Abstract
The coronavirus disease 2019 (COVID-19) was declared a pandemic in March 2020 by the World Health Organization (WHO). To date, there were > 163 million confirmed cases of COVID-19 and the disease has claimed > 3.3 million lives globally. As with many other diseases, inflammation is a key feature of COVID-19. When inflammation is overwhelming, it may lead to unfavorable outcomes or even death. Scientists all over the world are working tirelessly in search of therapeutic strategies to suppress or modulate inflammation in COVID-19. This review gives an overview of the role of inflammation in COVID-19. It also critically examines the various treatment approaches that target the immune system and inflammation in COVID-19, as well as highlights the key findings in the numerous studies conducted thus far.
Keywords: COVID-19, SARS-CoV-2, inflammation, cytokine storm, pharmacotherapy
Introduction
In December 2019, there were an increasing number of pneumonia cases in Wuhan, a city in China’s Hubei Province. However, the cause of the disease was unclear at that time. A month later, the Chinese Centre for Disease Control and Prevention announced the isolation of a novel coronavirus (CoV) from a patient with pneumonia in Wuhan [1]. The virus was different from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle-East Respiratory Syndrome coronavirus (MERS-CoV) previously reported in the published literature. This newly isolated CoV was initially called 2019-nCOV and is now widely known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This was followed by the declaration of COVID-19 as a pandemic by the WHO two months later, in March 2020 [2]. Since its emergence, COVID-19 has spread uncontrollably across the globe and SARS-CoV-2 has infected people in nearly every country in the world. As of 17th May 2021, there were > 163 million confirmed cases of COVID-19 with > 3.3 million people losing their lives to the disease (https://www.worldometers.info/coronavirus/).
Although pharmacotherapy plays a pivotal role in the treatment of many infectious diseases, the process of developing new drugs is usually very time-consuming, challenging and costly. Under normal circumstances, up to 15 years are needed from the development of the original idea to the drug reaching the patient [3]. In addition, pharmaceutical companies usually have to spend millions of dollars before promising results can be reached. Therefore, there is an urgent need to come up with a quick solution to treat patients with COVID-19, while researchers continue the quest for a curative treatment.
One of the key features of COVID-19 is the overwhelming inflammation observed in some patients, especially those who develop severe illness. An exaggerated immune response mediated by an array of cytokines plays a role in the pathogenesis of the disease. Studies have demonstrated that several types of immune cells and inflammatory mediators are involved in the disease process [4,5]. Hence researchers from all over the world have explored several anti-inflammatory and other pharmacotherapeutic agents to combat the deadly inflammation. This review gives an overview of the role of inflammation in the pathogenesis of COVID-19, critically examines the pharmacologic agents that target inflammation and the immune system in the treatment of COVID-19, as well as consolidates the important findings from the numerous studies carried out thus far.
Inflammation in COVID-19
There is increasing evidence that points to the association between one’s immune response and progression of disease in COVID-19, and abnormalities in immune cells, inflammatory markers and the cytokine storm have been implicated in disease severity and outcome. In addition, cytokine storm, hyperinflammation, and multi-organ failure have also been indicated in patients with severe disease. This section discusses the role of inflammation in COVID-19 from these perspectives.
Overview of pathogenesis of COVID-19
A basic understanding of the pathogenesis of COVID-19 and the immune response in SARS-CoV-2 infection is crucial in the understanding of the role of inflammation in COVID-19. SARS-CoV-2 is a coronavirus belonging to the Coronaviridae family and Coronavirinae subfamily. SARS-CoV-2 is an RNA virus. Its RNA is positive sense and single-stranded and the genome size of SARS-CoV-2 is 29.9 kb [6]. The structure of the virus has been widely studied since its emergence. The genetic materials of the virus are surrounded by an envelope. The structural proteins that are associated with SARS-CoV-2 include nucleocapsid (N) protein, envelope (E) protein, spike (S) protein, membrane (M) protein, and sixteen non-structural proteins (nsp1-16) [7].
The mechanisms of how SARS-CoV-2 enters the cells have been described. SARS-CoV-2’s entry into host cells requires the use of its spike protein. The virus first attaches its receptor binding domain (RBP) to the human angiotensin-converting enzyme 2 (hACE2) receptor with the help of human proteases. Research has found that the RBD of SARS-CoV-2 has a stronger binding affinity for hACE2 in comparison with that of SARS-CoV. However, the more potent RBD is less exposed to hACE2 than that of SARS-COV. Pre-activation of the spike by furin (a proprotein convertase) also plays a role in cell entry. These features contribute to efficient cell entry and help explain why the virus is highly infectious and is capable of spreading rapidly in the community [8].
In the respiratory system, the two main types of cells that SARS-CoV-2 targets are ciliated bronchial epithelial cells and type 2 pneumocytes. However, hACE2 is widely found in many other bodily systems such as the cardiovascular-, renal-, and gastrointestinal systems. Endothelial cell binding of SARS-CoV-2 may result in systemic vasculitis, disseminated intravascular coagulation and thromboembolism, whereas epithelial cell binding may result in diarrhea [9]. The virus replicates itself after gaining entry to cells before it becomes recognized and attacked by the immune system. Figure 1A and 1B shows the structure and the mechanism by which SARS-CoV-2 enters the host cell.
