Abstract
Objective
To characterize neurological involvement in multisystem inflammatory syndrome in children (MIS-C) related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
Retrospective analysis of the clinical, electroencephalographic, CSF and neuroradiological parameters recorded in seven children (3 males, aged 3–10 years) affected by MIS-C with acute neurological involvement.
Results
All cases presented acute encephalopathy with drowsiness, irritability, mood deflection and diffuse EEG slowing with periodic posterior complexes. Focal neurological signs, normal brain MRI and CSF, were present in four patients; these patients received intravenous methylprednisolone at 30 mg/kg/day for 3 days. In all cases, the clinical picture rapidly improved in the first three days, and all neurological symptoms and EEG abnormalities disappeared within 10 and 30 days respectively. The severity and duration of the EEG abnormalities was proportional to the extent of the neurological involvement.
Conclusions
Patients with MIS-C may present acute encephalitis characterized by rapid-onset encephalopathy and EEG abnormalities (slow wave activity and/or epileptic abnormalities), in some cases associated with focal neurological signs that disappear with immunomodulatory therapy. The detection through neurological evaluation of sentinel neurological signs and distinctive EEG patterns documentable at disease onset will allow timely diagnosis and treatment of these cases.
Keywords: COVID-19, SARS-CoV-2, Multisystem inflammatory syndrome in children (MIS-C), Encephalitis, Neurological symptoms, EEG abnormalities
Abbreviations: MIS-C, multisystem inflammatory syndrome in children; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IVIg, intravenous immunoglobulins; IVMP, intravenous methylprednisolone
1. Introduction
During the ongoing COVID-19 pandemic, a growing number of children worldwide are presenting multisystem inflammatory syndrome (MIS-C) in association with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [[1], [2], [3]]. This “Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19” is a new entity characterized by fever, multisystem organ involvement, laboratory evidence of inflammation, and laboratory or epidemiological evidence of SARS-CoV-2 infection, in individuals aged 0–19 years with no alternative diagnosis [4]. The etiology is not clear, but it has been suggested that it may be a post-infectious manifestation secondary to an abnormal immune response [2].
Neurological involvement in MIS-C, observed in up to 34 % of cases [2], has not been included in the diagnostic criteria for the condition and remains poorly characterized.
Most papers report non-specific symptoms, such as headache [3,5], meningism [3,5], drowsiness [1], irritability [1], hypotonia [1] and confusion [1,3,5]; dysarthria, cerebellar ataxia, muscle weakness and hyporeflexia, have been described anecdotally [5]. To date, EEG changes in the course of MIS-C, mostly described as focal or generalized slowing, epileptic abnormalities or, more rarely, seizures, have been reported in only a few cases [1,5]. A combination of EEG slowing and thalamic lesions has been described in a patient with encephalopathy [1].
In view of the poor characterization of this aspect of MIS-C, we set out to analyze the neurological profile of a sample of children presenting MIS-C with neurological signs.
2. Patients
Between October 1, 2020 and February 15, 2021, 34 children were hospitalized at Buzzi Children's Hospital in Milan with a diagnosis of MISC according to WHO criteria and American College of Rheumatology recommendations [3,4]. Other inflammatory and infectious diseases were excluded (including Kawasaki disease and principal viral causes of encephalitis). All the cases were treated with intravenous immunoglobulins (IVIg), 2 g/kg, and with intravenous methylprednisolone (IVMP), 2 mg/kg/day. Ten of them presented neurological symptoms, and of these, 7 underwent a complete neurological and EEG assessment. This report describes the neurological and electroclinical characteristics of these 7 patients. Fever was considered the onset symptom, and the first day with fever was taken as day 1.
Four of the children, presenting focal neurological signs, also underwent CSF (including SARS-CoV-2 PCR and neurotropic viral PCR) and brain MRI evaluation (T1-and T2-weighted, FLAIR and diffusion sequences).
The clinical and paraclinical features of the sample are summarized in the Table 1 .
Table 1.
