Abstract
Several studies have examined the functions of nucleic acids in small extracellular vesicles (sEVs). However, much less is known about the protein cargos of sEVs and their functions in recipient cells. This study demonstrates the presence of lysine‐specific demethylase 1 (LSD1), which is the first identified histone demethylase, in the culture medium of gastric cancer cells. We show that sEVs derived from gastric cancer cells and the plasma of patients with gastric cancer harbor LSD1. The shuttling of LSD1‐containing sEVs from donor cells to recipient gastric cancer cells promotes cancer cell stemness by positively regulating the expression of Nanog, OCT4, SOX2, and CD44. Additionally, sEV‐delivered LSD1 suppresses oxaliplatin response of recipient cells in vitro and in vivo, whereas LSD1‐depleted sEVs do not. Taken together, we demonstrate that LSD1‐loaded sEVs can promote stemness and chemoresistance to oxaliplatin. These findings suggest that the LSD1 content of sEV could serve as a biomarker to predict oxaliplatin response in gastric cancer patients.
Keywords: gastric cancer, LSD1, small extracellular vesicles, stemness
Subject Categories: Cancer; Membrane & Intracellular Transport; Post-translational Modifications, Proteolysis & Proteomics
Lysine specific demethylase 1 is not only a nuclear protein but is also secreted. Gastric cancer cells release sEVs that contain LSD1, thereby promoting cancer cell stemness and oxaliplatin resistance.

Introduction
Gastric cancer, which is the fifth most frequently diagnosed cancer and the third leading cause of cancer‐related deaths worldwide, accounted for more than one million new cases and 783,000 estimated deaths in 2018 (Bray et al, 2018). The incidence of gastric cancer and gastric cancer‐related mortality are declining globally. However, the incidence of gastric cancer is high in several parts of the world, especially in East Asia and South America (Van Cutsem et al, 2016). The therapeutic strategies for gastric cancer include surgery and chemotherapy (Takahashi et al, 2013). However, the current chemotherapy regimens for gastric cancer have limited efficacy as they do not mitigate tumor recurrence, which is partially due to the persistence of cancer stem cells (CSCs) (Enjoji et al, 2018; Huang et al, 2019). The molecular mechanisms underlying cancer stemness have not been completely elucidated.
In multicellular organisms, distant cells can interact through various molecules or extracellular vesicles (EVs) harboring unique proteins, lipids, and nucleic acids (Tkach & Thery, 2016). The secreted EVs are detected in the urine, amniotic fluid, bronchoalveolar lavage fluid, breast milk, saliva, and blood. Small EVs (sEVs) are the smallest subset of EVs with a size ranging from 50 to 150 nm (EL Andaloussi et al, 2013). Various proteins and nucleic acids are packed into the sEVs. The fusion of sEVs to the target cells enables the delivery of molecular cargos to the recipient cells. Hence, sEVs are important mediators of cell‐to‐cell communication.
Recent studies have suggested that the sEV‐related nucleic acids, such as microRNAs (miRNAs) and messenger RNAs (mRNAs), which mediate various signaling processes, are potential diagnostic and prognostic biomarkers for cancer (Valadi et al, 2007; Wang et al, 2010; Ono et al, 2014; Singh et al, 2014; Bao et al, 2018). However, limited studies have examined the proteins within sEVs. sEVs harbor several proteins, such as such as β‐catenin (Chairoungdua et al, 2010), EGFR (Zhang et al, 2017a), PD‐L1 (Chen et al, 2018), p65 and p53 (Yang et al, 2017), DNMT1 (Cao et al, 2017), Notch3 (Lin et al, 2019), Claudin (Li et al, 2009), and Wnt10b (Chen et al, 2017). Additionally, previous studies have reported that sEVs comprise several transcriptional regulators and that mRNAs and proteins within sEVs are involved in both the response to environmental stimuli and epigenetic modifications, especially histone modification (Ung et al, 2014; Qian et al, 2015). Studies on the functions of histone demethylases, which are one of the sEV cargos, are limited.
This study demonstrated that the histone demethylase LSD1, which functions as an oncogene in gastric cancer (Huang et al, 2007; Wang et al, 2009; Kontaki & Talianidis, 2010; Zheng et al, 2016b; Li et al, 2017), is enriched in sEVs from gastric cancer cells and plasma of patients with gastric cancer. The sEV cargos, including LSD1, can be transferred from the parent to recipient gastric cancer cells and enhance their stemness and suppress chemosensitivity in vitro and in vivo. Conversely, sEVs lacking LSD1 do not promote gastric cell stemness or suppressed chemosensitivity. The findings of this study demonstrated that LSD1 is delivered through sEVs. Additionally, this study elucidated a critical non‐canonical pathway of LSD1 that promotes gastric cancer carcinogenesis by functioning as a secreted protein instead of a nuclear protein.
Results
LSD1 is secreted through sEVs from gastric cancer cells
Cells secrete various growth factors or EVs during growth. Hence, the morphology of cells may change upon stimulation with cargos secreted into the cell culture medium. In this study, conditioned medium from the gastric cancer cell line MGC‐803 promoted sphere formation, which is a characteristic feature of cancer cell stemness, in the recipient gastric cancer cells (Fig 1A). Hence, we hypothesized that some specific components in conditioned medium may contribute to cancer cell stemness. sEVs are one of the potential components in the conditioned medium that contribute to cancer cell stemness as they have critical roles in intercellular communication (Chaput & Thery, 2011; Lee et al, 2012). To verify this hypothesis, a sphere formation assay was performed using GW4869 (10 µM), an inhibitor of sEV biogenesis and secretion (Jiang et al, 2017; Faict et al, 2018). Treatment with GW4869 mitigated the conditioned medium‐induced enhanced sphere formation to a level observed in the cells incubated in fresh medium (Fig 1B). Therefore, these data indicate that sEVs may contribute to gastric cancer cell stemness. To further confirm the role of sEVs in gastric cancer cell stemness, the MGC‐803 cells were treated with sEVs isolated using differential ultracentrifugation (sEV fraction) or the supernatant of differential ultracentrifugation (sEV‐lacking fraction). sEV‐treated cells exhibited enhanced sphere‐forming ability (Fig 1C). The sEV marker proteins were detected using Western blotting to examine the number of sEVs secreted from equal numbers of cells. GW4869 effectively inhibited the secretion of sEVs from the gastric cancer cells (Fig EV1A). The number of sEVs secreted from the GW4869‐treated cells was lower than that secreted from the untreated cells. Additionally, treatment with GW4869 decreased the number of sEVs released from the same number of cells and consequently inhibited sphere formation in the recipient cells (Fig EV1B). Furthermore, sEVs dose‐dependently promoted sphere formation in recipient cells (Fig EV1C). Thus, gastric cancer cell‐derived sEVs promoted gastric cancer cell stemness.
Figure 1. LSD1 is secreted from gastric cancer cells through small extracellular vesicles (sEVs).
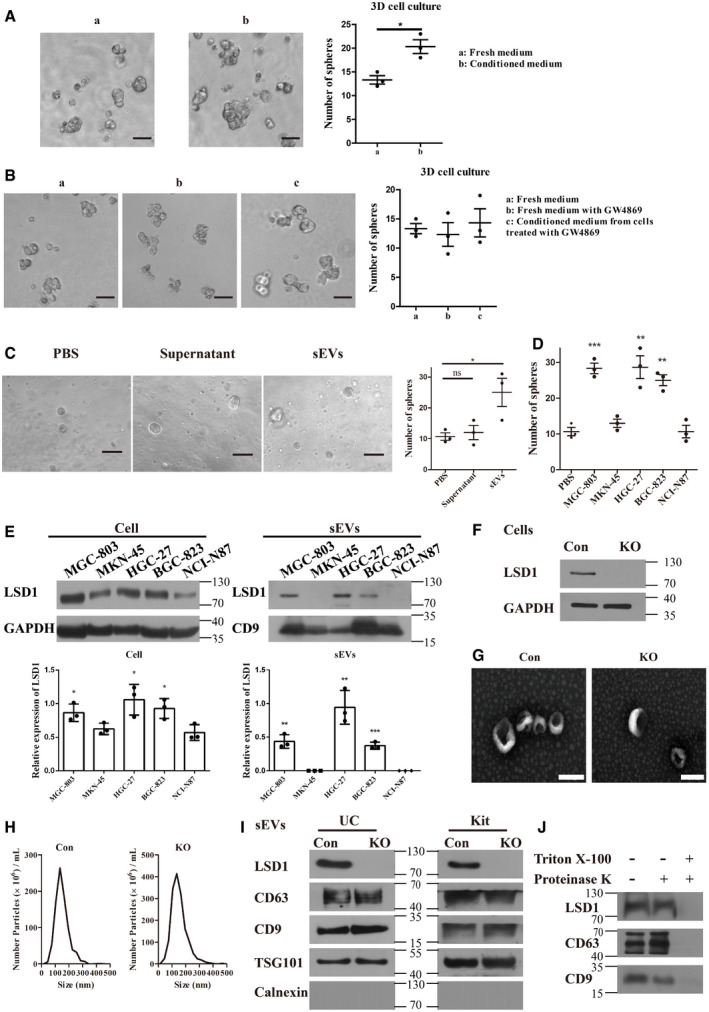
-
ASphere formation in MGC‐803 cells incubated with fresh medium or conditioned medium from MGC‐803 cells for 7 days. The number of spheres was quantified and indicated on the right. Scale bar = 100 µm (n = 3 biological replicates; mean ± standard error of mean (SEM); *P = 0.0146; two‐tailed unpaired Student’s t‐test).
-
BSphere formation in MGC‐803 cells with indicated treatment. The number of spheres was quantified and indicated on the right. Scale bar = 100 µm (n = 3 biological replicates; mean ± SEM; no significant differences; two‐tailed unpaired Student’s t‐test).
-
CSphere formation in MGC‐803 cells incubated with an equal volume of phosphate‐buffered saline, supernatant after differential centrifugation, or sEVs. Scale bar = 100 µm (n = 3 biological replicates; mean ± SEM; ns, no significant difference; *P = 0.0390; two‐tailed unpaired Student’s t‐test).
-
DSphere formation in MGC‐803 cells treated with sEVs (20 μg/ml) from five gastric cancer cell lines as indicated (n = 3 biological replicates; mean ± SEM; ***P = 0.0007 (MGC‐803), **P = 0.0061 (HGC‐27), and **P = 0.0018 (BGC‐823); two‐tailed unpaired Student’s t‐test).
-
EExpression levels of LSD1 in MGC‐803, MKN‐45, HGC‐27, BGC‐823, and NCI‐N87 cell lines and their corresponding sEVs. The samples with equal amounts of proteins were loaded (n = 3 biological replicates; mean ± SEM; compared with the NCI‐N87; *P = 0.0432 (cell/MGC‐803), *P = 0.0300 (cell/HGC‐27), **P = 0.0306 (cell/BGC‐823), **P = 0.0018 (sEVs/MGC‐803), **P = 0.0029 (sEVs/HGC‐27), and ***P = 0.0003 (sEVs/BGC‐823); two‐tailed unpaired Student’s t‐test; GAPDH was used as a loading control for cell lysis; CD9 was used as a loading control for sEV lysis).
-
FEstablishment of LSD1 knockout (KO) MGC‐803 cell line. Con indicates MGC‐803 cells, while KO indicates LSD1 KO MGC‐803 cells.
-
G, HTransmission electron microscopy images (G) and the size distribution (H) of sEVs from MGC‐803 and LSD1 KO MGC‐803 cells. Scale bar = 100 nm.
