CoV2 OR Coronavirus Infections OR COVID-19 Virus OR COVID-19 OR Coronavirus OR Severe Acute Respiratory Syndrome Coronavirus 2 OR 2019nCoV”. A mix of medical subject headings and free keywords were used. No language or time limiters were used. The search strategy was implemented and tested by two reviewers independently (JT and SKT); no discrepancy was found between the reviewers.
All the retrieved titles and abstracts were exported to a referencing software program (EndNote X9, Philadelphia, Clarivate). Any duplicates found were deleted. Two reviewers (JT and SKT) screened all the titles and abstracts independently; those that seemed suitable were considered for inclusion in the full-text review. When the information provided in the abstract and title were inadequate to determine eligibility, articles were included in the full-text review. Any discrepancy between the reviewers (SKT and JT) was resolved by discussion. A pre-piloted data extraction chart was used to extract the data from the included studies, which was done independently by two reviewers (JT and VR). All the extracted data was checked for accuracy by a third reviewer (SKT). The pre-piloted charts had data fields on Author, year, country of origin or study setting, source of SARS-COV-2 virus, active ingredient or mouth rinse molecule, formulation, concentration, contact time, comparison groups, outcomes assessed, method of evaluating the outcome, findings and overall conclusions.
A quantitative analysis was not feasible due to the heterogeneity in the molecules tested and the contact times used. Therefore, the included studies' background characteristics, the molecules they tested, and their outcomes are reported qualitatively. Findings have been organised according to the mouth rinse molecule tested.
The Risk of Bias Assessment was done using the Toxicological data reliability assessment tool (TOXRTOOL) [14]. For in vitro studies, it uses a set of 18 questions marking 1 or 0 against each criterion and simple arithmetic summation of all values to categorise as 1, 2, or 3. If the total score is ≥15, category one is assigned. For scores >11, category 2 is assigned, and for all scores <11, category 3 is assigned. Categories 1 and 2 represent that the data is reliable without and with restriction, respectively, while category 3 indicates that the data reported from the study is not reliable.
Results
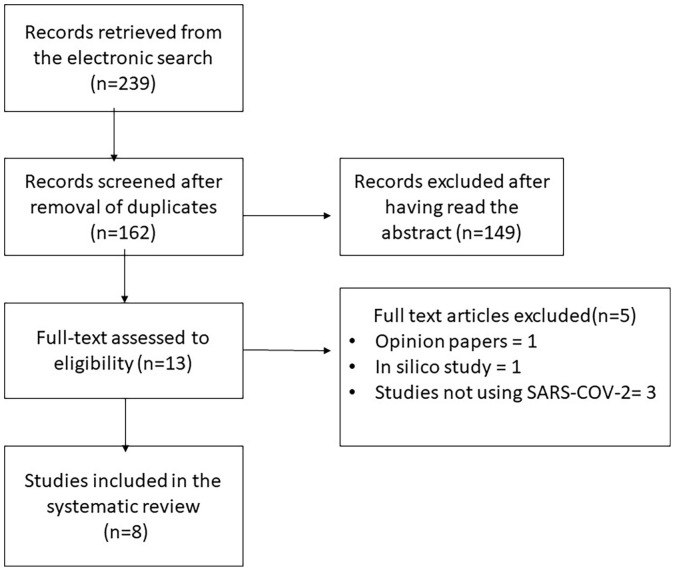
A total of 239 titles were retrieved from the electronic search; 162 remained after removal of duplicates and were screened by titles and abstracts (Fig. 1 ). Thirteen articles were found relevant for a full-text review, with eight articles being included in this review. Five articles were excluded, three assessed the effectiveness of mouth rinses on viruses other than SARS-COV-2 [[15], [16], [17]], one each was an in-silico study [18] and an opinion piece [19].
Fig. 1.
Flowchart demonstrating the number of studies, included and excluded with reasons.