Clinical and paraclinical features of the patients.
| Patient | Age (y), Sex | SARS-CoV-2 Timing of primary infection |
Evidence of infection (1) |
MIS-C |
EEG (3) | CSF (4) | MRI | Therapy (5) | Neurological Outcome |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 Laboratory or epidemiological evidence of infection |
Other plasma antibodies: IgM and IgG | Systemic manifestations (2) | Neurological manifestations |
||||||||
| #1 | 5,M | Patient: 8 weeks before MIS-C onset | Patient: asymptomatic, swab not performed, serology testing + Parents: paucisymptomatic, swab + |
No evidence of acute infections | Fever > 38 °C, asthenia (from day 1 to day 10) Gastrointestinal symptoms: abdominal pain, emesis, diarrhea (from day 1 to day 10) Cardiac involvement: mild dysfunction (EF 49–50 %, ↑ D-dimer, ↑ pro-BNP, normal Troponin T) (from day 2 to day 4) Alterations of blood tests: ↑ CRP, ↑procalcitonin, ↑ ESR, ↑ ferritin, ↑ IL-6, low platelets, hypoalbuminemia, hyponatremia, lymphocytopenia (from day 3 to day 13) |
Day 1: severe headache, drowsiness Day 4: severe irritability, mood deflection, sleep disorder, headache, photophobia, diffuse limb pain, oculomotor apraxia, lower limb weakness and areflexia, gait disorder, speech disorder |
Day 4: BA: diffuse delta slow activity (0.5–1 Hz) PRP: biphasic delta slow complexes EC: bilateral anterior theta rhythmic discharges. Day 7: BA: focal posterior slow activity (0.5–1 Hz) PRP: biphasic delta slow complex EC: bilateral anterior theta rhythmic discharges. Day 10: BA: slow posterior dominant rhythm EC: theta rhythmic discharges |
Day 4: normal | Day 4: Brain MRI: normal Spinal MRI: enhancement of the anterior roots of the cauda equina |
MIS-C therapy Add-on: IVMP 30 mg/kg/day (from day 4 to day 6) |
Day 10: full recovery without neurological sequelae Day 30: normal EEG |
| #2 | 3,F | Patient: unidentified Parents: unidentified |
Patient: asymptomatic, swab not performed, serology testing + Parents: asymptomatic, swab not performed |
No evidence of acute infections | Fever > 39 °C (from day 1 to day 5) Mucocutaneous signs: rash, conjunctivitis (from day 1 to day 2) Nephrological involvement: proteinuria and lower limb edema (from day 3 to day 5) Alterations of blood tests: ↑ CRP, ↑procalcitonin, ↑ ESR, ↑ IL-6, ↑ ferritin, low platelets, hypoalbuminemia, hyponatremia, lymphocytopenia (from day 3 to day 14) |
Day 7: severe irritability, mood deflection, drowsiness |
Day 7: BA: focal posterior slowing (1–1.5 Hz) PRP: biphasic delta slow complexes EC: bilateral anterior theta rhythmic discharges Day12: BA: focal posterior slowing (4 Hz) EC: focal bilateral anterior theta rhythmic discharges Day15: BA: focal posterior slowing (6 Hz) EC: focal bilateral anterior theta rhythmic discharges |
Day 7: normal | Day 7: Brain MRI: normal |
MIS-C therapy Add-on: IVMP 30 mg/kg/day (from day 8 to day 10) |
Day 12: full recovery without neurological sequelae Day 22: normal EEG |
| #3 | 3, F | Patient: unidentified Parents: unidentified |
Patient: asymptomatic, swab +, serology testing + Parents: asymptomatic, swab not performed |
No evidence of acute infections | Fever >39 °C (from day 1 to day 9) Gastrointestinal symptoms: abdominal pain, diarrhea (from day 1 to day 7) Cardiac involvement: severe dysfunction (EF < 35 %, ↑ Troponin T, ↑ pro-BNP, ↑ D-dimer) (from day 2 to day 5) Vascular involvement: femoral artery thrombosis Pulmonary involvement: subpleural thickenings and pleural effusion Metabolic involvement: acidosis with elevated lactates (from day 1 to day 5) Alterations of blood tests: ↑ PLT, neutrophilia, hypoalbuminemia, hyponatremia (from day 1 to day 21) Intensive care required |
Day 1: Generalized tonic-clonic seizures, irritability, drowsiness, hyporeactivity, mood deflection |
Day1: BA: diffuse delta slow activity (1.5 Hz) EC: bilateral anterior theta rhythmic discharges |
Day 1: normal | Day 2: Brain MRI: normal |
MIS-C therapy Add-on: IVMP 30 mg/kg/day (from day 2 to day 4) |
Day 21: full recovery without neurological sequelae Day 31: normal EEG |
| #4 | 7, F | Patient: unidentified Parents: 4 weeks before MIS-C onset |
Patient: asymptomatic, swab not performed, serology testing + Parents: paucisymptomatic, swab + |