-
IExpression levels of LSD1, CD63, CD9, TSG101, and calnexin in sEVs from MGC‐803 and LSD1 KO MGC‐803 cells. sEVs were extracted using two different extraction methods (ultracentrifugation (UC) and commercial kit). Calnexin is an sEV negative marker. UC, sEVs isolated using the ultracentrifugation method; Kit, sEVs isolated using the commercial kit.
-
JExpression levels of LSD1, CD63, and CD9 in sEVs with indicated treatment.
Source data are available online for this figure.
Figure EV1. Small extracellular vesicles (sEVs) promote sphere formation in recipient cells.
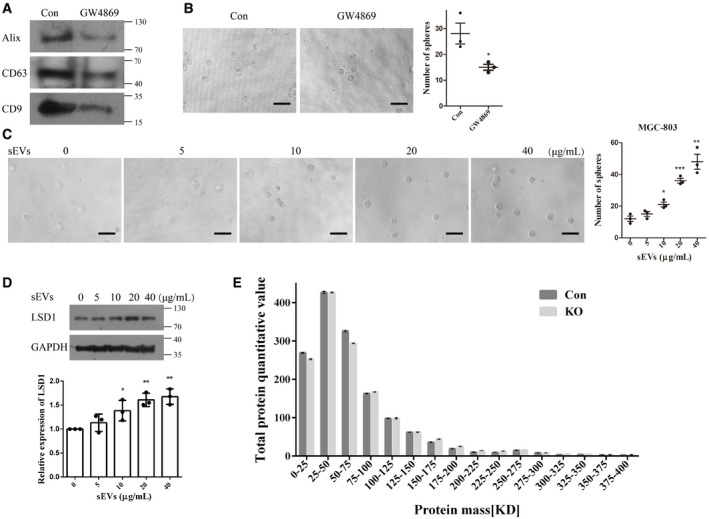
- sEV marker proteins were detected using Western blotting to determine the amount of sEVs secreted by equal numbers of cells (Con indicates sEVs isolated from MGC‐803 cells; GW4869 indicates sEVs isolated from GW4869‐treated MGC‐803 cells).
- Sphere formation assay results of MGC‐803 cells treated with sEVs (from equal number of cells) from control or GW4869‐treated cell culture medium. Scale bar = 100 µm (n = 3 biological replicates; mean ± standard error of mean (SEM); *P = 0.0365; two‐tailed unpaired Student’s t‐test).
- Sphere formation assay results of MGC‐803 cells treated with different doses of sEVs as indicated. Scale bar = 100 µm (n = 3 biological replicates; mean ± SEM; *P = 0.0176, ***P = 0.0005, and **P = 0.0020; two‐tailed unpaired Student’s t‐test).
- Expression level of LSD1 in MGC‐803 cells treated with different doses of sEVs as indicated (n = 3 biological replicates; mean ± SEM; *P(10 μg/ml) = 0.0362, **P(20 μg/ml) = 0.0017, and **P(40 μg/ml) = 0.0019; two‐tailed unpaired Student’s t‐test; GAPDH was used as a loading control for cell lysis).
- Protein contents in sEVs from control (Con) and LSD1 knockout (KO) cells were profiled using mass spectrometry (n = 3 biological replicates; mean ± SEM).
Source data are available online for this figure.
The ability of sEVs from diverse gastric cancer cells to promote sphere formation is unclear owing to their complexity and heterogeneity. Therefore, the MGC‐803 cells were treated with an equal number of sEVs (determined based on the protein levels) derived from five representative gastric cell lines. As shown in Fig 1D, only sEVs derived from MGC‐803, HGC‐27, and BGC‐823 cells promoted sphere formation in the recipient cells. The data shown in Fig 1E (left panel) indicate that these cell lines exhibit LSD1 overexpression, which is consistent with the results of previous studies (Zheng et al, 2013). LSD1, which is reported to promote cancer cell stemness (Amente et al, 2013; Lei et al, 2015), has been considered as a drug target (Zheng et al, 2016a; Sun et al, 2017; Zheng et al, 2017; Duan et al, 2018; Liu et al, 2019). Previous studies have predicted that LSD1 is a secretory protein using Phobius (Kall et al, 2007) and SPOCTOPUS (Viklund et al, 2008). However, LSD1 is localized to the nucleus (Shi et al, 2004). The correlation between sEVs and LSD1 is unclear. In this study, the expression of LSD1 in sEVs derived from MGC‐803, MKN‐45, HGC‐27, BGC‐823, and NCI‐N87 cell lines was examined (Fig 1E; right panel). sEVs derived from MGC‐803, HGC‐27, and BGC‐823 cells exhibited higher levels of LSD1 than those derived from other cell lines. This was consistent with the LSD1 expression levels in the cells. Consistently, sEVs derived from MGC‐803, HGC‐27, and BGC‐823 cells exhibited enhanced ability to promote sphere formation in the recipient cells (Fig 1D). In addition to promoting sphere formation in the recipient cells, sEVs dose‐dependently enhanced the levels of LSD1 in the recipient cells (Fig EV1C–D). Next, the role of the sEV cargo LSD1 in promoting the stemness of gastric cancer cells was examined.
sEVs from MGC‐803 and LSD1 knockout (KO) MGC‐803 cells (Fig 1F) were subjected to transmission electron microscopy (TEM). The size distribution was monitored using NanoSight particle tracking analysis (NTA) for quality control (Fig 1G–H). Additionally, the protein content in the sEVs was analyzed using mass spectrometry to examine the effect of LSD1 KO on sEV contents. As shown in Fig EV1E, the distribution of proteins with different masses was not significantly different between sEVs derived from MGC‐803 and those derived from LSD1 KO MGC‐803 cells. As shown in Fig 1I, the analysis of LSD1 levels revealed that LSD1 was enriched in sEVs derived from MGC‐803 but not in those derived from LSD1 KO MGC‐803 cells, no matter the sEVs isolated by ultracentrifugation or commercial kits. Meanwhile, the levels of sEV markers (CD63, TSG101, and CD9) were constant and the sEV negative marker calnexin was not detected. To further confirm the presence of LSD1 in sEVs, the MGC‐803 cell‐derived sEVs were treated with proteinase K, Triton X‐100, or their combination. As shown in Fig 1J, LSD1 was not detected upon treatment with the combination of proteinase K and Triton X‐100. This is because Triton X‐100 damages the structure of sEVs, which allows proteinase K penetration and consequently the digestion of proteins in sEVs. Treatment with proteinase K did not affect the LSD1 levels as proteinase K could not penetrate and damage the proteins in sEVs. This indicated that LSD1 was within the sEVs.
These results demonstrate that sEVs secreted from gastric cancer cells promote sphere formation and that LSD1 is secreted through sEVs. However, the delivery of sEVs harboring LSD1 to the recipient cells must be further clarified.
LSD1‐containing sEVs can deliver LSD1 to target cells
The ability of sEVs to fuse with recipient cells was examined. As shown in Fig 2A, sEVs (stained with PKH26; red) from MGC‐803 (Con sEVs) and LSD1 KO MGC‐803 cells (KO sEVs) fused with recipient MGC‐803 and MKN‐45 cells (cell membrane was stained with Dio; green and nuclei were stained with 4',6‐diamidino‐2‐phenylindole (DAPI)). Next, the LSD1 KO MGC‐803 and MKN‐45 cells were incubated with Con and KO sEVs. LSD1 was detected only in LSD1 KO MGC‐803 and MKN‐45 cells treated with Con sEVs but not in those treated with KO sEVs (Fig 2B). These results demonstrate that the nuclear protein LSD1 can be delivered into recipient gastric cancer cells through sEVs.
Figure 2. LSD1‐containing small extracellular vesicles (sEVs) fuse to the recipient cell and deliver LSD1.
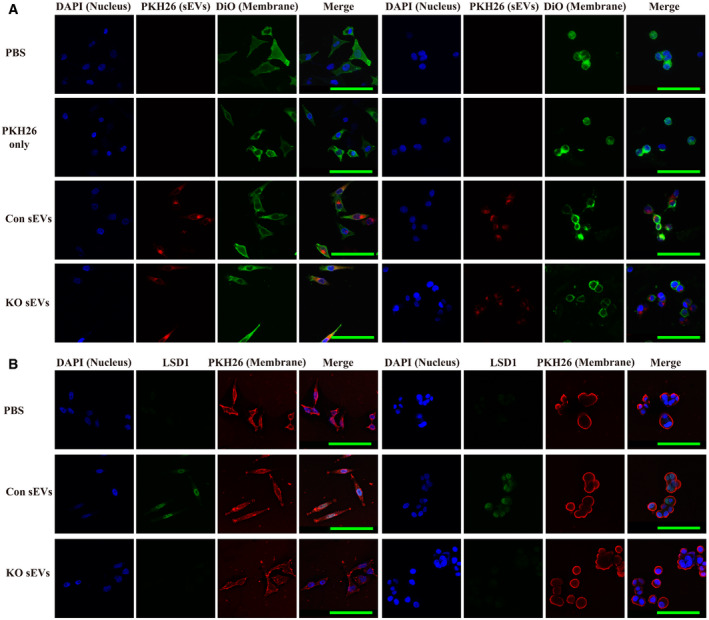
- Confocal microscopy image analysis of sEV fusion to MGC‐803 cells. The MGC‐803 (left side) and MKN‐45 (right side) cells were treated with sEVs derived from MGC‐803 cells (Con sEVs) or LSD1 knockout (KO) MGC‐803 cells (KO sEVs) and stained with PKH26 for 12 h. Additionally, the cell membrane was stained with Dio, while the nuclei were stained with 4',6‐diamidino‐2‐phenylindole (DAPI). Scale bar = 100 µm.
- Immunofluorescence confocal microscopy analysis of LSD1 (green) in LSD1 KO MGC‐803 cells (left panel) and LSD1 KO MKN‐45 cells (right panel) incubated with 20 μg/ml Con sEVs and KO sEVs for 12 h. The cell membrane was stained with PKH26, while the nuclei were stained with DAPI. Scale bar = 100 µm.
LSD1 promotes stemness by facilitating the accumulation of SOX2
Next, the role of LSD1 in gastric cancer was examined. The expression of LSD1 in gastric cancer tissues was analyzed using The Cancer Genome Atlas data from UALCAN (http://ualcan.path.uab.edu/index.html) (Chandrashekar et al, 2017). As shown in Fig 3A, the expression of LSD1 in gastric cancer tissues was upregulated when compared with that in the adjacent normal tissues. Furthermore, the expression of LSD1 in clinical specimens was analyzed using the data from Gene Expression Profiling Interactive Analysis (GEPIA) (http://gepia.cancer‐pku.cn/) (Tang et al, 2017). As shown in Fig 3B, the expression of LSD1 in the gastric cancer tissues was upregulated when compared with that in the adjacent non‐cancerous tissues. The overall survival analysis of data from GEPIA also confirmed that the expression of LSD1 in gastric cancer tissue was associated with poor prognosis (Fig 3C). These findings indicate that the enhanced expression of LSD1 in gastric cancer tissues is associated with poor clinical outcomes. However, the role of LSD1 in poor prognosis of gastric cancer is unknown.
Figure 3. LSD1 facilitates stemness and promotes the accumulation of SOX2.