General characteristics of the included studies
General characteristics of the included studies are presented in Table 1 . Eight in vitro studies were included in this systematic review. Four studies were conducted in the USA [[20], [21], [22], [23]] with one each in Malaysia [24], Singapore [25], Germany [26], and India [27]. In all studies, the outcome assessed was the virucidal activity of the active ingredient against SARS CoV-2. The typical active ingredient tested in all studies was Povidone Iodine (PVP-I). While five studies [[20], [21], [22],24,25] assessed Povidone Iodine (PVP-I) at different concentrations and formulations, the other three [23,26,27] assessed PVP along with other active ingredients, such as chlorhexidine, hydrogen peroxide, and Ethanol. The source of virus in the studies has varied according to the country of origin, while those from the USA have used USA-WA1/2020 strain, the study from Malaysia used SARS-COV-2/MY/UM/6-3;TIDREC [24]. Anderson et al. [25] from Singapore used hCoV-19/Singapore/2/2020 while Meister et al. [26] used BetaCoV/Germany/Ulm/01/2020 and BetaCoV/Germany/Ulm/02/2020. Throat swabs from an infected patient were also used by Jain et.al [27] and Meisteret.al [26]. The contact time in the studies ranged from as low as 15 seconds to 30 minutes. The most commonly tested contact time was 30 s, used in 6 studies [20,21,[24], [25], [26], [27]]. The lowest contact time tested was 15 s, used by 3 studies [20,21,24] and the longest contact time of 30 mins was tested by Xu et al. [23].
Table 1.
Characteristics of the included articles.
| Study | Year | Country | Source of Virus | Active Ingredient | Formulation | Concentration | Control | Method of assessment | Contact Time | Outcome assessed | Findings | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hassandarvish et al. 2020 [24] | 2020 | Malaysia | Infecting confluent monolayers of Vero E6 cells in DMEM medium (SARS-COV-2/MY/UM/6-3; TIDREC) | Povidone Iodine | Gargle/Mouthwash | 1% and 0.5% | Distilled water | Virus time-kill assay according to EN14476 method | 15,30,60s | Virucidal activity against SARS COV-2 | 1% PVP achieved >5 log 10 reduction (99.9% kill rate) in viral titres at 15,30,60s under both clean and dirty conditions whereas 0.5% PVP demonstrated >4 log10 at 15s and >5 log 10 at 30 and 60s | The activity of PVP-I is unlikely to be reduced in the presence of interfering substances such as biological tissue, and increasing the contact time would result in similar virucidal activity at undiluted and 50% dilution. |
| Bidra et al. 2020 [21] | 2020 | USA | Stock prepared by growing in Vero76 cells of USA-WA1/2020 strain | Povidone Iodine | Oral Antiseptic Rinse | 0.5%,1%, 1.5% | Ethanol (70%) and water | Standard endpoint dilution assay | 15,30s | Virucidal activity against SARS COV-2 | PVP I achieved >3 log 10 reduction in viral titres at 15 and 30s, whereas Ethanol achieved a 2.17 log 10 reduction at 15s and 3.33 log 10 reduction at 30s | The lowest concentration of 0 .5% PVP I has virucidal effects at minimum 15s contact |
| Anderson et al. 2020 [25] | 2020 | Singapore | Infecting confluent monolayers of Vero E6 cells in DMEM medium by hCoV-19/Singapore/2/2020 | Povidone Iodine | Antiseptic Solution | 10% | Not defined | Virus time-kill assay according to EN14476 method | 30s | Virucidal activity against SARS COV-2 | All four tested PVP-I products achieved >4 log 10 reduction in viral titres with a contact time of 30s | PVP-I products can be effectively used for infection control to augment health and hygiene measures. |
| Throat spray | 0.45% | |||||||||||
| Skin Cleanser | 7.5% | |||||||||||
| Gargle/Mouthwash | 1% | |||||||||||
| Pelletier et al. 2020 [22] | 2020 | USA | Stock prepared by growing in Vero76 cells of USA-WA1/2020 strain | Povidone Iodine | Nasal Antiseptic | 5%,2.5%,1% | Ethanol (70%), water | Standard endpoint dilution assay | 60s | Virucidal activity against SARS COV-2 | Nasal and oral rinse antiseptics at all concentrations achieved >4 log 10 reduction at 60s. It was similar for Ethanol also at 60s | PVP-I nasal and oral preparations can be effectively used in nasal passages, nasopharynx and oral cavities against SARS-CoV-2 |
| Oral Rinse | 3%,1.5%,1% | |||||||||||