No evidence of acute infections | Fever > 39 °C, asthenia (from day 1 to day 2) Gastrointestinal symptoms: abdominal pain, diarrhea (from day 1 to day 4) Mucocutaneous signs: rash (from day 2 to day 5) Alterations of blood tests: ↑ CRP, neutrophilia, ↑ ferritin, low platelets (from day 1 to day 13) |
Day 4: severe irritability, mood deflection, headache, diffuse limb pain, speech disorder, photophobia, meningism, drowsiness |
Day 4: BA: diffuse theta slow activity (4–6 Hz), PRP: delta activity (2–2.5 Hz) in posterior regions EC: absent Day 8: BA: 8 Hz posterior dominant rhythm PRP: focal posterior delta waves |
Day 4: normal |
Day 5: Brain MRI: normal |
MIS-C therapy Add-on: IVMP 30 mg/kg/day (from day 4–6) |
Day 8: full recovery without neurological sequelae Day 15: normal EEG |
| #5 | 10, M | Patient: 4 weeks before MIS-C onset | Patient: paucisymptomatic (ageusia), swab +, serology testing + Parents: asymptomatic, swab not performed |
No evidence of acute infections | Fever > 38 °C, asthenia (from day 1 to day 10) Gastrointestinal symptoms: abdominal pain, nausea (from day 1 to day 6) Mucocutaneous signs: conjunctivitis (from day 2 to day 8) Cardiac involvement: mild dysfunction (EF 48 %, ↑ pro-BNP, normal Troponin T) (from day 7 to day 10) Alterations of blood tests: low platelets (from day 7 to day 14) Intensive care required |
Day 1: headache, drowsiness Day 4: mild irritability, mood deflection, lower limb pain |
Day 5: BA: 8 Hz posterior dominant rhythm PRP: focal anterior biphasic delta waves EC: frontal spikes |
Not performed | Not performed | MIS-C therapy | Day 8: full recovery without neurological sequelae Day 21: normal EEG |
| #6 | 8, M | Patient: 3 weeks before MIS-C onset | Patient: paucisymtomatic (asthenia), swab +, serology testing + Parents: asymptomatic, swab - |
No evidence of acute infections | Fever > 39 °C (from day 1 to day 4) Gastrointestinal symptoms: emesis, abdominal pain (from day 1 to day 5) Pulmonary involvement: mild subpleural thickenings (from day 4 to day 8) Alterations of blood tests: ↑ CPR, neutrophilia, low platelets, lymphocytopenia, ↑ ferritin (from day 4 to day 13) |
Day 2: mild irritability, drowsiness, mood deflection |
Day 2: BA: 8 Hz posterior dominant rhythm PRP: focal posterior biphasic delta waves and diffuse slow waves EC: absent |
Not performed | Not performed | MIS-C therapy | Day 6: full recovery without neurological sequelae Day 20: normal EEG |
| #7 | 8, F | Patient: 4 weeks before MIS-C onset | Patient: asymptomatic, swab +, serology testing + Parents: asymptomatic, swab + |
No evidence of acute infections | Fever > 39 °C (from day 1 to day 10) Gastrointestinal symptoms: abdominal pain, diarrhea, emesis (from day 1 to day 10) Cardiac involvement: mild dysfunction (EF 48 %, ↑ D-dimer, ↑ pro-BNP, ↑ Troponin T) (from day 3 to day 10) Pulmonary involvement: subpleural thickenings (from day 3 to day 9) Mucocutaneous signs: Conjunctivitis (from day 4 to day 7) Metabolic involvement: Hyperglycemia (from day 3 to day 5) Alterations of blood tests: ↑ CRP, ↑ PCT, neutrophilia, ↑ IL-6, low platelets, lymphocytopenia (from day 3 to day 19) Intensive care required |
Day 1: mild irritability, drowsiness, mood deflection |
Day 3: BA: slow delta waves mixed with posterior dominant rhythm (8Hz) PRP: diffuse isolated delta waves EC: temporal spikes |
Not performed | Not performed | MIS-C therapy | Day 5: full recovery without neurological sequelae Day 17: normal EEG |
(1) Evidence of infection: Serologic anti-SARS Cov2 nucleocapsid antibodies test performed at the onset of fever (day 1). Plasma antibodies searched for: CMV, Coxsackie virus, EBV, HBV, HCV, HIV1-2 HSV1-2, Mycoplasma pneumoniae, Parvovirus.
(2) All patents were tested for: EF: ejection fraction; D-dimer, pro-BNP (B-type natriuretic peptide), Troponin T; WBC (white blood cell count), CRP (C Reactive Protein), ESR (Erythrocyte Sedimentation Rate), PCT (procalcitonin), IL-6, ferritin, LDH (lactate dehydrogenase), albumin, electrolytes, blood urea nitrogen, creatinine, glucose, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, fibrinogen, PT (prothrombin time), PTT (partial thromboplastin time). In Table only pathological findings are reported.