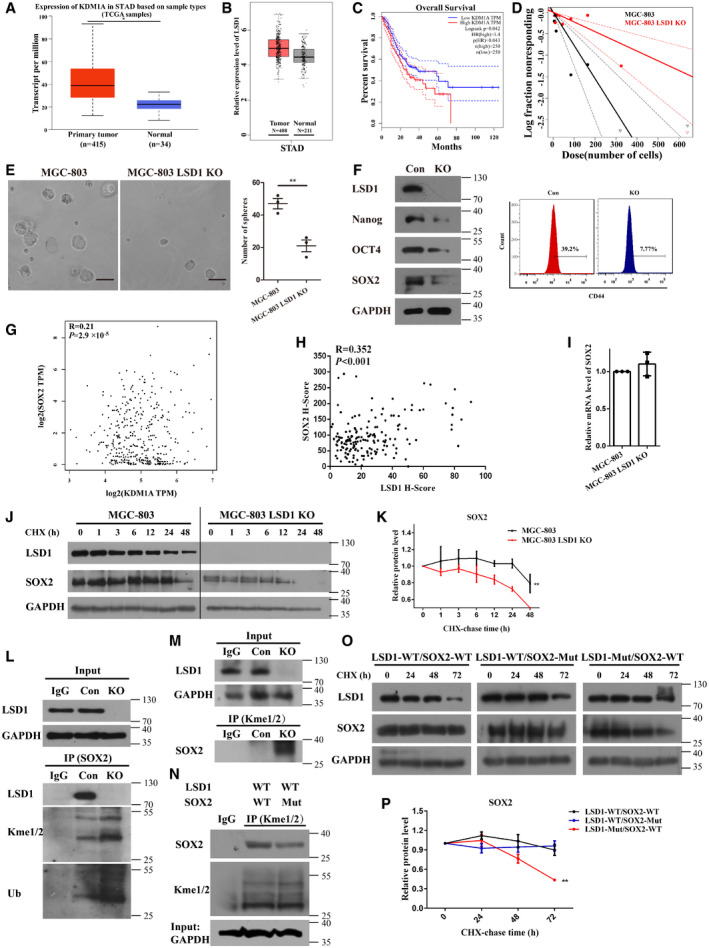
- Expression level of LSD1 (KDM1A) in gastric cancer and non‐cancerous tissues from UALCAN datasets (STAD, stomach adenocarcinoma; central band, boxes, and whiskers of the boxplot represent the median, first quartile, third quartile, minimum, and maximum values, respectively).
- Expression level of LSD1 (KDM1A) in gastric cancer or non‐cancerous tissues from Gene Expression Profiling Interactive Analysis (GEPIA) datasets (central band, boxes, and whiskers of the boxplot represent the median, first quartile, third quartile, minimum, and maximum values, respectively).
- Overall survival analysis using GEPIA datasets (log‐rank test; the solid line represents the survival curve, while the dashed line represents the 95% confidence interval).
- In vitro limiting dilution assay with MGC‐803 and LSD1 knockout (KO) MGC‐803 cells (the solid line represents the sphere formation ability curve, while the dashed line represents the 95% confidence interval).
- Sphere formation assay results of MGC‐803 and LSD1 KO MGC‐803 cells. Scale bar = 100 µm (n = 3 biological replicates; mean ± standard error mean (SEM); **P = 0.0058; two‐tailed unpaired Student’s t‐test).
- Expression levels of LSD1, Nanog, OCT4, SOX2, and CD44 in LSD1 KO MGC‐803 cells.
- Correlation between LSD1 and SOX2 mRNA levels analyzed using the GEPIA dataset (Pearson’s test).
- Correlation between LSD1 and SOX2 in 172 gastric cancer tissues (Pearson’s test).
- The mRNA levels of SOX2 in different cells were detected using quantitative real‐time polymerase chain reaction (n = 3 biological replicates; mean ± SEM).
- Stability of SOX2 in MGC‐803 and LSD1 KO MGC‐803 cells treated with cycloheximide (20 μM) at the indicated times.
- Relative intensity of SOX2 in (J) (n = 3 biological replicates, mean ± SEM).
- Immunoprecipitation of Kme1/2 and ubiquitin (Ub) with SOX2 in the presence or absence of LSD1. The cells were treated with MG132 (10 μM) for 8 h before analysis.
- Reverse immunoprecipitation of Kme1/2 on SOX2.
- Immunoprecipitation of Kme1/2 on SOX2 (WT indicates HEK293T cells co‐transfected with LSD1‐WT and SOX2‐WT; Mut indicates HEK293T cells co‐transfected with LSD1‐WT and SOX2‐Mut; WT, wild type; Mut, mutant).
- Stability of SOX2 in HEK293T cells co‐transfected with different plasmids (WT, wild type; SOX2‐Mut, K42R, and K117R mutations; LSD1‐Mut: K661A mutation).
- Relative intensity of SOX2 in (O) (n = 3 biological replicates; mean ± SEM; **P = 0.0055; two‐tailed unpaired Student’s t‐test).
Source data are available online for this figure.
Cancer stem cells, which are a small subgroup of cells capable of self‐renewal and differentiation (Clarke et al, 2006), contribute to tumor initiation, progression, therapeutic resistance, and tumor recurrence (Tanase et al, 2014). Hence, gastric CSCs (GCSCs) have piqued the interest of the scientific community. Some GCSC candidate markers are reported to be potential therapeutic targets for gastric cancer (Singh, 2013). Therefore, this study examined the role of LSD1 in gastric cancer cell self‐renewal ability and chemoresistance. The results of the in vitro limiting dilution assays (Fig 3D) suggested that LSD1 KO significantly inhibited the self‐renewal of gastric cancer cells. Additionally, the sphere number and size of MGC‐803 cells were higher than those of LSD1 KO MGC‐803 cells (Fig 3E). Meanwhile, LSD1 KO downregulated the expression of stemness markers, including OCT4, SOX2, Nanog, and CD44, which are core transcription factors that promote self‐renewal in the tumor cells (Fig 3F). The role of LSD1 in gastric cancer cell stemness was further evaluated by rescuing LSD1 expression in LSD1 KO MGC‐803 cells and treating MGC‐803 cells with the LSD1 inhibitor GSK‐LSD1. As shown in Fig EV2A–B, rescuing LSD1 expression effectively restored the sphere formation ability of LSD1 KO MGC‐803 cells, and treatment with GSK‐LSD1 significantly attenuated the sphere formation ability of MGC‐803 cells. These findings suggest that LSD1 is required for the self‐renewal of gastric cancer cells and that the inhibition of LSD1 suppresses the stemness of gastric cancer cells. Next, the mechanism underlying LSD1‐mediated regulation of gastric cancer cell stemness was examined.
Figure EV2. LSD1 expression is correlated with gastric cancer cell stemness.
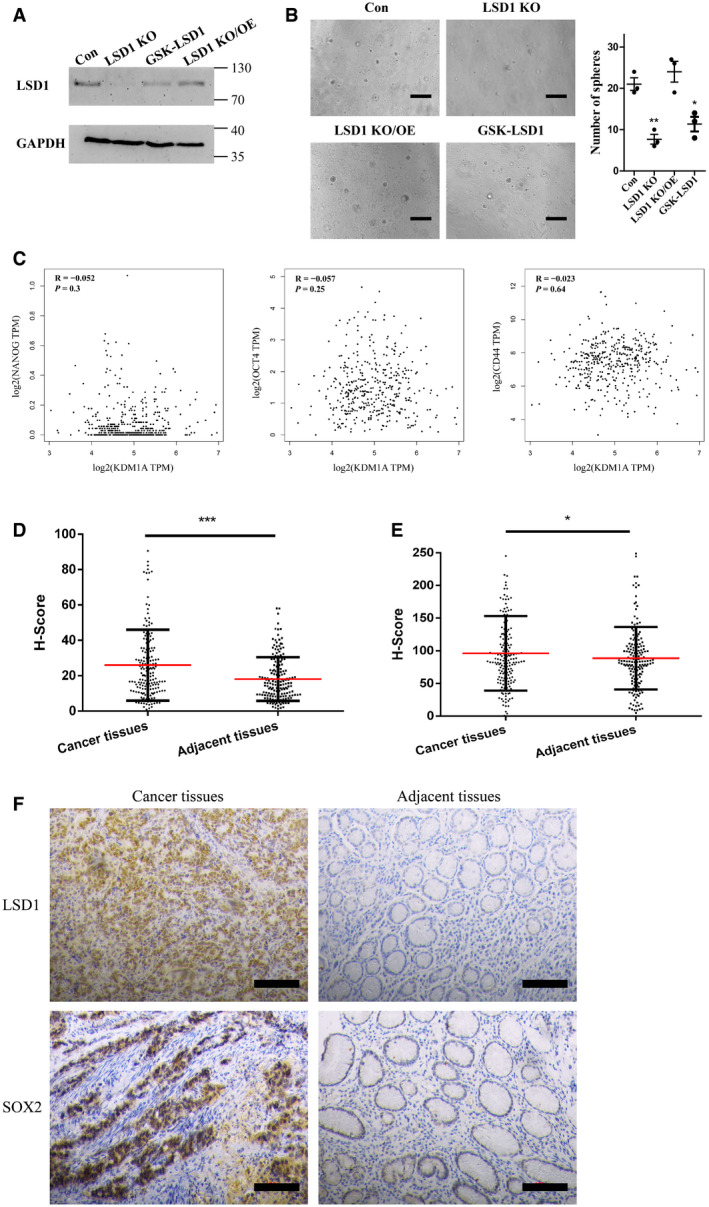
-
AExpression level of LSD1 in MGC‐803, LSD1 knockout (KO) MGC‐803, LSD1 KO MGC‐803 cells transfected with LSD1‐encoding plasmid, and GSK‐LSD1‐treated MGC‐803 cells. GAPDH was used as a loading control.
-
BSphere formation assay results of MGC‐803 cells subjected to different treatments as indicated in the figure. Scale bar = 100 µm (n = 3 biological replicates; mean ± standard error mean (SEM); **P = 0.0042 and *P = 0.0146; two‐tailed unpaired Student’s t‐test).
-
CCorrelation of LSD1 (KDM1A) with Nanog, OCT4, and CD44 determined using Gene Expression Profiling Interactive Analysis (GEPIA) datasets (Pearson’s test).
-
D, EExpression levels of LSD1 (D) and SOX2 (E) in 172 pairs of gastric cancer tissues and adjacent non‐cancerous tissues were examined using immunohistochemical analysis (n = 172 paired tissues; mean ± SEM; ***P < 0.0001 (LSD1) and *P = 0.0348 (SOX2); two‐tailed unpaired Student’s t‐test).
-
FRepresentative images are shown as indicated. Scale bar = 100 µm.
Source data are available online for this figure.
In this study, LSD1 KO decreased the levels of stemness markers. The correlation between LSD1 and stemness markers was examined using the data from GEPIA (Tang et al, 2017). LSD1 was not significantly correlated with OCT4, Nanog, and CD44 (Fig EV2C). However, LSD1 was significantly correlated with SOX2 (R = 0.21, P < 0.0001) in gastric cancer (Fig 3G). LSD1 is reported to demethylate SOX2 in ovarian cancer (Zhang et al, 2018). Thus, the regulatory effect of LSD1 on SOX2 in gastric cancer was investigated. Immunohistochemical analysis was performed on 172 pairs of gastric cancer tissues and adjacent non‐cancerous tissues. As shown in Fig EV2D–F, the expression levels of LSD1 and SOX2 were upregulated in gastric cancer tissues. Additionally, the expression of LSD1 was significantly and positively correlated with that of SOX2 (R = 0.352; P < 0.001) in gastric cancer specimens (Fig 3H).