Figure 1.
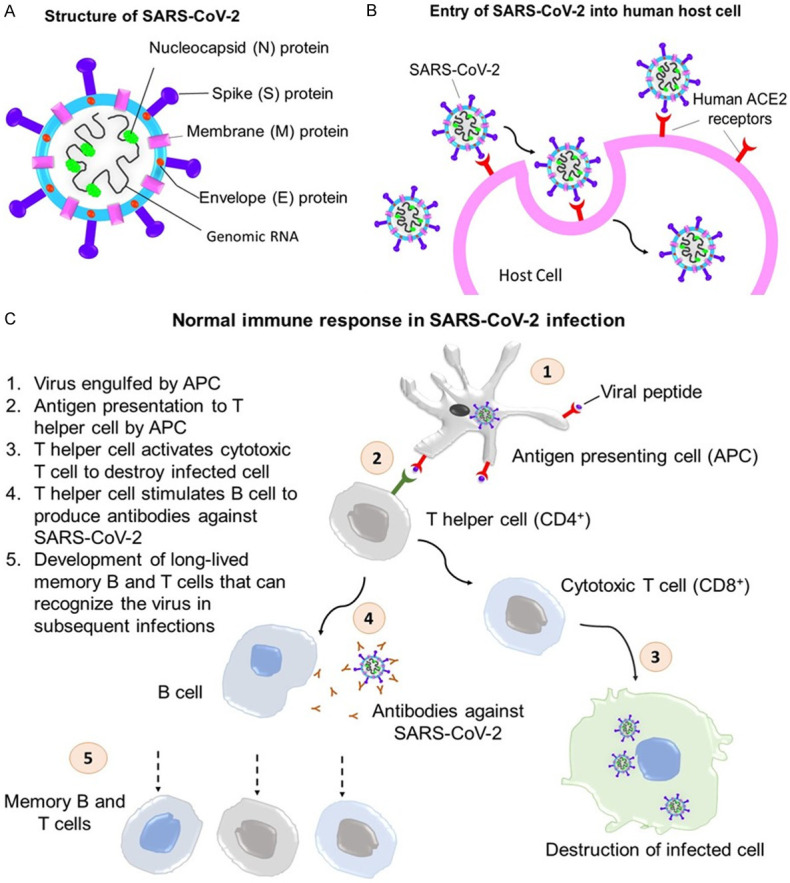
(A) Structure of SARS-CoV-2, (B) entry of SARS-COV-2 into human host cell and (C) normal immune response in SARS-CoV-2 infection.
Immune response in SARS-CoV-2 infection
The human body reacts to SARS-CoV-2 infection in two main phases. The first phase is called the innate immune response, which acts as a first line of immune defense. During this phase, innate immune cells secrete interferons and cytokines, which in turn, initiate the adaptive immune response. The innate immune response occurs in not just SARS-CoV-2 infection but any viral infections [10]. During this phase, the infected person may present with symptoms such as fever, muscle aches, and malaise. One of the factors that affects the severity of COVID-19 lies in the differential innate immune response. A delayed production of type I interferons in older patients, for instance, may lead to increased recruitment of inflammatory cells such as macrophages, monocytes and neutrophils, which in turn, leads to overwhelming inflammation [11].
As the adaptive immune response sets in, CD8+ cytotoxic T cells play a role in targeting the virus-infected cells while the CD4+ helper T cells stimulate B cells to produce antibodies that specifically target the virus [10]. Studies have shown that a maladaptive immune response, with an increase in innate immune cell lineages and reduction in lymphocytes (lymphopenia) are associated with more severe disease and poorer outcomes [4,5,12]. A delayed type I interferon response during the innate response phase may be the explanation for uninterrupted initial viral replication and delayed priming of the adaptive immune response. Therefore, the viral load becomes too high and severe disease that requires intensive intervention results, which is accompanied by a high risk of intubation and mortality [13].
During the adaptive response phase, CD4+ T cell response appears to be more prominent than the CD8+ T cell response as circulating CD8+ T cells specific to SAR-CoV-2 were detected in 70% of convalescent patients whereas circulating CD4+ T cells were detected in 100% of patients. CD4+ T cells mainly respond to S protein, whereas the N, S, and M proteins are each responsible for 11% to 27% of the total CD4+ response [14]. Tan et al demonstrated that an early induction of T cell immunity against SARS-CoV-2 was associated with viral clearance and a mild disease [15]. As for B cell immunity, many patients infected with SARS-CoV-2 were found to be seroconverted within 5 to 15 days and a 90% seroconversion was detected by the 10th day post symptom onset. The S protein is the main target of the neutralizing antibodies, with > 90% of them targeting the RBD of the S protein [16], although other antigens are also targeted. This partly explains why the development of many vaccines revolves around S protein. The normal innate and adaptive responses in viral infections are summarized in Figure 1C.
Cytokine storm in COVID-19
Although many infected patients are asymptomatic or having only mild to moderate symptoms, a small minority of patients have severe to life-threatening disease [17]. This is because the effects of the overactive immune response are more destructive than that of the infection itself, which result in massive and irreversible damage to the organs. This overwhelming immune response in COVID-19 has been linked to what is termed as a cytokine storm.