(3) EEG: BA: background activity; EC: epileptiform changes; PRP: periodic and rhythmic patterns.
(4) CSF analysis: physical chemical analysis, PCR SARS-CoV-2 test, viral PCR (HSV1-2, HZV, EBV, enterovirus), oligoclonal bands assay.
(5) IVIg (intra-venous immunoglobulins) 2 g/kg (max 100 g) and IVMP (intra-venous methylprednisolone) 2 mg/kg/day (max 60 mg/day).
2.1. Patients 1–4: severe phenotype
Patients 1,2,3 and 4 displayed signs of both focal and diffuse neurological involvement. They presented with severe irritability, mood deflection and drowsiness, variably associated with headache, meningism, photophobia, oculomotor apraxia, gait disorder, pain and slow, “whiny” and repetitive speech with reduced verbal output and preserved comprehension. Patient 3 presented with two generalized tonic-clonic seizures during fever.
EEG at the onset of the neurological symptoms was characterized by absence of physiological organization. Wake EEG was dominated by high-amplitude delta slow activity, diffuse or predominant over the posterior regions. During sleep, posterior delta biphasic complexes and focal bilateral anterior theta rhythmic discharges were detected.
In these children with encephalopathy, focal neurological signs and marked EEG slowing, IVMP was increased (30 mg/kg/day for 3 days). The symptomatology peaked in 2–3 days and thereafter showed a dramatic improvement.
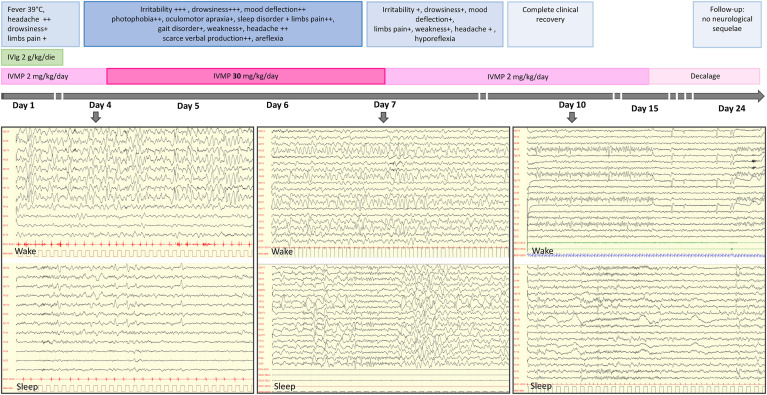
The Fig. 1 shows the clinical and EEG changes recorded over time in a representative patient (patient 1). On day 7 slow activity was still present over the posterior regions. It improved markedly by day 10, with the trace showing theta rhythmic discharges; progressive reorganization of the posterior rhythm was followed by recovery of physiological sleep spindles. A normal EEG was recorded between day 15 and day 24.
Fig. 1.
Timeline of patient 1's course. EEG day 4: wake: diffuse delta (0,5–1,5 Hz) activity; sleep: biphasic delta slow complexes. EEG day 7: wake: focal posterior delta (1,5-2 Hz) waves; sleep: lack of physiological organization, theta rhythmic discharges. EEG day 10: wake: reactive theta (6 Hz) posterior dominant rhythm; sleep: focal bilateral anterior discharges. IVIg: intra-venous immunoglobulins; IVMP: intra-venous methylprednisolone.
In the four children who underwent CSF analysis and brain MRI, these examinations were normal.
2.2. Patients 5–7: mild phenotype
Patients 5,6 and 7 showed mild symptoms of diffuse encephalopathy, namely irritability, drowsiness, mood deflection and headache. EEG in these patients documented focal or diffuse delta waves, high-amplitude biphasic delta waves in sleep (patients 5 and 6) and/or focal spikes (patients 5 and 7). All these patients recovered between days 5 and 8, and the EEG performed between days 15 and 21 was normal.
3. Discussion
Recent data from the Literature suggest that neurological involvement may be frequent in MIS-C patients, but the clinical syndrome is far from clearly defined.
Between 1 and 7 days from the onset of fever, all the patients in our series developed encephalopathy characterized by the simultaneous presence of drowsiness, irritability, and mood deflection, not fully justified by the systemic involvement. In four cases, focal neurological signs (oculomotor apraxia, speech impairment, gait disorder and seizures) were also present. When focal neurological signs were present, IVMP was increased to 30 mg/kg/day for three days. The clinical picture improved in all the cases, with all neurological symptoms disappearing completely within 10 days of their onset.
