Abstract
Accumulating evidence shows that Coronavirus Disease 19 (COVID-19) survivors may encounter prolonged mental issues, especially post-traumatic stress symptoms (PTSS). Despite manifesting a plethora of behavioral or mental issues in COVID-19 survivors, previous studies illustrated that static brain functional networks of these survivors remain intact. The insignificant results could be due to the conventional statistic network analysis was unable to reveal information that can vary considerably in different temporal scales. In contrast, time-varying characteristics of the dynamic functional networks may help reveal important brain abnormalities in COVID-19 survivors. To test this hypothesis, we assessed PTSS and collected functional magnetic resonance imaging (fMRI) with COVID-19 survivors discharged from hospitals and matched controls. Results showed that COVID-19 survivors self-reported a significantly higher PTSS than controls. Tapping into the moment-to-moment variations of the fMRI data, we captured the dynamic functional network connectivity (dFNC) states, and three discriminative reoccurring brain dFNC states were identified. First of all, COVID-19 survivors showed an increased occurrence of a dFNC state with heterogeneous patterns between sensorimotor and visual networks. More importantly, the occurrence rate of this state was significantly correlated with the severity of PTSS. Finally, COVID-19 survivors demonstrated decreased topological organizations in this dFNC state than controls, including the node strength, degree, and local efficiency of the supplementary motor area. To conclude, our findings revealed the altered temporal characteristics of functional networks and their associations with PTSS due to COVID- 19. The current results highlight the importance of evaluating dynamic functional network changes with COVID-19 survivors.
Keywords: Coronavirus disease 19 (COVID-19), Post-traumatic stress symptoms (PTSS), Functional magnetic resonance imaging (fMRI), Dynamic functional network connectivity (dFNC), Mental health
Abbreviations
- ADN
auditory network
- CBN
cerebellar network
- CCN
cognitive-control network
- COVID-19
Coronavirus Disease 2019
- dFNC
dynamic functional network connectivity
- DMN
default-mode network
- FDR
false-discovery rate
- GAD-7
Generalized Anxiety Disorder-7
- GSP
genomics superstruct project
- HCP
human connectome project
- ICA
independent component analysis
- IC
independent component
- PCL-5
post-traumatic stress disorder checklist for DSM-5
- PHQ-9
Patient Health Questionnaire-9
- PTSD
post-traumatic stress disorder
- PTSS
post-traumatic stress symptoms
- ROIs
Regions of interest
- sFNC
static FNC
- SCN
sub-cortical network
- SMA
supplementary motor area
- SPM12
Statistical Parametric Mapping
- SMN
sensorimotor network
- TCs
time-courses
- VSN
visual network
1. Introduction
Coronavirus Disease 2019 (COVID-19) is a global pandemic caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which has been spreading worldwide with more than 106 million confirmed cases and 2 million death as the end of March 2021 (Worldometer, 2021). Although primarily considered as respiratory disease, COVID-19 has become increasingly recognized as neurotropic; patients might experience some mild (e.g., headaches, loss of smell, and tingling sensations), as well as severe neurological symptoms (e.g., aphasia, strokes, and seizures) (Paterson et al., 2020; Pezzini and Padovani, 2020; Widge et al., 2020). Using structural magnetic resonance imaging, previous studies reported that half of the COVID-19 patients have brain structural abnormalities, including white matter hyperintensities (Afshar et al., 2020; Anzalone et al., 2020; Egbert et al., 2020; Kandemirli et al., 2020) and frontal-temporal gray matter volume abnormalities (Duan et al., 2021).
In addition to the aforementioned neurological symptoms, new research indicated COVID-19 survivors might demonstrate long-term mental problems, including fatigue, sleep difficulties, anxiety, depression, and more importantly, post-traumatic stress disorder (PTSD) (Huang et al., 2021). Considering the neuropsychiatric symptoms in COVID-19 survivors, it is plausible to hypothesize that they may experience the disruption of widespread brain functional networks, which can be investigated by examining functional connectivity between different brain regions using resting-state functional MRI (fMRI) (Biswal et al., 2010; Greicius et al., 2003; Norton et al., 2012). To date, although brain structural abnormalities have been described in the context of COVID-19 (Lu et al., 2020), brain functional studies are scarce. A recent case study using fMRI to evaluate static functional connectivity of the default mode network in a COVID-19 patient after surviving critical illness reported intact brain network function (Fischer et al., 2020). As far as the current authors’ knowledge, no researchers have investigated the disrupted functional networks of COVID-19 survivors.
Challenging the conventional assumption that the functional connectivity is temporally static during the resting-state fMRI scan, the dynamic functional network connectivity (dFNC) is an approach featuring dynamic changes in the integration and segregation of functional networks (Fu et al., 2019a; Lurie et al., 2020; Tu et al, 2019, 2020). Since dFNC is more sensitive to capture the dynamic adaptions to diverse cognitive and mental demands (Lurie et al., 2020; Preti et al., 2017), it enables a more sophisticated evaluation of the spontaneously fluctuating nature of neural signals in different temporal scales than static ones (Bonkhoff et al., 2020). Thereafter, researchers deem that dFNC holds great potential for revealing novel biomarkers for neuropsychiatric diseases (Fu et al., 2021; Kim et al., 2017; Tu et al, 2019, 2020). Therefore, the time-varying characteristics of dynamic functional networks captured by the dFNC may reveal important brain abnormalities in COVID-19 survivors that would be otherwise missed by static functional network connectivity.
To leverage this novel understanding of functional networks to test the above research hypothesis, we applied a novel analytical framework combining independent component analysis (ICA), sliding-window cross-correlation, k-means clustering, and graph-theory methods, to compare dFNC in resting-state fMRI data between COVID-19 survivors and matched controls. Since COVID-19 patients usually encounter issues of social isolation, physical discomfort, and fear for survival, are vulnerable to develop long-term mental health problems such as post-traumatic stress symptoms (PTSS) (Janiri et al., 2021; Mazza et al., 2020; Pfefferbaum and North, 2020; Xiong et al., 2020), we predicted that there could be discriminative the time-vary characteristics of the dynamic functional networks associated with PTSS in COVID-19 survivors.
2. Materials and methods
2.1. Participants and behavioral assessments
The present study included 50 hospitalized COVID-19 survivors (i.e., discharged between February and March 2020 from hospitals in Wuhan, China, about 6 months after discharging at the time of the testing) and 43 age- and sex-matched non-COVID-19 controls recruited from the local communities. All COVID-19 survivors of the current study were diagnosed and discharged based on the polymerase chain reaction result according to world health organization guidelines (Dennison Himmelfarb and Baptiste, 2020). All COVID-19 survivors did not have any COVID-19 related symptoms, e.g., fever and cough, at the time of this study. No subject in the control group had been clinically diagnosed as COVID-19. All participants provided written informed consent prior to the study, and the ethical protocols for conducting the current study were approved by the ethics committee of the local hospital.
