Abstract
Route of vaccine administration plays a role in extent and quality of immunogenicity. 790 military personnel accidentally received the first of two doses of Pfizer/BioNTech mRNA anti Covid-19 vaccine using a needle intended for subcutaneous administration. A serological blood test (on day 21, prior to the second intramuscular dose) was performed, analyzing whether immunological response was elicited. 98.2% demonstrated seroconversion. IgG titers were negatively correlated with age and did not correlate with BMI. Our results could help reassure providers confronted with a similar mistake and may even imply a possibly new and effective administration route.
Keywords: COVID 19, BNT162b2, mRNA vaccine, Seroconversion, SARS-CoV-2
1. Introduction
SARS-CoV-2 virus, first reported in December 2019, has rapidly spread worldwide, and declared a pandemic by the World Health Organization, with tremendous medical, social and economic consequences. BNT162b2 anti Covid-19 mRNA vaccine (Pfizer, New York, United States (US) and BioNTech, Mainz, Germany) [1] was authorized for emergency use by the Food and Drugs administration [2]. When administered intramuscularly, the vaccine elicits an IgG antibody response to SARS-CoV-2 spike protein. It had been shown, that the route of vaccine administration plays an important role in the extent and quality of immunogenicity, independent of the administered dose [3], [4].
On December 28th 2020, the Israeli Defense Forces Medical Corp (IDFMC) launched a vaccination program in 22 bases across Israel, using the BNT165b2 vaccine. Our goal was to rapidly vaccinate all medical crew and combatant soldiers. Needles and syringes were supplied with the vials. Medical personnel were instructed to use a 23-25gauge needles for vaccine administration.
On January 6th and 7th, 790 military personnel accidentally received the first of two doses using a 27gauge needle, intended for subcutaneous administration. The mistake was noted as medical officers from a different IDFMC unit reviewed a learning video from the vaccination site, and noticed the use of a shorter needle. Instructions were sent immediately to the unit’s physician and a report filed to the Israeli Ministry of Health (IMoH). Though studies in animal models suggest subcutaneous administration of vaccines elicit an effective immune response [5], [6], such information is not available specifically for BNT162b2. In coordination with the IMOH, serological blood sampling was performed prior to the time of the second vaccine dose administration (21 days after the first vaccination) to assess whether an appropriate immune response was elicited.
2. Methods
Presence of IgG antibodies was assessed using Abbott SARS-Cov-2 IgG II Quant on the ARCHITECT i System [7], used for the qualitative and quantitative determination of IgG antibodies to SARS-Cov-2 (spike receptor binding domain) in human serum and plasma. Seroconversion was defined as IgG levels above 50 Au/ml. Consent to a 5 ml blood draw was requested from all participants.
Data regarding age, gender, height and weight was drawn from IDFMC electronic medical records. Continuous variables were presented as median with interquartile range, and categorical variables were presented as counts and percentages. Correlation between IgG titers to age and body mass index (BMI) were calculated using Spearman test. Analyses were carried out with SPSS version 25.0 statistical package. For all analyses performed, a value of p < 0.05 was considered statistically significant.
3. Results
719 military personnel consented to blood withdrawal (Table 1 ). Of them, 594 (82.6%) were male, with a median age of 22 years (interquartile range- IQR 21–24) and a median BMI of 22.72 (IQR 20.98–25.1).
Table 1.
Demographic and serological characteristics (N = 719).
| Characteristics | Males | Females |
|---|---|---|
| No. of participants (%) | 594 (82.6) | 125 (17.4) |
| Median Age (IQR)- years | 22 (21–24) | 20 (19–21) |
| Median Body Mass Index (IQR) | 22.72 (20.98–25.1) | 21.99 (19.78–24.63) |
| Median IgG result (IQR)- Au/ml | 1885.5 (1079–3466.5) | 2578 (1454–3968) |
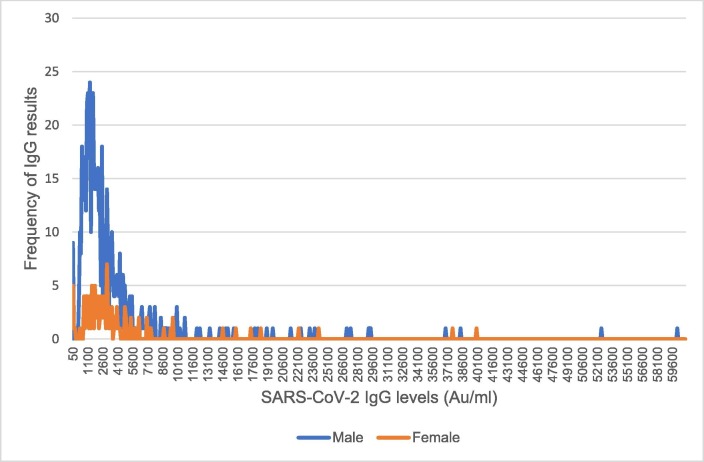
Quantitative IgG response range was 1.3- 60000Au/ml, with a median of 1989Au/ml (IQR 1112.8–3568 Au/ml). Females had a significantly higher serological response (median IgG levels 2578Au/ml, IQR 1454–3968 Au/ml) than males (median 1885.5 Au/ml, IQR 1079–3466.5 Au/ml, p = 0.01), as depicted in Fig. 1 . Overall, 706 subjects (98.2%) demonstrated seroconversion. 36 subjects (5%) had IgG titers above 10,000Au/ml, 16 were known to be recovering from Covid-19 illness, while another five experienced symptoms possibly related to Covid-19 in the last six months. IgG titers were negatively correlated with age (rs = -0.223, p < 0.001 for males; rs = -0.254, p = 0.004 for females), and did not correlate with BMI (rs = -0.028, p = 0.5 for males; rs = 0.13, p = 0.15 for females).