In the children with more severe encephalopathy with focal neurological signs and/or seizures, EEG showed absence of physiological organization, which was replaced by delta slowing, diffuse or predominant over the posterior regions. We documented posterior slow biphasic complexes in sleep and occasional anterior epileptiform abnormalities. This EEG pattern was evident at the onset of the neurological symptoms and persisted for a few days. After 3–4 days it began to improve, before normalizing some days after the disappearance of the neurological symptoms. In the clinically milder cases, we observed mainly focal slow waves.
We documented a correlation between the severity of neurological involvement and the extent and duration of the EEG abnormalities. The EEG picture evolved positively: gradual restoration of physiological background organization was followed by disappearance of the slow posterior complexes and then of the anterior theta rhythmic discharges. EEG normalization was complete within two weeks of the neurological recovery.
The four children with more severe neurological involvement underwent CSF assessment and brain MRI that showed no abnormalities. CSF has previously been studied in a small number of patients and almost always found to be normal [1,5], although cases of pleocytosis have been described [5]. Similarly, MRI is normal in most MIS-C cases, even though bilateral thalamic lesions [1] and signal changes in the splenium of the corpus callosum and centrum semiovale have been reported [5]. The SARS-CoV-2 virus was not detected in the 4 patients who underwent CSF analysis.
Patients with MIS-C may present acute neurological involvement clinically characterized by rapid onset of encephalopathy, with focal neurological signs, seizures or EEG abnormalities (slow wave activity and/or epileptic abnormalities), consistent with what is typically observed in cases of encephalitis arising in the course of multisystem inflammatory conditions.
All our cases recovered completely and were treated, in accordance with the guidelines on MIS-C, with IVIg and IVMP, two immunomodulatory agents also used in the treatment of encephalitis. In the four children with more severe neurological involvement, IVMP was increased.
As previously suggested [2], it is reasonable to suppose that the neurological involvement observed in our cases is due to an immune-mediated pathophysiological mechanism triggered by previous SARS-CoV-2 infection. This hypothesis is supported both by the fact that the neurological symptoms occurred in the course of a post-infectious multisystem inflammatory condition, and by the absence of SARS-CoV-2 and neurotropic viruses in the CSF.
However, this hypothesis needs to be confirmed through study of CSF antibody and cytokine profiles.
In conclusion in our experience, in some cases encephalitis can be part of the clinical picture of MIS-C. Our patients, with timely diagnosis and treatment, showed a benign evolution leading to complete recovery. However, the spectrum and frequency of the neurological involvement may be broader, and signs of it may be missed in many cases. Indeed, it seems likely that patients with mild and non-specific neurological symptoms are not adequately investigated for encephalitis, while neurological signs and symptoms appearing in those with multiorgan failure may be ignored, misunderstood or underestimated.
Neurological assessment and EEG evaluation, aimed at detecting sentinel neurological signs and distinctive EEG abnormalities, may ensure that this condition is not missed, and should therefore be included in the diagnostic work up.
As MIS-C is still a very new medical condition, long-term clinical and instrumental follow up is mandatory to understand its impact on neurodevelopment in affected children.
This study was conducted in a limited sample, and not all the children with neurological signs underwent full neurological evaluation. All investigations were carried out on the basis of good clinical practice; therefore, the four most serious patients underwent brain MRI and CSF assessment, while the other three were monitored clinically and through electroencephalography.
Study funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
The parents/guardians of the patients gave written consent for the analysis and publication of the data.
Ethics approval
The study complied with institutional regulations for anonymized retrospective studies and was approved by the ethics committee of Area 1 of Milan (2021/ST/004).
Acknowledgment
The authors thank Catherine Wrenn for English translation of the manuscript and OBM Onlus for funding the English language revision.
References
- 1.Abel D., Shen M.Y., Abid Z., et al. Encephalopathy and bilateral thalamic lesions in a child with MIS-C associated with COVID-19. Neurology. 2020;95:745–748. doi: 10.1212/WNL.0000000000010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T.-H. Neurological involvement associated with COVID-19 infection in children. J. Neurol. Sci. 2020;418:1–3. doi: 10.1016/j.jns.2020.117096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson L.A., Canna S.W., Friedman K.G., et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheum. 2020;72(11):1791–1805. doi: 10.1002/art41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO – World Health Organization Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Available at. Accessed.
- 5.Abdel-Mannan O., Eyre M., Lobel U., et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77(11):1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]