Lysine‐specific demethylase 1 demethylates lysine on SOX2, which leads to the deubiquitination and stabilization of SOX2 in CSCs (Zhang et al, 2013; Zhang et al, 2018; Zhang et al, 2019). Hence, the ability of LSD1 to stabilize SOX2 in gastric cancer was examined. The mRNA level of SOX2 in MGC‐803 and LSD1 KO MGC‐803 cells was examined. As shown in Fig 3I, LSD1 KO did not affect the SOX2 mRNA level. Next, the MGC‐803 and LSD1 KO MGC‐803 cells were treated with cycloheximide to inhibit mRNA translation. LSD1 increased the half‐life of SOX2 (Fig 3J–K), which suggested that LSD1 stabilizes SOX2 in gastric cancer (Fig 3J–K). Furthermore, the MGC‐803 and LSD1 KO MGC‐803 cells were subjected to immunoprecipitation assay using anti‐SOX2 antibodies to examine the effect of LSD1 KO on the levels of Kme1/2 (Fig 3L). Additionally, the anti‐Kme1/2 antibody was used as a bait for SOX2 (Fig 3M). LSD1 KO promoted the methylation of SOX2 (Fig 3L–M). Previous studies have reported that LSD1 demethylates K42 and K117 of SOX2 to inhibit proteolysis (Zhang et al, 2018; Zhang et al, 2019). In this study, K42R and K117R mutants of SOX2 were generated to further confirm the regulatory effect of LSD1 on SOX2 methylation in gastric cancer cells. The HEK293T cells were co‐transfected with LSD1‐wild type (WT) and SOX2‐WT or LSD1‐WT and SOX2 mutant (Mut). The results of the Kme1/2 immunoprecipitation assay (Fig 3N) revealed that K42R and K117R mutations significantly decreased the lysine methylation of SOX2. Compared with that in HEK293T cells co‐transfected with LSD1‐Mut and SOX2‐WT, SOX2 stability was significantly higher in cells co‐transfected with LSD1‐WT and SOX2‐WT or LSD1‐WT and SOX2‐Mut (Fig 3O–P). These results suggest that LSD1‐mediated demethylation or SOX2 mutations enhanced the stability of SOX2. However, the stability of SOX2 decreased upon mutation of LSD1. In summary, LSD1 functions as a demethylase to remove the methyl groups on the lysine residues of SOX2 and consequently prevents the methylation‐dependent proteolysis of SOX2. However, further studies are needed to confirm this finding.
LSD1‐containing sEVs promote gastric cancer cell stemness
Next, the ability of LSD1 delivered by sEVs to promote the stemness of recipient cells was examined. The MGC‐803 and MKN‐45 cells were incubated with Con and KO sEVs and subjected to sphere formation assay. As shown in Fig 4A, Con sEV‐treated cells exhibited enhanced sphere formation ability. Furthermore, the results of the limited dilution assay also demonstrated that Con sEVs increased the frequency of gastric cancer cell sphere formation (Fig 4B). Meanwhile, Con sEV‐treated cells exhibited upregulated expression levels of LSD1, OCT4, SOX2, and CD44 when compared with control cells. In contrast, the expression levels of LSD1, OCT4, SOX2, and CD44 were similar between KO sEV‐treated and control cells (Fig 4C–E). This indicated that sEVs from gastric cancer cells, which exhibit upregulated LSD1 expression, may promote the stemness of recipient gastric cancer cells. Next, the MGC‐803 and MKN‐45 cells were treated with sEVs from HEK293T cells, WT‐LSD1‐overexpressing HEK293T cells, and LSD1 K661A mutant (LSD1 K661A)‐overexpressing HEK293T cells (Fig EV3). sEVs derived from WT‐LSD1‐overexpressing HEK29T cells (WT‐LSD1 sEVs) but not those derived from LSD1 K661A‐overexpressing HEK293T cells promoted sphere formation in the MGC‐803 and MKN‐45 cells (Fig 4F). These results suggest that in addition to LSD1 in the cells, sEV‐delivered LSD1 can promote the stemness of gastric cancer cells in vitro.
Figure 4. LSD1‐containing small extracellular vesicles (sEVs) promote cancer cell stemness in vitro .
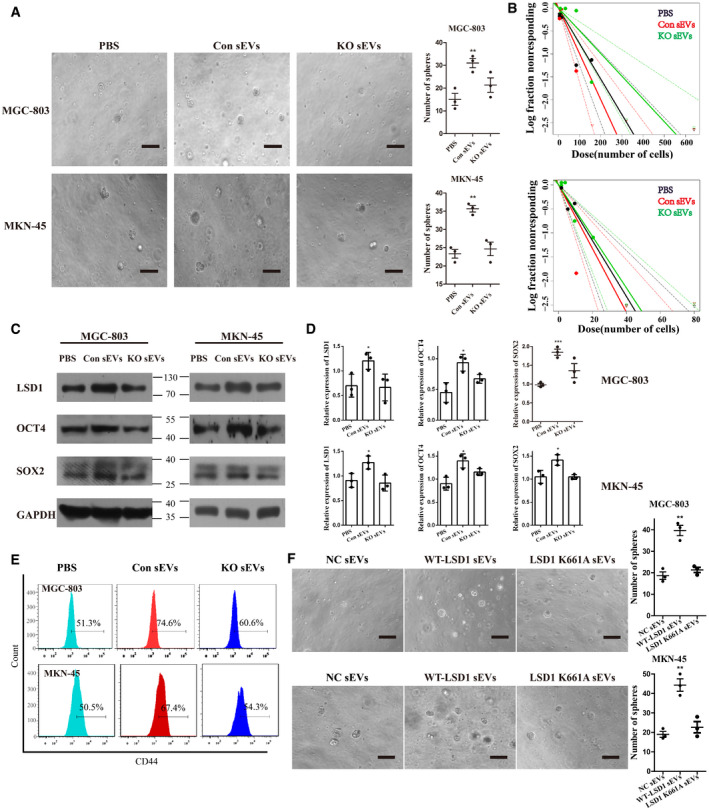
- Sphere formation assay of MGC‐803 and MKN‐45 cells incubated with 20 μg/ml sEVs from MGC‐803 or LSD1 knockout (KO) MGC‐803 cells for 7 days (n = 3 biological replicates; mean ± standard error of mean (SEM); **P = 0.0090 (MGC‐803) and **P = 0.0012 (MKN‐45); two‐tailed unpaired Student’s t‐test; scale bar = 100 µm).
- In vitro limiting dilution assays performed using MGC‐803 (upper panel) and MKN‐45 (bottom panel) cells incubated with 20 μg/ml sEVs from MGC‐803 or LSD1 KO MGC‐803 cells for 14 days (the solid line represents the sphere formation ability curve, while the dashed line represents the 95% confidence interval. The circles and triangles represent data from different groups).
- Expression levels of LSD1, OCT4, and SOX2 in MGC‐803 and MKN‐45 cells incubated with 20 μg/ml sEVs from MGC‐803 or LSD1 KO MGC‐803 cells for 48 h.
- Quantification of the results of (C) (n = 3 biological replicates; mean ± SEM; *P = 0.0385 (LSD1), *P = 0.0160 (OCT4), and ***P = 0.0006 (SOX2) for MGC‐803; *P = 0.0299 (LSD1), *P = 0.0147 (OCT4), and *P = 0.0258 (SOX2) for MKN‐45; two‐tailed unpaired Student’s t‐test).
- Expression level of CD44 in MGC‐803 and MKN‐45 cells incubated with 20 μg/ml sEVs from MGC‐803 or LSD1 KO MGC‐803 cells for 48 h.
- Sphere formation assay results of MGC‐803 (upper panel) and MKN‐45 (bottom panel) cells incubated with 20 μg/ml sEVs from HEK293T cells (left panel), WT‐LSD1 sEVs (middle panel), and LSD1 K661A sEVs (right panel) for 7 days. Scale bar = 100 µm (n = 3 biological replicates; mean ± SEM; **P = 0.0021 (MGC‐803) and **P = 0.0020 (MKN‐45); two‐tailed unpaired Student’s t‐test).
Source data are available online for this figure.
Figure EV3. Detection of LSD1 in HEK293T cells transfected with different vectors.
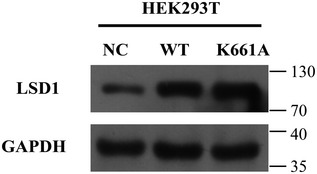
Expression levels of LSD1 in HEK293T cells transfected with the negative control, LSD1‐encoding, or LSD1 K661A mutant‐encoding plasmids.
Source data are available online for this figure.
The findings of in vitro studies were verified in vivo. The results of the in vivo limiting dilution assay revealed that Con sEVs promoted the tumor formation ability of MGC‐803 cells. In contrast, KO sEVs and BBI608, which inhibits stemness by selective inhibiting STAT3 (Li et al, 2015), decreased the tumor formation ability of MGC‐803 cells (Fig 5A–B). In contrast to KO sEVs and BBI608, Con sEVs enhanced tumor volume and weight (Fig 5C–D). After four weeks, the mice were sacrificed and the tumor was excised. The expression of stemness markers was examined in the tumor. As shown in Fig 5E, Con sEVs upregulated the expression of SOX2, which suggested that it increased tumor self‐renewal capacity. Moreover, the expression of CD44 and OCT4, which are well‐known transcription complexes, was upregulated in the cancer tissues derived from Con sEV‐treated mice. Meanwhile, the expression levels of SOX2, OCT4, and CD44 were downregulated in the KO sEV‐treated and BBI608‐treated groups, which was consistent with the phenotype in vivo. Immunofluorescence analysis further verified the regulatory effect of LSD1 on SOX2 in vivo (Fig 5F). Next, the tumor formation rate was investigated using a second‐generation tumor xenograft model. As shown in Fig 5G, LSD1 sEVs but not KO sEVs promoted second‐generation tumor formation. Meanwhile, Con sEVs significantly increased the second‐generation tumor weight, which further demonstrated that LSD1‐containing sEVs promoted gastric cancer cell stemness in vivo (Fig 5H).
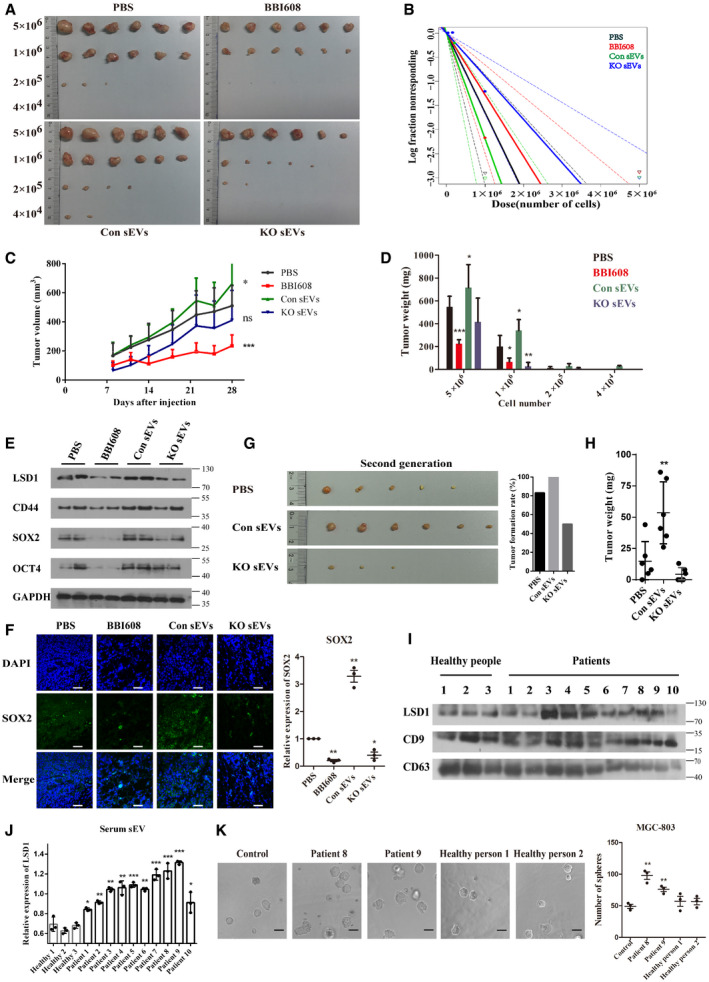
Figure 5. LSD1‐containing small extracellular vesicles (sEVs) promote gastric cancer cell stemness in vivo and clinical samples.