The PTSD checklist for DSM-5 (PCL-5) was administered (Blevins et al., 2015). It consists of 20 items tapping into 20 symptoms of PTSD (Blevins et al., 2015), clustered into four domains (i.e., intrusion, avoidance, cognition/mood, and arousal/reactivity). Each item is rated on a 5-point Likert scale (i.e., 0 = “Not at all” to 4 = “Extremely”). The total score of PCL-5 and scores of sub-domains were used in the following analyses. Meanwhile, participants also completed the Generalized Anxiety Disorder-7 (GAD-7) (Spitzer et al., 2006) and Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al., 2001), to measure their anxiety and depression levels, respectively.
2.2. MRI data acquisition
The MRI data of all participants were collected using a GE 3.0 T MR750 scanner with a standard 32-channel head coil at the radiology department of the Renmin Hospital of Wuhan University, between July and August 2020. Subjects were asked to stay awake and to keep their heads still during the scan, with their eyes open and ears plugged. High resolution brain structural images were acquired with a T1-weighted fast-spoiled gradient echo sequence (repetition time = 8.16 ms, echo time = 3.18 ms, flip angle = 12°, slice thickness = 1 mm, interslice gap = 1 mm, and field-of-view = 256). Eight-minute resting-state brain functional images were acquired with a T2-weighted gradient echo-planar imaging (repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, slice thickness = 3.5 mm, field-of-view = 256 mm, and 38 slices).
2.3. Preprocessing and quality control
fMRI data were preprocessed using Statistical Parametric Mapping (SPM12) toolbox under the MATLAB 2019 environment. The first five dummy scans were discarded before preprocessing to guarantee the remaining fMRI scans were collected when magnetization achieved a steady state. We performed a slice timing correction and then a functional realignment on the functional images. The fMRI data were subsequently warped into the standard Montreal Neurological Institute space using an echo-planar imaging template and were slightly resampled to 3 × 3 × 3 mm3 isotropic voxels. The spatial smoothing was finally performed to smooth the data with a 6-mm full-width at half-maximum Gaussian kernel.
Because image quality is very important for ICA as well as dFNC analysis, similar to our previous studies (Fu et al., 2019b, Fu et al., 2021), we only retained participants 1) with head motion ≤ 3° and ≤ 3 mm and 2) with good functional data providing near full brain successful normalization (by comparing the individual mask with the group mask, see supplementary materials).
2.4. Analysis framework
The framework to explore variations in dFNC in COVID-19 survivors and matched controls is provided in Fig. S1. This framework is based on a fully automated ICA-based pipeline called Neuromark (Du et al., 2020), which has been successfully applied to multiple neuroimaging studies and identified a wide range of brain connectivity abnormalities in neurological and psychiatric diseases (Fu et al., 2021; Fu et al., 2019a; Li et al., 2020; Tu et al., 2020). There are three major procedures in this framework: 1) apply Neuromark pipeline to extract corresponding functional regions and time-courses (TCs) for each individual; 2) calculate dFNC between the ICA TCs via a sliding-window approach and perform a k-mean clustering on dFNC estimates to identify reoccurring connectivity patterns (i.e., reoccurring states) across subjects and time; 3) calculate the fractional rate to measure the frequency of occurrences of different connectivity patterns, and calculate state-based dFNC and graph-theory measures to explore transient information transmission in large-scale functional networks.
Besides dFNC, we also calculated static FNC (sFNC) between TCs using Pearson's correlation to build static functional connectivity.
2.5. Neuromark
NeuroMark is a reliable ICA-based pipeline that automatically estimates functional regions adaptable to each individual subject and comparable across subjects by taking advantage of the reliable brain network templates extracted from 1828 healthy controls as guidance. Two large healthy datasets, i.e., the human connectome project (HCP, http://www.humanconnectomeproject.org/) and the genomics superstruct project (GSP, https://dataverse.harvard.edu/dataverse/GSP), were used for the construction of the templates. Group ICA was performed on the GSP and HCP datasets, respectively, and the identified independent components (ICs) from the two datasets were then matched by comparing their group-level spatial maps. The reproducible IC pairs were further evaluated by examining their peak activations and low-frequency fluctuations of their corresponding TCs. Regions of interest (ROIs) were defined based on their anatomical and functional prior knowledge and were then used as references to calculate spatial maps and TCs for the dataset in the present study. Technical details are described in the supplementary materials.
2.6. Dynamic functional network connectivity
For each subject, dFNC was estimated via a sliding-window approach. A tapered window, created by convolving a rectangle (width = 20 TRs = 40s) with a Gaussian (σ = 3 TRs), was used as the window to segment TCs of ROIs. To enhance the estimation accuracy, we applied a graphical least absolute shrinkage and selection operator method to estimate the regularized inverse covariance matrix (Allen et al., 2014; Friedman et al., 2008). After obtaining the dFNC matrices, we applied a k-means clustering algorithm to classify dFNC into different groups based on their spatial similarity. The cluster centroids were referred to as reoccurring “brain states”, in a conceptual analogy to electroencephalogram (EEG) microstates (Khanna et al., 2015). The optimal number of states was estimated by the elbow criterion, defining as the ratio of within-cluster distance to between-cluster distance (Allen et al., 2014).
2.7. State occurrences and state-based dFNC/graph-theory measures
To assess the occurrence of different dFNC states, we calculated the fractional rate of each brain state by dividing the number of the total windows by the number of windows assigned to each state. To investigate the dFNC pairs in each state, we calculated the state-based dFNC by averaging the dFNC estimates across time windows that were assigned to the same state. Graph-theory analysis was further applied to demonstrate the abnormal topological organizations in functional brain networks. Node strength (i.e., the sum of weights of links connected to a node), node degree (i.e., the number of links connected to a node), and local efficiency (i.e., the number of edges within neighbors of a node) of the component TCs were estimated using the dFNC matrices via the brain connectivity toolbox (https://sites.google.com/site/bctnet/) and then averaged across windows within each state. We threshold the dFNC matrices by setting the dFNC pairs to 0 if their absolute connectivity <0.2 and then calculated graph-theory measures based on absolutely weighted matrices.
2.8. Statistical analyses
Independent-samples t-tests were applied to compare the total score of PCL-5, sub-domains of PCL-5, GAD, and PHQ, between COVID-19 survivors and controls. The threshold for statistical significance was corrected for multiple comparisons using the false-discovery rate [FDR] procedure (i.e., PFDR<0.05).