In general, cytokines are small proteins that are released by cells, especially immune cells that regulate the immune response to diseases or inflammation. They include a broad array of chemicals e.g. chemokines (which play a role in chemotactic activities), lymphokines (cytokines released by the lymphocytes), interleukins (IL) (cytokines released by white blood cells that act on other white blood cells), and monokines (cytokines released by the monocytes). Other examples of cytokines include tumour necrosis factors (TNF) and interferons (IFN). Cytokines may act on the cells that secret them (autocrine effect), neighboring cells (paracrine effect) or cells that are distant from the site of secretion (endocrine effect). Some cytokines are pro-inflammatory while others are anti-inflammatory [18].
A cytokine storm (CS) refers to uncontrolled and massive cytokine release (or hypercytokaemia) by the innate immune system in the presence of infectious or non-infectious stimuli. Although the concept of a cytokine storm may have started before the coining of the term, the first mentioned of term cytokine storm can be dated back to 1993, in an article on graft-versus-host disease [19]. Cytokine storms are not uncommon in viral infections caused by MERS-CoV, SARS-CoV, influenza, and other viruses [20,21]. Impaired and delayed viral clearance, delayed type I interferon response, increased neutrophil extracellular traps (NETS) and pyroptosis are some proposed underlying mechanisms for CS in COVID-19 [22].
On the other hand, cytokine storm syndrome (CSS) encompasses a diverse set of conditions and were formerly known as familial or primary hemophagocytic lymphohistiocytosis (HLH) and secondary HLH. Other terms under the umbrella of CSS include macrophage activation syndrome (MAS), cytokine release syndrome (CRS), malignancy-associated haemophagocytic syndrome (MAHS), infection-associated haemophagocytic syndrome (IAHS) and cytokine storm (CS) [23].
Research has shown that CS contributes to hyperinflammation in the lungs and results in acute respiratory distress syndrome (ARDS). Postmortem findings of lungs affected by ARDS in COVID-19 demonstrated diffuse alveolar damage, bilateral interstitial mononuclear inflammatory infiltrates with a dominance of lymphocytes, accompanied by reduced peripheral blood levels of CD8+ and CD4+ T cells, as well as increased levels of proinflammatory Th17 helper cells [24]. Minimally invasive autopsies revealed a dominance of macrophages and monocytes in the alveolar infiltrates with moderate multinucleated giant cell infiltration and minimal neutrophil, eosinophil and lymphocyte infiltration. Other findings include increased proliferation of type II alveolar cells, congestion, edema and widening of alveolar septal blood vessels, presence of hyaline thrombi in microvessels, pulmonary interstitial fibrosis and lung tissue focal hemorrhages [25].
CS is also one of the chief causes of multi-organ damage in COVID-19. Other than the lungs, cytokine-induced injuries can occur in the heart, liver, kidney, and other organs of COVID-19 patients [26] whereas pathological changes have been observed in the spleen, heart, kidney, liver, blood vessels during autopsies [25]. If not treated, multi-organ damage may lead to multi-organ failure followed by death. The uncontrolled release of cytokines also causes vascular injury and leads to platelet activation, which partly explains the hypercoagulable states in many COVID-19 patients with severe disease [27]. The underlying mechanisms of cytokine storm and pathogenesis of multi-organ failure are summarized in Figure 2.
Figure 2.
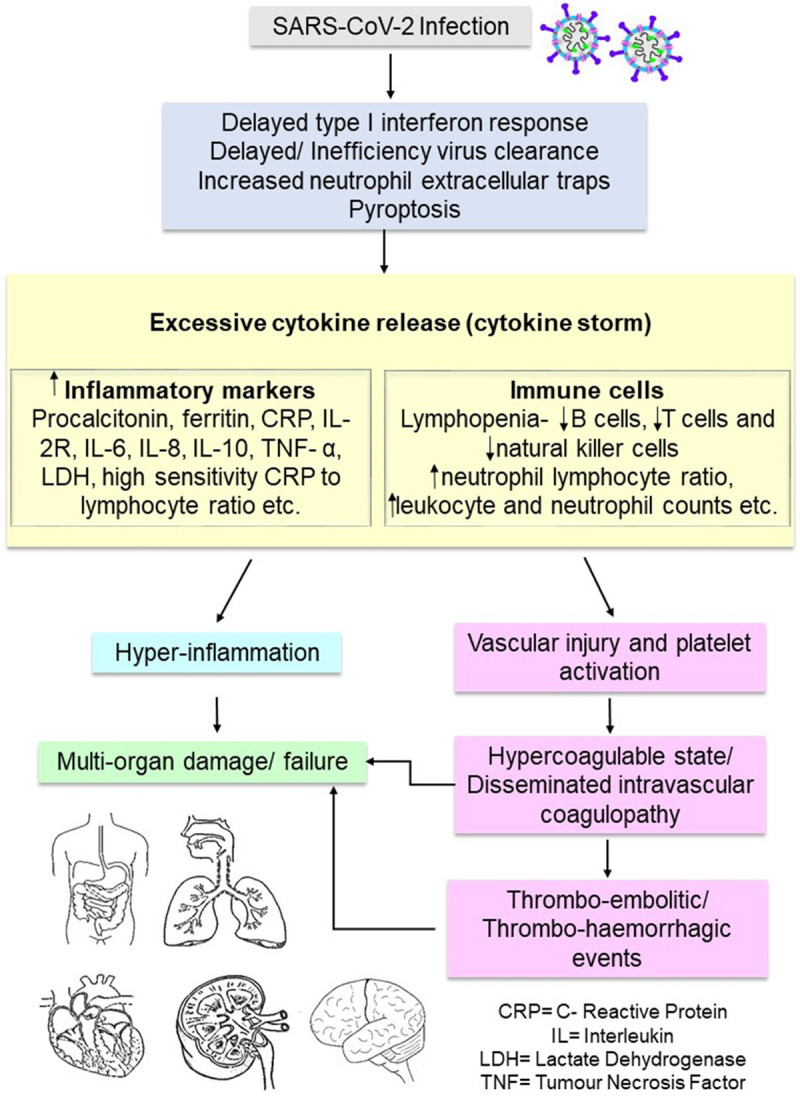
Underlying mechanisms of cytokine storm and pathogenesis of multi-organ failure.