-
A, BIn vivo limiting dilution assay results of MGC‐803 cells treated with phosphate‐buffered saline, BBI608, sEVs from control cells (Con sEVs), and sEVs from LSD1 knockout (KO) cells (KO sEVs) as indicated for 28 days in BALB/c‐nu mice. Representative images of tumors excised from the mice (A) and the frequency of tumor formation (B) are shown (the solid line represents the sphere formation ability curve, while the dashed line represents the 95% confidence interval. The circles and triangles represent data from different groups).
-
CTumor volume of each group subjected to in vivo limiting dilution assay with 5 × 106 cells with indicated treatment (n = 6 biological replicates; mean ± standard error of mean (SEM), *P = 0.0441 and ***P = 0.0002; two‐tailed unpaired Student’s t‐test).
-
DTumor weight of each group subjected to in vivo limiting dilution assay (n = 6 biological replicates; mean ± SEM; ***P < 0.0001, *P = 0.0285, *P = 0.0162, *P = 0.0498, and *P = 0.0049; two‐tailed unpaired Student’s t‐test).
-
EExpression levels of LSD1, CD44, SOX2, and OCT4 in tumor tissues subjected to in vivo limiting dilution assay performed with 5 × 106 cells.
-
FImmunofluorescence image and quantification of the expression of SOX2 in tumor tissues subjected to in vivo limiting dilution assay. Scale bar = 50 µm (n = 3 biological replicates; mean ± SEM; **P = 0.0024, **P = 0.0088, and *P = 0.0342; two‐tailed unpaired Student’s t‐test).
-
GRepresentative images of second‐generation tumors (left) and the tumor formation rate (right) in each group.
-
HSecond‐generation tumor weight in each group (n = 6 biological replicates; mean ± SEM; *P = 0.0091; two‐tailed unpaired Student’s t‐test).
-
I, JExpression levels of LSD1 in sEVs isolated from the plasma. CD9 and CD63 were used as markers of sEVs. CD9 was used as a loading control for sEV lysis (n = 3 biological replicates; mean ± SEM; *P = 0.0256 (patient 1), **P = 0.0068 (patient 2), **P = 0.0013 (patient 3), **P = 0.0031 (patient 4), ***P = 0.0009 (patient 5), **P = 0.0012 (patient 6), ***P = 0.0007 (patient 7), ***P = 0.0010 (patient 8), ***P = 0.0001 (patient 9), and *P = 0.0461 (patient 10); two‐tailed unpaired Student’s t‐test).
-
KSphere formation assay results of MGC‐803 cells treated with sEVs as indicated for 7 days. Scale bar = 100 µm (n = 3 biological replicates; mean ± SEM; **P = 0.0019 and **P = 0.0050; two‐tailed unpaired Student’s t‐test).
Source data are available online for this figure.
The clinical significance of LSD1‐containing sEVs was examined. sEVs from the plasma samples of 10 patients with gastric cancer who did not undergo chemotherapy and three healthy subjects were isolated using differential ultracentrifugation. The sEVs were subjected to TEM and NTA (Fig EV4A–B) for quality control. Additionally, one sample of sEVs was chosen to verify the localization of LSD1. Treatment with proteinase K and Triton X‐100 revealed that LSD1 was a component of the sEV cargo in the plasma samples (Fig EV4C). As shown in Fig 5I–J, the amount of LSD1 in sEVs isolated from the plasma samples of patients with gastric cancer was higher than that from the plasma samples of healthy subjects. Moreover, a sphere formation assay was performed with 20 μg/ml sEVs. sEVs derived from the plasma of patients with gastric cancer but not those derived from the plasma of healthy individuals promote sphere formation in MGC‐803 cells (Fig 5K), which further confirmed the importance of sEVs in delivering LSD1 in human subjects. To study the clinical relevance of sEVs‐LSD1, the expression of SOX2 in the tissues of 10 patients that were used to isolate sEVs was investigated using IHC. As shown in Fig EV4D–E, the amount of LSD1 in sEVs was positively correlated with the level of SOX2 in tissues. This indicates that sEVs‐LSD1 is closely related to the stemness of gastric cancer tissues. Thus, sEV‐delivered LSD1 plays a vital role in sEV‐induced gastric cancer cell stemness in vivo and clinical settings.
Figure EV4. Characterization of patient plasma‐derived small extracellular vesicles (sEVs).
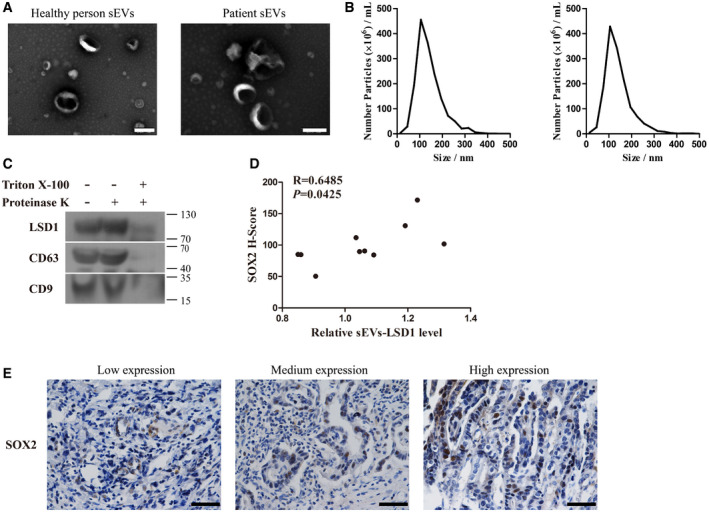
-
A, BTransmission electron microscopy image (A) and the size distribution (B) of sEVs from the plasma of healthy individuals (left panel) and patients with gastric cancer (right panel). Scale bar = 200 nm.
-
CExpression levels of LSD1, CD63, and CD9 in sEVs with indicated treatment.
-
DCorrelation between the amount of LSD1‐containing sEVs and SOX2 in tissues of 10 patients used to isolate sEVs (Pearson’s test).
-
ERepresentative images are shown as indicated. Scale bar = 50 µm.
Source data are available online for this figure.
LSD1‐containing sEV‐induced stemness mediates oxaliplatin resistance in gastric cancer cells
CSCs are associated with tumorigenicity, drug resistance, and self‐renewal (Shibue & Weinberg, 2017). Therefore, CSCs are considered to be the main cause of chemoresistance (Brabletz, 2012). Oxaliplatin, a third‐generation platinum‐based anticancer drug, is used both as adjuvant and palliative agents for gastric cancer chemotherapy. Hence, sEV‐induced resistance to oxaliplatin was examined. MKN‐45, NCI‐N87, and LSD1 KO MGC‐803 cells were chosen as references as they exhibit decreased LSD1 expression or do not exhibit LSD1 expression. As shown in Fig 6A–D, Con sEVs but not KO sEVs significantly decreased oxaliplatin sensitivity in MGC‐803, MKN‐45, NCI‐N87, and LSD1 KO MGC‐803 cells. Further in vivo experiments using a subcutaneous xenograft model also suggested that LSD1‐containing sEVs decreased the sensitivity of MGC‐803 cells to oxaliplatin. The Con sEV‐treated group exhibited higher tumor weight than KO sEV‐treated group (Fig 6E–F). Additionally, the Con sEV‐treated group exhibited significantly faster tumor growth than the KO sEV‐treated and phosphate‐buffered saline (PBS)‐treated groups (Fig 6G). Next, the clinical significance of LSD1‐containing sEVs was examined. A proliferation assay was performed using MGC‐803 cells treated with oxaliplatin and sEVs derived from the plasma of patients with gastric cancer and healthy individuals. As shown in Fig 5H, sEVs in the plasma from patients with gastric cancer upregulated LSD1 expression and significantly decreased the oxaliplatin sensitivity of MGC‐803 cells. However, the oxaliplatin sensitivity was not significantly different between the healthy plasma sEV‐treated groups and the PBS‐treated group (Fig 6H). These results indicate that LSD1‐containing sEVs can induce oxaliplatin resistance in gastric cancer cells in vitro and in vivo.
Figure 6. LSD1‐containing small extracellular vesicles (sEVs) induce oxaliplatin resistance in gastric cancer cells.
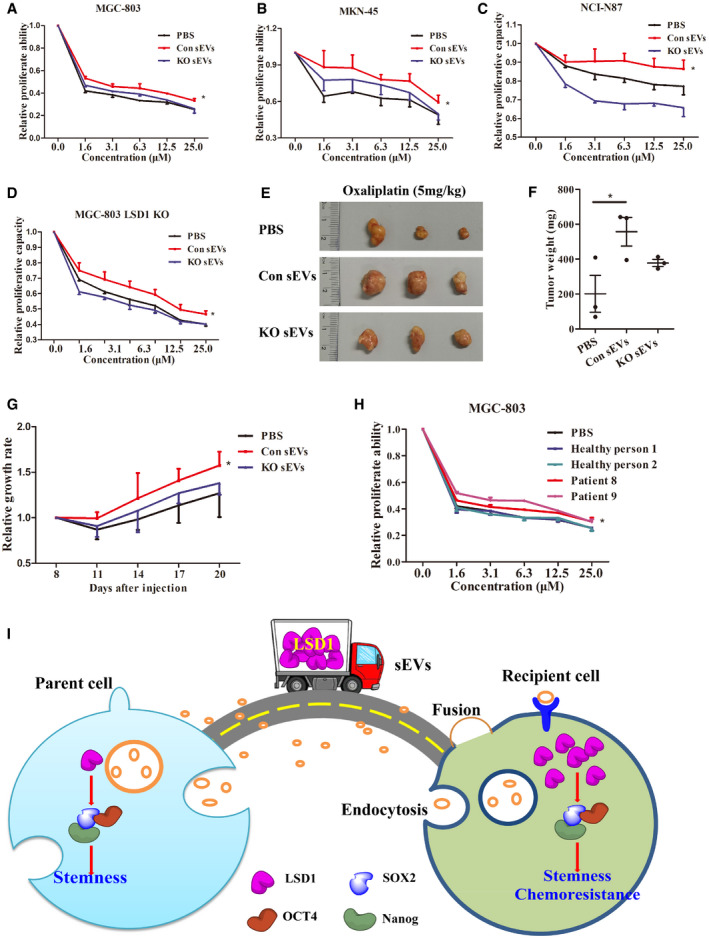
-
A–DProliferation assay results of MGC‐803 (A), MKN‐45 (B), NCI‐N87 (C), and LSD1 knockout (KO) MGC‐803 (D) cells treated with oxaliplatin along with indicated treatments (n = 3 biological replicates, mean ± standard error of mean (SEM); *P = 0.0204 (MGC‐803), *P = 0.0498 (MKN‐45), *P = 0.0473 (NCI‐N87), and *P = 0.0019 (LSD1 KO MGC‐803); two‐tailed unpaired Student’s t‐test).