| Bidra et al. 2020 [20] | 2020 | USA | Stock prepared by growing in Vero76 cells of USA-WA1/2020 strain | Povidone Iodine | Oral Antiseptic rinse | 0.5%,1.25%, 1.5% | Hydrogen Peroxide (1.5% and 3%), Ethanol (70%), water | Standard endpoint dilution assay | 15,30s | Virucidal activity against SARS COV-2 | PVP I achieved >4 log 10 reduction in viral titres at 15s and >3 log 10 at 30 seconds, whereas hydrogen peroxide achieved not more than 1.8 log10 reduction at 15 and 30s. Log reductions similar to PVP I were observed with Ethanol. | PVP I completely inactivated SARS CO V-2 at minimal concentration and contact times; however, hydrogen peroxide had minimal viricidal effect for as long as 30s. |
| Meister et al. 2020 [26] | 2020 | Germany | Strain 1- VeroE6 cells inoculated with throat swabs positive for SARS CoV-2) | Hydrogen Peroxide | Oral rinse | not defined | Medium control | Standard endpoint dilution assay | 30s | Virucidal activity against SARS COV-2 | Log reduction factor of 0.78,0.61 and 0.33 for strain 1, 2 and 3 respectively | Benzalkonium chloride, Povidone-iodine and Ethanol can significantly reduce viral infectivity to undetectable levels. |
| Strain 2 - Vero E6 cells inoculated with BetaCoV/Germany/Ulm/01/2020 | Chlorhexidine | Oral rinse | not defined | Log reduction factor of 1,.78 and 1.17 for strain 1, 2 and 3, respectively | ||||||||
| Strain 3 - VeroE6 cells inoculated with BetaCoV/Germany/Ulm/02/2020 | Dequalinium chloride, benzalkonium chloride | Oral rinse | not defined | Log reduction factor of >3.11,>2.78 and >2.61 for strain 1, 2 and 3, respectively | ||||||||
| Chlorhexidine | Oral rinse | 2% | Log reduction factor of 0.50, 0.56 and 0.50 for strain 1, 2 and 3 respectively | |||||||||
| Polyvidone iodine | Oral rinse | 1% | Log reduction factor of >3.11, >2.78 and >2.61 for strain 1,2 and 3 respectively | |||||||||
| Ethanol + essential oils (Listerine) | Oral rinse | not defined | Log reduction factor of >3.11, >2.78 and >2.61 for strain 1,2 and 3 respectively | |||||||||
| Octenidinedihydrochloride | Oral rinse | not defined | Log reduction factor of 1.11,.78 and .61 for strain 1, 2 and 3, respectively | |||||||||
| Polyaminopropylbiguanide | Oral rinse | not defined | Log reduction factor of.61,>1.78 and >1.61 for strain 1, 2 and, respectively | |||||||||
| Jain et al. 2021 [27] | 2021 | India | Stock prepared by growing in Vero E6 and SARS CoV-2 isolated from a patient | Chlorhexidine Di-gluconate | Mouthwash | .2%,.12% | Not Defined | DiAGSure nCOV-19 Detection Assay kit | 30s and 60s | Virucidal activity against SARS CoV-2 | Viral infectivity was reduced by 99.9% by .12% solution and >99.9% by .2% solution at both 30 and 60s | Chlorhexidine and povidone-iodine are effective against SARS CoV2. Chlorhexidine digluconate in 0.2% concentration inactivated more than 99.9% of SARS CoV 2 virus, in minimal contact time of 30s, and was considered as more efficacious than povidone-iodine 1% utilised for 30 and 60s |
| Povidone Iodine | Mouthwash | 1% | Viral infectivity was reduced by 99.8% at 30s and >99.9% at 60s | |||||||||
| Xu et al. 2020 [23] * | 2020 | USA | Stock prepared by growing in Vero E6 and SARS CoV-2 of USA-WA1/2020 strain | Hydrogen peroxide(Colgate Peroxyl) | Mouthwash | 1.5% | Medium control | 30 mins | Virucidal activity against SARS CoV-2 | Viral infectivity reduced by >99.99% | All mouth rinses have virucidal effects however cytotoxic effects of mouth rinses should be considered in assessing the antiviral activities. | |
| Povidone Iodine | Mouthwash | 10% solution (1% available iodine) | Viral infectivity reduced by >99.99% | |||||||||
| Chlorhexidine (Chlorhexidine gluconate) | Mouthwash | 0.12% | Viral infectivity reduced by >99.99% | |||||||||
| Ethanol, thymol, methyl salicylate,menthol,eucalyptol(Listerine) | Mouthwash | 20–30% Ethanol | Viral infectivity reduced by >99.99% | |||||||||
| Thymol 0.064% | ||||||||||||
| Methyl salicylate 0.06% | ||||||||||||
| Menthol (Racementhol) 0.042% | ||||||||||||
| Eucalyptol 0.092% |
Study is a pre-print published without peer-review. The peer-reviewed article with similar results were published subsequently, the citation of the published article is “Xu C, Wang A, Hoskin ER, Cugini C, Markowitz K, Chang TL, Fine DH. Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity in vitro. Pathogens. 2021 Mar 1;10(3):272. doi: 10.3390/pathogens10030272”. Peer-reviewed article couldn’t be included as it was published post the search date.