The differences of sFNC between COVID-19 survivors and controls were analyzed using a general linear model (GLM), controlling for age and gender. The statistically significant threshold was corrected for multiple comparisons (i.e., across all pairs of sFNC, N = 1378) using the FDR procedure.
The statistical comparisons of state occurrences and state-based dFNC/graph-theory measures between two groups were analyzed using GLMs, controlling for age and gender, and the statistically significant threshold was FDR corrected. In addition, we calculated the partial correlations between the dynamic characteristics (i.e., the state with abnormal occurrence in COVID-19 survivors) and the scores of PCL-5 (i.e., the total score and four sub-domain scores), controlling for age, gender, and group label.
2.9. Data availability
Raw data were generated at CAS Key Laboratory of Mental Health, Institute of Psychology, Beijing, China. Derived data supporting the findings of this study are available from the corresponding author on request.
3. Results
3.1. Behavioral results
After quality control, 44 COVID-19 survivors (average age: 53.11 ± 10.25; range: 25–69 years; male/female: 12/32) and 42 controls (average age: 52.21 ± 11.05; range: 25–67 years; male/female: 11/31) were included for further analyses. COVID-19 survivors and controls were matched by age, gender, and mean framewise displacement [FD] (age: P = 0.70; gender: P = 0.91, mean FD: P = 0.30).
Table 1 summarized scores of self-report assessments in COVID-19 survivors and controls. Using the independent-sample t-test, we found that COVID-19 survivors had significantly higher PCL-5 total scores than controls (COVID-19 survivors: 15.36 ± 12.05; controls: 7.19 ± 5.55; t84 = 4.01, P < 0.001). Similarly, COVID-19 survivors also showed significantly higher scores in all PCL-5 sub-domains, GAD, and PHQ (Table 1).
Table 1.
Self-report assessments in COVID-19 survivors and controls.
| Controls |
COVID-19 survivors |
Independent-sample t-test |
||
|---|---|---|---|---|
| (n = 42) | (n = 44) | P value | t value | |
| PCL-total | 7.19 ± 5.55 | 15.36 ± 12.05 | <0.001 | 4.01 |
| PCL-intrusion | 2.19 ± 1.99 | 3.89 ± 3.35 | 0.006 | 2.84 |
| PCL-avoidance | 0.57 ± 0.99 | 1.46 ± 1.28 | <0.001 | 3.56 |
| PCL-cognition/mood | 1.71 ± 2.02 | 5.18 ± 4.85 | <0.001 | 4.29 |
| PCL-arousal/reactivity | 2.71 ± 2.41 | 4.84 ± 3.44 | 0.001 | 3.30 |
| GAD | 2.50 ± 2.71 | 5.93 ± 5.20 | <0.001 | 3.81 |
| PHQ | 3.69 ± 3.45 | 7.84 ± 5.77 | <0.001 | 4.03 |
PCL = PTSD checklist; GAD = General Anxiety Disorder; PHQ = Patient Health Questionnaire. Scores were described as mean ± standard deviation.
3.2. Brain parcellation and static functional network connectivity
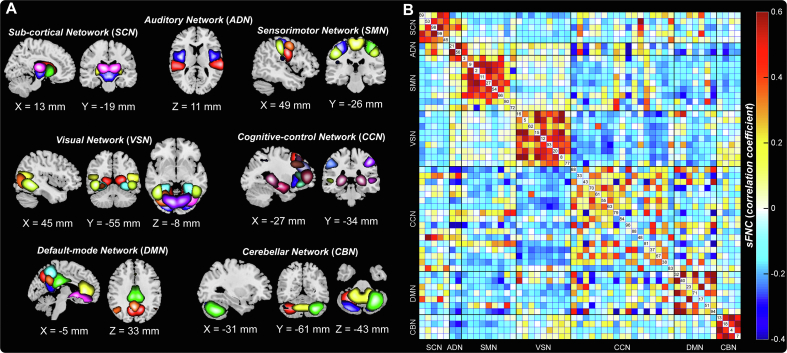
We parcellated the brain into 53 spatially independent components as ROIs, which covered almost the whole brain using the Neuromark pipeline. Based on their anatomical and functional prior knowledge, the 53 ROIs were arranged into seven functional networks (Fig. 1A): sub-cortical network (SCN), auditory network (ADN), sensorimotor network (SMN), visual network (VSN), cognitive-control network (CCN), default-mode network (DMN), and cerebellar network (CBN). Detailed labels and peak coordinates of each ROI were summarized in the supplementary materials Table S1. We calculated the Pearson's correlation coefficient between TCs of ROIs as the measure of sFNC for each subject. The averaged sFNC across participants was displayed in Fig. 1B, and we did not observe a significant difference in sFNC between COVID-19 survivors and controls (PFDR > 0.05).
Fig. 1.