-
E, FRepresentative images of tumors (E) and the tumor weight (F) of mice treated with oxaliplatin in the presence or absence of sEVs (n = 3 biological replicates; mean ± SEM; *P = 0.0488; two‐tailed unpaired Student’s t‐test).
-
GTumor growth rate in mice treated with oxaliplatin in the presence or absence of sEVs. Tumor growth rate was measured according to tumor volume (n = 3 biological replicates; mean ± SEM; *P = 0.0439; two‐tailed unpaired Student’s t‐test).
-
HProliferation assay results of MGC‐803 cells treated with oxaliplatin in the presence or absence of sEVs from the plasma of patients with gastric cancer or healthy individuals (n = 3 biological replicates; mean ± SEM; *P = 0.0487; two‐tailed unpaired Student’s t‐test).
-
ISchematic model for the shuttling of LSD1 from parent cells to recipient cells using sEVs as vehicles. The sEV‐delivered LSD1 promotes recipient gastric cancer stemness and chemoresistance.
Discussion
Globally, gastric cancer is one of the most common malignancies. However, chemotherapy for gastric cancer is associated with side effects, low response rates, and chemoresistance. Chemoresistance is a major challenge for patients with gastric cancer undergoing chemotherapy. The molecular mechanisms of chemoresistance in gastric cancer include decreased intracellular concentrations of drugs, cell stemness, and alterations in drug targets. However, there are no clinical strategies or biomarkers to predict the response to chemotherapy. Hence, there is a need to identify novel molecular mechanisms to mitigate chemoresistance or predict the response to chemotherapy (Baguley, 2010). The discovery of cancer stemness, which is the main cause for chemoresistance, has advanced our understanding of tumorigenesis and chemoresistance and may provide novel targets for cancer therapy (Shibue & Weinberg, 2017). Therefore, the mechanism underlying gastric cancer stemness must be elucidated and novel biomarkers must be identified to predict the response to chemotherapy in gastric cancer. In this study, conditioned medium of gastric cancer cells promoted sphere formation in the recipient gastric cancer cell lines. Treatment with GW4869, an inhibitor of sEVs biogenesis and release, mitigated gastric cancer cell conditioned medium‐induced sphere formation in the recipient cells. This indicated that some components in the conditioned medium may promote cancer cell stemness.
The cargos, including RNA, DNA, and proteins, of sEVs from the parent cells are delivered to the recipient cells. Hence, sEVs have a major role in cell–cell communication (Kalluri, 2016). The conditioned medium was divided into sEV fraction and non‐sEV fraction. Only the sEV fraction promoted sphere formation in the recipient cells, which suggested that cargos in sEVs promote the stemness of recipient cells.
Screening of a small panel of gastric cancer cell lines and their sEVs revealed the presence of LSD1 in sEVs. LSD1 is a FAD‐dependent demethylase that regulates embryonic development, cell differentiation, epithelial–mesenchymal transition, cell metastasis, and mitochondrial respiration in diverse cells (Wang et al, 2007; Sun et al, 2011; Hino et al, 2012; Zheng et al, 2013; Zheng et al, 2015; Thambyrajah et al, 2016; Hosseini & Minucci, 2017). However, LSD1 was localized to the cell nucleus. LSD1 is a potential therapeutic target for cancer. Previous studies have focused on the biological role of LSD1 as a nuclear protein. However, the findings of this study indicated that LSD1 can also be secreted through sEVs. This was also confirmed by analyzing the contents of the sEVs. The mechanism underlying the packaging of the nuclear protein LSD1 into sEVs has not been elucidated. The nuclear components can be loaded into sEVs through micronuclei (MN), which can be encapsulated into multivesicular bodies (MVBs) and consequently form a part of sEVs (Yokoi et al, 2019). LSD1 may be loaded into sEVs through similar mechanisms. Additionally, sEVs derived from gastric cells may fuse with target cells and deliver LSD1 to the recipient cells. sEV‐delivered LSD1 in recipient cells promoted the stemness and chemoresistance of gastric cancer cells both in vitro and in vivo. In addition to the sEVs derived from cell lines, the sEVs isolated from the plasma of patients with gastric cancer promoted the stemness and chemoresistance of recipient cells. This novel oncogenic mechanism of LSD1 explains the stemness and chemoresistance of gastric cancer. Thus, sEV‐delivered LSD1 may serve as a potential marker for predicting the clinical response of patients to oxaliplatin treatment.
In this study, the role of sEV‐delivered LSD1 in regulating the stemness of gastric cancer in vivo was examined. Interestingly, the tumor size slightly decreased in mice treated with KO sEVs. This suggests that in addition to LSD1, other components of sEVs may suppress the growth of cancer cells. LSD1 was reported to promote the expression of some key components of RNAi‐induced silencing complex (such as DICER, AGO2, and TRBP2) (Sheng et al, 2018). Therefore, miRNA in sEVs from LSD1 KO MGC‐803 cells may be dysregulated, which may contribute to the proliferation of recipient cells. Additionally, Nanog is reported to be secreted from high‐grade serous carcinoma cells into exosomes in effusion supernatants (Sherman‐Samis et al, 2019). LSD1 positively regulated Nanog in MGC‐803 cells. Hence, Nanog and LSD1‐containing sEVs may promote the stemness of recipient cells together. Additionally, LSD1 stabilized SOX2 through demethylation in MGC‐803 cells. The results of this study suggest that LSD1 functions as a demethylase to remove the methyl groups on lysine of SOX2 and consequently prevents the methylation‐dependent proteolysis of SOX2. However, further studies are needed to confirm these findings.
In summary, this study demonstrated that LSD1 is secreted through sEVs, which can be delivered to the recipient cells and consequently promote their stemness and chemoresistance (Fig 6I). LSD1 has been detected in some cancer cell‐derived sEVs (Liang et al, 2013; Skogberg et al, 2013; He et al, 2015). However, this is the first study to report the function and the clinical application prospects of LSD1‐containing sEVs in gastric cancer. The findings of this study provided novel insights into the role of LSD1 in gastric cancer cell stemness. Thus, LSD1 is a potential therapeutic target for cancer. Additionally, sEV‐delivered LSD1 can be a potential biomarker to predict oxaliplatin response in clinical settings.
Materials and Methods
Cells and cell culture conditions
The gastric cancer cell lines MGC‐803, BGC‐823, NCI‐N87, and HGC‐27 were purchased from the Cell Bank of the Shanghai Institute of Cell Biology, Chinese Academy of Sciences. MKN‐45 cells were purchased from the Shanghai Bogoo Biotechnology Company. The cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (BI, Israel) supplemented with 10% fetal bovine serum (BI, Israel). All cells were cultured in a humidified atmosphere at 5% CO2 and 37°C.
The lentiviral vector containing Lenti‐CAS9‐sgRNA was produced and packaged by Shanghai Genechem Co. Ltd., China. The sgRNA target site for deleting LSD1 was 5′‐CCGGCCCTACTGTCGTGCCT‐3′. For transfection with Lenti‐CAS9‐sgRNA, the MGC‐803 cells (5 × 104 cells/well) were seeded in 24‐well plates and cultured in RPMI‐1640 medium (BI, Israel) supplemented with 10% FBS (BI, Israel). The lentivirus was added to 500 μl complete medium at a final concentration of 107 TU/ml. After 20 h of incubation, the lentivirus‐containing medium was replaced with complete medium. At day 2 post‐lentiviral transfection, the cells were incubated with 0.5 μg/ml puromycin in culture medium for 3 days to select the stable LSD1 KO cell line.
Western blotting
Equal amounts (20 μg) of sEVs were resuspended in PBS and treated with 1 μg/ml proteinase K for 20 min at 37°C or 0.1% Triton X‐100 for 20 min, followed by treatment with 1 μg/ml proteinase K for 20 min at 37°C. Untreated sEVs served as a negative control. The sEVs were mixed with the loading buffer and denatured.
The whole‐cell lysates were prepared using radioimmunoprecipitation assay buffer. The lysates were mixed with the loading buffer and denatured. Next, 30 μg of cell lysate was loaded for Western blotting.
Western blotting was performed according to the standard methods. Approximately 30 μg of protein was subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS–PAGE) using a 10% gel. The resolved proteins were transferred to a 0.2‐μm nitrocellulose membrane (P/N66485, Pall, USA). The membrane was blocked with 5% milk in PBS for 2 h, following by incubation with anti‐LSD1 (ab129195, Abcam, England), anti‐CD9 (134403, CST, USA), anti‐CD63 (ab59479, Abcam, England), anti‐calnexin (ab22595, Abcam, England), anti‐OCT4 (ab181557, Abcam, England), anti‐SOX2 (14962, CST, USA), anti‐Nanog (ab21624, Abcam, England), and anti‐GAPDH (AB‐P‐R 001, Hangzhou Goodhere Biotechnology, China) antibodies overnight at 4°C. Next, the membrane was washed with PBS containing 0.05% Tween‐20 (PBST) at room temperature and incubated with peroxidase‐conjugated goat anti‐rabbit IgG (ZB‐2301, Zsbio, China) and peroxidase‐conjugated goat anti‐mouse IgG (ZB‐2305, Zsbio, China) for 2 h at room temperature. The membrane was then washed with PBST at room temperature and developed using an enhanced chemiluminescence reagent (34096, Thermo Fisher, USA).
sEV isolation
The cells were cultured in serum‐free medium for 36 h. The medium was centrifuged at 1,500 g for 30 min to remove cell debris, followed by centrifugation at 10,000 g for 30 min to remove large vesicles. Further, the samples were centrifuged at 100,000 g for 2 h. The supernatant was removed, and the pellet was resuspended in 2 ml of PBS. The resulting pellet was washed with PBS at 100,000 g for 2 h. The samples were centrifuged to obtain the sEVs. sEVs were resuspended in 200 μl PBS and stored at −80°C until use. Additionally, sEVs were filtered through a 0.22‐μm filter before use. In this study, sEVs were obtained using differential ultracentrifugation unless otherwise specified. For the isolation of plasma‐derived sEVs, the plasma was diluted 10 times before differential ultracentrifugation. Quantification of sEVs was performed using the bicinchoninic acid assay.
The kit used for sEV isolation was the total exosome isolation reagent (4478359, Invitrogen, USA). Briefly, the medium was collected and centrifuged at 1,500 g for 30 min to remove cell debris, followed by centrifugation at 10,000 g for 30 min to remove large vesicles. The supernatant was incubated with the exosome isolation reagent at a ratio of 3:1 (v/v) at 4°C overnight. The samples were centrifuged at 10,000 g, and the precipitate was collected (sEVs).
All sEVs in this study were obtained using differential ultracentrifugation, except those in Fig 1I, which were isolated using total exosome isolation reagent (4478359, Invitrogen, USA). The reagent was only used for verification of the presence of LSD1 in sEVs. All the sEVs in this study were filtered through 0.22‐µm membrane filters before functional experiments.
The relevant data from the experiments are submitted to the EV‐TRACK knowledgebase (EV‐TRACK ID: EV200198) (Van Deun et al, 2017).
sEV labeling
Purified sEVs were labeled using the PKH26 red fluorescent labeling kit (MINI26‐1KT, Sigma, Germany), following the manufacturer’s instructions. Briefly, sEVs were incubated with diluted PKH26 in a ratio of 1:1 (v/v) for 5 min. Size exclusion chromatography was performed to remove PKH26 micelles from the labeled sEVs. The PKH26‐labeled sEVs (20 μg/ml) were incubated with 1.2 × 104 target cells for 12 h. The target cell membrane was stained with Dio (C1038, Beyotime, China), while the nuclei were stained with DAPI (BS130A, Biosharp, China). PKH26‐labeled sEVs were examined using a confocal microscope (Nikon, Japan).