Povidone iodine-(PVP-I)
All the included studies tested the virucidal efficacy of PVP-I with other molecules or compared among differing PVP-I concentrations. Among four studies that tested the efficacy of PVP-I at varying concentrations, the virucidal activity remained the same irrespective of the concentration and formulation [[20], [21], [22],25]. One study observed that the log reduction factor differed based on the viral strains, with >3.11, >2.78 and >2.61 being reported for strain 1, 2 and 3, respectively [26]. Among the reviewed studies, a kill rate of >5 log 10 was reported at 15 s (1% PVP) and 30 s (0.5% PVP) by one study [24] >4 log 10 at 15 [20,24] and 60 s [22,27] was observed by two studies, while Anderson et al., also reported a kill rate of >4 log 10 at 30 s [25]. Three studies observed a kill rate of >3log 10 even at short contact times of 15 and 30 s [20,21,27]. Virucidal activity of >99.9% was found by one study that tested a contact time of 30 mins [23].
Hydrogen peroxide (H2O2)
Three studies assessed the virucidal activity of H2O2 [20,23,26]. A concentration of 1.5% was used in two studies. Meister et al. [26] reported differing levels of reductions with the three different strains, a log reduction of 0.78, 0.61, 0.31 for strain 1, 2 and 3, respectively, at 30 s, which was lower than other mouth rinse molecules (PVP-I, CHX, Ethanol + essential oils and Dequalinium chloride + benzalkonium chloride). Xu et al. reported a kill rate of >99.9% after 30 mins of contact time [23]. One study found Hydrogen peroxide to be more effective than PVP-I [20].
Chlorhexidine (CHX)
The CHX mouthwash was assessed by three studies [23,26,27]. One study assessed the effecticacy of CHX concentration at 0.2% and 0.12% [27], while another tested 0.12% concentration in comparison to other actives [23]. On the other hand, the third study tested two different concentrations but did not report the concentration of one of the products used in their study [26]. Further, all the three virus strains (1, 2 and 3) tested by Meister et al. had titres <2 log at both CHX concentrations after an exposure of 30 s, which was lower than that was observed for PVP-I, Ethanol + essential oils and Dequalinium chloride + benzalkonium chloride [26]. Jain et al. found that the virucidal activity of CHX remained the same at 30 and 60 s contact times, but it was slightly higher when used at a concentration of 0.2% (>99.9%) than at 0.12% (99.9%), they found CHX to be more effective than PVP-I [27]. Xu et al. also demonstrated >99.9% virucidal activity like other tested products, however, the contact time was 30 mins [23].
Ethanol
A 70% solution of Ethanol was used as positive control by three studies that had PVP-I as their primary test product, and they found Ethanol to be as effective as PVP-I [[20], [21], [22]].
Other compounds
Besides the above described active ingredients, essential oils were assessed in two studies by testing commercially available mouth rinses (i.e. Listerine, Listerine ultra), which proved to be as effective as PVP-I in reducing viral titre [23,26,]. A mouth rinse with Dequalinium chloride/benzalkonium chloride, two quaternary ammonium compounds, achieved a log reduction value of ≥3.11, ≥2.78 and ≥2.61 on the three different strains, thus mirroring the results of PVP-I., as reported by Meister et al. [26]. Studies also tested compounds such as Sodium Bicarbonate, Sodium chloride, Octenidine dihydrochloride, but in general, they had inferior virucidal activity compared to PVP-I.