Spatial maps of reproducible components as regions of interest (ROIs) and their static functional network connectivity (sFNC). (A) 7 functional networks, including 53 ROIs, were identified by two independent datasets. Each color represents a single component as a ROI. (B) Average sFNC across all participants, which was calculated by Pearson's correlation coefficient between time-courses (TCs) of ROIs. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Reoccurring dynamic brain states across COVID-19 survivors and controls
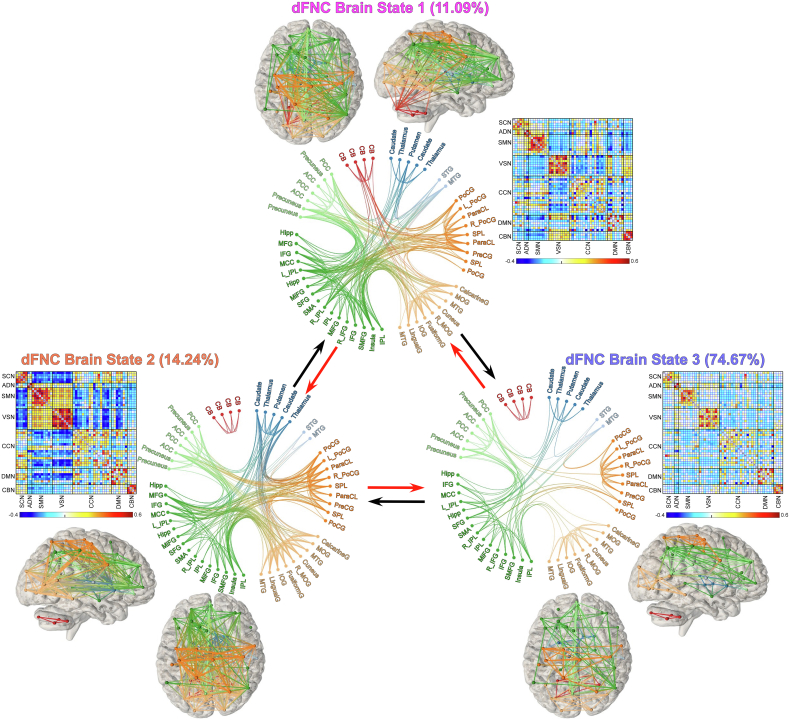
Reoccurring dynamic brain states were identified by clustering the windowed dFNC estimates based on their spatial similarity (Allen et al., 2014; Fu et al., 2018). Here, the term “brain states” refers to the dFNC patterns that reoccur across windows and subjects. We performed a cluster number validity analysis using the elbow criteria to determine the optimal number of clusters as 3, which was within the reasonable range of the number of clusters used in previous studies (Allen et al., 2014; Tu et al, 2019, 2020). Three highly structured dFNC brain states are displayed in Fig. 2. The circle panel displays the functional profile of each brain state that only retained strong connectivity (absolute connectivity strength > 0.2). The fractional rate was not uniformly distributed across brain states. States 1 and 2 occurred less frequently, showing strongly interconnected brain networks. The dFNC in state 1 showed less negative connectivity between SCN and SMN/VSN but more heterogeneous between SMN and VSN (i.e., negative correlations between these two networks) than dFNC in state 2. Interestingly, positive connectivity between CBN and VSN, as well as negative connectivity between CBN and SMN, was only observed in state 1. In state 2, strong negative connectivity between SCN and SMN/VSN, as well as strong within-network connectivity in VSN, were observed. In contrast, State 3 was a sparsely connected brain state with weak inter-network connectivity, but occurred most frequently (i.e., accounts for >70 % of all windows). Overall, the identified sparsely connected brain state (i.e., state 3) was more frequent, while the more strongly connected states (i.e., states 1 and 2) were less frequent, which were in line with previous findings in dynamic connectivity states (Allen et al., 2014; Kim et al., 2017; Tu et al., 2020), indicating the consistency of the time-varying characteristics of dFNC.
Fig. 2.
Reoccurring dynamic functional network connectivity (dFNC) brain states identified by clustering analysis. The dFNC patterns of brain states are displayed as the matrix form, accompanied by the functional profile of each centroid, showing the top 250 connectivity with strength >0.2 in each state. The connectivity between brain regions was also mapped to a brain template, with different colors indicating different functional networks. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Altered time-varying characteristics of dFNC states in COVID-19 survivors
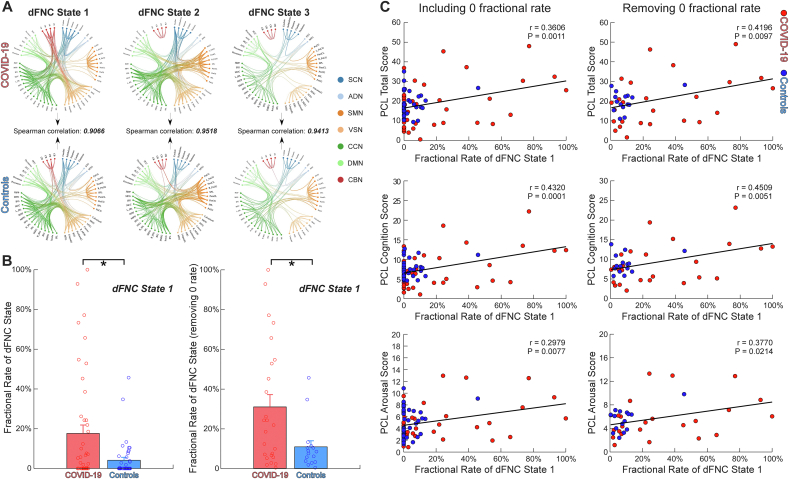
The global FNC patterns of the identified three dFNC states appeared similar between COVID-19 survivors and controls. We calculated the mean FNC patterns of dFNC states for each group respectively and then measured the Spearman correlations between the dFNC patterns of the two groups. The correlations shown in Fig. 3A were larger than 0.9 for all dFNC states (r = 0.9066, 0.9518, 0.9413 for each state, respectively), suggesting the intact global patterns of brain states in COVID-19 survivors. To further demonstrate that the global patterns of brain states did not differ between groups, we calculated the Euclidean distance from each time window to the cluster centroids in COVID-19 survivors and controls, respectively. Results showed no significant difference in distance from dFNC estimates to the cluster centroids between groups (P > 0.10)f
Fig. 3.
Intact global dynamic functional network connectivity (dFNC) patterns but altered temporal characteristic in dFNC state. (A) The spatial patterns of dFNC did not differ between Coronavirus Disease 2019 (COVID-19) survivors and controls (Spearman correlations: r = 0.9066, 0.9518, 0.9413, respectively). (B) The fractional rate of dFNC state 1 was significantly larger in COVID-19 survivors than controls, with and without excluding subjects without state 1. Bars represent the mean of fractional rate, and error bars represent the standard deviation of the mean. (C) The fractional rate of dFNC state 1 was significantly correlated with post-traumatic stress symptoms (PTSS). The correlation coefficients between the fractional rate and PTSS are shown using scatter plots, with different colors representing different groups. Asterisks * indicate significant group difference after false-discovery rate (FDR) correction. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Despite the intact global spatial patterns of brain states, we found an altered temporal characteristic of the dFNC state in COVID-19 survivors. Among the three identified dFNC states, we observed that COVID-19 survivors showed a significantly increased occurrence (measured by fractional rate) in dFNC state 1 (PFDR < 0.05). We then excluded subjects without state 1 (i.e., dFNC patterns in these subjects were not clustered into state 1) and repeated the statistical analysis. The occurrence of state 1 was still significantly different between the two groups (PFDR < 0.05). We further investigated the relationships between this abnormal temporal characteristic of dFNC state and PTSS in COVID-19 survivors. We found that with and without excluding subjects without state 1, the fractional rate of state 1 was significantly correlated with the PCL total score, PCL cognition score, and PCL arousal score, respectively (Fig. 3C; PFDR < 0.05).