Association between immune cells/inflammatory markers and disease outcome
In general, abnormalities in immune cell counts and marked increase in proinflammatory markers are more frequently seen in patients with severe COVID-19. Qin et al demonstrated that patients with COVID-19 had a lower level of B cells, T cells and natural killer (NK) cells [5]. Comparing the severe and non-severe groups, the former tended to have an even lower level of these cells. On the other hand, the neutrophil lymphocyte ratio (NLR), leukocyte and neutrophil counts were observed to be higher in the former, while a lower percentage of basophils, eosinophils and monocytes was observed. For the T cells, both the suppressor T cells and helper T cells were affected. A couple of infection-related and inflammatory markers were demonstrated to be higher in the severe COVID-19 cases, which included procalcitonin, C-reactive protein (CRP), serum ferritin, interleukin (IL)-2R, IL-6, IL-8, tumor necrosis factor (TNF)-α [5]. Similar findings were also shown in another study by Tan et al in which an overall lower level of B cells, NK cells and T cells (both CD4+ and CD8+ cells) and more marked levels of IL-6, IL-10, and CRP were detected in severe cases of COVID-19 [4].
Severe inflammation is not only associated with disease severity but also disease outcome. In a study with 283 patients with RT-PCR confirmed COVID-19, three inflammatory markers, i.e. serum CRP (86.36% sensitivity; 88.89% specificity), lactate dehydrogenase (LDH) (90.91% sensitivity; 80.56% specificity), and ferritin (95.45% sensitivity; 86.57% specificity) were shown to be useful in predicating mortality [28]. Zeng et al on the hand, showed that an array of inflammatory markers such as serum soluble IL-2 receptor, IL-6, IL-8, IL-10, TNF-α, procalcitonin, ferritin, LDH, high-sensitivity CRP, and high sensitivity CRP to lymphocyte ratio were all elevated in critically ill COVID-19 patients. IL-6 and LDH were independent predictors in disease severity whereas an early decline in inflammatory markers yielded better outcomes. Among the critically ill and deceased patients, no significant decline in inflammatory cytokines and markers was observed throughout the course of disease [29].
Therapeutic strategies that target the immune system and inflammation in COVID-19
While as many as 80% of the patients present with mild to moderate illness and probably do not need any therapy, it is important that treatment is started early and not until the patients are severely ill, especially when adverse outcomes are predicted in some cases. To date, there is no cure for COVID-19. Neither is there a drug that is specifically used for the disease. For patients in respiratory distress, supportive treatment such as oxygen therapy is the mainstay of patient management. Both non-invasive and invasive mechanical ventilation have been used in these patients and intensive care plays a crucial role in severe cases. Researchers have explored therapeutic options such as passive immunity by convalescent plasma transfusion, the use of corticosteroids and other agents to suppress the overwhelming inflammation in COVID-19. This section discusses the various strategies that target the immune system and inflammation in SARS-CoV-2 infection.
Convalescent plasma therapy
Some researchers have suggested that the convalescent plasma of those who have recovered from COVID-19 may be a potential treatment option. In convalescent plasma therapy, the acellular portion of the blood of a recovered individual is transfused to a patient with the infection. There are several possible explanations for why convalescent plasma may work in COVID-19. The antibodies (mainly IgM and IgG) contained in the convalescent plasma exert their antiviral effects by binding to the antigenic parts of the virus, particularly the S protein. These antibodies, together with anti-inflammatory cytokines from healthy donors who have recovered from COVID-19, also exert immunomodulatory effects such as neutralization of cytokines, autoantibodies, and complement and regulation of the hypercoagulable state, all of which are responsible for the heightened inflammation (reviewed by Rojas et al) [30].
It is noteworthy that both convalescent plasma and steroids had been used in the previous SARS-CoV outbreak. Soo et al reported in a retrospective study that patients who received ribavirin-steroid therapy and convalescent plasma (n=19) had a shorter duration of hospitalization (P=0.001) and a lower mortality (P=0.049) compared to patients who received ribavirin and methylprednisolone. Nevertheless, the sample size was small in this study and the fact that the control group received further pulsed methylprednisolone instead of convalescent plasma might have confounded the findings [31].