Immunoprecipitation
Immunoprecipitation kit was purchased from Thermo Fisher Scientific (26147, Thermo Fisher Scientific, USA). The Kme1/2 or SOX2 complexes were purified from 1–2 mg of total protein using the anti‐Kme1/2 (PTM602, PTM Biolabs, China) or anti‐SOX2 antibody (14962, CST, USA) coupled to protein A/G Dynabeads (26147, Thermo Fisher Scientific, USA). The protein‐bead complexes were washed and eluted. The sample was then heated with loading buffer at 95–100°C for 10 min. Next, the sample was cooled to room temperature and subjected to SDS–PAGE analysis.
Immunofluorescence
The cells were cultured in a 24‐well plate. The recipient cells were incubated with 20 μg/ml of sEVs or PBS for 12 h. The cells were fixed with 4% paraformaldehyde and permeabilized with 0.01% Triton X‐100 for 20 min. Next, the cells were probed with anti‐LSD1 (ab129195, Abcam, England) antibodies. After washing with PBS at room temperature, the cells were incubated with anti‐rabbit secondary antibodies (A32723, Life, USA) for 2 h at room temperature. The samples were treated with DAPI (BS130A, Biosharp, China) for staining cell nucleus and PKH26 (MINI26‐1KT, Sigma, Germany) for membrane staining. The cells were imaged using a Nikon C2 Plus confocal microscope (Nikon, Japan).
Extreme limiting dilution assay
A limiting dilution assay is an experimental technique for quantifying the proportion of biologically active components in a large population (Hu & Smyth, 2009). This assay is a type of dose‐response experiment in which each culture exhibits a negative or positive response. The rate of positive and negative responses at each dose allows the determination of the frequency of biologically active components. Stem cell assays reflect cell stemness (Zhang et al, 2017b).
The in vitro limiting dilution assay was performed as previously described (Zhou et al, 2016). Briefly, gastric cancer cells subjected to different treatments were digested, diluted to single‐cell suspensions, and plated in 96‐well plates at a cell number of 1, 2, 5, 10, 20, 40, 80,160, and 320 cells per well. Wells without spheres were counted after one week. Extreme limiting dilution assays were performed using the software available at http://bioinf.wehi.edu.au/software/elda/ (Hu & Smyth, 2009).
Three‐dimensional (3D) cell culture
Cell sphere formation experiments were performed using the 3D cell culture media (D112501, Sciobio, China). The cells in 3D cell culture medium were plated into a 96‐well plate. Next, the cells were incubated with 10 μl of cell complete medium. After the medium became gelatinous, the cell culture medium was added to each well. After one week, the spheres were counted and photographed using a microscope (Nikon Ts2, Nikon, Japan).
Flow cytometric analysis
The treated cells were resuspended in PBS and incubated with the anti‐CD44 antibody (555479, BD, USA) for 20 min on ice. Next, the cells were washed thrice with PBS and subjected to flow cytometric analysis using the LSRFortessaTM Cell Analyzer (Becton Dickinson, USA). Flow cytometric data were analyzed using FlowJo 7.6 software (FlowJo, USA).
Mouse tumor xenograft model
Five‐week‐old female BALB/c nude mice were purchased from the Jingda Laboratory Animal, Hunan, China. All animals were housed in a pathogen‐free environment, and the experimental protocols were approved by the Ethics Committee of Zhengzhou University Health Science Center. The in vivo limiting dilution assay was performed as previously described (Zhou et al, 2016). Briefly, the gastric cancer cells treated with different sEVs were digested and resuspended in sterile PBS. The cells were diluted to different concentrations of (5 × 106, 1 × 106, 2 × 105, and 4 × 104 per 200 μl). An aliquot (200 μl) of the cell suspension from each group was inoculated subcutaneously into mice. Tumor volume was monitored every 3 days using a digital caliper. The tumor volume was calculated as follows: tumor volume (mm3) = length × width2 × 0.5. The tumors were administered with sEVs (20 μg sEVs/tumor) twice a week after the tumor volume reached 100 mm3 (1 week). On day 28, the mice were euthanized and the tumors were excised and weighed.
For experiments evaluating drug sensitivity, MGC‐803 cells were used to construct a subcutaneous xenograft model. Three groups were intraperitoneally treated with oxaliplatin (5 mg/kg bodyweight; dissolved in PBS) in the presence or absence of sEVs. The tumor growth rate was measured according to the tumor volume.
Second‐generation tumor xenograft model
A first‐generation transplanted tumor was selected for each group. The tumor was excised, equally divided into blocks based on the volume, and transplanted into mice. The tumor formation rate was determined after two weeks. After the tumor volume reached 100 mm3 (one week), the tumor volume was quantified every 3 days using a digital caliper as follows: tumor volume (mm3) = length × width2 × 0.5.
CCK‐8 assay
Cell proliferation was quantified using the CCK‐8 method (HY‐K0301, MCE, USA). The cells were seeded in 96‐well plates and incubated with 10 μl CCK‐8 solution for 4 h. The absorbance of the mixture at 450 nm was measured using a microplate reader (Envision, PerkinElmer, USA).
Immunohistochemistry
The specimens were fixed in 10% buffered formalin solution and embedded in paraffin wax. The serial sections (5 μm) were cut from the tissue blocks, deparaffinized in xylene, and hydrated in an alcohol series (75, 85, 95, and 100%). The tissue sections were then incubated with anti‐LSD1 (ab129195; Abcam) and anti‐SOX2 (14962; CST) antibodies. Further, the tissue sections were incubated with peroxidase‐conjugated goat anti‐rabbit Ig (ZB‐2301; Zsbio, China) or peroxidase‐conjugated goat anti‐mouse IgG (ZB‐2305; Zsbio, China) for 2 h at room temperature. Immunoreactive bands were developed using the 3,3′‐diaminobenzidine kit (ZL1‐9018, ZSGB‐BIO, China). The sections were digitally scanned using an Aperio AT2 scanner (Leica Biosystems, Germany). The images were analyzed with Aperio Image Toolbox (Leica Biosystems, Germany) using a pathologist‐trained nuclear‐, cytoplasmic‐, nuclear and cytoplasmic‐, and cytoplasmic‐specific algorithms. Protein expression was evaluated according to the H‐score system. The percentage of staining intensity was scored as 0 (no staining), 1+ (weak staining), 2+ (moderate staining), and 3+ (strong staining). The degree of expression in each sample was reported as the percentage of positive cells (0 to 100%). The final score (H‐score) was then obtained by multiplying the intensity and reactivity extent values (range, 0–300).
Statistical analysis
Three independent trials were performed for each in vitro experiment. Pearson’s correlation coefficient was used to evaluate the correlation between the groups. The differences were considered significant at P < 0.05, and P < 0.01 was considered highly significant. All statistical analyses were performed using GraphPad 6.0 or SPSS 21.0. The data were analyzed using Student’s t‐test. *P < 0.05, **P < 0.01, ***P < 0.001.
Ethical approval
Gastric cancer tissues and adjacent tissues were obtained from the First Affiliated Hospital of Zhengzhou University. All human tissues were collected using protocols approved by the Ethics Committee of Zhengzhou University Health Science Center. The blood samples were collected from patients with gastric cancer at the First Affiliated Hospital of Zhengzhou University and approved by the Ethics Committee of Zhengzhou University Health Science Center.
Author contributions
LJZ prepared the manuscript. LJZ, YYL, and QQF performed the experiments. LJZ performed the immunohistochemical analysis and evaluated the results with WCC, HMR, JRP, DDS, and ZYW. LJZ, LFZ, and JWW performed the in vivo experiments. CZ and AM critically evaluated the manuscript. YTZ and JYZ revised the manuscript. HML and YCZ designed the study and finalized the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81602961, No. 81430085, and No. 21372206), the National Key Research Program (No. 2018YFE0195100, No. 2016YFA0501800, and No. 2017YFD0501401); Science and Technology Innovation Talents of Henan Provincial Education Department (19IRTSTHN001), Basic and Frontier Technology Research Project of Henan Province (No. 162300410119), and the Natural Science Foundation of Henan Province (No. 162300410292).
EMBO reports (2021) 22: e50922.
Contributor Information
Yi‐Chao Zheng, Email: yichaozheng@zzu.edu.cn.
Hong‐Min Liu, Email: liuhm@zzu.edu.cn.
Data availability
All data obtained and/or analyzed in this study are available from the corresponding authors upon reasonable request. The proteomics data in this publication have been deposited at the ProteomeXchange Consortium via the PRIDE (https://www.ebi.ac.uk/pride) (Perez‐Riverol et al, 2019) partner repository (dataset identifier: PXD021511).