3.5. State-based dFNC and graph-theory measures
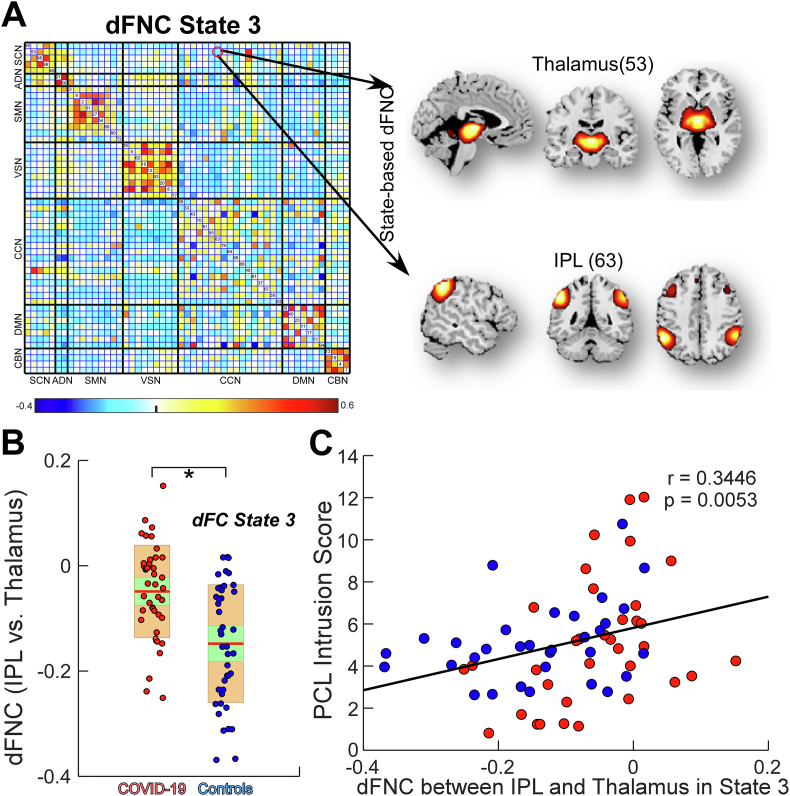
Although the global dFNC patterns are intact between COVID-19 survivors and controls, we hypothesized that local dFNC and topological organizations might reflect brain changes induced by PTSS in COVID-19 survivors. We found that the dFNC between thalamus and inferior parietal lobule (IPL) in state 3 shrank in COVID-19 survivors (closer to 0) and interestingly, this dFNC was positively correlated with the PCL-5 intrusion score (Fig. 4). Other state-based dFNC did not show any significant differences between groups (P > 0.05).
Fig. 4.
Abnormal state-based dynamic functional network connectivity (dFNC) in Coronavirus Disease (2019) (COVID-19) survivors. (A) dFNC between thalamus and inferior parietal lobule (IPL) within state 3. (B) dFNC between thalamus and IPL was lower (closer to zero) in COVID-19 survivors than healthy controls. Each dot represents an individual's connectivity strength and the color represents the group label. The green box represents 95 % of the standard deviation of mean and the yellow box represents the standard deviation. (C) State-based dFNC between thalamus and IPL was associated with post-traumatic stress disorder checklist for DSM-5 (PCL-5) intrusion score. Asterisks * indicate significant group difference after false-discovery rate (FDR) correction. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
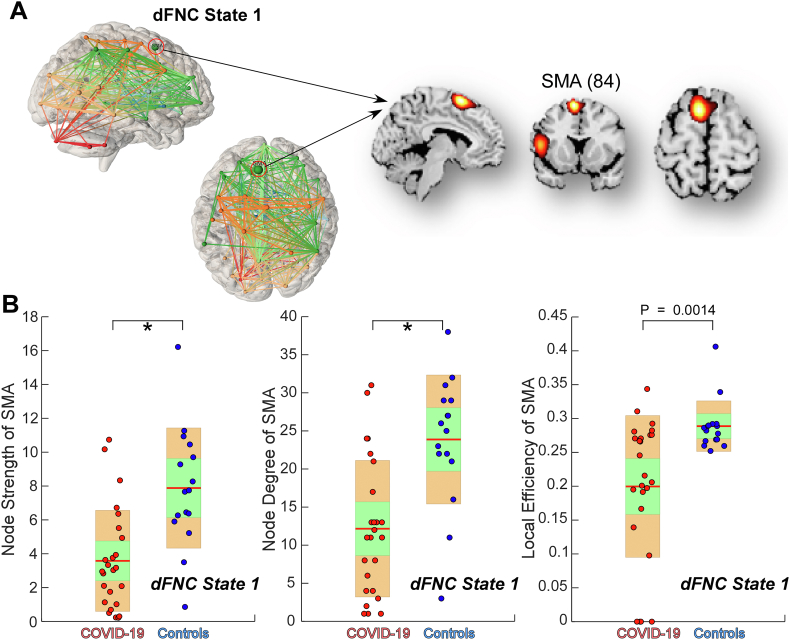
The graph-theory analysis showed that in state 1, the node degree and strength of the supplementary motor area (SMA) were significantly lower in COVID-19 survivors than controls (PFDR < 0.05; the local efficiency of the SMA was also lower in COVID-19 survivors, Puncorrected = 0.0014), suggesting that the function of the SMA in state 1 was disrupted in COVID-19 survivors (Fig. 5). The graph-theory measures did not show any significant differences in other brain regions (P > 0.05).
Fig. 5.
Topological measures of dynamic functional network connectivity (dFNC) states. (A) the 84th independent component (IC) of the spatial templates (model order = 100), i.e., the supplementary motor area (SMA) in dFNC state 1. (B) Group comparison in node strength, node degree, and local efficiency of the SMA are displayed using boxplots. Each dot represents an individual's value, and the color represents the group label. The green box represents 95 % of the standard deviation of the mean, and the yellow box represents the standard deviation. Asterisks * indicate significant group difference after false-discovery rate (FDR) correction. Only subjects who had the state 1 were used for the statistical analyses. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In the present study, we assessed PTSS and collected fMRI with COVID-19 survivors around six months after they were discharged from hospitals. Compared to non-COVID-19 controls, COVID-19 survivors had significantly higher PCL-5 total scores as well as scores of all PCL-5 sub-domains, GAD, and PHQ. Using a novel fMRI analytical pipeline, we found that COVID-19 survivors had abnormal time-varying characteristics and topological organizations in a reoccurring brain state, which was associated with the severity of their self-report PTSS.
Recent studies have demonstrated that COVID-19 survivors were vulnerable to develop PTSS after they were recovered from the infection (Janiri et al., 2021; Mazza et al., 2020; Tu et al., 2021). A cross-sectional study found a PTSD prevalence of 30.2 % patients who had recovered from COVID-19 infection within a short period (i.e., one to four months), and women reported more symptoms (Janiri et al., 2021). In line with the previous findings, our results showed that COVID-19 survivors could maintain a higher PTSS for a longer period (i.e., around six months). Due to the small number of males in both groups (N = 11 and N = 12 in control and COVID-19 survivor groups, respectively), sex was not considered as a factor in the present statistical analyses. Nevertheless, we observed that female survivors reported significantly higher PCL-5 scores than male survivors (t = 4.37, P < 0.001), suggesting females could be more vulnerable to PTSS due to COVID-19.