Joyner et al conducted a study on the safety of convalescent plasma transfusion in 5000 hospitalized patients whose condition was at risk of progressing to severe or life-threatening COVID-19 [32]. There were 36 reported serious adverse events (or < 1% of all transfusions) within the first four hours. Over the first 7 days, 602 mortalities were reported with an overall 7-day mortality rate of 14.9% (95% CI: 13.8%, 16.0%), which was no higher than the case fatality rate reported in the published literature for hospitalized patients (about 15-20%) [33,34] and patients who received intensive unit care (57%) [35]. The study concluded that given the deadly nature of the disease and the enormous number of patients with severe disease and comorbidities, the mortality rate in the study did not appear to be excessive and there was no indication of toxicity beyond the expected toxicity of plasma use in critically-ill patients.
On the other hand, Duan et al transfused 200 ml of convalescent plasma with neutralizing antibody titres of > 1:640 to 10 COVID-19 patients with severe disease, as an additional treatment on top of antiviral drugs and maximal supportive treatment. A median time of 15.5 days from illness onset to transfusion was recorded. In five patients, the neutralizing antibody level rapidly increased up to 1:640 post transfusion whereas four other patients were able to maintain a high level of neutralizing antibodies of 1:640 post transfusion. Within 3 days of transfusion, an improvement of clinical symptoms was observed with an increase in oxyhaemoglobin saturation, accompanied by an increase in lymphocyte count, a reduction in C-reactive protein, and radiologic improvement within 7 days. Seven patients with previous viremia demonstrated undetectable viral load post transfusion and no patient reported severe adverse effects due to transfusion [36].
In a randomized trial in which older adult COVID-19 patients were given either convalescent plasma containing high IgG titers against SARS-CoV-2 or placebo within 72 hours of the onset of mild symptoms, it was reported that 16% (13 out of 80) of patients given convalescent plasma and 31% (25 out of 80) patients given placebo developed severe respiratory disease (P=0.03). When patients (n=6) who reached primary end point prior to infusion of either placebo or convalescent plasma were excluded, a larger effect size was observed, suggesting that COVID-19 progression in mild disease was reduced by high-titer IgG convalescent plasma against SARS-CoV-2. The trial ended early at 76% of its targeted sample size due to reducing confirmed case number of COVID-19 in the trial region [37].
Monoclonal antibodies
Monoclonal antibodies are synthetic proteins that behave like antibodies in the immune system. Depending on how they are produced, they are divided into four main types, namely a) murine monoclonal antibodies (synthesized from mouse proteins with the drug name ending with “-omab”), b) chimeric monoclonal antibodies (synthesized from both mouse and human proteins with the drug name ending with “-imab”), c) humanized (synthesized from a small part of mouse protein, attaching to a human protein, with the drug name ending with “zumab”) and human (synthesized from purely human proteins, with the drug name ending with “-umab”) (https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/monoclonal-antibodies.html).
Chen et al investigated the effects of LY-CoV555 (bamlavinimab), a neutralizing antibody in a single intravenous (IV) infusion (using either 700 mg, 2800 mg or 7000 mg) versus placebo in 452 patients with mild or moderate COVID-19. The primary outcome assessed was the change of viral load from baseline at day 11. Findings of the interim analysis showed a significant difference in log viral load (-0.53) from baseline in the group receiving 2800 mg LYCoV555 and placebo (P=0.02). For the group receiving 700 mg and 7000 mg LYCoV555 versus placebo, the differences were -0.20 (P=0.38) and 0.09 (P=0.70) respectively. Less severe symptoms were observed in patients receiving LYCoV555 than those receiving placebo whereas less patients in the LYCoV555 group (1.6%) required hospitalization compared to the placebo group (6.3%). The study concluded that 2800 mg IV infusion of LYCoV555 appeared to speed up the natural decrease in viral load in mild or moderate COVID-19 cases [38].
Similar findings were reported by Weinreich et al in a study that investigated the effects of a neutralizing antibody cocktail, REGN-COV2 (consisting of imdevimab and casirivimab) on viral load in COVID-19 patients who were not hospitalized. The percentage of patients who required at least one medically-related visit among patients in the treatment group and placebo group was also analyzed. REGN-COV2 was demonstrated to lower viral load especially in patients with a high baseline viral load whereas the percentage of those required at least one medically-related visit was 6% for the placebo group and 3% for the treatment group [39]. Notably, the Food and Drug Administration (FDA) has authorized bamlanivimab and combination of casirivimab and imdevimab for emergency use in mild to moderate COVID-19 [40].
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial explored the efficacy and safety of tocilizumab, which is a monoclonal antibody used in treating rheumatoid arthritis. It binds to the IL-6 receptor and reduces inflammation. The clinical outcomes evaluated included mortality and need of invasive mechanical ventilation. Altogether, 4116 adult patients were enrolled in the trial, with 2022 patients treated with usual care plus tocilizumab and 2094 treated with usual care alone. It was reported that 31% (n=621) of the patients in the tocilizumab group and 35% (n=729) of the patients in the control group died within a period of 28 days (P=0.007). A significant mortality benefit was observed, especially in patients given systemic corticosteroids. A statistically significant difference in the likelihood of being discharged alive from the hospital between the tocilizumab group (57%, n=1150) and the control group (50%, n=1104; P < 0.0001) was reported. The patients in the tocilizumab group (35%, n=619, out of 1754) were also significantly less likely to require invasive mechanical ventilation or die when compared to the control group (42%, n=754, out of 1800; P < 0.0001). The study concluded that tocilizumab was beneficial in improving survival and clinical outcome, in addition to the beneficial effects of systemic corticosteroids [41].