Risk of Bias
In this review, six studies were found to have an overall score of ≥15 (category 1) demonstrating that data from these studies is reliable without restrictions. Two studies had a score of 14 and belonged to category 2. None of the studies had unreliable data (category 3). The scoring for all studies is shown in Table 2 .
Table 2.
Assessment of the risk of bias of the included studies.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hassandarvish et al. 2020 [24] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 15 |
| Bidra et al. 2020 [21] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 16 |
| Anderson et al. 2020 [25] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Pelletier et al. 2020 [22] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
| Bidra et al. 2020 [20] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
| Meister et al. 2020 [26] | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Jain et al. 2021 [27] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 14 |
| Xu et al. 2020 [23] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 14 |
Discussion
This systematic review attempted to provide a synthesised evidence on the efficacy of different mouth rinse molecules on the SARS-COV-2 virus and found promising results with PVP-I demonstrating better efficacy than other tested mouth rinses. The results indicate that pre-procedural mouth rinses could be a significant measure to prevent viral spread in the dental office.
Past literature indicates that PVP-I, a bactericidal and virucidal agent, is effective against SARS-CoV and MERS-CoV (Middle East Respiratory Syndrome), belonging to the Betacoronavirus genus and causative agents of the past pandemics, influenza A virus as well. PVP-I is thought to affect the virus's nucleic acid structures and its surface proteins, inhibiting viral binding to cells [16,28]. All the evaluated in vitro studies considered PVP-I, highlighting its reduction potential in inactivating SARS-CoV-2 even at 15 s contact and at low concentrations. However, cytotoxic effects of PVP-I must be taken into consideration since it is toxic for the oral and nasal mucosa in a concentration higher than 2.5% and 5%, respectively [21,29]. Even though commercial formulations do not reach those concentrations, cytotoxicity is an important parameter to evaluate that can influence the mouth rinse's effectiveness and must be taken into account [23]. Moreover, PVP-I mouth rinse cannot be used in pregnant women, subjects allergic to iodine, and patients with underlying thyroid disease or undergoing radioactive iodine therapy [21].
SARS-CoV-2, as other enveloped viruses (i.e. herpesviruses, other coronaviruses, paramyxoviruses and orthomyxoviruses), is known to be highly sensitive to a high concentration of Ethanol (60%–70%) used in surface decontamination since it interferes with the lipid envelope and causes the virus inactivation [30]. Commercial mouth rinses have a lower concentration of Ethanol. Still, studies proved them to be effective against different enveloped viruses (other than SARS-CoV-2) even at lower concentration (21%–27%) when combined with essential oils [31,32]. Ethanol was assessed in five examined studies; three used it as a positive control in high concentration (70%), while the remaining studies used Ethanol in a formulation with essential oils. Notably, this last combination proved to be effective, similarly to PVP-I, in reducing SARS-CoV-s viral titre and with less cytotoxicity [23,26]. Other actives, such as quaternary ammonium compounds like dequalinium chloride and benzalkonium chloride, were also assessed in the evaluated studies and were able to reduce SARS-COV-2 viral titre to undetectable levels but the complex mechanism behind viral inactivation is still not clear [23,26].
Another common molecule under evaluation is H2O2, with its known ability to inactivate influenza A and B viruses, adenoviruses and rhinoviruses, causing nucleic acid damages and increasing cell membrane permeability [7]. Despite these previous findings, H2O2 did not demonstrate great capacity in reducing viral titre [20,26]. Moreover, H2O2 proved to be more cytotoxic than PVP-I, even if diluted [23].
CHX is the most widely used active in mouthwashes for its bactericidal and bacteriostatic effects, thanks to its positive charge that allows it to interact with the microbial surface, which is negatively charged. In particular, it is effective against an enveloped viruses, such as Influenza A, herpesvirus 1, and cytomegalovirus [7,30]. The results from studies that tested CHX were mixed. While two studies found CHX to have better efficacy than PVP-I or other comparable actives, one study found it to perform poorly than other actives (PVP-I, Ethanol + essential oils and Dequalinium chloride + benzalkonium chloride) [23,26,27].