Although recent studies have demonstrated brain structural abnormalities in COVID-19 survivors (Lu et al., 2020), few previous studies tapped on the brain functional abnormalities, which might reveal the underlying pathophysiology of prolonged mental health symptoms in COVID-19 survivors. In line with a previous study showing intact brain network function in a COVID-19 patient surviving critical illness (Fischer et al., 2020), we did not find any disrupted sFNC by comparing the static functional connectivity/networks between COVID-19 survivors and controls. However, given the dynamic nature of brain activity and connectivity, time-varying characteristics of dFNC that may reveal neural pathophysiology of prolonged mental health symptoms in COVID-19 survivors cannot be discovered through sFNC alone (Chang and Glover, 2010; Hutchison et al., 2013; Liu et al., 2018). We, therefore, focused on investigating the dFNC of COVID-19 survivors and identified three reoccurring brain dFNC states characterized by different connectivity patterns.
Within each brain state, dFNC remained quasi-stable (Allen et al, 2014, 2018). Of the three dFNC states, COVID-19 survivors spent significantly more time in state 1, which was characterized by heterogeneous patterns between sensorimotor and visual networks and strong connectivities between cerebellar network and sensorimotor and visual networks. Importantly, the increased occurrence rate of this state was positively correlated with survivors’ PCL-5 scores, suggesting the link between PTSS and abnormalities in the time-vary characteristics of state 1. In addition, using graph-theory analysis, we observed the disrupted function of the SMA in state 1. Interestingly, we found that the local efficiency of SMA was negatively correlated with GAD and PHQ (results are provided in the supplementary materials). As a critical area for linking cognition to action (Nachev et al., 2008), SMA is believed to be involved in the psychomotor features of depression (Bracht et al., 2012; Sarkheil et al., 2020). Previous work has widely reported decreased SMA volumes in depressive subjects, suggesting a potential linkage between depressive symptoms and brain volume reduction in SMA (Exner et al., 2009). A recent study based on multimodal data from the UKBiobank COVID-19 database has also identified widespread loss of gray matter in COVID-19 patients, including the parahippocampal gyrus, frontal cortex, and insula, and these results extended to the anterior cingulate cortex, supramarginal gyrus, and temporal pole when examining the entire cortical surface (Douaud et al., 2021). Taken together these previous findings and our results, we speculate that COVID-19 survivors with depressive symptoms might show a reduction of brain volume within multiple brain regions linking with SMA, which will limit the local activities in these regions. The restricted local activities will further affect the information processing and integration, reflecting by transiently decreased regional efficiency.
State 2 was a regionally densely connected dFNC state characterized by highly positive within-network connectivity (i.e., subcortical, auditory, sensorimotor, and visual networks) and negative between-network connectivity (i.e., between subcortical-sensorimotor networks). Although this brain state has been identified abnormal in many neuropsychiatric disorders, including autism (Fu et al., 2019b), Parkinson's disease (Kim et al., 2017), schizophrenia (Du et al., 2018), and chronic pain (Tu et al., 2020) (these diseases share a common thalamocortical dysrhythmia model (Llinás et al., 1999; Vanneste et al., 2018)), typically with altered occurrence rate and disrupted cortical-subcortical connectivity, COVID-19 survivors and controls did not differ in the occurrence rate of this state. State 3 was the most frequent state that accounts for more than 70 % of the time. This brain state shared similar patterns with sFNC and was considered as the average of less variable dFNC states, which were not sufficiently distinct to be separated (Allen et al., 2014). Although the occurrence of this state was not aberrant in COVID-19 survivors, we observed lower dFNC (close to zero) between the thalamus and IPL than healthy controls. Such altered dFNC has been observed in many brain disorders, such as autism (Fu et al., 2019b) and schizophrenia (Damaraju et al., 2014). Since diffuse subcortical and parietal white matter hyperintensities were observed in COVID-19 survivors (Kandemirli et al., 2020; Parsons et al., 2020), the identified dFNC between thalamus and IPL might reflect the disrupted functional interaction caused by white matter abnormalities.
There are several limitations in this study. First, we only recruited participants from Wuhan city, the center of the outbreak of COVID-19 at the beginning of 2020. Cohort studies from other regions and countries are necessary for validation. Second, we only recorded brain imaging data in one session. Longitudinal observations will be insightful to observe the trajectories of PTSS and brain dysfunctions in COVID-19 survivors. Another limitation of this study is that we only investigated the associations between dynamic features and PTSS/GAD/PHQ. The investigation of correlations between dynamic features and other behavioral scores will provide more comprehensive information on COVID-19. For example, SMA is involved in motor preparation (Peng et al., 2015), simple voluntary movements (Colebatch et al., 1991), and higher motor processing functions (Rao et al., 1993). In future work, we would like to collect motor performance data for COVID-19 survivors to examine whether COVID-19 influences motor performance and whether it is associated with brain abnormalities in SMA. Finally, clinical records of COVID-19 survivors were not retrievable. Thus, we were not able to examine the relationship between clinical characteristics (e.g., the severity of the disease) and their PTSS/brain connectivity after 6 months.
In conclusion, we recorded PTSS and brain MRI in patients who survived from COVID-19 infection. We found that COVID-19 survivors had more severe PTSS than controls, and the PTSS were not associated with the sFNC, but with the disrupted dFNC state. Given the dynamic nature of brain activity and connectivity, we would like to highlight the great potential to investigate the underlying neural pathophysiology of prolonged mental health symptoms in COVID-19 survivors through the time-varying characteristics of dFNC.
CRediT authorship contribution statement
Zening Fu: Formal analysis, Methodology, Software, Writing – original draft. Yiheng Tu: Writing – review & editing, Funding acquisition. Vince D. Calhoun: Writing – review & editing, Funding acquisition. Yuqi Zhang: Investigation, Data curation. Qing Zhao: Investigation, Data curation. Jun Chen: Conceptualization, Project administration, Resources. Qingtao Meng: Conceptualization, Project administration, Resources. Zhijie Lu: Conceptualization, Project administration, Resources. Li Hu: Conceptualization, Project administration, Supervision, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [31822025 and 32071061 to LH], the Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences [E0CX521003 to YT], the Medical Science and Technology Youth Cultivation Project of PLA (21QNPY061 to ZJL), and National Institutes of Health (NIH) grants [R01EB006841, R01EB020407, and P20GM103472 to VC]. We also thank the patients and families who contributed to these studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100377.
Contributor Information
Qingtao Meng, Email: mengqingtao2018@126.com.
Zhijie Lu, Email: lzjwxyz@163.com.