References
- Amente S, Lania L, Majello B (2013) The histone LSD1 demethylase in stemness and cancer transcription programs. Biochem Biophys Acta 1829: 981–986 [DOI] [PubMed] [Google Scholar]
- Baguley BC (2010) Multidrug resistance in cancer. Methods Mol Biol 596: 1–14 [DOI] [PubMed] [Google Scholar]
- Bao L, You B, Shi S, Shan Y, Zhang Q, Yue H, Zhang J, Zhang W, Shi Y, Liu Y et al (2018) Metastasis‐associated miR‐23a from nasopharyngeal carcinoma‐derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene 37: 2873–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T (2012) EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell 22: 699–701 [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394–424 [DOI] [PubMed] [Google Scholar]
- Cao YL, Zhuang T, Xing BH, Li N, Li Q (2017) Exosomal DNMT1 mediates cisplatin resistance in ovarian cancer. Cell Biochem Funct 35: 296–303 [DOI] [PubMed] [Google Scholar]
- Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ (2010) Exosome release of beta‐catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 190: 1079–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce‐Rodriguez I, Chakravarthi B, Varambally S (2017) UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 19: 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N, Thery C (2011) Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 33: 419–440 [DOI] [PubMed] [Google Scholar]
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H et al (2018) Exosomal PD‐L1 contributes to immunosuppression and is associated with anti‐PD‐1 response. Nature 560: 382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zeng C, Zhan Y, Wang H, Jiang X, Li W (2017) Aberrant low expression of p85alpha in stromal fibroblasts promotes breast cancer cell metastasis through exosome‐mediated paracrine Wnt10b. Oncogene 36: 4692–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM (2006) Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 66: 9339–9344 [DOI] [PubMed] [Google Scholar]
- Duan Y, Qin W, Suo F, Zhai X, Guan Y, Wang X, Zheng Y, Liu H (2018) Design, synthesis and in vitro evaluation of stilbene derivatives as novel LSD1 inhibitors for AML therapy. Bioorg Med Chem 26: 6000–6014 [DOI] [PubMed] [Google Scholar]
- EL Andaloussi S, Mager I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12: 347–357 [DOI] [PubMed] [Google Scholar]
- Enjoji S, Yabe R, Tsuji S, Yoshimura K, Kawasaki H, Sakurai M, Sakai Y, Takenouchi H, Yoshino S, Hazama S et al (2018) Stemness is enhanced in gastric cancer by a SET/PP2A/E2F1 axis. Mol Cancer Res 16: 554–563 [DOI] [PubMed] [Google Scholar]
- Faict S, Muller J, De Veirman K, De Bruyne E, Maes K, Vrancken L, Heusschen R, De Raeve H, Schots R, Vanderkerken K et al (2018) Exosomes play a role in multiple myeloma bone disease and tumor development by targeting osteoclasts and osteoblasts. Blood Cancer J 8: 105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, Ngai SM, Chan TF, Wong N (2015) Hepatocellular carcinoma‐derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis 36: 1008–1018 [DOI] [PubMed] [Google Scholar]
- Hino S, Sakamoto A, Nagaoka K, Anan K, Wang Y, Mimasu S, Umehara T, Yokoyama S, Kosai K, Nakao M (2012) FAD‐dependent lysine‐specific demethylase‐1 regulates cellular energy expenditure. Nat Commun 3: 758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Minucci S (2017) A comprehensive review of lysine‐specific demethylase 1 and its roles in cancer. Epigenomics 9: 1123–1142 [DOI] [PubMed] [Google Scholar]
- Hu Y, Smyth GK (2009) ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods 347: 70–78 [DOI] [PubMed] [Google Scholar]
- Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T et al (2007) p53 is regulated by the lysine demethylase LSD1. Nature 449: 105–108 [DOI] [PubMed] [Google Scholar]
- Huang T, Song C, Zheng L, Xia L, Li Y, Zhou YJMC (2019) The roles of extracellular vesicles in gastric cancer development, microenvironment, anti‐cancer drug resistance, and therapy. Mol Cancer 18: 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Xiang L, He L, Yang G, Zheng J, Wang C, Zhang Y, Wang S, Zhou Y, Sheu T‐J et al (2017) Exosomes mediate epithelium‐mesenchyme crosstalk in organ development. ACS Nano 11: 7736–7746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer EL (2007) Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucleic Acids Res 35: W429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R (2016) The biology and function of exosomes in cancer. J Clin Investig 126: 1208–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontaki H, Talianidis I (2010) Lysine methylation regulates E2F1‐induced cell death. Mol Cell 39: 152–160 [DOI] [PubMed] [Google Scholar]
- Lee Y, El Andaloussi S, Wood MJ (2012) Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet 21: R125–R134 [DOI] [PubMed] [Google Scholar]
- Lei ZJ, Wang J, Xiao HL, Guo Y, Wang T, Li Q, Liu L, Luo X, Fan LL, Lin L et al (2015) Lysine‐specific demethylase 1 promotes the stemness and chemoresistance of Lgr5(+) liver cancer initiating cells by suppressing negative regulators of beta‐catenin signaling. Oncogene 34: 3188–3198 [DOI] [PubMed] [Google Scholar]
- Li J, Sherman‐Baust CA, Tsai‐Turton M, Bristow RE, Roden RB, Morin PJ (2009) Claudin‐containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer 9: 244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB, Li CJ (2015) Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci 112: 1839–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Liu XQ, Geng PF, Suo FZ, Ma JL, Yu B, Zhao TQ, Zhou ZQ, Huang CX, Zheng YC et al (2017) Discovery of [1,2,3]Triazolo[4,5‐d]pyrimidine derivatives as novel LSD1 inhibitors. ACS Med Chem Lett 8: 384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Peng P, Chen S, Li L, Zhang M, Cao D, Yang J, Li H, Gui T, Li X et al (2013) Characterization and proteomic analysis of ovarian cancer‐derived exosomes. J Proteomics 80: 171–182 [DOI] [PubMed] [Google Scholar]
- Lin X, Li S, Wang YJ, Wang Y, Zhong JY, He JY, Cui XJ, Zhan JK, Liu YS (2019) Exosomal Notch3 from high glucose‐stimulated endothelial cells regulates vascular smooth muscle cell calcification/aging. Life Sci 232: 116582 [DOI] [PubMed] [Google Scholar]
- Liu H‐M, Suo F‐Z, Li X‐B, You Y‐H, Lv C‐T, Zheng C‐X, Zhang G‐C, Liu Y‐J, Kang W‐T, Zheng Y‐C et al (2019) Discovery and synthesis of novel indole derivatives‐containing 3‐methylenedihydrofuran‐2(3H)‐one as irreversible LSD1 inhibitors. Eur J Med Chem 175: 357–372 [DOI] [PubMed] [Google Scholar]
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T (2014) Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7: ra63 [DOI] [PubMed] [Google Scholar]
- Perez‐Riverol Y, Csordas A, Bai J, Bernal‐Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M et al (2019) The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47: D442–D450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Shen Q, Yang X, Qiu Y, Zhang W (2015) The role of extracellular vesicles: an epigenetic view of the cancer microenvironment. Biomed Res Int 2015: 649161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W, LaFleur MW, Nguyen TH, Chen S, Chakravarthy A, Conway JR, Li Y, Chen H, Yang H, Hsu P‐H et al (2018) LSD1 ablation stimulates anti‐tumor immunity and enables checkpoint blockade. Cell 174: 549–563.e519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman‐Samis M, Onallah H, Holth A, Reich R, Davidson B (2019) SOX2 and SOX9 are markers of clinically aggressive disease in metastatic high‐grade serous carcinoma. Gynecol Oncol 153: 651–660 [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119: 941–953 [DOI] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA (2017) EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 14: 611–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Pochampally R, Watabe K, Lu Z, Mo YY (2014) Exosome‐mediated transfer of miR‐10b promotes cell invasion in breast cancer. Mol Cancer 13: 256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR (2013) Gastric cancer stem cells: a novel therapeutic target. Cancer Lett 338: 110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogberg G, Gudmundsdottir J, van der Post S, Sandström K, Bruhn S, Benson M, Mincheva‐Nilsson L, Baranov V, Telemo E, Ekwall O (2013) Characterization of human thymic exosomes. PLoS One 8: e67554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GQ, Ye P, Murai K, Lang M‐F, Li S, Zhang H, Li W, Fu C, Yin J, Wang A et al (2011) miR‐137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat Commun 2: 529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Peng JD, Suo FZ, Zhang T, Fu YD, Zheng YC, Liu HM (2017) Discovery of tranylcypromine analogs with an acylhydrazone substituent as LSD1 inactivators: design, synthesis and their biological evaluation. Bioorg Med Chem Lett 27: 5036–5039 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Saikawa Y, Kitagawa Y (2013) Gastric cancer: current status of diagnosis and treatment. Cancers 5: 48–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase CP, Neagu AI, Necula LG, Mambet C, Enciu AM, Calenic B, Cruceru ML, Albulescu R (2014) Cancer stem cells: involvement in pancreatic cancer pathogenesis and perspectives on cancer therapeutics. World J Gastroenterol 20: 10790–10801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45: W98–W102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambyrajah R, Mazan M, Patel R, Moignard V, Stefanska M, Marinopoulou E, Li Y, Lancrin C, Clapes T, Möröy T et al (2016) GFI1 proteins orchestrate the emergence of haematopoietic stem cells through recruitment of LSD1. Nat Cell Biol 18: 21–32 [DOI] [PubMed] [Google Scholar]
- Tkach M, Thery C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164: 1226–1232 [DOI] [PubMed] [Google Scholar]
- Ung TH, Madsen HJ, Hellwinkel JE, Lencioni AM, Graner MW (2014) Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci 105: 1384–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H (2016) Gastric cancer. Lancet 388: 2654–2664 [DOI] [PubMed] [Google Scholar]
- Van Deun J, Mestdagh P, Agostinis P, Akay Ö, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, Bertier L et al (2017) EV‐TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods 14: 228–232 [DOI] [PubMed] [Google Scholar]
- Viklund H, Bernsel A, Skwark M, Elofsson A (2008) SPOCTOPUS: a combined predictor of signal peptides and membrane protein topology. Bioinformatics 24: 2928–2929 [DOI] [PubMed] [Google Scholar]
- Wang J, Hevi S, Kurash JK, Lei H, Gay F, Bajko J, Su H, Sun W, Chang H, Xu G et al (2009) The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet 41: 125–129 [DOI] [PubMed] [Google Scholar]
- Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia‐Bassets I et al (2007) Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature 446: 882–887 [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang S, Weber J, Baxter D, Galas DJ (2010) Export of microRNAs and microRNA‐protective protein by mammalian cells. Nucleic Acids Res 38: 7248–7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JK, Song J, Huo HR, Zhao YL, Zhang GY, Zhao ZM, Sun GZ, Jiao BH (2017) DNM3, p65 and p53 from exosomes represent potential clinical diagnosis markers for glioblastoma multiforme. Ther Adv Med Oncol 9: 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi A, Villar‐Prados A, Oliphint PA, Zhang J, Song X, De Hoff P, Morey R, Liu J, Roszik J, Clise‐Dwyer K et al (2019) Mechanisms of nuclear content loading to exosomes. Sci Adv 5: eaax8849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Hoang N, Leng F, Saxena L, Lee L, Alejo S, Qi D, Khal A, Sun H, Lu F et al (2018) LSD1 demethylase and the methyl‐binding protein PHF20L1 prevent SET7 methyltransferase‐dependent proteolysis of the stem‐cell protein SOX2. J Biol Chem 293: 3663–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Leng F, Saxena L, Hoang N, Yu J, Alejo S, Lee L, Qi D, Lu F, Sun H et al (2019) Proteolysis of methylated SOX2 protein is regulated by L3MBTL3 and CRL4(DCAF5) ubiquitin ligase. J Biol Chem 294: 476–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Wang X, Yang H, Li J et al (2017a) Exosome‐delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun 8: 15016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bögler O et al (2017b) m(6)A Demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem‐like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31: 591–606.e596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lu F, Wang J, Yin F, Xu Z, Qi D, Wu X, Cao Y, Liang W, Liu Y et al (2013) Pluripotent stem cell protein Sox2 confers sensitivity to LSD1 inhibition in cancer cells. Cell Rep 5: 445–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YC, Chang J, Zhang T, Suo FZ, Chen XB, Liu Y, Zhao B, Yu B, Liu HM (2017) An overview on screening methods for Lysine Specific Demethylase 1 (LSD1) inhibitors. Curr Med Chem 24: 2496–2504 [DOI] [PubMed] [Google Scholar]
- Zheng Y‐C, Duan Y‐C, Ma J‐L, Xu R‐M, Zi X, Lv W‐L, Wang M‐M, Ye X‐W, Zhu S, Mobley D et al (2013) Triazole‐dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J Med Chem 56: 8543–8560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YC, Ma J, Wang Z, Li J, Jiang B, Zhou W, Shi X, Wang X, Zhao W, Liu HM (2015) A systematic review of histone lysine‐specific demethylase 1 and its inhibitors. Med Res Rev 35: 1032–1071 [DOI] [PubMed] [Google Scholar]
- Zheng Y‐C, Shen D‐D, Ren M, Liu X‐Q, Wang Z‐R, Liu Y, Zhang Q‐N, Zhao L‐J, Zhao L‐J, Ma J‐L et al (2016a) Baicalin, a natural LSD1 inhibitor. Bioorg Chem 69: 129–131 [DOI] [PubMed] [Google Scholar]
- Zheng YC, Yu B, Chen ZS, Liu Y, Liu HM (2016b) TCPs: privileged scaffolds for identifying potent LSD1 inhibitors for cancer therapy. Epigenomics 8: 651–666 [DOI] [PubMed] [Google Scholar]
- Zhou A, Lin K, Zhang S, Chen Y, Zhang N, Xue J, Wang Z, Aldape KD, Xie K, Woodgett JR et al (2016) Nuclear GSK3beta promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat Cell Biol 18: 954–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Data Availability Statement
All data obtained and/or analyzed in this study are available from the corresponding authors upon reasonable request. The proteomics data in this publication have been deposited at the ProteomeXchange Consortium via the PRIDE (https://www.ebi.ac.uk/pride) (Perez‐Riverol et al, 2019) partner repository (dataset identifier: PXD021511).