Despite most studies demonstrating a low risk of bias, this systematic review's results are limited because several methodological aspects and comparison groups varied between the studies. The findings support the scientific rationale for proposing pre-procedural mouthrinses based on the evidence gathered from in vitro studies. We anticipate that randomised controlled trials with a larger sample size will support or refute the findings of this review. Several clinical trials have already been registered, while a few articles with preliminary results have already been published [[33], [34], [35]].
In conclusion, PVP-I was the most widely tested active ingredient. Evidence from in vitro studies demonstrates that PVP-I has higher virucidal activity than other commonly used molecules. Although very few studies tested the effectiveness of dequalinium chloride + benzalkonium chloride and Ethanol (alone or with essential oils), they effectively reduced viral load comparable to PVP-I. Findings related to CHX were contrasting between the studies, while H2O2 was found to have inferior properties compared to other widely used mouth rinse molecules. However, caution is required as this finding comes from only one study. Clinical studies will be essential to confirm the effectiveness of mouth rinse molecules discussed in this review.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgements
Dr. Santosh Kumar Tadakamadla is supported by a National Health and Medical Research Council Early Career Fellowship, Australia.
References
- 1.Bajaj N., Granwehr B.P., Hanna E.Y., Chambers M.S. Salivary detection of SARS-CoV-2 (COVID-19) and implications for oral health-care providers. Head Neck. 2020;42(7):1543–1547. doi: 10.1002/hed.26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M.Y., Zhao R., Gao L.J., Wang D.P., Cao J.M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. Infection, prevention and control during health care when coronavirus disease (COVID-19) is suspected or confirmed. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Interim Infection Prevention and Control Guidance for Dental Settings During the Coronavirus Disease 2019 (COVID-19) Pandemic 2020 [Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html].
- 5.Xu H., Zhong L., Deng J., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. Published 2020 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergara-Buenaventura A., Catro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg. 2020;58(8):924–927. doi: 10.1016/j.bjoms.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Eng J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farronato M., Boccalari E., Del Rosso E., Lanteri V., Mulder R., Maspero C. A Scoping Review of Respirator Literature and a Survey among Dental Professionals. Int J Environ Res Public Health. 2020;17(16):5968. doi: 10.3390/ijerph17165968. Published 2020 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton M.J., Clarkson J.E., Goulao B., Glenny A.M., McBain A.J., Schilder A.G., et al. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database Syst Rev. 2020;9:Cd013626. doi: 10.1002/14651858.CD013626.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton M.J., Clarkson J.E., Goulao B., Glenny A.M., McBain A.J., Schilder A.G., Webster K.E., Worthington H.V. Antimicrobial mouthwashes (gargling) and nasal sprays to protect healthcare workers when undertaking aerosol-generating procedures (AGPs) on patients without suspected or confirmed COVID-19 infection. Cochrane Database Syst Rev. 1858;9(9):D013628. doi: 10.1002/14651858.CD013628.pub2. Sep 16 PMID: 32936947; PMCID: PMC8188293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton M.J., Clarkson J.E., Goulao B., Glenny A.M., McBain A.J., Schilder A.G., Webster K.E., Worthington H.V. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database Syst Rev. 2020;9(9):D013627. doi: 10.1002/14651858.CD013627.pub2. PMID: 32936948; PMCID: PMC8187985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider K., Schwarz M., Burkholder I., Kopp-Schneider A., Edler L., Kinsner-Ovaskainen A., et al. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol Lett. 2009;189(2):138–144. doi: 10.1016/j.toxlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Eggers M., Eickmann M., Zorn J. Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA) Infect Dis Ther. 2015;4(4):491–501. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggers M., Koburger-Janssen T., Eickmann M., Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther. 2018;7(2):249–259. doi: 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyers C., Robison R., Milici J., Alam S., Quillen D., Goldenberg D., et al. Lowering the transmission and spread of human coronavirus. J Med Virol. 2021;93(3):1605–1612. doi: 10.1002/jmv.26514. [DOI] [PubMed] [Google Scholar]