Li Hu, Email: huli@psych.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Afshar H., Yassin Z., Kalantari S., Aloosh O., Lotfi T., Moghaddasi M., Sadeghipour A., Emamikhah M. Evolution and resolution of brain involvement associated with SARS- CoV2 infection: a close Clinical – paraclinical follow up study of a case. Mult. Scler. Relat. Disord. 2020;43 doi: 10.1016/j.msard.2020.102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Eichele T., Wu L., Calhoun V.D. EEG signatures of dynamic functional network connectivity states. Brain Topogr. 2018;31:101–116. doi: 10.1007/s10548-017-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cerebr. Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone N., Castellano A., Scotti R., Scandroglio A.M., Filippi M., Ciceri F., Tresoldi M., Falini A. Multifocal laminar cortical brain lesions: a consistent MRI finding in neuro-COVID-19 patients. J. Neurol. 2020;267:2806–2809. doi: 10.1007/s00415-020-09966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.N., Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S., Dogonowski A.M., Ernst M., Fair D., Hampson M., Hoptman M.J., Hyde J.S., Kiviniemi V.J., Kötter R., Li S.J., Lin C.P., Lowe M.J., Mackay C., Madden D.J., Madsen K.H., Margulies D.S., Mayberg H.S., McMahon K., Monk C.S., Mostofsky S.H., Nagel B.J., Pekar J.J., Peltier S.J., Petersen S.E., Riedl V., Rombouts S.A.R.B., Rypma B., Schlaggar B.L., Schmidt S., Seidler R.D., Siegle G.J., Sorg C., Teng G.J., Veijola J., Villringer A., Walter M., Wang L., Weng X.C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.F., Zhang H.Y., Castellanos F.X., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.L. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J. Trauma Stress. 2015;28:489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- Bonkhoff A.K., Espinoza F.A., Gazula H., Vergara V.M., Hensel L., Michely J., Paul T., Rehme A.K., Volz L.J., Fink G.R., Calhoun V.D., Grefkes C. Acute ischaemic stroke alters the brain's preference for distinct dynamic connectivity states. Brain. 2020;143:1525–1540. doi: 10.1093/brain/awaa101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Federspiel A., Schnell S., Horn H., Höfle O., Wiest R., Dierks T., Strik W., Müller T.J., Walther S. Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PloS One. 2012;7 doi: 10.1371/journal.pone.0052238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Glover G.H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch J.G., Deiber M.P., Passingham R.E., Friston K.J., Frackowiak R.S.J. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J. Neurophysiol. 1991;65:1392–1401. doi: 10.1152/jn.1991.65.6.1392. [DOI] [PubMed] [Google Scholar]
- Damaraju E., Allen E.A., Belger A., Ford J.M., McEwen S., Mathalon D.H., Mueller B.A., Pearlson G.D., Potkin S.G., Preda A., Turner J.A., Vaidya J.G., Van Erp T.G., Calhoun V.D. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison Himmelfarb C.R., Baptiste D. Coronavirus disease (COVID-19) [WWW document] J. Cardiovasc. Nurs. 2020 doi: 10.1097/jcn.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Winkler A., Nichols T.E., Collins R., Matthews P.M., Allen N., Miller K.L., Smith S.M. medRxiv; 2021. Brain Imaging before and after COVID-19 in UK Biobank. 2021.06.11.21258690. [DOI] [Google Scholar]
- Du Y., Fryer S.L., Fu Z., Lin D., Sui J., Chen J., Damaraju E., Mennigen E., Stuart B., Loewy R.L., Mathalon D.H., Calhoun V.D. Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. Neuroimage. 2018;180:632–645. doi: 10.1016/j.neuroimage.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Fu Z., Sui J., Gao S., Xing Y., Lin D., Salman M., Abrol A., Rahaman M.A., Chen J., Hong L.E., Kochunov P., Osuch E.A., Calhoun V.D. NeuroMark: an automated and adaptive ICA based pipeline to identify reproducible fMRI markers of brain disorders. NeuroImage Clin. 2020;102375 doi: 10.1016/j.nicl.2020.102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Premi E., Pilotto A., Cristillo V., Benussi A., Libri I., Giunta M., Bockholt H.J., Liu J., Campora R., Pezzini A., Gasparotti R., Magoni M., Padovani A., Calhoun V. Alterations of frontal-temporal gray matter volume associate with clinical measures of older adults with COVID-19. Neurobiol. Stress. 2021;100326 doi: 10.1016/j.ynstr.2021.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbert A.R., Cankurtaran S., Karpiak S. Brain abnormalities in COVID-19 acute/subacute phase: a rapid systematic review. Brain Behav. Immun. 2020;89:543–554. doi: 10.1016/j.bbi.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner C., Lange C., Irle E. Impaired implicit learning and reduced pre-supplementary motor cortex size in early-onset major depression with melancholic features. J. Affect. Disord. 2009;119:156–162. doi: 10.1016/j.jad.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Fischer D., Threlkeld Z.D., Bodien Y.G., Kirsch J.E., Huang S.Y., Schaefer P.W., Rapalino O., Hochberg L.R., Rosen B.R., Edlow B.L. Intact brain network function in an unresponsive patient with COVID-19. Ann. Neurol. 2020;88:851–854. doi: 10.1002/ana.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Hastie T., Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008 doi: 10.1093/biostatistics/kxm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Caprihan A., Chen J., Du Y., Adair J.C., Sui J., Rosenberg G.A., Calhoun V.D. Altered static and dynamic functional network connectivity in Alzheimer's disease and subcortical ischemic vascular disease: shared and specific brain connectivity abnormalities. Hum. Brain Mapp. 2019;40:3203–3221. doi: 10.1002/hbm.24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Iraji A., Turner J.A., Sui J., Miller R., Pearlson G.D., Calhoun V.D. Dynamic state with covarying brain activity-connectivity: on the pathophysiology of schizophrenia. Neuroimage. 2021;224 doi: 10.1016/j.neuroimage.2020.117385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Tu Y., Di X., Du Y., Pearlson G.D., Turner J.A., Biswal B.B., Zhang Z., Calhoun V.D. Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: an application to schizophrenia. Neuroimage. 2018;180:619–631. doi: 10.1016/J.NEUROIMAGE.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Tu Y., Di X., Du Y., Sui J., Biswal B.B., Zhang Z., de Lacy N., Calhoun V.D. Transient increased thalamic-sensory connectivity and decreased whole-brain dynamism in autism. Neuroimage. 2019;190 doi: 10.1016/j.neuroimage.