Corticosteoirds
Research on corticosteroid administration in COVID-19 patients has yielded contradicting findings. In one study that investigated the effects of a short course of methylprednisolone given early in moderate to severe cases of COVID-19, a significantly shorter duration of median hospital stay was reported in patients who received methylprednisolone (5 days) when compared to those in the control group (8 days; P < 0.001) [42] whereas another study showed that corticosteroid treatment was not associated with virus clearance time, duration of hospital stay or symptom duration [43]. Yang et al conducted a meta-analysis using 15 studies with a total of 5270 patients infected with coronavirus (including MERS-CoV, SARS-CoV and SARS-CoV-2) and revealed that corticosteroids therapy is more likely to be used in critical patients and that its use is associated with a higher mortality, longer duration of hospitalization, and higher rate of bacterial infection [44].
A randomized trial on the use of inhaled budesonide in 146 COVID-19 patients recently has its data published. Patients with mild COVID-19 within 7 days of symptom onset were either assigned inhaled budesonide or usual care. The primary end point included emergency visit, assessment or hospitalization. With the budesonide group, one participant reached the primary outcome whereas the usual care group had 10 patients reaching the primary end point (P=0.004). The clinical recovery time for the budesonide group was significantly shorter than the usual care group (7 days vs 8 days; P=0.007). At day 14 and day 28, the proportion of those with fever or persistent symptoms was lower in the budesonide group. Therefore, the study concluded that inhaled budesonide decreased the probability of the need for emergency care and recovery time in patients in early COVID-19 [45].
The REMAP-CAP trial consisted of several arms (e.g. corticosteroids, antiviral drugs or immunoglobulin). There were 614 patients with COVID-19 (either suspected or confirmed) enrolled into at least one arm of the trial. For the corticosteroid arm, there were 137 patients randomized to a 7-day fixed-dose intravenous hydrocortisone, 146 patients to a shock-dependent course of hydrocortisone and 101 patients to no hydrocortisone (after excluding patients who withdrew). Within a timeframe of 21 days, the fixed-dose group and shock-dependent dose group gave a 93% and 80% probability of superiority respectively, in terms of the odds of improvement in the number of days that were organ-support free. The results were inconclusive as the trial was terminated early without any treatment meeting any of the predetermined statistical superiority criteria [46].
Non-steroidal anti-inflammatory drugs (NSAIDs)
Numerous studies have investigated the acute or chronic use of NSAIDs in COVID-19. Thus far, there is no evidence to suggest NSAIDs are related to less favourable clinical outcomes in COVID-19. A study on the use of NSAIDs among patients with COVID-19 demonstrated no increased mortality risk associated with acute ibuprofen use (n=40) of chronic NSAID use (n=96) when compared to non-NSAID users (n=357). Acute ibuprofen or chronic NSAID users also did not demonstrate a significant higher risk of hospitalization, severe COVID-19 or oxygen support compared to non-NSAID users [47]. Another study reported similar findings and suggested that ibuprofen use did no worsen clinical outcomes in COVID-19 with regards to mortality and the need for oxygen support [48]. Wong et al (2020) reported that there was no statistical difference in COVID-19 related death risk between current -and non-users of NSAIDs in the general population or patient with osteoarthritis or rheumatoid arthritis [49].
Other treatment strategies
Mesenchymal stem cells (MSCs) are known for their immunomodulatory properties. MSCs have been used in COVID-19 patients and demonstrated some beneficial effects. In China, intravenous injection of ACE2-negative and TMPRSS2-negative MSCs in seven patients with severe COVID-19 has demonstrated improvement in pulmonary symptoms and functions. An increase in peripheral lymphocyte count, a decline in proinflammatory markers and overactivated immune cells, as well as an increase in anti-inflammatory markers were reported in patients receiving MSC treatment in comparison with the placebo group [50]. On the other hand, human umbilical cord mesenchymal stem cells (hUM-MSCs) were given three times at three-day intervals to a 65-year-old severely ill COVID-19 patient. Laboratory- and CT-evident remission of inflammation was observed with good tolerance of the hUC-MSCs [51]. However, much is still unknown about the role of MSC treatment in COVID-19 and it should be used with caution under investigational situation. Table 1 gives a summary of the key findings regarding treatment strategies that target the immune system and inflammation in COVID-19.
Table 1.