- 18.Haridas M., Sasidhar V., Nath P., Abhithaj J., Sabu A., Rammanohar P. Compounds of Citrus medica and Zingiber officinale for COVID-19 inhibition: in silico evidence for cues from Ayurveda. Future J Pharm Sci. 2021;7(1):13. doi: 10.1186/s43094-020-00171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravo-Nguyen R. Reduce aerosol risk with preprocedural mouth rinse. Dimens Dent Hyg. 2020;18(10):16–18. [Google Scholar]
- 20.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J Prosthodont. 2020;29(7):599–603. doi: 10.1111/jopr.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J Prosthodont. 2020;29(6):529–533. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelletier J.S., Tessema B., Frank S., Westover J.B., Brown S.M., Capriotti J.A. Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) Ear Nose Throat J. 2020 doi: 10.1177/0145561320957237. [DOI] [PubMed] [Google Scholar]
- 23.Xu C., Wang A., Hoskin E.R., Cugini C., Markowitz K., Chang T.L., Fine D.H. Differential effects of antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro. bioRxiv [Preprint] 2020 doi: 10.3390/pathogens10030272. Dec 1:2020.12.01.405662. Update in: Pathogens. 2021 Mar 01;10(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassandarvish P., Tiong V., Mohamed N.A., Arumugam H., Ananthanarayanan A., Qasuri M., et al. In vitro virucidal activity of povidone iodine gargle and mouthwash against SARS-CoV-2: implications for dental practice. Br Dent J. 2020:1–4. doi: 10.1038/s41415-020-2402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson D.E., Sivalingam V., Kang A.E.Z., Ananthanarayanan A., Arumugam H., Jenkins T.M., et al. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect Dis Ther. 2020;9(3):669–675. doi: 10.1007/s40121-020-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222(8):1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain A., Grover V., Singh C., Sharma A., Das D., Singh P., et al. Chlorhexidine: an effective anticovid mouth rinse. J Indian Soc Periodontol. 2021;25(1):86–88. doi: 10.4103/jisp.jisp_824_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sriwilaijaroen N., Wilairat P., Hiramatsu H., Takahashi T., Suzuki T., Ito M., et al. Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities. Virol J. 2009;6:124. doi: 10.1186/1743-422X-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramezanpour M., Smith J.L.P., Psaltis A.J., Wormald P.J., Vreugde S. In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells. Int Forum Allergy Rhinol. 2020;10(10):1141–1148. doi: 10.1002/alr.22575. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell V.B., Thomas D., Stanton R., Maillard J.Y., Murphy R.C., Jones S.A., Humphreys I., Wakelam M.J.O., Fegan C., Wise M.P., Bosch A., Sattar S.A. Potential Role of Oral Rinses Targeting the Viral Lipid Envelope in SARS-CoV-2 Infection. Function (Oxf) 2020;1(1):zqaa002. doi: 10.1093/function/zqaa002. Epub 2020 Jun 5. PMID: 33215159; PMCID: PMC7239187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennison D.K., Meredith G.M., Shillitoe E.J., Caffesse R.G. The antiviral spectrum of Listerine antiseptic. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(4):442–448. doi: 10.1016/s1079-2104(05)80124-6. [DOI] [PubMed] [Google Scholar]
- 32.Meiller T.F., Silva A., Ferreira S.M., Jabra-Rizk M.A., Kelley J.I., DePaola L.G. Efficacy of Listerine Antiseptic in reducing viral contamination of saliva. J Clin Periodontol. 2005;32(4):341–346. doi: 10.1111/j.1600-051X.2005.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottsauner M.J., Michaelides I., Schmidt B., Scholz K.J., Buchalla W., Widbiller M., et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin Oral Investig. 2020;24(10):3707–3713. doi: 10.1007/s00784-020-03549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez Lamas L., Diz Dios P., Pérez Rodríguez M.T., Del Campo Pérez V., Cabrera Alvargonzalez J.J., López Domínguez A.M., et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. 2020 doi: 10.1111/odi.13526. https://onlinelibrary.wiley.com/doi/full/10.1111/odi.13526 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seneviratne C.J., Balan P., Ko K.K.K., Udawatte N.S., Lai D., Ng D.H.L., et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49(2):305–311. doi: 10.1007/s15010-020-01563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]