Fig. 1.
SARS-CoV-2 IgG response to subcutaneous Pfizer/BioNTech vaccine administration. Frequencies of SARS-CoV-2 IgG levels for participating males and females. Females had a significantly higher serological response (median IgG levels 2578Au/ml, IQR 1454–3968 Au/ml) than males (median 1885.5 Au/ml, IQR 1079–3466.5 Au/ml, p = 0.01).
4. Discussion
While the subcutaneous injection of the Pfizer/BioNTech vaccine was accidental, our data shows that a single shot resulted in high rates of seroconversion (98%). These results are in line with recent data on 514 healthcare workers in an Israeli hospital of which 92% had detectable IgG antibodies 21 days after an intramuscular first dose of BNT162b2 [8] and BNT162b2 phase I/II trial data [9]. Whether the difference in immunogenicity found is related to lower age in our study population (negatively correlated to IgG titers) or to route of administration merits further studies.
The amount of subcutaneous fat, as implied by BMI, did not play a role in the seroconversion rates. Females had higher IgG titers. Whether this is unique to the route of administration is yet to be discovered. Additionally, in correlation with previous publications [8], [9] we found that recovered Covid-19 patients had a 10 fold higher immune response following a single subcutaneous vaccination shot.
Our data further reassures the need for constant quality assurance, especially when launching a large vaccination program. After assessment, the IDFMC alerted all vaccinating teams and supplied appropriate syringes.
This study has limitations, mainly the lack of a control group assessing IgG titers after intramuscular administration and the small subject population. This is of course since this was not designed as a study. However, our IgG levels match published immunogenicity data on intramuscularly administered BNT162b2 [8], [9].
5. Conclusions
Despite intended for intramuscular administration, subcutaneous injection of the Pfizer/BioNTech vaccine, resulted in high immunogenicity, precluding the need for another vaccine dose. This could help reassure providers confronted with a similar mistake. The importance of regular quality control and learning processes cannot be over emphasized.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank IDFMC northern and central medical command for their assistance in blood collection and transportation for laboratory analysis.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Assessment Report Authorization for Temporary Supply COVID-19 mRNA Vaccine BNT162b2 (BNT162b2 RNA) concentrate for solution for injection. Department of Health and Social Care (DHSC) Pfizer Limited & BioNTech Manufacturing GmbH. December 11, 2020.
- 3.Belyakov I.M., Ahlers J.D. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183(11):6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 4.Mohanan D., Slütter B., Henriksen-Lacey M., Jiskoot W., Bouwstra J.A., Perrie Y., et al. Administration routes affect the quality of immune responses: a cross-sectional evaluation of particulate antigen-delivery systems. J Control Release [Internet]. 2010;147(3):342–349. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Mishraa H., Mishrab D., Mishrac P.K., Nahara M., Dubeya V., Jaina N.K. Evaluation of Solid Lipid Nanoparticles as Carriers for Delivery of Hepatitis B Surface Antigen for Vaccination Using Subcutaneous Route. J Pharm Pharmaceut. 2010;13(4):495–509. doi: 10.18433/j3xk53. [DOI] [PubMed] [Google Scholar]
- 6.Takasuka N, Fujii H, Takahashi Y, Kasai M, Morikawa S, Itamura S et al. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice, Int Immunol. 16(10):1423–1430. [DOI] [PMC free article] [PubMed]
- 7.https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2
- 8.Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C et al. Impact of age, gender, ethnicity and prior disease status on immunogenicity following administration of a single dose of the BNT162b2 mRNA Covid-19 Vaccine: real-world evidence from Israeli healthcare workers, Israel, December 2020 to January 2021; Euro Surveill:2021; 26(6). [DOI] [PMC free article] [PubMed]
- 9.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]