Summary of key findings on therapeutic strategies targeting the immune system and inflammation in COVID-19
| Therapeutic option | Key findings | Reference |
|---|---|---|
| Convalescent plasma therapy | Mortality rate did not appear to be excessive and no indication of toxicity beyond the expected toxicity of plasma use in critically-ill patients. | [32] |
| Convalescent plasma therapy | Improvement of clinical symptoms and viral load in severe cases of COVID-19. | [36] |
| Convalescent plasma therapy | COVID-19 progression in mild disease was reduced by high-titer IgG convalescent plasma against SARS-CoV-2. | [37] |
| Bamlavinimab (LY-CoV555) | IV infusion of single dose of 2800 mg LYCoV555 appeared to speed up the natural decrease in viral load in patients with mild or moderate COVID-19. | [38] |
| REGN-COV2 (combination of casirivimab and imdevimab) | Reduced viral load especially in patients with a high baseline viral load. | [39] |
| Tocilizumab | Beneficial in improving survival and clinical outcome, in addition to the beneficial effects of systemic corticosteroids (RECOVERY trial). | [41] |
| Methylprednisolone | A significantly shorter median hospital stay compared to control group. | [42] |
| Corticosteroids | Not associated with virus clearance time, duration of hospital stay, or symptom duration. | [43] |
| Corticosteroids | More likely to be used in critical patients. | [44] |
| Its use was associated with a higher mortality, longer duration of hospitalization and higher rate of bacterial infection. | ||
| Inhaled budesonide | Decreased the probability of the need for emergency care and recovery time in patients in early COVID-19. | [45] |
| Intravenous hydrocortisone | Results were inconclusive. | [46] |
| Trial was terminated early without any treatment meeting any of the prespecified criteria for statistical superiority (REMAP-CAP trial). | ||
| Non-steroidal anti-inflammatory drugs (NSAIDs) | No significant higher risk of mortality, hospitalization, severe COVID-19 or oxygen support was demonstrated among acute ibuprofen and chronic NSAID users when compared to non-NSAID users. | [47] |
| Ibuprofen | Use of ibuprofen did not worsen clinical outcomes in COVID-19 patients in terms of mortality and need for oxygen support. | [48] |
| NSAIDs | No difference in COVID-19 related death risk between current- and non-users of NSAIDs in general population and patients with osteoarthritis or rheumatoid arthritis. | [49] |
| Mesenchymal stem cell (MSC) injection | Improvement of pulmonary symptoms and functions, reduction in proinflammatory markers and overactivated immune cells and increase in anti-inflammatory markers in seven COVD-19 patients given IV MSC injection. | [50] |
| Human umbilical cord MSCs | Laboratory- and CT-evident remission of inflammation in a case of severe COVID-19. | [51] |
Conclusions
Given the high number of reported cases and deaths on a daily basis, there is a desperate need to come up with effective treatment strategies for patients with COVID-19. Although supportive and intensive care play a crucial role, especially in severe cases, it is important that the overwhelming inflammation associated with severe and critical cases is addressed, as there are no specific or curative drugs for COVID-19 thus far. The therapeutic options that target inflammation and the immune system discussed in this review are some of the many examples of strategies that have been investigated and used for the treatment of COVID-19. While some of these strategies have shown promising results, others have been found to be ineffective.
In a study that aimed to examine the potential drugs for COVID-19 through large-scale compound repurposing, nearly 12,000 clinical-stage or FDA-approved small molecules have been screened. Among these, 100 molecules were identified to exhibit viral replication inhibition and 21 out the 100 molecules are known drugs demonstrating dose-response relationships [52]. These findings suggest that there is still much room for further research into pharmacologic treatment for COVID-19, amidst the race to break the chain of transmission through the global vaccination campaigns. Having said that, combating inflammation in COVID-19 remains an important aspect of the management of COVID-19. Future research should focus on the underlying mechanisms of these anti-inflammatory strategies used in COVID-19.
Disclosure of conflict of interest
None.
References
- 1.Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12:135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K, Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160:261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MY, Zhao R, Gao LJ, Gao XF, Wang DP, Cao JM. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol. 2020;10:587269. doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albini A, Di Guardo G, Noonan DM, Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern Emerg Med. 2020;15:759–766. doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller SN, Rouse BT. Immune responses to viruses. Clin Immunol. 2008:421–431. [Google Scholar]
- 11.Mishra KP, Singh AK, Singh SB. Hyperinflammation and immune response generation in COVID-19. Neuroimmunomodulation. 2020;27:80–86. doi: 10.1159/000513198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A Yale IMPACT Team. Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magleby R, Westblade LF, Trzebucki A, Simon MS, Rajan M, Park J, Goyal P, Safford MM, Satlin MJ. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. 2020:ciaa851. doi: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, Sette A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, Zhuang Y, Tham CYL, Chia A, Smith GJD, Young B, Kalimuddin S, Low JGH, Lye D, Wang LF, Bertoletti A. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34:108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong RSY. The SARS-CoV-2 outbreak: an epidemiological and clinical perspective. SN Compr Clin Med. 2020:1–9. doi: 10.1007/s42399-020-00546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrara JL, Abhyankar S, Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25:1216–1217. [PubMed] [Google Scholar]