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Yeming, Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Yanping, Li C., Peng L., Li Yong, Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Ying, Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A., Bandettini P.A., Calhoun V.D., Corbetta M., Della Penna S., Duyn J.H., Glover G.H., Gonzalez-Castillo J., Handwerker D.A., Keilholz S., Kiviniemi V., Leopold D.A., de Pasquale F., Sporns O., Walter M., Chang C. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri D., Carfì A., Kotzalidis G.D., Bernabei R., Landi F., Sani G. Posttraumatic stress disorder in patients after severe COVID-19 infection. JAMA Psychiatry. 2021 doi: 10.1001/jamapsychiatry.2021.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D., Kaya Y., Yildirim D., Tuzuner F., Yildirim M.S., Ozluk E., Gucyetmez B., Karaarslan E., Koyluoglu I., Demirel Kaya H.S., Mammadov O., Ozdemir I.K., Afsar N., Yalcinkaya B.C., Rasimoglu S., Guduk D.E., Jima A.K., Ilksoz A., Ersoz V., Eren M.Y., Celtik N., Arslan S., Korkmazer B., Dincer S.S., Gulek E., Dikmen I., Yazici M., Unsal S., Ljama T., Demirel I., Ayyildiz A., Kesimci I., Deveci S.B., Tutuncu M., Kizilkilic O., Telci L., Zengin R., Dincer A., Akinci I.O., Kocer N. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;297:E232–E235. doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A., Pascual-Leone A., Michel C.M., Farzan F. Microstates in resting-state EEG: current status and future directions. Neurosci. Biobehav. Rev. 2015;49:105–113. doi: 10.1016/j.neubiorev.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Criaud M., Cho S.S., Díez-Cirarda M., Mihaescu A., Coakeley S., Ghadery C., Valli M., Jacobs M.F., Houle S., Strafella A.P. Abnormal intrinsic brain functional network dynamics in Parkinson's disease. Brain. 2017;140:2955–2967. doi: 10.1093/brain/awx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Fu Z., Luo X., Zeng Q., Huang P., Zhang M., Calhoun V. The influence of cerebral small vessel disease on static and dynamic functional network connectivity in subjects along Alzheimer’s disease continuum. Brain Connect. 2020 doi: 10.1089/brain.2020.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang N., Chang C., Duyn J.H. Co-activation patterns in resting-state fMRI signals. Neuroimage. 2018 doi: 10.1016/j.neuroimage.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R.R., Ribary U., Jeanmonod D., Kronberg E., Mitra P.P. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Li X., Geng D., Mei N., Wu P.Y., Huang C.C., Jia T., Zhao Y., Wang D., Xiao A., Yin B. Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study: a brief title: cerebral changes in COVID-19. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie D.J., Kessler D., Bassett D.S., Betzel R.F., Breakspear M., Kheilholz S., Kucyi A., Liégeois R., Lindquist M.A., McIntosh A.R., Poldrack R.A., Shine J.M., Thompson W.H., Bielczyk N.Z., Douw L., Kraft D., Miller R.L., Muthuraman M., Pasquini L., Razi A., Vidaurre D., Xie H., Calhoun V.D. Questions and controversies in the study of time-varying functional connectivity in resting fMRI. Netw. Neurosci. 2020;4:30–69. doi: 10.1162/netn_a_00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Norton L., Hutchison R.M., Young G.B., Lee D.H., Sharpe M.D., Mirsattari S.M. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology. 2012;78:175–181. doi: 10.1212/WNL.0b013e31823fcd61. [DOI] [PubMed] [Google Scholar]
- Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020;267:2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D., Liddle E.B., Iwabuchi S.J., Zhang C., Wu Z., Liu J., Jiang K., Xu L., Liddle P.F., Palaniyappan L., Fang Y. Dissociated large-scale functional connectivity networks of the precuneus in medication-naïve first-episode depression. Psychiatry Res. Neuroimaging. 2015;232:250–256. doi: 10.1016/j.pscychresns.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum B., North C.S. Mental health and the covid-19 pandemic. N. Engl. J. Med. 2020;383:510–512. doi: 10.1056/nejmp2008017. [DOI] [PubMed] [Google Scholar]
- Preti M.G., Bolton T.A., Van De Ville D. The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage. 2017;160:41–54. doi: 10.1016/j.neuroimage.2016.12.061. [DOI] [PubMed] [Google Scholar]
- Rao S.M., Binder J.R., Bandettini P.A., Hammeke T.A., Yetkin F.Z., Jesmanowicz A., Lisk L.M., Morris G.L., Mueller W.M., Estkowski L.D., Wong E.C., Haughton V.M., Hyde J.S. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–2318. doi: 10.1212/wnl.43.11.2311. [DOI] [PubMed] [Google Scholar]
- Sarkheil P., Odysseos P., Bee I., Zvyagintsev M., Neuner I., Mathiak K. Functional connectivity of supplementary motor area during finger-tapping in major depression. Compr. Psychiatr. 2020;99:152166. doi: 10.1016/j.comppsych.2020.152166. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Tu Y., Fu Z., Mao C., Falahpour M., Gollub R.L., Park J., Wilson G., Napadow V., Gerber J., Chan S.T., Edwards R.R., Kaptchuk T.J., Liu T., Calhoun V., Rosen B., Kong J. Distinct thalamocortical network dynamics are associated with the pathophysiology of chronic low back pain. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-17788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Fu Z., Zeng F., Maleki N., Lan L., Li Z., Park J., Wilson G., Gao Y., Liu M., Calhoun V., Liang F., Kong J. Abnormal thalamocortical network dynamics in migraine. Neurology. 2019;92 doi: 10.1212/WNL.0000000000007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Zhang Y., Li Y., Zhao Q., Bi Y., Lu X., Kong Y., Wang L., Lu Z., Hu L. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol. Psychiatr. 2021:1–6. doi: 10.1038/s41380-021-01223-w. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., Song J.-J., De Ridder D. Thalamocortical dysrhythmia detected by machine learning. Nat. Commun. 2018;9:1103. doi: 10.1038/s41467-018-02820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O'Dell S., Schmidt S.D., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.-V., Floyd K., Suthar M.S., Buchanan W., Luke C.J., Ledgerwood J.E., Mascola J.R., Graham B.S., Beigel J.H. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2032195. NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worldometer . 2021. Coronavirus update (live): cases and deaths from COVID-19 virus pandemic. [WWW Document]. Worldometers. [Google Scholar]
- Xiong J., Lipsitz O., Nasri F., Lui L.M.W., Gill H., Phan L., Chen-Li D., Iacobucci M., Ho R., Majeed A., McIntyre R.S. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J. Affect. Disord. 2020 doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were generated at CAS Key Laboratory of Mental Health, Institute of Psychology, Beijing, China. Derived data supporting the findings of this study are available from the corresponding author on request.
Data will be made available on request.