- 20.Wong JP, Viswanathan S, Wang M, Sun LQ, Clark GC, D’Elia RV. Current and future developments in the treatment of virus-induced hypercytokinemia. Future Med Chem. 2017;9:169–178. doi: 10.4155/fmc-2016-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yongzhi X. COVID-19-associated cytokine storm syndrome and diagnostic principles: an old and new Issue. Emerg Microbes Infect. 2021;10:266–276. doi: 10.1080/22221751.2021.1884503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 26.Mustafa MI, Abdelmoneim AH, Mahmoud EM, Makhawi AM. Cytokine storm in COVID-19 patients, its impact on organs and potential treatment by QTY code-designed detergent-free chemokine receptors. Mediators Inflamm. 2020;2020:8198963. doi: 10.1155/2020/8198963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du F, Liu B, Zhang S. COVID-19: the role of excessive cytokine release and potential ACE2 down-regulation in promoting hypercoagulable state associated with severe illness. J Thromb Thrombolysis. 2021;51:313–329. doi: 10.1007/s11239-020-02224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arshad AR, Khan I, Shahzad K, Arshad M, Haider SJ, Aslam MJ. Association of inflammatory markers with mortality in COVID-19 infection. J Coll Physicians Surg Pak. 2020;30:158–163. doi: 10.29271/jcpsp.2020.supp2.S158. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Z, Yu H, Chen H, Qi W, Chen L, Chen G, Yan W, Chen T, Ning Q, Han M, Wu D. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit Care. 2020;24:525. doi: 10.1186/s13054-020-03255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, Rojas-Villarraga A, Ramírez-Santana C, Díaz-Coronado JC, Manrique R, Mantilla RD, Shoenfeld Y, Anaya JM. Convalescent plasma in COVID-19: possible mechanisms of action. Autoimmun Rev. 2020;19:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soo YO, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK, Ng MH, Chan P, Cheng G, Sung JJ. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–8. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, Wiggins CC, Senefeld JW, Klompas AM, Hodge DO, Shepherd JRA, Rea RF, Whelan ER, Clayburn AJ, Spiegel MR, Baker SE, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Herasevich V, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, van Buskirk CM, Winters JL, Stubbs JR, van Helmond N, Butterfield BP, Sexton MA, Diaz Soto JC, Paneth NS, Verdun NC, Marks P, Casadevall A, Fairweather D, Carter RE, Wright RS. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95:1888–97. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, Zhang Z, Yu M, Tao Y, Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020;46:1472–1474. doi: 10.1007/s00134-020-06047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW the Northwell COVID-19 Research Consortium. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y, Fernández Viña V, Álvarez Paggi D, Esperante S, Ferreti A, Ofman G, Ciganda Á, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A, Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, López Castelo R, Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D, Aranda SS, Sánchez Yanotti C, De Luca J, Jares Baglivo S, Laudanno S, Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos J, González M, Perez E, Kreplak N, Pastor Argüello S, Gibbons L, Althabe F, Bergel E, Polack FP Fundación INFANT-COVID-19 Group. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen MS. Monoclonal antibodies to disrupt progression of early Covid-19 infection. N Engl J Med. 2021;384:289–291. doi: 10.1056/NEJMe2034495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, Miller J, Kenney RM, Alangaden G, Ramesh MS Henry Ford COVID-19 Management Task Force. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020;71:2114–2120. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zha L, Li S, Pan L, Tefsen B, Li Y, French N, Chen L, Yang G, Villanueva EV. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81:e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramakrishnan S, Nicolau DV Jr, Langford B, Mahdi M, Jeffers H, Mwasuku C, Krassowska K, Fox R, Binnian I, Glover V, Bright S, Butler C, Cane JL, Halner A, Matthews PC, Donnelly LE, Simpson JL, Baker JR, Fadai NT, Peterson S, Bengtsson T, Barnes PJ, Russell REK, Bafadhel M. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The Writing Committee for the REMAP-CAP Investigators. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu Esba LC, Alqahtani RA, Thomas A, Shamas N, Alswaidan L, Mardawi G. Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study. Infect Dis Ther. 2021;10:253–268. doi: 10.1007/s40121-020-00363-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26:1259.e5–1259.e7. doi: 10.1016/j.cmi.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong AY, MacKenna B, Morton CE, Schultze A, Walker AJ, Bhaskaran K, Brown JP, Rentsch CT, Williamson E, Drysdale H, Croker R, Bacon S, Hulme W, Bates C, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, Tomlinson L, Mathur R, Wing K, Forbes H, Eggo RM, Parry J, Hester F, Harper S, Evans SJ, Smeeth L, Douglas IJ, Goldacre B OpenSAFELY Collaborative. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis. 2021;80:943–951. doi: 10.1136/annrheumdis-2020-219517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, Xiao M, Nie P, Gao Y, Qian C, Hu M. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine (Baltimore) 2020;99:e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riva L, Yuan S, Yin X, Martin-Sancho L, Matsunaga N, Pache L, Burgstaller-Muehlbacher S, De Jesus PD, Teriete P, Hull MV, Chang MW, Chan JF, Cao J, Poon VK, Herbert KM, Cheng K, Nguyen TH, Rubanov A, Pu Y, Nguyen C, Choi A, Rathnasinghe R, Schotsaert M, Miorin L, Dejosez M, Zwaka TP, Sit KY, Martinez-Sobrido L, Liu WC, White KM, Chapman ME, Lendy EK, Glynne RJ, Albrecht R, Ruppin E, Mesecar AD, Johnson JR, Benner C, Sun R, Schultz PG, Su AI, García-Sastre A, Chatterjee AK, Yuen KY, Chanda SK. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


